1. Introduction
The large-scale use of chemical substances in agriculture to maintain soil fertility and prevent the harmful effects of pests and diseases on major crops has fostered high yields and led to the food security that modern agriculture exhibits today [
1]. For the first time, there were enough food supplies, at least from production, to nourish the growing global population [
2]. Uncontrolled and overused chemical agents harm agriculture's long-term sustainability and global food security [
3,
4].
Although very effective, some chemical substances may also cause various health disorders for humans and the surrounding flora and fauna. Additionally, the effectiveness of some pesticides declined over time as the pests they were combating mutated and acquired various resistance genes that allowed them to survive contact with such agents.
Imidacloprid, a widely used popular insecticide, effectively controls many insects, many of which are pests. However, studies have shown that workers who handle this chemical insecticide are at serious risk of health issues due to prolonged exposure [
5]. It causes the near annihilation of a significant group of beneficial insects, such as the honeybee. [
6,
7]. Raising awareness and acting could work towards a future where these risks are minimised.
While botanical pesticides may not be as potent at stopping pests that harm crops, they show promise for the future of agriculture [
8,
9,
10]. They have the advantage of having a lower impact on human health and a less adverse effect on agroecosystems. In some cases, they have been incorporated into integrated pest management programs, aiming to reduce the negative impact of agrochemicals without significantly affecting current agricultural yields [
11,
12].
One of the examples of botanical insecticides that has caught attention is based on the use of essential oils from citrus peels [
13,
14]. These peels contain a relative abundance of monoterpenes, such as limonene and linalool, which have proven insecticidal action [
14].
In a previous study, essential oil from the peels of tangerines (
Citrus reticulata L.) controlled whiteflies (
Trieurodes vaporariorum W.) [
15]. These extracts were obtained with nonpolar organic solvents (petroleum ether and n-hexane), whose toxicity and flammability make them challenging to use in broader contexts.
The work will use ethanol extracts from the tangerine peel (
Citrus reticulata L.) with a new, safer solvent-ethyl alcohol. This choice of solvent is much less toxic, flammable, and safer than petroleum ether and n-hexane, providing reassurance about the safety of the research methods [
16].
Other authors have also reported that essential oils obtained from C. reticulata possess antimicrobial activity [
17,
18,
19,
20,
21], including some insect-transmitted infections, such as ‘cucumber wilt’, a serious illness caused by the bacteria
Erwinia tracheiphila [
22].
Additionally, alcoholic extracts of
C. reticulata peel have been found to contain some flavonoids, especially poly-methoxy-flavonoids (PMFs), such as tangeretin and nobiletin, which are attributed antifungal properties against certain fungal pests like
Botrytis cinerea,
Sclerotinia sclerotiorum, and
Fusarium oxysporum [
22,
23].
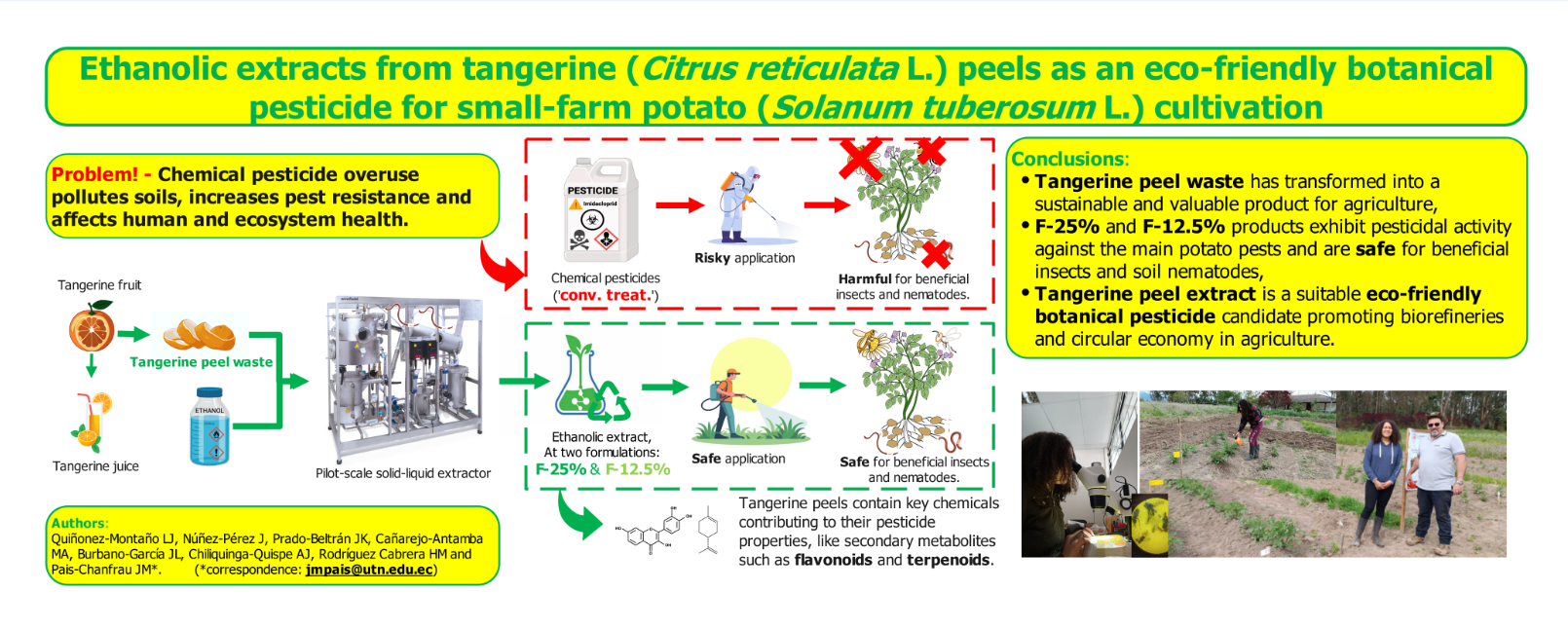
This study used potatoes (Solanum tuberosum L.) as a model crop. The study will be conducted in a small field plot under controlled conditions, using two levels of ethanolic extract of tangerine. The impact of the mentioned formulations will be meticulously evaluated and contrasted with the conventional chemical treatment and with a variant that did not receive any pesticide treatment. The dynamics of the different groups of insects in the agroecosystem will be observed with the utmost care, and the yields obtained from the potato will be compared.
2. Materials and Methods
2.1. Raw Materials
Citrus reticulata L. var. ‘Clementine’ employed here was obtained from the Pimampiro Canton (0°24′0″N, 77°58′12″W), situated in northern Ecuador. The gathered fruits reached maximum ripeness when the skin detached rapidly from the pulp [
24].
Before peeling, the tangerines were cleansed with abundant tap water to eliminate dust collected during shipping. Each fruit was manually dried using a cloth. Following peeling, the objects were cubed into small pieces (1-2 cm) to optimise ethanolic extraction, as suggested by previous studies [
15,
24].
2.2. Pilot-Plant Extraction-Concentration Ethanolic-Extract of Tangerine Peels
The tangerine peel extract was extracted and concentrated in a pilot unit for discontinuous solvent extraction, coupled with a condenser for solvent separation (Armfield Limited, Hampshire, England BH24 1DY, UK.
http://www.armfield.co.uk).
Near 10 kg of fresh, washed mandarin peel was weighed. Then, the material was mixed with 20 litres of 96 % ethyl alcohol, technical grade (for a solvent/solid ratio of about 2 l/kg), and left in contact overnight for about 12 hours.
The next day, the ethanolic extract began to evaporate, condense, and recirculate for about 5-6 hours in the pilot unit. Afterwards, the alcohol was evaporated and separated from the extract for 2 hours, ensuring the condenser temperature remained below 90 °C. The operation was halted when the temperature rose above 90 °C, concluding the material concentration process and the safe removal of the solvent.
The material, resulting from a precise and controlled process, was stored in a 1-litre amber bottle at 4 °C, ensuring its preservation until its use in field experiments and analyses.
2.3. Characterization of Ethanolic-Extract of Tangerine Peels
2.3.1. Phytochemical Screening of Ethanolic Tangerine Peel Extract
The ethanolic extract was evaluated for its phytochemical analysis using standard procedures reported in the literature with minor modifications.
2.3.1.1. Protein and Amino Acids Determinations (Ninhydrin Assay)
A mixture of equal volumes of tangerine peel ethanolic extract and 0.2 % (m/v) ninhydrin solution (freshly prepared) in a test tube was heated for 1-2 minutes, resulting in a blue to dark purple product, demonstrating the existence of amino acids and proteins [
25].
2.3.1.2. Test for Phenols and Tannins
In a test tube, equal amounts of ethanolic extract and 2 % (m/v) FeCl
3 solution were mixed to give a blue-green or black colouration, indicating that phenols and tannins are present [
25].
2.3.1.3. Test for Carbohydrates
An equal volume of ethanolic extract was combined with Benedict's reagent in a test tube and subsequently heated to boiling. The emergence of a reddish-brown precipitate signifies the presence of carbohydrates [
26].
