Submitted:
18 March 2025
Posted:
19 March 2025
You are already at the latest version
Abstract
Keywords:
Introduction
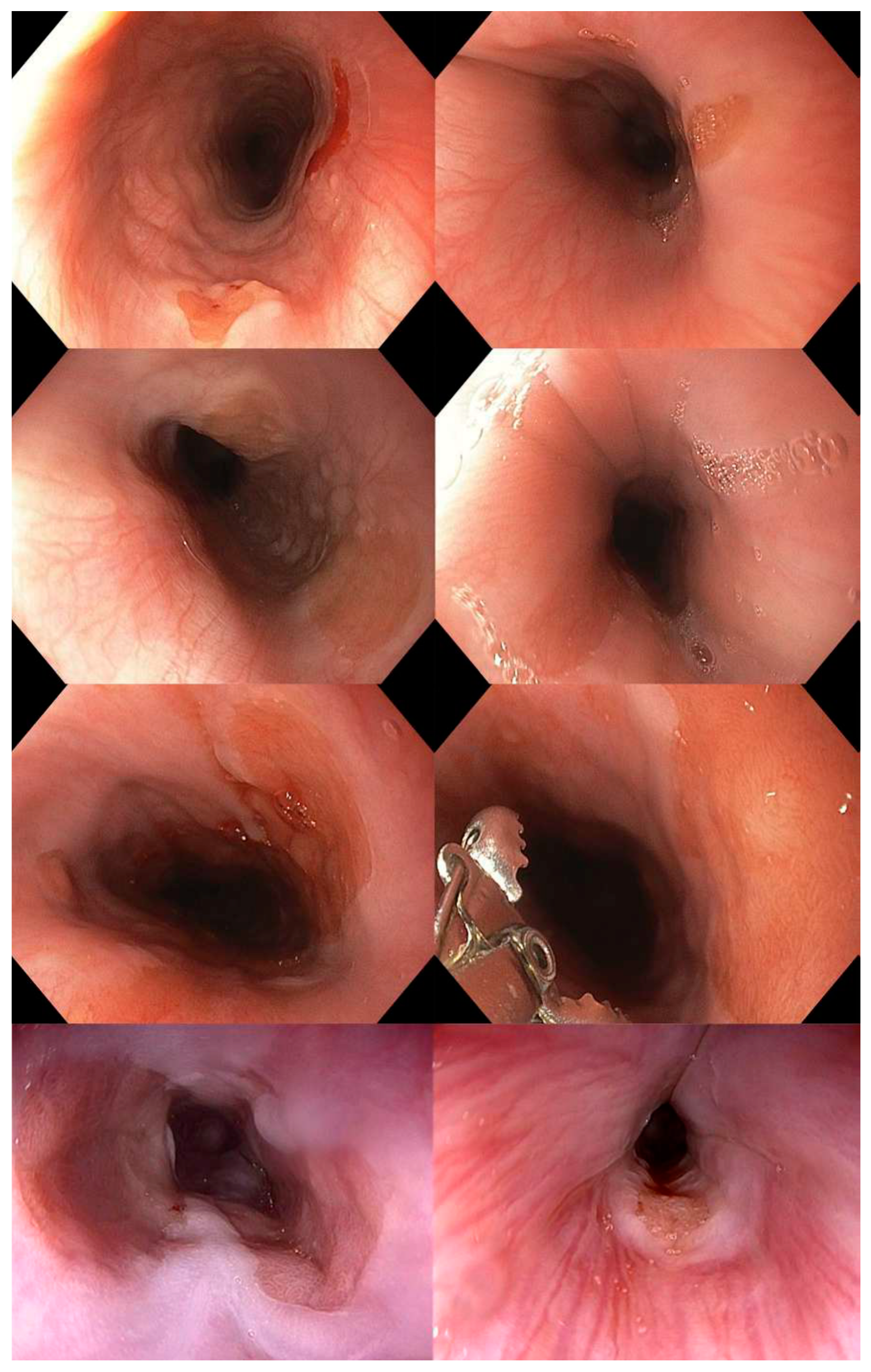
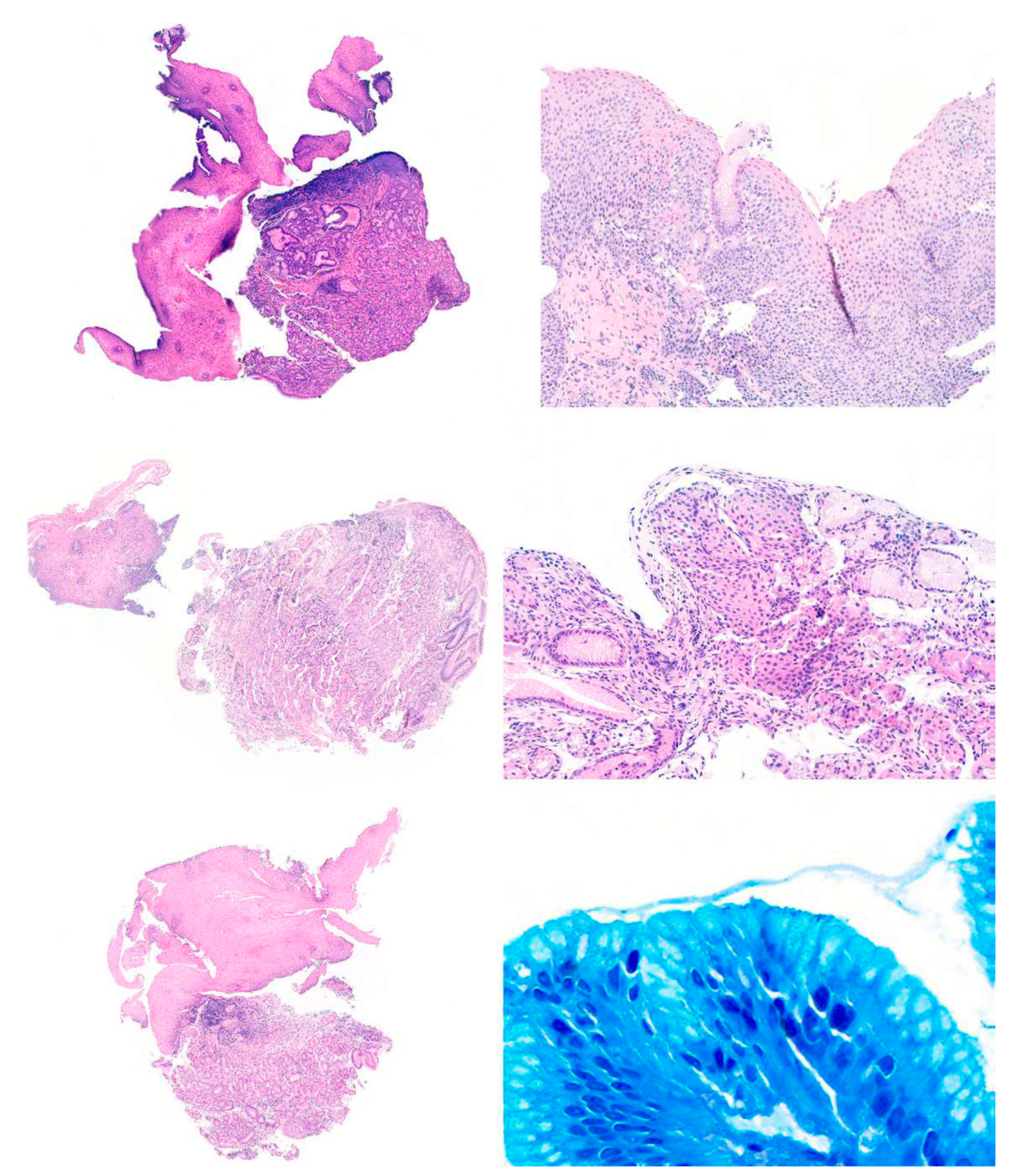
Material and Methods
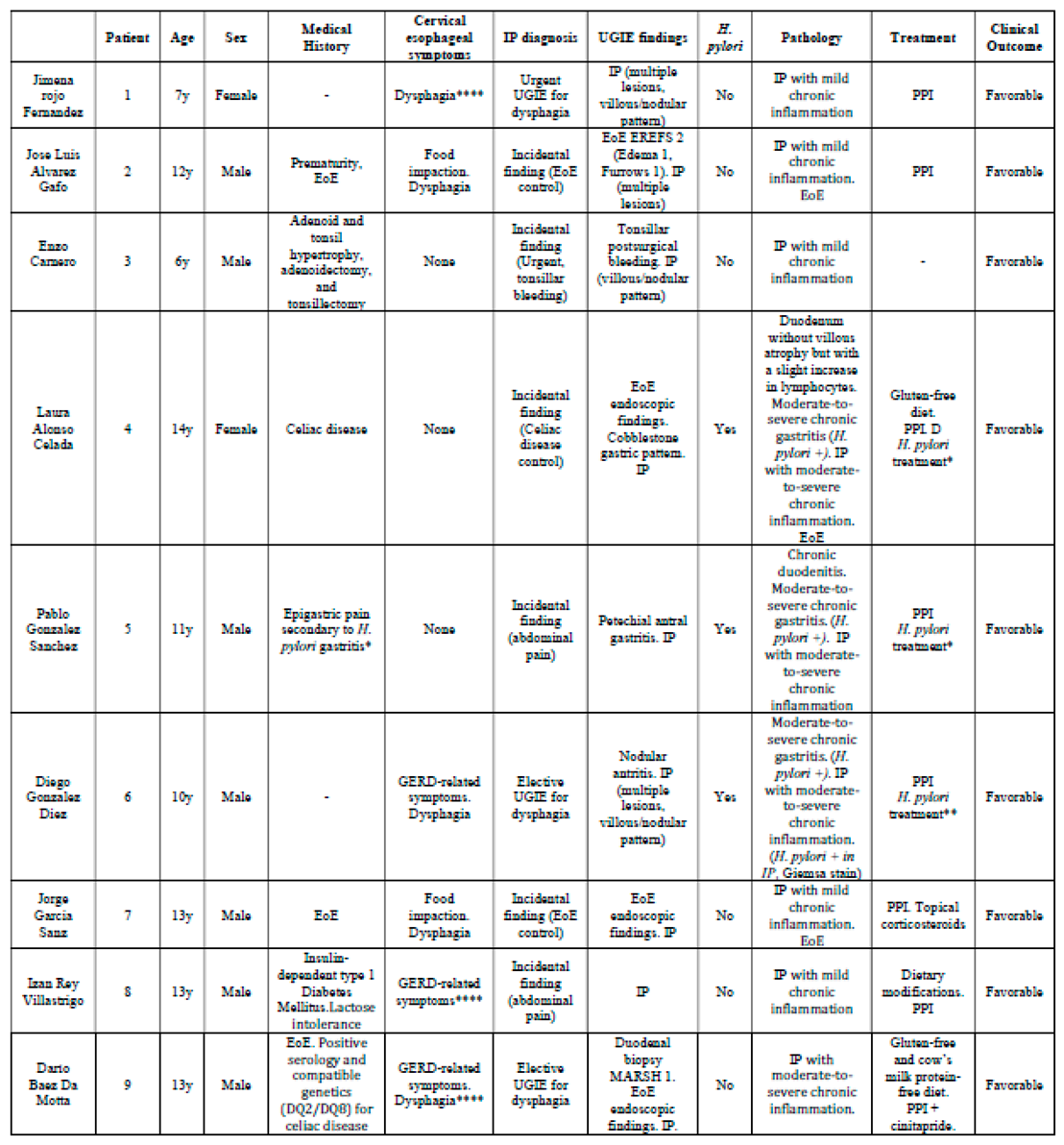
Results
Discussion
Author Contributions
Funding
Conflicts of Interest
Ethical Approval
Statement of Availability of the Data Used During the Study
References
- Rodríguez-Martínez A, Salazar-Quero JC, Tutau-Gómez C, Espín-Jaime B, Rubio-Murillo M, Pizarro-Martín A. Heterotopic gastric mucosa of the proximal oesophagus (inlet patch): endoscopic prevalence, histological and clinical characteristics in paediatric patients. Eur J Gastroenterol Hepatol. 2014, 26, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Georges A, Coopman S, Rebeuh J, Molitor G, Rebouissoux L, Dabadie A, Kalach N, Lachaux A, Michaud L. Inlet patch: clinical presentation and outcome in children. J Pediatr Gastroenterol Nutr. 2011, 52, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo G, Cremon C, Bertelli L, Oliva S, De Giorgio R, Pagano N. Esophageal Inlet Patch: An Under-Recognized Cause of Symptoms in Children. J Pediatr. 2016, 176, 99–104.e1. [Google Scholar] [CrossRef] [PubMed]
- Borhan-Manesh F, Farnum JB. Incidence of heterotopic gastric mucosa in the upper oesophagus. Gut. 1991, 32, 968–972. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Akar T, Aydın S. The true prevalence of cervical inlet patch in a specific center dealing with esophageal diseases. Eur Rev Med Pharmacol Sci. 2022, 26, 3127–3131. [Google Scholar] [CrossRef] [PubMed]
