1. Introduction
Liquid biopsy has emerged as a powerful and minimally invasive approach for cancer diagnosis and monitoring, offering an attractive complement or alternative to traditional tissue biopsies [
1]. In this approach, various tumor-derived components, such as circulating tumor DNA, circulating tumor cells, extracellular vesicles, circulating RNAs, and more recently, tumor-educated platelets (TEPs), have been analyzed to detect or characterize cancers [
2]. TEPs are blood platelets whose RNA and protein profiles as well as their functional states are altered through interactions with tumor cells and the tumor microenvironment [
3]. Traditionally recognized for their roles in hemostasis and thrombosis, platelets are anucleate, short-lived (7–10 days) cell fragments shed from mature megakaryocytes and represent the second most abundant cell type in blood. Beyond clotting, they actively participate in inflammation, tissue repair, and tumor progression [
4].
Through direct crosstalk with tumor cells, platelets can take up tumor-associated molecules and undergo modifications in RNA splicing and protein secretion, effectively transforming them into reservoirs of tumor information [
5]. Early studies have revealed that alterations in platelet counts and content in cancer patients offer valuable diagnostic clues [
6]. Best et al. demonstrated that the mRNA profiles of platelets could distinguish patients with cancer from healthy individuals with high accuracy, sparking intense research on TEP-based diagnostics [
7]. Historical observations such as thrombocytosis noted in patients with cancer since 1964 have been reinforced by clinical evidence linking elevated platelet counts to poor prognosis [
8]. Preclinical studies further underscore the multifaceted role of platelets: by binding to tumor cells, they form microaggregates that shield tumor cells from immune attack, while the release of soluble factors like transforming growth factor-β (TGFβ) and platelet factor 4 (PF4) enhances tumor cell invasion and metastasis [
9]. In response, tumor cells secrete cytokines such as interleukin-6 (IL-6) and chemokine ligand 5 (CCL5) to stimulate platelet production or activation, perpetuating a cycle that promotes angiogenesis, metastasis, and proliferation [
10].
This dynamic intercellular communication facilitates tumor progression and positions TEPs as promising targets for diagnostic and therapeutic applications. Despite the encouraging potential of TEP-based liquid biopsy, challenges persist, particularly regarding precise targeting and monitoring of platelets in clinical settings [
11]. In this review, we comprehensively summarized the biological alterations in platelets induced by tumor interactions and their emerging roles in cancer diagnosis and prognosis. Here, we discuss recent advances in platelet RNA and protein profiling, evaluate the diagnostic performance of TEPs, and explore their potential as dynamic biomarkers for treatment monitoring. Furthermore, we address the current technical and clinical challenges, outlining future directions for integrating TEP-based liquid biopsy into routine oncology practice.
2. Literature Search and Selection Methodology
We searched PubMed, Web of Science, Scopus, and Google Scholar for publications published from January 2015 to December 2024. We used keywords including “tumor-educated platelets,” “platelet RNA cancer,” “platelet proteomics tumor,” “liquid biopsy platelets,” “platelet RNA diagnostics,” and “platelet prognostic cancer,”.
We included clinical and laboratory research articles, systematic reviews, and meta-analyses on the diagnostic and prognostic applications of platelets/TEPs in cancer. Studies focusing solely on basic platelet biology, small case reports, non-English publications, and conference abstracts without complete data were excluded. For each study, we recorded key details such as cancer type, platelet-derived biomarkers (RNA, protein, count, etc.), study size, diagnostic accuracy metrics (sensitivity, specificity, area under the curve [AUC]), and prognostic endpoints (e.g., survival correlations). The evidence was then thematically synthesized by discussing mechanistic insights, diagnostic applications by biomarker type, prognostic applications, and comparative analyses across studies.
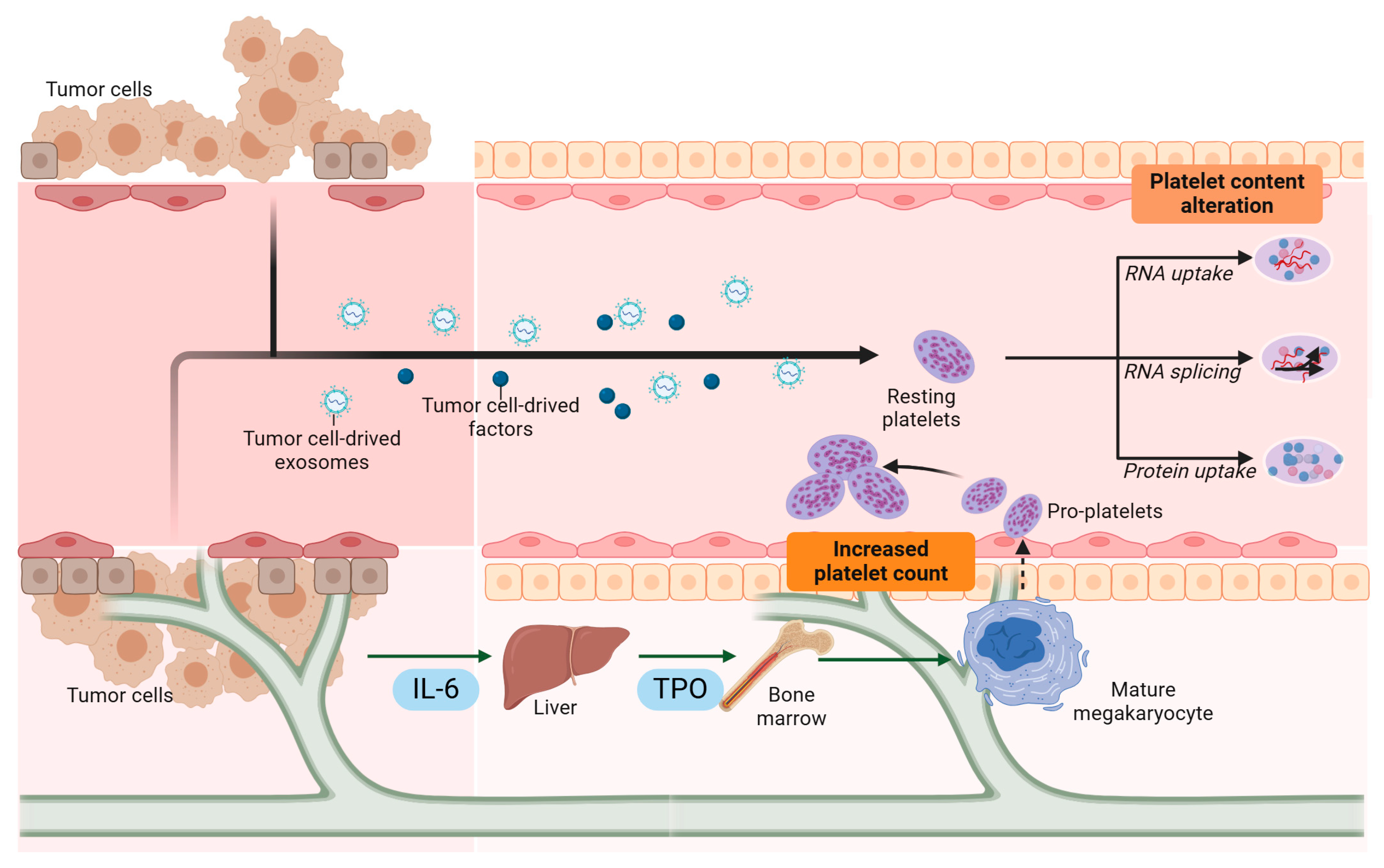
3. Tumor-Driven Platelet Education and Modulation
Platelets are anucleated cell fragments derived from megakaryocytes in the bone marrow that circulate for approximately 7–10 days. Under normal conditions, they play fundamental roles in coagulation, wound healing, and immune responses [
11]. However, in patients with cancer, platelets undergo both quantitative and qualitative changes as they interact with tumor cells and the tumor microenvironment. These changes transform platelets into what is termed “tumor-educated platelets” (TEPs). Several interconnected mechanisms underlie this phenomenon, including sequestration of tumor-derived biomolecules, modulation of platelet RNA content, and alterations in thrombopoiesis at the megakaryocyte level. In addition, tumor cells can directly induce platelet activation and increase platelet count (thrombocytosis), further affecting cancer progression and patient outcomes [
7]. Platelets are not simply passive cell fragments; rather, they engage in active crosstalk with tumor cells. As illustrated in
Figure 1, tumors release soluble factors, extracellular vesicles, and cytokines that directly influence platelet function and RNA content, while activated platelets in turn support tumor progression.
3.1. Sequestration of Tumor-Derived Biomolecules
One mechanism of tumor-driven platelet education is the direct uptake of circulating tumor-derived factors. As tumor cells proliferate, they release various biomolecules, such as proteins, DNA/RNA fragments, and extracellular vesicles (microparticles and exosomes), into the bloodstream. Platelets avidly absorb and store these molecules, effectively acting as “sponges” that collect the molecular signatures of the tumor [
12]. For example, mutant EGFRvIII mRNA from glioblastoma has been detected in platelet RNA following exposure to tumor cells [
13]. By sequestering and storing these factors, platelets carry key oncogenic messages that reflect the molecular state of the tumor [
11].
3.2. Modulation of Platelet RNA Content
Unlike most other cell types, platelets contain no nucleus yet harbor a residual pool of mRNAs and the cellular machinery for splicing pre-mRNAs into mature transcripts. Tumor-secreted signals, including thrombin, chemokines, and growth factors, can bind to platelet surface receptors and influence platelet RNA processing [
14]. This can result in the selective loading or splicing of particular RNAs, generating tumor-specific “RNA fingerprints.” Platelets from patients with cancer commonly display distinct RNA profiles and altered splicing events compared to those from healthy controls [
15]. Platelets also can synthesize certain proteins from these spliced RNAs, and tumor-derived stimuli may lead to the de novo production of oncogenic or pro-angiogenic factors within platelets, thereby enhancing tumor progression [
11].
3.3. Megakaryocyte Education and Thrombopoiesis Alteration
Tumor-driven changes in platelet function may originate even before platelets enter the circulation. Inflammatory cytokines, such as interleukin-6 (IL-6), are often elevated in cancer and can drive excess thrombopoietin (TPO) production in the liver. Thereafter, TPO binds to its receptor, c-Mpl, on megakaryocytes in the bone marrow, causing increased megakaryocyte proliferation and platelet production, a phenomenon known as reactive thrombocytosis. A high platelet count (thrombocytosis) is frequently observed in multiple cancers (e.g., ovarian cancer), and this paraneoplastic effect is associated with a worse prognosis [
16]. Moreover, tumors can induce qualitative shifts in megakaryocytes, leading to newly formed platelets “pre-loaded” with specific RNAs or proteins. Mouse models have shown altered platelet protein profiles in tumor-bearing hosts, implying that systemic factors secreted by tumors can reshape platelet formation at the source [
17].
3.4. Increased Platelet Levels (Thrombocytosis)
Clinical studies have reinforced the idea that thrombocytosis can lead to malignancy. In one cohort of patients who presented with elevated platelet counts, nearly one-third of those later diagnosed with lung or colorectal cancer exhibited thrombocytosis as the only initial sign of malignancy within a 2-year window [
18]. Thrombocytosis is prevalent in ovarian, lung, and gastric cancers, and is correlated with hematogenous metastasis and recurrence. In advanced-stage ovarian cancer, the incidence of thrombocytosis can reach as high as 65% [
19]. This suggests that thrombocytosis may serve not only as a predictor of certain cancers but also as an indicator of tumor progression.
In ovarian cancer, tumor-induced thrombocytosis is largely driven by the release of IL-6 into the bloodstream by malignant cells. IL-6 increases hepatic TPO production, which, in turn, accelerates megakaryocyte maturation and platelet release. Murine models of ovarian cancer and colitis-associated colorectal cancer have shown that genetic ablation of IL-6 in tumor cells or inhibition of IL-6 with antibodies significantly reduces paraneoplastic thrombocytosis and enhances the sensitivity of tumor cells to paclitaxel [
20]. Interestingly, in breast cancer, the association between thrombocytosis and reduced survival is particularly strong in inflammatory tumor subtypes, indicating that the interplay between tumor cell-derived cytokines, immune cells, and megakaryocytes can differ by tumor type. Additionally, other factors secreted by tumor and stromal cells, such as granulocyte–macrophage colony-stimulating factor (GM–CSF), CCL5, and platelet factor 4 (PF4), have been implicated in platelet production [
21]. Blocking these factors suppresses megakaryocyte proliferation and platelet production in mouse models of breast cancer [
20].
3.5. Tumor Cells Activate Platelets
In addition to simply elevating platelet counts, tumor cells also induce functional changes in platelets. Platelets from patients with metastatic ovarian, pancreatic, and breast cancers exhibit a heightened aggregation response to agonists, such as adenosine diphosphate (ADP), adrenaline, and collagen, compared to platelets from healthy donors [
22]. Tumor cells secrete potent stimulatory molecules, such as ADP [
26] and immunoglobulin G (IgG), and release functional proteins and RNAs that reprogram platelet proteomic and transcriptomic profiles. Recent studies demonstrate that platelets in circulation actively internalize tumor-derived proteins (e.g., platelet-derived growth factor [PDGF], transforming growth factor-β [TGFβ], and matrix metalloproteinase 1 [MMP1]) as well as tumor-associated mRNAs (e.g., EGFRvIII in glioma, PCA3 in prostate cancer) [
23].
This tumor-mediated reprogramming is reflected in platelet morphology. Electron cryotomography has revealed disintegrated platelet microtubules and increased mitochondrial numbers [
35], although the precise mechanisms underlying these structural changes remain unclear. Notably, such alterations often correlate with hyperactive platelets in vivo, as indicated by elevated platelet activation markers (e.g., soluble CD40 ligand and soluble P-selectin) in patient blood samples [
24].
3.6. Molecular Pathways of Platelet–Tumor Interactions
Tumor cell-induced platelet activation is increasingly being recognized as distinct from classical hemostatic activation. For instance, platelet surface receptors containing immunoreceptor tyrosine-based activation motifs (ITAMs)—such as glycoprotein VI (GPVI), C-type lectin-like receptor 2 (CLEC2), and FcγRIIa—play an outsized role in platelet activation in response to tumor-secreted molecules [
25]. Tumor cells that express the surface ligand podoplanin bind CLEC2 on platelets, triggering ITAM phosphorylation, recruitment of spleen tyrosine kinase (Syk), and downstream signaling through protein kinase C and integrin αIIbβ3. This cascade culminates in platelet granule release, aiding tumor progression and metastasis, and can also precipitate thrombotic events that increase mortality in patients with cancer [
23].
