1. Introduction
Adenocarcinoma is the most common subtype of non-small cell lung cancer (NSCLC), representing approximately 40% of all NSCLC lung cancer cases [
1]. Stage 0 disease, adenocarcnoma in situ (AIS), is characterized by lesions less than 3 cm with a non-invasive lepidic growth pattern [
2]. It is typically detected on imaging as a ground-glass nodule (GGN) and tends to grow slowly and not immediately suggestive of malignancy. Accordingly, current guidelines for managing GGNs recommend routine follow-up with low-dose CT scans every 1-2 years for up to five years after initial detection, making surgical resection of GGNs an unnecessarily invasive treatment in many cases [
3].
Still, stage 0 AIS of the lung has consistently been reported to achieve a 100% five-year overall survival (OS) rate following surgical resection, irrespective of surgical approach [
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15]. However, a vast majority of these findings stem from studies conducted in Asia, which limits their generalizability to populations in the United States. Further, in the last five years, only a few small studies from the United States [
10], Europe [
12], and Australia [
14] have addressed OS outcomes for stage 0 AIS patients undergoing surgical resection. Moreover, there are no data to demonstrate that surgical intervention is needed to achieve satisfactory long-term survival rates. To address this gap in evidence, we aimed to identify the most effective treatment modality for stage 0 AIS of the lung in the United States by comparing the five-year OS rates of patients who underwent surgical treatment to those who received routine surveillance and no treatment.
2. Materials and Methods
2.1. Ethics Statement
This study used publicly available, de-identified patient information. Therefore, our analysis is not considered to involve human subjects, constitute as human subject research, and is exempt from Institutional Review Board approval.
2.2. Data Source
For this study, we accessed data collected from the National Cancer Database (NCDB), a data source managed by the American College of Surgeons (ACS), Commission on Cancer (CoC) and the American Cancer Society. The NCDB captures approximately 70% of all newly diagnosed lung cancer cases in the United States and Puerto Rico, gathering data from more than 1,500 cancer centers and containing more than 30 million patient records [
