Submitted:
17 March 2025
Posted:
19 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Data Collection
2.3. Data Analysis
2.4. Operational Definitions
3. Results
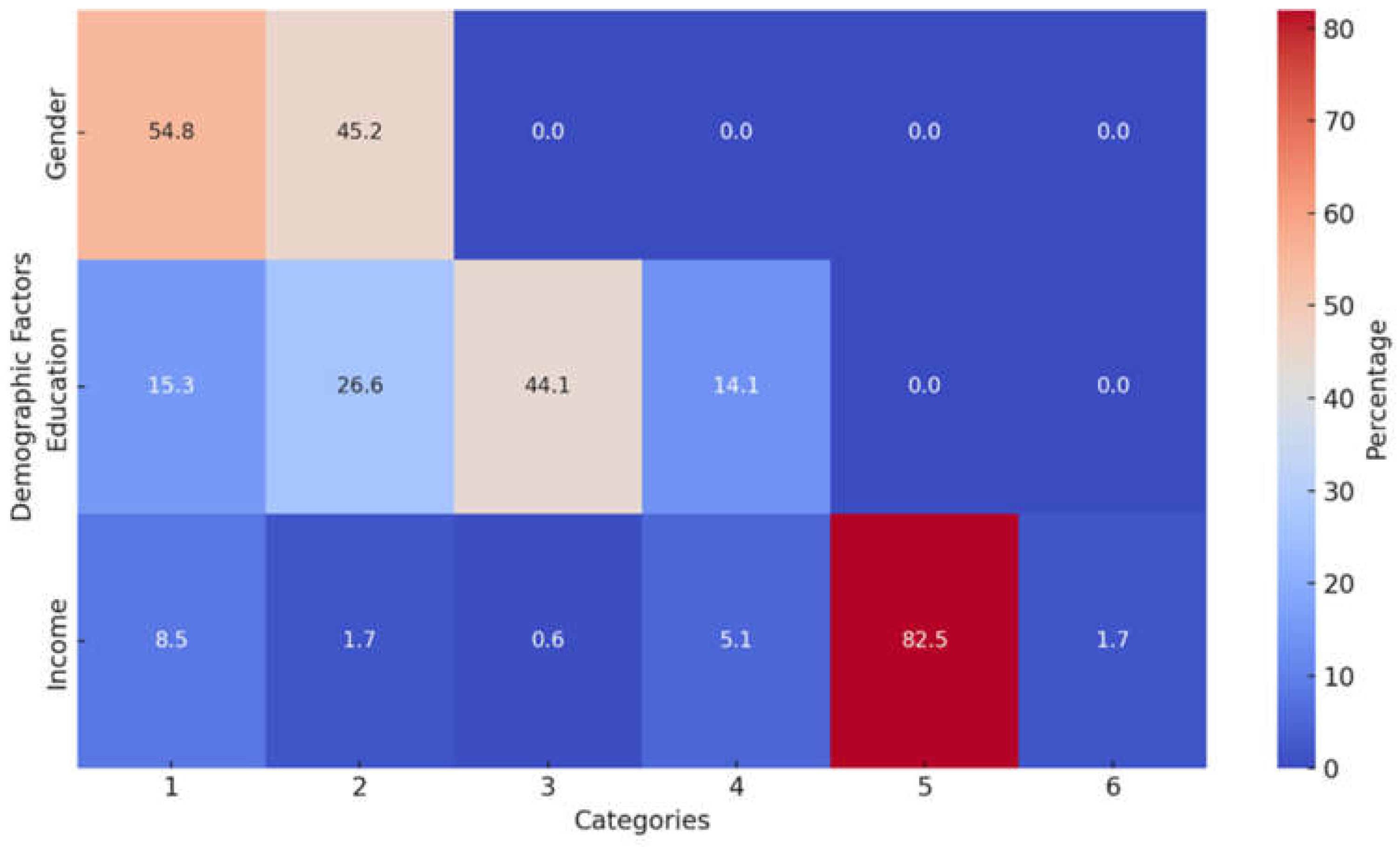
3.1. Demographic Analysis
3.2. Comorbidity and Sputum Conversion
3.3. Social History Factors and Sputum Conversion Times
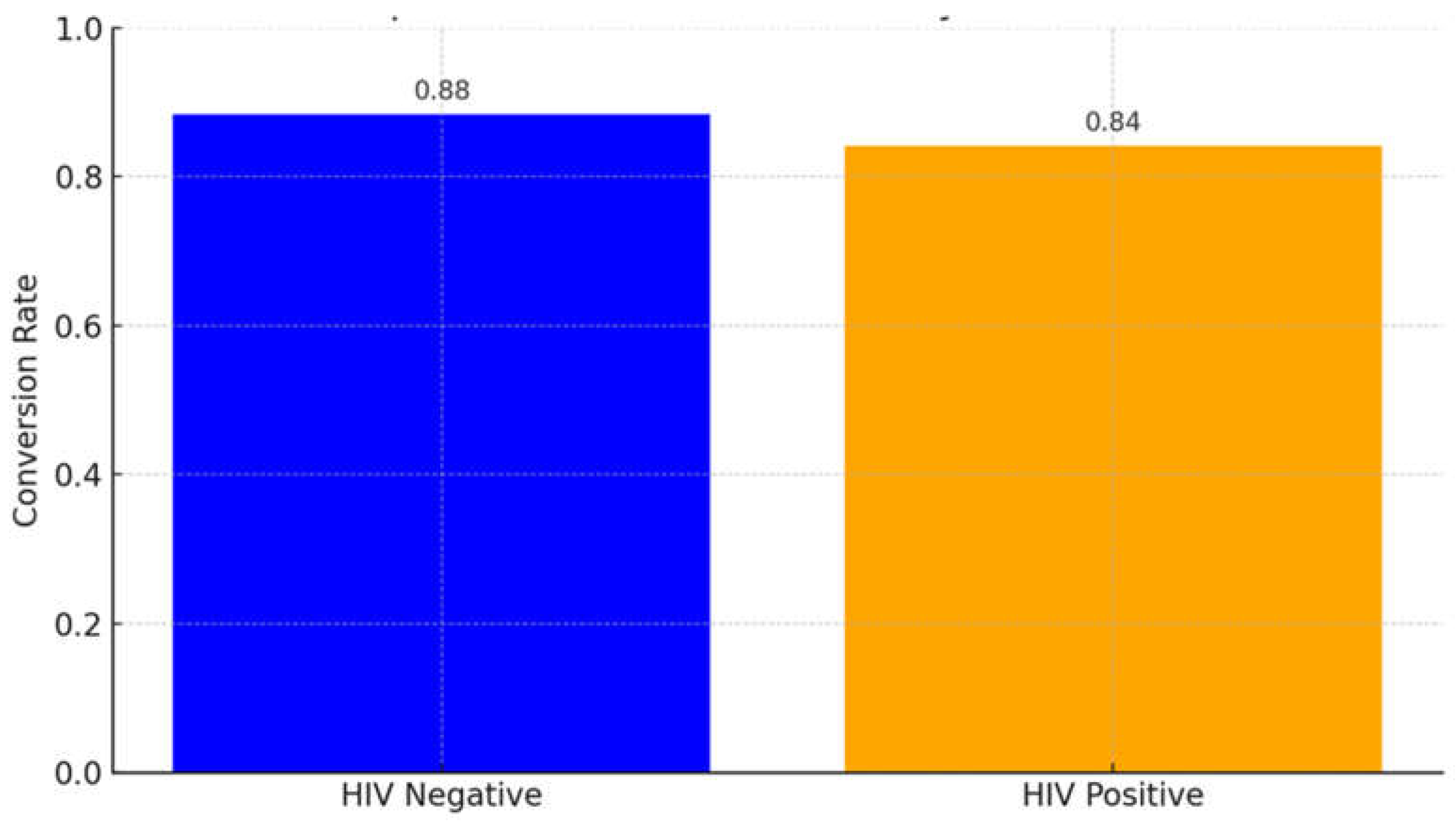
3.4. HIV Status and Sputum Conversion
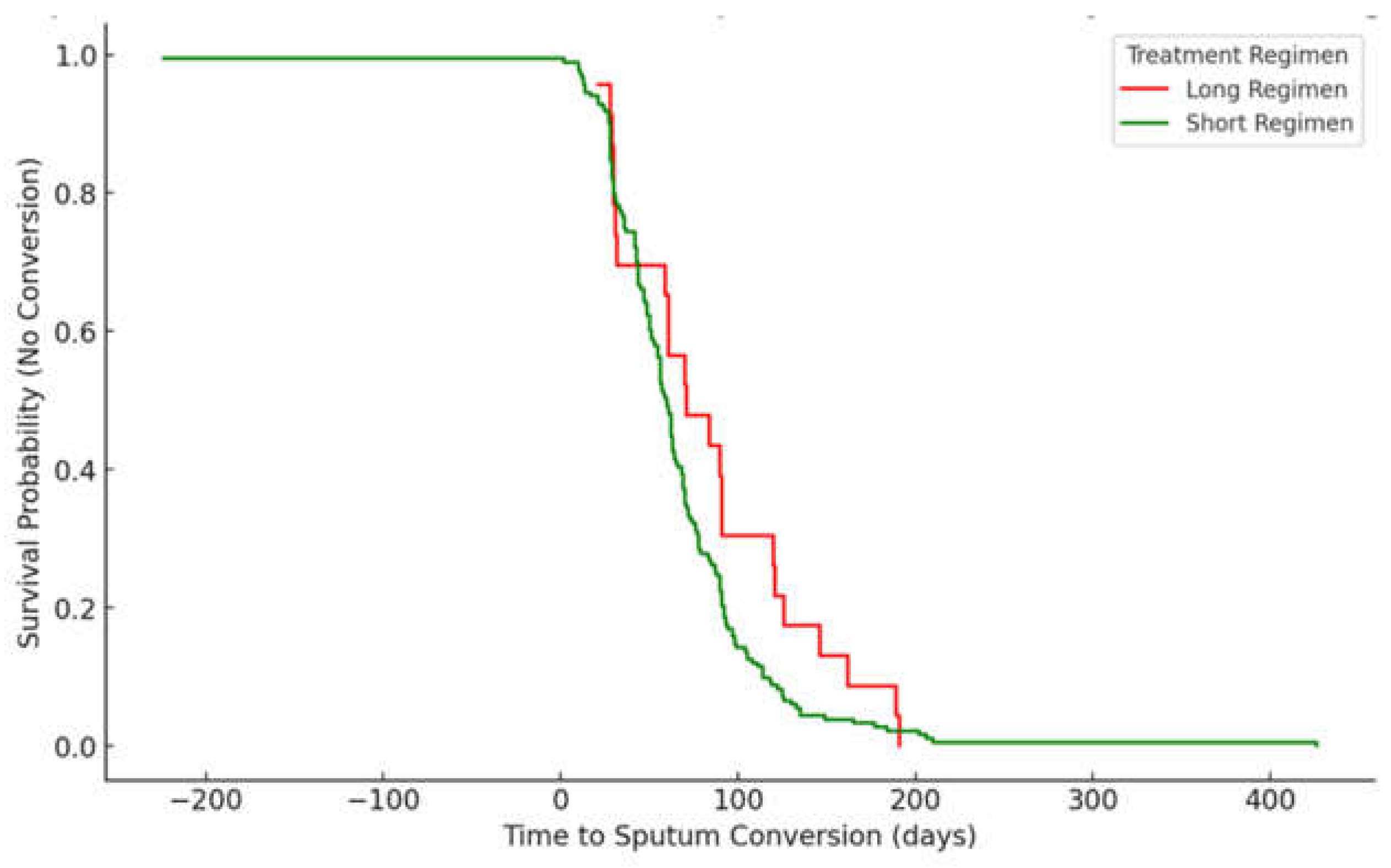
3.5. Time to Sputum Conversion Stratified by HIV Status (Kaplan-Meier Survival Curve)