2.3.1.4. Flavonoid Assays (Alkaline Reagent Test)
An equal volume of ethanolic extract was combined with a 2% NaOH solution in a test tube, resulting in a pronounced yellow colouration. The presence of flavonoids results in a colourless solution upon the addition of several drops of concentrated hydrochloric acid [
27].
2.3.1.5. Flavonoid Assays (Shinoda Test)
In a test tube, 1 ml of ethanolic extract and 10 drops of diluted hydrochloric acid were mixed, followed by 500 mg of magnesium. The presence of flavonoids results in the production of reddish, pink, or brown colouration [
28].
2.3.1.6. Test for Steroids (Libermann Test)
In a test tube, 1 ml of ethanolic extract was combined, and 1 ml of ethanolic extract and 2 ml chloroform + 2 ml acetic acid. Then, the mixture was cooled using ice, and 0.5 ml of concentrated sulphuric acid was added incrementally and precisely. If, after that, the colour turns into violet, blue, or green, it implies the presence of steroidal molecules [
27].
2.3.1.7. Test for Cardiac Glycosides
In a test tube, an equal volume of ethanolic extract from tangerine peels was combined with glacial acetic acid, which contained two drops of a 2 % (m/v) FeCl
3 solution. The solution was transferred to a distinct test tube containing 1 ml of strong sulphuric acid. The presence of glycosides is indicated by a brown ring at the interphase or a blue colouration in the acetic acid layer, along with a red colour in the interphase of the two acids [
25].
2.3.2. FTIR Study of Ethanolic Tangerine Peel Extract
Ethanolic extract from tangerine peels was analysed using infrared spectrometry (Agilent Cary 630 FTIR, Agilent Technologies Inc., CA, USA) across a wavenumber range of 400 to 4,000 cm−1, performing 32 scans at a resolution of 4 cm−1. Additionally, an ATR sampling method was employed to analyse a single rebound diamond crystal.
2.3.3. Reversed-Phase HPLC
The samples were analysed using the RP-HPLC system (Ultimate 3000 HPLC, with a reverse-phase C-18 column (150 × 4.6 mm) from HPLC Hypersil GOLDTM, an autosampler, a Thermo Scientific™ quaternary pump, a column compartment, and a photodiode array detector (PAD)). The chromatograms were produced at a wavelength of 205 nm using a linear gradient of H2O/MeCN (95:5) to (40:60) for 8 min.
2.4. Toxicity Evaluation on Caenorhabditis Elegans Model
2.4.1. Caenorhabditis Elegans Strain Culture
C. elegans wild-type N2 strain worms were cultured on NGM agar plates using
Escherichia coli strain OP50 as feed. The nematode culture was maintained at 20 °C and sub-cultured every two weeks [
29]. Each culture was synchronised by bleaching with sodium hypochlorite solution treatment and then culture for two days at 20 °C until the L2 nematodes stage. [
30,
31].
2.4.2. Toxicity Assay and LC50 Estimation
Toxicity assays were performed in 96-well culture plates in a final volume of 200 μL of liquid S-basal culture media dosed with 1, 6, 12.5, 25 and 75 % of the tangerine peel ethanolic-extract (Eth-E) [
29]. Twenty L2 nematodes were collected individually with a worm picker in each well. Four wells were prepared for each treatment in triplicate on one culture plate. Ivermectin (6.0 mg/mL) was used as a positive control. Liquid S-basal culture media was used as the negative control, and ARPON solution (0.99 mg/mL) was used as the solvent control [
32].
Plates were incubated at 20 °C for 24, 48 and 72 hours. After exposure, the live and dead worms were counted by visual inspection under a dissection microscope. The dead condition was considered when the worms did not respond to light and mechanical stimuli. Results were presented as the mean survival percentage of three replicates with t-student analysis for independent samples. Finally, the lethal concentration (LC50) was calculated for PROBIT analysis.
2.5. Field Experiments on Small Potato (Solanum tuberosum L.) Cultivation
The field experiments were conducted between January 2023 and April 2023 on the grounds of the experimental farm “La Pradera”, belonging to the “Universidad Técnica del Norte” and located in the canton of Chaltura, province of Imbabura, Ecuador (0°22’31.3" N; 78°21’20.5" W). During a rainy period, the most persistent insect and fungal pests proliferate over the crops.
The “Capiro” variety of Solanum tuberosum L. was used for the small-scale potato cultivation experiment. A completely randomised block design of experiments was used. Four treatments (formulae at 12.5 % (v/v) (F-12.5%) and 25.0 % (v/v) (F-25%), conventional management (‘conv. treat’), and ‘untreated’) were carried out, with three blocks per treatment. In each block, three rows of five plants were planted, separating the rows by about 0.4 m.
The ethanolic extract formulated at 12.5 % (v/v) and 25 % (v/v) (F-12.5% and F-25%) was applied every seven days. To each formulation, 1 ml·l-1 of polyether polymethyl-siloxane was added as an adjuvant to promote the formation of a homogeneous emulsion. Adjuvant was also included in the conventional chemical treatment.
Each treatment was supplemented with fertilisers during the experiment, as described in
Table 1.
Direct and indirect monitoring were implemented to measure the variables. In indirect monitoring tracking, the number of eggs and nymphs of Bactericera cockerelli S., aphids, and Lepidoptera larvae were counted on the 15 plants. Each plant was divided into three-thirds, and in each third, the number of these insects was counted on three leaves.
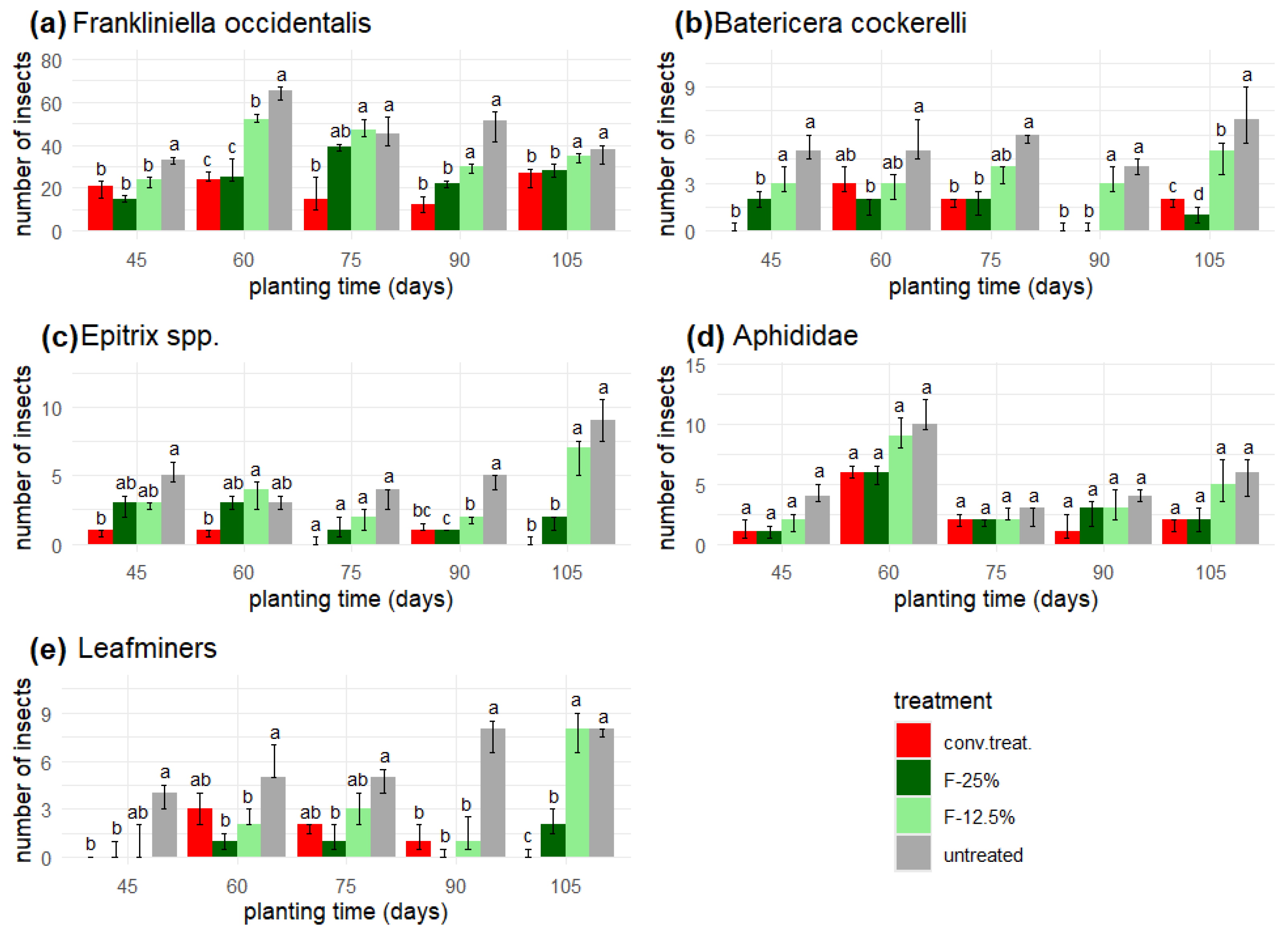
Yellow traps (dim. 10 x 25 cm) were used to monitor the units. These were changed every 15 days, and the number of F. occidentalis, B. cockerelli, Epitrix spp., aphids, and leaf miners were recorded.