- Variend S, Howat AJ. Upper oesophageal gastric heterotopia: a prospective necropsy study in children. J Clin Pathol. 1988, 41, 742–745. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yin Y, Li H, Feng J, Zheng K, Yoshida E, Wang L, Wu Y, Guo X, Shao X, Qi X. Prevalence and Clinical and Endoscopic Characteristics of Cervical Inlet Patch (Heterotopic Gastric Mucosa): A Systematic Review and Meta-Analysis. J Clin Gastroenterol. 2022, 56, e250–e262. [Google Scholar] [CrossRef] [PubMed]
- Bogomoletz WV, Geboes K, Feydy P, et al. Mucin histochemistry of heterotopic gastric mucosa of the upper esophagus in adults: possible pathogenic implications. Hum Pathol. 1988, 19, 1301–1306. [Google Scholar] [CrossRef]
- Lauwers GY, Mino M, Ban S, Forcione D, Eatherton DE, Shimizu M, Sevestre H. Cytokeratins 7 and 20 and mucin core protein expression in esophageal cervical inlet patch. Am J Surg Pathol. 2005, 29, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Meliț LE, Dincă AL, Borka Balas R, Mocanu S, Mărginean CO. Not Every Dyspepsia Is Related to Helicobacter pylori-A Case of Esophageal Inlet Patch in a Female Teenager. Children (Basel). 2023, 10, 229. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trippel M, Casaulta C, Sokollik C. Heterotopic gastric mucosa: Esophageal inlet patch in a child with chronic bronchitis. Dig Endosc. 2016, 28, 688. [Google Scholar] [CrossRef] [PubMed]
