1. Introduction
Although vitamin B12 (cobalamin) is synthesized primarily by plant organisms, its natural sources in the human diet are predominantly meat, eggs, and dairy products [
1]. It is absorbed in the terminal ileum in the presence of Castle factor, which is produced by the parietal cells of the stomach [
2]
. It is particularly important to obtain adequate amounts of the aforementioned vitamin through food during pregnancy and breastfeeding, as it is essential for optimal functioning of the body, including maintaining the health of the nervous system of the mother and the child. Insufficient cobalamin can affect the body indirectly. A deficiency in this vitamin during pregnancy has been linked to an elevated risk of metabolic disorders in the mother, including obesity and insulin resistance These conditions have the potential to negatively impact fetal development [
3,
4]. Low levels of vitamin B12 have been demonstrated to suppress critical processes involved in the normal development of the fetal nervous system. These processes include reduced activity of methylation DNA, RNA, histones, and important enzymes necessary for myelin synthesis. These findings suggest that cobalamin deficiency may have adverse effects on fetal development, including neural tube defects and impaired neurological development. [
5,
6,
7]. A prolonged deficiency of vitamin B12 can result in demyelination or damage to the myelin sheaths that surround nerve fibers in the brain and spinal cord. This can lead to the development of motor disorders, motor coordination problems, and impaired sensory function [
8]. This underscores the importance of supplementation of this nutrient during key periods of a woman's life. Health Canada guidelines, for prenatal nutrition, recommend a vitamin B12 intake of 2.6 µg per day for pregnant women and 2.8 µg per day for breastfeeding women [
9].
From a clinical point of view, the symptoms of severe and long-term cobalamin deficiency and autism spectrum disorder (ASD) are similar. ASD is a neurodevelopmental disorder marked by challenges in social communication and interaction, as well as repetitive behavioral patterns. In both long-term cobalamin deficiency and autism spectrum disorder, neurological symptoms may occur, including motor, cognitive, and mood-related impairments [
1,
10,
11]. An increasing number of studies are indicating the possibility of a correlation between vitamin B12 deficiency and the emergence of ASD [
12,
13,
14].
This narrative review analyzes the latest literature on the relationship between vitamin B12 deficiency and excess and the risk and development of autism. Both the results and limitations of available studies were taken into account to indicate directions for future research that could provide more conclusive answers about the role of this vitamin in the etiology and course of ASD.
2. Materials and Methods
The aim of this narrative review is to explore the potential role of vitamin B12 in autism spectrum disorder, with a particular focus on its involvement in neurodevelopment, gut microbiota regulation, and neurotransmitter metabolism. Given the growing interest in biological factors contributing to ASD, this review examines existing evidence on B12 levels in individuals with ASD, its potential as a biomarker for disease progression, and the implications for therapeutic strategies, including supplementation.
Studies included were published between 2000 and 2024. The literature search was conducted on PubMed, Web of Science and Google Scholar in December 2024, covering studies from 2000 to 2024 using relevant search terms, including “vitamin B12, cobalamin, ASD, autism spectrum disorder, gut microbiota, neurodevelopment, neurotransmitters, inflammation, biomarkers, supplementation”. Both original research and review articles were considered during the initial screening. Mendeley software was used to remove duplicates that appeared due to overlapping search terms.
Since this work is a narrative review, no strict inclusion criteria were established for the selection of articles. During the screening process, studies addressing the role of vitamin B12 in autism were included, along with selected studies exploring this issue in animal models. Based on titles and abstracts, conference abstracts were excluded. Studies not written in English were also omitted. Additionally, supporting literature on potential therapeutic approaches was included. It is essential to note that review is not systematic, and despite attempts to cover all studies, one should keep in mind significant limitations.
3. The Role of Vitamin B12 in Nervous System Development and Metabolism During the Prenatal and Early Childhood Periods
The appropriate development of the nervous system in children results from a multifaceted process, during which both the prenatal and early years of life are paramount importance [
14]. Pregnancy is a period of significant metabolic changes, necessitating an enhanced intake of specific nutrients [
15]. The early childhood period is distinguished by the rapid growth and organization of the nervous system, which remains susceptible to external environmental factors and nutritional inputs [
16]. Among these nutrients, vitamin B12 is particularly important for normal neurological development and thus merits detailed discussion [
5,
14,
17].
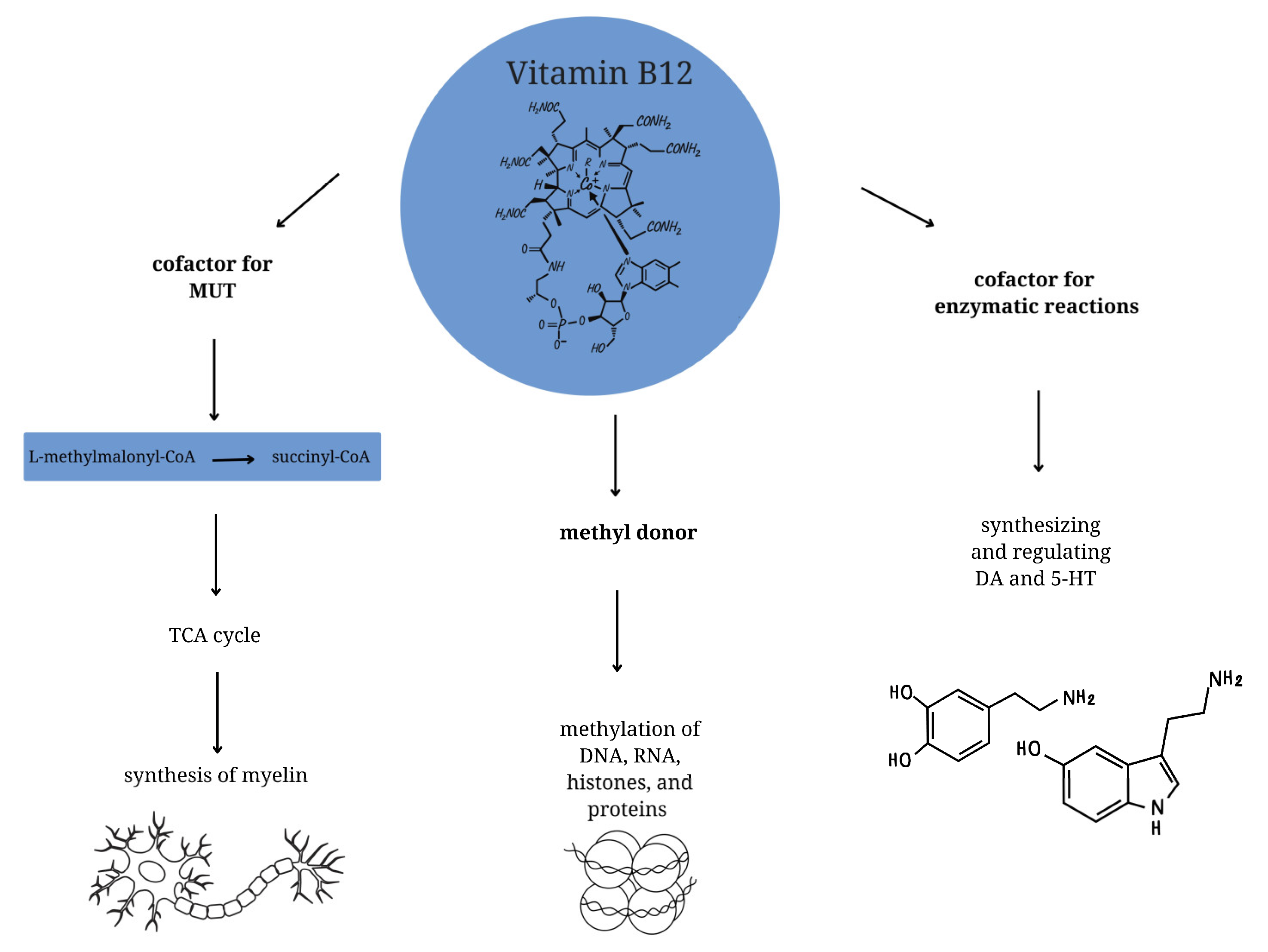
Vitamin B12 is a complex compound comprising a central cobalt atom surrounded by a corrin ring and bound to various ligands [
5]. At the cellular level, it functions as a cofactor for enzymatic reactions and is involved in synthesizing and regulating dopaminergic and serotonergic neurotransmitters [
18]. Furthermore, it plays a crucial role in the production of hemoglobin, as it catalyzes the conversion of methylmalonic acid to succinyl-CoA, which is essential for optimal erythropoiesis [
18,
19]. In regard to neurodevelopment, vitamin B12 is functioning as a methyl donor in one-carbon metabolism, which facilitates the proper methylation of DNA, RNA, histones, and proteins [
20,
21]. Deficiencies in cobalamin at this level can result in the reduction of DNA synthesis and methylation levels [
8]
. Furthermore, vitamin B12 functions as a cofactor for methylmalonyl-CoA mutase (MUT) [
22,
23,
24]. MUT employs cobalamin in the form of adenosylcobalamin to facilitate the conversion of L-methylmalonyl-CoA to succinyl-CoA, which subsequently enters the tricarboxylic acid (TCA) cycle. A deficiency of vitamin B12 has been demonstrated to result in impaired MUT activity, which can in turn lead to defective synthesis of myelin, and thus to the development of neuropathy and nerve atrophy [
25] (
Figure 1).