High-mobility group box 1 protein (HMGB1) released by tumor cells similarly activates platelets via Toll-like receptor 4 (TLR4)-mediated pathways. In addition, tumor-secreted procoagulant factors (e.g., thrombin and tissue factor–bearing extracellular vesicles) can further enhance platelet activation [
25]. Super-resolution imaging studies have shown that, in the presence of tumor cells, platelet membrane proteins, including P-selectin and several SNARE components, undergo nanoscale reorganization that is not observed under normal physiological stimulation (e.g., ADP and thromboxane A2). These findings suggest that tumor-driven platelet activation proceeds via unique mechanisms that are distinct from those of classical hemostasis [
23]. These interactions involve unique signaling cascades distinct from classical hemostasis.
Figure 2 depicts the molecular pathways, including CLEC2–podoplanin and ITAM–Syk signaling, which culminate in platelet granule release and reinforcement of the metastatic process.
Multiple interconnected mechanisms underlie how tumors reprogram platelets. As shown in
Table 1, each mechanism—from direct biomolecule uptake to altered thrombopoiesis—can impart substantial, quantifiable changes in platelet composition and behavior, ultimately facilitating tumor progression.
3.7. Clinical Implications
Through sequestration of tumor signals, reprogramming of RNA content, and altered thrombopoiesis, cancer imprints its molecular and functional signatures on platelets. TEPs may carry mutant tumor RNA, exhibit unique mRNA splicing profiles, contain increased levels of oncogenic proteins, and display altered morphology and hyperactivation [
26]. These changes not only foster tumor progression —by promoting angiogenesis, immune evasion, and metastatic spread—but also provide a minimally invasive window into tumor biology [
23].
Numerous studies have explored the potential of TEPs as liquid biopsy tools. RNA sequencing, protein profiling, and functional assays of platelets have revealed cancer-specific signatures that correlate with tumor type and stage [
27]. Identifying the precise molecular mechanisms by which tumors re-educate platelets is essential for two reasons: (1) identifying new therapeutic targets that could curb tumor-promoting platelet functions without compromising normal hemostasis and (2) refining liquid biopsy approaches that leverage TEPs as biomarkers for early cancer detection, treatment monitoring, and prognostic assessment [
27].
In summary, platelets are not merely bystanders of malignancy. They are dynamic participants in which tumor cells actively recruit, modify, shaping the metastatic landscape. A deeper understanding of platelet–tumor crosstalk holds promise for novel diagnostic tools and interventions aimed at improving clinical outcomes in cancer patients.
4. Diagnostic Applications of Tumor-Educated Platelets
One of the most explored uses of TEPs is for cancer diagnostics, which involves detecting the presence of cancer or even determining its characteristics through a blood sample. Several types of platelet alterations have been studied as diagnostic biomarkers, including platelet count and size, platelet-derived RNA profiles (both coding and noncoding RNAs), and platelet protein content [
28].
4.1. Platelet Count and Volume as Diagnostic Clues
Basic platelet indices such as platelet count and mean platelet volume (MPV) are routinely measured in clinical blood tests. Changes in these parameters have long been observed in patients with cancer. Many malignancies are associated with an elevated platelet count (thrombocytosis), which is hypothesized to result from tumor-driven inflammation and thrombopoietic factors. For example, approximately 31% of patients with epithelial ovarian cancer present with thrombocytosis, a condition correlated with advanced disease and poor outcomes [
29]. Likewise, platelet volume (MPV) may shift in the presence of cancer, although findings have been inconsistent; some studies report MPV increases in early-stage cancer and decreases in late-stage cancer, while others find an overall lower MPV in cancer patients compared to healthy individuals [
30]. One study observed that patients with early-stage lung cancer had a higher MPV, whereas those with advanced lung cancer had a lower MPV, suggesting that MPV may reflect disease stage [
30]. In contrast, another study found that patients with lung cancer generally had a lower MPV than cancer-free controls [
29]. This discrepancy could be due to differences in cancer types, stages, or concurrent conditions affecting platelets, highlighting the fact that platelet count and size alone are not sufficiently specific for reliable cancer diagnosis. Many non-cancerous conditions, such as infection, inflammation, and autoimmune diseases, can also alter platelet count and volume [
30]. Therefore, although abnormal platelet counts or volumes in a patient might raise suspicion and have some utility as part of a broader diagnostic workup, they are generally insufficient as standalone diagnostic tests. The greater utility may be associated with a better prognosis. In the context of TEP diagnostics, the platelet count and MPV are considered supportive markers or part of multiparametric models rather than definitive cancer indicators on their own [
18].
4.2. Platelet RNA Signatures for Cancer Detection
The RNA repertoire of platelets is a rich source of potential diagnostic biomarkers. Platelets contain thousands of mRNA transcripts and diverse non-coding RNAs such as microRNAs, long non-coding RNAs, and circular RNAs. Importantly, these RNA profiles are significantly altered in the presence of cancer. The concept of using platelet RNA profiles for cancer detection was dramatically advanced in a landmark study in 2015 (the ThromboSeq study). In that study, RNA sequencing of platelets from cancer patients and healthy individuals allowed the classification of samples with 96% accuracy in distinguishing cancer from non-cancer and even enabled the identification of the tumor type with approximately 71% accuracy across six different cancer types [
11]. Moreover, platelet RNA profiles revealed specific oncogenic mutations present in the tumor (e.g., EGFR or KRAS mutations) by detecting their RNA signals in platelets [
11]. This high diagnostic potential shows that platelets are a promising liquid biopsy target, on par with or, in some ways, superior to circulating tumor DNA . Subsequent studies reinforced and refined these findings.
4.2.1. Specific mRNA Biomarkers
Individual platelet mRNA markers associated with cancer have been reported previously. For example, platelet levels of ITGA2B mRNA were found to be significantly higher in patients with non-small cell lung cancer (NSCLC) than in controls, helping to differentiate early-stage (Stage I) lung cancer from benign lung nodules [
28]. In colorectal cancer, platelets contain elevated TIMP1 mRNA, which not only serves as a biomarker but can also be functionally transferred to tumor cells to promote growth. Similarly, TPM3 mRNA levels were higher in the platelets of patients with breast cancer and could be shuttled via platelet-derived particles to breast cancer cells, enhancing their migration [
28]. These examples illustrate that tumors impart distinct mRNA profiles to platelets and that the detection of such mRNAs can signal the presence of a tumor. Practically, assays such as RT-PCR can be developed for panels of these mRNAs. In lung cancer patients, one study identified a 3-gene platelet mRNA set (MAX, MTURN, and HLA-B) that was significantly upregulated; this 3-gene signature achieved an AUC of 0.825 for distinguishing patients from controls, proving to be particularly effective in female patients [
28].
4.2.2. Whole Transcriptome Signatures and Machine Learning
Instead of using single markers, many studies have used high-throughput sequencing of platelet RNA combined with machine learning algorithms to classify samples. Best et al.’s 2015 study is one such approach, and subsequent work further showed that in an independent cohort, platelet RNA profiles detected early-stage NSCLC with approximately 81% accuracy and late-stage NSCLC with approximately 88% accuracy, remaining robust across different ages, smoking statuses, and inflammatory conditions [
11]. Another study on endometrial cancer compared platelet RNA-seq to circulating tumor DNA analyses and found that a platelet-based classifier (with AI assistance) had a higher accuracy and AUC for detecting endometrial cancer than the ctDNA-based approach [
31]. These findings suggest that TEP RNA profiles outperform traditional liquid biopsy analytes in certain settings. Researchers have also explored advanced feature selection. For instance, one study applied a particle swarm optimization (PSO) algorithm to identify optimal RNA splicing isoform differences in platelets, which improved the detection of early NSCLC [
11]. Deep learning models are being tested as well; recent reports on ovarian cancer in 2022 used neural network models (“TEPOC” and “DeepCox”) on platelet gene expression to predict ovarian cancer and patient survival, highlighting that relatively small gene panels (around 100 genes) can be sufficient for good performance [
32].
4.2.3. Pan-Cancer vs. Cancer-Specific Models
An important question is whether a single “pan-cancer” platelet RNA signature can detect any cancer or whether assays must be tailored to each cancer type. The 2015 ThromboSeq study suggested pan-cancer potential [
7]; however, later research had some limitations. For instance, when a pan-cancer platelet RNA model trained on common tumors was applied to an external cohort of patients with renal cell carcinoma, it yielded an accuracy of only approximately 49% (with an AUC of approximately 0.615) in detecting that cancer [
33]. This indicates that, while many cancers induce overlapping platelet RNA changes, there are unique aspects by cancer type; a model built on one set of cancers may not be directly generalized to others. Thus, current efforts are aimed at incorporating more data from diverse tumor types to improve the generalizability of platelet RNA diagnostic models. A 2022 study made significant progress by analyzing platelet RNA from a large cohort of patients with multiple cancers [
34]. They reported that a TEP RNA panel could detect the presence of cancer in approximately 66% of 1,096 patients with cancer (across stages I–IV), with an impressive specificity of 99% in asymptomatic individuals (and approximately 78% in symptomatic controls). Even among early-stage (I–III) tumors, approximately half were correctly detected using the platelet RNA test [
34]. These results from a large multicenter study provide strong evidence that platelet RNA signatures are a viable approach for cancer detection, especially given the high specificity which is critical for a screening tool. However, the sensitivity (detecting two-thirds of all cancers and only half of early cancers) suggests that further improvements or a multi-modal combination may be needed to capture a greater fraction of early malignancies.
4.2.4. Non-Coding RNAs
Beyond mRNAs, platelet non-coding RNAs have also shown diagnostic relevance. Platelet microRNAs (miRNAs) are abundant and remarkably stable. In some cases, changes in platelet miRNA profiles are tumor-specific. For example, in nasopharyngeal carcinoma (NPC), two platelet miRNAs (miR-34c-3p and miR-18a-5p) were significantly elevated compared to those in healthy individuals, and a combined miRNA panel achieved an AUC of 0.954 for diagnosing NPC [
35]. Interestingly, these miRNAs were not elevated in the plasma of the same patients, suggesting that the tumor “educates” platelets in a way not detectable by conventional circulating miRNA analysis. This highlights the unique information captured by the platelets. Other studies have identified platelet long non-coding RNAs (lncRNAs) that can serve as biomarkers; one study found that four lncRNAs (LNCAROD, SNHG20, LINC00534, and TSPOAP-AS1) were upregulated in platelets of patients with colorectal cancer, with a logistic model using these achieving an AUC of approximately 0.78 for CRC detection [
36]. Circular RNAs (circRNAs) in platelets represent another emerging class; for instance, circNRIP1 has been reported as a potential diagnostic marker for NSCLC and is present at higher levels in patient platelets. Exploration of non-coding RNAs expands the TEP biomarker landscape and may provide additional layers of diagnostic accuracy or cancer-type specificity. In addition to global transcriptome profiling, numerous studies have pinpointed individual platelet RNA molecules that correlate strongly with cancer presence.
Table 2 highlights some of the most frequently reported platelet-derived RNAs, along with their diagnos-tic accuracies and hypothesized biological roles.
In summary, platelet RNA-based diagnostics have demonstrated moderate-to-high accuracy in numerous studies. A systematic review and meta-analysis focusing on lung cancer, which pooled data from 10 studies (involving 7,858 patients and 6,632 controls), found an overall sensitivity of 80% and a specificity of 69% for platelet-based tests in diagnosing lung cancer, with a summary ROC curve AUC of approximately 0.85, indicating good overall accuracy [
37]. This meta-analysis underscores that across different settings, TEP assays tend to perform within a useful range, albeit not perfectly. It also highlighted that the differences in study design (cancer stage distribution, types of RNAs measured, and technical platforms) contributed to heterogeneity in the results. Nonetheless, a consistent finding is that cancer patients have distinct platelet RNA profiles that can be harnessed for detection. As techniques are refined and standardized, TEP RNA diagnostics could become part of clinical practice, particularly for early detection or for patients who cannot undergo tissue biopsy. The advantages of platelet RNA tests include the relative abundance of platelets (yielding sufficient material), the stability of many platelet RNAs, and the routine and safe nature of blood draws. However, challenges, such as ensuring high-purity platelet preparations (to avoid leukocyte RNA contamination) and the availability of sequencing technology in clinical laboratories, remain unresolved.
Numerous clinical studies have evaluated the utility of TEP-based biomarkers for detecting various malignancies, often stratifying patients by disease stage and comparing against diverse control groups.
Table 3 summarizes selected investigations, highlighting cancer types, biomarker approaches, performance metrics, and key findings.
4.3. Platelet-Derived Protein Markers for Cancer
Platelets carry a wide array of proteins, within their granules or on their surface, which are released upon activation triggered by tumor interactions. These bioactive proteins include growth factors, such as vascular endothelial growth factor (VEGF) and PDGF; chemokines, such as platelet factor 4 (PF4 or CXCL4), angiogenic regulators such as thrombospondin-1 (TSP-1), and various proteases. Tumors can induce quantitative changes in these proteins, making the study of the platelet proteome a valuable complement to RNA-based analyses of TEPs [
38].
Studies have shown that the platelet proteome is significantly altered in patients with cancer. For example, Sabrkhany et al. identified 85 proteins that differed markedly in the platelets of patients with early-stage lung and pancreatic cancers compared to those of healthy controls. Notably, after surgical tumor removal, the levels of 81 of these proteins returned to normal, suggesting a direct causal link between tumor presence and the observed changes in platelet protein composition [
29].
Among the proteins of interest, platelet VEGF and PDGF levels are often elevated in early-stage lung cancers. In patients with colorectal cancer, elevated concentrations of VEGF, PF4, and PDGF distinguish patients from controls with high diagnostic power (AUC = 0.893, p < 0.001). These factors, which play critical roles in tumor angiogenesis and growth, likely reflect the sequestration of tumor-derived or tumor-induced molecules by platelets [
29,
39].
