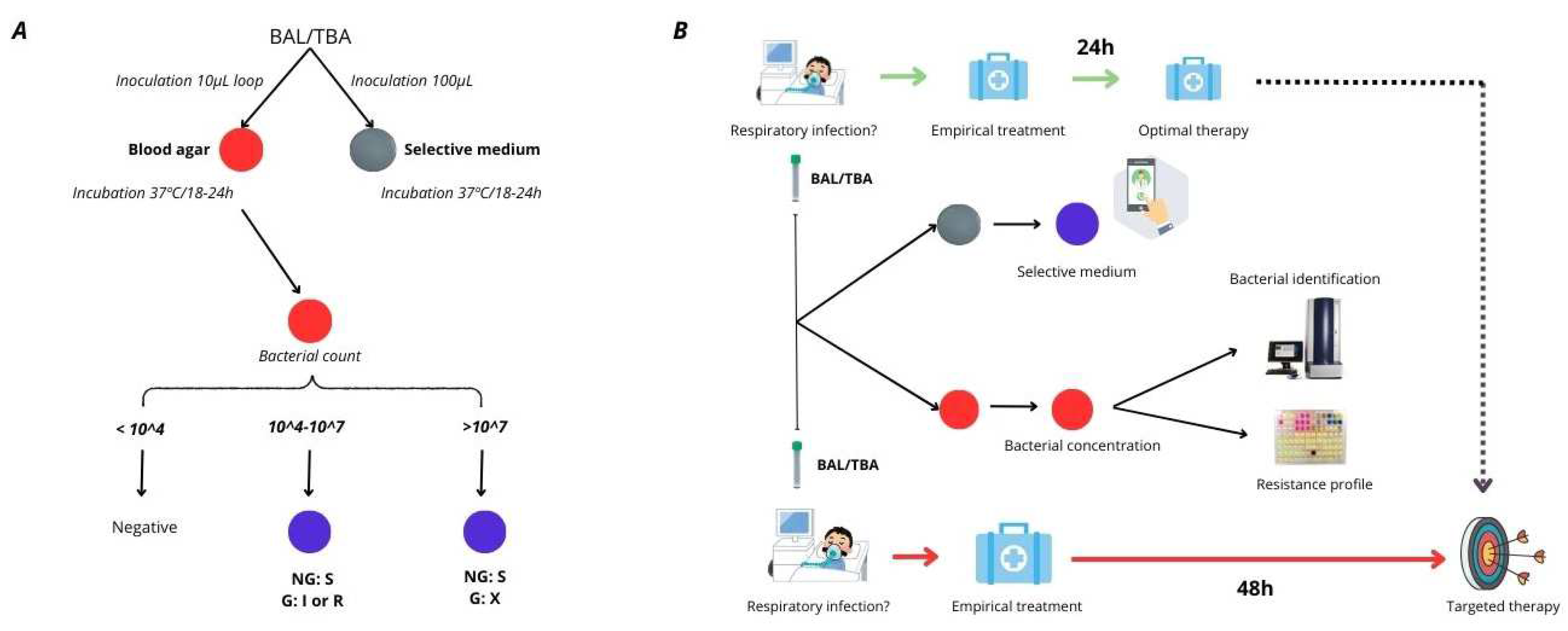
1. Introduction
Meropenem (MER) is frequently used as a first-line treatment for infections caused by
Pseudomonas aeruginosa due to its broad-spectrum activity and efficacy against Gram-negative bacteria. The Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS) recommend piperacillin-tazobactam (TZP), cefepime (FEP)/ceftazidime (CAZ), or MER for the empiric treatment of ventilator-associated pneumonia and hospital-acquired pneumonia in units where double antipseudomonal coverage is appropriate. [
1].
However, the rapid emergence of resistance in
P. aeruginosa is a significant concern, particularly in intensive care units (ICUs). Prolonged hospital stays, invasive procedures, mechanical ventilation, and extensive antibiotic exposure are key factors contributing to the selection and dissemination of resistant strains in ICU patients. These factors increase the risk of colonization and infection with multidrug-resistant organisms, including
P. aeruginosa. The frequent use of broad-spectrum antibiotics, such as meropenem, in these settings further exacerbates the problem by applying selective pressure promoting the survival and proliferation of resistant strains [
2,
3,
4].