4. The Relationship Between Vitamin B12 Deficiency and Neurological Development
Numerous studies emphasize the necessity of maintaining sufficient levels of vitamin B12 from the beginning of pregnancy to facilitate its proper course and optimal neurological development of the infant, although the results of many of them do not allow for clear conclusions.
One such study is a prospective cohort study that assessed the relationship between the mother's vitamin B12 level at the beginning and end of pregnancy and the neurological development of infants 40 days after birth. The study's findings indicated that newborns whose mothers had an average serum cobalamin concentration of 312 to 408 pg/ml during pregnancy demonstrated superior motor skills, gross motor skills, language, and cognitive functions [
26]. It has been demonstrated in other studies that infants born to mothers with vitamin B12 deficiency during pregnancy exhibit substandard performance on cognitive assessments in comparison to infants born to mothers with adequate cobalamin levels. On the other hand, clear deficits in cognitive development were observed in the cohort of mothers who showed simultaneous deficiencies of vitamin B12 and B6, which may indicate the additional contribution of deficiencies of other vitamins in the etiology of cognitive disorders in infants [
27]. However, it should be noted that both of the above-mentioned studies were primarily observational, which excludes the possibility of drawing clear conclusions from them regarding the impact of vitamin B12 deficiency in pregnant women on the development of neurodevelopmental dysfunction in children. This assertion is particularly salient in light of the findings of another prospective study by Wu et al., which failed to establish a correlation between the total vitamin B12 or holotranscobalamin (the active form of vitamin B12) levels in the mother's blood and the cognitive development of infants. It is imperative to acknowledge the limitations inherent in this study, particularly the absence of data regarding cobalamin levels in children and the modest sample size [
28]. It is important to acknowledge the limitations of this study, particularly the absence of data regarding cobalamin levels in children and the modest sample size.
Similarly, five selected prospective cohort studies that were conducted in different countries and concerned the impact of maternal vitamin B12 supplementation on children's cognitive development yielded inconclusive results. The studies analyzed data from mothers and their children, including indicators of cognitive, linguistic, and/or motor development. Some of them suggested a link between vitamin B12 deficiency and poorer cognitive development in children; however, others did not confirm these observations [
29,
30,
31,
32,
33]. Therefore, it is worth paying attention to the differentiating and converging factors in the selected group of publications. The first point of divergence is geographical diversity, which is important given that vitamin B12 deficiency in both children and mothers can manifest itself with different frequencies depending on the region and the dietary patterns typical of the population that inhabits it. These studies were conducted in India, Singapore, the United Kingdom and Spain. In addition, the researchers used different methods to assess cobalamin levels, and the specific time points at which measurements were taken varied considerably between studies. However, despite the numerous discrepancies, most studies, based on the sources provided, confirm the negative impact of low vitamin B12 levels in pregnant women on the subsequent neurological development of children.
The most relevant and reliable randomized controlled trials (RCTs) also do not provide a clear answer. Two RCTs were conducted by the same research team, but differed significantly in the age at which the cognitive development of the children was assessed [
34,
35]. The first study (2017) used the Bayley Scales of Infant Development III (BSID-III) to assess the cognitive development of children aged 9 months. The results showed no statistically significant differences in the scores on the aforementioned scale between infants born to mothers who received vitamin B12 supplementation and those whose mothers received a placebo. However, a significant correlation was found between elevated maternal serum homocysteine levels assessed in three trimesters of pregnancy and poorer outcomes among infants in some BSID-III subscales [
34]. The second study was a randomized controlled clinical trial conducted on the same group of participants, but this time the children were 30 months old (2019). The results indicated that children of mothers who received vitamin B12 supplementation during pregnancy demonstrated higher scores in the expressive language domain compared to children of mothers who received a placebo. Consistent with the preceding study, a substantial correlation was identified between elevated homocysteine levels during the second and third trimesters of pregnancy and diminished expressive language and gross motor skills development in children [
35]. Vitamin B12 deficiency is associated with hyperhomocysteinemia, which results in excessive accumulation of homocysteine in the body and consequent cell damage, and this condition is associated as a risk factor for neurodegenerative diseases [
36]. The reason for the discrepancies in the studies may be that in the first study, vitamin B12 supplementation was stopped at six weeks postpartum, which could have led to a decrease in cobalamin levels in both mothers and infants. Furthermore, the second study had a larger number of participants. It should also be noted that the assessment of cognitive function in infants is less reliable compared to older children.
Despite these discrepancies, the studies suggest that long-term vitamin B12 supplementation during pregnancy may have a positive effect on the development of expressive language in children. Subsequent studies analyzing the effect of vitamin B12 deficiency on the development of neurological disorders in children should also take homocysteine levels into account. Previous studies have shown that elevated homocysteine levels during pregnancy, like vitamin B12 deficiency, are risk factors for neural tube defects and related neurological complications [
37].
5. Consequences of Excess Levels of Vitamin B12
In newborns whose mothers had adequate levels of vitamin B12, it stores at birth in serum are approximately 25 µg [
38]. In the case of infants born to mothers with a cobalamin deficiency, their endogenous reserves may be significantly reduced [
39]. As described above, vitamin B12 is a vital nutrient for the optimal functioning of the nervous system and plays a pivotal role in DNA methylation processes [
40,
41,
42]. Conversely, there is a paucity of evidence to suggest that an excess of cobalamin can also have adverse effects [
43,
44].
The study by Yuan et al. revealed a correlation between elevated (>95 percentile) maternal vitamin B12 levels and an increased risk of pregnancy complications, including intrahepatic cholestasis of pregnancy (ICP), preeclampsia (PE), and gestational diabetes mellitus (GDM). Additionally, high vitamin B12 levels have been shown to disrupt the natural development of the fetus, resulting in higher birth weight and was associated with a higher risk of large-for-gestational-age (LGA) newborns [
43]. This suggests that the optimal vitamin B12 and other vitamin levels for pregnant women need to be determined. To achieve this goal, further research is needed based on a large group of participants to obtain the most conclusive results. This will allow for evidence-based recommendations on the use of this vitamin during pregnancy, so that the dose taken is safe for the fetus.
