1. Introduction
The underlying arrhythmogenic mechanism of idiopathic ventricular arrhythmias (IVAs) originating from the ventricular outflow tracts (OT) (and adjacent anatomical structures like e.g. aortic sinuses of Valsalva, aorto-mitral continuity) has generally been accepted to be triggered activity induced by cAMP-mediated delayed afterdepolarization (DAD) [
1,
2,
3,
4,
5,
6]. (Note: beside the above-mentioned structures a minority of triggered-activity-mediated focal IVAs have also been described to originate from more distant locations like the papillary muscles, the moderator band and epicardial locations). However, despite significant research efforts in the field, it still remains elusive why this focal mechanism originates mainly in the ventricular outflow tracts (and the above-mentioned anatomic locations) in structurally normal hearts, and what specific factors can be held responsible for initiating arrhythmogenesis. The presumably more complex nature of the arrhythmogenic substrate in OT-related IVA is also supported by observations that describe the relative frequent co-existence of supraventricular tachycardia (mainly AVNRT) with IVA [
7,
8,
9]; along with others that suggest the involvement of preferential pathways of conduction tissue in arrhythmogenesis (based on observations of discrete prepotentials preceding the ventricular signals within this region) [
10,
11,
12,
13]. Moreover, we recently reported on the abolishment of OT-related premature ventricular contractions (PVCs) that occurred in parallel with successful ablation of accessory pathways at the atrioventricular annuli of both the left and right side of the heart [
14]. In the current case series, we add yet another interesting piece to this puzzle, by describing the unique findings of the electrophysiology study of six patients with idiopathic VA. All of these patients were referred to our center for catheter ablation of symptomatic PVCs originating from the ventricular outflow tracts (or adjacent perivalvular structures). We demonstrate here that stimulation at specific atrial location in the vicinity of the atrioventricular annulae was capable to evoke OT-related PVCs with an electrocardiographical morphology virtually identical to the clinical PVCs. Based on these findings we hypothesize that specific pathways with preferential conduction might exist between certain atrial locations (e.g., periannular network of nodal-type-tissue) and the OT regions, which might play a role in the genesis of OT-related IVA. (Such connections also seem to be possible in the case of perivalvular IVAs, which originate either around the mitral or the tricuspid annulus.)
2. Methods
Six patients were included in this study. All patients were referred for catheter ablation because of symptoms of palpitations and a significant PVC burden as detected by 24-hour Holter monitoring. A 12-lead surface ECG was performed to assess the origin of the PVCs (
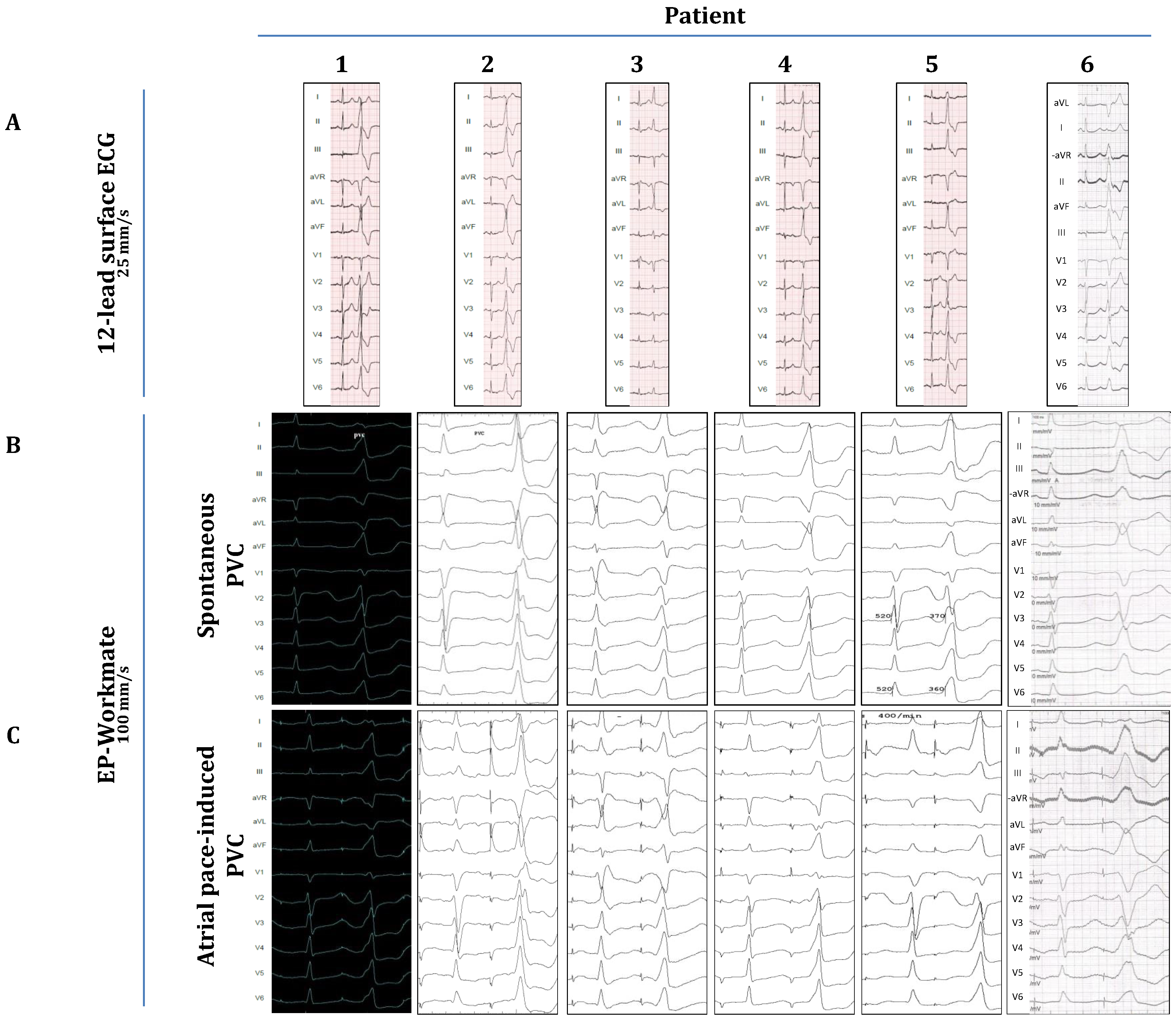
Figure 1.A.).