The mechanisms of meropenem resistance in
P. aeruginosa are diverse and include overexpression of efflux pumps (e.g., MexAB-OprM), loss of outer membrane porins (e.g., OprD downregulation), and production of carbapenemases (e.g., VIM, IMP, KPC, NDM). These resistance mechanisms significantly limit treatment options, often requiring combination therapies with agents such as amikacin or colistin to improve clinical outcomes. The increasing prevalence of carbapenem-resistant
P. aeruginosa underscores the need for rapid diagnostic tests to detect resistance mechanisms promptly and guide appropriate therapy [
5,
6]. Molecular techniques based on multiplex PCR have been developed in recent years to identify respiratory pathogens directly from the samples [
7]. However, molecular techniques present a limitation due to the discrepancy between genotype and phenotype, especially in
P. aeruginosa, which could lead to misinterpreting the results [
8].
Studies have shown that inappropriate initial antimicrobial therapy for
P. aeruginosa infections is associated with increased mortality and longer hospital stays, emphasizing the need for rapid resistance testing to ensure timely and effective treatment adjustments. Rapid identification of resistance could help in optimizing antimicrobial therapy, reducing the use of ineffective antibiotics, and improving patient outcomes. It also aids in antimicrobial stewardship by minimizing the unnecessary use of broad-spectrum antibiotics, thereby reducing the selection pressure for resistant strains [
9,
10,
11,
12]. Several studies emphasize the critical role of rapid diagnostic tests in improving outcomes for patients with
P. aeruginosa pneumonia. Sun et al. (2011) highlight that inappropriate initial antimicrobial therapy is a major contributor to increased mortality and extended hospital stays, underscoring the importance of quickly detecting resistance mechanisms to guide treatment and improve clinical outcomes [
13]. Similarly, El Solh and Alhajhusain (2009) highlighted that empirical antibiotic therapy is often ineffective due to resistance patterns, worsening patient prognosis, and emphasizing the need for prompt resistance testing [
14]. Lynch and Zhanel (2022) also note the association between inadequate initial therapy and higher mortality, particularly in ventilator-associated pneumonia, and advocate for rapid resistance testing to optimize therapy [
15]. Finally, Kalil et al. (2016) in the IDSA and ATS guidelines also associated inappropriate therapy to increased mortality and recommend rapid diagnostic tests to ensure timely adjustments to treatment [
1].
These studies collectively highlight the importance of timely and accurate resistance testing in improving patient outcomes with
P. aeruginosa infections, particularly those involving the respiratory tract. The problem in achieving this, is that traditional antimicrobial susceptibility testing (AST) methods, such as broth microdilution or automated systems, can take 48-72 hours, delaying appropriate treatment. Previously, our team developed a selective culture medium for screening TZP/FEP-resistant
P. aeruginosa in respiratory samples [
16]. In response to the diagnostic challenges in those samples, we identified the need to develop also a selective medium for detecting meropenem-resistant
P. aeruginosa. Thus, we aimed to design this medium based on the previously developed formulation and ensuring that encompassed the three empiric treatment options recommended by clinical guidelines for respiratory tract infections [
16] [
Figure 1].
2. Results
According to the Minimal Inhibitory Concentration (MIC) assay, this set included 85 MER-susceptible and 45 MER-resistant/intermediate (susceptible at increased exposure) isolates (
Supplementary Table S1). The results showed that 44 of 45 resistant isolates (97,8%) were detected on the meropenem medium. On the other hand, for susceptible strains, concordance slightly decreased due to the unexpected growth of some strains in the selective medium. The highest number of false positives was observed at the 1 x 10
8 CFU/mL dilution, with 16 susceptible strains exhibiting growth on the medium. This was followed by the 1 x 10
7 CFU/mL, 1 x 10
6 CFU/mL, 1 x 10
5 CFU/mL, and 1 x 10
4 CFU/mL dilutions, which presented 9, 7, 6, and 6 false positives, respectively.
These findings allowed us to establish the sensitivity and specificity of the medium for each dilution tested. The sensitivity values exceed 97% for all bacterial concentrations, with the majority reaching 100%. In contrast, specificity values varied across the different dilutions being slightly reduced; however, they remained around 90% except at 1 x 10^8 CFU/mL, where it decreased to 81,2%. (
Table 1).