It is of significant importance to consider both deficiency and excess of vitamin B12 when administering vitamin B12 supplementation. It has been demonstrated that both infrequent (≤2 times per week) and frequent (>5 times per week) supplement intake is associated with an increased risk of ASD. Conversely, moderate multivitamin supplement intake (3-5 times per week) during pregnancy has been linked to a decreased risk of ASD [
45]. The findings of this study are groundbreaking in that they address a gap in the existing literature by examining the potential impact of over-supplementation with vitamin B12, which is a topic that has not been explored in previous studies. The only limitation of this study is the lack of analysis of vitamin B12 levels in children. The findings of this research provide a foundation for future research aimed at identifying optimal levels of essential nutrients, such as cobalamin, that are crucial for normal fetal neurological development and child health after birth.
6. Impact of Maternal Vitamin B12 Levels During Pregnancy on ASD Risk in Offspring
The available literature contains only a limited number of studies that directly examine the relationship between vitamin B12 levels during pregnancy and the risk of developing autism spectrum disorder in offspring. The three months prior to conception and the first month of pregnancy have been identified as critical periods for the development of the fetal nervous system due to the closure of the neural tube. A disruption of this process can result in the development of autism, at least in some cases [
46].
One study that evaluated the effect of vitamin B12 deficiency on the development of autism is a long-term clinical-control study conducted by Sourander et al., which included 1,558 children diagnosed with ASD and the same number of children in the control group. The results were inconclusive because no significant relationship was found between the level of vitamin B12 in the mother's serum and the risk of autism spectrum disorder in the offspring. However, both elevated vitamin B12 levels (≥81st percentile) and reduced levels (<20th percentile) in the first trimester of pregnancy were associated with an increased risk of childhood autism in offspring [
44]. The most important limitations of the study are the lack of routine vitamin B12 measurements in newborns and the fact that the study was conducted on people with strongly expressed ASD traits, which limits the possibility of generalizing the results.
On the other hand, Schmidt et al. have shown that prenatal intake of vitamins, including vitamin B12, in the preconception period can potentially reduce the risk of autism in a child, especially in mothers and children with specific variants of one-carbon metabolism genes (maternal
MTHFR 677 TT,
CBS rs234715 GT + TT, and child
COMT 472 AA genotypes) [
46]. However, the study has several limitations. The vitamin intake was based on the mothers' self-assessment. Since their dietary habits were not analyzed, it cannot be ruled out that the information provided did not always correspond to the actual level of supplementation. Another limitation is the lack of vitamin B12 analysis in children.
In another study by Hollowood-Jones, a significant relationship was found only between low vitamin B12 levels and autism. Two to five years after giving birth, the metabolic profiles of mothers of children with autism spectrum disorder (ASD-M). were analyzed and compared with the profiles of mothers of typically developing children (TD-M). All participants had not consumed folate, vitamin B12, or multivitamin supplements for two months prior to sampling. The results showed that ASD-M had some significantly different abnormalities associated with low vitamin B12 levels compared to TD-M. Moreover, vitamin B12 levels were significantly lower in the ASD-M group compared to the TD-M group, confirming the link between low vitamin B12 levels in mothers and autism [
47].
Other studies of comparable significance suggest that, in addition to B12, other vitamins, including A, D, and K, may also contribute to an elevated risk of autism in offspring [
48]. Chen et al. found a significant correlation between lower levels of vitamin D in the mother's serum during the first trimester of pregnancy and an increased risk of autism spectrum disorders in the offspring [
49]. However, the researchers did not confirm a link between cobalamin levels and the occurrence of autism. Noteworthy limitations of the study include its relatively small sample size, which hinders the reliability of the findings.
7. Effect of Vitamin B12 Deficiency on Symptoms Severity in Children with Autism Spectrum Disorder
For a considerable period, scientists have been making intensive efforts to the identification of metabolic biomarkers that have the potential to serve as diagnostic tools for children with ASD, with the objective of differentiating them from healthy children [
50]. The result of these efforts is a number of studies that point to low levels of vitamins, important for the development of the nervous system, as a potential differentiating factor. One study that examined this relationship is a comparative study by Zou et al. in which a reduced vitamin B12 level compared to normal was the differentiating factor between the group of children with ASD and the healthy group [
51]. Li et al. obtained similar results, showing that both the vitamin B12 level was lower in children diagnosed with ASD, but also that they scored higher on the Childhood Autism Rating Scale (CARS) compared to the control group [
52]. Concurrently, a team of researchers led by Belardo et al. conducted a study to assess the potential impact of co-occurring deficiencies of vitamins B6, B9, and B12 on specific phenotypic characteristics associated with autism spectrum disorders. The researchers employed a metabolomic and methylomic approach to analyze urine samples from children with ASD who exhibited deficiencies in these vitamins [
53]. Another particularly important study that analyzed the relationship between vitamin B12 levels and specific characteristics related to neurodevelopmental processes is the study conducted by Wu et al. The authors used the Gesell Developmental Scale to assess the functions that indicate the level of maturity of a child in the most important areas of his or her behavior. In children aged 2-4 years, serum vitamin B12 levels showed a positive correlation with language development quotients, while in the 4-6 age group, with adaptive, motor and social behavior skills [
54]. While the aforementioned study suggests a potential correlation between vitamin B12 levels and autism, there are other studies, such as the observational study by Shi et al., which found no statistically significant difference in vitamin B12 levels between the ASD group and the typically developing control group. The study included a relatively small number of participants (132 children with ASD and 132 children with TD), which may have reduced the likelihood of detecting subtle differences in vitamin B12 levels between the groups [
55]. Similarly, no significant differences in vitamin B12 levels were found between children with ASD and TD in another case-control study conducted in Haikou, China, by Guo et al [
56].
The studies discussed thus far have concentrated on examining the relationship between vitamin B12 levels and the severity of autism symptoms in groups of children with full-blown ASD. However, there is now a growing necessity to extend the scope of the study to encompass children exhibiting so-called "subdiagnostic" autism symptoms. The term refers to a situation in which a child exhibits some of the characteristics of ASD, but the presentation is not severe enough to meet the full diagnostic criteria for the disorder. This is the group that Arija et al. chose as their research target when assessing the intake of nutrients important for development among children with full autism and those with subdiagnostic autism symptoms. It turned out that the diet of both groups of children with neurodevelopmental disorders was poorer in terms of vitamin B12 content compared to children developing normally [
57].
8. Possible Causes of Low Vitamin B12 Levels in ASD
Some studies show that children with autistic traits have relatively lower vitamin B12 levels compared to children without these traits, with the differences becoming more pronounced in later childhood. A nutrient-poor diet may be one of the factors responsible for cobalamin deficiencies. The dietary patterns of autistic individuals may affect their overall health and well-being, which is of particular importance in the context of their neurological development [
58,
59,
60]. Vitamin B12 intake was shown to be significantly lower among children and adolescents with autistic traits compared to those without. The study's authors suggest that the reduced intake of vitamin B12 may be related to the reduced consumption of animal products, fish and legumes among those with autistic traits [
61].
Another reason for a lack of cobalamin, which is often suggested by researchers, is malabsorption syndrome [
62]. Erden et al. conducted a study that examined the association between malabsorption, vitamin B12 deficiency, and the emergence of autism in children. The authors observed a substantial decrease in serum vitamin B12 levels in children diagnosed with autism. However, they did not establish a correlation between cobalamin levels and the presence of anti-phosphatidylserine antibodies (APCA) [
40]. Therefore, special attention should be paid to the diet of children with ASD, and monitoring of nutrient intake should be recommended throughout their development, rather than at specific points in time, as at each stage of development, the intake of adequate nutrients has a significant impact on the functioning of the nervous system.