Changes in TSP-1 levels have also been observed. In mouse models, platelet TSP-1 levels increase with tumor growth and decrease after tumor resection, whereas human studies have similarly reported changes in TSP-1 along with other factors, such as PF4 and CTAP-III, particularly in advanced cancers. For instance, in late-stage lung cancer, the levels of PF4, CTAP-III, and TSP-1 are lower than those in controls, in contrast to the increases observed in early-stage cases. In pancreatic cancer, specifically tumors in the head of the pancreas, only VEGF was significantly elevated, suggesting that the most effective marker panel may depend on the tumor type and stage [
29].
PF4, a chemokine stored in platelet alpha granules and released upon activation, has been noted to increase early during tumor development in animal studies; however, in some advanced cases, its levels may drop, possibly due to consumption or platelet exhaustion. Moreover, combining multiple platelet protein measurements, such as platelet count, platelet volume, and specific protein levels, can improve diagnostic accuracy. One study identified a panel of platelet proteins that distinguished benign from malignant ovarian tumors with high sensitivity and specificity [
29].
Although platelet protein biomarkers have been less extensively studied than platelet RNA, their potential is growing. Protein assays such as ELISA are routinely used in clinical laboratories, which can simplify the translation of these findings into diagnostic tests. However, specificity remains a challenge, as changes in platelet protein profiles can also result from systemic inflammation, cardiovascular diseases, and factors such as IL-6-mediate stimulation of megakaryocytes that elevate platelet VEGF. Therefore, careful validation of appropriate control groups, including patients with inflammatory but non-cancerous conditions, is essential [
38].
Despite being largely in the research phase with no standardized panel yet accepted for clinical diagnosis, the consistent observation that platelet proteins are remarkably altered in cancer continues to drive the discovery of active biomarkers. These insights may eventually lead to the development of robust diagnostic tools that exploit the unique properties of the platelet proteome for cancer detection [
29,
38].
4.4. Diagnostic Performance and Comparative Analysis
Considering the above evidence, TEPs have demonstrated notable diagnostic performance across various studies and cancer types. Several key findings have emerged from the literature.
Multicancer platelet RNA-seq (ThromboSeq) studies have reported approximately 95% sensitivity and over 90% specificity overall in initial studies, with approximately 71% accuracy for identifying cancer types. In the context of lung cancer, platelet RNA panels achieved 80–88% accuracy in non-small cell lung cancer, with higher accuracy noted in late-stage cases, and a meta-analysis showed 80% pooled sensitivity and 69% specificity [
15]. In a large pan-cancer study conducted in 2022, approximately 66% sensitivity for all cancers (and 50% for early-stage cancers) was observed, with 99% specificity in an asymptomatic population [
11].
Specific miRNA and lncRNA markers have also been evaluated, with an NPC miRNA panel achieving an AUC of approximately 0.95 and a colorectal cancer platelet lncRNA panel reaching an AUC of approximately 0.78. In addition, platelet protein panels, such as the triad of VEGF, PF4, and PDGF for colorectal cancer, demonstrated an AUC of approximately 0.89 [
40], and various protein changes were able to correctly classify early lung and pancreatic cancers while normalizing post-surgery [
41].
Collectively, these studies indicate that TEP-based tests can achieve AUCs in the 0.8–0.95 range [
11], which is comparable with many other liquid biopsy approaches and even some tissue biomarkers [
42]. However, the reported performance varies across studies. Early proof-of-concept studies sometimes showed very high accuracy (often above 90%) [
11], whereas broader or more recent studies reported moderate accuracy (70–85%) [
15]. This discrepancy may reflect an overfitting or optimistic bias in the initial smaller studies, whereas larger cohorts and meta-analyses provide a more realistic picture. The variability in findings necessitates a closer look at the consistent and conflicting results, which will be addressed in a later section on the comparative analysis of the studies [
11,
43].
Another important consideration is how TEPs compare with other liquid biopsy components such as circulating tumor DNA (ctDNA), circulating tumor cells (CTCs), and classical serum protein markers. TEPs offer practical advantages; for example, platelets are extremely abundant, making tests sensitive even for early tumors [
44], and platelet RNA is stable because it is protected within the platelets, whereas ctDNA is prone to degradation [
45]. Moreover, platelet isolation is a fast process that does not require ultra-specialized equipment. In contrast, ctDNA provides direct genetic information (such as specific mutations), and CTCs allow for cytological analysis, capabilities that platelet profiling does not directly offer. Instead of serving as a competitor, TEP analysis could be complementary. Several authors have proposed combined approaches that integrate platelet RNA profiles with ctDNA mutations or protein tumor markers to build a multidimensional diagnostic test, potentially yielding a higher sensitivity than any single approach [
46].
In conclusion, the diagnostic value of TEPs is supported by a growing body of evidence, with the latest research solidifying the notion that platelets carry tumor signals that can be harnessed for cancer detection.
5. Prognostic and Monitoring Applications of TEPs
Beyond diagnosis, an important aspect of cancer care is prognosis: understanding how a disease is likely to progress and how a patient might respond to therapy. Platelets have been studied as prognostic biomarkers in cancer for decades, initially through simple measures, such as platelet count. More recently, using the concept of TEPs, platelet molecular profiles have been investigated for their ability to predict outcomes such as survival, recurrence, or treatment response [
47].
5.1. Platelet Count and Indices as Prognostic Markers
Although the platelet count and MPV have limitations in early diagnosis, they show more consistent trends in prognosis. Thrombocytosis or a high platelet count is a well-established adverse prognostic factor in several cancers. For instance, patients with ovarian cancer and elevated platelet counts often present with more advanced disease and worse overall survival, and the platelet count has been proposed as an independent prognostic factor [
48]. A meta-analysis by Li et al. (2018) involving various advanced cancers found that an elevated platelet-to-lymphocyte ratio (PLR) was associated with decreased overall survival and progression-free survival, whereas, in non-small cell lung cancer, a high PLR has been correlated with poorer prognosis [
49].
Similarly, changes in MPV, which can reflect the platelet activation status, have prognostic implications. Some studies have linked higher MPV with worse outcomes in certain cancers, although the data are mixed. A recent analysis reported that among patients with non-small cell lung cancer treated with immune checkpoint inhibitors, those who experienced an increase in MPV after one cycle of treatment had significantly different outcomes than those who did not. Specifically, an increase in MPV after the first dose of pembrolizumab was associated with longer overall survival and a higher occurrence of immune-related side effects, suggesting that MPV may serve as an early indicator of a patient’s response to immunotherapy [
50]. Other studies have indicated that MPV may help stratify early versus late disease, although conflicting results have been reported [
51].
In general, persistently high or rising platelet counts during the course of the disease often indicate disease progression or recurrence. Platelets may increase in response to tumor growth via IL-6 and thrombopoietin feedback loops; therefore, monitoring platelet trends could provide clues about tumor trajectory. In some cancers, such as colorectal and pancreatic cancers, thrombocytosis resolves after successful surgery and may increase if the cancer recurs, making platelets a potential surrogate marker of tumor burden [
52].
It is important to note that platelet count and volume are indirect and nonspecific markers. Their prognostic power is moderate and they are usually considered alongside other factors. They may be most effective when combined with more specific platelet biomarkers, such as RNA or proteins, or when integrated with other clinical variables in prognostic models [
53].
5.2. Platelet RNA Signatures and Gene Expression for Prognosis
Just as platelet RNA profiles can detect the presence of cancer, they may also contain information about the aggressiveness of a tumor or its likely outcomes. Recent studies have used platelet gene expression for the risk stratification of patients.
Ovarian Cancer Prognostic Signatures: Two notable studies in 2022 examined platelet mRNA profiles in patients with ovarian cancer and identified gene expression patterns that correlated with patient survival [
54]. In one approach, a model termed tumor-educated platelet ovarian cancer (TEPOC) was developed, and a deep learning model known as DeepCox was employed. These models were used in the order of 100 platelet-derived genes to predict the outcomes. Both studies reported that platelet gene-based models could significantly stratify patients with ovarian cancer according to survival risk and serve as effective prognostic biomarkers. An advantage was that these models used relatively small gene sets, making them more clinically tractable than earlier attempts that required over 1000 genes. The results were promising; patients in the validation cohorts could be separated into high-risk and low-risk groups with significant differences in survival based on their platelet RNA profiles. However, the authors caution that additional data from larger independent populations are required to validate these prognostic signatures [
55].
Platelet RNA Changes with Treatment: Another aspect of prognostication is monitoring how platelet RNA patterns change during therapy, which might predict treatment response. Preliminary evidence suggests that certain platelet RNAs can serve as early markers of treatment efficacy. For example, if a therapy is effectively attacking a tumor, the “education” signals to platelets might diminish (leading to a decrease in tumor-associated RNAs), whereas if a tumor is resistant, those signals might persist or even intensify [
40]. Although a concrete example has not yet been established, ongoing trials are likely to explore this dynamic response. An indirect example is the observed change in the MPV during immunotherapy, which implies that platelets respond dynamically in the treatment context [
56].
General Prognostic Relevance of Platelet Gene Expression: A recurring theme is that worse outcomes often correlate with more pronounced platelet activation or “education” by the tumor. Platelets that support tumors —by shielding circulating tumor cells or aiding angiogenesis —would logically worsen prognosis. Thus, if a patient’s platelets exhibit very high levels of pro-tumor signals, the patient might have a more aggressive disease course [
15]. For example, an increase in certain platelet-derived pro-angiogenic factors, such as VEGF, TSP-1, and PDGF, might correlate with higher metastatic potential and shorter survival [
57]. Thrombocytosis is associated with poor survival, and there is evidence that platelet RNA signatures can refine prognostic stratification even within the same platelet count. In ovarian cancer, one study found that even after accounting for platelet count, the gene expression profile of platelets provided independent prognostic information. This suggests that qualitative platelet features, such as RNA and protein content, can provide prognostic insights beyond quantitative measures, such as count and volume [
58].
In summary, the prognostic value of TEPs is an emerging research area. Traditional platelet metrics, including platelet count, platelet-to-lymphocyte ratio, and MPV, are already known to be significant prognostic indicators of many cancers. Building on this, platelet molecular profiling is poised to enhance prognostication by capturing how actively a tumor engages platelets. Early data on ovarian cancer were among the first to demonstrate this in a solid, data-driven manner, and similar approaches can be extended to other cancers. Recent studies have emphasized that platelets are readily available for both detection and prognostication, reflecting a growing consensus in the field.
5.3. Platelets in Disease Monitoring and Therapeutic Decision-Making
Platelets offer a practical means of monitoring disease status over time, as blood tests can be repeated frequently to enable the real-time tracking of tumor dynamics.
Early Detection of Recurrence: In theory, if a patient is in remission after treatment, their platelet profile should revert to a normal, non-educated state. If the tumor begins to recur, subtle changes in platelet RNA or protein content may be detectable even before clinical or radiographic evidence of relapse. Some case studies have noted that platelet counts can increase months before imaging-detected relapses, such as in colorectal cancer. However, larger trials are needed to validate whether platelet-based assays can consistently predict relapse earlier than conventional methods [
59].
Therapy Selection: Platelet profiles may guide therapeutic choices. For instance, if platelet RNA indicates the presence of certain mutations or pathways, such as MET or HER2 status, it could help in selecting targeted therapies for that patient [
60]. While this represents more of a diagnostic use case, it directly affects prognosis by tailoring treatment strategies. Additionally, if a platelet signature suggests that a tumor is highly angiogenesis-driven (for example, with high VEGF levels), clinicians might opt for anti-angiogenic therapy, whereas, if platelet markers indicate an inflammatory tumor microenvironment, immunotherapy might be considered [
61]. Although these possibilities are speculative, they become reasonable as more data are accumulated.
Predicting and Monitoring Treatment Response: Some researchers have proposed that dynamic changes in TEPs could serve as biomarkers of treatment efficacy. For example, following chemotherapy or targeted therapy, a shift in the platelet RNA signature toward a “healthy” profile might predict a good response, whereas the persistence of a “cancer” signature could signal treatment resistance [
62]. A concrete example is the observed change in the MPV during immunotherapy, which has been linked to outcomes [
63]. Another study demonstrated that certain platelet protein levels normalized after curative surgery, and failure of such normalization could indicate residual disease [
60].
Overall, the integration of TEPs into prognostic and monitoring algorithms is still in development but holds promise for personalized patient management. If platelet biomarkers can reliably identify high-risk patients or detect early relapse, interventions can be timely to improve patient survival. Moreover, as an accessible “real-time biopsy,” platelets could reduce the need for repeated tissue biopsies to assess tumor evolution [
64].
6. Consistency of Findings and Comparative Analysis of Studies
Despite variations in individual studies, the overall body of research has consistently demonstrated that platelets from cancer patients exhibit significant differences from those from healthy individuals in terms of RNA profiles, protein content, and counts. Elevated platelet counts, which have been robustly linked to poor prognoses in cancers such as lung, ovarian, renal, and colorectal cancers, often normalize after tumor resection, reinforcing the causal relationship between tumor presence and altered platelet profiles [
65]. Moreover, the technical feasibility of isolating platelets and performing analyses such as RNA sequencing (using standardized protocols such as ThromboSeq) has been successfully demonstrated across multiple laboratories, further supporting the reproducibility of TEP diagnostics [
65,
66].
At the same time, methodological differences contributed to variability in the results. Studies have used a range of endpoints, ranging from specific mRNA targets to global RNA profiles or from miRNAs to proteins, and the choice of control groups (healthy donors versus patients with benign conditions) can significantly impact specificity. Additionally, the degree of platelet alteration appears to depend on the cancer stage and tumor type; for instance, late-stage cancers tend to induce more pronounced changes than early-stage cancers. Confounding factors, including comorbid conditions, infections, and medications, as well as differences in sample handling and technical protocols, further contribute to the inconsistencies among studies [
67,
68].
Nevertheless, large-scale clinical studies and meta-analyses, such as a 2023 lung cancer analysis including nearly 14,000 patients, have validated the diagnostic and prognostic utility of TEPs, supporting the notion that the aggregate pattern of multiple biomarkers is a robust indicator of tumor presence [
69]. However, certain discrepancies, such as the reported MPV paradox, can be attributed to heterogeneity in study designs and patient populations, stratified by cancer type and stage, generally revealing clear, consistent patterns [
66]. Overall, accumulating evidence strongly supports the use of TEPs as reliable indicators for cancer detection and prognosis, while ongoing efforts to standardize protocols and control confounding factors are essential to translate these findings into clinical practice.