16]. Disease subtype and staging data are directly recorded in the NCDB using the American Joint Committee on Cancer 8th edition TNM classifications for our given study period. International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) histology and topography codes were used for data extraction. Patients who underwent surgical resection were identified using surgical procedure of the primary site codes. Research manuscripts reporting large datasets that are deposited in a publicly available database should specify where the data have been deposited and provide the relevant accession numbers. If the accession numbers have not yet been obtained at the time of submission, please state that they will be provided during review. They must be provided prior to publication.
2.3. Study Design
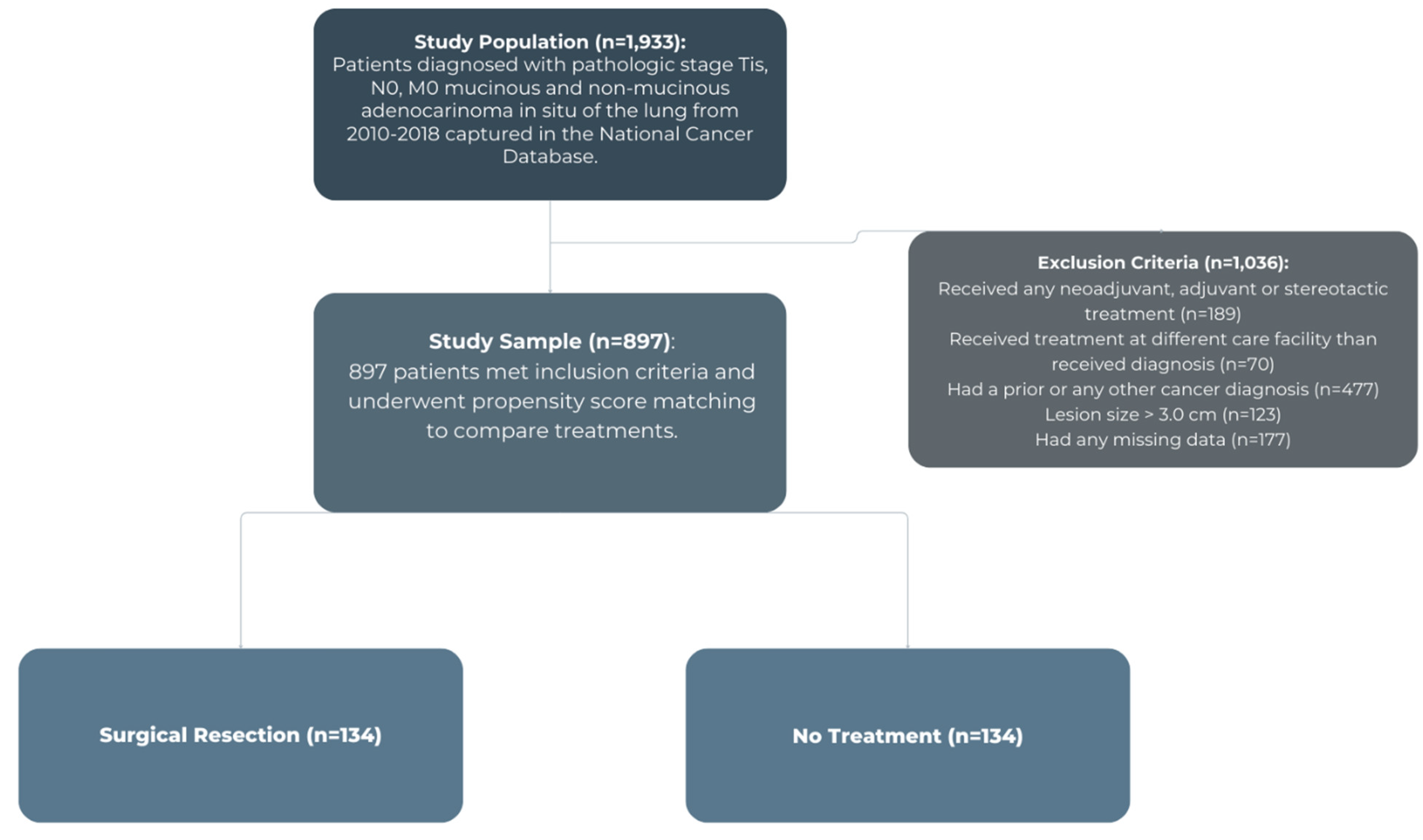
Data were extracted from all patients diagnosed with stage 0 (TIS, N0, M0) AIS from 2010 to 2018. Patients were excluded if they received any neoadjuvant or adjuvant treatment, stereotactic body radiotherapy, had missing data, a prior cancer diagnosis, or a concomitant cancer diagnosis during the study period. Complete TNM staging according to the 8th addition was initially included in data analysis, but all patients were found to be N0 and M0 and thus only tumor size was kept in the final analysis. Patients were divided into two groups: patients who did not receive surgical treatment or any adjuvant therapy and those who underwent surgical resection and did not receive any adjuvant therapy.
The primary outcome was overall survival at five years, assessed from the time of diagnosis to the time of death or last known follow-up. Cox proportional hazards modeling and propensity score-matched analysis were used to evaluate OS across the two treatment groups. Covariates associated with patients undergoing surgery were evaluated using multivariable logistic regression. Survival outcomes based on surgical approach were evaluated in a propensity score-matched subgroup analysis that compared wedge resection against segmentectomy and wedge resection against lobectomy.
2.4. Statistical Analysis
Patients diagnosed with stage 0 (TIS, N0, M0) with lesions no greater than 3 cm that were classified as mucinous or non-mucinous adenocarcinoma in situ on histology from 2010 to 2018 were grouped based on treatment modality: surgical resection versus no treatment, as shown in
Figure 1. Baseline characteristics and unadjusted outcomes were analyzed using Pearson’s chi-square test for categorical variables and Wilcoxon Rank Sum test for continuous variables. Covariates associated with surgical treatment were assessed using a multivariable logistic regression model that included: age, sex, race, insurance status, income, education, facility type, distance from facility, year of diagnosis, Charlson Deyo Comorbidity (CDCC) score, and tumor size. An adjusted multivariable Cox proportional hazards model was then used to assess differences in overall survival between the two groups.