2.6. Post-Harvest Analysis
Finally, after harvesting the three blocks of each variant, the yields of each treatment block (in ton·ha-1) were determined. Additionally, a random sample of 10 potato tubers from each block was taken to determine which showed observable external damage, thus determining the percentage of damaged tubers in the sample. The damaged tubers were also carefully cut to count the white worms they contained.
2.7. Statistical Analysis of Experiments
The statistical language R (RStudio 2024.12.0+467) was used for statistical analysis and part of the graphs. The Tukey test was used to compare normally distributed samples, while the non-parametric Friedmann and Dunn tests were used to compare non-normal samples.
3. Results
3.1. Pilot-Scale Solid-Liquid Extraction
In the extraction-concentration process, 992 mL of tangerine peel ethanolic extract (with a dark brown colour and characteristic smell) was obtained from 9.8 kg of initial tangerine peels and 20 litres of ethanol at 96 % (technical degree’ ethanol). The whole extraction-concentration yield of the process was 9.9 % (w/w).
3.2. Characterization of Ethanolic Extract from Tangerine Peels
Phytochemical screening of tangerine peel ethanolic extract was conducted through various chemical assays to illustrate the existence of secondary metabolites (e.g., phenols, tannins, flavonoids, proteins, amino acids, reducing sugars, saponins, carbohydrates, steroids, terpenoids, cardiac glycosides, alkaloids, etc.) (
Table 2). Certain substances have demonstrated beneficial bioactivity for human health and plant protection.
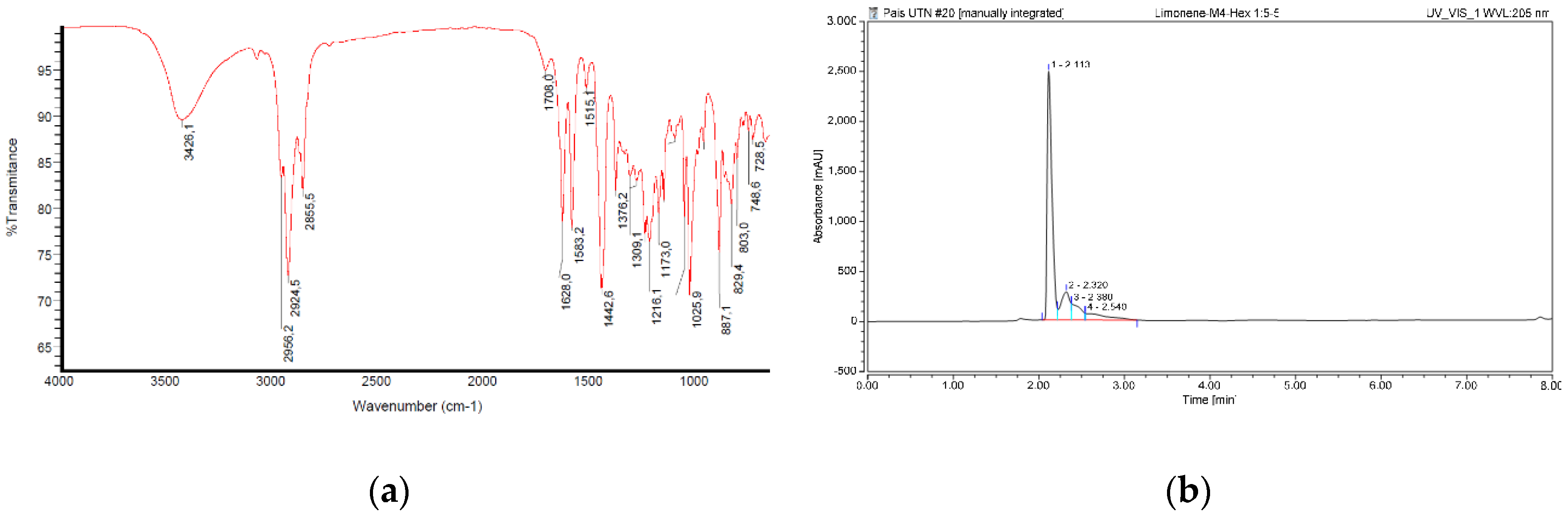
The FTIR spectral peaks indicate the primary interactions among the atoms concerning the extract composition (
Figure 1a). The initial peak is around 3,426 cm
-1, indicative of the stretching vibration of the O-H bond. The 3,000 to 2,800 cm−1 peaks correspond to the asymmetric stretching of aliphatic C-H bonds in ethyl (-CH
3) and methylene (-CH
2) groups. The pronounced peak at 1,627 cm
−1 indicates the C = C bond stretching, likely associated with the cyclohexene limonene ring. Peaks at roughly 1,442 cm
−1 indicate the doublet stretching of the C-H bond in the methylene groups.
As a result, the ethanolic extract of tangerine peel records the presence of functional groups commonly found in secondary metabolites such as limonene, phenols, flavonoids, and steroids.
The HPLC detects significant compounds susceptible to UV/Vis in the ethanolic tangerine peel extract (
Figure 1b).
The HPLC chromatogram shows one prominent peak in the sample at retention times of 2.113 minutes, representing 70 % of this compound in the entire sample. The remaining peaks in the chromatogram profile represent 30 % of the mixture content.
3.3. Toxicity Evaluation of Ethanolic-Extracts of Tangerine Peels on Caenorhabditis elegans Model
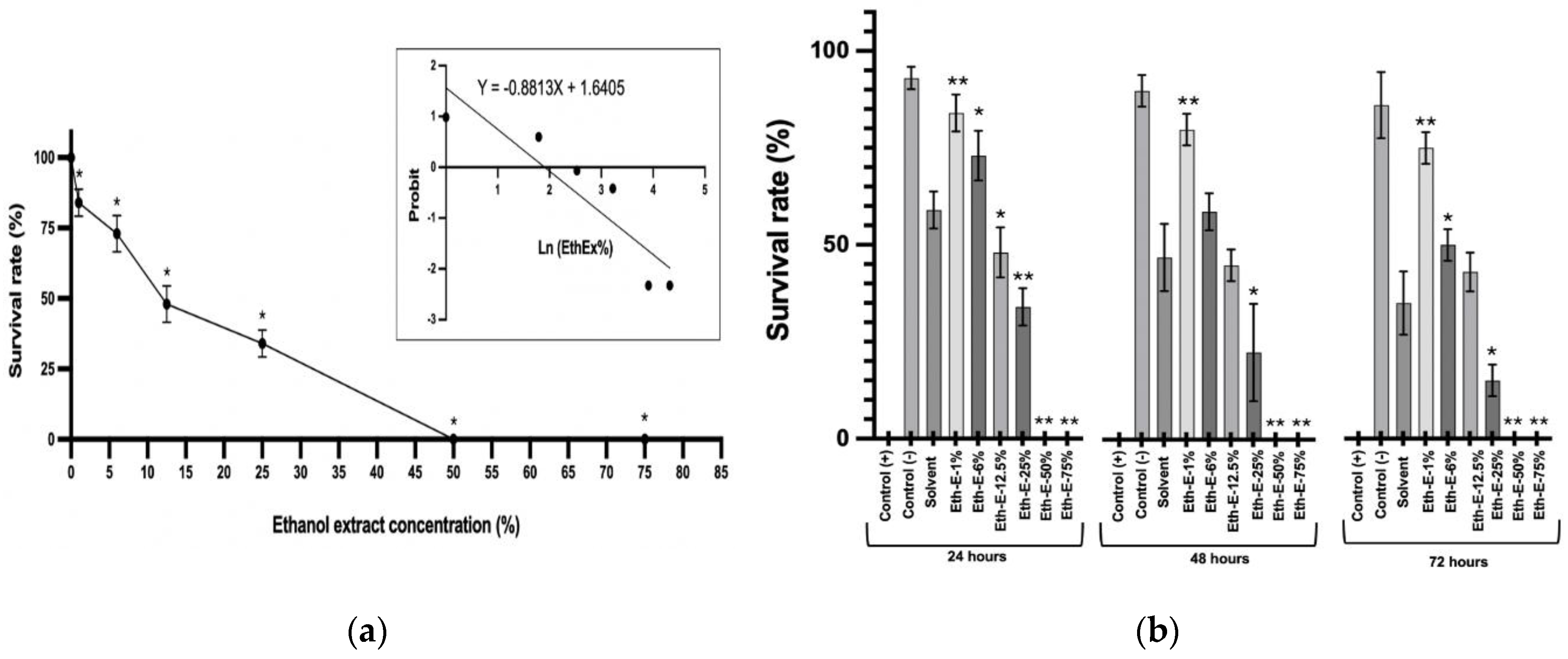
The results exhibited a dose-dependent effect of the ethanolic extract (solved on ARPON) on the L2-nematodes' mortality after 24 hours of exposure, as shown in
Figure 2a. The PROBIT analysis demonstrated an LC
50 value of 6.43 %.