7. Limitations and Challenges in TEP Research
Although the potential of TEPs is evident, several challenges and limitations must be overcome before platelet-based liquid biopsies are fully integrated into clinical practice. These range from technical hurdles in sample processing to fundamental questions regarding specificity and standardization. Despite promising findings, numerous technical and clinical hurdles must be overcome to integrate TEP-based liquid biopsies into rou-tine oncology practice.
Table 4 outlines key limitations and discusses potential solutions.
7.1. Technical and Methodological Challenges
7.1.1. Platelet Isolation and Purity
Obtaining platelet-rich plasma free of contamination by other blood cells is a critical step because even a small number of leukocytes can release nucleic acids that confound platelet RNA analyses. One centrifugation-based protocol yields approximately 1–5 white blood cells per million platelets [
70]. However, not all studies have achieved this level of rigor. Standardized isolation methods, perhaps even automated devices, are needed so that any clinic or laboratory can prepare samples with consistent purity [
71]. Additionally, preventing platelet activation during blood drawing using proper anticoagulants and gentle handling is crucial, as activation can cause platelets to transiently release or take up RNAs and proteins, thereby skewing the results [
60,
70].
7.1.2. Low Input and Analytical Sensitivity
Because platelets are anucleated, they contain much less RNA than whole cells. Although platelets are abundant, the total RNA yield per microliter of blood remains low. Therefore, the detection of subtle changes, especially in early cancer, may require deep sequencing or highly sensitive PCR techniques [
72]. The cost and complexity of such deep profiling can be high, and low-input RNA sequencing of platelet samples is not routinely performed in clinical laboratories [
72]. Developing targeted assays that focus on a defined panel of key platelet RNAs or proteins could alleviate this challenge; however, these panels still need to be defined and validated [
73,
74].
7.1.3. Data Processing and Algorithms
Interpreting platelet-omics data often involves advanced computational algorithms, such as machine learning classifiers, which must be both robust and interpretable for clinical use. One challenge is that a model trained on one cohort may not directly apply to another, highlighting the need for algorithms that can adapt to or be trained on large and diverse datasets to capture the generalizable features of TEP profiles [
73]. Moreover, regulatory approval of AI-driven diagnostics requires consistency and clarity in decision-making, which can be difficult if the algorithms function as black boxes. Researchers are actively addressing these issues by simplifying models, for example, by reducing the number of genes from approximately 1000 to approximately 100, and by combining multiple cohorts for training [
74].
7.1.4. Reproducibility and Standardization
This field requires agreed-upon standard operating procedures and reference materials. A standardized set of reference platelet samples, perhaps pooled from donors, could be used to calibrate assays across laboratories so that the results of each study are comparable rather than siloed. Recent meta-analyses and systematic reviews are helping shape best practices, and guidelines from organizations such as the International Society of Thrombosis and Hemostasis or relevant oncology consortia are expected to be established to ensure that platelet-based liquid biopsy assays for reproducibility [
29,
74].
7.2. Biological and Clinical Challenges
7.2.1. Specificity to Cancer
Platelets respond to many stimuli besides tumors. Infections, inflammatory diseases, tissue injury, and intense exercise can alter platelet behavior. This raises concerns about false positives, that is, non-cancer conditions being misinterpreted as cancer owing to platelet changes. For example, chronic infections can cause elevated platelet counts and inflammatory RNA signatures in platelets [
29,
75]. To mitigate this, a TEP-based test may need to be combined with other clinical information or biomarkers to ensure specificity (e.g., checking C-reactive protein levels for inflammation alongside the TEP test to contextualize the results). To date, a large study conducted in 2022 showed very high specificity in asymptomatic controls, which is encouraging; however, careful validation in populations with benign diseases is required [
29,
76].
7.2.2. Heterogeneity Among Cancers
Not all tumors educate their platelets to the same degree. Some cancer types might shed a lot of material into the blood (for example, lung or colon cancers that are highly vascularized), whereas others, such as certain brain tumors, may not because of barriers, such as the blood-brain barrier, even though some brain tumor signals, such as EGFRvIII, reach platelets in glioma patients [
65,
75]. Additionally, individual patient differences in tumor biology, such as varying levels of platelet activation induced by the tumor, indicate that a one-size-fits-all platelet biomarker panel may overlook a subset of cancers. Addressing this issue requires either the use of very broad panels or personalized baseline comparisons if a precancer platelet profile is available, which is not typically the case in practice [
11,
53].
7.2.3. Dynamic Changes and Timing
Platelet education is a dynamic process, and the “snapshot” provided by a single blood draw may be influenced by transient factors. For example, a patient with cancer who has undergone surgery or an invasive diagnostic procedure may exhibit platelet changes due to that event rather than due to the tumor itself [
23,
77]. Timing sample collection, perhaps by collecting multiple samples over time, might provide a more stable readout of tumor-related changes by averaging out transient noise. This represents a logistical consideration for implementing TEP tests [
77].
7.2.4. Unknown Mechanisms
However, some aspects of how tumors alter platelets remain unclear. Although many factors are known to be involved, the reasons why certain platelet proteins increase in early-stage cancer and decrease in late-stage cancer are not yet fully understood. Without a clear understanding of the underlying mechanisms, selecting appropriate biomarkers may be as challenging as searching for a needle in a haystack. This uncertainty also implies that interventions aimed at blocking platelet education, which could theoretically serve as a therapeutic strategy, are not yet feasible. More basic research is needed to map the tumor-platelet communication pathways in detail. This knowledge will ultimately help determine which platelet biomarkers are most directly linked to tumor presence versus secondary effects [
23,
77].
7.3. Validation and Clinical Trial Challenges
7.3.1. Need for Large-Scale Validation
Many TEP biomarkers are still in the discovery and early validation stages. Therefore, large prospective trials are needed for clinical testing. For example, a prospective screening trial in a high-risk population (such as heavy smokers with lung cancer) using a platelet RNA test is needed to accurately measure its performance in terms of sensitivity, specificity, and predictive value in a real screening scenario. Although such trials are expensive and time-consuming, they are essential. Similarly, for prognostic use, trials embedding platelet biomarker analysis in treatment protocols can determine whether using that information improves patient management; for instance, whether adjusting therapy based on a platelet marker leads to better outcomes [
11,
78].
7.3.2. Regulatory Approval and Standardization
TEP-based tests have not received regulatory approval for routine use. To achieve this, a specific test, such as a kit measuring a set of five platelet RNAs, needs to be finalized, manufactured under strict quality controls, and validated in clinical laboratories to demonstrate reproducibility. However, the process can take several years to complete. The field may eventually converge to a few key assays, perhaps varying by cancer type, to move through this pipeline. For instance, a platelet RNA test for the early detection of lung cancer could become a candidate if further validated, or a platelet protein panel for colon cancer might emerge as another option. Although various academic groups are currently exploring different assays, eventual consensus and partnerships with industry are required to bring a test to market. There is growing interest from industry, as platelets are increasingly recognized as a "holy grail" of cancer biomarkers due to their accessibility and the richness of information they contain [
78].
7.3.3. Integration with Existing Workflows
Even if a TEP test proves effective, practical questions remain regarding how it will fit into patient care. Will it complement or replace imaging techniques such as CT? Can oncologists trust platelet test results enough to decide whether to perform a biopsy? These considerations indicate that clinical guidelines and physicians’ awareness must be matched with this research. Early adoption may occur in scenarios in which current methods fall short. For example, in cases of cancer of unknown primary origin, a platelet RNA signature might help to pinpoint the tissue of origin when metastases are present but no obvious primary tumor is found, given that platelets can carry tissue-specific RNAs. Another scenario could involve patients who are unfit for invasive biopsy, in whom a platelet test might provide valuable diagnostic clues non-invasively [
11]. Demonstrating clear clinical utility in such situations is the key to driving adoption [
79].
8. Future Directions and Potential Solutions
The challenges outlined above are being actively addressed in ongoing research. There are several noteworthy future research directions and solutions. Significant strides are being made to address the current challenges of TEP-based diagnostics, with numerous clinical trials and techno-logical innovations underway.
Table 5 outlines some of these ongoing efforts and emerging directions.
8.1. Multi-Modal Liquid Biopsy Approaches
As suggested in recent reviews, combining platelet biomarkers with other liquid biopsy markers may yield optimal results. Future cancer diagnostic tests may simultaneously analyze TEP RNA, circulating tumor DNA mutations, and circulating proteins to provide a more comprehensive picture. Machine learning can integrate these diverse data types into final diagnostic outputs. This multi-modal approach can compensate for the limitations of any single analyte; for example, if a tumor does not shed much DNA, it might still educate platelets, or vice versa. One study indicated that integrating platelet data improved circulating tumor DNA alone for endometrial cancer detection [
80,
81].
8.2. Refinement of Biomarker Panels
Ongoing large-scale sequencing of platelets from diverse patient cohorts will help identify the most robust and universal biomarkers. In recent years, researchers have expected to converge on a set of biomarkers, whether RNAs or proteins, that consistently perform well. These markers can be incorporated into targeted tests that are faster and more cost-effective than whole-transcriptome sequencing. For instance, if a handful of miRNAs or mRNAs repeatedly proved to be top-performing across studies, a multiplex PCR or NanoString assay could be developed to measure them [
82].
8.3. Automation and Clinical Laboratory Development
Automation is key to the routine clinical use of TEPs. The development of dedicated devices is expected to isolate platelets at the point of care and perhaps even perform on-chip analyses of specific platelet biomarkers. Analogous to automated complete blood count machines that measure platelet counts, future devices may measure platelet RNA markers and output a “cancer risk score.” Early prototypes and workflows for such automation are likely to be explored in research settings [
82,
83].
8.4. Standardizing Pre-Analytical Variables
Efforts to standardize the collection and processing of blood samples for platelet analysis are essential for enhancing consistency. For example, a consensus may emerge that fasting morning blood draws using citrate tubes and analysis within two hours is optimal. Establishing standardized pre-analytical variables will help codify trial protocols, which will eventually be incorporated into routine laboratory manuals [
84].
8.5. Understanding Mechanisms
Research continues into the biogenesis of TEP signals. Studies have investigated how tumors induce specific splicing events in platelets and how tumor-derived extracellular vesicles selectively package certain RNAs that end up in platelets. Unraveling these processes could identify druggable targets to interrupt tumor-platelet crosstalk and reveal whether certain platelet changes are causative of worse outcomes or merely correlate with tumor presence. If proven to be causative, interventions such as antiplatelet drugs (e.g., low-dose aspirin) may reduce metastasis. Future trials should combine platelet-targeting strategies with standard cancer treatments to assess improvements in patient outcomes [
23,
85].
8.6. Clinical Trials and Real-World Evidence
More clinical trials that explicitly incorporate TEP analyses are expected. For example, a trial could test whether platelet RNA testing during follow-up visits can detect recurrence earlier than conventional imaging. Another trial may stratify patients for treatment based on platelet biomarkers. Additionally, as some tests become available for research use, real-world data will accumulate, such as if academic medical centers begin offering experimental platelet tests to patients with undiagnosed cancer. This real-world evidence provides further validation and may uncover new clinical applications [
86,
87].
8.7. Interdisciplinary Collaboration
The field of TEPs is at the intersection of oncology, hematology, and bioinformatics. Collaborative networks and cross-institutional consortia are essential for accelerating progress. Sharing data among multiple centers will enable the construction of large datasets necessary to refine algorithms that generalize well, ultimately advancing both science and clinical translation [
88].
The path forward for TEPs involves both improving scientific understanding and translating it into practical diagnostic tools. The progress made in the past 5–6 years, from an intriguing idea to large validation studies, demonstrates significant potential. If these challenges are met, TEP-based tests could become a standard component of the liquid biopsy toolkit, offering clinicians a new noninvasive window into the status of cancer through a simple blood sample. Researchers have even referred to platelets as a possible “holy grail” in cancer blood biomarker research, realizing that promise will depend on the outcomes of ongoing research and development efforts [
82].
9. Conclusion
TEPs represent a frontier in cancer diagnostics and prognostics, embodying the concept that “blood can tell the story of a tumor.” In this expanded review, we outlined how platelets, far from passive bystanders, actively communicate with cancer cells and, in the process, acquire tumor-specific information. The scientific rationale for TEPs has been well established by mechanistic studies demonstrating the transfer of tumor molecules into platelets and the alteration of platelet behavior by tumor-secreting factors. Building on this, a growing body of evidence has shown that analyzing platelet counts and RNA and protein profiles can aid in early cancer detection, diagnosis, disease monitoring, and prognostication.
We discuss how multiple independent investigations have achieved promising diagnostic accuracy using TEPs across cancer types, with recent large-cohort studies confirming that the signal is real and not limited to small experimental settings. TEP-based assays have achieved sensitivity and specificity that approach clinical utility, and in some contexts (such as early-stage cancer or minimal residual disease detection), they may offer advantages over traditional biomarkers. Platelets have long been linked to worse outcomes when elevated. However, platelet-derived molecular signatures now offer a more nuanced stratification of patients, as seen in recent ovarian cancer studies and other emerging data.
However, this review also underscores the importance of critical evaluation and refinement of TEP approaches. We highlight that not all studies were in complete agreement; differences in methodology and patient cohorts have led to variability in the reported markers and accuracies. A comparison of the findings, we can see suggests that the overarching concept holds; however, standardization and validation are required to reconcile discrepancies and ensure reliability. Challenges such as ensuring specificity (as platelets also respond to non-cancer conditions) and solving technical issues (such as isolating platelets purely and affordably sequencing their contents) remain to be fully addressed.
The path forward involves multidisciplinary efforts, including larger clinical trials to validate TEP biomarkers, improvements in bioinformatics to create robust predictive models, and collaborative standard settings so that results are reproducible across different laboratories and hospitals. Current trends show movement in this direction, with meta-analyses, cross-validation studies, and accelerated technological development. The integration of TEP analysis with other liquid biopsy methods and imaging could eventually lead to the development of composite diagnostic algorithms that could significantly improve early cancer detection rates and patient stratification.