- Fernández-García A, Sáez Álvarez S, González-Lamuño Sanchis C, Iglesias Blázquez C, Rodríguez Ruiz M, Arredondo Montero J. Esophageal inlet patch in a 7-year-old girl with subacute dysphagia. Pediatr Neonatol. 2024, 65, 603–604. [Google Scholar] [CrossRef] [PubMed]
- Macha S, Reddy S, Rabah R, Thomas R, Tolia V. Inlet patch: heterotopic gastric mucosa--another contributor to supraesophageal symptoms? J Pediatr. 2005, 147, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto K, Shibagaki K, Nonomura S, Sumi S, Fukuda N, Takahashi Y, Kotani S, Okimoto E, Oshima N, Kawashima K, Ishimura N, Ishihara S. Heterotopic Gastric Mucosa in Middle Esophagus Complicated with Esophageal Ulcers. Intern Med. 2022, 61, 2735–2740. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alberty JB, Chanis R, Khoshoo V. Symptomatic gastric inlet patches in children treated with argon plasma coagulation: a case series. J Interv Gastroenterol. 2012, 2, 91–93. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ajmal S, Young JS, Ng T. Adenocarcinoma arising from cervical esophageal gastric inlet patch. J Thorac Cardiovasc Surg. 2015, 149, 1664–1665. [Google Scholar] [CrossRef] [PubMed]
- Probst A, Schaller T, Messmann H. Adenocarcinoma arising from ectopic gastric mucosa in an esophageal inlet patch: treatment by endoscopic submucosal dissection. Endoscopy. 2015, 47 (Suppl. 1), E337–E338. [Google Scholar] [CrossRef] [PubMed]
- Carrie, A. Adenocarcinoma of the upper end of the esophagus arising from ectopic gastric epithelium. Br J Surg. 1950, 37, 474. [Google Scholar] [CrossRef]
