1. Introduction
At present, head and neck cancer ranks sixth among the most common malignant tumors worldwide, and it is increasingly affecting elderly invidivuals [
1,
2,
3]. Maintaining good overall condition is crucial for a stable cancer treatment over a long period. However, elderly patients often have reduced physiological and biological functions and various coexisting diseases; thus, they have low resistance to general cancer treatments [
4].
Despite the development of various anticancer drugs, cisplatin (CDDP) remains the key drug in chemoradiotherapy (CRT) for head and neck squamous cell carcinoma (HNSCC). The antitumor effect of CDDP occurs via DNA damage-induced apoptosis in tumor cells [
5,
6]. Although CDDP exerts remarkable therapeutic effects, its adverse events, such as bone marrow suppression, kidney toxicity, gastrointestinal symptoms (e.g., nausea and vomiting), and hearing impairment, are problematic. It has been reported that serious complications are more likely to occur in elderly than in younger patients [
7]. This can lead to unplanned treatment interruptions and deterioration of the patient’s overall condition, which may result in poor prognosis. Therefore, management of these complications is extremely important.
Supportive therapies, such as moisturizing treatment for radiation dermatitis, active use of opioids for stomatitis and mucositis, and oral care, are considered to be effective measures against the adverse events of CRT [
8,
9,
10]. For bone marrow suppression and other organ toxicities, symptomatic interventions are mainly performed after the occurrence of adverse events. To reduce the risk of these serious adverse events, treatment intensity is adjusted by reducing the dose of anticancer drugs or changing the regimen based on the decision of each facility individually.
Meanwhile, some screening tools are used to evaluate the overall function of elderly patients, such as the geriatric 8 (G8) functional assessment tool, peripheral blood neutrophil-to-lymphocyte ratio (NLR), and modified Glasgow prognostic score (mGPS). The G8 tool was developed to quickly evaluate the overall condition of elderly patients with cancer, and various cutoff values have been reported to result in differences in the incidence of adverse events and prognosis [
2,
11,
12,
13]. The NLR reflects the immune and inflammatory states in cancer tissue, and high NLR values are reportedly associated with worse prognosis and treatment resistance [
14,
15,
16]. The mGPS is an evaluation index that combines serum C-reactive protein (CRP), an inflammatory indicator, and serum albumin, a nutritional indicator. mGPS has been reported to be a better prognostic marker than performance states or cancer stages [
17,
18].
Few studies have investigated the appropriate administration method specific to elderly individuals receiving CRT using the aforementioned screening tools. We hypothesized that the CDDP dosage per cycle required for adequate chemotherapy differs between elderly and nonelderly patients. We investigated the long-term differences between these individuals receiving CDDP-based chemotherapy at the same intensity.
2. Materials and Methods
Participants and Treatments
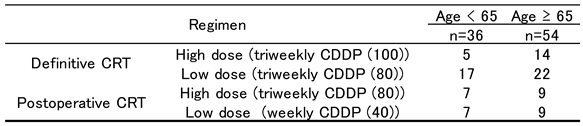
This study enrolled patients who were newly diagnosed with locally advanced HNSCC (LA-HNSCC) in our department between 2014 and 2023 and received CDDP-based CRT. Those who received induction chemotherapy (ICT) before CRT were excluded, whereas those who received definitive and postoperative CRT were included. The definitive CRT regimens were triweekly CDDP (100) and triweekly CDDP (80), and the postoperative CRT regimens were triweekly CDDP (80) and weekly CDDP (40). We used the triweekly CDDP (80) regimen for both definitive and postoperative CRT until June 2020. From July 2020, we used the triweekly CDDP (100) and weekly CDDP (40) regimens for definitive and postoperative CRT, respectively. Patients who received CRT with the TPF regimen (i.e., docetaxel, CDDP, and 5-fluorouracil) for external ear canal cancer and alternating CRT for nasopharyngeal cancer were excluded. For primary tumors, the radiation doses were set to 70 and 60–70 Gy for definitive and postoperative CRT, respectively. For cervical lymph nodes, the dose was set to 40 Gy for both definitive and postoperative CRT.
Evaluation
We defined patients aged <65 years as nonelderly and those aged ≥65 years as elderly. We divided the patients into two groups based on CDDP dosage per cycle: the high-dose group, which included patients receiving a triweekly CDDP (100) regimen for definitive CRT and a triweekly CDDP (80) regimen for postoperative CRT and the low-dose group, which included patients receiving a triweekly CDDP (80) regimen for definitive CRT and a weekly CDDP (40) regimen for postoperative CRT. We compared overall survival (OS), progression free survival (PFS), radiation dose, total CDDP dose, and toxicity between high- and low-dose groups for nonelderly and elderly patients.
Regarding toxicity, we defined severe adverse events according to the JCOG1008 study19 criteria and the Common Terminology Criteria for Adverse Events, version 5.0. The criteria were as follows: grade 4 or higher bone marrow suppression, grade 3 or higher febrile neutropenia, and renal toxicity indicated by estimated glomerular filtration rate <40. We also included patients as having severe toxicity if CDDP administration was postponed for >2 weeks from the scheduled administration date due to specified administration postponement criteria: grade 3 or higher leukopenia and neutropenia, grade 2 or higher thrombocytopenia, renal toxicity indicated by serum creatinine level > 1.5 mg/dL, hepatic toxicity indicated by AST and ALT levels > 100 IU/L and total bilirubin > 2.5 mg/dL, as well as grade 4 or higher mucositis and dermatitis.