In the analysis of the 130 clinical strains collected from BAL and TBA, 13
P. aeruginosa isolates were recovered using the conventional methods employed by the Microbiology Service at the University Hospital Virgen del Rocío (Seville). All isolates obtained during the clinical sample validation were subsequently tested using the MicroScan WalkAway system (Beckman Coulter, USA) and the gold standard method (BMD). When compared to the BMD reference method, the plates demonstrated a high degree of categorical agreement (CA), with a value of 92,3%. The reduction in CA was due to one major error (ME), which was a false positive result (
Supplementary Table S2). Despite the relatively small number of
P. aeruginosa isolates recovered, the data obtained from the plates and BMD enabled the calculation of the sensitivity and specificity of the medium. The sensitivity was 100% (CI: (100% - 100%) and specificity 80% (CI: 45-100%) (
Table 2).
Moreover, the medium detected the growth of other meropenem-resistant Gram-negative bacteria, which were subsequently identified by MALDI-TOF (Bruker, USA) (
Supplementary Table S2). Notably, after 24 hours of incubation, no growth of competing microorganisms, including Gram-positive bacteria or fungi, was observed. These findings indicate that the medium demonstrates strong specificity and selectivity for MER-resistant Gram-negative bacteria.
3. Discussion
In this study, we have developed a selective medium that has shown excellent sensitivity and specificity. A study conducted in European ICUs, including Spain, found that 24.9% of
P. aeruginosa isolates were resistant to meropenem, particularly in critically ill patients
[18]. In 2019, another study reported that 17.3% of
P. aeruginosa isolates were extensively drug-resistant (XDR), including resistance to meropenem
[19]. At our institution,
P. aeruginosa resistance to meropenem was observed in 15% of cases in 2023. This indicates that 15% of patients were at risk of receiving ineffective treatment. This issue could be mitigated through the use of rapid antimicrobial resistance test such as this selective culture medium. Moreover, the remaining 85% of patients would also benefit from the early determination of antimicrobial susceptibility, allowing for the timely de-escalation of therapy within the first 24 hours. This strategy not only enhances antimicrobial stewardship but also minimizes unnecessary exposure to broad-spectrum antibiotics, thereby reducing the risk of selecting for resistant strains and improving overall clinical outcomes.
In 2021, Fournier et al. developed screening plates for carbapenem-.resistant
Pseudomonas; however, these set the cut-off at 10¹–10² CFU/mL and the breakpoint at 8 mg/L, making them unsuitable for respiratory samples due to a microorganism is considered an infection causative agent at concentrations >10⁴, and treatment differs when the MIC is between 2 and 8 mg/L [
17]. Unlike the latter screening medium, our plates have been clinically validated for respiratory samples such as BAL and BAS. Its use is particularly valuable for identifying resistant
P. aeruginosa in intensive care unit patients, who are highly susceptible to these infections due to mechanical ventilation. Furthermore, the common respiratory flora present in these samples did not interfere with the test, as it was effectively removed from the culture medium, ensuring complete selectivity. Additionally, the implementation of this culture medium would be highly cost-effective, as its price is less than 1€ per plate. Given its ability to rapidly identify meropenem-resistant
P. aeruginosa, its use could lead to significant cost savings by reducing the duration of ineffective treatments, preventing complications associated with inadequate therapy, and decreasing the length of hospital stays. Furthermore, by enabling early de-escalation of broad-spectrum antibiotics in susceptible cases, it contributes to more efficient resource utilization and helps mitigate the economic burden of antimicrobial resistance in healthcare settings.
However, certain limitations have been found when classifying strains as susceptible or resistant, particularly in strains that were susceptible by MIC but showed growth in our medium. This has been given primarily to dilution of 1 x 10^8 CFU/mL, when the strains had a MIC of 2 (breakpoint). This observation is illustrated in
Table 1, where at that dilution, sensitivity reaches 100%, while specificity decrease to approximately 80%, indicating a higher number of strains classified as resistant compared to the reference method. Moreover, our selective medium is not able to discriminate between resistant and intermediate (susceptible at increased exposure). Therefore, when our plates yield a positive result, the physician must decide whether to seek an alternative empirical treatment until the full resistance phenotype is known or, if no other options are available, administer high-dose meropenem. Nevertheless, a positive aspect of this method is its high negative predictive value, almost 100% across all dilutions, indicating that it is highly unlikely for a resistant strain to fail to grow in our medium. This is crucial, as such an omission would represent a significant error, potentially causing the patient to receive ineffective treatment or suboptimal doses for their infection.
