1. Introduction
Modern implant designs have undergone extensive improvement over the past decades, with particular emphasis on optimizing surface morphology, chemistry, and macro-geometry to promote osseointegration and enhance clinical outcomes [
1,
2,
3]. Among the critical determinants of successful osseointegration, surface topography and surface chemistry are recognized as pivotal factors modulating the complex interplay between the implant and surrounding biological tissues [
4,
5,
6]. Numerous surface modification strategies have been developed to promote and accelerate the integration of titanium implants with host bone, including grit blasting, acid etching, anodization, calcium phosphate deposition, and functionalization with bioactive molecules [
7,
8]. These approaches are primarily designed to enhance early cell attachment, promote osteoblastic differentiation, and facilitate the formation of a stable bone-implant interface. In addition to the critical role of micro- and nano-topography in promoting mechanical interlocking and favorable biological responses, surface chemistry has emerged as an equally crucial factor in achieving successful implant integration. Specifically, the oxidation state and chemical composition of the titanium surface play critical roles in influencing protein adsorption, cell adhesion, and subsequent bone healing [
9]. It is well established that oxidized titanium surfaces demonstrate superior osteoconductive properties compared to untreated metallic titanium. However, a significant limitation arises from the phenomenon of time-dependent surface degradation, often referred to as "biologic aging", whereby titanium surfaces progressively accumulate hydrocarbons and organic contaminants from the ambient environment [
10,
11]. This accumulation leads to the deterioration of surface bioactivity, reducing the surface hydrophilicity and its capacity to promote optimal osseointegration, independent of its initial roughness or topography [
12].
Biologic aging is characterized by a gradual shift from a super-hydrophilic to a hydrophobic surface state, which diminishes the surface ability to interact with water and biological fluids, thereby impairing protein adsorption, osteogenic cell adhesion, and early bone healing events [
13,
14]. The phenomenon of photocatalytic activation of titanium dioxide (TiO₂) by ultraviolet (UV) light, first identified in 1997 [
15], has been shown to effectively reverse surface hydrophobicity and restore super-hydrophilic properties. This effect is attributed to the photoinduced decomposition of hydrocarbon contaminants and the concomitant densification of the oxide layer, resulting in a highly reactive titanium surface [
16].
In addition to UV photofunctionalization, plasma-based surface modification techniques have been extensively investigated for their ability to enhance titanium surface properties. Plasma treatments utilize ionized gases (e.g., oxygen, argon, nitrogen, or air) within a vacuum system to generate plasma, which effectively removes organic contaminants improving hydrophilicity [
17]. Plasma-treated titanium surfaces have demonstrated significantly enhanced in vitro biological performance, including improved adhesion and proliferation of osteoblasts, fibroblasts, and mesenchymal stem cells, as well as increased extracellular matrix protein deposition [
18,
19]. Furthermore, plasma activation has been shown to upregulate osteogenic signaling pathways, including the enhanced release of cytokines and growth factors conducive to bone regeneration and wound healing [
20].
Despite the evidence supporting plasma-induced surface modifications
in vitro, a significant gap remains regarding the translation of these findings into clinical practice, as few studies have examined the
in vivo effects of plasma-treated surfaces in human subjects under controlled conditions [
21]. Therefore, the present split-mouth randomized controlled clinical trial was designed to evaluate the impact of plasma-induced surface modification on the stability pattern of titanium implants placed in human subjects. In this study, each participant received both plasma-activated and non-activated implants in a controlled intra-individual comparison. Implant stability was assessed using standardized measurements over a 90-day period following insertion, allowing for longitudinal evaluation of both primary and secondary stability. The null hypothesis (H₀) tested in this investigation was that no statistically significant differences in primary or secondary stability would be observed between plasma-treated and untreated titanium implants throughout the 90-day follow-up period.
2. Materials and Methods
2.1. Study Design
This study was designed as a multicenter, single-blind, split-mouth, randomized controlled clinical trial with balanced (1:1) randomization. It was conducted by three experienced operators, who enrolled and treated patients between June and November 2024. The trial was reported in accordance with the CONSORT (CONsolidated Standards of Reporting Trials) guidelines. The study protocol was developed following the ethical principles outlined in the Fortaleza revision (2013) of the Declaration of Helsinki for research involving human subjects. Ethical approval was granted by the relevant committee (Comitato Etico Unico Regionale Friuli Venezia Giulia n. CEUR-2024-IND-9), and the trial was retrospectively registered in a public clinical trial registry (
www.clinicaltrials.gov - NCT06808724). Prior to the study initiation, a calibration meeting was held among all participating centers to discuss and standardize the operative protocols. Written instructions detailing the collection of experimental parameters were provided to each clinician to ensure consistent inter-examiner reliability. All participants received comprehensive information about the study protocol, the proposed treatment and its alternatives, as well as any potential risks. Informed consent was obtained in writing from all patients, who also authorized the use of their data for research purposes.