The patients’ general condition at the start of primary treatment was evaluated based on the G8 score, NLR, and mGPS. As regards the mGPS criteria, following a previous report, a score of 0 indicates CRP ≤ 0.5 and Alb ≥ 3.5; a score of 1, CRP > 0.5 or Alb < 3.5; and a score of 2, CRP > 0.5 and Alb < 3.5 [
20].
Statistical Analysis
Fisher’s exact test was employed to test dichotomous variables, such as the high- or low-dose group and presence or absence of severe toxicity. Kaplan–Meier curves were constructed, and the log-rank test was employed to compare OS and PFS. For continuous variables, such as total CDDP dose and G8 score, the F test were employed to test for equal variance, followed by the Student’s t-test, or Welch’s t-test. Statistical analyses were conducted using EZR version 1.60 (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [
21], and P-values < 0.05 were considered statistically significant.
3. Results
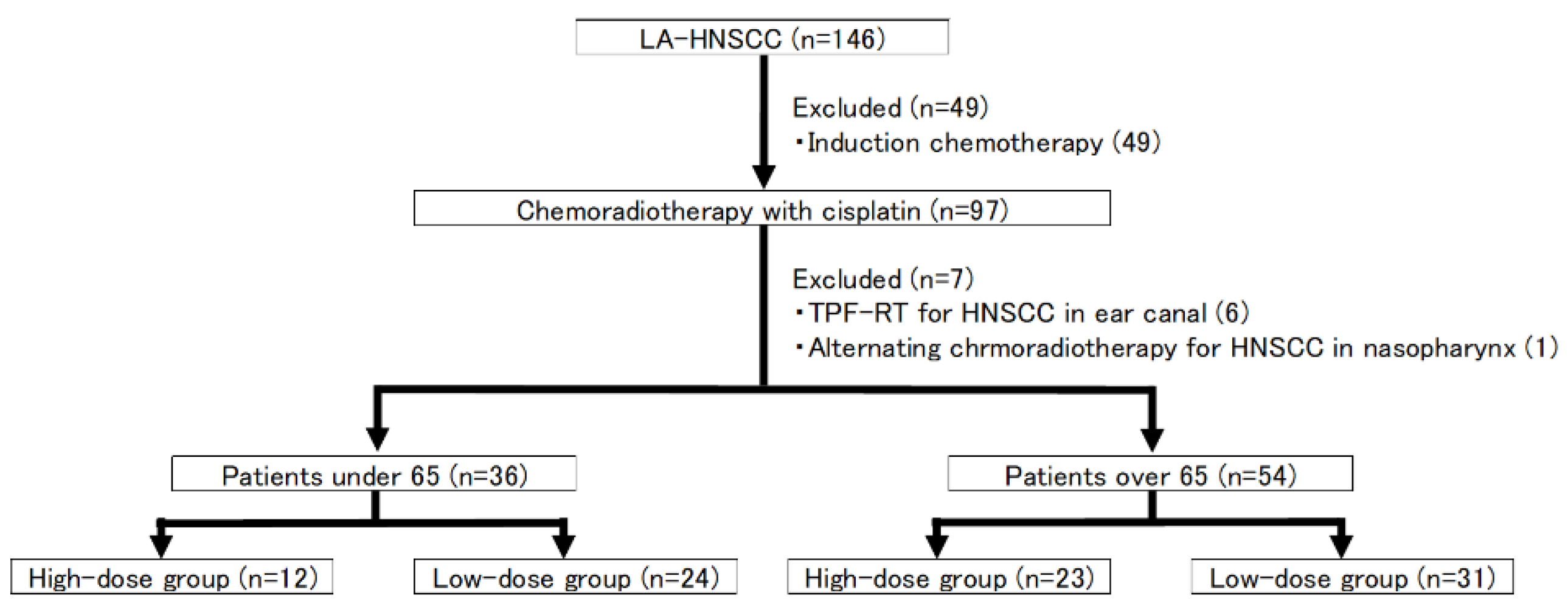
3.1. Patient Characteristics (Figure 1)
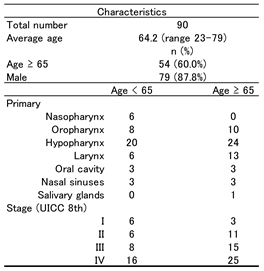
This study enrolled 146 patients with LA-HNSCC who received CRT. Of them, 49 who received ICT before CRT were excluded, along with 6 patients who received TPF-RT for external ear canal cancer and 1 who received alternating therapies for nasopharyngeal cancer. Ultimately, 90 patients were included in the final analysis. The included patients were divided into the nonelderly (n = 36 patients) and elderly (n = 54 patients). Both groups exhibited a high proportion of patients with advanced cancers (stages 3 and 4) (nonelderly patients, 24 in 36 patients; elderly patients, 40 in 54 patients). The nonelderly patients had a high proportion of patients with nasopharyngeal cancer (nonelderly patients, 6 in 36 patients; elderly patients, 0 in 54 patients) and stage 1 p16-positive oropharyngeal cancer (nonelderly patients, 6 in 36 patients; elderly patients, 3 in 54 patients) (
Table 1).
All patient in stage I were p16+ squamous cell carcinoma in oropharynx.
3.2. Treatment
A total of 58 patients received definitive CRT as the first-line treatment and 32 received postoperative CRT. A total of 12 nonelderly and 23 elderly patients were administered high-dose CDDP, whereas 24 nonelderly and 31 elderly patients were administered low-dose CDDP (
Figure 1) (
Table 2).
Figure 1.