9. Vitamin B12 Supplementation in ASD
Some studies show that children with autistic traits have relatively lower vitamin B12 levels compared to children without these traits, with the differences becoming more pronounced in later childhood. A nutrient-poor diet may be one of the factors responsible for cobalamin deficiencies. The dietary patterns of autistic individuals may affect their overall health and well-being, which is of particular importance in the context of their neurological development [
58,
59,
60]. Vitamin B12 intake was shown to be significantly lower among children and adolescents with autistic traits compared to those without. The study's authors suggest that the reduced intake of vitamin B12 may be related to the reduced consumption of animal products, fish and legumes among those with autistic traits [
61].
Another reason for a lack of cobalamin, which is often suggested by researchers, is malabsorption syndrome [
62]. Erden et al. conducted a study that examined the association between malabsorption, vitamin B12 deficiency, and the emergence of autism in children. The authors observed a substantial decrease in serum vitamin B12 levels in children diagnosed with autism. However, they did not establish a correlation between cobalamin levels and the presence of anti-phosphatidylserine antibodies (APCA) [
40]. Therefore, special attention should be paid to the diet of children with ASD, and monitoring of nutrient intake should be recommended throughout their development, rather than at specific points in time, as at each stage of development, the intake of adequate nutrients has a significant impact on the functioning of the nervous system.
10. Vitamin B12 Supplementation in ASD
An intriguing result emerged from a study conducted by Hendren et al., which sought to assess the potential of methyl-B12, a pivotal enhancer of methylation responses, in alleviating autism symptoms [
63]. The study was based on the hypothesis that children with autism may have a limited ability to methylate DNA, as well as impaired antioxidant processes associated with the diagnosis of autism spectrum disorder [
64]. A total of 57 autistic children aged 3 to 7 years with an IQ above 50 were randomly assigned to an 8-week treatment regimen of methyl-B12 at a dose of 75 µg/kg body weight or a placebo with saline solution. The clinical improvement achieved among children with ASD treated with methyl-B12, as compared to the control group, was positively correlated with an increase in methionine concentration in blood plasma, a decrease in S-adenosyl-l-homocysteine (SAH) concentration, and an improvement in the S-adenosylmethionine (SAM) to SAH ratio [
63]. The results obtained indicate an increase in the ability to methylate DNA and cellular antioxidant potential under the influence of methyl-B12, as well as their potentially positive impact on the symptoms of autism.
On the other hand, a study conducted by Bertoglio et al. did not show an overall improvement in the group of children with autism receiving methylcobalamin, but 30% of the children showed a clinically significant change in behavior and an increase in glutathione (GSH) levels. These results suggest that methyl B12 supplementation may benefit children with autism, especially those with impaired methionine metabolism and/or increased oxidative stress. In Bertgolio's study, all participants received both methylcobalamin and placebo for six weeks, but in a different order [
65]. In contrast, the study by Hendren et al. was a parallel study, meaning that participants were randomly assigned to one of two groups: a group receiving methylcobalamin vitamin B12 or a placebo group [
63]. Slight differences in the study design can have a significant impact on the results. In cross-over studies, there is a possibility of a carry-over effect, in which the effects of the initial treatment phase can influence the results of the subsequent phase. In parallel group studies, by implementing interventions in different groups simultaneously, this risk is reduced, increasing the reliability of the results. Both studies, despite different methodologies, indicate the potential effectiveness of methylcobalamin supplementation of vitamin B12 in some children with autism.
A meta-analysis evaluating the efficacy of cobalamin treatment in ASD demonstrated improvements in methylation capacity and the total glutathione redox ratio resulting from vitamin B12 supplementation. These improvements were associated with clinical improvements in ASD symptoms, including communication, interpersonal skills, and functioning in daily life. Additionally, improvements were observed in sleep, gastrointestinal symptoms, hyperactivity, tantrums, non-verbal IQ, eye contact, echolalia, stereotypies, anemia, and bedwetting. The most commonly observed adverse effects of the therapeutic intervention under examination were hyperactivity, irritability, sleep disturbances, aggression and behavioral deterioration. However, these were few and mild in intensity, and moreover, they did not differ significantly from those observed in the placebo group [
66].
11. Interaction of Vitamin B12 with Gut Microflora and Autism
Some studies indicate that autism may be a consequence of a distinct composition of gut microbiota (dysbiosis) compared to healthy individuals, through its impact on the gut-brain axis, especially since the gut microbiota produces nearly 40% of all human metabolites [
67,
68,
69]. Meta-analysis showed that children with ASD exhibit dysbiosis compared to neurotypical children, with specific bacterial groups examined [
70]. The microbiota of children with ASD, as evaluated in the study, exhibited an increased abundance of
Bacteroides,
Parabacteroides,
Clostridium,
Faecalibacterium, and
Phascolarctobacterium, in comparison to a neurotypically developed control group. Conversely, a decreased abundance of
Coprococcus and
Bifidobacterium was observed. The alterations in the microbiota were linked not only to comorbid gastrointestinal issues but also to elevated autistic symptoms. However, this meta-analysis is not without limitations. The results of the studies are inconclusive and often contradictory. The groups analyzed were heterogeneous in terms of age, gender, methodology and study design. Moreover, it was not possible to assess bacterial diversity at the species level. It is worth noting that there is no optimal and universal composition of the gut microbiota; rather, it is the balance and diversity in the bacterial population that is crucial for the proper functioning of the immune and nervous systems [
70].
Furthermore, a growing body of research indicates that not only the infant's microbiota, but also the mother's gut dysbiosis during pregnancy may be important in the development of autism. The reason for this may be the state of maternal immune activation (MIA), which can potentially alter microglia function [
71]. Dysbiosis may weaken the gut barrier, increasing the risk of inflammation and neurotoxic effects from bacterial metabolites [
72]. Physiologically antimicrobial peptides produced by the intestinal epithelium are concentrated in the inner layer of the mucus covering the epithelium, where they destroy bacteria that have managed to penetrate the deeper layers of the mucus. This prevents damage to the bacterial cells of the microbiota that inhabit the outer layer of mucus. In addition, secretory IgA (sIgA) is more concentrated in the outer layer of mucus, protecting the intestinal microbiota from an immune response [
73]. The intestinal barrier serves a protective function against the entry of pathogenic microorganisms and their metabolites, which could stimulate an immune response in the form of increased production of pro-inflammatory interleukins, including IL-1, IL-6, and IL-8. Studies have demonstrated that elevated levels of pro-inflammatory interleukins are a hallmark of children diagnosed with ASD, with increases in IL-1B, IL-6, IL-4, IFN-γ, and TGF-β observed [
71].