2.2. Patients Selection
All partially edentulous patients requiring the placement of two contralateral implants in pristine bone within the same arch were screened at the clinical centers for potential inclusion in this trial.
2.2.1. Inclusion Criteria
indication for two contralateral implants in the same arch, based on a thorough diagnosis and treatment plan;
presence of a residual alveolar ridge with a minimum available bone height of 8 mm and a minimum width of 6 mm at the planned implant sites, ensuring adequate dimensions for implant placement;
the alveolar ridge must be fully healed (at least 6 months after the loss or extraction of the corresponding tooth);
absence of regenerated bone;
plaque index lower than 25% and bleeding index lower than 20%;
buccolingual width of keratinized gingiva ≥ 4 mm;
patient age > 18 years;
patients must be able to review and understand the study protocol;
signed informed consent.
2.2.2. General Exclusion Criteria
myocardial infarction within the past 6 months;
uncontrolled coagulation disorders;
uncontrolled metabolic disorders;
radiotherapy in the head and neck region within the past 24 months;
current or past treatment with antiresorptive medications;
pregnancy;
poor motivation or unwillingness to attend follow-up visits;
alcohol or substance abuse.
2.2.3. Local Exclusion Criteria
2.Vacuum Plasma Surface Treatment
Implants allocated to the test group underwent vacuum plasma treatment immediately prior to placement. Each implant was retrieved from its sterile vial using a surgical mount and placed within a sealed plastic chamber of a specifically designed device (Plasma X Motion, MegaGen, Gyeongbuk, South Korea). A vacuum pump reduced the internal pressure to below 10 torr to remove air and surface contaminants. Once the vacuum was established, the implant was automatically connected to a high-voltage electrode delivering up to 3 kV in a dielectric barrier discharge configuration, generating plasma under low-pressure conditions (<13 mbar). Each plasma treatment cycle lasted approximately 50 seconds, during which plasma discharge further cleaned and activated the implant surface. The system operated in a closed environment, without the introduction of external gases.
2.Surgical Procedures
All patients received antibiotic and analgesic therapy, consisting of 2 g of amoxicillin administered 1 hour prior to surgery, followed by 1 g of amoxicillin every 12 hours for 6 days (for penicillin-allergic patients, clarithromycin 250 mg twice daily was prescribed). Ketoprofen 80 mg was administered as needed for pain control.
Under local anesthesia (4% articaine with adrenaline 1:100.000) a mid-crestal longitudinal incision was performed, ensuring the preservation of keratinized tissue. Implant osteotomies were performed at a minimum distance of 3.0 mm from adjacent teeth or implants, ensuring that at least 1 mm of bone surrounded the implants on both buccal and lingual aspects. The same drilling sequence was applied to both sites, with preparation for subcrestal placement of 2 mm. After opening the randomization envelopes, two identical titanium grade 4 implants were placed (AnyRidge 4.0x7 mm, 4.0x8.5 mm, 4.0x10 mm, or 4.0x11.5 mm; MegaGen, Gyeongbuk, South Korea). The implant surface treatment consisted in the incorporation of calcium ions into a sandblasted, large-grit, acid-etched (SLA) titanium substrate, generating a nanostructured calcium titanate layer chemically bonded to the implant surface (XPEED, MegaGen, Gyeongbuk, South Korea). The test implant was treated immediately prior to insertion with a vacuum plasma device as previously described (
Figure 1), while the control implant was inserted without any additional treatment.
The final insertion torque was recorded using a surgical motor (Implantmed, W&H, Bürmoos, Austria). If an implant showed an insertion torque below 25 Ncm, the patient was excluded from the study. Immediately after implant placement, straight transepithelial abutments (OCTA, MegaGen, Gyeongbuk, South Korea) measuring 3 mm in height were connected to the fixture with a torque of 25 Ncm. An independent assessor measured Implant Stability Quotient (ISQ) values at the abutment level in mesio-distal, disto-mesial, bucco-lingual, and linguo-buccal directions using a resonance frequency analysis device (Osstell Beacon, Osstell, Gothenburg, Sweden) with a specific transducer tightened to 5 Ncm (SmartPeg #74). For each implant, two ISQ values were recorded: a mesial value, calculated as the average of mesio-distal and disto-mesial measurements, and a buccal value, calculated as the average of bucco-lingual and linguo-buccal measurements. The flap was then sutured around the transepithelial abutments caps with single stitches and the Sentineri technique [
22] using a synthetic monofilament, to allow for non-submerged healing.