Table 1 contains the demographic data and the findings of echocardiography and cardiac MRI.
Table 2 shows the individual PVC burdens together with the morphological analysis of all PVCs on ECG. All patients underwent standard preprocedural preparations: Antiarrhythmic drugs (beta-blockers or calcium-channel-blockers, see
Table 1.) were discontinued 5 half-lives before the procedure. Local anesthesia (1% lidocaine) was applied to the subinguinal region, before vascular access was established. No oral or intravenous sedatives were used during the electrophysiology study in any of the cases described in this study. A radiofrequency ablation catheter (specific type left at the operator’s discretion) was placed into the heart either alone (single-catheter approach) or together with a steerable decapolar catheter (Inquiry, Abbott Laboratories, Abbott Park, Illinois, USA). The ablation catheter was guided either manually or with magnetic navigation (Stereotaxis MNS; Stereotaxis, Inc., Saint Louis, MO, USA). Despite significant pre-procedural PVC burdens, all patients included in this study, exhibited a fairly low PVC-frequency (≤1 PVC/3-5 min) during the first phase of the EP procedure, when intravenous isoproterenol (10-20µg/min) and programmed ventricular stimulation (maximum three ventricular extra stimulus at two different drive-trains from two different locations in the right ventricle, using the ablation catheter) was utilized to induce the arrhythmia. Despite these significant efforts we did not observe OT-related ventricular tachycardia in any of these patients nor did we detect the increase of their PVC burden. Due to this low PVC-frequency, activation-mapping was not attempted. Nevertheless, anatomical maps of the OTs were created (
Figure 2A, right panel) with the CARTO system (Biosense Webster, Inc., Diamond Bar, CA, USA) and pace-mapping was done in the OTs to approximate the PVC-origins. Subsequently, as part of the attempts to induce clinical PVCs (of OT-origin) programmed atrial stimulation (atrial incremental bipolar pacing at several cycle lengths (CL) between 350-600 ms at an output between 2,5-15 mA) was implemented, using either the ablation catheter or the decapolar catheter. Atrial stimulation was performed at distinct locations around the mitral annulus (within the coronary sinus, starting at the posteroseptal aspects, and then moving sequentially along the mitral annulus towards the posterolateral, anterolateral and anteroseptal aspects by advancing the ablation/decapolar catheter further inside the coronary sinus/great cardiac vein) and at the septal aspects of the tricuspid annulus (anteroseptal, midseptal and posteroseptal). Atrial-pacing-induced PVCs were recorded and their morphology was compared with spontaneous PVCs (recorded on pre-procedural ECGs and with the EP-Workmate system;
Figure 1). The specific periannular sites where OT-related PVCs could be elicited were marked on the fluoroscopic image (
Figure 2.).
Figure 1.
Comparison of spontaneous and atrial-pace-induced PVCs. A) Spontaneous PVCs on pre-procedural ECG. B) and C) Spontaneous and atrial-pace-induced PVCs as recorded during the procedure with the EP-Workmate system.
Figure 1.
Comparison of spontaneous and atrial-pace-induced PVCs. A) Spontaneous PVCs on pre-procedural ECG. B) and C) Spontaneous and atrial-pace-induced PVCs as recorded during the procedure with the EP-Workmate system.
Figure 2.
Intracardiac electrograms (EGMs) during atrial-pace-induced PVCs. A) Positions of the decapolar catheter (CS) and the ablation catheter (Abl.) as depicted on fluoroscopy (LAO projection) and on the anatomical map of the CARTO system (in patient 2). Patient 1: the decapolar catheter is located in the distal CS/great cardiac vein, and pacing occurs from CS 9,10. Patient 2: decapolar catheter is located more proximally in the CS (CS 9,10 at CS-ostium), and the ablation catheter is at the superior mitral annulus/aorto-mitral-continuity; pacing occurs from CS 1,2. B) 12-lead EGMs and intracardiac EGMs from the decapolar/ablation catheters (the latter only for Patient 2.) as recorded by the EP-Workmate system during an atrial-pace-induced PVC. Timing of the pacing stimulus is depicted at the bottoms of each panel. The “atrial-stimulus-to-normal-QRS time” and the “atrial-stimulus-to-PVC time” are depicted on both recordings. Note: “stimulus-to-PVC time” is shorter in both cases than the “stimulus-to-normal-QRS time”. *Represent the atrial activation signals and † designate the ventricular activation signals on both panels. Inset) Magnified image of the framed part of the EGM on „Abl1-2” depicting the atrial (*) and ventricular (†) activation signals and a (putative) pre-potential (PP) between them.
Figure 2.
Intracardiac electrograms (EGMs) during atrial-pace-induced PVCs. A) Positions of the decapolar catheter (CS) and the ablation catheter (Abl.) as depicted on fluoroscopy (LAO projection) and on the anatomical map of the CARTO system (in patient 2). Patient 1: the decapolar catheter is located in the distal CS/great cardiac vein, and pacing occurs from CS 9,10. Patient 2: decapolar catheter is located more proximally in the CS (CS 9,10 at CS-ostium), and the ablation catheter is at the superior mitral annulus/aorto-mitral-continuity; pacing occurs from CS 1,2. B) 12-lead EGMs and intracardiac EGMs from the decapolar/ablation catheters (the latter only for Patient 2.) as recorded by the EP-Workmate system during an atrial-pace-induced PVC. Timing of the pacing stimulus is depicted at the bottoms of each panel. The “atrial-stimulus-to-normal-QRS time” and the “atrial-stimulus-to-PVC time” are depicted on both recordings. Note: “stimulus-to-PVC time” is shorter in both cases than the “stimulus-to-normal-QRS time”. *Represent the atrial activation signals and † designate the ventricular activation signals on both panels. Inset) Magnified image of the framed part of the EGM on „Abl1-2” depicting the atrial (*) and ventricular (†) activation signals and a (putative) pre-potential (PP) between them.