An adjusted propensity score-matched analysis of those undergoing surgical resection and those not receiving surgery was then performed. Propensity scores were developed, defined as the probability of patients who did not receive any treatment and those who underwent surgical resection for stage 0 AIS, conditional on sociodemographic and prognostic variables. Covariates in our propensity score-matched analysis included: age, sex, race, insurance status, income, education, facility type, distance from facility, year of diagnosis, CDCC score, and tumor size. Covariates were determined a priori to be clinically relevant. Propensity score-matching was used over inverse probability weighting to better estimate the average treatment effect for patients.
Using a greedy 1:1 matching scheme without replacement and a radius-matching caliper of 0.01 the most appropriately matched pairs were identified. After matching, balance was assessed using absolute standardized differences. Following propensity-score matching, Kaplan–Meier analysis was used to assess overall long-term survival of the two groups. Overall survival was measured from the time of diagnosis to the time of death or last known follow-up. This model was then applied to a secondary subgroup analysis that assessed overall survival within the surgical cohort across different surgical approaches (wedge resection versus segmentectomy and wedge resection versus lobectomy). Model balance and diagnostics were evaluated with no violation of major assumptions identified. For all comparisons, a two-sided p-value of 0.05 was used to define statistical significance. Statistical analysis was performed using Stata/MP software, version 13.1 for Mac (StataCorp, College Station, TX).
3. Results
3.1. Predictors of Surgical Intervention and Overall Survival
A total of 716 (79.8%) patients of 897 patients who met study inclusion criteria underwent surgical resection for stage 0 AIS from 2010-2018. Baseline patient characteristics by treatment modality are represented in
Table 1. In univariate analysis, patients undergoing surgical resection were more likely to be younger, female, and have smaller tumors. No significant differences were seen across race, income, education, mean distance from the treating facility, or comorbidity score.
Multivariable analysis evaluating independent predictors of patients with stage 0 AIS to receive surgery are presented in
Table 2. Females and younger patients were more likely to receive surgery and those with larger tumors were less likely to receive surgery. Factors associated with survival among patients with stage 0 AIS who received surgery are also shown in
Table 2. Decreased age and female sex were associated with a survival benefit, while black race was associated with an increased risk of mortality compared to white race. Among the entire cohort with stage 0 AIS multivariable Cox proportional hazards analysis revealed that surgery was associated with improved overall survival (0.25; CI: 0.16-0.40, p <0.001).
3.2. Propensity Score-Matched Analysis
Propensity-score matching was performed to create two groups of 134 patients diagnosed with AIS who either underwent surgical resection or did not receive surgery. Both groups were well-balanced after propensity-score matching as shown in
Table 3. All absolute standardized mean differences were less than or equal to 10.2.