Sutures were removed 7 days after surgery and ISQ measurements were repeated by the same assessor at 7, 14, 21, 28, 42, 60, and 90 days postoperatively, following the same standardized protocol. After 4 months, implants were restored with screw-retained prostheses by a prosthodontist. Following prosthetic rehabilitation, patients received personalized oral hygiene instructions and were enrolled in a maintenance program with periodic recall visits to ensure peri-implant health.
2.5. Predictor and Outcome Variables
The primary independent variable was pre-operative implant surface activation using vacuum plasma device (treatment vs. no treatment).
Primary outcome measures:
implant osseointegration;
pattern of implant secondary stability assessed over the first 90 days following implant placement, as measured by ISQ values.
Secondary outcome measure:
2.6. Sample Size and Randomization
Sample size calculation was performed using a web-based software tool (
https://app.sampsize.org.uk). A total of 9 patients (18 implants) was determined to be necessary to detect a statistically significant difference in implant stability (ISQ values) at 4 weeks between the two groups (α = 0.05; power = 0.95), based on the expected difference in ISQ values derived from a recent study (3.64 ± 2.48) [
23]. Randomization was conducted by an independent investigator (AR), not involved in patient selection or treatment. A computer-generated randomization sequence was created using a balanced, randomly permuted block method (
www.random.org) to assign the two implants placed in each patient to either the test group or the control group. Implants allocated to the test group underwent vacuum plasma surface activation immediately before placement, while implants assigned to the control group were inserted without any surface treatment. Randomization codes were enclosed in numbered, identical, sealed, opaque envelopes, which were opened only after implant site preparation to ensure allocation concealment. The investigator performing the ISQ measurements was blinded to the allocation of implants to ensure unbiased assessment throughout the study period.
2.7. Statistical Analysis
Data analysis was performed by an independent investigator (AR) using SPSS software (version 26.0, IBM Corp., Armonk, NY, USA). Since no significant differences were detected between mesial and buccal ISQ values at any time point, implant stability was expressed as a single value, calculated as the mean of mesial and buccal measurements. The Shapiro–Wilk test was used to assess data normality. Longitudinal intra-group comparisons were analyzed using the Friedman test, while inter-group comparisons were performed using the two-sample Wilcoxon rank-sum test. For torque values, which followed a normal distribution, inter-group differences were evaluated using the T-test for independent samples. A p-value < 0.05 was considered indicative of statistical significance.
3. Results
3.1. Study Population
A total of 31 consecutive patients were screened for eligibility, and after the application of inclusion and exclusion criteria, 24 patients (18 males and 6 females; age range: 39–78 years; mean age: 58.1 ± 10.3 years; 11 smokers and 13 non-smokers) were enrolled in the present study. Each participant received two identical implants, for a total of 48 implants placed. Of these, 20 implants were inserted in the maxilla and 28 in the mandible. Regarding implant lengths, 2 patients received 7 mm-long implants, 9 patients received 8.5 mm-long implants, 12 patients received 10 mm-long implants, and 1 patient received 11.5 mm-long implants (
Table 1).
All surgical procedures were performed by three experienced clinicians (CS: 10 patients; MM: 6 patients; TL: 8 patients). No dropouts or losses to follow-up were recorded throughout the study period.
3.2. Clinical Outcomes
At 90 days post-placement, all 48 implants achieved successful osseointegration and were referred for prosthetic rehabilitation. No failures, complications, or adverse events, either local or systemic, were reported throughout the study period.
The mean peak insertion torque for all implants was 49.4 ± 14.0 Ncm (range: 25–80 Ncm). In the test group, the mean insertion torque was 49.7 ± 13.9 Ncm (range: 27–80 Ncm), while in the control group it was 49.0 ± 14.4 Ncm (range: 25–80 Ncm). T-test for independent samples revealed no statistically significant difference between groups (p = 0.86).