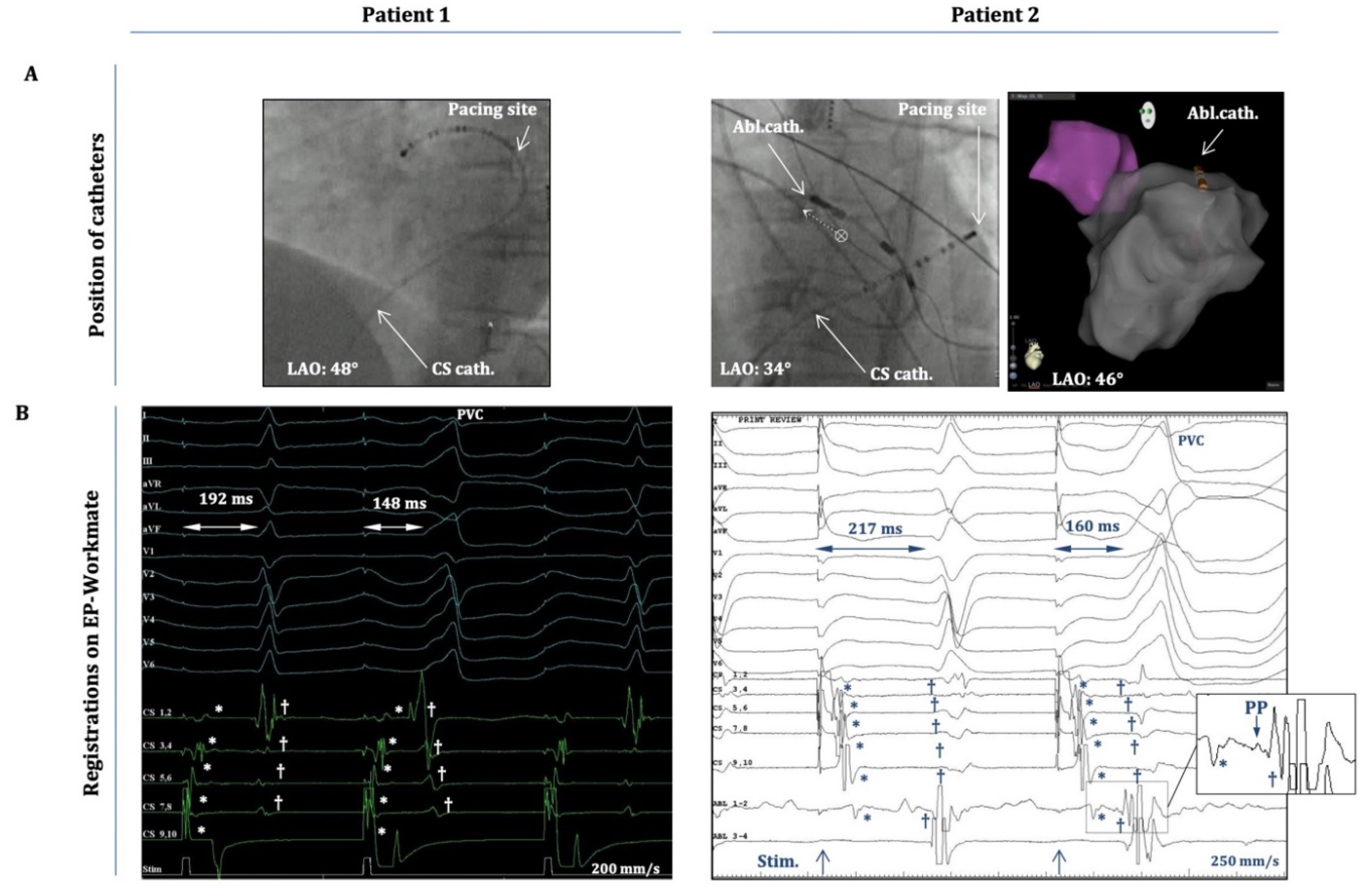
Table 1.
Patient characteristics and echocardiographic/cardiac-MRI findings.
Table 1.
Patient characteristics and echocardiographic/cardiac-MRI findings.
| |
Age |
Sex (m/f) |
LV systolic function |
Structural Heart Disease |
Antiarrhythmic medication |
| Case 1 |
52 |
m |
Mild dysfunction |
None* |
Beta-blocker |
| Case 2 |
57 |
f |
Mild to moderate dysfunction |
None* |
Beta-blocker |
| Case 3 |
58 |
f |
Normal |
None |
Calcium channel blocker |
| Case 4 |
57 |
f |
Normal |
None |
Calcium channel blocker |
| Case 5 |
71 |
m |
Moderate dysfunction |
None* |
Beta-blocker |
| Case 6 |
43 |
f |
Normal |
None |
Beta-blocker |
Table 2.
ECG/Holter findings.
Table 2.
ECG/Holter findings.
| |
PVC burden |
|
PVC morphology |
Possible PVC
origin |
| Percentage |
>10.000
/24h |
BBB pattern |
Horizontal axis |
Precordial transition zone |
| Case 1 |
17% |
yes |
|
LBBB |
Left inferior |
V2-V3 |
outflow tract |
| Case 2 |
12% |
yes |
|
RBBB |
Right inferior |
none |
aortomitral continuity/ superior mitral annulus |
| Case 3 |
16% |
yes |
|
LBBB |
Horizontal |
V3-V4 |
parahisian region |
| Case 4 |
16% |
yes |
|
LBBB |
Left inferior |
V1-V2 |
outflow tract |
| Case 5 |
19% |
yes |
|
LBBB |
Left inferior |
V2-V3 |
outflow tract |
| Case 6 |
18% |
yes |
|
LBBB |
Inferior |
V2-V3 |
outflow tract |
3. Results
All patients in this study were referred to our department with symptomatic VA and a high PVC- burden (12-19%; ≥10.000 PVC/24h), despite antiarrhythmic therapy (beta-blocker or calcium- channel-blocker). No patient exhibited clinically relevant valvular disease. Three patients had normal LV systolic function, whereas the other three patients exhibited mild-to-moderate systolic dysfunction. However, in this latter group cardiac MRI did not reveal structural heart disease (as evidenced also by the lack of late-gadolinium enhancement), hence all patients were diagnosed to have idiopathic VA, and the latter group was diagnosed to have PVC-induced-cardiomyopathy (as the most feasible condition responsible for their LV dysfunction).