3.6. Treatment Regimen
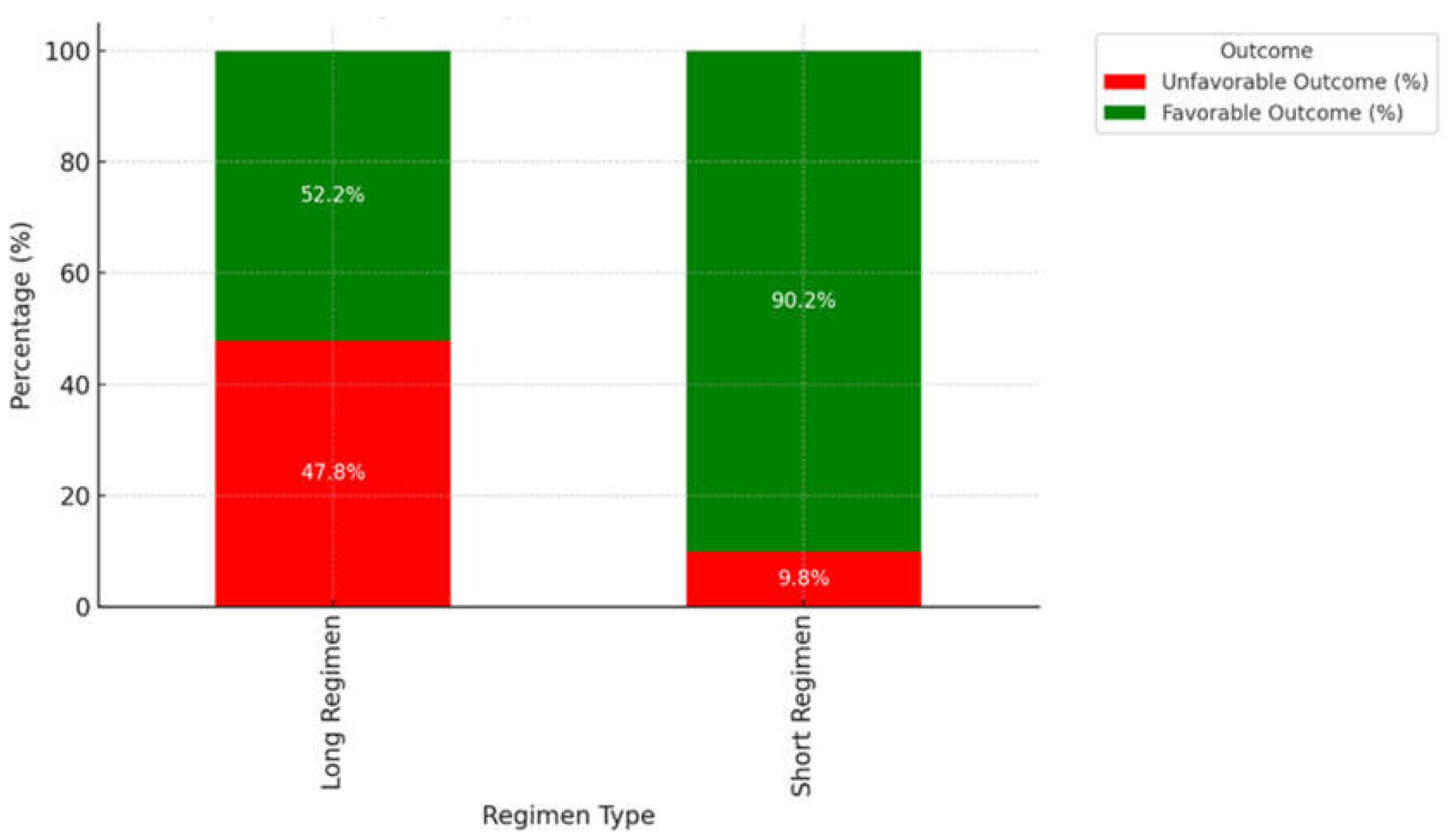
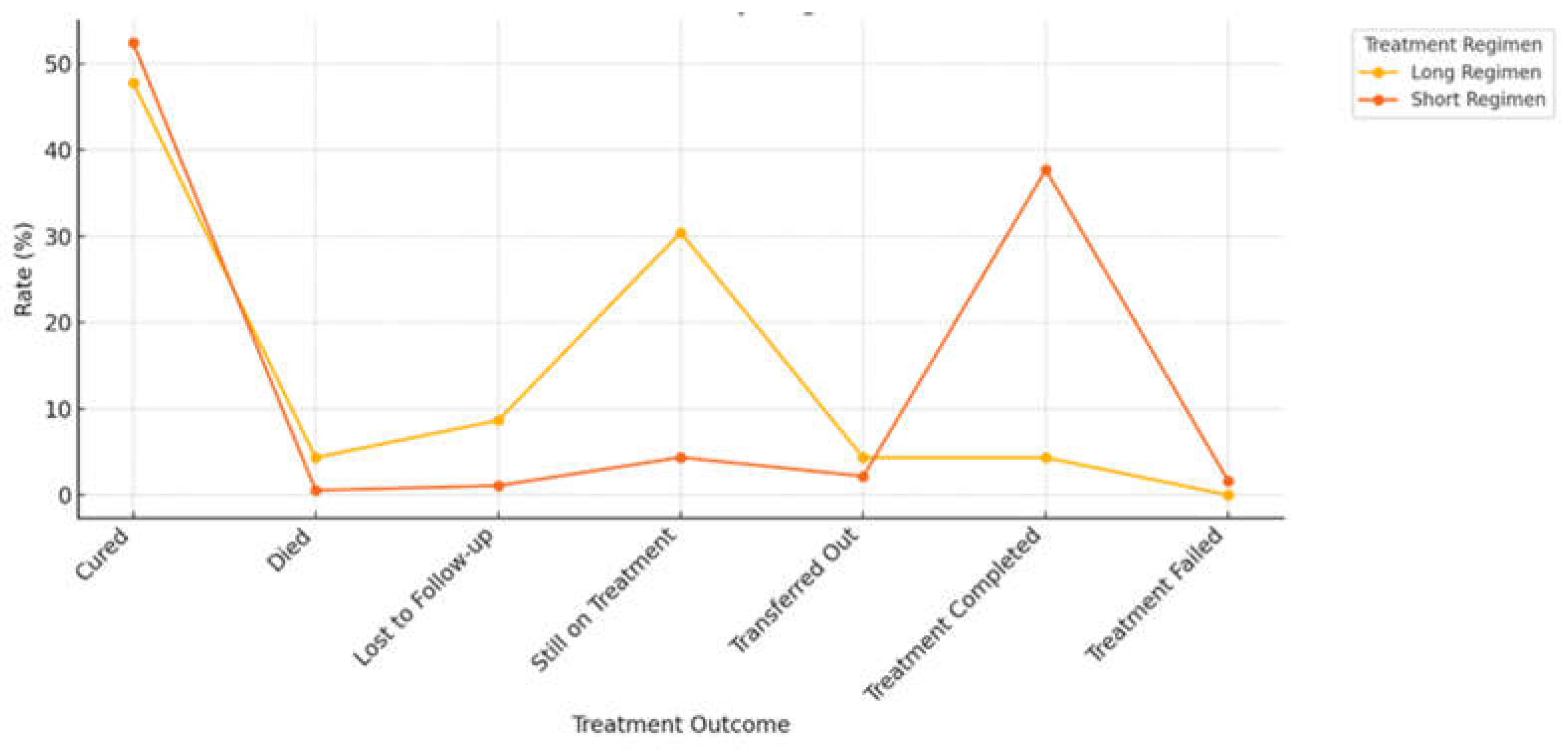
3.7. Impact of Regimen Type on Treatment Outcome
3.8. Treatment Outcomes Based on Different Types of Drug Regimen
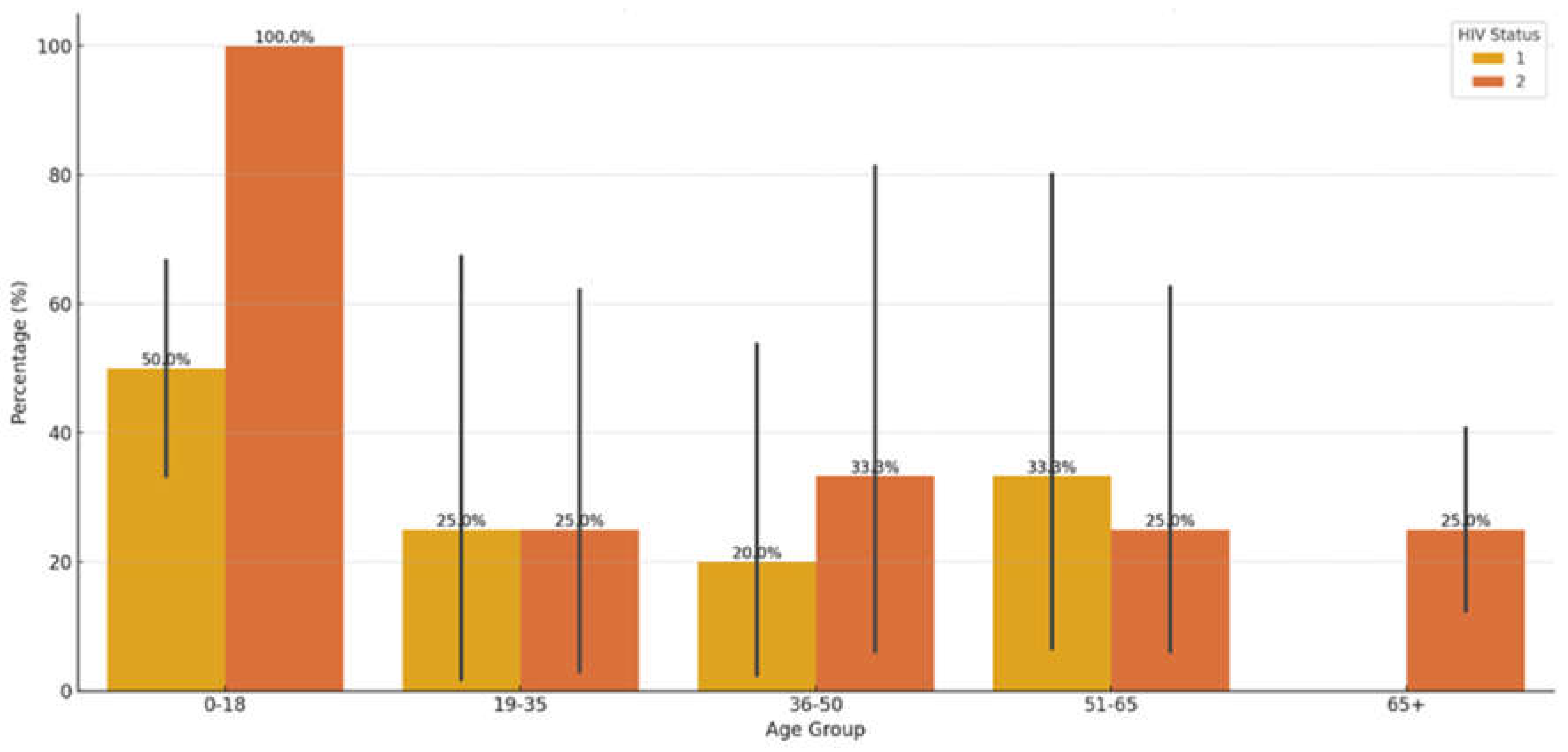
3.9. Relationship Between Age, HIV-Status and Comorbidities
3.10. Logistic Regression Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TB | Tuberculosis |
| MDR-TB | Multidrug-resistant tuberculosis |