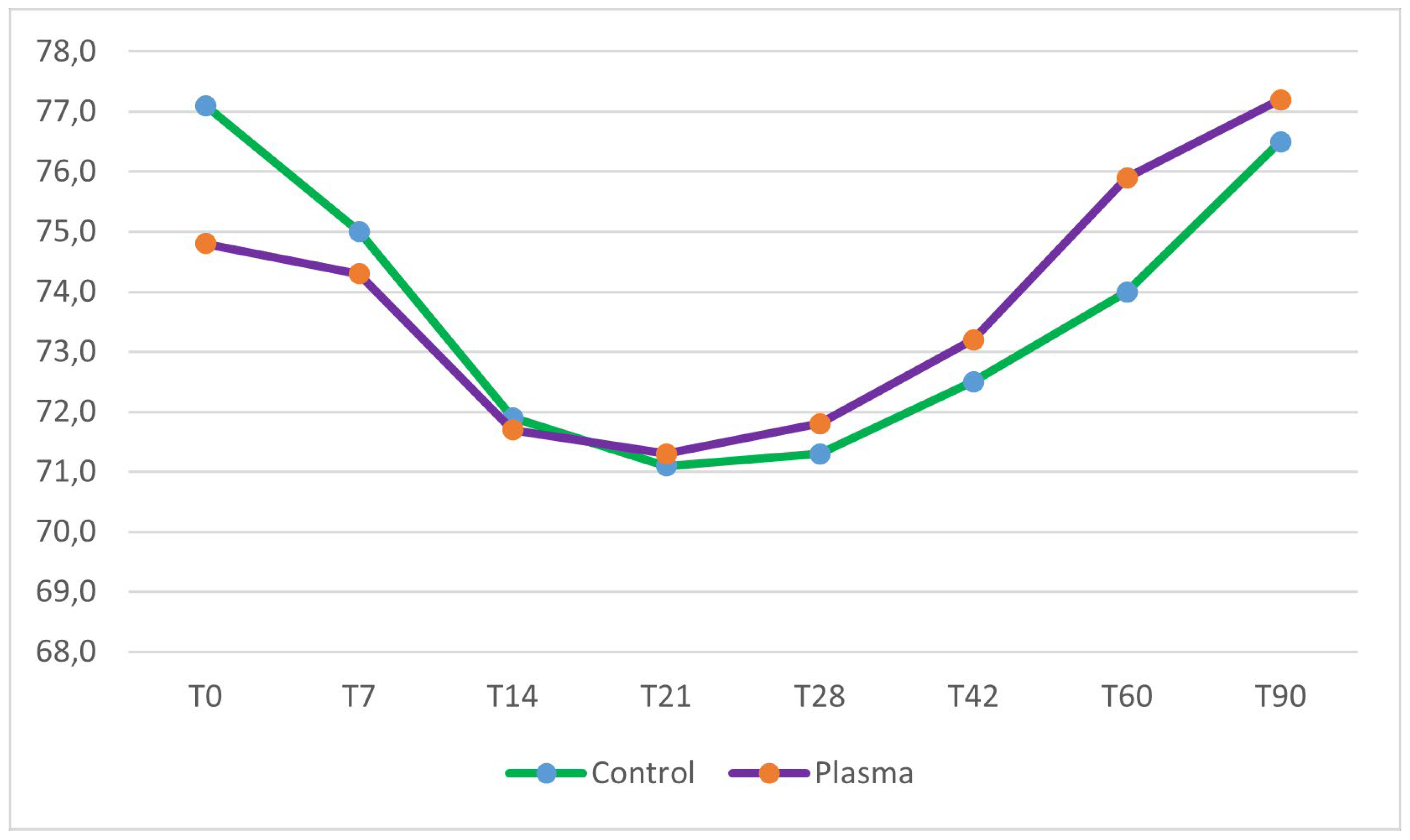
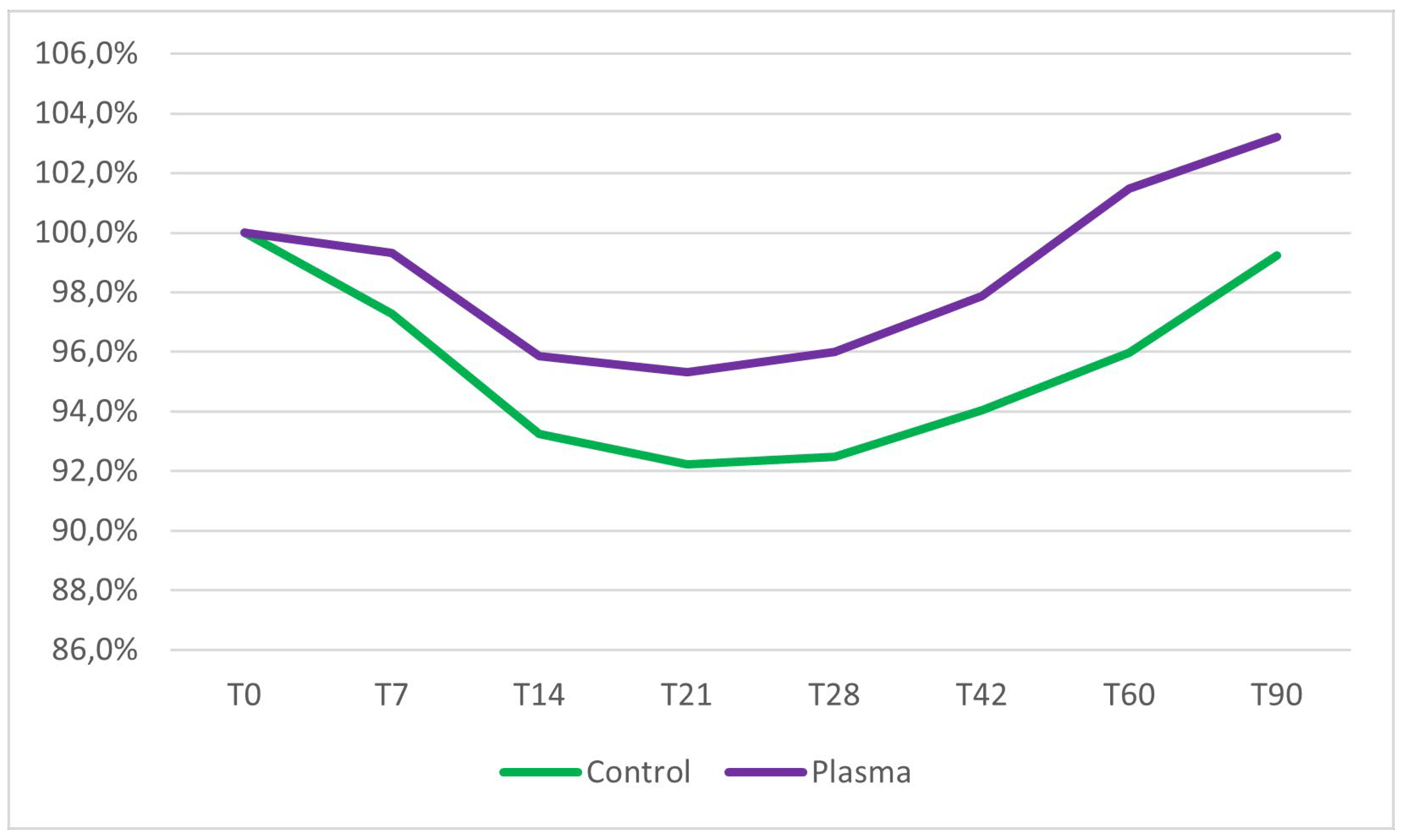
No significant differences in mean ISQ values at baseline were observed between the test group (74.8 ± 10.5) and the control group (77.1 ± 9.0) (two sample Wilcoxon rank-sum test, p = 0.41). Implant stability decreased in both groups during the first three weeks of healing. The lowest stability was observed at 21 days post-insertion, with a mean ISQ of 71.3 ± 11.2 in the test group, representing a 4.7% reduction from the mean primary stability, and a mean ISQ of 71.1 ± 14.2 in the control group, corresponding to a 7.8% reduction from the mean primary stability. Following the third week, implant stability progressively increased in both groups. However, only the test group implants demonstrated ISQ values at 60 and 90 days that were higher than their initial baseline stability (
Figure 2 and
Figure 3).
Inter-group comparisons, assessed using the two-sample Wilcoxon rank-sum test, revealed no statistically significant differences in ISQ values between the test and control groups from baseline (day 0) to day 42. However, at 60 and 90 days post-placement, ISQ values in the test group were significantly higher than those in the control group (p = 0.04 and p = 0.03, respectively). Intra-group analyses, performed using the Friedman test, identified significant changes in ISQ values over time within both groups throughout the entire observation period. A detailed summary of the results is provided in
Table 2.
4. Discussion
The present split-mouth randomized controlled clinical trial aimed to evaluate the effect of vacuum plasma treatment on early implant stability in partially edentulous patients. The findings demonstrated that while both plasma-treated and untreated implants exhibited a characteristic initial decrease in stability during the first three weeks post-placement [
24], implants subjected to plasma activation showed a superior recovery pattern, with statistically significant higher ISQ values at 60 and 90 days compared to control implants. Importantly, all implants achieved successful osseointegration at 90 days, with no adverse events reported, confirming the high predictability of implant integration in both plasma-treated and untreated sites. Therefore, based on the observed results, the null hypothesis of no significant difference in implant stability between plasma-treated and untreated implants was rejected at 60 and 90 days.