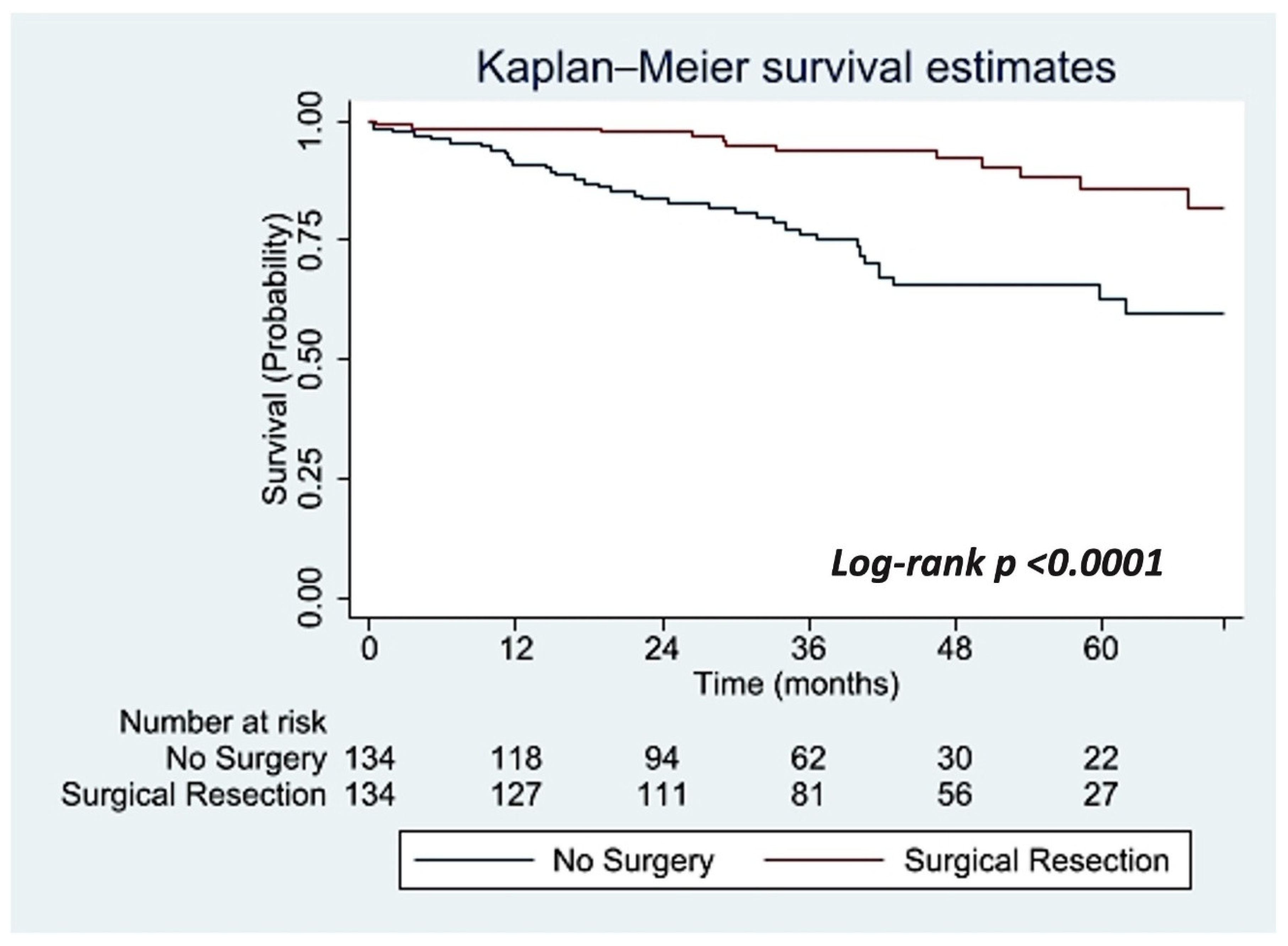
Overall survival was significantly improved at 24-, 36-, 48-, and 60-months in the surgical group compared to the no treatment group. OS at 24-months in the surgical group was 97.7% (95% CI: 92.9-99.2%) compared to the no treatment group at 83.6% (95% CI: 75.9-88.9%). OS at 36-months in the surgical group was 93.8% (95% CI: 87.3-97.0%) compared to the no treatment group at 76.3% (95% CI: 67.3-83.1%). OS at 48-months in the surgical group was 92.2% (95% CI: 84.6-96.1%) compared to the no treatment group at 65.7% (95% CI:54.7-74.6%). Most notably, overall survival at five years was significantly improved in the surgical group at 85.8% (95% CI: 74.2-92.4%) compared to the no treatment group at 62.8% (95% CI: 50.1-72.7%) (log-rank, p <0.0001) as is illustrated in
Figure 2.