| XDR-TB | Extensively drug-resistant tuberculosis |
| AFB | Acid-fast bacilli |
| DCSPs | Delayed sputum conversion patients |
| PTB | Pulmonary tuberculosis |
| STR | short treatment regimen |
| LTR | longer treatment regimen |
| SCC | sputum culture conversion |
| SLR | standard longer regimen |
| ART | Antiretroviral therapy |
References
- Ordonez, A.A.; Tucker, E.W.; Anderson, C.J.; Carter, C.L.; Ganatra, S.; Kaushal, D.; Kramnik, I.; Lin, P.L.; Madigan, C.A.; Mendez, S.; et al. Visualizing the dynamics of tuberculosis pathology using molecular imaging. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef] [PubMed]
- Shah, M., Dansky, Z., Nathavitharana, R., Behm, H., Brown, S., Dov, L., Fortune, D., Gadon, N.L., Gardner Toren, K., Graves, S, Haley, C.A., 2024. NTCA Guidelines for Respiratory Isolation and Restrictions to Reduce Transmission of Pulmonary Tuberculosis in Community Settings. Clin. Infect. Dis. 2024, ciae199.
- Weldemhret, L.; Atsbaha, A.H.; Bekuretsion, H.; Desta, A.; Legesse, L.; Kahsay, A.G.; Hagos, D. Time to Sputum Culture Conversion and Its Predictors Among Multidrug Resistant Tuberculosis Patients in Tigray, Northern Ethiopia: Retrospective Cohort Study. Infect. Drug Resist. 2023, 16, 3671–3681. [Google Scholar] [CrossRef]
- Global tuberculosis report 2024. Geneva: World Health Organization; 2024. Licence: CC BY-NC-SA 3.0 IGO (Accessed 5 January 2025).
- World Health Organization. Global tuberculosis report 2021. Geneva,Switzerland: World Health Organization; 2021 (Accessed 5 January 2025).