Data exhibits that the tangerine peel ethanol extract causes an interesting anthelmintic effect in C. elegans models. It produces significant mortality from 25 to 75 % at 24 hours of testing and progressively increases over time. Qualitative microscopic analysis of the digestive nematode integrity revealed interesting disruptions in its structure and functionality. These findings suggest that the extract and the solvent generate a direct acute toxic effect on the digestive system and the cuticle of the nematodes.
Interestingly, the solvent used in the assay reduces nematode survival (compared to control (-) with solvent; p = 6.88·10
-5). Some evidence suggests that the silicones in ARPON (polyether polymethyl siloxane copolymer) can inflame and irritate epithelia in the long term [
33], justifying the effect on the cuticle observed in
C. elegans (
Figure 2b). However, the combined use of tangerine essential oil shows a protective effect, improving nematode survival (Eth-E-1% and Eth-E-6%).
The observed effect could be due to the antioxidant and anti-inflammatory capacity reported for essential oils from plants, including different species of citrus [
34,
35,
36,
37].
Finally, the toxic effect of the ethanolic extracts is significant, starting from the F-25% dose in combination with the solvent at all the experimental times, highlighting the safe use of tangerine peel ethanolic extract from F-25% in the agricultural field.
3.4. Small-Scale Field Experiments in Potato Cultivation (Solanum tuberosum L.)
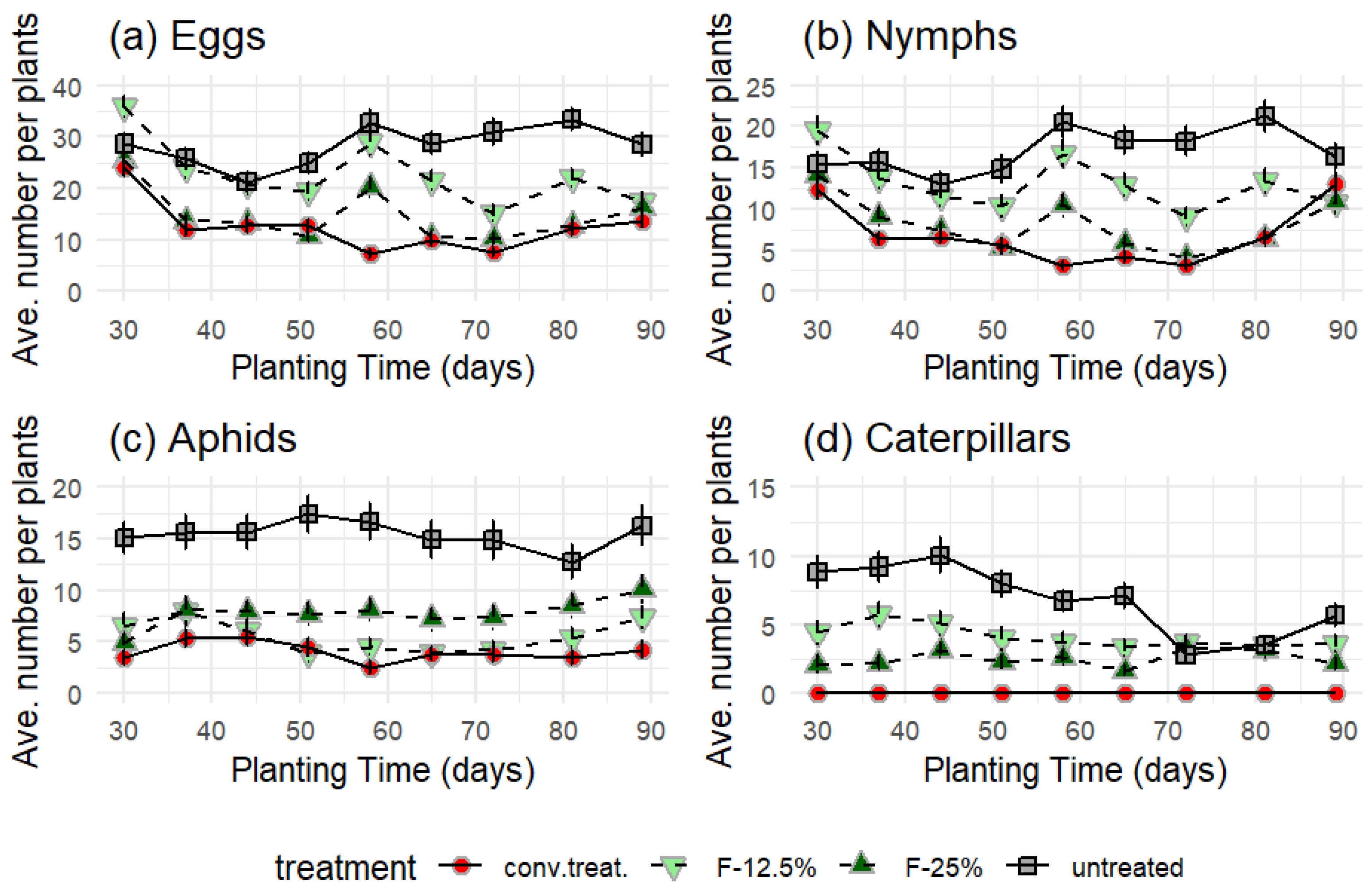
The application results with the formulations show more Bactericera cockerelli eggs than the other pests. Additionally, in the treatment with the ethanol extract formulation at 12.5% (v/v) (F-12.5%) and the untreated variant (further as 'untreated'), a greater quantity was observed compared to the other treatments throughout the entire experiment.
The four treatments had significantly different average numbers of eggs, nymphs, aphids, and caterpillars (according to the Friedmann test, p < 0.001).
In all cases, the lowest values were always from the conventional treatment ('conv. treat.') and the highest from the 'untreated' variant. The intermediate ranges were the F-12.5% and F-25% treatments, with the latter being closer to 'conv. treat.', except for the aphids (
Figure 3c), where F-12.5% was closer to 'conv. treat.'.
However, the most significant difference between these two treatments was observed on day 72, when the control surpassed the F-12.5% treatment with double the oviposition. In contrast, F-25% reached 21 eggs, and the conventional treatment (‘conv. treat.’) exceeded 23 eggs.
On the other hand, the results were similar for the F-25% treatment and the 'conv. treat.' both show the lowest oviposition (
Figure 3a).
The number of nymphs about the number of eggs was reduced by 45 % with the application of F-25% and 'conv. treat.' while with the application of F-12.5%, it was only 42 % and with the 'untreated', it was reduced by 40 %.
The effect of treatments on nymphs was very similar to that on eggs, with F-25% and 'conv. treat.' showing the lowest number of nymphs (
Figure 4b).
The insect pest with the highest presence in the yellow traps was thrips, which showed a lower population with the 'conv. treat.' In some weeks, it was identical to the application of F-25%.
Similar populations were observed for the aphids on days 75 and 90 when applying 'conv. treat.', F-12.5%, and F-25%, while for the last count, a lower population was noted with F-25% and 'conv. treat.'. Similarly, the population of
Bactericera cockerelli was reduced by F-25% and 'conv. treat.' in the last three measurements (
Figure 4b).
Unlike
Epitrix spp., the most significant effect on its population was achieved with the applications of F-12.5% and the 'conv. treat.'. The application of F-25% surpassed these last two by around 5-6 insects, while compared to the 'untreated', it surpassed them by around 13-15 individuals (
Figure 4c).