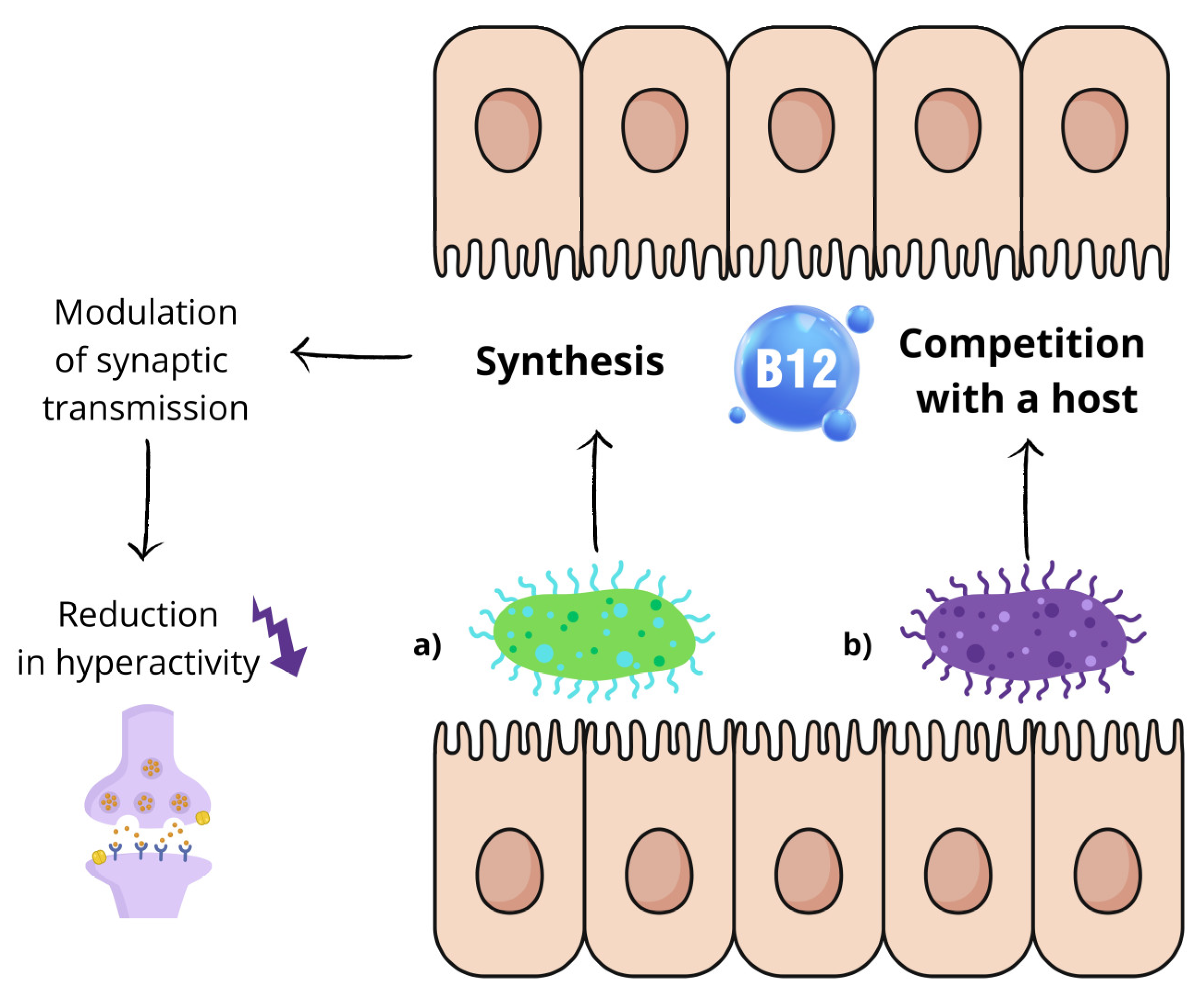
Living in symbiosis with the human body, commensal microorganisms require a variety of vitamin combinations, with cobalamin serving as an essential enzyme cofactor for methionine synthesis, nucleotide metabolism, carbon and nitrogen metabolism, and other cellular processes. The synthesis and transport mechanisms utilized by different bacterial species vary [
74]. As both humans and bacteria require exogenous cobalamins, it can be postulated that the gut microbiota may be in direct competition with its host for cobalamin [
75]. It has also been shown that the production of vitamin B12 by bacteria colonizing the intestine enables them to modulate excitatory synaptic transmission (
Figure 2) [
76]. Kang et al. used nematodes C. elegans with the unc-2/CaV2α(gf) mutation in presynaptic voltage-gated calcium channels as an animal model to study this relationship. Their goal was to recreate the conditions of increased excitatory transmission and imbalance between excitation and inhibition, which are likely to underlie autism. When the nematodes were given a medium containing bacteria that synthesize vitamin B12 (
Comamonas aquatica, Pseudomonas putida), a significant reduction in hyperactivity was observed. This effect did not occur with bacterial strains that did not produce vitamin B12 [
77].
On the other hand, it has been shown that cobalamin reduces cholinergic signaling in the nervous system by changing the methionine (Met)/S-adenosylmethionine (SAM) cycle in the intestines, which consequently reduces the availability of free choline used by neurons to synthesize acetylcholine [
77]. It is worth noting that previous studies have shown that low levels of acetylcholine may be a contributing factor to the onset of autism-related symptoms, and acetylcholinesterase inhibitors have emerged as a potential avenue for therapeutic intervention in ASD [
78].
Two further animal studies that investigated the relationship between the microbiome, vitamin B12 and autism are particularly noteworthy. The first is an experimental study by Abujamel et al. on male Sprague Dawley rats. The authors investigated the effect of altering the intestinal microbiota – by administering
Bifidobacterium longum (BF) and fecal microbiota transplantation (FT) – on vitamin B12 biosynthesis in the intestine. Supplementation with
Bifidobacterium longum increased intestinal vitamin B12 biosynthesis and restored the behavioral phenotype of autistic rats to levels observed in the control group. [
79]. Equally important is the study by Alfawaz et al. on male Western Albino rats with propionic acid-induced autism (PPA) [
80]. Inducing autistic traits in animals with propionic acid is a widely used research method for modeling the behavioral and neurological aspects of autism spectrum disorders in laboratory conditions. The study aimed to evaluate the effect of vitamin B12 supplementation on oxidative stress, lipid metabolism and gut microbiota composition. Vitamin B12 supplementation showed beneficial effects in rats with PPA-induced autism. These effects included alleviation of oxidative stress, improvement of lipid metabolism and modulation of the composition of intestinal microflora [
80].
Conversely, a number of studies conducted among the human population have demonstrated that vitamin B12 supplementation in patients with vitamin B12 deficiency results in a substantial increase in bacterial diversity, marked by the proliferation of
Firmicutes and the decrease of
Bacteroidetes [
81]. In contrast, the results of a study conducted on exclusively breastfed infants with both normal and reduced vitamin B12 levels showed no statistically significant differences between the gut microbiota composition of the study group and the control group. The limitation of this study is the relatively short duration of vitamin B12 deficiency in clinically asymptomatic infants, which may have prevented the observation of its impact on the gut microbiota. Furthermore, the cutoff point for deficiency, 203 pg/ml (150 pmol/l), was based on studies in older patients, which may not be directly applicable to infants [
82].
Although knowledge about the influence of cobalamin on the microbiota and its composition on the level of this vitamin already provides us with important information regarding potential mechanisms involved in the etiopathogenesis of autistic disorders, many issues remain to be clarified. First of all, there is a lack of extensive research among children diagnosed with ASD. It seems equally important to study the bacterial flora in pregnant women, as maternal dysbiosis may play a significant role in the development of autism in children.
12. Conclusions
Vitamin B12 may play a key role in maintaining the health of the gut microbiota, whose imbalance is associated with the development of low-grade inflammation and changes in the gut-brain axis. Disruptions of this axis can affect the functioning of the nervous system and be potentially related to the development of autism spectrum disorder. In addition, cobalamin is involved in the metabolism of neurotransmitters such as serotonin and dopamine and in the myelination of neurons, which is crucial for proper brain development. People with ASD often show abnormalities in their intestinal microbiota and in their levels of B vitamins, including B12, which suggests that deficiencies of this vitamin may be one of the risk factors for ASD.