- Paradkar, M.S.; Pradhan, N.N.; Balaji, S.; Gaikwad, S.N.; Chavan, A.; Dharmashale, S.N.; Sahasrabudhe, T.; Lokhande, R.; Deshmukh, S.A.; Barthwal, M.; et al. Early Microbiologic Markers of Pulmonary Tuberculosis Treatment Outcomes. Ann. Am. Thorac. Soc. 2023, 20, 1760–1768. [Google Scholar] [CrossRef]
- Abebe, M.; Atnafu, A.; Tilahun, M.; Sero, N.; Neway, S.; Alemu, M.; Tesfaye, G.; Mihret, A.; Bobosha, K.; Wan, C. Determinants of sputum culture conversion time in multidrug-resistant tuberculosis patients in ALERT comprehensive specialized hospital, Addis Ababa, Ethiopia: A retrospective cohort study. PLOS ONE 2024, 19, e0304507. [Google Scholar] [CrossRef]
- Assemie, M.A.; Alene, M.; Petrucka, P.; Leshargie, C.T.; Ketema, D.B. Time to sputum culture conversion and its associated factors among multidrug-resistant tuberculosis patients in Eastern Africa: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020, 98, 230–236. [Google Scholar] [CrossRef]
- Ncha, R.; Variava, E.; Otwombe, K.; Kawonga, M.; Martinson, N.A. Predictors of time to sputum culture conversion in multi-drug-resistant tuberculosis and extensively drug-resistant tuberculosis in patients at Tshepong-Klerksdorp Hospital. South. Afr. J. Infect. Dis. 2019, 34, 1–8. [Google Scholar] [CrossRef]
- Lee, H.H.; Jo, K.-W.; Yim, J.-J.; Jeon, D.; Kang, H.; Shim, T.S. Interim treatment outcomes in multidrug-resistant tuberculosis patients treated sequentially with bedaquiline and delamanid. Int. J. Infect. Dis. 2020, 98, 478–485. [Google Scholar] [CrossRef]
- Djouma, F.N.; Noubom, M.; Ateudjieu, J.; Donfack, H. Delay in sputum smear conversion and outcomes of smear-positive tuberculosis patients: a retrospective cohort study in Bafoussam, Cameroon. BMC Infect. Dis. 2015, 15, 139–139. [Google Scholar] [CrossRef] [PubMed]
- Kateete, D.P.; Mbabazi, M.M.; Nakazzi, F.; Katabazi, F.A.; Kigozi, E.; Ssengooba, W.; Nakiyingi, L.; Namiiro, S.; Okwera, A.; Joloba, M.L.; et al. Sputum microbiota profiles of treatment-naïve TB patients in Uganda before and during first-line therapy. Sci. Rep. 2021, 11, 24486. [Google Scholar] [CrossRef]
- Bisognin, F.; Amodio, F.; Lombardi, G.; Reggiani, M.L.B.; Vanino, E.; Attard, L.; Tadolini, M.; Re, M.C.; Monte, P.D. Predictors of time to sputum smear conversion in patients with pulmonary tuberculosis under treatment. New Microbiologica 2018, 42, 171–175. [Google Scholar]
- Ibrahim, M.N.; Husain, N.R.N.; Daud, A.; Chinnayah, T. Epidemiology and Risk Factors of Delayed Sputum Smear Conversion in Malaysian Aborigines with Smear-Positive Pulmonary Tuberculosis. Int. J. Environ. Res. Public Heal. 2022, 19, 2365. [Google Scholar] [CrossRef]
- Gamachu, M.; Deressa, A.; Birhanu, A.; Ayana, G.M.; Raru, T.B.; Negash, B.; Merga, B.T.; Alemu, A.; Ahmed, F.; Mohammed, A.; et al. Sputum smear conversion and treatment outcomes among drug-resistant pulmonary tuberculosis patients in eastern Ethiopia: A 9-years data analysis. Front. Med. 2022, 9, 1007757. [Google Scholar] [CrossRef]
- Pang, M.; Dai, X.; Wang, N.; Yi, J.; Sun, S.; Miao, H.; Zhang, J.; Zhang, H.; Li, J.; Ding, B.; et al. A study on factors influencing delayed sputum conversion in newly diagnosed pulmonary tuberculosis based on bacteriology and genomics. Sci. Rep. 2024, 14, 18550. [Google Scholar] [CrossRef]
- Asemahagn, M.A. Sputum smear conversion and associated factors among smear-positive pulmonary tuberculosis patients in East Gojjam Zone, Northwest Ethiopia: a longitudinal study. BMC Pulm. Med. 2021, 21, 118–210. [Google Scholar] [CrossRef] [PubMed]
- Hansoti, B.; Mishra, A.; Rao, A.; Chimoyi, L.; Redd, A.D.; Reynolds, S.J.; Stead, D.F.; Black, J.; Maharaj, R.; Hahn, E.; et al. The geography of emergency department-based HIV testing in South Africa: Can patients link to care? eClinicalMedicine 2021, 40, 101091. [Google Scholar] [CrossRef] [PubMed]
- UNICEF commemorates World AIDS Day 2024 at launch of Eastern Cape Global Alliance chapter. Available online at: https://www.unicef.org/southafrica/press-releases/unicef-commemorates-world-aids-day-2024-launch-eastern-cape-global-alliance-chapter#:~:text=The%20Eastern%20Cape%20province%20is,Eastern%20Cape%20province%20in%202023 [accessed 17 March 2025].
- Peltzer, K.; Davids, A. Lay Counsellors' Experiences of Delivering HIV Counselling Services in Public Health Facilities in a Eastern Cape Province District of South Africa. J. Psychol. Afr. 2011, 21, 53–61. [Google Scholar] [CrossRef]
- Statistics South Africa Available online: https://www.statssa.gov.za/?p=16760 [accessed 10 March 2025].