In conclusion, this method offers several notable advantages when combined with the usual culture media. Firstly, it significantly reduces the time required to determine the susceptibility to this first-line antibiotic in severe infections. Secondly, it is easy to prepare, making it a practical choice for clinical settings. Most importantly, it demonstrates high sensitivity and specificity, with particular emphasis on its excellent negative predictive value, ensuring reliable identification of resistant strains. All of this may help to optimize the empirical treatment, where the delay of the appropriate therapy is crucial for the outcome of the patients. Nevertheless, further studies are needed to assess its real impact on antimicrobial treatment strategies and patient outcomes in clinical practice.
4. Materials and Methods
Bacterial Strains and Study Design: a total of 130
P. aeruginosa isolates were obtained from clinical samples, including bronchoalveolar lavage (BAL), tracheobronchial aspirates (TBA), and blood cultures. These samples were collected from the Microbiology Service at the University Hospital Virgen del Rocío in Seville, Spain. Among them, 21 isolates exhibited resistance to meropenem (MER), 14 were intermediate (susceptible at increased exposure), while 85 were classified as susceptible (
Supplementary Table S1). To evaluate the plates under clinical conditions, additional BAL and TBA samples (130 specimens) were tested over a one-month period. The
P. aeruginosa PAO1 strain served as a negative control, whereas one MER-resistant clinical isolate was used as positive control. Furthermore, other bacterial species, including
Stenotrophomonas maltophilia, Acinetobacter baumannii, Klebsiella pneumoniae, and
Escherichia coli, were incorporated to assess the chromogenic response of different species when cultured on plates.
Antimicrobial Susceptibility Testing: The minimum inhibitory concentration (MIC) of meropenem was determined using two methods: MicroScan WalkAway System (Beckman Coulter, USA), and Broth Microdilution Method (performed according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines) [
20]. All susceptibility tests were conducted in triplicate, using freshly prepared plates and bacterial inocula for each experiment. MIC results were interpreted based on EUCAST clinical breakpoints, classifying isolates with MIC values of ≤ 2 mg/L as susceptible and those with MICs > 2 mg/L as resistant (although > 2 to 8 mg/L is classified as intermediate, susceptible at increased exposure, we classify it as resistant).
Selective medium for MER resistance: For optimal bacterial screening, CLED agar (Biomerieux, Paris, France) was prepared following the manufacturer’s instructions. The medium was supplemented with meropenem (Sigma-Aldrich, St. Louis, MO, USA) at a final concentration of 0.425 mg/L to facilitate the selective growth of resistant isolates. To minimize contamination by Gram-positive bacteria and fungi, vancomycin (Duchefa Biochemie, Haarlem, Netherlands) and amphotericin B (Acros Organics, New Jersey, USA) were incorporated at final concentrations of 20 μg/mL and 5 μg/mL, respectively. These additions effectively inhibited the growth of
Enterococcus spp.,
Streptococcus spp.,
Staphylococcus spp., and fungal species. For visual differentiation of BIChromET medium [
16], resazurin (1%) was included in the formulation. This allowed distinction between lactose-fermenting bacteria, which appeared as light beige colonies, and non-fermenting bacteria, which formed purple colonies. The stock solutions of meropenem, vancomycin, and amphotericin B were prepared as described in
Table 1. CLED powder was dissolved in distilled water and sterilized by autoclaving at 121°C for 30 minutes. Once the medium cooled to 56°C, the antibiotic stock solutions were added (
Table 3). The prepared plates were stored at 4°C, protected from direct light, and remained viable for up to two weeks before use.