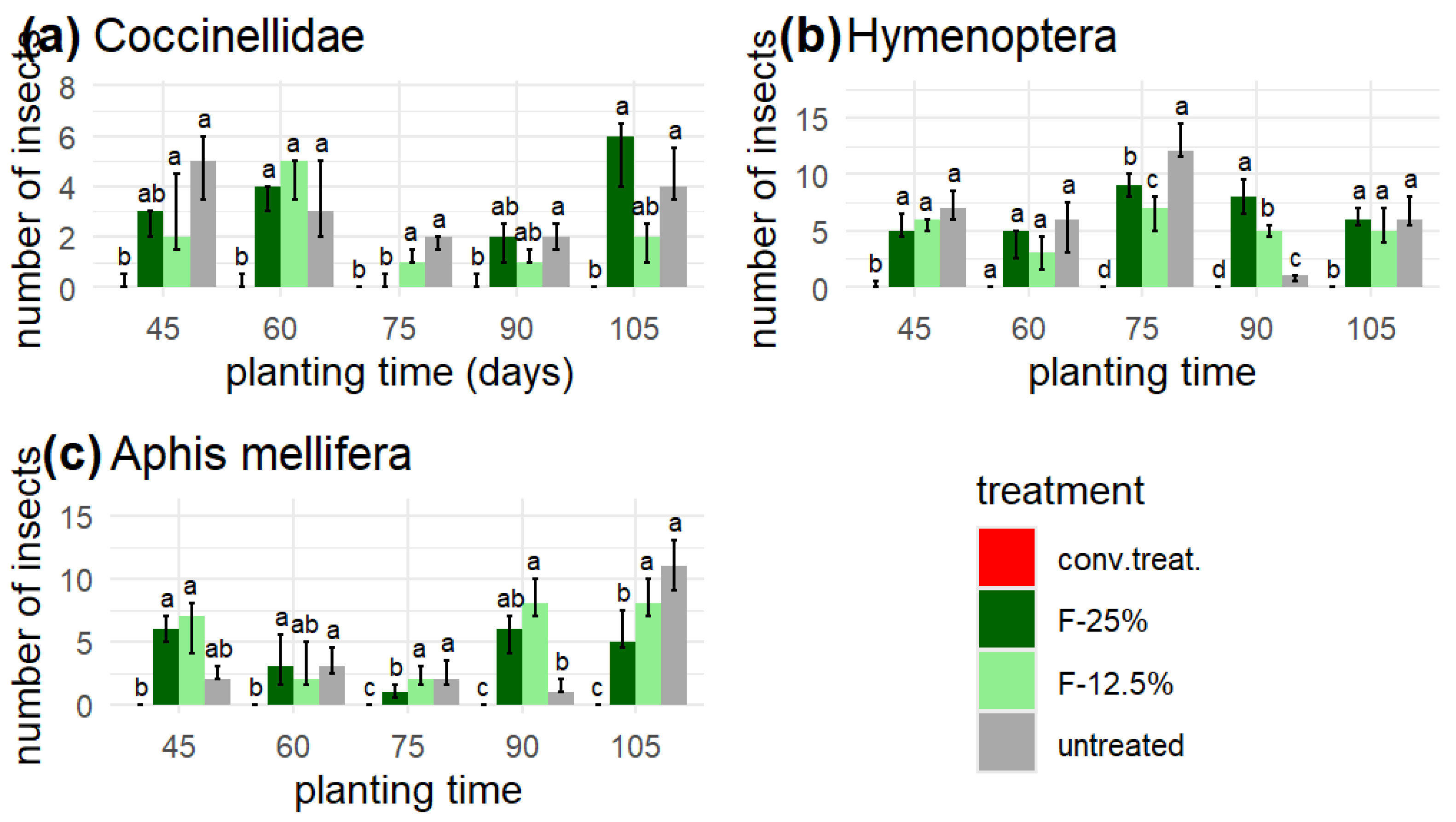
The results show that in the plots with 'conv. treat.' fewer than three individuals of ladybugs and wasps were present, and honeybees (Aphis mellifera) were absent from this treatment.
However, the extracts' application did not affect the populations of beneficial insects, with the F-25% and 'untreated' doses showing eight more specimens of Hymenoptera compared to the F-12.5% applications (
Figure 5).
Unlike honeybees, their population showed no difference between applying extracts and without treatments. The number of ladybugs was lower; however, the oil application reduced their population by 4 to 5 specimens compared to the 'untreated' group.
3.5. Postharvest Analysis
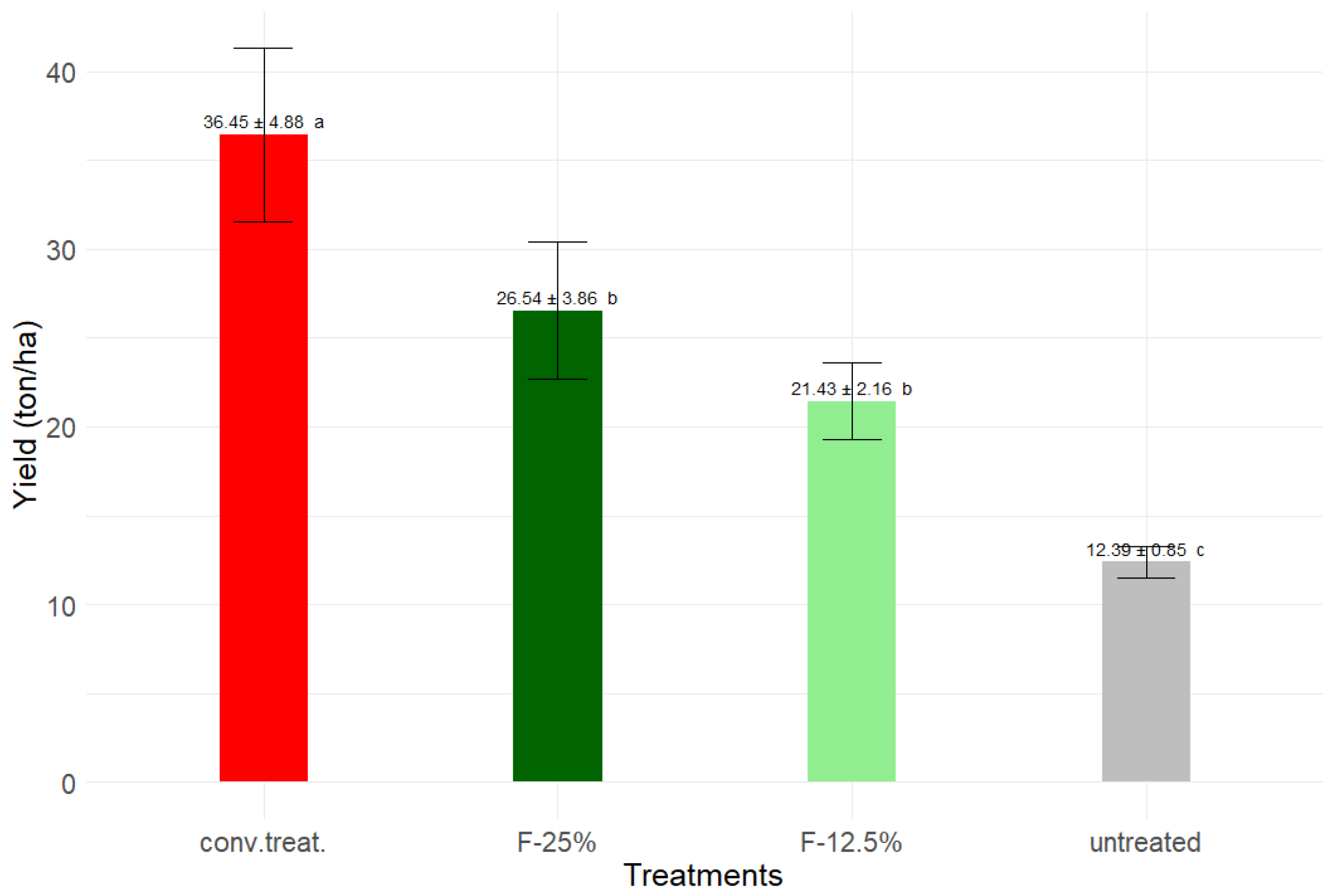
Conventional management reached ~36 tons·ha
-1, the highest yielding unit, surpassing the experimental units F-25% and F-12.5% by more than 10 tons·ha
-1. The plots without applications showed 24 tons·ha
-1 less than conventional management, demonstrating the damage caused by different pests on yield (
Figure 6).
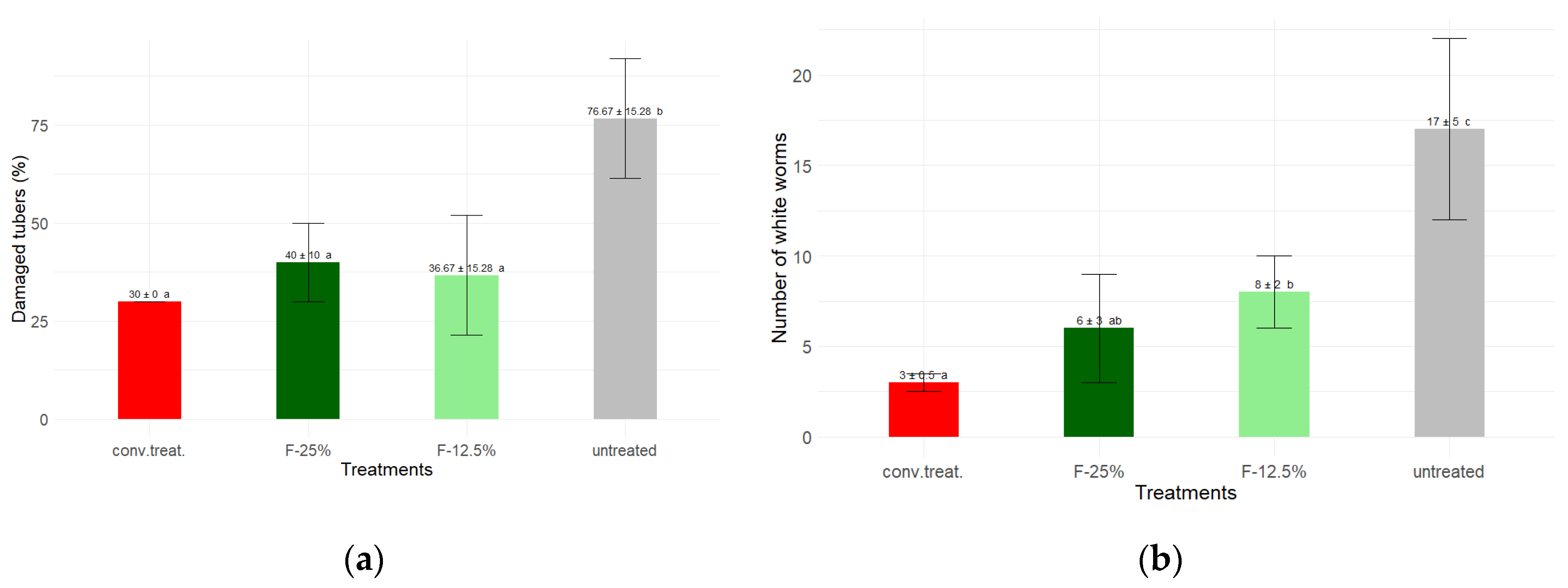
Finally, the selected samples of tubers (n = 10) from each treatment block were thoroughly examined and cut. The number of damaged tubers was detected, and then the number of white grub larvae (
Premnotrypes vorax Hustache (Coleoptera: Curculionidae)) present in each damaged tuber was counted, determining the average number of white grub larvae in each treatment (
Figure 7).