- Kim, J.; Kwak, N.; Lee, H.Y.; Kim, T.S.; Kim, C.-K.; Han, S.K.; Yim, J.-J. Effect of drug resistance on negative conversion of sputum culture in patients with pulmonary tuberculosis. Int. J. Infect. Dis. 2016, 42, 64–68. [Google Scholar] [CrossRef]
- Tierney, D.B.; Franke, M.F.; Becerra, M.C.; Virú, F.A.A.; Bonilla, C.A.; Sánchez, E.; Guerra, D.; Muñoz, M.; Llaro, K.; Palacios, E.; et al. Time to Culture Conversion and Regimen Composition in Multidrug-Resistant Tuberculosis Treatment. PLOS ONE 2014, 9, e108035. [Google Scholar] [CrossRef]
- Rieu, R.; Chang, C.; Collin, S.M.; Fazekas, J.; Dassanaike, S.; Abbara, A.; Davidson, R.N. Time to detection in liquid culture of sputum in pulmonary MDR-TB does not predict culture conversion for early discharge. J. Antimicrob. Chemother. 2016, 71, 803–806. [Google Scholar] [CrossRef]
- Putri, F.A.; Burhan, E.; Nawas, A.; Soepandi, P.Z.; Sutoyo, D.K.; Agustin, H.; Isbaniah, F.; Dowdy, D.W. Body mass index predictive of sputum culture conversion among MDR-TB patients in Indonesia. Int. J. Tuberc. Lung Dis. 2014, 18, 564–570. [Google Scholar] [CrossRef]
- Parikh, R.; Nataraj, G.; Kanade, S.; Khatri, V.; Mehta, P. Time to sputum conversion in smear positive pulmonary TB patients on category I DOTS and factors delaying it. J. Assoc. Physicians India 2012, 60, 22–26. [Google Scholar]
- Shah, N.S.; Pratt, R.; Armstrong, L.; Robison, V.; Castro, K.G.; Cegielski, J.P. Extensively Drug-Resistant Tuberculosis in the United States, 1993-2007. JAMA 2008, 300, 2153–2160. [Google Scholar] [CrossRef] [PubMed]
- Alzarea, A.I.; Saifullah, A.; Khan, Y.H.; Alanazi, A.S.; Alatawi, A.D.; Algarni, M.A.; Almalki, Z.S.; Alahmari, A.K.; Alhassan, H.H.; Mallhi, T.H. Evaluation of time to sputum smear conversion and its association with treatment outcomes among drug-resistant tuberculosis patients: a retrospective record-reviewing study. Front. Pharmacol. 2024, 15, 1370344. [Google Scholar] [CrossRef]
- Holtz, T.H.; Sternberg, M.; Kammerer, S.; Laserson, K.F.; Riekstina, V.; Zarovska, E.; Skripconoka, V.; Wells, C.D.; Leimane, V. Time to Sputum Culture Conversion in Multidrug-Resistant Tuberculosis: Predictors and Relationship to Treatment Outcome. Ann. Intern. Med. 2006, 144, 650–659. [Google Scholar] [CrossRef]
- Rodriguez, M.; Monedero, I.; Caminero, J.A.; Encarnación, M.; Dominguez, Y.; Acosta, I.; Muñoz, E.; Camilo, E.; Martinez-Selmo, S.; Santos, S.d.L.; et al. Successful management of multidrug-resistant tuberculosis under programme conditions in the Dominican Republic. Int. J. Tuberc. Lung Dis. 2013, 17, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Basit, A.; Ahmad, N.; Khan, A.H.; Javaid, A.; Sulaiman, S.A.S.; Afridi, A.K.; Adnan, A.S.; Haq, I.U.; Shah, S.S.; Ahadi, A.; et al. Predictors of Two Months Culture Conversion in Multidrug-Resistant Tuberculosis: Findings from a Retrospective Cohort Study. PLOS ONE 2014, 9, e93206. [Google Scholar] [CrossRef]
- Velayutham, B.; Nair, D.; Kannan, T.; Padmapriyadarsini, C.; Sachdeva, K.S.; Bency, J.; Klinton, J.S.; Haldar, S.; Khanna, A.; Jayasankar, S.; et al. Factors associated with sputum culture conversion in multidrug-resistant pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 2016, 20, 1671–1676. [Google Scholar] [CrossRef]
- Joseph, P.; Desai, V.B.R.; Mohan, N.S.; Fredrick, J.S.; Ramachandran, R.; Raman, B.; Wares, F.; Ramachandran, R.; Thomas, A. Outcome of standardized treatment for patients with MDR-TB from Tamil Nadu, India. 2011, 133, 529–534.
- Singla, R.; Sarin, R.; Khalid, U.; Mathuria, K.; Singla, N.; Jaiswal, A.; Puri, M.M.; Visalakshi, P.; Behera, D. Seven-year DOTS-Plus pilot experiencein India: results, constraints and issues. Int J Tuberc Lung Dis, 2009, 13: 976–981.
- Brust, J.C.M.; Lygizos, M.; Chaiyachati, K.; Scott, M.; van der Merwe, T.L.; Moll, A.P.; Li, X.; Loveday, M.; Bamber, S.A.; Lalloo, U.G.; et al. Culture Conversion Among HIV Co-Infected Multidrug-Resistant Tuberculosis Patients in Tugela Ferry, South Africa. PLOS ONE 2011, 6, e15841. [Google Scholar] [CrossRef]
- Akalu, T.Y.; Muchie, K.F.; Gelaye, K.A. Time to sputum culture conversion and its determinants among Multi-drug resistant Tuberculosis patients at public hospitals of the Amhara Regional State: A multicenter retrospective follow up study. PLOS ONE 2018, 13, e0199320. [Google Scholar] [CrossRef]
- Shibabaw, A.; Gelaw, B.; Wang, S.-H.; Tessema, B. Time to sputum smear and culture conversions in multidrug resistant tuberculosis at University of Gondar Hospital, Northwest Ethiopia. PLOS ONE 2018, 13, e0198080. [Google Scholar] [CrossRef]
- Wenlu, Y.; Xia, Z.; Chuntao, W.; Qiaolin, Y.; Xujue, X.; Rong, Y.; Dan, S.; Xi, Y.; Bin, W. Time to sputum culture conversion and its associated factors among drug-resistant tuberculosis patients: a systematic review and meta-analysis. BMC Infect. Dis. 2024, 24, 169. [Google Scholar] [CrossRef]
- Kurbatova, E.V.; Cegielski, J.P.; Lienhardt, C.; Akksilp, R.; Bayona, J.; Becerra, M.C.; Caoili, J.; Contreras, C.; Dalton, T.; Danilovits, M.; et al. Sputum culture conversion as a prognostic marker for end-of-treatment outcome in patients with multidrug-resistant tuberculosis: a secondary analysis of data from two observational cohort studies. Lancet Respir. Med. 2015, 3, 201–209. [Google Scholar] [CrossRef]
- Senkoro, M.; Mfinanga, S.G.; Mørkve, O. Smear microscopy and culture conversion rates among smear positive pulmonary tuberculosis patients by HIV status in Dar es Salaam, Tanzania. BMC Infect. Dis. 2010, 10, 210. [Google Scholar] [CrossRef]
- Hafkin, J.; Modongo, C.; Newcomb, C.; Lowenthal, E.; MacGregor, R.R.; Steenhoff, A.P.; Friedman, H.; Bisson, G.P. Impact of the human immunodeficiency virus on early multidrug-resistant tuberculosis treatment outcomes in Botswana. Int. J. Tuberc. Lung Dis. 2013, 17, 348–353. [Google Scholar] [CrossRef]
- Alakaye, O.J. Time to sputum culture conversion of Multi-Drug Resistant Tuberculosis in HIV positive versus HIV negative patients in Lesotho. University of Pretoria (South Africa). 2018.