Patient enrollment. Among the 146 patients with HNSCC who were enrolled in the study, those who underwent induction chemotherapy before chemoradiotherapy, chemoradiotherapy combined with drugs other than cisplatin, and alternating chemoradiotherapy were excluded. Ultimately, 90 (36 nonelderly and 54 elderly) patients were included in the analysis.
Figure 1.
Patient enrollment. Among the 146 patients with HNSCC who were enrolled in the study, those who underwent induction chemotherapy before chemoradiotherapy, chemoradiotherapy combined with drugs other than cisplatin, and alternating chemoradiotherapy were excluded. Ultimately, 90 (36 nonelderly and 54 elderly) patients were included in the analysis.
The course of the treatment is presented in
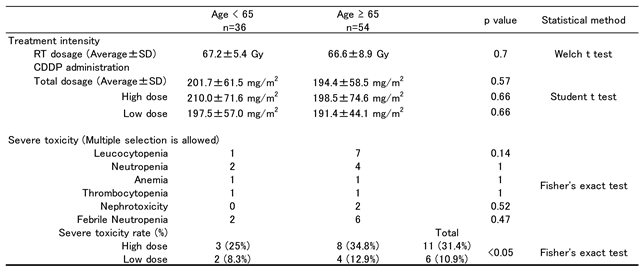
Table 3. Radiation was completed in 89 patients, except for 1 patient aged 78 years who finished at 22 Gy due to progressive disease during definitive CRT for hypopharyngeal cancer. The RT dosage in the nonelderly and elderly patients were 67.2 ± 5.4 and 66.6 ± 8.9 Gy, respectively P = 0.70). The total CDDP doses were 201.7 ± 61.5 and 194.4 ± 58.5 mg/m
2 in the nonelderly and elderly patients, respectively (P = 0.57). In both nonelderly and elderly patients, the total CDDP dose did not significantly differ between the high- and low-dose groups (nonelderly patients, 210.0 ± 71.6 mg/m
2 in the high-dose group vs. 197.5 ± 57.0 mg/m
2 in the low-dose group, P = 0.66; elderly patients, 198.5 ± 74.6 mg/m
2 in the high-dose group vs. 191.4 ± 44.1 mg/m
2 in the low-dose group, P = 0.66).
The patients in the high-dose group had a significantly higher incidence of severe toxicity than that in low-the groups (31.4% (11 in 35 patients) in the high-dose group vs. 10.9% (6 in 55 patients) in the low-dose group, P < 0.05). Notably, the proportion of elderly patients who developed severe toxicity in the high-dose group was 34.8% (8 in 23 patients), which was higher than that in any other groups. The most common severe toxicities in both groups were leukopenia and neutropenia.
3.3. Prognosis
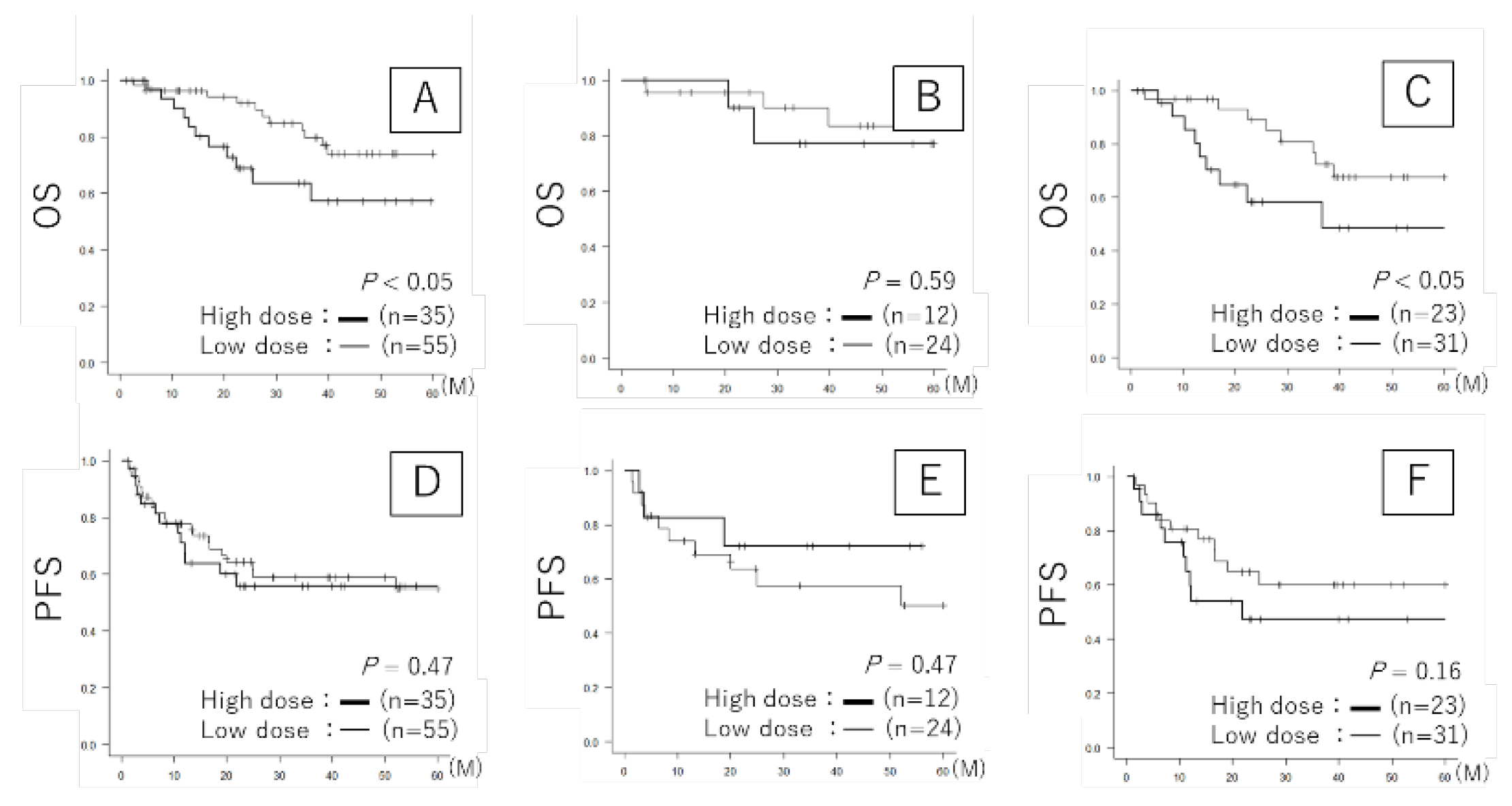
The prognosis after primary therapy is illustrated in
Figure 2. For OS, among all patients, the median survival time and 3-year survival rate were 86.0 months and 63.4% in the high-dose group, and not available (NA) and 79.7% in the low-dose group, respectively (P < 0.05). Among nonelderly patients, the median survival time and 3-year survival rate were NA and 77.1% in the high-dose group, and NA and 90.0% in the low-dose group, respectively (P = 0.59). Among the elderly patients, the median survival time and 3-year survival rate were 36.7 months and 58.3% in the high-dose group and NA and 72.4% in the low-dose group, respectively (P < 0.05) (