Figure 1A demonstrates the clinical PVC of each patient as depicted on the preprocedural ECGs, and
Table 2 summarizes the morphological characteristics of these PVCs. In five cases the clinical PVCs presented with an LBBB morphology, and five clinical PVCs showed a clear inferior axis (except for case 3; here the axis was more horizontal). Based on these characteristics we concluded that the PVC-origins were in the RVOT or the LVOT (depending on transition zones in the precordial leads); except for cases 2 and 3, where they originated from adjacent anatomical structures (case 2: superior mitral annulus/aorto-mitral-continuity; case 3: parahisian-region of the RV-inflow tract); as also confirmed by pace-mapping during the procedure.
Figure 1B shows the relevant PVCs as detected by the EP-Workmate-system at a recording speed of 100 mm/s. On Figure1.C we show representative images of atrial-pacing-induced-PVCs. Their morphology closely resembled that of spontaneous PVCs (
Figure 1B,C), i.e., the pacing-induced-PVCs showed the same type of BBB-morphology, the same frontal-plane axis, and identical transition zones.
Importantly, these PVCs occurred after apparent atrial capture, hence they were not elicited by inadvertent ventricular capture. This is clearly shown in
Figure 2, which demonstrates (case 1 and 2), that the atrial activation sequence on the decapolar electrodes (within the CS) occurs before the onset of the „atrial-pacing-induced” PVC, during pacing from CS 9-10 (case 1) or from CS 1-2 (case 2). It is also evident that there is an isoelectric line between the atrial and ventricular local activation signals. In addition, for case2 (inset in Figure2.B) a small pre-potential between the atrial and ventricular signals on Abl1-2 could also be appreciated (with the ablation catheter located at the spot of earliest ventricular activation). Moreover, the „atrial-stimulus-to-PVC-interval” (from the atrial pacing stimulus till the PVC-onset) was consistently shorter than the „atrial-stimulus-to-normal-QRS-interval” (from the pacing stimulus till the onset of the normal QRS) (
Figure 2B); and the duration of the „atrial-stimulus-to-PVC-interval” (together with local A-to-V intervals on the electrograms from the decapolar/ablation catheters) remained essentially the same when the OT-PVCs were elicited with the same pacing CL during consecutive drivetrains of atrial pacing (the duration of the stimulus-to-PVC-intervals did not change more than 5-10%.)
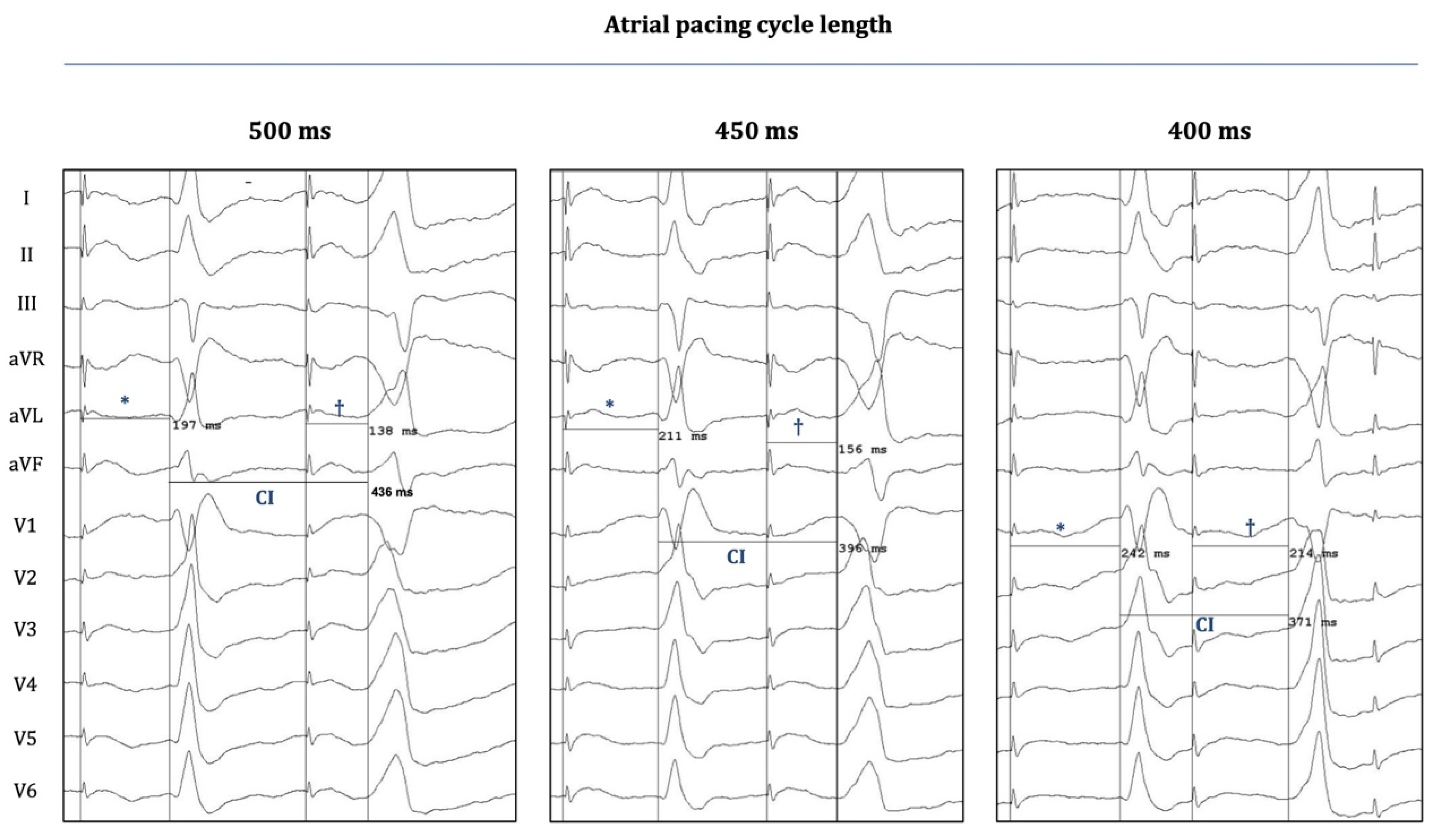
Figure 3 demonstrates another important phenomenon that was observed when OT-PVCs were elicited with progressively shorter atrial pacing CL: The coupling intervals of the PVCs became progressively shorter when the CL was decreased from 500 to 400 ms. On the other hand, decremental conduction was also observed (over the hypothetical preferential pathway between the atrium and the OTs), as the "atrial-stim-to-PVC-interval" was consistently prolonging with progressively shortened CLs; a phenomenon similar to the characteristic behavior of the normal AV conduction over the AV node (which can also be seen in the case of the "normally conducted" atrial pacing stimuli at the left sides of the panels of
Figure 3).
