1. Introduction
Autoimmune thyroid disease (ATD) and insulin-dependent diabetes mellitus (Type 1 diabetes, T1D) are due to target cell destruction by autoreactive T lymphocytes [
1]. The combination is polyglandular syndrome Type 3 variant (APS3v) [
2]. There is an increased incidence of T1D worldwide especially in children under 5 years of age, likely associated with ATD (APS3v) [
3]. The substitutive administration of the deficient hormones i.e. insulin and levo-thyroxine (L-T4) is the standard treatment that, however, does not halt the autoimmune process and does not rescue the residual hormone producing cells [
4].
Identification of innovative therapeutic interventions, especially aimed to preserve the esidual cells, is of crucial importance in the expectation of quality of life in pediatric patients [
5]. Family and population studies have shown that APS3v has a strong genetic bacground [
2]. Recently, among several gene variations that underly the pathogenesis of ATD and T1D, particular interest was generated by the potential pathophysiological role played in different autoimmune conditions including T1D and APS3v by the PTPN22 (protein tyrosine phosphatase N22 gene) C1858T mutation [
6], which changes amino acid residue 620 from Arg (R) to Trp (W) (R620W) in the lymphoid tyrosine phosphatase Lyp protein [
6]. Lyp is a negative regulator of T cell antigen receptor (TCR) signaling, acting in concert with C-terminal Src kinase (CSK). R620W variant leads to a gain of function mutation with paradoxical reduced T cell activation [
7]. Peripheral blood T lymphocytes (PBMC) of T1D patients are indeed hyporesponsive to in vitro stimulation with monoclonal antibodies (mAbs) to CD3 [aCD3]. Subtle TCR signaling defects induced by Lyp variant could have implications at the level of thymocyte tolerization and escape of autoreactive T lympho-cytes, through positive selection of otherwise negatively selected autoimmune T cells [
7]. The variant has effects on both innate and adaptive immune responses. We also observed altered B cell homeostasis and Toll-Like receptor (TLR) 9-driven response in T1D carriers of the
PTPN22 C1858T allelic variant [
8]. In the light of this, LypR620W may be a valid drug target for the treatment of T1D and APS3v patients. In this regard, one of the approaches to immunotherapy is gene silencing that can be achieved by using antisense oligonucleotides (ASO), ribozymes, DNAzymes or RNA interference (RNAi). siRNAs, constituted by two antisense strands intended to recognize a target RNA, have proven to be a more robust technology to achieve effective silencing in cultured cells [
9]. Systemic injection of ASO was already shown to be a feasible approach for treating disease-related genes. However, with specific reference to immunotherapies, the utility of antisense technology already presented limitations not only for molecules high extent of degradation but also for their inefficient transport across the plasma membranes of immunocytes, in particular T and B lymphocytes. We implemented a novel immunotherapy based on the use of siRNA causing variant allele selective inhibition instead of complete gene knockdown [
10,
11]. A crucial goal in the development of a feasible immunotherapy is the realization of efficient and safe delivery agents of siRNAs. Thus, in order to improve delivery, siRNAs were loaded into liposomes (lipoplexes). We already demonstrated the feasibility of the approach of using lipoplexes to obtain inhibition of C1858T allelic variant of
PTPN22 in T1D peripheral blood mononuclear cells (PBMC) [
12]. Furthermore, we implemented lipoplexes through functionalization to improve selective delivery to specific immunocytes, in particular we explore the possibility to expose Fab of monoclonal antibodies targeting CD20 (Rituximab) to improve delivery to B lymphocytes [
13]. Based on the experience carried out with Fab of Rituximab, siRNA-lipoplexes will be improved by conjugation with aCD3 FDA approved mAbs to achieve specific silencing in cytotoxic T cells. An alternative strategy of functionalization to target several immunocytes in the peripheral blood was exploited using high affinity Siglec-10 sialoside mimetic (SAM, F9-PEG-lipid) [
14,
15]. The Siglec family of sialic-acid binding proteins is primarily expressed on cells of the immune system which mediate innate and adaptive functions [
15]. In view of the prospective use of the prepared and characterized lipoplexes for im munotherapy of T1D and APS3v, a fundamental step forward is to estimate the biodistri-bution of lipoplexes and of their functional derivatives by injections in C57BL/6 mice. It is also essential to assess their safety and efficacy in delaying or halting the disease’s development in the NOD mouse model of T1D which harbor the R619W variant of
Ptpn22, equivalent to the human R620W
PTPN22 variant. In the light of the foregoing in this manuscript we aimed to optimize the formulations of lipoplexes functionalized with F9-PEG by testing their physical and chemical properties to warrant best performance for in vivo delivery.
2. Materials and Methods
2.1. siRNA Design
Authentic siRNA sequences were designed to specifically target C1858T PTPN22 gene variant (Rosetta Inpharmatics, Sigma-Aldrich Chemical Co., Saint Louis, MO, US) as previously described [
11] (Italian Patent 102018000005182 released on 26.6.2020; Europe, USA and China extended PCT/IT2019/050095 filed on 8.5.2019, Inventor: Dr. Alessandra Fierabracci).
2.2. Liposome/ Lipoplex Preparation and Characterization
2.2.1. General Liposome and Lipoplex Preparations
Additional details are provided in previously published manuscripts [
11,
12,
13] referring to protocols for liposome preparations. Briefly, gemini surfactant 2R,3S-2,3-di-methoxy-1,4-bis (N-hexadecyl-N, N-dimethylammonium)-butane dibromide (gemini) was prepared as previously described [
16,
17] (
Figure S1). The aqueous dispersion of DMPC (purity>99%, Avanti Polar Lipids Inc. (Alabaster, AL) and gemini mixed liposome was prepared according to a previously described procedure [
18]. Briefly, a thin film of lipids was prepared on the inside wall of a round-bottom flask by evaporation of a CHCl
3 solution containing the proper amounts of DMPC and gemini to obtain the 50/50 molar percentage mixture. The obtained film was dried overnight (O/N) under high vacuum, and hydrated with a buffer solution (5mM HEPES, 0.1 mM EDTA, pH 7.4 (Sigma-Aldrich, Chemical Company (Co.), St Louis, MO) to obtain 1.0 mM overall lipid dispersion. The solution was vortex-mixed and then freeze-thawed six times from liquid nitrogen to 313K. The dispersion was then extruded (10 times) through a 100 nm polycarbonate membrane (Whatman Nuclepore, Toronto, ON, Canada). The extrusions were carried out at 40°C, well above the transition temperature of DMPC (24.2°C), using a 10 mL extruder (Lipex Biomembranes, Vancouver, Canada). For the preparation of lipoplexes, a fixed volume of a siRNA solution (2.6 μM in buffer) was added to an equal volume of a diluted liposome solution (200 μM) to have the final concentrations: [siRNA] = 1.3 μM, [DMPC] = 50 μM, [gemini] =50 μM, corresponding to a lipid/siRNA charge ratio +/- = 2.
2.2.2. Optimization of Lipid/siRNA Ratio
To understand the effect of a different amount of siRNA on the formation of lipoplexes and on their biological activity, compared to previously published studies where the positive (gemini in liposome)/negative (siRNA) ratio was +2/-1 [
11,
12,
13], here we explored also ratios such as +4/-1, + 3/-1, +1/-1 and +1/-2. Lipoplexes at different ratios were obtained by adding a volume of 2.6 μM siRNA solution to an equal volume of liposome solution at a total lipid concentration of 200, 150 and 50, 25 μM, respectively.