In conclusion, TEPs offer a compelling and scientifically grounded avenue for effective cancer management. They exemplify the concept of a “biosensor” circulating in our blood—platelets capture tumor signals and thus can be read out to reveal the presence and stage of cancer. As research continues to address current limitations, TEPs are poised to transition from a predominantly research topic to clinical reality, potentially improving outcomes by enabling earlier diagnosis, personalized prognostic assessments, and better monitoring of treatment responses. The coming years will be critical in determining how this innovative liquid biopsy approach can be harnessed in routine oncology practice; however, the evidence amassed thus far provides a strong foundation and optimism that TEPs will become a valuable component of precision cancer medicine.
Author Contributions
Conceptualization, W.A.K., E.Y.A., and T.J.A; writing—original draft preparation, W.A.K. and E.Y.A; writing—review and editing, W.A.K., E.Y.A., H.Y.K., Y.S.S., M.K.L., and T.J.A.; supervision, W.A.K., Y.S.S., and M.K.L. All authors contributed substantially to the discussion of content and have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lone, S.N.; Nisar, S.; Masoodi, T.; Singh, M.; Rizwan, A.; Hashem, S.; El-Rifai, W.; Bedognetti, D.; Batra, S.K.; Haris, M.; et al. Liquid biopsy: a step closer to transform diagnosis, prognosis and future of cancer treatments. Molecular Cancer 2022, 21, 79. [Google Scholar] [CrossRef]
- Pantel, K.; Alix-Panabières, C. Liquid biopsy and minimal residual disease — latest advances and implications for cure. Nature Reviews Clinical Oncology 2019, 16, 409–424. [Google Scholar] [CrossRef]
- Best, M.G.; Vancura, A.; Wurdinger, T. Platelet RNA as a circulating biomarker trove for cancer diagnostics. J Thromb Haemost 2017, 15, 1295–1306. [Google Scholar] [CrossRef]
- Ding, S.; Dong, X.; Song, X. Tumor educated platelet: the novel BioSource for cancer detection. Cancer Cell International 2023, 23, 91. [Google Scholar] [CrossRef]
- Trivanović, D.; Mojsilović, S.; Bogosavljević, N.; Jurišić, V.; Jauković, A. Revealing profile of cancer-educated platelets and their factors to foster immunotherapy development. Translational Oncology 2024, 40, 101871. [Google Scholar] [CrossRef]
- Best, M.G.; Wesseling, P.; Wurdinger, T. Tumor-Educated Platelets as a Noninvasive Biomarker Source for Cancer Detection and Progression Monitoring. Cancer Res 2018, 78, 3407–3412. [Google Scholar] [CrossRef]
- Best, M.G.; Sol, N.; Kooi, I.; Tannous, J.; Westerman, B.A.; Rustenburg, F.; Schellen, P.; Verschueren, H.; Post, E.; Koster, J.; et al. RNA-Seq of Tumor-Educated Platelets Enables Blood-Based Pan-Cancer, Multiclass, and Molecular Pathway Cancer Diagnostics. Cancer Cell 2015, 28, 666–676. [Google Scholar] [CrossRef]
- Gu, M.; Zhai, Z.; Huang, L.; Zheng, W.; Zhou, Y.; Zhu, R.; Shen, F.; Yuan, C. Pre-treatment mean platelet volume associates with worse clinicopathologic features and prognosis of patients with invasive breast cancer. Breast Cancer 2016, 23, 752–760. [Google Scholar] [CrossRef]
- Schlesinger, M. Role of platelets and platelet receptors in cancer metastasis. Journal of Hematology & Oncology 2018, 11, 125. [Google Scholar] [CrossRef]
- Haemmerle, M.; Stone, R.L.; Menter, D.G.; Afshar-Kharghan, V.; Sood, A.K. The Platelet Lifeline to Cancer: Challenges and Opportunities. Cancer Cell 2018, 33, 965–983. [Google Scholar] [CrossRef]
- Antunes-Ferreira, M.; D’Ambrosi, S.; Arkani, M.; Post, E.; In ‘t Veld, S.G.J.G.; Ramaker, J.; Zwaan, K.; Kucukguzel, E.D.; Wedekind, L.E.; Griffioen, A.W.; et al. Tumor-educated platelet blood tests for Non-Small Cell Lung Cancer detection and management. Scientific Reports 2023, 13, 9359. [Google Scholar] [CrossRef]
- Zhuang, T.; Wang, S.; Yu, X.; He, X.; Guo, H.; Ou, C. Current status and future perspectives of platelet-derived extracellular vesicles in cancer diagnosis and treatment. Biomarker Research 2024, 12, 88. [Google Scholar] [CrossRef]
- Figueroa, J.M.; Skog, J.; Akers, J.; Li, H.; Komotar, R.; Jensen, R.; Ringel, F.; Yang, I.; Kalkanis, S.; Thompson, R.; et al. Detection of wild-type EGFR amplification and EGFRvIII mutation in CSF-derived extracellular vesicles of glioblastoma patients. Neuro Oncol 2017, 19, 1494–1502. [Google Scholar] [CrossRef]
- D'Ambrosi, S.; Nilsson, R.J.; Wurdinger, T. Platelets and tumor-associated RNA transfer. Blood 2021, 137, 3181–3191. [Google Scholar] [CrossRef]
- Xiang, Y.; Xiang, P.; Zhang, L.; Li, Y.; Zhang, J. A narrative review for platelets and their RNAs in cancers: New concepts and clinical perspectives. Medicine (Baltimore) 2022, 101, e32539. [Google Scholar] [CrossRef]
- Zhou, L.; Wu, D.; Zhou, Y.; Wang, D.; Fu, H.; Huang, Q.; Qin, G.; Chen, J.; Lv, J.; Lai, S.; et al. Tumor cell-released kynurenine biases MEP differentiation into megakaryocytes in individuals with cancer by activating AhR–RUNX1. Nature Immunology 2023, 24, 2042–2052. [Google Scholar] [CrossRef]
- Gaertner, F.; Ishikawa-Ankerhold, H.; Stutte, S.; Fu, W.; Weitz, J.; Dueck, A.; Nelakuditi, B.; Fumagalli, V.; van den Heuvel, D.; Belz, L.; et al. Plasmacytoid dendritic cells control homeostasis of megakaryopoiesis. Nature 2024, 631, 645–653. [Google Scholar] [CrossRef]
- Giannakeas, V.; Narod, S.A. Incidence of Cancer Among Adults With Thrombocytosis in Ontario, Canada. JAMA Network Open 2021, 4, e2120633–e2120633. [Google Scholar] [CrossRef]
- Hufnagel, D.H.; Cozzi, G.D.; Crispens, M.A.; Beeghly-Fadiel, A. Platelets, Thrombocytosis, and Ovarian Cancer Prognosis: Surveying the Landscape of the Literature. International Journal of Molecular Sciences 2020, 21, 8169. [Google Scholar] [CrossRef]
- Amer, H.; Kampan, N.C.; Itsiopoulos, C.; Flanagan, K.L.; Scott, C.L.; Kartikasari, A.E.R.; Plebanski, M. Interleukin-6 Modulation in Ovarian Cancer Necessitates a Targeted Strategy: From the Approved to Emerging Therapies. Cancers 2024, 16, 4187. [Google Scholar] [CrossRef]
- Kumar, A.; Taghi Khani, A.; Sanchez Ortiz, A.; Swaminathan, S. GM-CSF: A Double-Edged Sword in Cancer Immunotherapy. Front Immunol 2022, 13, 901277. [Google Scholar] [CrossRef]
- Oncul, S.; Cho, M.S. Interactions between Platelets and Tumor Microenvironment Components in Ovarian Cancer and Their Implications for Treatment and Clinical Outcomes. Cancers (Basel) 2023, 15. [Google Scholar] [CrossRef]
- Dudiki, T.; Veleeparambil, M.; Zhevlakova, I.; Biswas, S.; Klein, E.A.; Ford, P.; Podrez, E.A.; Byzova, T.V. Mechanism of Tumor-Platelet Communications in Cancer. Circulation Research 2023, 132, 1447–1461. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, C.; Pan, F.; Chen, Y.; Xiong, L.; Li, Y.; Chu, X.; Huang, G. Platelets in the tumor microenvironment and their biological effects on cancer hallmarks. Front Oncol 2023, 13, 1121401. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, C.; Pan, F.; Chen, Y.; Xiong, L.; Li, Y.; Chu, X.; Huang, G. Platelets in the tumor microenvironment and their biological effects on cancer hallmarks. Frontiers in Oncology 2023, 13. [Google Scholar] [CrossRef]
- Eslami-S, Z.; Cortés-Hernández, L.E.; Glogovitis, I.; Antunes-Ferreira, M.; D’Ambrosi, S.; Kurma, K.; Garima, F.; Cayrefourcq, L.; Best, M.G.; Koppers-Lalic, D.; et al. In vitro cross-talk between metastasis-competent circulating tumor cells and platelets in colon cancer: a malicious association during the harsh journey in the blood. Frontiers in Cell and Developmental Biology 2023, 11. [Google Scholar] [CrossRef]
- Morales-Pacheco, M.; Valenzuela-Mayen, M.; Gonzalez-Alatriste, A.M.; Mendoza-Almanza, G.; Cortés-Ramírez, S.A.; Losada-García, A.; Rodríguez-Martínez, G.; González-Ramírez, I.; Maldonado-Lagunas, V.; Vazquez-Santillan, K.; et al. The role of platelets in cancer: from their influence on tumor progression to their potential use in liquid biopsy. Biomark Res 2025, 13, 27. [Google Scholar] [CrossRef]
- Zhang, Q.; Song, X.; Song, X. Contents in tumor-educated platelets as the novel biosource for cancer diagnostics. Frontiers in Oncology 2023, 13. [Google Scholar] [CrossRef]
- Chen, M.; Hou, L.; Hu, L.; Tan, C.; Wang, X.; Bao, P.; Ran, Q.; Chen, L.; Li, Z. Platelet detection as a new liquid biopsy tool for human cancers. Frontiers in Oncology 2022, 12. [Google Scholar] [CrossRef]
- Detopoulou, P.; Panoutsopoulos, G.I.; Mantoglou, M.; Michailidis, P.; Pantazi, I.; Papadopoulos, S.; Rojas Gil, A.P. Relation of Mean Platelet Volume (MPV) with Cancer: A Systematic Review with a Focus on Disease Outcome on Twelve Types of Cancer. Curr Oncol 2023, 30, 3391–3420. [Google Scholar] [CrossRef]
- Jopek, M.A.; Pastuszak, K.; Sieczczyński, M.; Cygert, S.; Żaczek, A.J.; Rondina, M.T.; Supernat, A. Improving platelet-RNA-based diagnostics: a comparative analysis of machine learning models for cancer detection and multiclass classification. Mol Oncol 2024, 18, 2743–2754. [Google Scholar] [CrossRef]
- Hajjar, M.; Albaradei, S.; Aldabbagh, G. Machine Learning Approaches in Multi-Cancer Early Detection. Information 2024, 15, 627. [Google Scholar] [CrossRef]
- Xiao, R.; Liu, C.; Zhang, B.; Ma, L. Tumor-Educated Platelets as a Promising Biomarker for Blood-Based Detection of Renal Cell Carcinoma. Front Oncol 2022, 12, 844520. [Google Scholar] [CrossRef]
- In 't Veld, S.; Arkani, M.; Post, E.; Antunes-Ferreira, M.; D'Ambrosi, S.; Vessies, D.C.L.; Vermunt, L.; Vancura, A.; Muller, M.; Niemeijer, A.N.; et al. Detection and localization of early- and late-stage cancers using platelet RNA. Cancer Cell 2022, 40, 999–1009.e1006. [Google Scholar] [CrossRef]
- Yuan, M.; Jia, Y.; Xing, Y.; Wang, Y.; Liu, Y.; Liu, X.; Liu, D. Screening and validation of platelet activation-related lncRNAs as potential biomarkers for prognosis and immunotherapy in gastric cancer patients. Frontiers in Genetics 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wei, X.; Wu, B.; Su, J.; Tan, W.; Yang, K. Tumor-educated platelet miR-34c-3p and miR-18a-5p as potential liquid biopsy biomarkers for nasopharyngeal carcinoma diagnosis. Cancer Manag Res 2019, 11, 3351–3360. [Google Scholar] [CrossRef] [PubMed]
- D'Ambrosi, S.; Visser, A.; Antunes-Ferreira, M.; Poutsma, A.; Giannoukakos, S.; Sol, N.; Sabrkhany, S.; Bahce, I.; Kuijpers, M.J.E.; Oude Egbrink, M.G.A.; et al. The Analysis of Platelet-Derived circRNA Repertoire as Potential Diagnostic Biomarker for Non-Small Cell Lung Cancer. Cancers (Basel) 2021, 13. [Google Scholar] [CrossRef]
- Tavukcuoglu, Z.; Butt, U.; Faria, A.V.S.; Oesterreicher, J.; Holnthoner, W.; Laitinen, S.; Palviainen, M.; Siljander, P.R. Platelet-derived extracellular vesicles induced through different activation pathways drive melanoma progression by functional and transcriptional changes. Cell Commun Signal 2024, 22, 601. [Google Scholar] [CrossRef]