Figure 3.
Twelve-lead surface electrograms during atrial-pace-induced PVCs. Surface EGMs (EP-Workmate) are depicted during the occurrence of an atrial-pace-induced-PVC (Patient 3) at three different atrial pacing cycle lengths (CL). “Stimulus-to-normal-QRS times” and “stimulus-to-PVC times” are labelled with * and †, respectively; the corresponding durations are indicated on each panel. The coupling intervals (CI) of PVCs are also indicated. Shortening the pacing CL results in a progressive shortening of the CI, whereas it also leads to a prolongation of both the “stimulus-to-normal-QRS time” and the “stimulus-to-PVC time”. The latter finding suggests that the putative pathway between the atrium and the OT-locations (entry and exit sites) could possess decremental properties, similarly to AV nodal conduction.
Figure 3.
Twelve-lead surface electrograms during atrial-pace-induced PVCs. Surface EGMs (EP-Workmate) are depicted during the occurrence of an atrial-pace-induced-PVC (Patient 3) at three different atrial pacing cycle lengths (CL). “Stimulus-to-normal-QRS times” and “stimulus-to-PVC times” are labelled with * and †, respectively; the corresponding durations are indicated on each panel. The coupling intervals (CI) of PVCs are also indicated. Shortening the pacing CL results in a progressive shortening of the CI, whereas it also leads to a prolongation of both the “stimulus-to-normal-QRS time” and the “stimulus-to-PVC time”. The latter finding suggests that the putative pathway between the atrium and the OT-locations (entry and exit sites) could possess decremental properties, similarly to AV nodal conduction.
Based on these, we concluded that peri-annular atrial pacing was capable to evoke OT-PVCs by presumably capturing the "atrial entry-sites" of certain preferential pathways (between atria and OTs), and due to the decremental behavior of this conduction it might also be plausible that these unique pathways consist of nodal-type-tissue.
4. Discussion
4.1. Clinical Relevance of OT-VA
Although OT-VAs are generally considered to follow a benign clinical course, there have been reports on
i) clinical cases with more malignant subtypes of OT-VA, capable of causing syncope and even sudden cardiac death, and
ii) incessant forms accounting for tachycardia-induced cardiomyopathy and/or PVC-induced cardiomyopathy [
2,
3,
4]. In addition, high PVC/non-sustained VT burden can render many patients highly symptomatic and therefore significantly lower their quality of life. Thus, according to current guidelines, therapy is recommended in symptomatic cases and in asymptomatic individuals for whom LV dysfunction is suspected to be attributed to PVC-/tachycardia-induced cardiomyopathy [
2,
3,
4,
6,
15]. In most cases this means eventual catheter ablation, since pharmacological therapy often has limited efficacy [
4]. In fact, all of the patients included in this report were referred for catheter ablation due to a high PVC burden with symptoms not amenable to medical therapy and 50% of these patients were suspected to have LV dysfunction due to PVC-induced cardiomyopathy.
Patients referred to catheter ablation of idiopathic VA represent a significant population in the field of invasive electrophysiology (EP), accounting for around 10% of all referrals to EP centers [
16]. The reports on the procedural outcome of OT-VA ablations have been somewhat conflicting with regards to acute success rates and long-term recurrence rates: some studies claim that the acute and long-term success would nearly 100% [
17,
18], while others report on more humble outcomes [
6,
15,
19,
20]. These varying success rates might reflect differences in follow-up methods, definitions of success and also inclusion bias, but it has to be acknowledged that ablation-failure in an unselected clinical population is far from being negligible and there is still room for further improvements. Technical issues, such as 1) non-inducibility of tachycardia, 2) arrhythmia foci near critical cardiac structures, 3) epicardial origin; might account for a significant part of ablation failures; however, another important aspect to consider is our limited understanding of the actual arrhythmia substrate underlying IVA.
4.2. Arrhythmogenic Substrate for OT-VA: Current Concepts
Delayed afterdepolarization (DAD)-mediated Ca
2+ overload and subsequent triggered activity (from a focal source in the OT) has generally been accepted to represent the underlying substrate for OT-VA [
1,
2,
3,
4,
5]. However, the exact etiology of cAMP-increase and subsequent Ca
2+- accumulation is not well understood. Moreover, another unresolved issue is why the OTs would contain the foci of triggered activity in around 80% of the cases of IVA [
6]. What is so special about them (compared to other areas of the ventricles) that would make them more prone to generate triggered-activity mediated PVCs/VTs?