2.2.3. Functionalization of Lipoplexes with Siglec-10 Ligand F9
The size, size distribution, ζ -potential and stability over time of liposomes and lipoplexes were investigated by dynamic and dielectrophoretic light scattering (DLS, DELS) measurements. Circular dichroism spectroscopy (CD) spectra were also recorded on lipoplexes to further check the stability over time. All the details of preparation and characterization are described in previously published manuscripts [
11,
12,
13]. Lipoplexes functionalized with the Siglec-10 ligand F9 were prepared using 3 different protocols
(I, G, H) (
Figure S2). In protocol
I (already described in [
13], liposomes functionalized with the Siglec-10 ligand -F9 (Sig10L, [
15]) were prepared following the same procedure reported above. F9-PEG-lipid was added in the film in a 2.8% molar percentage with respect to the overall lipid composition to obtain liposomes L2. Lipoplexes were prepared starting from L2 liposomes, already containing the F9-PEG-lipid, as described in the previous paragraph. In preparation protocol
G, after extrusion of the DMPC/gemini liposomes (L1), F9- PEG-lipid was added in half the amount of preparation
I and incubated for 30 minutes at 40°C to obtain the functionalized liposomes (L1+). Finally, lipoplexes were prepared following the same procedure as described above, adding a diluted siRNA solution to an equal volume of liposomes L1+. In preparation protocol
H, after extrusion of the DMPC/gemini liposomes (L1), siRNA was added and incubated for 1 hour at room temperature to obtain lipoplex
F. F9- PEG-lipid was then added to the lipoplex suspension in an amount that is half that of preparation
I and incubated for 30 minutes at 40°C to obtain lipoplex
H. In addition, we explored the stability of the lipoplexes over time, from day 0 (preparation of the lipoplexes) to day 7, considering the different protocols
(I, G, H) for functionalization.
2.2.4. Labelling of Lipoplexes with ATTO740 Fluorescent Dye
For fluorescently labeled lipoplexes to be used in in vitro experiments, ATTO740 fluorescent dye (ATTO-TEC GmbH, Martinshardt, Siegen, Germany) was added in the film preparation step to obtain after hydration a suspension of DMPC/gemini/F9- PEG/ATTO740 liposomes consisting of [DMPC] = [gemini]= 100 μM, [F9-PEG-lipid] = 5.6 μM (2.8% of total lipid), [ATTO740] 0.2 μM (0.1%). Fluorescent lipoplexes were obtained following protocol I, by adding a proper volume of siRNA to an equal volume of fluorescent liposomes L2.
2.2.5. Fluorescent Liposomes and Lipoplexes for In Vivo Experiments
Fluorescent liposomes (DMPC/ gemini) and lipoplexes (DMPC/ gemini /siRNA) were prepared for in vivo experiments. The protocol of preparation was the same as reported above (L1), the PKH26 (Invitrogen, MA, USA) fluorescent probe was added in the film preparation step (PHK26 was added as 0.1% moles with respect to total lipids) and the final concentrations of the components were: [DMPC]= [gemini] = 1.45 mM, [siRNA] = 37.6 μM, [PKH26] = 2.9 μM).
2.3. Binding Efficiency of siRNA to Liposomes
2.3.1. Electrophoresis
Lipoplexes were analyzed by agarose gel (2%) electrophoresis followed by ethidium bromide staining to visualize bound and free siRNA. The binding efficiency of siRNA to liposomes was investigated by adding 10μL of lipoplex formulation into the wells. Free siRNA was used as positive control, while the liposomes were used as negative control. Lipoplexes formulations were analyzed at Day 0 (time of preparation), 1, 3, 7 where Day 3=48 hours following their preparation. Electrophoresis was run for 30 minutes at 37°C. The siRNA bands were then visualized using a real-time UV transilluminator (Invitrogen, MA, USA). The same protocol was employed to analyze DMPC/gemini/F9-PEG-Lipid/siRNA and DMPC/gemini/F9-PEG-Lipid/ATTO740/siRNA lipoplexes.
2.3.2. HPLC Determination
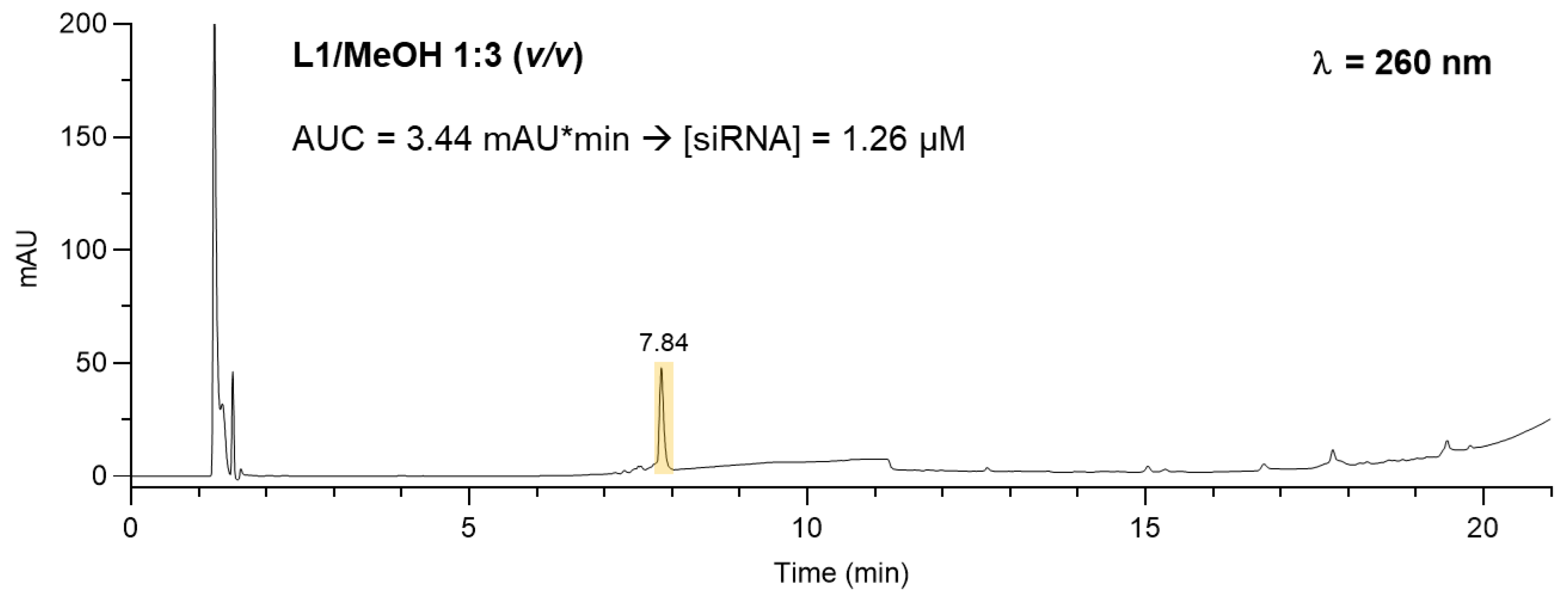
The amount of siRNA in each lipoplex sample was determined by high-pressure liquid performance chromatography (HPLC). For calibration curve construction, a stock solution of siRNA in water (100 µM) was dissolved in a buffer solution containing 5 mM HEPES, 0.1 mM EDTA, pH 7.4 to obtain different siRNA concentrations (0.25, 0.5, 1, 2, 4, and 8 µM). For stability measurements, a 4 µM siRNA in 5 mM HEPES, 0.1 mM EDTA, pH 7.4 was incubated for 0.5, 1, 2, 4, 8, 24, and 48 h at 25 °C and 37 °C prior to HPLC injection. Lipoplex formulations were treated with methanol to a 1:3 (v/v) ratio in order to ensure the rupture of the lipoplex prior to HPLC analysis. The optimized sample/methanol v/v ratio was assessed by DLS analysis (NanoZetaSizer, Malvern UK). HPLC analysis was performed in a Dionex UltiMate 3000 UHPLC (Thermo Fisher) system equipped with an automatic injector, column heater, and coupled with a DAD-3000 Diode Array Detector (Thermo Fisher). The column used was a Waters Acquity UPLC BEH C18 1.7 µm (2.1 x 150 mm) which was kept at a constant temperature of 75 °C. The eluents were A) 0.1 M triethylammonium acetate buffer (pH 7.0) in H2O and B) acetonitrile. Gradient elution: 0- 2 min: 5% B; 9 min 45% B; 12 min 45% B; 17 min 95% B; 30 min 95% B. Flow rate was 0.3 mL/min. The injection volume was 10 µl and the UV detection was at 260 nm. Data were acquired and processed using Chromeleon 7.2 SR5 and GraphPad Prism 8.0. Concentrations of siRNA after lipoplex rupture were found by linear regression analysis of the calibration curve.