Figure 2A–C). For PFS, among all patients, the median survival time and 3-year survival rate were 62.7 months and 55.8% in the high-dose group, and 72.7 months and 59.0% in the low-dose group, respectively (P = 0.47). Among nonelderly patients, the median survival time and 3-year survival rate were NA and 72.2% in the high-dose group and NA and 57.4% in the low-dose group (P = 0.47). Among elderly patients, the median survival time and 3-year survival rate were 21.8 months and 47.4% in the high-dose group and 72.7 months and 60.2% in the low-dose group (P = 0.16) (
Figure 2D–F). For OS, a statistically significant worse prognosis was observed in among all patients and elderly patients. Moreover, although no significant difference was observed, for PFS, in nonelderly group, high-dose administration of CDDP had a good prognosis than low-dose administration of CDDP, whereas in elderly group, low-dose administration of CDDP tended to have a good prognosis than high-dose administration of CDDP.
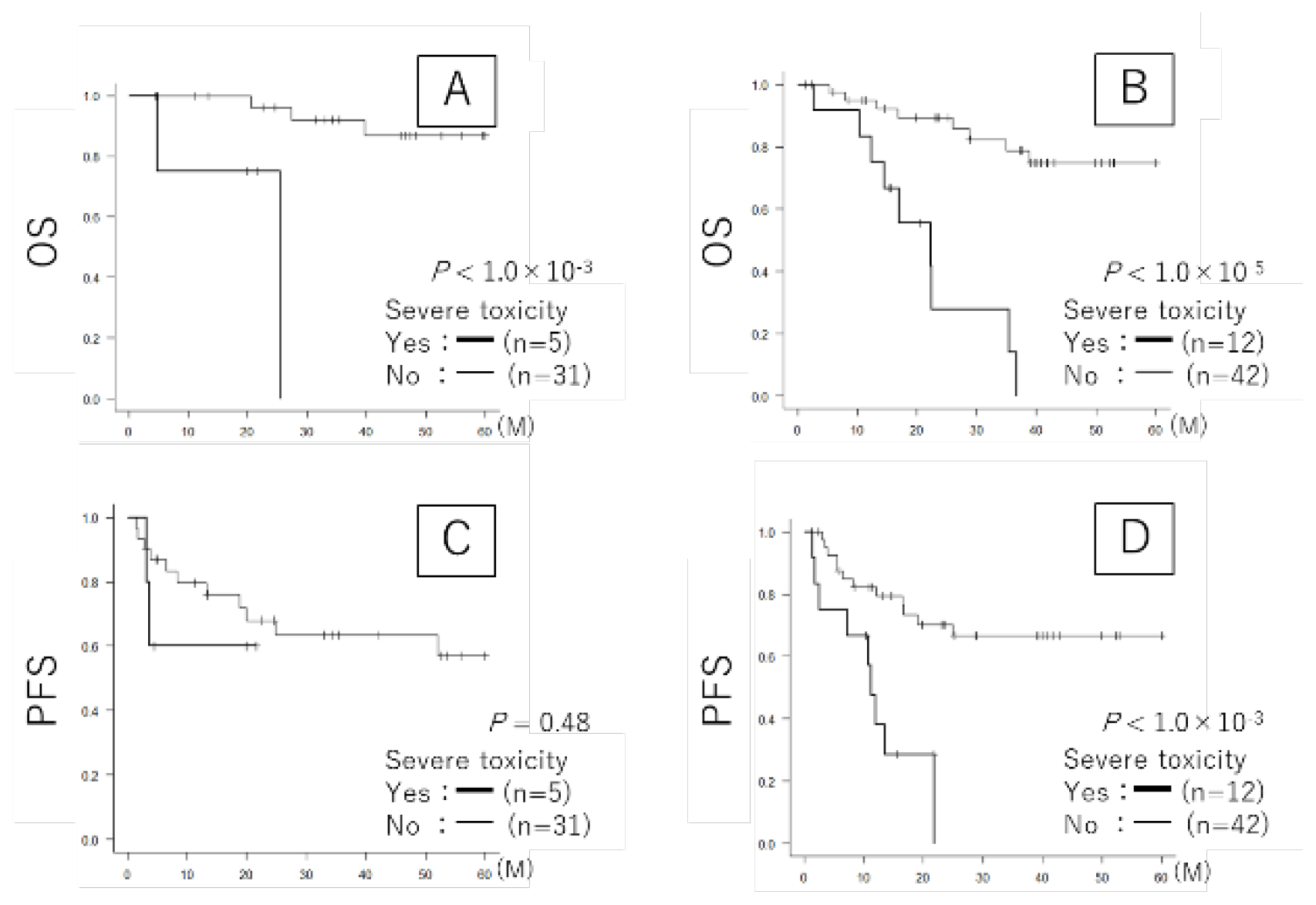
3.4. Severe Toxicity and Prognosis (Table 4 and Figure 3)
When focusing on the presence or absence of severe toxicity, the total CDDP dose was lower significantly in those who developed severe toxicity than in those who did not among the nonelderly and elderly patients (nonelderly patients: 136.0 ± 76.7 mg/m
2 vs. 212.3 ± 52.8 mg/m
2, P < 0.05; elderly patients: 153.3 ± 71.7 mg/m
2 vs. 206.2 ± 49.1 mg/m
2, P < 0.05). As regards prognosis, both the nonelderly and elderly patients who developed severe toxicity showed worse OS (nonelderly patients: the median survival time was 25.5 months and 3-year survival rate was NA in severe toxicity cases vs. the median survival time was NA and 3-year survival rate was 92.0% in no severe toxicity cases, respectively, P < 1.0×10
-3; elderly patients: the median survival time was 22.4 months and 3-year survival rate was 13.9% in severe toxicity cases vs. the median survival time was 86.0 months and 3-year survival rate was 78.8% in no severe toxicity cases, respectively, P < 1.0×10
-5) (
Figure 3A,B). For PFS, a statistically significant worse prognosis was observed only in elderly patients. In nonelderly patients, the median survival time and 3-year survival rate were NA in severe toxicity cases compared to a median survival time of NA and 3-year survival rate of 63.4% in cases without severe toxicity (P = 0.48). In contrast, among elderly patients, the median survival time was 11.2 months with NA for the 3-year survival rate in severe toxicity cases compared to 62.7 months and 3-year survival rate of 66.6% in cases without severe toxicity (P < 1.0×10
-3) (
Figure 3C,D).