The results of studies conducted so far suggest that vitamin B12 can serve as a biomarker not only for predicting the course of the disease, but also for evaluating the effectiveness of therapy. Due to the inconsistency of the results obtained, additional studies, such as longitudinal studies, are required to determine whether there is a causal relationship between vitamin B12 levels and the severity of ASD symptoms, which will allow for monitoring changes over a longer period of time. It should be emphasized that long-term observation is essential to understand the dynamics of the relationship between vitamin B12 levels and ASD symptoms. Single-point measurements cannot fully account for the inherent variability in children's health, nor do they account for potential external influences. Changes in vitamin levels and their impact on neurodevelopment may result from interactions with other factors, such as diet, environment or therapeutic interventions. Longitudinal study would facilitate the tracking of these changes and increase the understanding of the potential impact of vitamin B12 levels on the neurological development of children with ASD at different stages of life.
The study of vitamin levels, including B12, should be part of a broader research initiative aimed at identifying biological factors that may affect the neurological development and behavior of children with ASD. An essential step in further research is the identification of specific biochemical markers to effectively select children who are most likely to benefit from vitamin B12 supplementation. This approach may facilitate earlier detection of disorders and contribute to the development of effective intervention strategies that can improve the quality of life of children affected by these disorders. Furthermore, the identification of vitamin B12 deficiencies in the ASD child population emphasizes the need for increased public awareness and education on prevention, which may help to reduce the prevalence of this disorder. In this context, it is necessary to encourage healthcare professionals to implement preventive measures, such as dietary modifications and the use of supplements, to provide effective support for children with ASD and their families.
Author Contributions
Conceptualization, M.Z., M.S.; methodology, M.Z., M.S.; validation, B.K., N.W.; resources, B.K. and N.W..; data curation, A.J.O.; writing—original draft preparation, M.Z., M.S., J.P., K.M. and A.J.O.; writing—review and editing, M.Z., M.S., J.P., K.M., A.J.O., B.K. and N.W..; visualization, M.S. and K.M.; supervision, B.K. and N.W.; project administration, M.Z., M.S. and B.K.; funding acquisition, N.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Medical University of Bialystok, grant number B.SUB.25.445. The financial sponsor played no role in the design, execution, analysis, and interpretation of data.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- D. O. Kennedy, “B Vitamins and the Brain: Mechanisms, Dose and Efficacy--A Review,” Nutrients, vol. 8, no. 2, Jan. 2016. [CrossRef]
- G. Ågren, “Nature of the Anti-anæmic Factor (Castle),” Nature 1944 154:3909, vol. 154, no. 3909, pp. 430–431, 1944. [CrossRef]
- G. V. Krishnaveni et al., “Low plasma vitamin B12 in pregnancy is associated with gestational ‘diabesity’ and later diabetes,” Diabetologia, vol. 52, no. 11, pp. 2350–2358, Nov. 2009. [CrossRef]
- B. A. Knight et al., “Lower Circulating B12 Is Associated with Higher Obesity and Insulin Resistance during Pregnancy in a Non-Diabetic White British Population,” PLoS One, vol. 10, no. 8, Aug. 2015. [CrossRef]
- M. R. Pepper and M. M. Black, “B12 in fetal development,” Semin Cell Dev Biol, vol. 22, no. 6, pp. 619–623, 2011. [CrossRef]
- M. M. Black, “Effects of vitamin B12 and folate deficiency on brain development in children,” Food Nutr Bull, vol. 29, no. 2 Suppl, 2008. [CrossRef]
- J. L. Finkelstein, A. J. Layden, and P. J. Stover, “Vitamin B-12 and Perinatal Health,” Adv Nutr, vol. 6, no. 5, pp. 552–563, 2015. [CrossRef]
- F. Zeeshan, A. Bari, S. Farhan, U. Jabeen, and A. W. Rathore, “Correlation between maternal and childhood VitB12, folic acid and ferritin levels,” Pak J Med Sci, vol. 33, no. 1, p. 162, Jan. 2017. [CrossRef]
- “Nutrition for a healthy pregnancy : national guidelines for the childbearing years,” p. 130, 1999.
- A. Hunt, D. Harrington, and S. Robinson, “Vitamin B12 deficiency,” BMJ, vol. 349, Sep. 2014. [CrossRef]
- Ç. Yektaş, M. Alpay, and A. E. Tufan, “Comparison of serum B12, folate and homocysteine concentrations in children with autism spectrum disorder or attention deficit hyperactivity disorder and healthy controls,” Neuropsychiatr Dis Treat, vol. 15, pp. 2213–2219, 2019. [CrossRef]
- Y. Tan et al., “Correlation between Vitamin B12 and Mental Health in Children and Adolescents: A Systematic Review and Meta-analysis,” Clin Psychopharmacol Neurosci, vol. 21, no. 4, pp. 617–633, 2023. [CrossRef]
- Y. M. Al-Farsi et al., “Low folate and vitamin B12 nourishment is common in Omani children with newly diagnosed autism,” Nutrition, vol. 29, no. 3, pp. 537–541, Mar. 2013. [CrossRef]
- B. R. Vohr, E. P. Davis, C. A. Wanke, and N. F. Krebs, “Neurodevelopment: The Impact of Nutrition and Inflammation During Preconception and Pregnancy in Low-Resource Settings,” Pediatrics, vol. 139, no. Suppl 1, pp. S38–S49, Apr. 2017. [CrossRef]
- R. J. Schmidt et al., “Prenatal vitamins, one-carbon metabolism gene variants, and risk for autism,” Epidemiology, vol. 22, no. 4, pp. 476–485, Jul. 2011. [CrossRef]
- A. M. Alex et al., “A global multicohort study to map subcortical brain development and cognition in infancy and early childhood,” Nat Neurosci, vol. 27, no. 1, pp. 176–186, Jan. 2024. [CrossRef]
- S. Heland, N. Fields, S. J. Ellery, M. Fahey, and K. R. Palmer, “The role of nutrients in human neurodevelopment and their potential to prevent neurodevelopmental adversity,” Front Nutr, vol. 9, Nov. 2022. [CrossRef]
- L. M. Young, A. Pipingas, D. J. White, S. Gauci, and A. Scholey, “A Systematic Review and Meta-Analysis of B Vitamin Supplementation on Depressive Symptoms, Anxiety, and Stress: Effects on Healthy and ‘At-Risk’ Individuals,” Nutrients, vol. 11, no. 9, Sep. 2019. [CrossRef]
- F. Bellazzi and M. Bertolaso, “Emergence in Complex Physiological Processes: The Case of Vitamin B12 Functions in Erythropoiesis,” Systems 2024, Vol. 12, Page 131, vol. 12, no. 4, p. 131, Apr. 2024. [CrossRef]