2.4. Preliminary In Vivo Biodistribution Experiment in Mice
PKH26 labelled lipoplexes containing 30ug of siRNA were injected in 100ul of HEPES buffer through tail vein of a 2-month-old C57BL/6 male mice. After 48 hours from injection mice was sacrificed and organ samples were taken and snap-frozen. Peripheral blood mononuclear cell (PBMC) samples were also taken and cytospin slides stored at - 80°C. Tissue cryosections (5 μm) and PBMC samples were rehydrated in phosphate buffer solution (PBS), then formaldehyde fixed and permeabilized with 0,1 % PBS-Triton X-100 (Sigma-Aldrich Co.) for 5 min; after incubation with Wheat Germ Agglutinin (WGA, diluted 1:200) conjugated to Oregon Green (ThermoFisher, Invitrogen) for plasma membrane labelling, nuclei were counterstained with Dapi (Invitrogen, 1 μg/ml). Confocal microscopy imaging was performed using Olympus Fluoview FV1000 confocal microscope equipped with 405nm, 488nm, 543nm and 633 nm lasers, using a 60X (1.40 Numerical Aperture) oil immersion objective and FV10-ASW version 2.0 software. Optical sections were acquired with a scanning mode format of 1024×1024 pixels, sampling speed of 125 μs/pixel, and Z-reconstructions of serial single optical sections (0,5 μm z-stack) were carried out with an electronic zoom at 2X.
3. Results
3.1. Experiment 1. Definition of Optimal Lipid/siRNA Ratio
The optimal positive (lipid)/negative (siRNA) charge ratio, with respect to size, ζ-pot and sample stability was estimated in the range +3/-1 and +2/-1 confirming previously published data where the +2/-1 ratio was used for lipoplex preparation [
11,
12]. The +2/-1 ratio was therefore selected for the subsequent optimization of the lipoplex formulations. As shown in
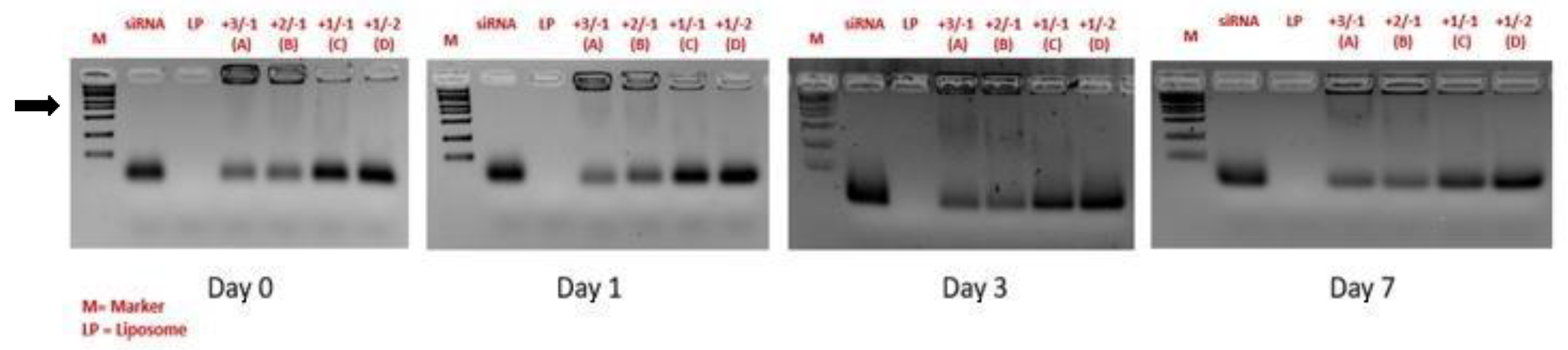
Figure 1, for +2/-1 and +3/-1 lipid/siRNA ratios the band corresponding to the free siRNA has a lower intensity with respect to +1/-1 and +1/-2 lipid/siRNA ratios.
Most importantly, these results indicate that for +2/-1 and +3/-1 ratios the amount of free siRNA in the formulation is negligible compared to the corresponding lipoplex band in the same run, which instead remains locked in the sample well. In addition, the ratio between complexed siRNA and free siRNA for the various formulations seems to remain constant during the time course, thus suggesting the stability of the lipoplexes with respect to degradation phenomena. siRNA remains mostly complexed to lipoplexes in wells over a period of 48 hours after the preparation.
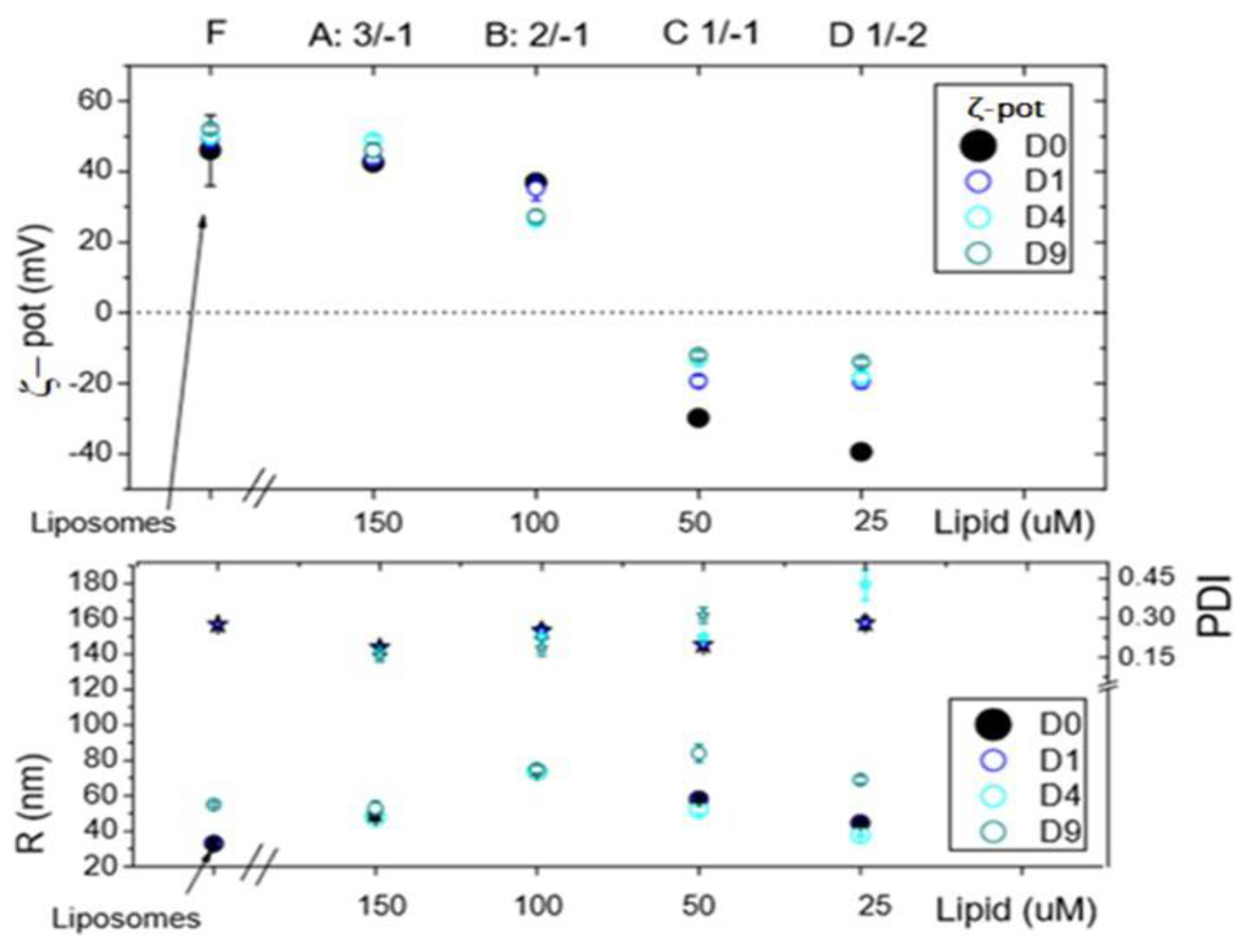
In
Figure 2, DLS analysis revealed that lipoplexes formulated with +2/-1 ratio as well as those with +3/-1 charge ratio features a positive ζ-potential. The ζ-potential remains stable over time as well as particle size and PDI. For charge ratios +1/-1 and +1/-2, a negative ζ-potential was observed, as expected. These two formulations showed lower stability, probably due to a progressive reorganization of the aggregates after preparation as highlighted by variation of PDI, size, and ζ-potential over time.