These findings align with existing preclinical and
in vitro studies indicating that plasma treatment of the implant surface can have a positive effect on osseointegration by increasing hydrophilicity of the implant [
17,
18]. The hydrophilicity of the implant is a crucial factor to promote osseointegration, as a hydrophilic surface has a higher affinity for proteins than a hydrophobic surface. Stable adsorption of proteins can affect the ability of cells to adhere and migrate to the implant surface. In addition, high hydrophilicity can promote differentiation and maturation of osteoblasts, thereby promoting osseointegration [
25].
Plasma activation restores the hydrophilicity of titanium surfaces by removing hydrocarbon contaminants and generating a more reactive oxide layer; this surface rejuvenation is particularly relevant for overcoming the effects of "biologic aging," a phenomenon characterized by the progressive accumulation of organic contaminants that compromise the bioactivity and osteoconductive potential of titanium over time [
10,
11,
16,
19]. The present clinical data confirm that plasma activation may counteract the negative effects of biologic aging, as evidenced by the significant increase in ISQ values in the test group compared to controls during the later stages of healing. Interestingly, the test implants not only recovered from the initial stability dip but also surpassed baseline ISQ values by 60 and 90 days, indicating an accelerated and potentially higher-quality osseointegration process. This finding is in agreement with the recent histological human study by Makary et al. [
21], which demonstrated that plasma-treated implants exhibited a significantly higher bone-to-implant contact (BIC) rate of 38.7%, compared to 22.4% for untreated implants, thus confirming the enhanced osseointegration potential associated with plasma activation. This suggests that plasma-treated implants may achieve higher secondary stability within a shorter healing window, which could have meaningful implications for reducing treatment times and supporting early loading protocols in clinical practice. Moreover, the lack of significant differences in implant insertion torque between the two groups confirms that the observed effects on stability were not influenced by different primary mechanical anchorage but likely attributable to surface chemical modifications induced by plasma activation.
However, some limitations should be acknowledged. First, although the sample size was calculated based on expected differences in ISQ, the relatively limited number of patients may still affect the generalizability of the results. Second, the follow-up period was restricted to 90 days, and longer-term evaluations are necessary to determine whether the observed benefits of plasma activation persist over time and translate into improved long-term survival and success rates. Third, the ISQ differences between test and control implants, despite reaching statistical significance, were limited to a few points. Although this suggests a measurable biological effect, its clinical relevance may be limited, as both groups achieved stability levels consistent with successful osseointegration, as also demonstrated in previous animal studies [
26]. Furthermore, it is important to note that this study specifically evaluated vacuum plasma treatment on a nanostructured calcium-incorporated titanium surface. Therefore, these findings should not be generalized to implants with different surface treatments, which may elicit divergent biological outcomes. Future large-scale, multicenter clinical trials with extended follow-up are warranted to determine whether these differences translate into meaningful clinical advantages, such as earlier loading or improved long-term outcomes.
As a future perspective, it could be an interesting and promising approach to consider that implant stability patterns are influenced not only by implant surface modifications but also by site preparation techniques. For example, ultrasonic implant site preparation has been shown to preserve bone integrity and minimize surgical trauma, potentially enhancing early stability and reducing the typical post-placement stability dip [
27,
28,
29,
30]. Future studies could explore whether combining plasma activation with minimally invasive site preparation methods may further optimize early implant stability, particularly in more challenging clinical scenarios.
Author Contributions
Conceptualization, C.S. and T.L.; methodology, C.S.; software, A.R.; validation, R.M., and T.L.; formal analysis, A.R.; investigation, C.S., M.M., and T.L.; resources, A.R.; data curation, R.M.; writing—original draft preparation, C.S., and A.R.; writing—review and editing, R.M., M.M., and T.L.; visualization, R.M.; supervision, C.S.; project administration, T.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Regional Ethical Committee of Friuli Venezia Giulia (protocol code: CEUR-2024-IND-9; date of approval: 11/06/2024).