The 'untreated' variant was observed always to have significantly (p > 0.05) higher values than the other treatments. Additionally, no differences were observed between the 'conv. treat.', F-12.5% and F-25% treatments regarding the average value of damaged tubers, while, concerning the average number of counted white worms, no differences existed between the 'conv. treat.' and F-25%, nor between the F-25% and F-12.5% treatments, although there were differences between the latter and the 'conv. treat.' (
Figure 7).
This result suggests that the treatments studied based on alcoholic extracts of mandarin peel can extend their protective action to harmful soil insects such as P. vorax, the cause of the 'Andean potato weevil'.
4. Discussion
As was expected [
38], the phytochemical analysis confirmed the presence of several secondary metabolites, such as limonene, phenols, steroids, and flavonoids.
IR analysis allowed us to corroborate the phytochemical studies by detecting the most representative functional groups for the compounds in the extracts. The signals of peaks at 3,426, 2,924, 2,855, and 1,627 cm
-1 suggest the presence of limonene and flavonoids, which show the existence of -OH, -CH
3, and -C=C—groups present in molecules of the terpene family (such as limonene) and flavonoids (
Figure 1a).
According to the previous research [
15], the RP-HPLC spectrum peaks at 2.113 min, corresponding to limonene as the significant primary compound (70 %). The rest of the mixture probably corresponds to the other secondary metabolites (flavonoids, tannins, and steroids) (
Figure 1b).
The existence of mixtures within the extract is confirmed since the natural complexity of the plant constituents generates the detection of multiple peaks in the RP-HPLC profile and FTIR chromatogram (
Figure 1a,b).
Some authors have identified the presence of D-limonene with the insecticidal activity of the ethanolic extract [
39,
40,
41,
42,
43] and the flavonoids with the antifungal activity of ethanolic extract of
Citrus reticulate peels [
19,
22,
23,
44].
Besides, cardiac glycosides are promising therapeutics in treating and managing congestive heart failure, arrhythmias and heart failure [
45].
The results suggest that the overall toxicity of the F-12.5% and F-25% formulations is significantly lower than that of conventional chemical treatment, as evidenced by the almost negligible presence of beneficial pollinator insects in the yellow traps of this treatment. A similar result has been reported for other botanical insecticides [
12,
46,
47,
48].
Despite the lower yields, reaching roughly 73 % of the yield of the chemical ‘conv. treat.’ obtained, a formulation of 25 % ethanolic extract (F-25%) could be a good candidate for a botanical pesticide and could partially replace the use of chemical insecticides and fungicides.
Under the concepts of circular bioeconomy [
49,
50], utilisation and valorisation of agro-industrial waste can not only provide innovative solutions to the by-products and waste of agro-industry but also, as in this case, allow for the production of a botanical pesticide that is more environmentally friendly and effective against a group of insects and fungal pests that damage agricultural products, such as potatoes, and reduce their yields.
It could be a good alternative for small farms that wish to produce healthy organic products and organic bee honey [
51] on their farms [
9,
52].
Various experiments are underway to validate the results shown here. Some are planned to be carried out in the "dry" season (from May to September), where the rainfall regime is less intense and the climate consequently less humid. Additionally, a more thorough characterisation of the different chemical compounds and their concentration in the ethanolic extract of tangerine peels, which were only qualitatively characterised here, is planned.
5. Conclusions
The formulation based on ethanolic extract from tangerine peels (F-25%) demonstrated considerable efficacy as an environmentally sustainable botanical pesticide. It attains pest control levels comparable to conventional chemical methods while preserving beneficial insect populations, including Coccinellidae and Aphis mellifera. This formulation significantly reduced infestations of key pests, including Bactericera cockerelli and Frankliniela occidentalis, in alignment with sustainable pest control goals.
Phytochemical analyses indicated that limonene, flavonoids, and polymethoxy-flavonoids are crucial bioactive constituents that augment their pesticide and antifungal properties.
The F-25% treatment attained 73 % of conventional production levels, maintaining identical tuber quality and mitigating damage by Premnotrypes vorax larvae. Its low environmental toxicity, validated by Caenorhabditis elegans assays, underscores its safety for agroecosystems.
This approach improves the utilisation of citrus waste, conforming to circular bioeconomy principles and offering small-scale Andean farmers a sustainable substitute for petrochemical pesticides in organic potato cultivation and honey production.
Future research must increase extraction efficiency and field application procedures to enhance yield equivalency with existing methods. Integrating tangerine peel extracts into pest management systems may reduce chemical dependence, improve ecological resilience, and promote sustainable agricultural practices globally.
Author Contributions
Conceptualization, J.M.P.C. and J.K.P.B.; methodology, J.M.P.C., M.A.C.A. and J.K.P.B.; software, J.M.P.C.; validation, J.K.P.B., L.J.Q.M. and M.A.C.A.; formal analysis, L.J.Q.M., A.J.C.Q. and H.M.R.C.; investigation, L.J.Q.M., J.L.B.G. and J.N.P.; resources, J.L.B.G., A.J.C.Q. and H.M.R.C.; data curation, J.K.P.B.; writing—original draft preparation, J.K.P.B. and J.M.P.C.; writing—review and editing, J.M.P.C. and H.M.R.C.; visualization, J.M.P.C. and H.M.R.C.; supervision, J.M.P.C.; project administration, J.M.P.C.; funding acquisition, J.M.P.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgements
The authors thank Dr Marcelo Cevallos, dean of FICAYA, for supporting this research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jeschke, P. Progress of Modern Agricultural Chemistry and Future Prospects. Pest Manag Sci 2016, 72. [CrossRef]
- Kutluay Sahin, D.; Şahin, L. Economic Value of the Use of Chemicals in Agriculture: The Case of European Countries. Hacettepe Üniversitesi İktisadi ve İdari Bilimler Fakültesi Dergisi 2023, 41. [CrossRef]
- Kaur, R.; Choudhary, D.; Bali, S.; Bandral, S.S.; Singh, V.; Ahmad, M.A.; Rani, N.; Singh, T.G.; Chandrasekaran, B. Pesticides: An Alarming Detrimental to Health and Environment. Science of the Total Environment 2024, 915.
- Carvalho, F.P. Pesticides, Environment, and Food Safety. Food Energy Secur 2017, 6, 48–60. [CrossRef]
- Belguet, A.; Dahamna, S.; Abdessemed, A.; Ouffroukh, K.; Guendouz, A. Assessment Of Human Health Risk Associated With The Imidacloprid Pesticide. Indian Journal of Environmental Protection 2020, 40.
- Suchail, S.; Guez, D.; Belzunces, L.P. Toxicity of Imidacloprid and Its Metabolites in Apis Mellifera. Hazards of Pesticides to Bees 2001.
- Rondeau, G.; Sánchez-Bayo, F.; Tennekes, H.A.; Decourtye, A.; Ramírez-Romero, R.; Desneux, N. Delayed and Time-Cumulative Toxicity of Imidacloprid in Bees, Ants and Termites. Sci Rep 2014, 4. [CrossRef]
- Laxmishree, C.; Nandita, S. Botanical Pesticides – a Major Alternative to Chemical Pesticides: A Review. International J. of Life Sciences 2017, 5.
- Nagarjuna Reddy, D.; Al-Rajab, A.J. Botanical Pesticides for Organic Farming and Sustainable Agriculture. In Biopesticides in Organic Farming; 2021.
- Damalas, C.A.; Koutroubas, S.D. Botanical Pesticides for Eco-friendly Pest Management: Drawbacks and Limitations. Pesticides in Crop Production: Physiological and Biochemical Action 2020.
- Riyaz, M.; Mathew, P.; Zuber, S.M.; Rather, G.A. Botanical Pesticides for an Eco-Friendly and Sustainable Agriculture: New Challenges and Prospects. In Sustainable Agriculture; 2022.
- Ahmed, N.; Alam, M.; Saeed, M.; Ullah, H.; Iqbal, T.; Awadh Al-Mutairi, K.; Shahjeer, K.; Ullah, R.; Ahmed, S.; Abd Aleem Hassan Ahmed, N.; et al. Botanical Insecticides Are a Non-Toxic Alternative to Conventional Pesticides in the Control of Insects and Pests. In Global Decline of Insects; 2022.