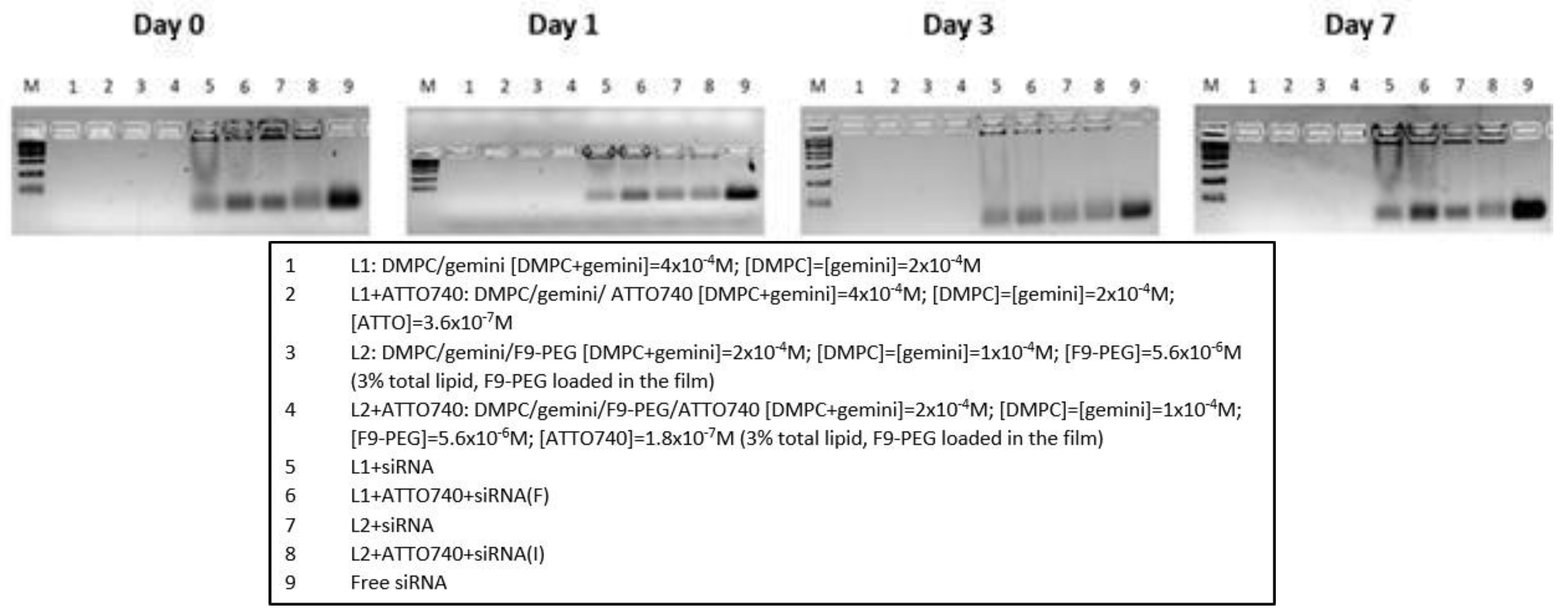
3.2. Experiment 2. Effect of F9-PEG-Lipid on Lipoplexes Stability
As already reported, the addition of F9-PEG-lipid to the liposome formulation causes an increase in size and PDI [
13]. We evaluated the effect of the addition of F9-PEG-lipid in lipoplexes as a function of the preparation protocols. In the protocol previously described [
13], F9-PEG-lipid was added in the film preparation step (here named protocol
I), then we explored also the addition of F9-PEG-lipid by incubation soon after liposome preparation (protocol
G), and just after lipoplex preparation (protocol
H). The best protocol was selected by evaluating the colloidal stability by DLS and DELS measurements and by checking the extent of siRNA complexation by agarose gel electrophoresis.
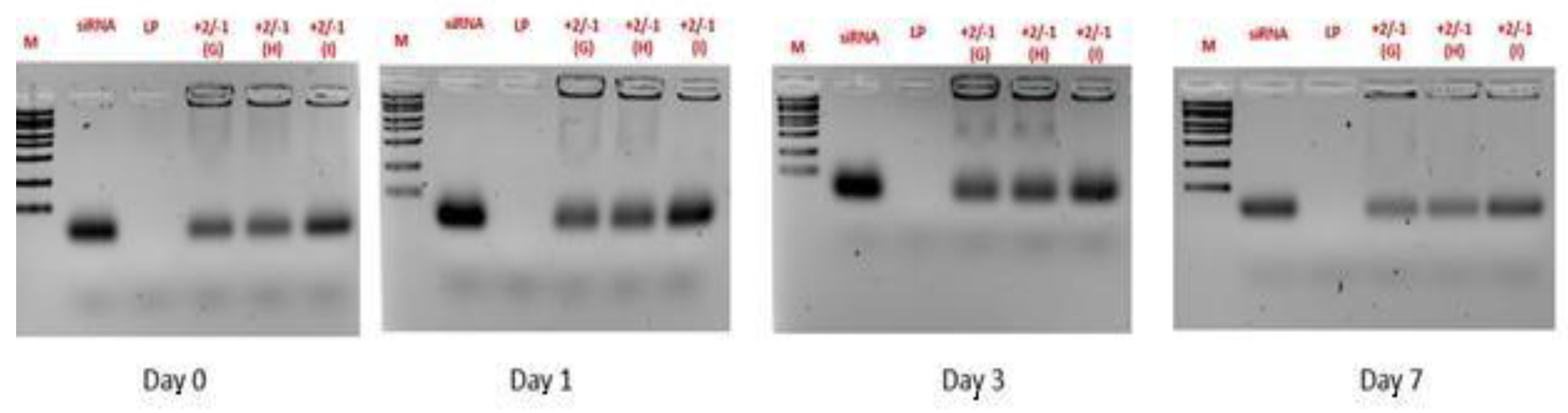
All the 3 protocols show a low amount of free siRNA, with the highest amount observed in Protocol
I (
Figure 3). The electrophoresis shows similar results over the time course of the experiment.