- Wahid, A.; Ghafoor, A.; Khan, A.W.; Al-Worafi, Y.M.; Latif, A.; Shahwani, N.A.; Atif, M.; Saleem, F.; Ahmad, N. Comparative effectiveness of individualized longer and standardized shorter regimens in the treatment of multidrug resistant tuberculosis in a high burden country. Front. Pharmacol. 2022, 13, 973713. [Google Scholar] [CrossRef]
- Abidi, S.; Achar, J.; Neino, M.M.A.; Bang, D.; Benedetti, A.; Brode, S.; Campbell, J.R.; Casas, E.C.; Conradie, F.; Dravniece, G.; et al. Standardised shorter regimens versus individualised longer regimens for rifampin- or multidrug-resistant tuberculosis. Eur. Respir. J. 2020, 55, 1901467. [Google Scholar] [CrossRef] [PubMed]
- Karnan, A.; Jadhav, U.; Ghewade, B.; Ledwani, A.; Shivashankar, P. A Comprehensive Review on Long vs. Short Regimens in Multidrug-Resistant Tuberculosis (MDR-TB) Under Programmatic Management of Drug-Resistant Tuberculosis (PMDT). Cureus 2024, 16, e52706. [Google Scholar] [CrossRef]
- Lotz, J.-D.K.; Porter, J.; Conradie, H.; Boyles, T.; Gaunt, B.; Dimanda, S.; Cort, D. Treating drug-resistant tuberculosis in an era of shorter regimens: Insights from rural South Africa. South Afr. Med J. 2023, 113, 47–56. [Google Scholar] [CrossRef]
- Mleoh, L.; Mziray, S.R.; Tsere, D.; Koppelaar, I.; Mulder, C.; Lyakurwa, D. Shorter regimens improved treatment outcomes of multidrug-resistant tuberculosis patients in Tanzania in 2018 cohort. Trop. Med. Int. Heal. 2023, 28, 357–366. [Google Scholar] [CrossRef]
- Hayre, K.; Takele, M.K.; Birri, D.J. Tuberculosis treatment outcomes and associated factors at Alemgena Health Center, Sebeta, Oromia, Ethiopia. PLOS ONE 2024, 19, e0303797. [Google Scholar] [CrossRef]
- Leketa, M.M.; Zondi, S.; Cele, L.; Mathibe, M.; Ngwepe, P. Factors associated with unfavourable treatment outcomes among tuberculosis patients at health facilities of Maseru, Lesotho. South Afr. Fam. Pr. 2024, 66, 6–e6. [Google Scholar] [CrossRef]
- Massud, A.; Khan, A.H.; Sulaiman, S.A.S.; Ahmad, N.; Shafqat, M.; Ming, L.C. Unsuccessful treatment outcome and associated risk factors. A prospective study of DR-TB patients from a high burden country, Pakistan. PLOS ONE 2023, 18, e0287966. [Google Scholar] [CrossRef]
- Osório, D.; Munyangaju, I.; Nacarapa, E.; Nhangave, A.-V.; Ramos-Rincon, J.-M. Predictors of unfavourable tuberculosis treatment outcome in Bilene District, Gaza Province, Mozambique: A retrospective analysis, 2016 - 2019. South Afr. Med J. 2022, 112, 234–239. [Google Scholar] [CrossRef]
- Limenh, L.W.; Kasahun, A.E.; Sendekie, A.K.; Seid, A.M.; Mitku, M.L.; Fenta, E.T.; Workye, M.; Simegn, W.; Ayenew, W. Tuberculosis treatment outcomes and associated factors among tuberculosis patients treated at healthcare facilities of Motta Town, Northwest Ethiopia: a five-year retrospective study. Sci. Rep. 2024, 14, 7695. [Google Scholar] [CrossRef]
- Hosu, M.C.; Faye, L.M.; Apalata, T. Comorbidities and Treatment Outcomes in Patients Diagnosed with Drug-Resistant Tuberculosis in Rural Eastern Cape Province, South Africa. Diseases 2024, 12, 296. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