Abnormal β-adrenergic signaling (increased sympathetic activation or selective dysregulation of β-receptors) within these regions has been implicated to play a crucial role in the pathomechanism [
1,
5]. Other studies report on subtle structural abnormalities of the RVOT in patients with OTVA, suggesting a possible link to arrhythmogenic right ventricular cardiomyopathy. In contrast, Lermann
et al. found no evidence for such structural abnormalities and they suggest that the unique embryological development of the OT would account for their arrhythmogenic potential. According to this theory the adult OT is formed by the incorporation of embryonic OT into the working myocardium of the ventricles, which possesses characteristics resembling that of the primary myocardium (slow conduction, spontaneous depolarization), therefore non-matured remnants of this embryonic phenotype in the adult OT would represent foci with nodal-tissue-like electrical properties, thereby forming the basis for arrhythmogenesis [
5,
21,
22,
23].
4.3. Conduction Tissue Remnants: Could They Have a Role in Arrhythmogenesis?
Intriguingly, such nodal-tissue-like remnants in the OT have also been suggested to derive from the embryologic atrioventricular conduction system. Conduction-tissue-specific immuno-markers have been described to be expressed in the OTs during the early stages of cardiac development [
24]. Several reports suggest the possible involvement of remnants of this primitive conduction system in the generation of OT-related IVA; based on the observations that discrete pre-potentials occur within the OT (or adjacent structures, e.g., aortic sinuses of Valsalva), which precede the local activation signal when recorded at the spot of the earliest ventricular activation [
10,
11,
12,
13]. These reports implicate that preferential pathways (insulated from the surrounding myocardium) might exist within these regions, which could connect the origins of the ventricular arrhythmia with their presumable exit/breakout site(s) within the OTs. These studies hypothesize that the anatomical basis for the existence of such insulated pathways (with slow-conduction and possible decremental properties) could be conduction tissue that fails to regress during the maturation process of the AV conduction system.
In fact, the entire AV conduction system has been shown to develop from a specialized interventricular ring, and during further steps of the early developmental stages this primitive conduction tissue would encircle both the junctions of the developing ventricles at the AV orifices (designated as e.g., right atrioventricular ring bundle), as well as the roots of the great arteries (designated as septal branch, a.k.a. “dead-end-tract”; and subaortic root branch). In the later phases of the maturation process these segments around the AV-junctions and the great arteries eventually disappear, giving rise to the mature conduction system which only consists of the AV node, the His bundle and the bundle branches [
24]. However, in case any part of the above-mentioned structures fails to regress completely, this remnant conduction-tissue could represent an arrhythmogenic focus at any of the above locations (including the OTs).
Other studies even describe sleeves of nodal-type-tissue around the tricuspid and the mitral annuli in the normal adult heart, which are also believed to represent remnants of the primitive conduction system [
25]. This AV-junctional network of nodal-type-tissue exhibits histological characteristics similar to those of atrial tissue, but their cellular electrophysiology rather resembles the characteristics of nodal tissue (action-potential and conduction properties, adenosine response, relative lack of gap-junctional protein connexin-43). Although their actual function remains rather elusive, studies have shown that certain parts of these AV-junctional sleeves can be electrically dissociated from the surrounding atrial tissue; therefore, they might be insulated from the atrial myocardium. Moreover, the posterior approaches of this system towards the AV node have been implicated to be the actual substrate of the slow AV nodal pathway, which represents the essential component of the re-entry circle in AVNRT [
25]. Some studies report on the relatively frequent co-existence of AVNRT and OT-VAs [
7,
8,
9]. Intriguingly, Kautzner et al. even describe cases where AVNRT was observed to initiate runs of OT-VT spontaneously and repeatedly. Moreover, in one case termination of RVOT-VT during RF application led to an abrupt transition into typical AVNRT [
8]. Is it therefore conceivable that the above-mentioned network of AV-junctional tissue is interconnected with conduction tissue remnants in the OTs, and that these remnants would represent the anatomical substrate for both of these disease entities?
4.4. PVC Ablation from the Atrium?
We recently reported on the unique electrophysiological findings of patients with co-existent WPW syndrome and OT-PVCs. The successful ablation of accessory pathways (APs) at the atrioventricular annuli simultaneously abolished the occurrence of the concomitant PVCs originating from the OTs [
14]. These observations suggested that during the ablation of the APs we might have also ablated parts of the above-mentioned network of nodal-type-tissue within the AV-junctional sleeves; thereby involuntarily disrupting the possible “entry”-sites of those preferential pathways (of conduction tissue remnants) that have been suggested to have their exit sites in the OTs. A similar explanation might also account for the unique results that Garcia
et al. present in their report, which describes (for the first time in the literature) the approach of idiopathic VA ablation from the right atrium. The authors suggest that the feasibility of targeting specific PVCs (arising from the posterior-superior-process of the left ventricle) from the right atrium lies in the fact that these structures are in close proximity to each other [
26]. However, an alternative explanation for these unique findings could be the ablation of the “entry”-sites of the above-mentioned specific pathways in the AV-junctional network at the tricuspid annulus.
4.5. Atrial-Pacing Induced OT-PVCs: Implications on Novel Mechanistic Insights into Arrhythmogenesis
Following the logic of this hypothesis in the current report we show that atrial pacing around the AV annuli was capable of evoking OT-PVCs (
Figure 1,
Figure 2 and
Figure 3) by presumably capturing specific fibers within the network of nodal-type-tissue of the AV-junctional sleeves. Although these OT-PVCs did not follow each atrial pacing stimulus, they occurred relatively frequently during consecutive drivetrains of pacing at certain locations in the periannular regions. Based on our recordings it was evident that the PVCs did not occur due to an inadvertent ventricular capture, but instead after apparent atrial activation (
Figure 1 and
Figure 2). Although the possibility for the concomitant occurrence of
spontaneous PVCs during the pacing episodes may not be excluded unambiguously, the following characteristics might argue for a causative interrelation between atrial pacing and the induction of OT-PVCs: 1) The selected patients exhibited a fairly low frequency (≤1 PVC/3-5 min) of spontaneous PVCs throughout the whole course of the EP study (as mentioned above in the Methods section), whereas with atrial pacing we could reproducibly induce PVCs (and hence significantly enhance their frequency of occurrence). 2) The duration of the "atrial-stimulus-to-PVC interval" remained relatively constant when pacing with the same cycle-length. 3) Subtle morphological differences could indeed be detected between spontaneous and pace-induced PVCs (
Figure 2B,C). 4) The coupling intervals of the pace-induced PVC (from the preceding normal QRS) showed a progressive shortening with decreasing pacing cycle lengths, whereas we observed a fairly constant coupling interval (at a certain range of heart rate) in the case of spontaneous OT-PVCs. Taken together all these findings suggested that the observed PVCs could indeed be elicited as a result of prior atrial stimulation and that co-incidental occurrence of spontaneous PVCs during atrial pacing might be excluded (although the latter scenario cannot be completely excluded either).