- E. Villamor, S. L. Rifas-Shiman, M. W. Gillman, and E. Oken, “Maternal intake of methyl-donor nutrients and child cognition at 3 years of age,” Paediatr Perinat Epidemiol, vol. 26, no. 4, pp. 328–335, Jul. 2012. [CrossRef]
- S. H. Zeisel, “Choline, Other Methyl-Donors and Epigenetics,” Nutrients, vol. 9, no. 5, May 2017. [CrossRef]
- C. Briani et al., “Cobalamin deficiency: clinical picture and radiological findings,” Nutrients, vol. 5, no. 11, pp. 4521–4539, Nov. 2013. [CrossRef]
- T. Takahashi-Iñiguez, E. García-Hernandez, R. Arreguín-Espinosa, and M. E. Flores, “Role of vitamin B12 on methylmalonyl-CoA mutase activity,” J Zhejiang Univ Sci B, vol. 13, no. 6, pp. 423–437, Jun. 2012. [CrossRef]
- K. Halczuk, J. Kaźmierczak-Barańska, B. T. Karwowski, A. Karmańska, and M. Cieślak, “Vitamin B12-Multifaceted In Vivo Functions and In Vitro Applications,” Nutrients, vol. 15, no. 12, Jun. 2023. [CrossRef]
- P. Wongkittichote et al., “Tricarboxylic acid cycle enzyme activities in a mouse model of methylmalonic aciduria,” Mol Genet Metab, vol. 128, no. 4, pp. 444–451, Dec. 2019. [CrossRef]
- M. C. Cortés-Albornoz, D. P. García-Guáqueta, A. Velez-Van-meerbeke, and C. Talero-Gutiérrez, “Maternal Nutrition and Neurodevelopment: A Scoping Review,” Nutrients, vol. 13, no. 10, Oct. 2021. [CrossRef]
- J. S. Lai et al., “Maternal plasma vitamin B12 concentrations during pregnancy and infant cognitive outcomes at 2 years of age,” Br J Nutr, vol. 121, no. 11, pp. 1303–1312, Jun. 2019. [CrossRef]
- B. T. F. Wu, R. A. Dyer, D. J. King, K. J. Richardson, and S. M. Innis, “Early second trimester maternal plasma choline and betaine are related to measures of early cognitive development in term infants,” PLoS One, vol. 7, no. 8, Aug. 2012. [CrossRef]
- V. K. Bhate et al., “Vitamin B12 and folate during pregnancy and offspring motor, mental and social development at 2 years of age,” J Dev Orig Health Dis, vol. 3, no. 2, pp. 123–130, Apr. 2012. [CrossRef]
- J. S. Lai et al., “Maternal plasma vitamin B12 concentrations during pregnancy and infant cognitive outcomes at 2 years of age,” Br J Nutr, vol. 121, no. 11, pp. 1303–1312, Jun. 2019. [CrossRef]
- J. Golding et al., “Maternal prenatal vitamin B12 intake is associated with speech development and mathematical abilities in childhood,” Nutr Res, vol. 86, pp. 68–78, Feb. 2021. [CrossRef]
- C. Bonilla et al., “Vitamin B-12 status during pregnancy and child’s IQ at age 8: a Mendelian randomization study in the Avon longitudinal study of parents and children,” PLoS One, vol. 7, no. 12, Dec. 2012. [CrossRef]
- J. Cruz-Rodríguez, A. Díaz-López, J. Canals-Sans, and V. Arija, “Maternal Vitamin B12 Status during Pregnancy and Early Infant Neurodevelopment: The ECLIPSES Study,” Nutrients, vol. 15, no. 6, Mar. 2023. [CrossRef]
- K. Srinivasan et al., “Effects of maternal vitamin B12 supplementation on early infant neurocognitive outcomes: a randomized controlled clinical trial,” Matern Child Nutr, vol. 13, no. 2, Apr. 2017. [CrossRef]
- S. Thomas et al., “Effect of Maternal Vitamin B12 Supplementation on Cognitive Outcomes in South Indian Children: A Randomized Controlled Clinical Trial,” Matern Child Health J, vol. 23, no. 2, pp. 155–163, Feb. 2019. [CrossRef]
- A. Waligóra, S. Waligóra, M. Kozarska, A. Damasiewicz-Bodzek, P. Gorczyca, and K. Tyrpień-Golder, “Autism spectrum disorder (ASD) - biomarkers of oxidative stress and methylation and transsulfuration cycle,” Psychiatr Pol, vol. 53, no. 4, pp. 771–788, 2019. [CrossRef]
- D. Gąsiorowska, K. Korzeniowska, A. Jabłecka Zakład Farmakologii Klinicznej, K. Kardiologii, and U. K. Medyczny im Marcinkowskiego Poznaniu, “Homocysteina Homocysteine,” vol. 1, pp. 169–175, 2008.
- A. J. McPhee, G. P. Davidson, M. Leahy, and T. Bearei, “Vitamin B12 deficiency in a breast fed infant,” Arch Dis Child, vol. 63, no. 8, pp. 921–923, 1988. [CrossRef]
- D. K. Dror and L. H. Allen, “Effect of vitamin B12 deficiency on neurodevelopment in infants: current knowledge and possible mechanisms,” Nutr Rev, vol. 66, no. 5, pp. 250–255, May 2008. [CrossRef]
- S. Erden, B. Akbaş İleri, Ç. Sadıç Çelikkol, K. Nalbant, İ. Kılınç, and A. Yazar, “Serum B12, homocysteine, and anti-parietal cell antibody levels in children with autism,” Int J Psychiatry Clin Pract, vol. 26, no. 1, pp. 8–13, 2022. [CrossRef]
- A. Belardo, F. Gevi, and L. Zolla, “The concomitant lower concentrations of vitamins B6, B9 and B12 may cause methylation deficiency in autistic children,” J Nutr Biochem, vol. 70, pp. 38–46, Aug. 2019. [CrossRef]
- C. X. Li et al., “Association Between MTHFR C677T Polymorphism and Susceptibility to Autism Spectrum Disorders: A Meta-Analysis in Chinese Han Population,” Front Pediatr, vol. 9, Mar. 2021. [CrossRef]
- X. Yuan et al., “Association of folate and vitamin B12 imbalance with adverse pregnancy outcomes among 11,549 pregnant women: An observational cohort study,” Front Nutr, vol. 9, Jul. 2022. [CrossRef]
- A. Sourander et al., “Maternal Serum Vitamin B12 during Pregnancy and Offspring Autism Spectrum Disorder,” Nutrients, vol. 15, no. 8, Apr. 2023. [CrossRef]
- R. Raghavan et al., “Maternal Multivitamin Intake, Plasma Folate and Vitamin B12 Levels and Autism Spectrum Disorder Risk in Offspring,” Paediatr Perinat Epidemiol, vol. 32, no. 1, pp. 100–111, Jan. 2018. [CrossRef]
- R. J. Schmidt et al., “Prenatal vitamins, one-carbon metabolism gene variants, and risk for Autism,” Epidemiology, vol. 22, no. 4, pp. 476–485, Jul. 2011. [CrossRef]
- K. Hollowood-Jones et al., “Altered metabolism of mothers of young children with Autism Spectrum Disorder: a case control study,” BMC Pediatr, vol. 20, no. 1, Dec. 2020. [CrossRef]
- R. Ribeiro, J. R. Nicoli, G. Santos, and J. Lima-Santos, “Impact of vitamin deficiency on microbiota composition and immunomodulation: relevance to autistic spectrum disorders,” Nutr Neurosci, vol. 24, no. 8, pp. 601–613, 2021. [CrossRef]
- J. Chen, K. Xin, J. Wei, K. Zhang, and H. Xiao, “Lower maternal serum 25(OH) D in first trimester associated with higher autism risk in Chinese offspring,” J Psychosom Res, vol. 89, pp. 98–101, Oct. 2016. [CrossRef]
- A. M. Khemakhem, R. E. Frye, A. El-Ansary, L. Al-Ayadhi, and A. Ben Bacha, “Novel biomarkers of metabolic dysfunction is autism spectrum disorder: potential for biological diagnostic markers,” Metab Brain Dis, vol. 32, no. 6, pp. 1983–1997, Dec. 2017. [CrossRef]