Table 5 presents a comparison of general condition scores at the start of the treatment, evaluated by G8, NLR, and mGPS between elderly patients either they developed severe toxicity or not. Compared with elderly patients who did not develop severe toxicity, those who did had lower G8 score (13.4 ± 1.8 in severe toxicity cases vs. 14.6 ± 1.7 in no severe toxicity cases, P = 0.054), higher NLR (3.9 ± 4.3 in severe toxicity cases vs. 2.1 ± 0.7 in no severe toxicity cases, P = 0.18), and higher mGPS (0.7 ± 0.8 in severe toxicity cases vs. 0.2 ± 0.4 in no severe toxicity cases, P = 0.051) at the treatment initiation.
Figure 3.
Prognosis based on severe toxicity. A: OS <65 years. B: OS ≥65 years. C: PFS <65 years. D: PFS ≥65 years. Thick curve: Severe toxicity group. Thin curve: Nonsevere toxicity group. A total of 5 patients aged <65 years developed severe toxicity, and 31 patients aged <65 years did not develop severe toxicity. Furthermore, 12 patients aged ≥65 years developed severe toxicity, and 42 patients aged ≥65 years did not develop severe toxicity (log-rank test).
Figure 3.
Prognosis based on severe toxicity. A: OS <65 years. B: OS ≥65 years. C: PFS <65 years. D: PFS ≥65 years. Thick curve: Severe toxicity group. Thin curve: Nonsevere toxicity group. A total of 5 patients aged <65 years developed severe toxicity, and 31 patients aged <65 years did not develop severe toxicity. Furthermore, 12 patients aged ≥65 years developed severe toxicity, and 42 patients aged ≥65 years did not develop severe toxicity (log-rank test).
Table 4.
Treatment intensity and Toxicity.
Table 4.
Treatment intensity and Toxicity.
Table 5.
General condition score at the start of the treatment in the elderly.
Table 5.
General condition score at the start of the treatment in the elderly.
4. Discussion
Regarding to the planning of chemoradiotherapy, keeping the adverse events within a tolerable limit is as crucial in achieving a good long-term prognosis as suppressing cancer. In the Long-term results of RTOG 91-11 [
22], a comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer, the results of a comparison of the prognosis of CDDP concomitant radiation therapy and radiation therapy alone revealed that the 10-year locoregional control was significantly better with CDDP concomitant radiation therapy, but no significant difference was observed in OS, and the rate of deaths unrelated to cancer was nearly twice as high with CDDP concomitant radiation therapy. In our study, elderly patients receiving high-dose CDDP per cycle had the highest rate of developing severe toxicity and demonstrated significantly worse OS. Although there were similar tendencies due to severe toxicity such as dose reduction of CDDP and worsening OS in both elderly and nonelderly patients, elderly patients seemed to be more affected the high administration of chemotherapy. These findings imply that high-dose administration per cycle may be excessive for elderly patients, potentially compromising adequate chemotherapy delivery.