Evaluation Assay: The sensitivity and specificity cut-off values for detecting MER-resistant
P. aeruginosa were established at 1 x 10^3 CFU/mL, considering the positive results of only the sample, of which the isolates were recovered onto the selective medium, which were plated at concentrations corresponding to >1 x 10^3 CFU/mL. This cutoff value was fixed considering that BAL and TBA are positive when the 1 x 10^4 CFU/mL bacteria are recovered from the clinical sample. Starting with a 0.5 McFarland standard (an inoculum of 1.5 x 10^8 CFU/mL), serial 10-fold dilutions were made in 0.85% saline solution, and 100 μL aliquots of each dilution from 10^4 to 10^8 CFU/mL were plated onto the selective medium. To quantify the viable bacteria in each dilution step, tryptic soy agar plates were inoculated concomitantly with 100 μL of each suspension and incubated overnight at 37
oC. Viable colonies were counted the following day. When no growth was observed after 18 h, incubation was extended up to 48 h to assess the negativity of the culture. The medium was designed to detect resistant
P. aeruginosa from 10^4 to 10^8 CFU/mL based on the IDSA guidelines [
21]. It established that a BAL culture was positive when more than 10^4 CFU/mL bacteria were recovered from the BAL. After evaluation with a collection of 130
P. aeruginosa, the results were analyzed for each dilution of bacterial concentration used in this study.
Validation with Clinical Samples: To assess the performance of the selective medium in clinical settings, a total of 130 respiratory samples, including 62 BAL and 68 TBA specimens collected from the University Hospital Virgen del Rocío, were analyzed. Each sample (100 μL) was inoculated onto plates and incubated at 37°C overnight. Following incubation, colonies displaying distinct morphology, size, and pigmentation were selected for further characterization. Identification was performed via mass spectrometry, while antimicrobial resistance profiling for non-
P. aeruginosa isolates was assessed using gradient strips (
Supplementary Table S2). For
P. aeruginosa isolates, meropenem resistance was confirmed through broth microdilution (BMD) testing. The results were interpreted according to the 2024 EUCAST breakpoints and compared with susceptibility data obtained from the Microbiology Service using the MicroScan WalkAway system (Beckman Coulter, USA).
Statistical analysis: To evaluate the performance of the selective medium, specificity (the proportion of MER-susceptible isolates correctly identified) and sensitivity (the proportion of MER-resistant isolates correctly detected) were calculated for each bacterial concentration used in the evaluation. This analysis helped determine the medium’s limitations, assess its performance across different bacterial loads, and identify potential strategies to optimize its accuracy. Additionally, 95% confidence intervals (CIs), positive predictive value (PPV), and negative predictive value (NPV) were estimated to further assess the reliability of the medium. For the validation phase using clinical specimens (BAL and TBA), sensitivity, specificity, and their respective 95% CIs were calculated to determine the medium’s effectiveness in detecting meropenem resistance in P. aeruginosa. Broth microdilution was used as the gold standard method for comparison. In the clinical evaluation step, true positive results were considered when a MER-resistant or intermediate P. aeruginosa (BMD MIC) growth in the plates, while the absence of growth in the plates when a MER-susceptible P. aeruginosa (BMD MIC) were present in the BAL/TBA samples were considered true negative. Moreover, the endpoints were considered in categorical agreement when the results were in the same susceptibility category (regardless of the MIC) for P. aeruginosa. VME is a very major error (false susceptibility), and ME is a major error (false resistance).
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Figure S1: Colour change of the media with different fermenter and non-fermenter species. Left plate picture correspond to the original colour of the plate before bacterial growth. (A) Stenotrophomonas maltophilia, (B) Acinetobacter baumannii, (C) Pseudomonas aeruginosa, (D) Klebsiella pneumoniae, and (E) Escherichia coli.; Table S1: Raw data of the evaluation step for meropenem; Table S2: Raw data of the clinical evaluation with 130 clinical specimens (TBA and BAL).