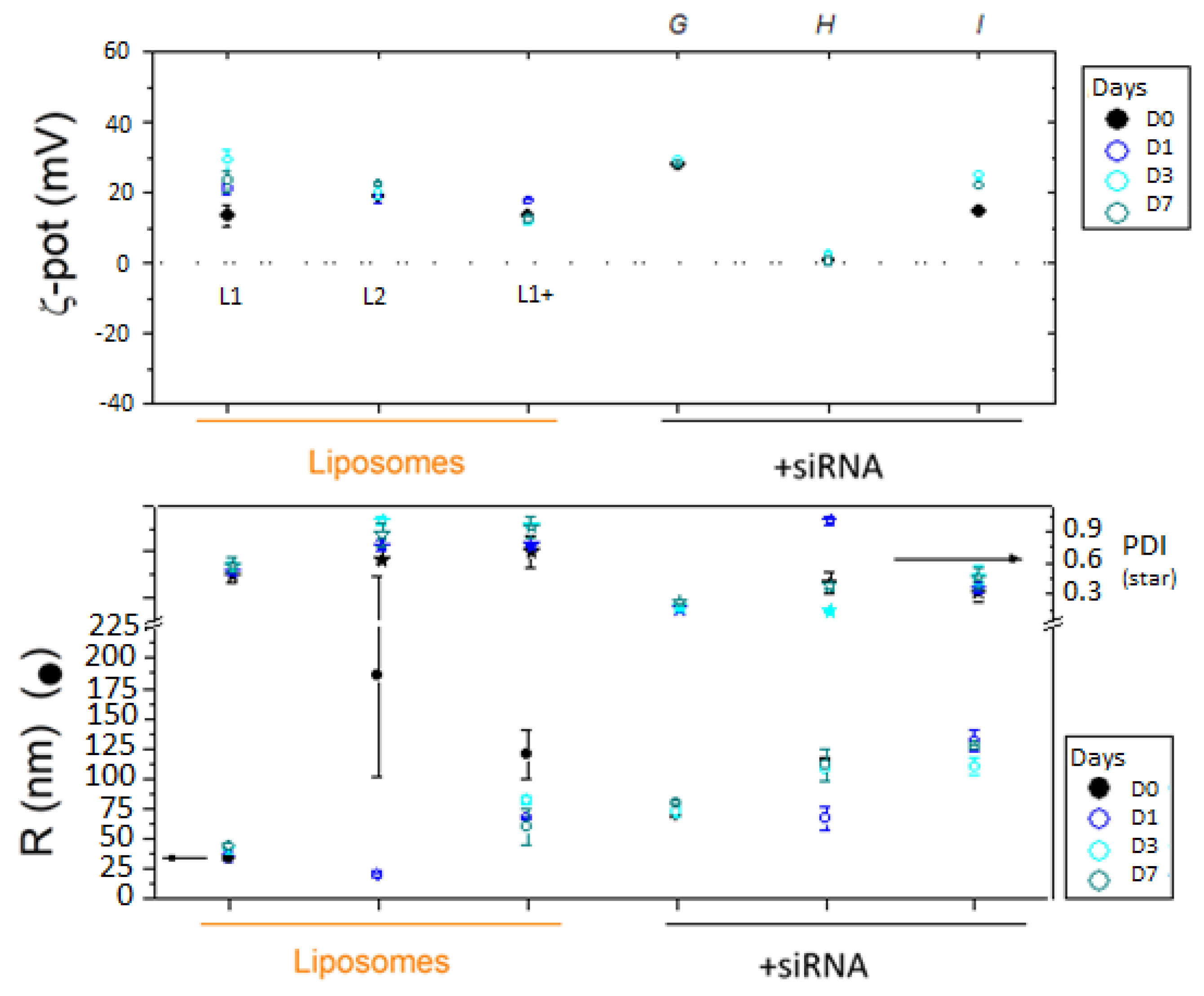
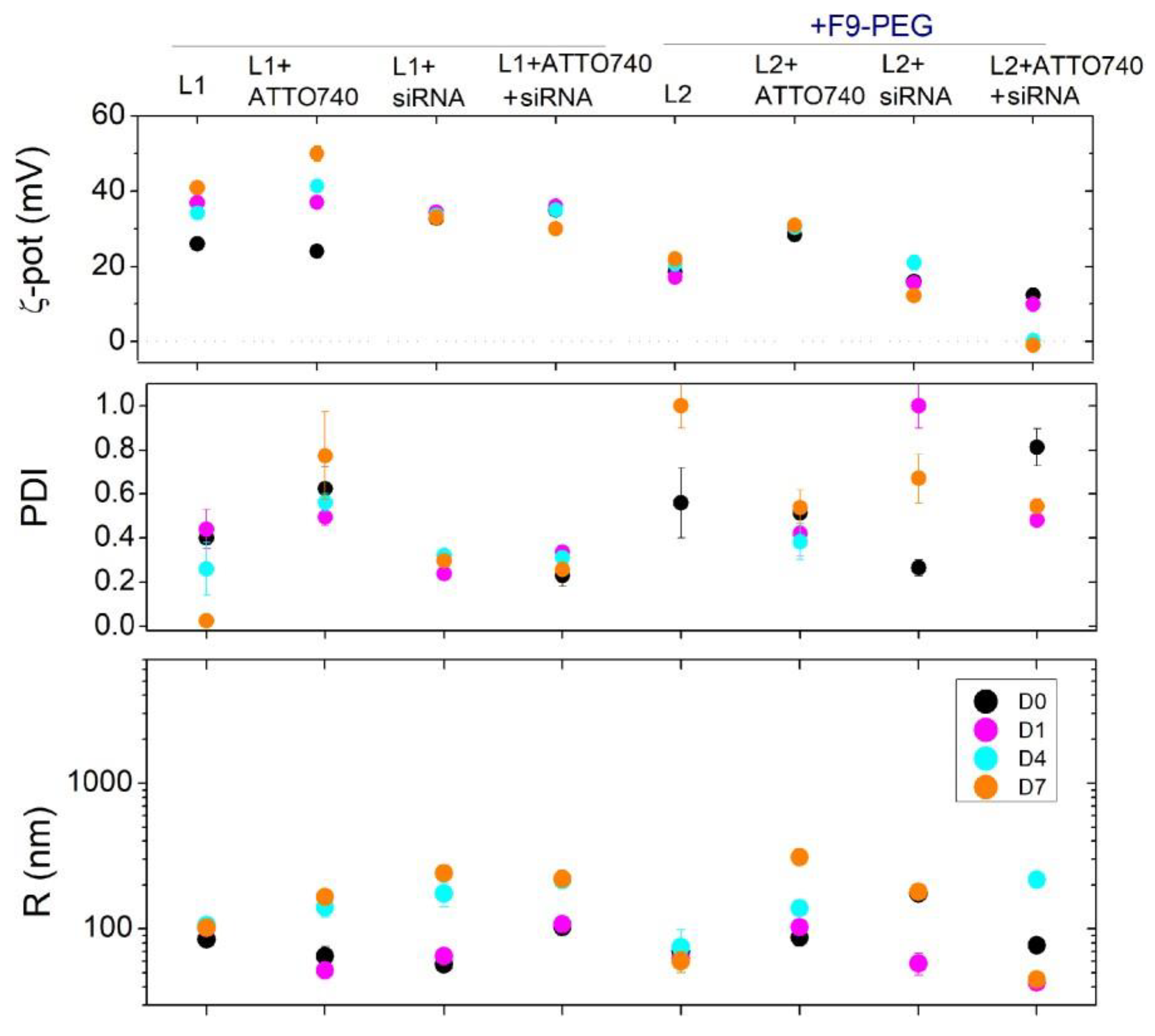
DLS analysis revealed the lower PDI for lipoplexes prepared according
I and
G protocols (approximately 0.3÷0.4) than H lipoplexes (
Figure 4), thus indicating a larger size homogeneity accompanied to a minor tendency to aggregation, which evidences the more presence of stable lipoplexes.
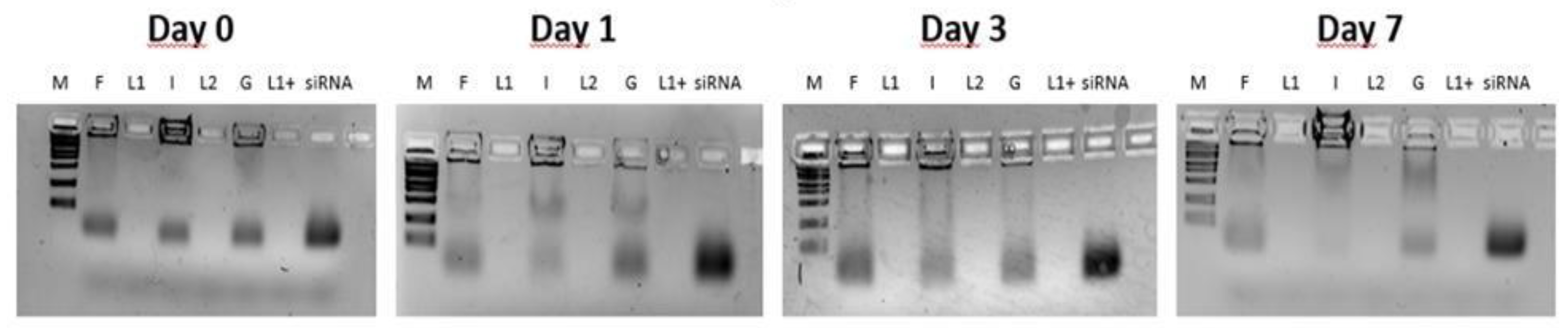
3.3. Experiment 3. Effect of Atto740 on Lipoplex Stability
Figure 5 and
Figure 6 report the effect of ATTO740 on the two protocols
G and
I in comparison with the formulation without F9-PEG lipid. As shown in
Figure 5, the addition of ATTO740 does not affect siRNA binding to liposome.