The efficacy of definitive CRT with triweekly CDDP (100) in HNSCC has been reported in previous large-scale clinical trials [
23,
24]. However, a detailed review of the metabolic pathways of CDDP suggests that this regimen is not appropriate for all patients. More than 90% of intravenously administered CDDP binds to albumin in the blood, with the remainder existing as free CDDP. Renal damage is primarily associated with free CDDP and is believed to be dose-dependent [
25]. Therefore, to avoid CDDP-induced nephrotoxicity, it is useful to avoid high concentrations of free CDDP remaining in the kidney for extended periods and to promote rapid elimination [
26]. Frederic et al. reported that high albumin levels in the blood before the first chemotherapy dose correlate with a high total cumulative CDDP dose achieved [
27]. This result may be related to reduction of free CDDP due to high albumin levels in the blood. The elderly are particularly susceptible to toxicities due to age-related declines in organ function, which may increase their risk of developing severe nephrotoxicity due to CDDP and other adverse events, such as severe bone marrow suppression due to delayed CDDP metabolism. In this study, elderly patients with a worse mGPS receiving a high-dose CDDP tended to have a worse OS, which may reflect the severe impact of toxicity from free CDDP on patients with lower albumin in the blood.
Recently, the efficacy and safety of CDDP-based CRT regimens with reduced dosage per cycle for HNSCC have been explored. JCOG1008 [
19], a large-scale clinical trial led by Kiyota et al., showed that the weekly CDDP (40) regimen was noninferior to the triweekly CDDP (100) regimen for postoperative CRT and exhibited a favorable safety profile. Chatterjee et al
. reported that in several meta-analyses, the weekly CDDP (40) regimen was noninferior to the triweekly CDDP (100) regimen for definitive CRT in terms of efficacy and toxicity [
28,
29,
30].
Certainly, reducing the treatment intensity simply because of only old age is not desirable. The use of screening tools, such as G8, NLR, and mGPS, to evaluate patients’ overall function may be useful in setting the appropriate cancer treatment intensity for elderly patients. This study demonstrated that elderly patients with severe toxicity had worse score in these screening tools. These findings may lead to the treatment strategy that predicts the risk of developing severe toxicity prior to treatment and decides appropriate CDDP dosage for patients.
This study has several limitations. First, we divided them into two groups, nonelderly and elderly, with the age limit of 65 years. However, as aforementioned, considering that patients with HNSCC who are treated are getting older every year, it may be more appropriate to increase the number of study participants and set the elderly standard to 70 or 75 years old. Second, the triweekly CDDP (100) regimen group in definitive CRT and the triweekly CDDP (80) regimen group in postoperative CRT were defined as the same high-dose group, whereas the triweekly CDDP (80) regimen group in definitive CRT and the weekly CDDP (40) regimen group in postoperative CRT were defined as the same low-dose group. However, it is considered to be more appropriate to compare the high- and the low-dose groups in definitive and postoperative CRT, respectively. Third, as this is a retrospective study with a limited number of participants, selection bias may have occurred in the setting of the study participants. To obtain results with sufficient evidence, a prospective randomized controlled trial with a larger number of participants is warranted.
5. Conclusions
In CRT for elderly patients with HNSCC, treatment with the same regimen as that for nonelderly patients may result in excessive dosage per cycle, leading to severe toxicity. This may decrease the total dose of CDDP and worsen OS. The triweekly CDDP (100) for definitive CRT and triweekly CDDP (80) for postoperative CRT may be excessive for some elderly patients with poor G8 and mGPS score. Particularly for elderly patients, setting the treatment intensity using various prognostic tools is useful for ensuring sufficient tolerance to long-term treatment and achieving a good prognosis.
Author Contributions
Conceptualization, H.O. and T.O..; methodology, H.O.; software, H.S.; validation, N.U, K.M. and T.O. formal analysis, H.O.; investigation,H.O. and M.K.; resources, H.O.; data curation, T.O.; writing—original draft preparation, H.O.; writing—review and editing, T.O.; visualization, R.I..; supervision, T.O.; project administration, H.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of Gifu University Graduate School of Medicine (Approval number: 2022-158 and 2022/10/5).
Informed Consent Statement
Patient consent was waived due to retrospective study. Since the study involves the analysis of existing patient data, direct informed consent from participants was not required. Instead, approval was obtained from the Institutional Review Board (IRB) at Gifu University Graduate School of Medicine (Approval number: 2022-158), which waived the need for individual consent due to the retrospective nature of the research and the use of anonymized data. Patient confidentiality and privacy were rigorously protected. All personal identifiers were removed from the data, and the information was anonymized to ensure that participants could not be identified. Data were handled in accordance with institutional and national guidelines for the protection of personal data. Furthermore, participants had the option to opt-out of the study if they wished, although no identifiable information was used, and the data analysis was entirely based on existing records.