- Kato-Noguchi, H.; Kato, M. Pesticidal Activity of Citrus Fruits for the Development of Sustainable Fruit-Processing Waste Management and Agricultural Production. Plants 2025, 14, 754. [CrossRef]
- Papanastasiou, S.A.; Ioannou, C.S.; Papadopoulos, N.T. Oviposition-Deterrent Effect of Linalool – a Compound of Citrus Essential Oils – on Female Mediterranean Fruit Flies, Ceratitis Capitata (Diptera: Tephritidae). Pest Manag Sci 2020, 76. [CrossRef]
- Flores, N.; Prado, J.; Espin, R.; Rodríguez, H.; Pais-Chanfrau, J.-M. Laboratory Evaluation of a Bio-Insecticide Candidate from Tangerine Peel Extracts against Trialeurodes Vaporariorum (Homoptera: Aleyrodidae). PeerJ 2024, 1–17.
- Capello, C.; Fischer, U.; Hungerbühler, K. What Is a Green Solvent? A Comprehensive Framework for the Environmental Assessment of Solvents. Green Chemistry 2007, 9. [CrossRef]
- Boudries, H.; Loupassaki, S.; Ladjal Ettoumi, Y.; Souagui, S.; Bachir Bey, M.; Nabet, N.; Chikhoune, A.; Madani, K.; Chibane, M. Chemical Profile, Antimicrobial and Antioxidant Activities of Citrus Reticulata and Citrus Clementina (L.) Essential Oils. Int Food Res J 2017, 24.
- Jayaprakasha, G.K.; Negi, P.S.; Sikder, S.; Mohanrao, L.J.; Sakariah, K.K. Antibacterial Activity of Citrus Reticulata Peel Extracts. Zeitschrift fur Naturforschung - Section C Journal of Biosciences 2000, 55. [CrossRef]
- Shahzad, K.; Nawaz, S.; Ahmad, R.; Akram, N.; Iqbal, Z. Evaluation of Antbacterial, Antifungal and Antioxidant Activity of Essentail Oil of Citrus Reticulata Fruit (Tangerine Fruit Peel). Pharmacologyonline 2009, 3.
- Tao, N.G.; Liu, Y.J.; Tang, Y.F.; Zhang, J.H.; Zhang, M.L.; Zeng, H.Y. Essential Oil Composition and Antimicrobial Activity of Citrus Reticulata. Chem Nat Compd 2009, 45. [CrossRef]
- Khaing, T.; Win, K.H.; Khaing, Y.K. Phytochemical Screening, Antimicrobial Activities and Extraction of Essential Oil from the Peel of Citrus Reticulata Blanco. International Journal of Scientific and Research Publications (IJSRP) 2019, 9. [CrossRef]
- Wang, T.; Li, Q.; Zhang, H.; Chen, J. Flavonoids from Citrus Reticulata: Inhibitory Activity against Pathogenic Fungi and Biocontrol Potential. Physiol Mol Plant Pathol 2024, 131. [CrossRef]
- Chutia, M.; Deka Bhuyan, P.; Pathak, M.G.; Sarma, T.C.; Boruah, P. Antifungal Activity and Chemical Composition of Citrus Reticulata Blanco Essential Oil against Phytopathogens from North East India. LWT - Food Science and Technology 2009, 42. [CrossRef]
- Flores-Mediavilla, N.A.; Prado-Beltrán, J.K.; Espín-Valladares, R.C.; Rodríguez-Cabrera, H.M.; Pais-Chanfrau, J.M. Bio-Insecticidal Potential of Tangerine Peel Oil Against Greenhouse Whitefly : A Green Biopesticide Candidate. Preprints (Basel) 2023, 04.
- Bagheri, G.; Martorell, M.; Ramírez-Alarcón, K.; Salehi, B.; Sharifi-Rad, J. Phytochemical Screening of Moringa Oleifera Leaf Extracts and Their Antimicrobial Activities. Cell Mol Biol 2020, 66. [CrossRef]
- Yadav, R.; Agarwala, M. Phytochemical Analysis of Some Medicinal Plants | Journal of Phytology. Journal of Phytology 2011, 3.
- Usman, A.; Abdulrahman, F.I.; Usman, A. Qualitative Phytochemical Screening and in Vitro Antimicrobial Effects of Methanol Stem Bark Extract of Ficus Thonningii (Moraceae). African Journal of Traditional, Complementary and Alternative Medicines 2009, 6. [CrossRef]
- Patil, D.K.; Jain, A.P. Extraction, Qualitative and Quantitative Determination of Secondary Metabolites of Corchorus Olitorius. Journal of Drug Delivery and Therapeutics 2019, 9. [CrossRef]
- Stiernagle, T. Maintenance of Caenorhabditis Elegans; 2015; Vol. 16.
- Mishra, S.; Gaur, A.V.; Agarwal, R. Standardization of Synchronization Procedure to Collect the Similar Aged C. Elegans. 2020. [CrossRef]
- Porta-de-la-Riva, M.; Fontrodona, L.; Villanueva, A.; Cerón, J. Basic Caenorhabditis Elegans Methods: Synchronization and Observation. Journal of Visualized Experiments 2012. [CrossRef]
- Hart, A. Behavior. WormBook 2006. [CrossRef]
- Tiwari, A.; Soucek, M.D.; Pieńkowska, K. Safety and Toxicity Aspects of Polysiloxanes (Silicones) Applications; 2014.
- Yang, J.; Lee, S.Y.; Jang, S.K.; Kim, K.J.; Park, M.J. Anti-Inflammatory Effects of Essential Oils from the Peels of Citrus Cultivars. Pharmaceutics 2023, 15. [CrossRef]
- Rocha, P. dos S. da; Orué, S.L.; Ferreira, I.C.; Espindola, P.P. de T.; Rodrigues, M.V.B.; Carvalho, J.T.G. de; Baldivia, D. da S.; Leite, D.F.; Santos, H.F. dos; Oliveira, A.S.; et al. Lipid-Lowering and Anti-Inflammatory Effects of Campomanesia Adamantium Leaves in Adipocytes and Caenorhabditis Elegans. Pharmaceuticals 2024, 17. [CrossRef]
- Fuentes, C.; Verdú, S.; Fuentes, A.; Ruiz, M.J.; Barat, J.M. Effects of Essential Oil Components Exposure on Biological Parameters of Caenorhabditis Elegans. Food and Chemical Toxicology 2022, 159. [CrossRef]
- D’Almeida, R.E.; Sued, N.; Arena, M.E. Citrus Paradisi and Citrus Reticulata Essential Oils Interfere with Pseudomonas Aeruginosa Quorum Sensing in Vivo on Caenorhabditis Elegans. Phytomedicine Plus 2022, 2. [CrossRef]
- Budiarto, K.; Andrini, A.; Budiyati, E.; Mariana, B.D.; Chaireni; Martasari; Mas’udah, S.; Yulia, N.D.; Ikarini, I.; Yulianti, F. Bioactive Phytochemical Contents on Fruit Peel of Several Citrus Species. In Proceedings of the BIO Web of Conferences; 2024; Vol. 91.
- Gomes da Câmara, C.; Ramos de Melo, J.; Correia da Silva, M. Insecticidal Activity of Melaleuca Leucadendron and Citrus Reticulata Essential Oils against Larvae of Plutella Xylostella. Rev Prot Veg 2015, 30.
- Author, C.; Kosar Abbas, S.; Ahmad, F.; Sagheer, M.; Yasir, M.; Ahmad, S.; Muhammad, W. Insecticidal and Growth Inhibition Activities of Citrus Paradisi and Citrus Reticulata Essential Oils Against Lesser Grain Borer, Rhyzopertha Dominica (F.) (Coleoptera: Bostrichidae). World Journal of Zoology 2012, 7. [CrossRef]
- Safavi, S.A.; Mobki, M. Fumigant Toxicity of Essential Oils from Citrus Reticulata Blanco Fruit Peels against Tribolium Castaneum Herbst (Coleoptera: Tenebrionidae). J Crop Prot 2012, 1.
- Fouad, H.A.; da Camara, C.A.G. Chemical Composition and Bioactivity of Peel Oils from Citrus Aurantiifolia and Citrus Reticulata and Enantiomers of Their Major Constituent against Sitophilus Zeamais (Coleoptera: Curculionidae). J Stored Prod Res 2017, 73. [CrossRef]
- Safavi, S.A.; Mobki, M. Susceptibility of Tribolium Castaneum (Herbst, 1797) Larvae to Essential Oils of Citrus Reticulata Blanco Fruit Peels and the Synergist, Diethyl Maleate. Biharean Biol 2016, 10.
- Rane Zab Anish Kumar, P.; Bhaskar, A. Determination of Bioactive Components from the Ethanolic Peel Extract of Citrus Reticulata by Gas Chromatography - Mass Spectrometry. International Journal of Drug Development and Research 2012, 4.