- Yu, L.; Guo, Y.; Chang, Z.; Zhang, D.; Zhang, S.; Pei, H.; Pang, J.; Zhao, Z.J.; Chen, Y. Bidirectional Interaction Between Cancer Cells and Platelets Provides Potential Strategies for Cancer Therapies. Frontiers in Oncology 2021, 11. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, Q.; Li, D.Z.; Zhou, X.; Yu, D.S.; Zhong, J. TIMP1 mRNA in tumor-educated platelets is diagnostic biomarker for colorectal cancer. Aging (Albany NY) 2019, 11, 8998–9012. [Google Scholar] [CrossRef]
- Hu, Y.; Zeng, C.; Li, J.; Ren, S.; Shao, M.; Lei, W.; Yi, J.; Han, W.; Cao, J.; Zou, J.; et al. TRIM27 revealing by tumor educated platelet RNA-sequencing, as a potential biomarker for malignant ground-glass opacities diagnosis mediates glycolysis of non-small cell lung cancer cells partially through HOXM1. Transl Lung Cancer Res 2024, 13, 2307–2325. [Google Scholar] [CrossRef]
- Nicolò, E.; Gianni, C.; Pontolillo, L.; Serafini, M.S.; Munoz-Arcos, L.S.; Andreopoulou, E.; Curigliano, G.; Reduzzi, C.; Cristofanilli, M. Circulating tumor cells et al.: towards a comprehensive liquid biopsy approach in breast cancer. Translational Breast Cancer Research 2024, 5. [Google Scholar] [CrossRef]
- Mondal, D.; Shinde, S.; Sinha, V.; Dixit, V.; Paul, S.; Gupta, R.K.; Thakur, S.; Vishvakarma, N.K.; Shukla, D. Prospects of liquid biopsy in the prognosis and clinical management of gastrointestinal cancers. Frontiers in Molecular Biosciences 2024, 11. [Google Scholar] [CrossRef]
- She, W.; Garitaonaindia, Y.; Lin, Y. The latest advances in liquid biopsy for lung cancer-a narrative review. Transl Lung Cancer Res 2024, 13, 3241–3251. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xu, Y.; Tong, L.; Zhang, X.; Zhang, S. Recent progress of exosomal lncRNA/circRNA–miRNA–mRNA axis in lung cancer: implication for clinical application. Frontiers in Molecular Biosciences 2024, 11. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, Y.; Song, X.; Ren, J. The mechanisms and diagnostic potential of lncRNAs, miRNAs, and their related signaling pathways in cervical cancer. Frontiers in Cell and Developmental Biology 2023, 11. [Google Scholar] [CrossRef]
- Du, X.; Zang, X.; Zhang, H.; Liu, L.; Xu, Y.; Li, X.; Mou, R.; Xu, H.; Zhu, J.; Xie, R. Mean platelet volume/platelet count ratio can predict the recurrence-free survival rate of patients after complete resection of gastrointestinal stromal tumors. Frontiers in Oncology 2024, 14. [Google Scholar] [CrossRef]
- Yoshikawa, N.; Matsukawa, T.; Hattori, S.; Iyoshi, S.; Yoshida, K.; Yoshihara, M.; Tamauchi, S.; Shimizu, Y.; Ikeda, Y.; Yokoi, A.; et al. Mean platelet volume as a potential biomarker for survival outcomes in ovarian clear cell carcinoma. Int J Clin Oncol 2023, 28, 1680–1689. [Google Scholar] [CrossRef]
- Qi, X.; Chen, J.; Wei, S.; Ni, J.; Song, L.; Jin, C.; Yang, L.; Zhang, X. Prognostic significance of platelet-to-lymphocyte ratio (PLR) in patients with breast cancer treated with neoadjuvant chemotherapy: a meta-analysis. BMJ Open 2023, 13, e074874. [Google Scholar] [CrossRef]
- Li, X.; Ren, H.; Peng, L.; Li, J. Prognostic value of pretreatment platelet count, fibrinogen and d-dimer levels in osteosarcoma patients: A meta-analysis. Medicine 2024, 103, e38463. [Google Scholar] [CrossRef]
- Shi, H.; Wang, H.; Pan, J.; Liu, Z.; Li, Z. Comparing prognostic value of preoperative platelet indexes in patients with resectable gastric cancer. Scientific Reports 2022, 12, 6480. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhong, H.; Ye, L.; Li, Q.; Fang, S.; Gu, W.; Qian, Y. Prognostic value of pretreatment platelet counts in lung cancer: a systematic review and meta-analysis. BMC Pulm Med 2020, 20, 96. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tao, L.; Qiu, J.; Xu, J.; Yang, X.; Zhang, Y.; Tian, X.; Guan, X.; Cen, X.; Zhao, Y. Tumor biomarkers for diagnosis, prognosis and targeted therapy. Signal Transduction and Targeted Therapy 2024, 9, 132. [Google Scholar] [CrossRef]
- Liu, C.J.; Li, H.Y.; Gao, Y.; Xie, G.Y.; Chi, J.H.; Li, G.L.; Zeng, S.Q.; Xiong, X.M.; Liu, J.H.; Shi, L.L.; et al. Platelet RNA signature independently predicts ovarian cancer prognosis by deep learning neural network model. Protein Cell 2023, 14, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Giannakeas, V.; Narod, S.A.; Akbari, M.R. Emerging applications of tumour-educated platelets in the detection and prognostication of ovarian cancer. Protein Cell 2023, 14, 556–559. [Google Scholar] [CrossRef]
- Hu, H.; Song, H.; Han, B.; Zhao, H.; He, J. Tumor-educated platelet RNA and circulating free RNA: emerging liquid biopsy markers for different tumor types. Frontiers in Bioscience-Landmark 2024, 29, 80. [Google Scholar] [CrossRef]
- Hu, M.S.; Jiang, M.; Wang, Y.J.; Xu, S.F.; Jiang, F.Y.; Han, Y.T.; Liu, Z.W.; Yu, H. Platelet-related gene risk score: a predictor for pancreatic cancer microenvironmental signature, chemosensitivity and prognosis. Am J Cancer Res 2023, 13, 6113–6124. [Google Scholar]
- Li, Q.; Zhang, C.; Ren, Y.; Qiao, L.; Xu, S.; Li, K.; Liu, Y. A novel platelets-related gene signature for predicting prognosis, immune features and drug sensitivity in gastric cancer. Front Immunol 2024, 15, 1477427. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, A.; Zeller, J.; Peter, K.; McFadyen, J.D. Decoding the role of platelets in tumour metastasis: enigmatic accomplices and intricate targets for anticancer treatments. Frontiers in Immunology 2023, 14. [Google Scholar] [CrossRef]
- Huber, L.T.; Kraus, J.M.; Ezić, J.; Wanli, A.; Groth, M.; Laban, S.; Hoffmann, T.K.; Wollenberg, B.; Kestler, H.A.; Brunner, C. Liquid biopsy: an examination of platelet RNA obtained from head and neck squamous cell carcinoma patients for predictive molecular tumor markers. Explor Target Antitumor Ther 2023, 4, 422–446. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, N.; Zhang, Y.; Zhang, D.; Sun, S.; Yu, H.; Lin, Y.; Zhao, X.; Wang, H.; Wu, X.; et al. PD-L1 mRNA derived from tumor-educated platelets as a potential immunotherapy biomarker in non-small cell lung cancer. Translational Lung Cancer Research 2024, 13, 345–354. [Google Scholar] [CrossRef]
- Garcia-Leon, M.J.; Liboni, C.; Mittelheisser, V.; Bochler, L.; Follain, G.; Mouriaux, C.; Busnelli, I.; Larnicol, A.; Colin, F.; Peralta, M.; et al. Platelets favor the outgrowth of established metastases. Nature Communications 2024, 15, 3297. [Google Scholar] [CrossRef]
- Xu, L.; Li, X.; Li, X.; Wang, X.; Ma, Q.; She, D.; Lu, X.; Zhang, J.; Yang, Q.; Lei, S.; et al. RNA profiling of blood platelets noninvasively differentiates colorectal cancer from healthy donors and noncancerous intestinal diseases: a retrospective cohort study. Genome Med 2022, 14, 26. [Google Scholar] [CrossRef]
- Post, E.; Sol, N.; Best, M.G.; Wurdinger, T. Blood platelets as an RNA biomarker platform for neuro-oncological diseases. Neuro-Oncology Advances 2022, 4, ii61–ii65. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Song, X.; Song, X. Contents in tumor-educated platelets as the novel biosource for cancer diagnostics. Front Oncol 2023, 13, 1165600. [Google Scholar] [CrossRef] [PubMed]
- Mai, S.; Inkielewicz-Stepniak, I. Pancreatic Cancer and Platelets Crosstalk: A Potential Biomarker and Target. Front Cell Dev Biol 2021, 9, 749689. [Google Scholar] [CrossRef]
- Walraven, M.; Sabrkhany, S.; Knol, J.C.; Dekker, H.; de Reus, I.; Piersma, S.R.; Pham, T.V.; Griffioen, A.W.; Broxterman, H.J.; Oude Egbrink, M.; et al. Effects of Cancer Presence and Therapy on the Platelet Proteome. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- Gerber, D.E.; Singh, H.; Larkins, E.; Ferris, A.; Forde, P.M.; Selig, W.; Basu Roy, U. A New Approach to Simplifying and Harmonizing Cancer Clinical Trials-Standardizing Eligibility Criteria. JAMA Oncol 2022, 8, 1333–1339. [Google Scholar] [CrossRef]
- Wiyarta, E.; Nugraha, D.A.; Ramadani, M.I.; Gustya, G.F.; Ammar, M.F.; Edwar, H.D.; Kheirizzad, N.; Mukhlisah, M.N.; Burhan, E.; Syahruddin, E. Clinical utility and diagnostic value of tumor-educated platelets in lung cancer: a systematic review and meta-analysis. Front Oncol 2023, 13, 1201713. [Google Scholar] [CrossRef]
- Sakai, K.; Ohara, S.; Tanaka, J.; Suda, K.; Muramatsu, T.; Uematsu, C.; Tsutani, Y.; Mitsudomi, T.; Nishio, K. Improved platelet separation performance from whole blood using an acoustic fluidics system. Cancer Sci 2024, 115, 3795–3803. [Google Scholar] [CrossRef]
- Jin, J.; Shao, Y.; Zhang, J.; Cao, J.; Tao, Z.; Hu, X. High-purity isolation platelets by gradient centrifugation plus filtration. International Journal of Laboratory Hematology 2023, 45, 187–194. [Google Scholar] [CrossRef]
- Collinson, R.J.; Boey, D.; Wilson, L.; Ng, Z.Y.; Mirzai, B.; Chuah, H.; Leahy, M.F.; Howman, R.; Linden, M.; Fuller, K.; et al. PlateletSeq: A novel method for discovery of blood-based biomarkers. Methods 2023, 219, 139–149. [Google Scholar] [CrossRef]
- Karp, J.M.; Modrek, A.S.; Ezhilarasan, R.; Zhang, Z.-Y.; Ding, Y.; Graciani, M.; Sahimi, A.; Silvestro, M.; Chen, T.; Li, S.; et al. Deconvolution of the tumor-educated platelet transcriptome reveals activated platelet and inflammatory cell transcript signatures. JCI Insight 2024, 9. [Google Scholar] [CrossRef]
- Cygert, S.; Pastuszak, K.; Górski, F.; Sieczczyński, M.; Juszczyk, P.; Rutkowski, A.; Lewalski, S.; Różański, R.; Jopek, M.A.; Jassem, J.; et al. Platelet-Based Liquid Biopsies through the Lens of Machine Learning. Cancers (Basel) 2023, 15. [Google Scholar] [CrossRef]
- Antunes-Ferreira, M.; Koppers-Lalic, D.; Würdinger, T. Circulating platelets as liquid biopsy sources for cancer detection. Mol Oncol 2021, 15, 1727–1743. [Google Scholar] [CrossRef]
- Walke, V.; Das, S.; Mittal, A.; Agrawal, A. Tumor Educated Platelets as a Biomarker for Diagnosis of Lung cancer: A Systematic Review. Asian Pac J Cancer Prev 2024, 25, 1911–1920. [Google Scholar] [CrossRef]
- Roweth, H.G.; Battinelli, E.M. Lessons to learn from tumor-educated platelets. Blood 2021, 137, 3174–3180. [Google Scholar] [CrossRef]
- Goossens, N.; Nakagawa, S.; Sun, X.; Hoshida, Y. Cancer biomarker discovery and validation. Translational Cancer Research 2015, 4, 256–269. [Google Scholar]
- Salwei, M.E.; Reale, C. Workflow analysis of breast cancer treatment decision-making: challenges and opportunities for informatics to support patient-centered cancer care. JAMIA Open 2024, 7, ooae053. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Guo, H.; Zhao, Y.; Liu, Z.; Wang, C.; Bu, J.; Sun, T.; Wei, J. Liquid biopsy in cancer: current status, challenges and future prospects. Signal Transduction and Targeted Therapy 2024, 9, 336. [Google Scholar] [CrossRef] [PubMed]
- Nicolò, E.; Gianni, C.; Pontolillo, L.; Serafini, M.S.; Munoz-Arcos, L.S.; Andreopoulou, E.; Curigliano, G.; Reduzzi, C.; Cristofanilli, M. Circulating tumor cells et al.: towards a comprehensive liquid biopsy approach in breast cancer. Transl Breast Cancer Res 2024, 5, 10. [Google Scholar] [CrossRef]