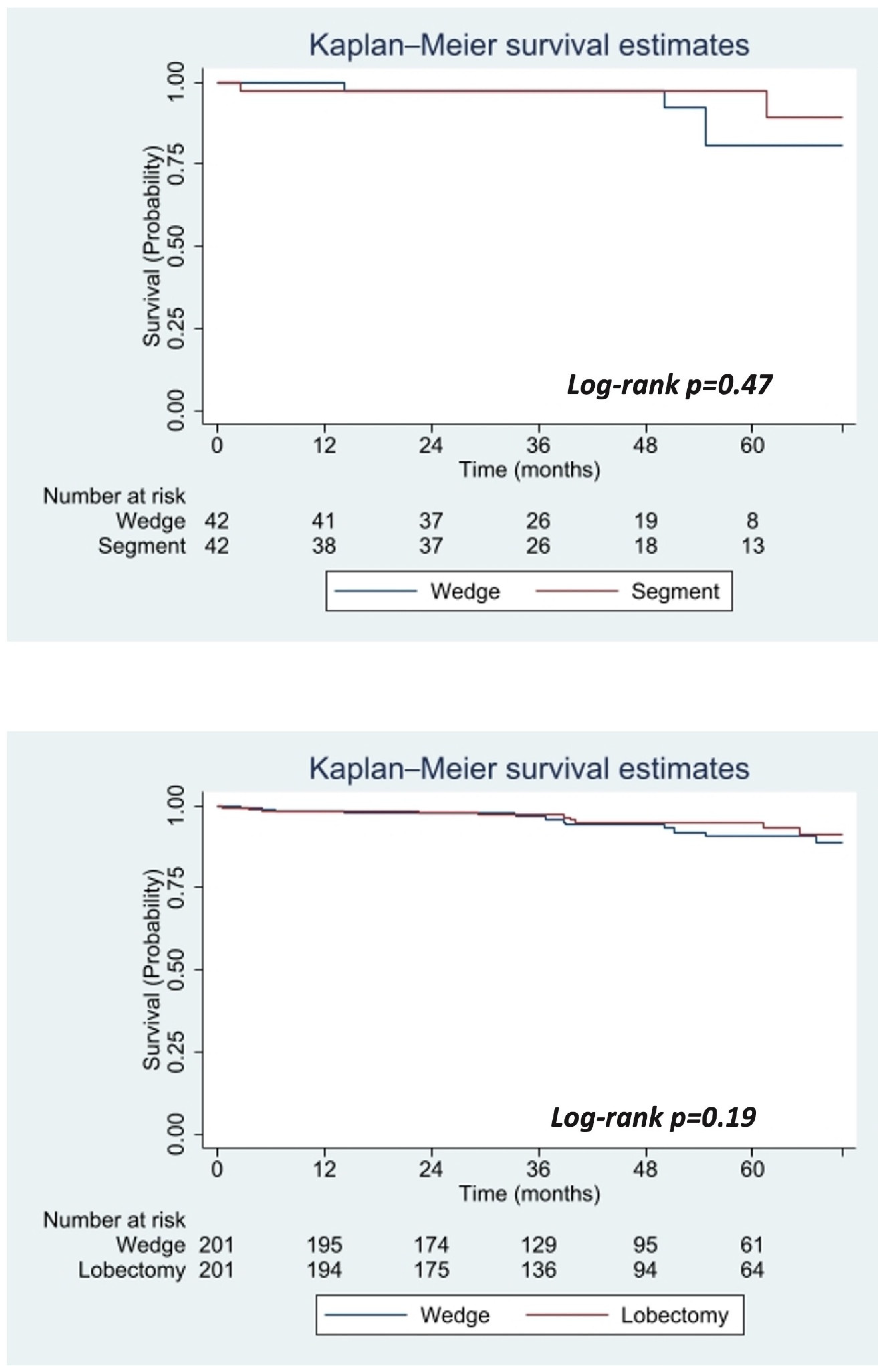
Two additional propensity score-matched analyses were performed among stage 0 AIS patients who received surgery to detect a survival benefit across different surgical approaches as demonstrated in
Figure 3. In this subgroup analysis patients who underwent a wedge resection were compared to those who received a segmentectomy and against those who underwent a lobectomy. Among two groups of 42 patients with stage 0 AIS who underwent surgical resection there was no significant difference in OS at five years between those who underwent a wedge resection 80.1% (95% CI: 54.7-92.6%) compared to a segmentectomy 97.6% (95% CI: 83.9-99.7%) (log-rank, p=0.47). Further, among two groups of 201 stage 0 AIS patients who underwent surgical resection there was no significant difference in OS at five-years between those who underwent a wedge resection 90.8% (95% CI: 83.8-94.8) compared to a lobectomy 94.9% (95% CI: 89.9-97.4%) (log-rank, p=0.19).