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study, who also authorized the use of their data for research purposes.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
The following abbreviations are used in this manuscript:
| ISQ |
Implant Stability Quotient |
| UV |
Ultraviolet |
| SLA |
Sandblasted, Large-grit, Acid-etched |
| BIC |
Bone-to-Implant Contact |
References
- Esposito, M.; Ardebili, Y.; Worthington, H.V. Interventions for Replacing Missing Teeth: Different Types of Dental Implants. Cochrane Database Syst Rev 2014, CD003815. [Google Scholar] [CrossRef] [PubMed]
- Shayeb, M.A.; Elfadil, S.; Abutayyem, H.; Shqaidef, A.; Marrapodi, M.M.; Cicciù, M.; Minervini, G. Bioactive Surface Modifications on Dental Implants: A Systematic Review and Meta-Analysis of Osseointegration and Longevity. Clin Oral Investig 2024, 28, 592. [Google Scholar] [CrossRef]
- Buser, D.; Sennerby, L.; De Bruyn, H. Modern Implant Dentistry Based on Osseointegration: 50 Years of Progress, Current Trends and Open Questions. Periodontol 2000 2017, 73, 7–21. [Google Scholar] [CrossRef]
- Sul, Y.-T.; Kang, B.-S.; Johansson, C.; Um, H.-S.; Park, C.-J.; Albrektsson, T. The Roles of Surface Chemistry and Topography in the Strength and Rate of Osseointegration of Titanium Implants in Bone. J Biomed Mater Res A 2009, 89, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Mesa-Restrepo, A.; Byers, E.; Brown, J.L.; Ramirez, J.; Allain, J.P.; Posada, V.M. Osteointegration of Ti Bone Implants: A Study on How Surface Parameters Control the Foreign Body Response. ACS Biomater Sci Eng 2024, 10, 4662–4681. [Google Scholar] [CrossRef]
- Insua, A.; Galindo-Moreno, P.; Miron, R.J.; Wang, H.-L.; Monje, A. Emerging Factors Affecting Peri-Implant Bone Metabolism. Periodontol 2000 2024, 94, 27–78. [Google Scholar] [CrossRef] [PubMed]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface Treatments of Titanium Dental Implants for Rapid Osseointegration. Dent Mater 2007, 23, 844–854. [Google Scholar] [CrossRef]
- Marenzi, G.; Impero, F.; Scherillo, F.; Sammartino, J.C.; Squillace, A.; Spagnuolo, G. Effect of Different Surface Treatments on Titanium Dental Implant Micro-Morphology. Materials (Basel) 2019, 12, 733. [Google Scholar] [CrossRef]
- Tsukimura, N.; Kojima, N.; Kubo, K.; Att, W.; Takeuchi, K.; Kameyama, Y.; Maeda, H.; Ogawa, T. The Effect of Superficial Chemistry of Titanium on Osteoblastic Function. J Biomed Mater Res A 2008, 84, 108–116. [Google Scholar] [CrossRef]
- Att, W.; Hori, N.; Takeuchi, M.; Ouyang, J.; Yang, Y.; Anpo, M.; Ogawa, T. Time-Dependent Degradation of Titanium Osteoconductivity: An Implication of Biological Aging of Implant Materials. Biomaterials 2009, 30, 5352–5363. [Google Scholar] [CrossRef]
- Hori, N.; Att, W.; Ueno, T.; Sato, N.; Yamada, M.; Saruwatari, L.; Suzuki, T.; Ogawa, T. Age-Dependent Degradation of the Protein Adsorption Capacity of Titanium. J Dent Res 2009, 88, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Minamikawa, H.; Att, W.; Ikeda, T.; Hirota, M.; Ogawa, T. Long-Term Progressive Degradation of the Biological Capability of Titanium. Materials (Basel) 2016, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.L.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A Review on the Wettability of Dental Implant Surfaces II: Biological and Clinical Aspects. Acta Biomater 2014, 10, 2907–2918. [Google Scholar] [CrossRef] [PubMed]
- Rupp, F.; Gittens, R.A.; Scheideler, L.; Marmur, A.; Boyan, B.D.; Schwartz, Z.; Geis-Gerstorfer, J. A Review on the Wettability of Dental Implant Surfaces I: Theoretical and Experimental Aspects. Acta Biomater 2014, 10, 2894–2906. [Google Scholar] [CrossRef]
- Wang, R.; Hashimoto, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Light-Induced Amphiphilic Surfaces. Nature 1997, 388, 431–432. [Google Scholar] [CrossRef]