Author Contributions
Conceptualization, J.M.O.d.l.R. and Á.R.-V.; Methodology, J.M.O.d.l.R., Á.R.-V. and G.M.-G.; Validation, J.M.O.d.l.R., G.M.-G., and C.C.M.; Investigation, J.M.O.d.l.R., G.M.-G., and C.C.M.; Resources, J.M.O.d.l.R., G.M.-G., Á.R.-V., J.M.C. and J.A.L.; Writing—original draft, C.C.M .,and J.M.O.d.l.R.; Writing—review and editing, Á.R.-V., G.M.-G., J.M.C. and J.A.L.; Supervision, J.A.L.; Funding acquisition, J.M.O.d.l.R., G.M.-G., Á.R.-V., J.M.C. and J.A.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study has been funded by Instituto de Salud Carlos III (ISCIII) through the projects “PI22/01464 and PI23/01760” and co-funded by the European Union. JMOR is supported by the Subprograme Sara Borrell, Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Ciencia, Innovación y Universidades, Spain (CD21/00098). A.R.V. is supported by the Subprograme Juan Rodés, Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Ciencia, Innovación y Universidades, Spain (JR20/00023). GM-G has received funding from the Andalusia Government in the grants for human resources reinforcement in the research activity (Acción B de refuerzos de larga duración).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; El Solh, A.A. Executive Summary: Management of Adults with Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Ong, D.S.Y.; Jongerden, I.P.R.; Buiting, A.G.; Hall, M.A.L.-V.; Ben Speelberg, B.; Kesecioglu, J.; Bonten, M.J.M. Antibiotic exposure and resistance development in Pseudomonas aeruginosa and Enterobacter species in intensive care units*. Crit. Care Med. 2011, 39, 2458–2463. [Google Scholar] [CrossRef] [PubMed]
- Solé, M.; Fàbrega, A.; Cobos-Trigueros, N.; Zamorano, L.; Ferrer-Navarro, M.; Ballesté-Delpierre, C.; Reustle, A.; Castro, P.; Nicolás, J.M.; Oliver, A.; et al. In vivoevolution of resistance ofPseudomonas aeruginosastrains isolated from patients admitted to an intensive care unit: mechanisms of resistance and antimicrobial exposure. J. Antimicrob. Chemother. 2015, 70, 3004–3013. [Google Scholar] [CrossRef] [PubMed]
- Farrington, N.; Dubey, V.; Johnson, A.; Horner, I.; Stevenson, A.; Unsworth, J.; Jimenez-Valverde, A.; Schwartz, J.; Das, S.; Hope, W.; et al. Molecular pharmacodynamics of meropenem for nosocomial pneumonia caused by Pseudomonas aeruginosa. mBio 2024, 15, e0316523. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, D.; Das Talukdar, A.; Choudhury, M.D.; Maurya, A.P.; Paul, D.; Chanda, D.D.; Chakravorty, A.; Bhattacharjee, A. Transcriptional Analysis of MexAB-OprM Efflux Pumps System of Pseudomonas aeruginosa and Its Role in Carbapenem Resistance in a Tertiary Referral Hospital in India. PLOS ONE 2015, 10, e0133842. [Google Scholar] [CrossRef] [PubMed]
- Wheatley, R.; Caballero, J.D.; Kapel, N.; de Winter, F.H.R.; Jangir, P.; Quinn, A.; del Barrio-Tofiño, E.; López-Causapé, C.; Hedge, J.; Torrens, G.; et al. Rapid evolution and host immunity drive the rise and fall of carbapenem resistance during an acute Pseudomonas aeruginosa infection. Nat. Commun. 2021, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Lee, N.; Cilloniz, C.; Vila, J.; Van der Eerden, M. Laboratory diagnosis of pneumonia in the molecular age. Eur. Respir. J. 2016, 48, 1764–1778. [Google Scholar] [CrossRef] [PubMed]
- Weinmaier, T.; Conzemius, R.; Bergman, Y.; Lewis, S.; Jacobs, E.B.; Tamma, P.D.; Materna, A.; Weinberger, J.; Beisken, S.; Simner, P.J. Validation and Application of Long-Read Whole-Genome Sequencing for Antimicrobial Resistance Gene Detection and Antimicrobial Susceptibility Testing. Antimicrob. Agents Chemother. 2023, 67, e0107222. [Google Scholar] [CrossRef] [PubMed]