M =marker
F =Lipoplexes/ATTO740
L1 =Liposome used to prepare F
I =Lipoplexes/ATTO740/F9-PEG (F9-PEG is added following extrusion) L1+ = Liposome used to prepare G
As determined by DELS and DLS, the
I preparation shows a higher ζ-potential and lower diameter over time, despite the relatively high PDI levels (
Figure 6).
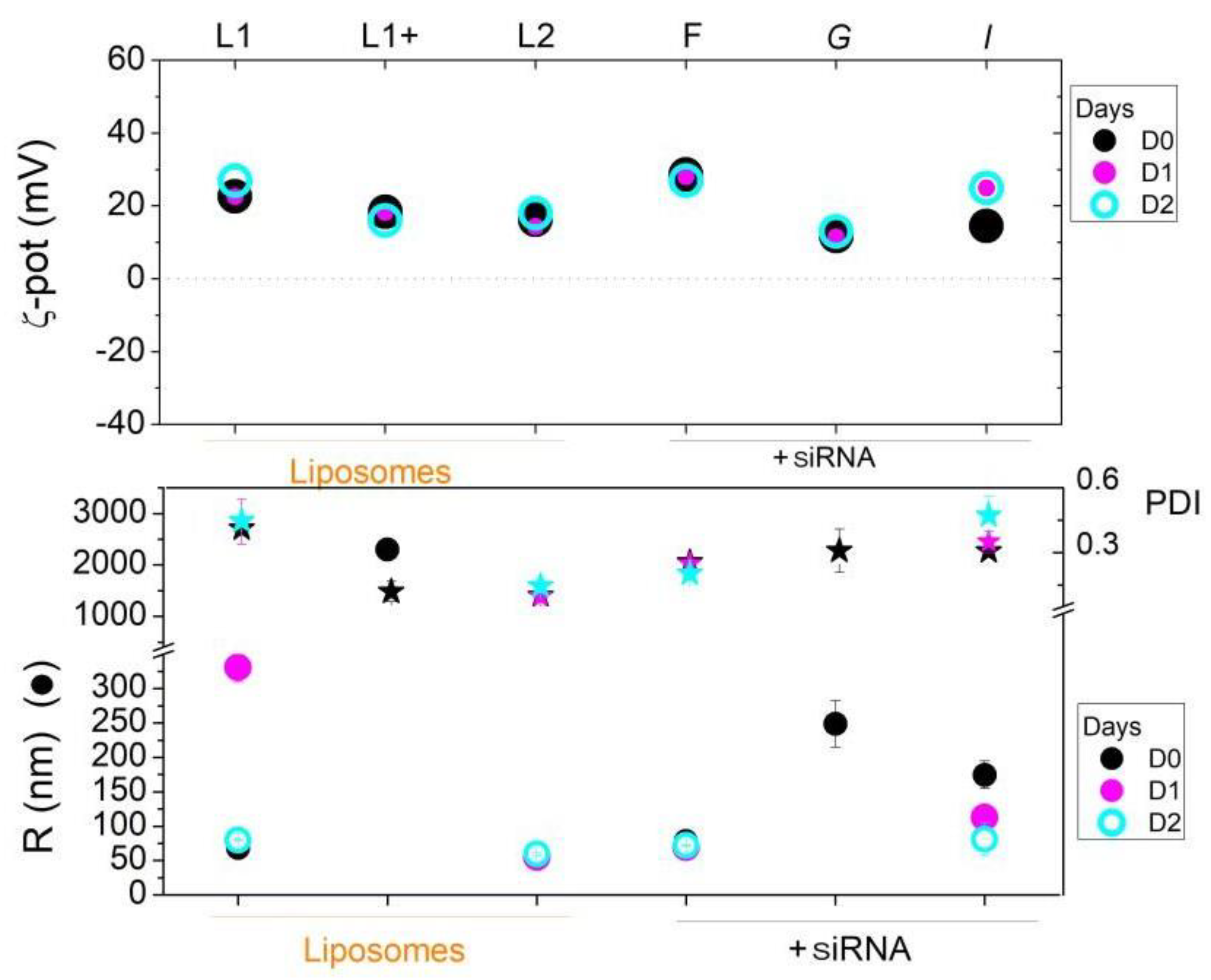
In
Figure 7 and
Figure 8, the effect of ATTO740 in all the formulations (both liposomes and lipoplexes) prepared by the
I protocol and those without F9-PEG-lipid are compared. Specifically, the effect on stability of the addition of siRNA to the different formulations functionalized with F9-PEG lipid and labelled/unlabeled with ATTO740 fluorescent dye was further sequentially evaluated with respect to the formulations without F9-PEG function alization (lipoplexes
F and liposomes L1).
As observed in
Figure 7, overall, the addition of the ATTO740 dye does not reduce siRNA binding to the liposome in both the F9-PEG-functionalised and non-functionalised formulations.
As revealed by DLS analysis (
Figure 8), the formulation functionalized with F9-PEG-lipid and labelled with ATTO740 exhibits a lower ζ-potential and better properties both respect to siRNA binding and lipoplex formation.
In this case, it is not surprising that the low ζ-pot is not associated to a minor colloidal stability, as usually occurs. In fact, here the lower ζ-potentials are not associated to a reduction of the overall surface charge of the lipoplexes but are more likely connected to the presence of F9-PEG-lipid which causes an increased distance of shear plane (where ζ -pot is determined, by definition) from lipoplex surface, which is reflected in the decrease of measured values. Furthermore, in 48 hours the PDI reduced approximately to values of a monodispersed system, at the same time also the size decreases, with a tendency to reduce its variability with respect to ATTO740 or the F9-PEG formulations.
3.4. HPLC Analyses of Free siRNA and Lipoplex Preparations
The concentration of siRNA in each lipoplex preparation was determined by HPLC analysis under denaturing conditions. After obtaining a calibration curve (y = 7.78 x + 0.99, R2 = 0.9981) by analyzing increasing concentrations of siRNA (0.25 to 8 μM) (
Figure S3), we tested the stability of free siRNA at 25 °C and 37 °C at increasing incubation times (
Figure S4). The peak area corresponding to the siRNA resulted approximately constant over time and the formation of additional peaks in the gradient elution was not highlighted, suggesting high stability of siRNA at the investigated temperatures. We then quantified the amount of incorporated siRNA in the L1, L1-ATTO740, L2, and L2- ATTO740 lipoplex preparations. To this end, we diluted each sample with methanol (1:3
v/v) in order to break the lipoplexes and then verified the effective siRNA release in the external medium by DLS evaluation. We also compared the count rate of each lipoplexsample before and after the 1:3 dilution with either the HEPES/EDTA buffer or methanol. The stability of siRNA in the presence of ethanol had previously been investigated to rule out its degradation: notably, the samples showed count rate values of ~500 when diluted in 5mM HEPES, 0.1 mM EDTA at pH 7.4, and values ~10 after dilution with methanol at the same ratio. These results confirm that methanol is capable of solubilizing the lipoplex components and the possibility of using this solution to detect the released siRNA. The HPLC analysis revealed that the concentration of siRNA in all samples re382 mained approximately at the initial value of 1.3 μM, thereby suggesting that there was no significant loss or degradation of siRNA during lipoplex preparation (
Figure 9 and
Figure S5).