Data Availability Statement
Data is unavailable due to privacy or ethical restrictions.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| HNSCC |
Head and neck squamous cell carcinoma |
| CRT |
Chemoradiotherapy |
| ICT |
Induction chemotherapy |
| OS |
Overall survival |
| PFS |
Progression free survival |
| JCOG |
Japan Clinical Oncology Group |
| CTCAE |
Common Terminology Criteria for Adverse Events |
| CRP |
C-reactive protein |
| AST |
Aspartate aminotransferase |
| ALT |
Alanine aminotransferase |
| NLR |
Neutrophil-to-lymphocyte ratio |
| mGPS |
modified Glasgow Prognostic Score |
References
- Grénman, R.; Chevalier, D.; Gregoire, V.; Myers, E.; Rogers, S. Treatment of head and neck cancer in the elderly: European consensus (panel 6) at the EUFOS congress in Vienna 2007. Eur Arch Otorhinolaryngol. 2010, 267, 1619–1621. [Google Scholar] [PubMed]
- Szturz, P.; Bossi, P.; Vermorken, J.B. Systemic treatment in elderly head and neck cancer patients: recommendations for clinical practice. Curr Opin Otolaryngol Head Neck Surg. 2019, 27, 142–150. [Google Scholar] [PubMed]
- Vigneswaran, N.; Williams, M.D. Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral Maxillofac Surg Clin North Am. 2014, 26, 123–141. [Google Scholar]
- Okuda, H.; Shibata, H.; Watanabe, T.; Terazawa, K.; Mori, K.; Ueda, N.; Ohashi, T.; Ogawa, T. Nonsurgical treatment strategies for elderly head and neck cancer patients: an emerging subject worldwide. Cancers. 2022, 14, 5689. [Google Scholar] [PubMed]
- Makovec, T. Cisplatin and beyond: molecular mechanisms of action and drug resistance development in cancer chemotherapy. Radiol Oncol. 2019, 53, 148–158. [Google Scholar]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014, 740, 364–378. [Google Scholar]
- Marty, M.; Pouillart, P.; Scholl, S.; Droz, J.P.; Azab, M.; Brion, N.; Pujade-Lauraine, E.; Paule, B.; Paes, D.; Bons, J. Comparison of the 5-hydroxytryptamine3 (serotonin) antagonist ondansetron (GR 38032F) with high-dose metoclopramide in the control of cisplatin-induced emesis. N Engl J Med. 1990, 322, 816–821. [Google Scholar] [CrossRef]
- Zenda, S.; Ishi, S.; Kawashima, M.; Arahira, S.; Tahara, M.; Hayashi, R.; Kishimoto, S.; Ichihashi, T. A dermatitis control program (DeCoP) for head and neck cancer patients receiving radiotherapy: a prospective phase II study. Int J Clin Oncol. 2013, 18, 350–355. [Google Scholar]
- Zenda, S.; Matsuura, K.; Tachibana, H.; Homma, A.; Kirita, T.; Monden, N.; Iwae, S.; Ota, Y.; Akimoto, T.; Otsuru, H.; Tahara, M.; Kato, K.; Asai, M. Multicenter phase II study of an opioid-based pain control program for head and neck cancer patients receiving chemoradiotherapy. Radiother Oncol. 2011, 101, 410–414. [Google Scholar] [CrossRef]
- Elting, L.S.; Cooksley, C.; Chambers, M.; Cantor, S.B.; Manzullo, E.; Rubenstein, E.B. The burdens of cancer therapy. Clinical and economic outcomes of chemotherapy-induced mucositis. Cancer. 2003, 98, 1531–1539. [Google Scholar]
- Neve, M.; Jameson, M.B.; Govender, S.; Hartopeanu, C. Impact of geriatric assessment on the management of older adults with head and neck cancer: a pilot study. J Geriatr Oncol. 2016, 7, 457–462. [Google Scholar] [PubMed]
- Pottel L, Lycke M, Boterberg T, Pottel H, Goethals L, Duprez F, Rottey S, Lievens Y, Van Den Noortgate N, Geldhof K, Buyse V, Kargar-Samani K, Ghekiere V, Debruyne PR. G-8 indicates overall and quality-adjusted survival in older head and neck cancer patients treated with curative radiochemotherapy. BMC Cancer. 2015, 15, 875.
- Ishii, R.; Ogawa, T.; Ohkoshi, A.; Nakanome, A.; Takahashi, M.; Katori, Y. Use of the Geriatric-8 screening tool to predict prognosis and complications in older adults with head and neck cancer: a prospective, observational study. J Geriatr Oncol. 2021, 12, 1039–1043. [Google Scholar] [PubMed]
- Mei, Z.; Shi, L.; Wang, B.; Yang, J.; Xiao, Z.; Du, P.; Wang, Q.; Yang, W. Prognostic role of pretreatment blood neutrophil-to-lymphocyte ratio in advanced cancer survivors: a systematic review and meta-analysis of 66 cohort studies. Cancer Treat Rev. 2017, 58, 1–53. [Google Scholar] [PubMed]
- Chua, W.; Charles, K.A.; Baracos, V.E.; Clarke, S.J. Neutrophil/lymphocyte ratio predicts chemotherapy outcomes in patients with advanced colorectal cancer. Br J Cancer. 2011, 104, 1288–1295. [Google Scholar]
- Piciucchi, M.; Stigliano, S.; Archibugi, L.; Zerboni, G.; Signoretti, M.; Barucca, V.; Valente, R.; Fave, G.; Capurso, G. The neutrophil/lymphocyte ratio at diagnosis is significantly associated with survival in metastatic pancreatic cancer patients. Int J Mol Sci. 2017, 18, 730. [Google Scholar]