- Luyt, C.-E.; Aubry, A.; Lu, Q.; Micaelo, M.; Bréchot, N.; Brossier, F.; Brisson, H.; Rouby, J.-J.; Trouillet, J.-L.; Combes, A.; et al. Imipenem, Meropenem, or Doripenem To Treat Patients with Pseudomonas aeruginosa Ventilator-Associated Pneumonia. Antimicrob. Agents Chemother. 2014, 58, 1372–1380. [Google Scholar] [CrossRef] [PubMed]
- Garcinuño, P.; Santibañez, M.; Gimeno, L.; Sánchez-Bautista, A.; Coy, J.; Sánchez-Paya, J.; Boix, V.; Merino, E.; Portilla, J.; Rodríguez, J.C. Empirical monotherapy with meropenem or combination therapy: the microbiological point of view. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1851–1855. [Google Scholar] [CrossRef] [PubMed]
- Filho, L.S.; Eagye, K.J.; Kuti, J.L.; Nicolau, D.P. Addressing resistance evolution in Pseudomonas aeruginosa using pharmacodynamic modelling: application to meropenem dosage and combination therapy. Clin. Microbiol. Infect. 2007, 13, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Oshima, K.; Nakamura, S.; Iwanaga, N.; Takemoto, K.; Miyazaki, T.; Yanagihara, K.; Miyazaki, Y.; Mukae, H.; Kohno, S.; Izumikawa, K. Efficacy of High-Dose Meropenem (Six Grams per Day) in Treatment of Experimental Murine Pneumonia Induced by Meropenem-Resistant Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2017, 61, e02056–16. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.-Y.; Fujitani, S.; Quintiliani, R.; Yu, V.L. Pneumonia Due to Pseudomonas aeruginosa: part II: antimicrobial resistance, pharmacodynamic concepts, and antibiotic therapy. Chest 2011, 139, 1172–1185. [Google Scholar] [CrossRef] [PubMed]
- El Solh, A.A.; Alhajhusain, A. Update on the treatment of Pseudomonas aeruginosa pneumonia. J. Antimicrob. Chemother. 2009, 64, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P.; Zhanel, G.G. Pseudomonas aeruginosa Pneumonia: Evolution of Antimicrobial Resistance and Implications for Therapy. Semin. Respir. Crit. Care Med. 2022, 43, 191–218. [Google Scholar] [CrossRef] [PubMed]
- de la Rosa, J.M.O.; Rodríguez-Villodres, Á.; Martín-Gutiérrez, G.; Mairal, C.C.; Escobar, J.L.G.; Gálvez-Benítez, L.; Cisneros, J.M.; Lepe, J.A. BIChromET: A Chromogenic Culture Medium for Detection of Piperacillin/Tazobactam and Cefepime Resistance in Pseudomonas aeruginosa. Antibiotics 2023, 12, 1573. [Google Scholar] [CrossRef]
- Nordmann, P.; Fournier, C.; Poirel, L. A Selective Culture Medium for Screening Carbapenem Resistance in Pseudomonas spp. Microb. Drug Resist. 2021, 27, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Torrens, G.; van der Schalk, T.E.; Cortes-Lara, S.; Timbermont, L.; del Barrio-Tofiño, E.; Xavier, B.B.; Zamorano, L.; Lammens, C.; Ali, O.; Ruzin, A.; et al. Susceptibility profiles and resistance genomics of Pseudomonas aeruginosa isolates from European ICUs participating in the ASPIRE-ICU trial. J. Antimicrob. Chemother. 2022, 77, 1862–1872. [Google Scholar] [CrossRef]
- del Barrio-Tofiño, E.; Zamorano, L.; Cortes-Lara, S.; López-Causapé, C.; Sánchez-Diener, I.; Cabot, G.; Bou, G.; Martínez-Martínez, L.; Oliver, A.; GEMARA-SEIMC/REIPI Pseudomonas study Group; et al. Spanish nationwide survey on Pseudomonas aeruginosa antimicrobial resistance mechanisms and epidemiology. J. Antimicrob. Chemother. 2019, 74, 1825–1835. [Google Scholar] [CrossRef] [PubMed]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters.Version 15.0, 2025. https://www.eucast.
- Mandell, L.A.; Wunderink, R.G.; Anzueto, A.; Bartlett, J.G.; Campbell, G.D.; Dean, N.C.; Dowell, S.F.; File, T.M., Jr.; Musher, D.M.; Niederman, M.S.; et al. Infectious Diseases Society of America/American Thoracic Society Consensus Guidelines on the Management of Community-Acquired Pneumonia in Adults. Clin. Infect. Dis. 2007, 44 (Suppl. 2), S27–S72. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).