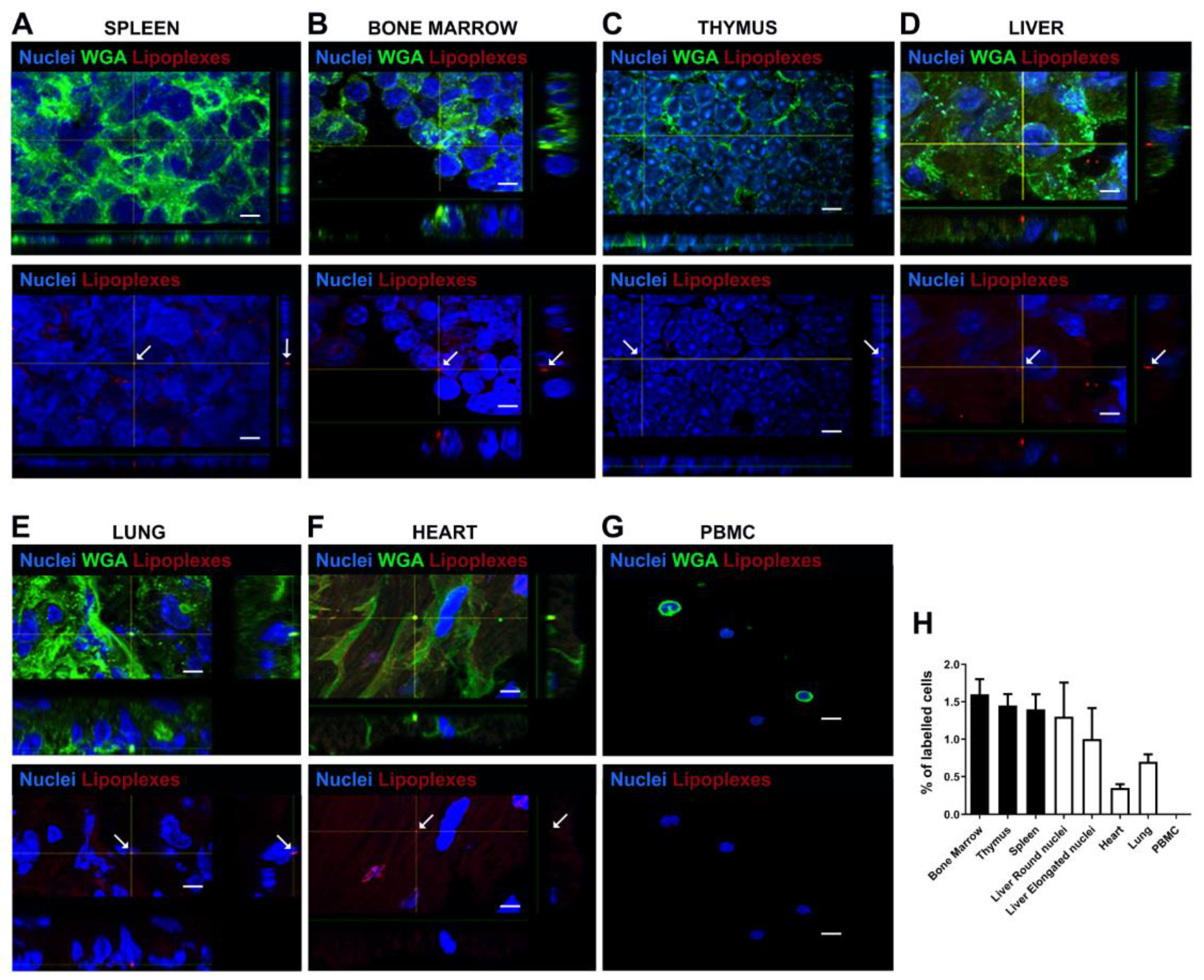
3.5. Biodistribution of PKH26-Labelled Lipoplexes
Under confocal microscopy examination lipoplexes internalization was observed in histological sections of spleen, bone marrow, thymus, liver, lung, heart organs while lipoplexes were not detected in the PBMC (
Figure 10).
4. Discussion
The efficacy of validated and emerging drug molecules has been improved by advanced devices and vehicles such as liposomal carriers being explored for delivery of therapeutic payloads at significant amounts to specific sites. In order to optimize the lipoplexes formulations for the delivery of a novel immunotherapy we first verified the effect of the charge ratio (lipid positively charged/siRNA negatively charged) on formation and stability of lipoplexes and confirmed the optimal efficiency of siRNA binding to liposomes by means of electrophoresis, DLS and DELS analyses and HPLC determination. The ratio +2 (lipid)/-1 (siRNA) was confirmed as optimal as in our previous reports [
11,
12]. DLS and DELS measurements allowed to check their stability over time. Further the efficiency of functionalization with F9-PEG was verified in different protocols, confirming that the addition of F9-PEG in the film preparation procedure results in a higher stability of the resulting lipoplex. This protocol also allows siRNA in lipoplex to be promptly available for cellular uptake and internalization. The effect of the addition of ATTO740-dye and F9-PEG promoted and increased the stability with respect to the F9-PEG formulation and the ATTO740-labelled lipoplexes as evidenced by DLS and DELS analysis. HPLC analysis confirmed that free siRNA was stable at 25°C and 37°C at increasing incubation times. The concentration of siRNA in all samples remained approximately at the initial value of 1.3 μM suggesting that there was no significant loss or degradation of siRNA during lipoplex preparation and upon storage. After these investigations into the stability of lipoplexes formulations functionalized with F9-PEG, which allowed us to confirm protocol
I as the most suitable for their preparation, and having already demonstrated that these formulations are efficiently taken up over time by PBMCs in vitro [
19], we further designed a protocol to evaluate in preliminary in vivo experiments the biodistribution of lipoplexes labelled with the PKH26 dye in C57BL/6 mice. Firstly, we were able to estimate the appropriate dose, lipoplex formulation and route of administration and then by immunohistochemistry we revealed the presence of lipoplexes in mice organs after 48 hours from the injection. This preliminary evaluation set the stage for future animal testing of biodistribution with lipoplexes labelled with ATTO740 and carrying murine siRNA comparing the results obtained with or without F9-PEG functionalization. Further ATTO740 will allow an improved imaging of the biodistribution in PBMC through the blood stream by means of IVIS technology and in targeted lymphoid organs [
20]. We would expect to visualize a valid uptake especially in PBMC where the siRNA is expected to produce its effect of variant inhibition within the first 24 hours from injection. This evaluation is fundamental before proceeding to the injection of functionalized lipoplexes in the NOD mouse model of disease harbouring the
Ptpn22 variant R619W equivalent to the human R620W. To this extent we already provided evidence that siRNA directed against the murine variant in the murine transfected L929 line can efficiently inhibit its expression at the equivalent dose range of inhibition obtained with human siRNA.
5. Conclusions
The results of this investigation demonstrate that F9-PEG lipoplexes are versatile nanocarriers for siRNA, offering potential for effective biomolecules selective release to obtain efficient mRNA variant Ptpn22 suppression. These formulations could be used for a novel personalized immunotherapeutic strategy for prevention and treatment of endocrine autoimmunity, especially in T1D and APS3v patients harbouring C1858T PTPN22 variant.
Patent
Italian Patent 102018000005182 released on 26.6.2020; Europe, USA and China extended PCT/IT2019/050095 filed on 8.5.2019, Inventor: Dr. Alessandra Fierabracci
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Figure S1: Gemini structure; Figure S2: Chart illustrates the lipoplexes functionalization procedure with F9-PEG-lipid; Figure S3: A) HPLC trace of free siRNA at 4 μM concentration. B) Calibration curve correlating siRNA concentration and the measured area under the curve (AUC) of each HPLC analysis; Figure S4: A) Evaluation of the stability of free siRNA (4 μM) by HPLC after 0.5-48 h of incubation at 25 °C. B) Evaluation of the stability of free siRNA (4 μM) by HPLC after 0.5-48 h of incubation at 37 °C; Figure S5: A) HPLC analysis of the F9-PEG-lipidlipoplex preparation (protocol I) following dilution with methanol. B) HPLC analysis of the ATTO740-labelled lipoplex preparation (protocol F) following dilution with methanol. C) HPLC analysis of the ATTO740-labelled F9-PEG-lipid-lipoplex preparation (protocol I) following dilution with methanol.
Author Contributions
Conceptualization, F.C., S.S., C.B., D.V.D., D.R. and A.F.; methodology, G.P., F.C., S.P., I.M., S.S., C.B., F.F. and J.C.P.; investigation, G.P., F.C., I.M., S.S., C.B., F.F., C.M., A.C., D.R. and A.F.; data curation, F.C., S.P., S.S., C.B., C.M., and A.C.; writing—original draft preparation, F.C., S.S. C.B. and A.F.; writing—review and editing A.F., F. C.; supervision, A.F.; project administration, A.F.; funding acquisition, A.F.
Funding
This research was funded by the Italian Ministry of Health with funding of Ricerca Finalizzata Grant RF-2019-12369889 to Alessandra Fierabracci and current research founds.