- Akinwumi, I.A.; Ambali, O.A. Cardiac Glycosides from African Medicinal Plants as Promising Therapeutics. Tropical Journal of Phytochemistry & Pharmaceutical Sciences 2024, 3, 158–167.
- Xavier, V.M.; Message, D.; Picanço, M.C.; Chediak, M.; Santana Júnior, P.A.; Ramos, R.S.; Martins, J.C. Acute Toxicity and Sublethal Effects of Botanical Insecticides to Honey Bees. Journal of Insect Science 2015, 15. [CrossRef]
- El Aalaoui, M.; Bouharroud, R.; Sbaghi, M.; El Bouhssini, M.; Hilali, L.; Dari, K. Comparative Toxicity of Different Chemical and Biological Insecticides against the Scale Insect Dactylopius Opuntiae and Their Side Effects on the Predator Cryptolaemus Montrouzieri. Archives of Phytopathology and Plant Protection 2019, 52. [CrossRef]
- Campos, E.V.R.; Proença, P.L.F.; Oliveira, J.L.; Bakshi, M.; Abhilash, P.C.; Fraceto, L.F. Use of Botanical Insecticides for Sustainable Agriculture: Future Perspectives. Ecol Indic 2019, 105. [CrossRef]
- Wagh, M.S.; S, S.; Nath, P.C.; Chakraborty, A.; Amrit, R.; Mishra, B.; Mishra, A.K.; Mohanta, Y.K. Valorisation of Agro-Industrial Wastes: Circular Bioeconomy and Biorefinery Process – A Sustainable Symphony. Process Safety and Environmental Protection 2024, 183.
- Sharma, P.; Gaur, V.K.; Sirohi, R.; Varjani, S.; Hyoun Kim, S.; Wong, J.W.C. Sustainable Processing of Food Waste for Production of Bio-Based Products for Circular Bioeconomy. Bioresour Technol 2021, 325.
- Ndakidemi, B.; Mtei, K.; Ndakidemi, P.A. Impacts of Synthetic and Botanical Pesticides on Beneficial Insects. Agricultural Sciences 2016, 07. [CrossRef]
- Ngegba, P.M.; Cui, G.; Khalid, M.Z.; Zhong, G. Use of Botanical Pesticides in Agriculture as an Alternative to Synthetic Pesticides. Agriculture 2022, Vol. 12, Page 600 2022, 12, 600. [CrossRef]
Figure 1.
Spectral characterisation of ethanolic extract of tangerine peels. (a) Scanning of FTIR between 4000 – 400 cm-1; (b) RP-HPLC profile at 205 nm.
Figure 1.
Spectral characterisation of ethanolic extract of tangerine peels. (a) Scanning of FTIR between 4000 – 400 cm-1; (b) RP-HPLC profile at 205 nm.
Figure 2.
Toxicology effect of ethanolic extract of tangerine peels on Caenorhabditis elegans. (a) Concentration-effect curve of the nematodes exposed to extract for 24 h. Each point represents the average of three experiments. (*) represents a significant difference between each treatment and the control (-). 1% (p = 0.00032), 6% (p = 0.01411), 12.5% (p = 0.03117), 25% (p = 0.00032), 50% (p = 0.00015) and 75% (p = 0.00015) according to Student’s t-test. The Probit regression analysis reflects R2 = 0.8044 and p = 0.0154. (b) Survival of C. elegans in different concentrations of extract at different experimental times. (*) p < 0.05, (**) p < 0.01 represents a significant difference compared to each treatment with the solvent.
Figure 2.
Toxicology effect of ethanolic extract of tangerine peels on Caenorhabditis elegans. (a) Concentration-effect curve of the nematodes exposed to extract for 24 h. Each point represents the average of three experiments. (*) represents a significant difference between each treatment and the control (-). 1% (p = 0.00032), 6% (p = 0.01411), 12.5% (p = 0.03117), 25% (p = 0.00032), 50% (p = 0.00015) and 75% (p = 0.00015) according to Student’s t-test. The Probit regression analysis reflects R2 = 0.8044 and p = 0.0154. (b) Survival of C. elegans in different concentrations of extract at different experimental times. (*) p < 0.05, (**) p < 0.01 represents a significant difference compared to each treatment with the solvent.
Figure 3.
Direct count of (a) eggs, (b) nymphs, (c) aphids, and (d) caterpillars observed before each treatment. The value represents the average of three treatment blocks, and the bar represents the standard deviation.
Figure 3.
Direct count of (a) eggs, (b) nymphs, (c) aphids, and (d) caterpillars observed before each treatment. The value represents the average of three treatment blocks, and the bar represents the standard deviation.
Figure 4.
Yellow traps: Insect pests. (a) Franklinella occidentalis, (b) Bactericera cockerelli, (c) Epitrix spp., (d) Aphididae, (e) Leafminers in yellow traps: insect pests. The bar above each column represents the interquartile range, and the unequal letters represent statistically different median values (p < 0.05) within each planting time, according to the Friedmann test.
Figure 4.
Yellow traps: Insect pests. (a) Franklinella occidentalis, (b) Bactericera cockerelli, (c) Epitrix spp., (d) Aphididae, (e) Leafminers in yellow traps: insect pests. The bar above each column represents the interquartile range, and the unequal letters represent statistically different median values (p < 0.05) within each planting time, according to the Friedmann test.
Figure 5.
Yellow traps: beneficial insects or natural enemies. (a) Coccinellidae, (b) Hymenoptera (except Aphis mellifera), and (c) Aphis mellifera. In concordance with the Friedman test, identical letters within each planting time indicate no significant differences (p > 0.05).
Figure 5.
Yellow traps: beneficial insects or natural enemies. (a) Coccinellidae, (b) Hymenoptera (except Aphis mellifera), and (c) Aphis mellifera. In concordance with the Friedman test, identical letters within each planting time indicate no significant differences (p > 0.05).
Figure 6.
The average potato harvest yields per treatment. The values represent the average ± standard deviation of three blocks of experiments. According to the Tukey test, the letters denote statistically significant differences (p > 0.05).
Figure 6.
The average potato harvest yields per treatment. The values represent the average ± standard deviation of three blocks of experiments. According to the Tukey test, the letters denote statistically significant differences (p > 0.05).
Figure 7.
Post-harvest analysis of samples (n = 10 per block and three blocks per treatment) of the different treatments. (a) Percentage of damaged tubers; (b) Average white worm (P. vorax H.) larvae detected in the damaged tubers. According to the Dunn test, unequal letters indicate significant differences (p > 0.05).
Figure 7.
Post-harvest analysis of samples (n = 10 per block and three blocks per treatment) of the different treatments. (a) Percentage of damaged tubers; (b) Average white worm (P. vorax H.) larvae detected in the damaged tubers. According to the Dunn test, unequal letters indicate significant differences (p > 0.05).
Table 1.
Different chemicals are used for ‘conv. treat.’.
Table 1.
Different chemicals are used for ‘conv. treat.’.
| Name |
Function |
‘conv. treat.’ |
F-25% |
F-12.5% |
‘untreated’ |
Dose |
Frequency |
Mode of action1
|
| ‘Biol’ |
organic fert. |
✓ |
✓ |
✓ |
✓ |
5 l |
Every month |
- |
| Compost |
organic fert. |
✓ |
✓ |
✓ |
✓ |
300 kg |
Start of cultivation |
- |
| Calcium carbonate |
Soil pH adjust. |
✓ |
✓ |
✓ |
✓ |
50 kg |
Start of cultivation |
- |
| NPK (13-40-13) |
inorganic fert. |
✓ |
✓ |
✓ |
- |
7.46 kg |
Every two months |
- |
| Imidacloprid |
insecticide |
✓ |
- |
- |
- |
0.5 ml/l |
Every two weeks |
(i) |
| Thiamethoxam + lambda cyhalothrin |
insecticide |
✓ |
- |
- |
- |
125 ml/l |
(ii) |
| Acephate |
insecticide |
✓ |
- |
- |
- |
100 g/l |
(iii) |
| Fipronil |
insecticide |
✓ |
- |
- |
- |
2 ml/l |
(iv) |
| Methomyl |
insecticide |
✓ |
- |
- |
- |
3 ml/l |
(iii) |
| Malathion |
insecticide |
✓ |
- |
- |
- |
2 ml/l |
(iii) |
| Fluopicolide + Propamocarb chlorhydrate |
fungicide |
✓ |
- |
- |
- |
1.6 ml/l |
Every two weeks |
(v) |
| Propineb + Fluopicolide |
fungicide |
✓ |
- |
- |
- |
2 ml/l |
(vi) |
| Carboxin + Captan |
fungicide |
✓ |
- |
- |
- |
3 g/l |
(vii) |
Table 2.
Phytochemical analysis of tangerine peel ethanolic extract.
Table 2.
Phytochemical analysis of tangerine peel ethanolic extract.
| Test |
Tangerine peel ethanolic extract |
| Amino acids and Proteins |
- |
| Phenols and Tannins |
+ |
| Carbohydrates |
- |
| Flavonoids (Alkaline test) |
+ |
| Flavonoids (Shinoda test) |
+ |
| Steroids |
+ |
| Cardiac Glycosides |
+ |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).