- Minamikawa, H.; Ikeda, T.; Att, W.; Hagiwara, Y.; Hirota, M.; Tabuchi, M.; Aita, H.; Park, W.; Ogawa, T. Photofunctionalization Increases the Bioactivity and Osteoconductivity of the Titanium Alloy Ti6Al4V. J Biomed Mater Res A 2014, 102, 3618–3630. [Google Scholar] [CrossRef]
- Hauser, J.; Zietlow, J.; Köller, M.; Esenwein, S.A.; Halfmann, H.; Awakowicz, P.; Steinau, H.U. Enhanced Cell Adhesion to Silicone Implant Material through Plasma Surface Modification. J Mater Sci Mater Med 2009, 20, 2541–2548. [Google Scholar] [CrossRef]
- Canullo, L.; Genova, T.; Pesce, P.; Nakajima, Y.; Yonezawa, D.; Mussano, F. Surface Bio-Functionalization Using Plasma of Argon Could Alter Microbiological and Topographic Surface Analysis of Dental Implants? Ann Anat 2020, 230, 151489. [Google Scholar] [CrossRef]
- Canullo, L.; Genova, T.; Chinigò, G.; Iacono, R.; Pesce, P.; Menini, M.; Mussano, F. Vacuum Plasma Treatment Device for Enhancing Fibroblast Activity on Machined and Rough Titanium Surfaces. Dent J (Basel) 2024, 12, 71. [Google Scholar] [CrossRef]
- Silva, N.; Marques, J.; da Cruz, M.B.; Luís, H.; Sério, S.; Mata, A. The Applications of Cold Atmospheric Plasma in Dentistry. Plasma Processes and Polymers 2023, 20, e2300067. [Google Scholar] [CrossRef]
- Makary, C.; Menhall, A.; Lahoud, P.; Yang, K.R.; Park, K.B.; Razukevicius, D.; Traini, T. Bone-to-Implant Contact in Implants with Plasma-Treated Nanostructured Calcium-Incorporated Surface (XPEEDActive) Compared to Non-Plasma-Treated Implants (XPEED): A Human Histologic Study at 4 Weeks. Materials (Basel) 2024, 17, 2331. [Google Scholar] [CrossRef] [PubMed]
- Sentineri, R.; Lombardi, T.; Berton, F.; Stacchi, C. Laurell-Gottlow Suture Modified by Sentineri for Tight Closure of a Wound with a Single Line of Sutures. Br J Oral Maxillofac Surg 2016, 54, e18–19. [Google Scholar] [CrossRef]
- Hung, Y.-W.; Chen, H.-L.; Lee, L.-T.; Tung, K.-C.; Bau, D.-T.; Wong, Y.-K. Effects of Non-Thermal Plasma on Sandblasted Titanium Dental Implants in Beagle Dogs. J Chin Med Assoc 2018, 81, 920–925. [Google Scholar] [CrossRef]
- Raghavendra, S.; Wood, M.C.; Taylor, T.D. Early Wound Healing around Endosseous Implants: A Review of the Literature. Int J Oral Maxillofac Implants 2005, 20, 425–431. [Google Scholar]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of Dental Implant Surface Modifications on Osseointegration. Biomed Res Int. 2016, 2016, 6285620. [Google Scholar] [CrossRef] [PubMed]
- Kahm, S.H.; Lee, S.H.; Lim, Y.; Jeon, H.J.; Yun, K.I. Osseointegration of Dental Implants after Vacuum Plasma Surface Treatment In Vivo. J Funct Biomater. 2024, 15, 278. [Google Scholar] [CrossRef]
- Preti, G.; Martinasso, G.; Peirone, B.; Navone, R.; Manzella, C.; Muzio, G.; Russo, C.; Canuto, R.A.; Schierano, G. Cytokines And Growth Factors Involved In The Osseointegration Of Oral Titanium Implants Positioned Using Piezoelectric Bone Surgery Versus A Drill Technique: A Pilot Study In Minipigs. J Periodontol. 2007, 78, 716–722. [Google Scholar] [CrossRef]
- Stacchi, C.; Vercellotti, T.; Torelli, L.; Furlan, F.; Di Lenarda, R. Changes in Implant Stability Using Different Site Preparation Techniques: Twist Drills versus Piezosurgery. A Single-Blinded, Randomized, Controlled Clinical Trial. Clin Implant Dent Relat Res 2013, 15, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Stacchi, C.; Bassi, F.; Troiano, G.; Rapani, A.; Lombardi, T.; Jokstad, A.; Sennerby, L.; Schierano, G. Piezoelectric Bone Surgery for Implant Site Preparation Compared with Conventional Drilling Techniques: A Systematic Review, Meta-Analysis and Trial Sequential Analysis. Int J Oral Implantol (Berl) 2020, 13, 141–158. [Google Scholar]
- Stacchi, C.; Troiano, G.; Montaruli, G.; Mozzati, M.; Lamazza, L.; Antonelli, A.; Giudice, A.; Lombardi, T. Changes in Implant Stability Using Different Site Preparation Techniques: Osseodensification Drills versus Piezoelectric Surgery. A Multi-Center Prospective Randomized Controlled Clinical Trial. Clin Implant Dent Relat Res 2023, 25, 133–140. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).