Data Availability Statement
The original contributions presented in this study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
We thank Alessia Palma for technical support in electrophoresis studies and James C. Paulson (Department of Immunology and Microbiology, The Scripps Research Institute, La Jolla, CA, United States) jpaulson@scripps.edu (J.C.P.) for providing the ligand.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
ATD Autoimmune thyroid disease
T1D Insulin-dependent diabetes mellitus
APS3v Polyglandular syndrome Type 3 variant
L-T4 Levo-thyroxine
PTPN22 protein tyrosine phosphatase N22 gene
TCR T cell antigen receptor
CSK C-terminal Src kinase
PBMC Peripheral blood T lymphocytes
mAbs Monoclonal antibodies
TLR Toll-Like receptor
ASO Antisense oligonucleotides
RNAi RNA interference
SAM Affinity Siglec-10 sialoside mimetic
DMPC Dimyristoylphosphatidylcholine
O/N Overnight
DLS Dynamic Light Scattering
DELS Dielectrophoretic Light Scattering
CD Circular dichroism spectroscopy
Sig10L Siglec-10 ligand
L2 Liposome + F9-PEG-lipid (2.8%)
L1+ Functionalized liposomes, Liposome used to prepare G
L1 DMPC/gemini liposomes, Liposome used to prepare F
F Lipoplexes/ATTO740
HPLC High-pressure liquid chromatography
PDI Polydispersity index
R Hydrodynamic size
LP Liposome
M Marker
References
- Redondo, M.J.; Morgan, N.G. Heterogeneity and endotypes in type 1 diabetes mellitus. Nat. Rev. Endocrinol. 2023, 19, 542–554. [Google Scholar] [PubMed]
- Dittmar, M.; Kahaly, G.J. Genetics of the autoimmune polyglandular syndrome type 3 variant. Thyroid. 2010, 20, 737–743. [Google Scholar] [PubMed]
- Norris, J.M.; Johnson, R.K.; Stene, L.C. Type 1 diabetes-early life origins and changing epidemiology. Lancet Diabetes Endocrinol. 2020, 8, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Rapini, N.; Schiaffini, R.; Fierabracci, A. Immunotherapy strategies for the prevention and treatment of distinct stages of type 1 diabetes: an overview. Int. J. Mol. Sci. 2020, 21, 2103. [Google Scholar] [CrossRef] [PubMed]
- Woittiez, N.J.; Roep, B.O. Impact of disease heterogeneity on treatment efficacy of immunotherapy in type 1 diabetes: different shades of gray. Immunotherapy. 2015, 7, 163–174. [Google Scholar] [PubMed]
- Gianchecchi, E.; Palombi, M.; Fierabracci, A. The putative role of the C1858T polymorphism of protein tyrosine phosphatase PTPN22 gene in autoimmunity. Autoimmun. Rev. 2013, 12, 717–725. [Google Scholar] [PubMed]
- Vang, T.; Congia, M.; Macis, M.D.; Musumeci, L.; Orrù, V.; Zavattari, P.; Nika, K.; Tautz, L.; Taskén, K.; Cucca, F.; Mustelin, T.; Bottini, N. Autoimmune-associated lymphoid tyrosine phosphatase is a gain-of-function variant. Nat. Gen. 2005, 37, 1317–1319. [Google Scholar]
- Gianchecchi, E.; Crino, A.; Giorda, E.; Luciano, R.; Perri, V.; Lo Russo, A.; Cappa, M.; Rosado, M.M.; Fierabracci, A. Altered B cell homeostasis and toll-like receptor 9-driven response in type 1 diabetes carriers of the C1858T PTPN22 allelic variant: implications in the disease pathogenesis. PLoS One 2014, 9, 110755. [Google Scholar]
- Scherer, L.J.; Rossi, J.J. Approaches for the sequence-specific knockdown of mRNA. Nat. Biotech. 2003, 21, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Bottini, N.; Musumeci, L.; Alonso, A.; Rahmouni, S.; Nika, K.; Rostamkhani, M.; Rostamkhani, M.; MacMurray, J.; Meloni, G.F.; Lucarelli, P.; Pellecchia, M.; Eisenbarth, G.S.; Comings, D.; Mustelin, T. A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat. Gen. 2004, 36, 337–338. [Google Scholar] [CrossRef] [PubMed]
- Perri, V.; Pellegrino, M.; Ceccacci, F.; Scipioni, A.; Petrini, S.; Gianchecchi, E.; Lo Russo, A.; De Santis, S.; Mancini, G.; Fierabracci, A. Use of short interfering RNA delivered by cationic liposomes to enable efficient down-regulation of PTPN22 gene in human T lymphocytes. PLoS One 2017, 12, 0175784. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, M.; Ceccacci, F.; Petrini, S.; Scipioni, A.; De Santis, S.; Cappa, M.; Mancini, G.; Fierabracci, A. Exploiting novel tailored immunotherapies of type 1 diabetes: Short interfering RNA delivered by cationic liposomes enables efficient down regulation of variant PTPN22 gene in T lymphocytes. Nanomed. 2019, 18, 371–379. [Google Scholar]
- Arena, A.; Belcastro, E.; Accardo, A.; Sandomenico, A.; Pagliarosi, O.; Rosa, E.; Petrini, S.; Conti, L.A.; Giorda, E.; Corsetti, T.; Schiaffini, R.; Morelli, G.; Fierabracci, A. Preparation and in vitro evaluation of RITUXfab-decorated lipoplexes to improve delivery of siRNA targeting C1858T PTPN22 variant in B lymphocytes. Int. J. Mol. Sci. 2021, 23, 408. [Google Scholar]
- Büll, C.; Heise, T.; Adema, G.J.; Boltje, T.J. Sialic acid mimetics to target the sialic acid–Siglec axis. Trends Biochem. Sci. 2016, 41, 519–531. [Google Scholar] [PubMed]
- Rillahan, C.D.; Schwartz, E.; McBride, R.; Fokin, V.V.; Paulson, J.C. Click and pick: Identification of sialoside analogues for siglec-based cell targeting. Angew. Chem. Int. Ed. 2012, 51, 11014–11018. [Google Scholar]
- Bello, C.; Bombelli, C.; Borocci, S.; di Profio, P.; Mancini, G. Role of the spacer stereochemistry on the aggregation properties of cationic gemini surfactants. Langmuir 2006, 22, 9333–9338. [Google Scholar] [PubMed]
- Seebach, D.; Kalinowski, H.-O.; Bastani, B.; Crass, G.; Daum, H.; Dörr, H.; DuPreez, N. P.; Ehrig, V.; Langer, W.; Nüssler, C.; Oei, H.-A.; Schmidt, M. Preparation of auxiliaries for asymmetric synthesis from tartaric acid. Addition of butyllithium to aldehydes in chiral media. Helv. Chim. Acta 1977, 60, 301–325. [Google Scholar]
- Hope, M.J.; Nayar, R.; Mayer, L.D.; Cullis, P.R. Reduction of liposome size and preparation of unilamellar vesicles by extrusion techniques. Liposome Technol. 1993, 1, 123–139. [Google Scholar]
- Arena, A.; Belcastro, E.; Ceccacci, F.; Petrini, S.; Conti, L.A.; Pagliarosi, O.; Giorda, E.; Sennato, S.; Schiaffini, R.; Wang, P.; Paulson, G.C.; Mancini, G.; Fierabracci, A. Improvement of lipoplexes with a sialic acid mimetic to target the C1858T PTPN22 variant for immunotherapy in endocrine autoimmunity. Front. Immunol. 2022, 13, 838331. [Google Scholar]
- Tu, S.-H.; Hsieh, Y.-C.; Huang, L.-C.; Lin, C.-Y.; Hsu, K.-W.; Hsieh, W.-S.; Chi, W.-M.; Lee, C.-H. A rapid and quantitative method to detect human circulating tumor cells in a preclinical animal model. Cancer Sci. 2025, 116, 345–357. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
 = lipoplex band locked in the sample well in the same run of free siRNA in the formulation.
= lipoplex band locked in the sample well in the same run of free siRNA in the formulation.
 = lipoplex band locked in the sample well in the same run of free siRNA in the formulation.
= lipoplex band locked in the sample well in the same run of free siRNA in the formulation.