- Bravaccini, S.; Boldrin, E.; Gurioli, G.; Tedaldi, G.; Piano, M.A.; Canale, M.; Curtarello, M.; Ulivi, P.; Pilati, P. The use of platelets as a clinical tool in oncology: opportunities and challenges. Cancer Lett 2024, 607, 217044. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, Y.; Hu, H. Advances and challenges in platelet counting: evolving from traditional microscopy to modern flow cytometry. Journal of Laboratory Medicine 2025, 49, 2–13. [Google Scholar] [CrossRef]
- Hindle, M.S.; Cheah, L.T.; Yates, D.M.; Naseem, K.M. Preanalytical conditions for multiparameter platelet flow cytometry. Res Pract Thromb Haemost 2023, 7, 102205. [Google Scholar] [CrossRef]
- Liao, K.; Zhang, X.; Liu, J.; Teng, F.; He, Y.; Cheng, J.; Yang, Q.; Zhang, W.; Xie, Y.; Guo, D.; et al. The role of platelets in the regulation of tumor growth and metastasis: the mechanisms and targeted therapy. MedComm (2020) 2023, 4, e350. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, A.; Jin, M.; Li, S.; Duan, Y. TEP RNA: a new frontier for early diagnosis of NSCLC. J Cancer Res Clin Oncol 2024, 150, 97. [Google Scholar] [CrossRef]
- Orcutt, X.; Chen, K.; Mamtani, R.; Long, Q.; Parikh, R.B. Evaluating generalizability of oncology trial results to real-world patients using machine learning-based trial emulations. Nature Medicine 2025, 31, 457–465. [Google Scholar] [CrossRef]
- Arora, A.; Alderman, J.E.; Palmer, J.; Ganapathi, S.; Laws, E.; McCradden, M.D.; Oakden-Rayner, L.; Pfohl, S.R.; Ghassemi, M.; McKay, F.; et al. The value of standards for health datasets in artificial intelligence-based applications. Nat Med 2023, 29, 2929–2938. [Google Scholar] [CrossRef]
- Yang, S.; Wang, L.; Wu, Y.; Wu, A.; Huang, F.; Tang, X.; Kantawong, F.; Anuchapreeda, S.; Qin, D.; Mei, Q.; et al. Apoptosis in megakaryocytes: Safeguard and threat for thrombopoiesis. Front Immunol 2022, 13, 1025945. [Google Scholar] [CrossRef]
- Hufnagel, D.H.; Cozzi, G.D.; Crispens, M.A.; Beeghly-Fadiel, A. Platelets, Thrombocytosis, and Ovarian Cancer Prognosis: Surveying the Landscape of the Literature. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef]
- Isingizwe, Z.R.; Meelheim, B.A.; Benbrook, D.M. Elevated Platelet Aggregation in Patients with Ovarian Cancer: More than Just Increased Platelet Count. Cancers 2024, 16, 3583. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Zhao, Z.; Yang, Y.; Meng, Z.; Qin, L. Effects of the interactions between platelets with other cells in tumor growth and progression. Frontiers in Immunology 2023, 14. [Google Scholar] [CrossRef]
- Xing, S.; Zeng, T.; Xue, N.; He, Y.; Lai, Y.Z.; Li, H.L.; Huang, Q.; Chen, S.L.; Liu, W.L. Development and Validation of Tumor-educated Blood Platelets Integrin Alpha 2b (ITGA2B) RNA for Diagnosis and Prognosis of Non-small-cell Lung Cancer through RNA-seq. Int J Biol Sci 2019, 15, 1977–1992. [Google Scholar] [CrossRef]
- Dudiki, T.; Veleeparambil, M.; Zhevlakova, I.; Biswas, S.; Klein, E.A.; Ford, P.; Podrez, E.A.; Byzova, T.V. Mechanism of Tumor-Platelet Communications in Cancer. Circ Res 2023, 132, 1447–1461. [Google Scholar] [CrossRef]
- Qiu, X.; Quan, G.; Ou, W.; Wang, P.; Huang, X.; Li, X.; Shen, Y.; Yang, W.; Wang, J.; Wu, X. Unraveling TIMP1: a multifaceted biomarker in colorectal cancer. Front Genet 2023, 14, 1265137. [Google Scholar] [CrossRef]
- Wang, S.; Claret, F.-X.; Wu, W. MicroRNAs as Therapeutic Targets in Nasopharyngeal Carcinoma. Frontiers in Oncology 2019, 9. [Google Scholar] [CrossRef]
- Lin, Y.; Zhao, W.; Lv, Z.; Xie, H.; Li, Y.; Zhang, Z. The functions and mechanisms of long non-coding RNA in colorectal cancer. Front Oncol 2024, 14, 1419972. [Google Scholar] [CrossRef]
- Mondal, D.; Shinde, S.; Sinha, V.; Dixit, V.; Paul, S.; Gupta, R.K.; Thakur, S.; Vishvakarma, N.K.; Shukla, D. Prospects of liquid biopsy in the prognosis and clinical management of gastrointestinal cancers. Front Mol Biosci 2024, 11, 1385238. [Google Scholar] [CrossRef]
- Li, X.; Ma, N.; Zhang, Y.; Wei, H.; Zhang, H.; Pang, X.; Li, X.; Wu, D.; Wang, D.; Yang, Z.; et al. Circular RNA circNRIP1 promotes migration and invasion in cervical cancer by sponging miR-629-3p and regulating the PTP4A1/ERK1/2 pathway. Cell Death & Disease 2020, 11, 399. [Google Scholar] [CrossRef]
- Sabrkhany, S.; Kuijpers, M.J.E.; Knol, J.C.; Olde Damink, S.W.M.; Dingemans, A.C.; Verheul, H.M.; Piersma, S.R.; Pham, T.V.; Griffioen, A.W.; Oude Egbrink, M.G.A.; et al. Exploration of the platelet proteome in patients with early-stage cancer. J Proteomics 2018, 177, 65–74. [Google Scholar] [CrossRef]
- Shen, Y.; Yang, W.; Liu, J.; Zhang, Y. Minimally invasive approaches for the early detection of endometrial cancer. Mol Cancer 2023, 22, 53. [Google Scholar] [CrossRef]
- Chebbo, M.; Assou, S.; Pantesco, V.; Duez, C.; Alessi, M.C.; Chanez, P.; Gras, D. Platelets Purification Is a Crucial Step for Transcriptomic Analysis. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Banerjee, M.; Rowley, J.W.; Stubben, C.J.; Tolley, N.D.; Freson, K.; Nelson, B.; Nagy, B., Jr.; Fejes, Z.; Blair, A.M.; Turro, E.; et al. Prospective, international, multisite comparison of platelet isolation techniques for genome-wide transcriptomics: communication from the SSC of the ISTH. J Thromb Haemost 2024, 22, 2922–2934. [Google Scholar] [CrossRef]
- Ju, M.; Fan, J.; Zou, Y.; Yu, M.; Jiang, L.; Wei, Q.; Bi, J.; Hu, B.; Guan, Q.; Song, X.; et al. Computational Recognition of a Regulatory T-cell-specific Signature With Potential Implications in Prognosis, Immunotherapy, and Therapeutic Resistance of Prostate Cancer. Frontiers in Immunology 2022, 13. [Google Scholar] [CrossRef]
- Wen, X.; Yang, G.; Dong, Y.; Luo, L.; Cao, B.; Mengesha, B.A.; Zu, R.; Liao, Y.; Liu, C.; Li, S.; et al. Selection and Validation of Reference Genes for Pan-Cancer in Platelets Based on RNA-Sequence Data. Frontiers in Genetics 2022, 13. [Google Scholar] [CrossRef]
- Wilfinger, W.W.; Eghbalnia, H.R.; Mackey, K.; Miller, R.; Chomczynski, P. Whole blood RNA extraction efficiency contributes to variability in RNA sequencing data sets. PLoS One 2023, 18, e0291209. [Google Scholar] [CrossRef]
- Phallen, J.; Leal, A.; Woodward, B.D.; Forde, P.M.; Naidoo, J.; Marrone, K.A.; Brahmer, J.R.; Fiksel, J.; Medina, J.E.; Cristiano, S.; et al. Early Noninvasive Detection of Response to Targeted Therapy in Non-Small Cell Lung Cancer. Cancer Res 2019, 79, 1204–1213. [Google Scholar] [CrossRef]
- Park, C.K.; Lee, S.W.; Cho, H.J.; Oh, H.J.; Kim, Y.C.; Kim, Y.H.; Ahn, S.J.; Cho, J.H.; Oh, I.J. Blood-Based Biomarker Analysis for Predicting Efficacy of Chemoradiotherapy and Durvalumab in Patients with Unresectable Stage III Non-Small Cell Lung Cancer. Cancers (Basel) 2023, 15. [Google Scholar] [CrossRef]
- Mamdani, H.; Matosevic, S.; Khalid, A.B.; Durm, G.; Jalal, S.I. Immunotherapy in Lung Cancer: Current Landscape and Future Directions. Front Immunol 2022, 13, 823618. [Google Scholar] [CrossRef]
Figure 1.
Tumor-derived exosomes and cytokines drive platelet number and function alterations. Tumor-derived interleukin-6 (IL-6) stimulates the liver to secrete thrombopoietin (TPO), which drives megakaryocyte maturation in the bone marrow, leading to elevated platelet production (right lower panel). Concurrently, tumor cell–derived exosomes and soluble factors are released into circulation, where they influence platelets to take up and splice RNA, as well as incorporate proteins (right upper panel). These changes modify platelet function and can promote cancer progression and metastasis. Thus, the dual role of the IL-6–TPO axis and tumor cell–derived components augments both platelet numbers and platelet activation, further enhancing immunomodulatory and pro-thrombotic processes within the tumor microenvironment. Created with BioRender.com.
Figure 1.
Tumor-derived exosomes and cytokines drive platelet number and function alterations. Tumor-derived interleukin-6 (IL-6) stimulates the liver to secrete thrombopoietin (TPO), which drives megakaryocyte maturation in the bone marrow, leading to elevated platelet production (right lower panel). Concurrently, tumor cell–derived exosomes and soluble factors are released into circulation, where they influence platelets to take up and splice RNA, as well as incorporate proteins (right upper panel). These changes modify platelet function and can promote cancer progression and metastasis. Thus, the dual role of the IL-6–TPO axis and tumor cell–derived components augments both platelet numbers and platelet activation, further enhancing immunomodulatory and pro-thrombotic processes within the tumor microenvironment. Created with BioRender.com.
Figure 2.
Direct platelet–tumor cell receptor engagement and indirect growth factor signaling mediate platelet activation and tumor progression. Tumor cells express platelet-derived growth factor receptor (PDGFR) and podoplanin, as well as produce soluble growth factors (e.g., EGF, PDGF, TGFβ) and the carbohydrate-binding protein galectin-3. Platelet glycoprotein VI (GPVI) can be clustered by galectin-3, initiating intracellular signaling via the Fc receptor (FcR) γ-chain and its immunoreceptor tyrosine-based activation motif (ITAM). This activates downstream kinases (e.g., Fyn/Lyn, Syk, PI3K), leading to phosphorylation of adaptor proteins (LAT, SLP-76) and enzymes (PLCγ2) that culminate in platelet activation, spreading, and release of additional growth factors. Meanwhile, the platelet CLEC-2 receptor recognizes podoplanin through its hemiITAM motif, also triggering signaling cascades involving Src and Syk. Tumor-derived PDGF ligands bind PDGFR on the tumor cell surface, activating Akt and promoting processes such as epithelial–mesenchymal transition (EMT), migration, invasion, and proliferation. Thus, both platelets and tumor cells engage in reciprocal crosstalk, enhancing tumor progression. Created with BioRender.com.
Figure 2.
Direct platelet–tumor cell receptor engagement and indirect growth factor signaling mediate platelet activation and tumor progression. Tumor cells express platelet-derived growth factor receptor (PDGFR) and podoplanin, as well as produce soluble growth factors (e.g., EGF, PDGF, TGFβ) and the carbohydrate-binding protein galectin-3. Platelet glycoprotein VI (GPVI) can be clustered by galectin-3, initiating intracellular signaling via the Fc receptor (FcR) γ-chain and its immunoreceptor tyrosine-based activation motif (ITAM). This activates downstream kinases (e.g., Fyn/Lyn, Syk, PI3K), leading to phosphorylation of adaptor proteins (LAT, SLP-76) and enzymes (PLCγ2) that culminate in platelet activation, spreading, and release of additional growth factors. Meanwhile, the platelet CLEC-2 receptor recognizes podoplanin through its hemiITAM motif, also triggering signaling cascades involving Src and Syk. Tumor-derived PDGF ligands bind PDGFR on the tumor cell surface, activating Akt and promoting processes such as epithelial–mesenchymal transition (EMT), migration, invasion, and proliferation. Thus, both platelets and tumor cells engage in reciprocal crosstalk, enhancing tumor progression. Created with BioRender.com.
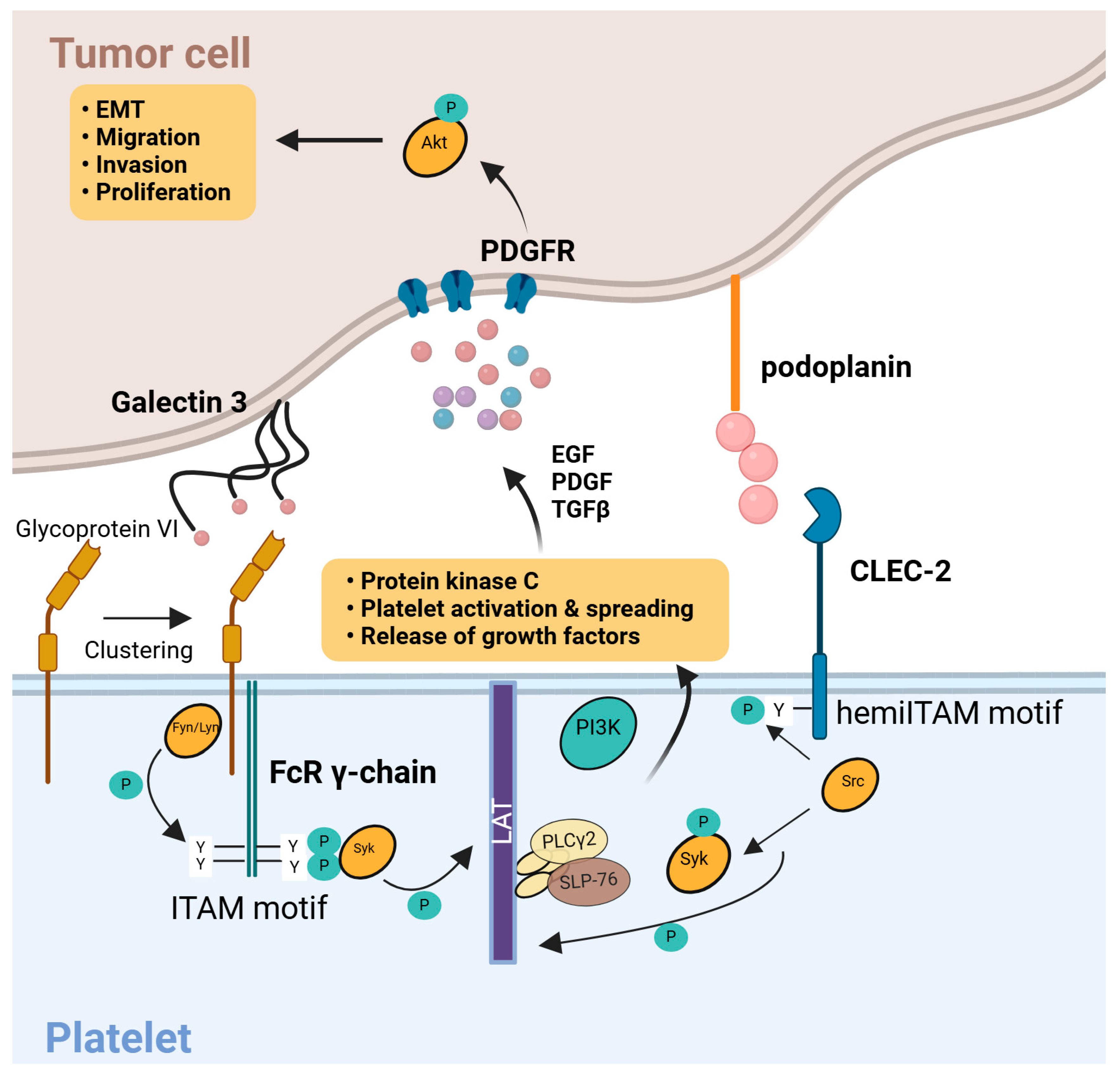
Table 1.