- Christensen WN, Sternberg SS. Adenocarcinoma of the upper esophagus arising in ectopic gastric mucosa: two case reports and review of the literature. Am J Surg Pathol. 1987, 11, 397–402. [Google Scholar] [CrossRef]
- Clemente, C. A case of adenocarcinoma of the upper third of the esophagus arising on ectopic gastric tissue. Tumori. 1974, 60, 17–24. [Google Scholar] [CrossRef]
- Sakamoto G, Nakamura K, Saito K, et al. Primary adenocarcinoma of the esophagus arising from heterotopic gastric glands. Gan No Rinsho. 1970, 16, 1105–1110. [Google Scholar]
- Schmidt H, Riddell RH, Walther B, et al. Adenocarcinoma of heterotopic gastric mucosa in the proximal esophagus. Leber Magen Darm. 1985, 15, 144–147. [Google Scholar]
- Tanaka M, Ushiku T, Ikemura M, Shibahara J, Seto Y, Fukayama M. Esophageal adenocarcinoma arising in cervical inlet patch with synchronous Barrett’s esophagus-related dysplasia. Pathol Int. 2014, 64, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Khatri R, Patel J, Song J, Malik Z, Smith MS, Parkman HP. Esophageal Inlet Patch: Association with Barrett’s Esophagus. Dig Dis Sci. 2023, 68, 3671–3678. [Google Scholar] [CrossRef] [PubMed]
- Jabbari M, Goresky CA, Lough J, Yaffe C, Daly D, Côté C. The inlet patch: heterotopic gastric mucosa in the upper esophagus. Gastroenterology. 1985, 89, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Kim EA, Kang DH, Cho HS, Park DK, Kim YK, Park HC, Kim JH. Acid secretion from a heterotopic gastric mucosa in the upper esophagus demonstrated by dual probe 24-hour ambulatory pH monitoring. Korean J Intern Med. 2001, 16, 14–17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