- Toiyama, Y.; Miki, C.; Inoue, Y.; Tanaka, K.; Mohri, Y.; Kusunoki, M. Evaluation of an inflammation-based prognostic score for the identification of patients requiring postoperative adjuvant chemotherapy for stage II colorectal cancer. Exp Ther Med. 2011, 2, 95–101. [Google Scholar]
- Abe, S.; Nozawa, H.; Kawai, K.; Sasaki, K.; Murono, K.; Emoto, S.; Kishikawa, J.; Ozawa, T.; Yokoyama, Y.; Nagai, Y.; Anzai, H.; Sonoda, H.; Ishihara, S. Poor nutrition and sarcopenia are related to systemic inflammatory response in patients with rectal cancer undergoing preoperative chemoradiotherapy. Int J Colorectal Dis. 2022, 37, 189–200. [Google Scholar]
- Kiyota, N.; Tahara, M.; Mizusawa, J.; Kodaira, T.; Fujii, H.; Yamazaki, T. Weekly cisplatin plus radiation for postoperative head and neck cancer (JCOG1008): a multicenter, noninferiority, Phase II/III randomized controlled trial. J Clin Oncol. 2022, 40, 1980–1990. [Google Scholar]
- Boukovala, M.; Modest, D.P.; Ricard, I.; Fischer Von Weikersthal, L.; Decker, T.; Vehling-Kaiser, U.; Uhlig, J.; Schenk, M.; Freiberg-Richter, J.; Peuser, B.; Denzlinger, C.; Peveling Genannt Reddemann, C.; Graeven, U.; Schuch, G.; Schwaner, I.; Heinrich, K.; Neumann, J.; Jung, A.; Held, S.; Stintzing, S.; Heinemann, V.; Michl, M. Evaluation of the inflammation-based modified Glasgow Prognostic Score (mGPS) as a prognostic and predictive biomarker in patients with metastatic colorectal cancer receiving first-line chemotherapy: a post hoc analysis of the randomized phase III XELAVIRI trial (AIO KRK0110). ESMO Open. 2024, 9, 103374. [Google Scholar]
- Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar]
- Arlene A Forastiere, Qiang Zhang, Randal S Weber, Moshe H Maor, Helmuth Goepfert, Thomas F Pajak, et al. Long-term results of RTOG 91-11: a comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J Clin Oncol. 2013, 31, 845–852. [Google Scholar]
- Adelstein, D.J.; Li, Y.; Adams, G.L.; Wagner, H.; Kish, J.A.; Ensley, J.F.; Schuller, D.E.; Forastiere, A.A. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol. 2003, 21, 92–98. [Google Scholar]
- Forastiere, A.A.; Zhang, Q.; Weber, R.S.; Maor, M.H.; Goepfert, H.; Pajak, T.F.; Morrison, W.; Glisson, B.; Trotti, A.; Ridge, J.A.; Thorstad, W.; Wagner, H.; Ensley, J.F.; Cooper, J.S. Long-term results of RTOG 91-11: a comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J Clin Oncol. 2013, 31, 845–852. [Google Scholar] [PubMed]
- Sasaki, Y.; Tamura, T.; Eguchi, K.; Shinkai, T.; Fujiwara, Y.; Fukuda, M.; Ohe, Y.; Bungo, M.; Horichi, N.; Niimi, S.; Minato, K. Pharmacokinetics of (glycolate-0,0′)-diammine platinum (II), a new platinum derivative, in comparison with cisplatin and carboplatin. Cancer Chemother Pharmacol. 1989, 23, 243–246. [Google Scholar] [PubMed]
- Ando, Y.; Nishiyama, H.; Shimodaira, H.; Takano, N.; Sakaida, E.; Matsumoto, K.; Nakanishi, K.; Sakai, H.; Tsukamoto, S.; Komine, K.; Yasuda, Y. Chapter 3: Management of kidney injury caused by cancer drug therapy, from clinical practice guidelines for the management of kidney injury during anticancer drug therapy 2022. Int J Clin Oncol. 2023, 28, 1315–1332. [Google Scholar] [PubMed]
- Frederic Jungbauer, Lena Huber, Sonja Ludwig, Nicole Rotter, Beatrice Walter, Lena Zaubitzer, et al. Prognostic Factors for the Therapeutic Performance of Cisplatin in Head and Neck Malignancies. Front Oncol. 2022, 12, e778380.
- Chatterjee, S.; Kiyota, N.; Vaish, R.; Sharma, A.; Tahara, M.; Noronha, V.; Prabhash, K.; D’Cruz, A. Weekly versus 3-weekly cisplatin along with radiotherapy for locoregionally advanced non-nasopharyngeal head and neck cancers: is the equipoise in literature addressed yet? Head Neck. 2023, 45, 1594–1603. [Google Scholar]
- Mohamed, A.; Twardy, B.; Zordok, M.A.; Ashraf, K.; Alkhoder, A.; Schrapp, K.; Steuer, C.; Chen, Z.; Pakkala, S.; Pillai, R.; Trad Wadsworth, J.; Higgins, K.; Beitler, J.J.; Ramalingam, S.S.; Owonikoko, T.K.; Khuri, F.R.; Shin, D.M.; Behera, M.; Saba, N.F. Concurrent chemoradiotherapy with weekly versus triweekly cisplatin in locally advanced squamous cell carcinoma of the head and neck: comparative analysis. Head Neck. 2019, 41, 1490–1498. [Google Scholar]
- De Felice, F.; Belgioia, L.; Alterio, D.; Bonomo, P.; Maddalo, M.; Paiar, F.; Denaro, N.; Corvo, R.; Merlotti, A.; Bossi, P.; Pappagallo, G.L. Survival and toxicity of weekly cisplatin chemoradiotherapy versus three-weekly cisplatin chemoradiotherapy for head and neck cancer: a systematic review and meta-analysis endorsed by the Italian Association of Radiotherapy and Clinical Oncology (AIRO). Crit Rev Oncol Hematol. 2021, 162, 103345. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).