- M. Zou et al., “Fisher discriminant analysis for classification of autism spectrum disorders based on folate-related metabolism markers,” J Nutr Biochem, vol. 64, pp. 25–31, Feb. 2019. [CrossRef]
- H. Li, Y. Dang, and Y. Yan, “Serum interleukin-17 A and homocysteine levels in children with autism,” BMC Neurosci, vol. 25, no. 1, Dec. 2024. [CrossRef]
- A. Belardo, F. Gevi, and L. Zolla, “The concomitant lower concentrations of vitamins B6, B9 and B12 may cause methylation deficiency in autistic children,” J Nutr Biochem, vol. 70, pp. 38–46, Aug. 2019. [CrossRef]
- Y. Wu et al., “[Serum folate and vitamin B12 levels and their association with neurodevelopmental features in preschool children with autism spectrum disorder],” Zhongguo Dang Dai Er Ke Za Zhi, vol. 26, no. 4, pp. 371–377, Apr. 2024. [CrossRef]
- A. Shi et al., “Serum binding folate receptor autoantibodies lower in autistic boys and positively-correlated with folate,” Biomed Pharmacother, vol. 172, Mar. 2024. [CrossRef]
- M. Guo et al., “Vitamin and mineral status of children with autism spectrum disorder in Hainan Province of China: associations with symptoms,” Nutr Neurosci, vol. 23, no. 10, pp. 803–810, Oct. 2020. [CrossRef]
- V. Arija, P. Esteban-Figuerola, P. Morales-Hidalgo, C. Jardí, and J. Canals-Sans, “Nutrient intake and adequacy in children with autism spectrum disorder: EPINED epidemiological study,” Autism, vol. 27, no. 2, pp. 371–388, Feb. 2023. [CrossRef]
- S. Marí-Bauset, I. Zazpe, A. Mari-Sanchis, A. Llopis-González, and M. Morales-Suárez-Varela, “Food selectivity in autism spectrum disorders: a systematic review,” J Child Neurol, vol. 29, no. 11, pp. 1554–1561, Nov. 2014. [CrossRef]
- W. G. Sharp et al., “Feeding problems and nutrient intake in children with autism spectrum disorders: a meta-analysis and comprehensive review of the literature,” J Autism Dev Disord, vol. 43, no. 9, pp. 2159–2173, Sep. 2013. [CrossRef]
- J. R. Ledford and D. L. Gast, “Feeding Problems in Children With Autism Spectrum Disorders,”, vol. 21, no. 3, pp. 153–166, Aug. 2006. [CrossRef]
- H. Tsujiguchi et al., “Relationship between Autistic Traits and Nutrient Intake among Japanese Children and Adolescents,” Nutrients, vol. 12, no. 8, pp. 1–15, Aug. 2020. [CrossRef]
- R. Langan and A. J. Goodbred, “Vitamin B12 Deficiency: Recognition and Management.,” Am Fam Physician, 2017.
- R. L. Hendren, S. J. James, F. Widjaja, B. Lawton, A. Rosenblatt, and S. Bent, “Randomized, Placebo-Controlled Trial of Methyl B12 for Children with Autism,” J Child Adolesc Psychopharmacol, vol. 26, no. 9, pp. 774–783, Nov. 2016. [CrossRef]
- S. Melnyk et al., “Metabolic imbalance associated with methylation dysregulation and oxidative damage in children with autism,” J Autism Dev Disord, vol. 42, no. 3, pp. 367–377, Mar. 2012. [CrossRef]
- K. Bertoglio, S. Jill James, L. Deprey, N. Brule, and R. L. Hendren, “Pilot study of the effect of methyl B12 treatment on behavioral and biomarker measures in children with autism,” J Altern Complement Med, vol. 16, no. 5, pp. 555–560, May 2010. [CrossRef]
- D. A. Rossignol and R. E. Frye, “The effectiveness of cobalamin (B12) treatment for autism spectrum disorder: A systematic review and meta-analysis,” J Pers Med, vol. 11, no. 8, p. 784, Aug. 2021. [CrossRef]
- S. M. Finegold et al., “Pyrosequencing study of fecal microflora of autistic and control children,” Anaerobe, vol. 16, no. 4, pp. 444–453, Aug. 2010. [CrossRef]
- H. E. Vuong and E. Y. Hsiao, “Emerging Roles for the Gut Microbiome in Autism Spectrum Disorder,” Biol Psychiatry, vol. 81, no. 5, pp. 411–423, Mar. 2017. [CrossRef]
- P. Vernocchi, F. Del Chierico, and L. Putignani, “Gut Microbiota Profiling: Metabolomics Based Approach to Unravel Compounds Affecting Human Health,” Front Microbiol, vol. 7, no. JUL, Jul. 2016. [CrossRef]
- L. Iglesias–vázquez, G. V. G. Riba, V. Arija, and J. Canals, “Composition of Gut Microbiota in Children with Autism Spectrum Disorder: A Systematic Review and Meta-Analysis,” Nutrients 2020, Vol. 12, Page 792, vol. 12, no. 3, p. 792, Mar. 2020. [CrossRef]
- M. Suprunowicz, N. Tomaszek, A. Urbaniak, K. Zackiewicz, S. Modzelewski, and N. Waszkiewicz, “Between Dysbiosis, Maternal Immune Activation and Autism: Is There a Common Pathway?,” Nutrients 2024, Vol. 16, Page 549, vol. 16, no. 4, p. 549, Feb. 2024. [CrossRef]
- Y. Kinashi and K. Hase, “Partners in Leaky Gut Syndrome: Intestinal Dysbiosis and Autoimmunity,” Front Immunol, vol. 12, Apr. 2021. [CrossRef]
- H. M. A. Fakhoury et al., “Vitamin D and intestinal homeostasis: Barrier, microbiota, and immune modulation,” J Steroid Biochem Mol Biol, vol. 200, p. 105663, Jun. 2020. [CrossRef]
- E. E. Putnam and A. L. Goodman, “B vitamin acquisition by gut commensal bacteria,” PLoS Pathog, vol. 16, no. 1, p. e1008208, 2020. [CrossRef]
- P. H. Degnan, M. E. Taga, and A. L. Goodman, “Vitamin B12 as a modulator of gut microbial ecology,” Cell Metab, vol. 20, no. 5, p. 769, Nov. 2014. [CrossRef]
- R. Gao and P. Penzes, “Common Mechanisms of Excitatory and Inhibitory Imbalance in Schizophrenia and Autism Spectrum Disorders,” Curr Mol Med, vol. 15, no. 2, p. 146, Mar. 2015. [CrossRef]
- W. K. Kang et al., “Vitamin B12 produced by gut bacteria modulates excitatory neurotransmission,” bioRxiv, p. 2022.09.06.506833, Sep. 2022. [CrossRef]
- A. Ure, G. R. Cox, R. Haslam, and K. Williams, “Acetylcholinesterase inhibitors for autistic spectrum disorders,” Cochrane Database Syst Rev, vol. 2023, no. 6, p. CD013851, Jun. 2023. [CrossRef]
- T. S. Abujamel et al., “Different Alterations in Gut Microbiota between Bifidobacterium longum and Fecal Microbiota Transplantation Treatments in Propionic Acid Rat Model of Autism,” Nutrients, vol. 14, no. 3, Feb. 2022. [CrossRef]
- H. Alfawaz et al., “Comparative study on the independent and combined effects of omega-3 and vitamin B12 on phospholipids and phospholipase A2 as phospholipid hydrolyzing enzymes in PPA-treated rats as a model for autistic traits,” Lipids Health Dis, vol. 17, no. 1, Aug. 2018. [CrossRef]
- H. M. Guetterman, S. L. Huey, R. Knight, A. M. Fox, S. Mehta, and J. L. Finkelstein, “Vitamin B-12 and the Gastrointestinal Microbiome: A Systematic Review,” Advances in Nutrition, vol. 13, no. 2, p. 530, Mar. 2021. [CrossRef]
- P. Boran, H. E. Baris, E. Kepenekli, C. Erzik, A. Soysal, and D. M. Dinh, “The impact of vitamin B12 deficiency on infant gut microbiota,” Eur J Pediatr, vol. 179, no. 3, pp. 385–393, Mar. 2020. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).