4. Discussion
This study compared OS among patients with stage 0 AIS of the lung who received surgical treatment to those who received no treatment and found that OS was significantly improved at 24-, 36-, 48-, and 60-months in the group who received surgery. No significant differences in OS were found across varying surgical approaches: wedge resection versus segmentectomy and wedge resection versus lobectomy for stage 0 AIS of the lung. Further, comorbidity score was not found to be a significant predictor of OS for patients who underwent surgery.
These findings suggest that surgical resection improves OS in stage 0 AIS of the lung and are consistent with other international studies that indicate a survival benefit associated surgical resection for stage 0 AIS, regardless of surgical approach [
4,
5,
7,
12,
14]. This study contributes to the existing literature by demonstrating a survival benefit in stage 0 AIS patients when directly compared to patient who underwent surveillance and received no treatment.
Still, there are several notable limitations of this study. While we aimed to reduce bias and confounding by performing a multivariable prediction model for surgical intervention and survival, this study is retrospective and still has the potential for confounding and selection bias. There is also a possibility that patients in the nonsurgical group had missed invasive disease and incorrectly staged with AIS of the lung. That is, patients who underwent surgery likely had a more comprehensive pathologic evaluation of their tumor compared to those who did not receive surgery, thus there is a higher potential for nonsurgical patients to have missed invasive disease. However, we attempted to reduce this likelihood by excluding all patients who received any cancer diagnosis during the period of the study, as it would be unlikely that nonsurgical patients diagnosed with stage 0 AIS of the lung would have an unrecognized invasive component or missed upstaging long-term.
Additionally, it is important to recognize that nonsurgical patients with stage 0 AIS of the lung may be underreported in the NCDB, especially in cases where patients are diagnosed by interventional pulmonologists rather than surgeons, as the NCDB is primarily directed for use among surgeons. Other limitations of the NCDB include lack of data on pulmonary function testing, smoking, frailty, cancer recurrence, and the use of a comorbidity index rather than providing individual comorbidities.
This study was limited by small sample sizes as this clinical situation, while becoming more common, is still fairly unique. This is especially notable for the surgical subgroup that underwent segmentectomy versus wedge resection for stage 0 AIS, which showed no difference in treatment modalities. However, we note that a difference may be appreciated if analyzed under a larger sample size. Lastly, the follow-up period in this study was relatively short for an indolent disease and additional studies with longer follow-up periods would add to the evidence presented in this study.
Still, this study is the first of its kind to shed light on survival outcomes based on treatment modality for Stage 0 AIS of the lung in a United States population. Accordingly, this data may be more appropriately generalized to the U.S. patient population when compared to the current literature. Our findings support long-term survival benefits for patients with Stage 0 AIS of the lung who undergo surgical resection compared to those who do not receive treatment. These results also demonstrate the ability to achieve long-term survival benefits by performing a wedge resection when compared to more aggressive operations such as a lobectomy for these patients.
5. Conclusion
In this national analysis of patients with stage 0 AIS of the lung, surgery was associated with improved OS at 24-, 36-, 48-, and 60-months compared to those who underwent routine surveillance and received no treatment. While surgical treatment for stage 0 AIS has been increasing, it is still not a widely adopted practice. This study supports surgeons performing surgical resections for stage 0 AIS patients of the lung and suggests that a wedge resection achieves similar long-term survival benefits when compared to a lobectomy among this population.
Funding
This research received no external funding.