We therefore speculate that unique connections (conduction tissue remnants?) might exist between specific atrial locations and the OTs, the activation of which could result in triggering PVCs (or repetitive ventricular activation) from the presumed exit-point of these pathways in the OTs. In the presence of insulation defects, such special “atrium-to-outflow-tract” pathways could represent the pathophysiological substrate of OT-VA. Since this premature conduction-tissue-network has previously been indicated: i) to be partially insulated from the surrounding (atrial) myocardium and ii) to possess distinct conduction properties, it is plausible to assume that they could serve as preferential pathways between the outflow tract and the atria; and once excited (either spontaneously or by pacing) their activation wave-front might be able to occasionally "leak out" towards the outflow tract regions, and exit there in the form of an OT-PVC. (The above mentioned subtle morphological differences between the spontaneous and the pace-induced PVCs might be explained by the existence of multiple adjacent exit points for the network of these conduction fibers, located within close proximity to each other within the same region of the OTs, and activated somewhat differently during pacing than during spontaneous PVCs.)
4.6. Limitations of the Study
Beside the relatively small sample size, an important limitation of this study is that we cannot completely exclude the possibility that atrial pacing had a non-specific effect on the induction of PVCs. Such non-specific effect could be the generally increased sympathetic tone, which is caused by the rapid heart rate during fast atrial pacing, which feels unusual and uncomfortable for the patient. This could then lead to increased beta-adrenergic signaling, which would in turn increase the possibility of triggered activity. However, in such a scenario it would still be controversial why PVCs would only originate from the outflow tracts and not from any other part of the heart. Nevertheless, we would like to emphasize that further research is required in order to find compelling evidence for the existence of these unique atrial-to-outflow-tract pathways and their potential role in the pathogenesis of IVAs.
By describing the intriguing phenomenon of atrial-pace induced OT-PVCs in our patients we wished to add an important "puzzle-piece" to these research efforts. We also believe, that the importance of our present report lies in the fact that (together with previous observations) it could have the potential to transform our current approach of catheter-ablation of OT-VA; since it might introduce a novel strategy for extending our mapping efforts into the atria, in order to identify and ablate the “entry-sites” of these special “atrium-to-outflow-tract” pathways.
Funding Sources
none. The data that support the findings of this study are available from the corresponding author (T. Sz-T.) upon reasonable request.
Abbreviations
| AVNRT |
atrioventricular nodal reentrant tachycardia |
| CS |
coronary sinus |
| cAMP |
Cyclic adenosine monophosphate |
| DAD |
delayed afterdepolarization |
| IVA |
idiopathic ventricular arrhythmias |
| ECG |
Electrocardiogram |
| L/RVOT |
Left/Right Ventricular Outflow Tract |
| OT |
outflow tract |
| LV |
left ventricle |
| LBBB |
left bundle branch block |
| RBBB |
right bundle branch block |
| BBB |
bundle branch block |
| PVC |
premature ventricular contraction |
| EP |
electrophysiology |
| AP |
accessory pathway |
| VA/T |
Ventricular Arrhythmia/Tachycardia |
References
- Lerman BB: Mechanism of outflow tract tachycardia. Heart Rhythm 2007, 4, 973–976. [CrossRef] [PubMed]
- Priori, S.G.; Blomstrom-Lundqvist, C.; Mazzanti, A.; Blom, N.; Borggrefe, M.; Camm, J.; Elliott, P.M.; Fitzsimons, D.; Hatala, R.; Hindricks, G.; et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2015, 36, 2793–2867. [Google Scholar] [PubMed]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2017.
- Pedersen, C.T.; Kay, G.N.; Kalman, J.; Borggrefe, M.; Della-Bella, P.; Dickfeld, T.; Dorian, P.; Huikuri, H.; Kim, Y.H.; Knight, B.; et al. EHRA/HRS/APHRS expert consensus on ventricular arrhythmias. Europace 2014, 16, 1257–1283. [Google Scholar] [CrossRef]
- Lerman, B.B. Outflow tract ventricular arrhythmias: An update. Trends Cardiovasc Med 2015, 25, 550–558. [Google Scholar] [CrossRef]
- Tanawuttiwat, T.; Nazarian, S.; Calkins, H. The role of catheter ablation in the management of ventricular tachycardia. Eur Heart J 2016, 37, 594–609. [Google Scholar] [CrossRef]
- Hasdemir, C.; Alp, A.; Simsek, E.; Kose, N.; Aydin, M.; Payzin, S. Spontaneous atrioventricular nodal reentrant tachycardia in patients with idiopathic ventricular arrhythmias: the incidence, clinical, and electrophysiologic characteristics. J Cardiovasc Electrophysiol 2013, 24, 1370–1374. [Google Scholar] [CrossRef]
- Kautzner, J.; Cihak, R.; Vancura, V.; Bytesnik, J. Coincidence of idiopathic ventricular outflow tract tachycardia and atrioventricular nodal reentrant tachycardia. Europace 2003, 5, 215–220. [Google Scholar] [CrossRef]