Key Mechanisms of Tumor-Driven Platelet Education.
Table 1.
Key Mechanisms of Tumor-Driven Platelet Education.
| Mechanism |
Representative Evidence/Studies |
Major Consequences for Platelet Function |
References |
| 1. Sequestration of tumor-derived biomolecules |
- Glioblastoma Example: Platelets can internalize mutant EGFRvIII mRNA in approximately 40–60% of patients with EGFRvIII-positive tumors, reflecting tumor molecular status.
- Colorectal Cancer: Platelet RNA content shows a 2–3-fold increase in tumor-specific transcripts (e.g., PCA3) compared to healthy controls.
- Tumor-secreted EVs (exosomes) containing integrins and oncogenic miRNAs can be absorbed by platelets, creating a “mirror” of the tumor’s genetic material. |
- Platelets become reservoirs of tumor-specific signals, enabling liquid biopsy approaches (e.g., detection of oncogenic mutations in platelets).
- Potential amplification of pro-tumor pathways if sequestered molecules (e.g., growth factors) are released at metastatic sites. |
[34] |
| 2. Modulation of platelet RNA content |
- RNA Splicing Events: Tumor-derived signals (e.g., thrombin, TGF-β, IL-6) induce alternative splicing in 200–300 platelet transcripts, leading to distinct cancer-associated RNA “fingerprints.”
- ITGA2B & PCA3: Reports indicate 2–5-fold elevated levels of these RNAs in platelets from lung and prostate cancer patients, respectively.
- Some studies showed that platelets can de novo synthesize proteins (e.g., VEGF variants) from tumor-influenced transcripts. |
- Emergence of unique “tumor-educated” RNA signatures that can distinguish cancer from non-cancer with high accuracy (~80–90% in some studies).
- Platelet-driven local release of oncogenic or pro-angiogenic factors, promoting tumor cell proliferation and vascularization. |
[7] |
| 3. Megakaryocyte education and thrombopoiesis alteration |
- Cytokine-Driven: Elevated IL-6 (~2–3× normal) in cancer patients stimulates hepatic TPO production, boosting megakaryocyte proliferation.
- Reactive Thrombocytosis: In ovarian cancer, up to 65% of advanced cases present with high platelet counts (>400×10^9/L).
- Mouse models: Blocking IL-6 reduces paraneoplastic thrombocytosis by ~50%, correlating with slower tumor growth. |
- Increased platelet production leads to higher circulating platelet counts, which can promote metastatic spread through “platelet shielding” of tumor cells.
- Newly formed platelets may be “pre-loaded” with cancer-related transcripts, amplifying tumor-specific signals in circulation. |
[89,90] |
| 4. Increased platelet levels (thrombocytosis) |
- Clinical Observations: A prospective cohort showed ~31% of patients with elevated platelets were later diagnosed with lung or colorectal cancer, making thrombocytosis a potential early warning sign.
- Ovarian Cancer: ~50–65% incidence of thrombocytosis, correlating with advanced stage and worse prognosis. |
- Heightened platelet count is consistently linked to poor prognosis in multiple tumor types (lung, ovarian, gastric, etc.).
- Elevated platelets enhance the risk of hematogenous metastasis and recurrent disease. |
[52,91] |
| 5. Tumor cell–induced platelet activation |
- In Vitro Aggregation: Tumor-derived ADP, IgG, or podoplanin can cause 20–30% higher platelet aggregation than in healthy controls.
- Morphological Changes: Electron cryotomography reveals disrupted microtubules and increased mitochondrial mass in up to ~65% of platelets from patients with metastatic cancer. |
- Hyperresponsive platelets form microthrombi around tumor cells, aiding immune evasion and extravasation.
- Elevated levels of activation markers (soluble P-selectin, sCD40L) correlate with metastatic potential. |
[35] |
| 6. Molecular pathways of platelet–tumor interactions |
- CLEC2–Podoplanin Axis: Podoplanin-expressing tumors (e.g., certain squamous cell carcinomas) bind CLEC2 on platelets, activating ITAM–Syk signaling, which can increase thrombotic risk by 2–3×.
- TLR4–HMGB1: Tumor cells release HMGB1 that triggers a TLR4-mediated cascade, elevating platelet granule release. |
- Unique, tumor-specific pathways shift platelet activation from normal hemostasis to pro-metastatic activity.
- Enhanced opportunity for therapeutic intervention: blocking CLEC2–podoplanin or TLR4–HMGB1 can reduce tumor-related thrombosis and metastasis in preclinical models. |
[25,92] |
Table 2.
Common Platelet RNA Biomarkers for Cancer Detection.
Table 2.
Common Platelet RNA Biomarkers for Cancer Detection.
| RNA Name |
Cancer Type(s) |
Diagnostic Accuracy / Observed Fold Change |
Mechanistic or Biological Role |
References |
| ITGA2B (Integrin αIIb) mRNA |
Non-small cell lung cancer (NSCLC) |
Elevated ~2–5× in platelets from NSCLC vs. healthy controls;
AUC ~0.80–0.85 in some studies |
Encodes a platelet membrane glycoprotein important for aggregation;
increased expression may reflect platelet hyper-reactivity in tumors |
[76,93] |
| PCA3 mRNA |
Prostate cancer |
Upregulated 3–4-fold in platelets from metastatic cases;
high specificity (≥85%) when combined with other markers |
Known prostate cancer–associated non-coding RNA;
possibly involved in androgen receptor signaling and disease progression |
[7,94] |
| TIMP1 mRNA |
Colorectal cancer (CRC) |
AUC ~0.78–0.85 for detecting CRC;
often 2–3-fold higher in platelets of advanced CRC patients |
Tissue inhibitor of metalloproteinases;
elevated TIMP1 in platelets may reflect increased tumor invasion and remodeling activity |
[40,95] |
| miR-34c-3p & miR-18a-5p |
Nasopharyngeal carcinoma (NPC) |
Combined panel AUC ~0.95;
significant elevation in patient platelets, not always mirrored in plasma miRNA |
Tumor suppressor (miR-34c) and oncogenic (miR-18a) pathways;
reflect complex tumor–platelet interactions unique to NPC |
[36,96] |
| LINC00534, TSPOAP-AS1, SNHG20 |
Colorectal, gastric, and other GI malignancies |
Each shows 2–4-fold higher expression in tumor-educated platelets;
combined panel AUC ~0.78–0.82 |
Long non-coding RNAs implicated in regulating cell cycle and metastatic processes;
higher levels correlate with more aggressive GI tumors |
[97,98] |
| circNRIP1 |
Non-small cell lung cancer (NSCLC) |
Elevated circNRIP1 in platelets correlated with ~75% sensitivity;
potential synergy with other circRNAs in multi-marker panels |
Circular RNA modulating multiple signaling pathways (e.g., PI3K/AKT);
may promote epithelial–mesenchymal transition in lung tumors |
[37,99] |
Table 3.
Overview of Diagnostic Studies Using Tumor-Educated Platelets.
Table 3.
Overview of Diagnostic Studies Using Tumor-Educated Platelets.
| Study (Year) |
Cancer Type(s) |
Stage of Disease |
Type of Controls |
Biomarker / Approach |
Cohort Size |
Performance Metrics |
Key Findings |
| Best et al. (2015) [7] |
Multi-cancer (lung, breast, colon, etc.) |
Stages I–IV (mixed) |
Healthy individuals (n=55) |
RNA-seq of platelet mRNA (ThromboSeq) |
228 patients + 55 healthy |
Sensitivity ~96%, Specificity ~90% |
Platelet RNA profiles accurately distinguished cancer vs. non-cancer and identified tumor types with ~71% accuracy. |
| In ’t Veld et al. (2022) [34] |
Multi-cancer (pan-cancer approach) |
Stages I–IV (mixed) |
Asymptomatic controls, symptomatic controls |
RNA-seq of platelet mRNA (expanded ThromboSeq) |
1,096 patients + asymptomatic + symptomatic |
Overall Sensitivity ~66%, Specificity ~99%
(in asymptomatic) |
Large multicenter study showing robust specificity; sensitivity higher in late-stage disease than early-stage. |
| Sabrkhany et al. (2018) [100] |
Lung and pancreatic |
Early-stage (I–II) and various stages |
Healthy donors (n=50) |
Platelet proteomics (LC–MS/MS) |
131 patients + 50 healthy |
AUC ~0.85–0.90 |
Identified >80 proteins with significant dysregulation; protein levels normalized after tumor resection in most early-stage patients. |
| Li et al. (2023) [76,86] |
Non-small cell lung cancer (NSCLC) |
Stage I–III (predominantly early/mid) |
Benign pulmonary nodules (n=150), healthy (n=100) |
Targeted mRNA panel + machine learning |
210 NSCLC + 250 controls total |
Sensitivity 80%, Specificity 73%, AUC 0.85 |
A 3-gene signature (MAX, MTURN, HLA-B) was highly upregulated in NSCLC platelets; strong accuracy for early-stage lung cancer detection. |
| Liu et al. (2022) [101] |
Endometrial cancer |
Stages I–III |
Benign endometrial lesions (n=80) |
Combined TEP RNA profiling + ctDNA |
90 endometrial cancer + 80 benign |
TEP AUC ~0.88 vs. ctDNA AUC ~0.75 |
TEP RNA performed better than ctDNA alone for cancer detection; a combined signature further improved sensitivity in early-stage disease. |
Table 4.
Current Limitations and Challenges in TEP Research.
Table 4.
Current Limitations and Challenges in TEP Research.
| Type of Challenge |
Description / Examples |
Potential Solutions |
References |
| Pre-analytical |
- Platelet contamination by leukocytes or RBCs can confound RNA/protein assays
- Sample handling (e.g., multiple freeze/thaw cycles) alters platelet activation status |
- Standardized platelet isolation protocols (e.g., dual-spin centrifugation, gentle pipetting)
- Use of automated devices to minimize contamination and activation |
[70,102,103,104] |
| Analytical Sensitivity |
- Limited RNA content per platelet requires deep sequencing or highly sensitive qPCR
- Variability in RNA extraction efficiency across labs |
- Development of targeted multiplex assays focusing on robust biomarker panels
- Rigorous training sets and QC standards for RNA isolation |
[72,73,74,105,106] |
| Data Analysis & Algorithmic |
- Complex machine learning models may be “black boxes” for clinicians
- Overfitting risk when training on small cohorts |
- Large, multi-institutional datasets to improve generalizability
- Explainable AI approaches or simplified gene panels for clinical interpretation |
[73,74] |
| Specificity to Cancer |
- Platelets respond to inflammation, infections, and other non-cancer conditions
- Elevated platelet counts can be non-cancerous (e.g., reactive thrombocytosis) |
- Include control groups with inflammatory or autoimmune diseases
- Combine TEP data with other biomarkers (ctDNA, imaging) for multi-modal specificity |
[29,75,76] |
| Inter-cancer Heterogeneity |
- Not all tumors “educate” platelets to the same extent
- Certain cancers (e.g., less vascularized, behind blood–brain barrier) may release fewer signals into circulation |
- Large-scale “pan-cancer” studies to capture diverse tumor profiles
- Cancer-type–specific panels if broad-based signatures prove insufficient |
[11,28,53,75] |
| Dynamic Changes & Timing |
- Single time-point sampling may not capture transient tumor–platelet interactions
- Recent chemotherapy or surgery can alter platelet states |
- Serial sampling at multiple time points
- Standardized timing of blood draw in relation to treatment (e.g., pre- vs. post-chemotherapy) |
[77,94] |
| Regulatory & Clinical Translation |
- No FDA-approved TEP test to date
- Need for consensus on validated biomarkers and standardized protocols |
- Large prospective clinical trials with consistent SOPs
- Collaboration between academic, regulatory, and industry stakeholders for test standardization |
[78] |
Table 5.
Potential Future Directions and Ongoing Clinical Efforts in TEP Research.
Table 5.
Potential Future Directions and Ongoing Clinical Efforts in TEP Research.
| Direction / Trial Name |
Focus / Rationale |
Study Population / Design |
Biomarker(s) Under Investigation |
Primary Endpoints |
Status / Expected Completion |
References |
| Multi-modal Liquid Biopsy Trials |
Integrating TEP RNA signatures with ctDNA and circulating proteins for comprehensive tumor profiling |
Prospective cohort studies combining blood-based assays; e.g., advanced lung or ovarian cancer cohorts |
Platelet RNA panels, ctDNA mutation load, tumor-specific proteins (VEGF, CEA, etc.) |
Diagnostic accuracy (AUC, sensitivity, specificity), early detection of recurrence |
Ongoing; projected results within next 1–2 years |
[42,80] |
| Automated Platelet Isolation Devices |
Development of point-of-care technologies to standardize platelet separation and reduce contamination |
Pilot feasibility studies in large hospitals; testing 100–200 blood samples from cancer & non-cancer patients |
Concentration and purity of platelet isolates, reproducibility of TEP RNA/protein measurements |
Turnaround time for test results, reproducibility across multiple clinics |
Prototypes in testing; commercial rollouts anticipated |
[82,83] |
| Serial Sampling for Treatment Monitoring |
Evaluating dynamic changes in TEPs over multiple time points to detect early therapeutic response or relapse |
Phase II trials in lung, breast, and GI cancers, collecting pre-, mid-, and post-therapy platelet samples |
Platelet RNA/protein panels (e.g., TIMP1, VEGF, IL-6 mRNA), morphological changes (MPV) |
Early detection of relapse vs. imaging alone, improved patient stratification for personalized therapy |
Trials expected to complete in 1–3 years |
[107,108] |
| Combination of TEP with Immunotherapy Biomarkers |
Improving patient selection for immune checkpoint inhibitors (ICIs) by integrating platelet activation signatures |
Single-arm or randomized trials combining PD-1/PD-L1 inhibitors with TEP-based monitoring in NSCLC or UC |
Platelet activation markers (sP-selectin, sCD40L), immune checkpoint protein transcripts in platelets |
Objective response rate, correlation with platelet markers and tumor burden, immune-related adverse events |
Early-phase clinical studies; expansions planned |
[61,109] |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).