Institutional Review Board Statement
This study used publicly available, de-identified patient information. Therefore, our analysis is not considered to involve human subjects, constitute as human subject research, and is exempt from Institutional Review Board approval.
Data Availability Statement
The data underlying this article are available from the National Cancer Database. The datasets were derived from sources in the public domain using deidentified Participant User Data Profiles that are compliant with the Health Insurance and Portability and Accountability Act and submitted to the Commission on Cancer’s National Cancer Database and can be found at:
https://www.facs.org/quality-programs/cancer-programs/national-cancer-database/
Conflicts of Interest
The authors declare that they have no known conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ACS: |
American College of Surgeons |
| AIS: |
Adenocarcinoma in Situ |
| CDCC: |
Charlson Deyo Comorbidity |
| CoC: |
Commission on Cancer |
| GGN: |
Ground-Glass Nodule |
| ICD-O-3: |
International Classification of Diseases for Oncology, 3rd edition |
| NCDB: |
National Cancer Database |
| NSCLC: |
Non-Small Cell Lung Cancer |
| OS: |
Overall Survival |
References
- Du, J. , et al., The novel circular RNA circ-CAMK2A enhances lung adenocarcinoma metastasis by regulating the miR-615-5p/fibronectin 1 pathway. Cell Mol Biol Lett. 2019.
- Travis, W.D. , et al., International association for the study of lung cancer/American thoracic society/European respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol, p: 6(2).
- MacMahon, H. , et al., Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology, p: 284(1).
- Liu, S. , et al., Precise Diagnosis of Intraoperative Frozen Section Is an Effective Method to Guide Resection Strategy for Peripheral Small-Sized Lung Adenocarcinoma. J Clin Oncol.
- Ito, M. , et al., Prediction for prognosis of resected pT1a-1bN0M0 adenocarcinoma based on tumor size and histological status: relationship of TNM and IASLC/ATS/ERS classifications. Lung Cancer.
- Ishida, H. , et al., Distinctive clinicopathological features of adenocarcinoma in situ and minimally invasive adenocarcinoma of the lung: A retrospective study. Lung Cancer.
- Behera, M. , et al., Lung Adenocarcinoma Staging Using the 2011 IASLC/ATS/ERS Classification: A Pooled Analysis of Adenocarcinoma in Situ and Minimally Invasive Adenocarcinoma. Clin Lung Cancer.
- Zhang, Y. , et al., Surgery for pre- and minimally invasive lung adenocarcinoma. J Thorac Cardiovasc Surg. 2020.
- Chen, C. , et al., Cancer-associated fibroblasts, matrix metalloproteinase-9 and lymphatic vessel density are associated with progression from adenocarcinoma in situ to invasive adenocarcinoma of the lung. Oncol Lett. 2020.
- Miyazawa, T. , et al., PD-L1 Expression in Non-Small-Cell Lung Cancer Including Various Adenocarcinoma Subtypes. Ann Thorac Cardiovasc Surg, p: 25(1).
- Boland, J.M. , et al., Adenocarcinoma in situ, minimally invasive adenocarcinoma, and invasive pulmonary adenocarcinoma--analysis of interobserver agreement, survival, radiographic characteristics, and gross pathology in 296 nodules. Hum Pathol.
- Tsubokawa, N. , et al., Prognostic significance of vascular invasion in intermediate-grade subtype of lung adenocarcinoma. Jpn J Clin Oncol. 1015. [Google Scholar]
- Carretta, A. , et al., Prognostic role of positron emission tomography and computed tomography parameters in stage I lung adenocarcinoma. Radiol Oncol.
- Aokage, K. , et al., Influence of Ground Glass Opacity and the Corresponding Pathological Findings on Survival in Patients with Clinical Stage I Non-Small Cell Lung Cancer. J Thorac Oncol.
- Russell, P.A. , et al., Does lung adenocarcinoma subtype predict patient survival? A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol. 1496. [Google Scholar]
- Bilimoria, K.Y. , et al., The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol, 2008. 15(3): p. 683-90.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).