- Chen, J.; Hoff, P.I.; Rossvoll, O.; De Bortoli, A.; Solheim, E.; Sun, L.Z.; Schuster, P.; Larsen, T.; Ohm, O.J. Ventricular arrhythmias originating from the aortomitral continuity: an uncommon variant of left ventricular outflow tract tachycardia. Europace 2012, 14, 388–395. [Google Scholar] [CrossRef]
- Shirai, Y.; Goya, M.; Isobe, M.; Hirao, K. Preferential Pathway Pacing within the Aortic Sinus of Valsalva: Strong Evidence for the Existence of Preferential Conduction with Different Exit Sites Traversing the Ventricular Septum. J Cardiovasc Electrophysiol 2015, 26, 805–808. [Google Scholar] [CrossRef]
- Yamada, T.; Murakami, Y.; Yoshida, N.; Okada, T.; Shimizu, T.; Toyama, J.; Yoshida, Y.; Tsuboi, N.; Muto, M.; Inden, Y.; et al. Preferential conduction across the ventricular outflow septum in ventricular arrhythmias originating from the aortic sinus cusp. J Am Coll Cardiol 2007, 50, 884–891. [Google Scholar] [PubMed]
- Hai, J.J.; Chahal, A.A.; Friedman, P.A.; Vaidya, V.R.; Syed, F.F.; DeSimone, C.V.; Nanda, S.; Brady, P.A.; Madhavan, M.; Cha, Y.M.; et al. Electrophysiologic characteristics of ventricular arrhythmias arising from the aortic mitral continuity-potential role of the conduction system. J Cardiovasc Electrophysiol 2015, 26, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Hachiya, H.; Yamauchi, Y.; Iesaka, Y.; Yagishita, A.; Sasaki, T.; Higuchi, K.; Kawabata, M.; Sugiyama, K.; Tanaka, Y.; Kusa, S.; et al. Discrete prepotential as an indicator of successful ablation in patients with coronary cusp ventricular arrhythmia. Circ Arrhythm Electrophysiol 2013, 6, 898–904. [Google Scholar] [PubMed]
- Szili Torok, T.L.; Ozcan, E.E.; Hasdemir, C.; Kis, Z.; Kardos, A.; Geczy, T.; Kovacs, I.; Benedek, I.; Oosterwerff, E.; Hendriks, A.A.; et al. Disappearance of Idiopathic Outflow Tract Premature Ventricular Contractions After Catheter Ablation of Overt Accessory Pathways. J Cardiovasc Electrophysiol 2017, 28, 78–84. [Google Scholar]
- Latchamsetty, R.; Bogun, F. Premature Ventricular Complexes and Premature Ventricular Complex Induced Cardiomyopathy. Curr Probl Cardiol 2015, 40, 379–422. [Google Scholar]
- Brooks, R.; Burgess, J.H. Idiopathic ventricular tachycardia. A review. Medicine (Baltimore) 1988, 67, 271–294. [Google Scholar]
- Joshi, S.; Wilber, D.J. Ablation of idiopathic right ventricular outflow tract tachycardia: current perspectives. J Cardiovasc Electrophysiol 2005, 16, S52–S58. [Google Scholar] [CrossRef]
- Tada, H.; Ito, S.; Naito, S.; Kurosaki, K.; Kubota, S.; Sugiyasu, A.; Tsuchiya, T.; Miyaji, K.; Yamada, M.; Kutsumi, Y.; et al. Idiopathic ventricular arrhythmia arising from the mitral annulus: a distinct subgroup of idiopathic ventricular arrhythmias. J Am Coll Cardiol 2005, 45, 877–886. [Google Scholar] [CrossRef]
- Tada, H.; Tadokoro, K.; Ito, S.; Naito, S.; Hashimoto, T.; Kaseno, K.; Miyaji, K.; Sugiyasu, A.; Tsuchiya, T.; Kutsumi, Y.; et al. Idiopathic ventricular arrhythmias originating from the tricuspid annulus: Prevalence, electrocardiographic characteristics, and results of radiofrequency catheter ablation. Heart Rhythm 2007, 4, 7–16. [Google Scholar]
- Valk, S.D.; de Groot, N.M.; Szili-Torok, T.; Van Belle, Y.L.; Res, J.C.; Jordaens, L. Clinical characteristics and acute results of catheter ablation for outflow tract ventricular tachycardia or premature beats. J Interv Card Electrophysiol 2012, 35, 301–309. [Google Scholar]
- Tandri, H.; Macedo, R.; Calkins, H.; Marcus, F.; Cannom, D.; Scheinman, M.; Daubert, J.; Estes, M.; Wilber, D.; Talajic, M.; et al. Role of magnetic resonance imaging in arrhythmogenic right ventricular dysplasia: Insights from the North American arrhythmogenic right ventricular dysplasia (ARVD/C) study. Am Heart J 2008, 155, 147–153. [Google Scholar] [PubMed]
- Markowitz, S.M.; Weinsaft, J.W.; Waldman, L.; Petashnick, M.; Liu, C.F.; Cheung, J.W.; Thomas, G.; Ip, J.E.; Lerman, B.B. Reappraisal of Cardiac Magnetic Resonance Imaging in Idiopathic Outflow Tract Arrhythmias. J Cardiovasc Electr 2014, 25, 1328–1335. [Google Scholar]
- Boukens, B.J.; Christoffels, V.M.; Coronel, R.; Moorman, A.F. Developmental basis for electrophysiological heterogeneity in the ventricular and outflow tract myocardium as a substrate for life-threatening ventricular arrhythmias. Circ Res 2009, 104, 19–31. [Google Scholar]
- Moorman, A.F.; de Jong, F.; Lamers, W.H. Development of the conduction system of the heart. Pacing Clin Electrophysiol 1997, 20, 2087–2092. [Google Scholar]
- McGuire, M.A.; de Bakker, J.M.; Vermeulen, J.T.; Moorman, A.F.; Loh, P.; Thibault, B.; Vermeulen, J.L.; Becker, A.E.; Janse, M.J. Atrioventricular junctional tissue. Discrepancy between histological and electrophysiological characteristics. Circulation 1996, 94, 571–577. [Google Scholar]
- Santangeli, P.; Hutchinson, M.D.; Supple, G.E.; Callans, D.J.; Marchlinski, F.E.; Garcia, F.C. Right Atrial Approach for Ablation of Ventricular Arrhythmias Arising From the Left Posterior-Superior Process of the Left Ventricle. Circ Arrhythm Electrophysiol 2016, 9. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).