1. Introduction
Antimicrobial textile is the functionalized textiles which inhibit the growth of microorganism during usage. Different methods were used for the preparation of antimicrobial textiles. Hydrophobically treated textile also help in keeping the surface clean. Among various methods using nanoparticles based coatings are very common to impart antimicrobial property to natural as well as synthetic textiles. Silver nanomaterials are very efficient in imparting self cleaning and antimicrobial properties to textiles [
1,
2]. Other than silver many metal oxides and metal nanoparticles like Cu, Au, Zinc and tin have also been used for the development of medical textiles. CuO coated textiles have shown broad spectrum antimicrobial activity [
3]. Nano selenium treated textiles exhibited activity against basicillus sublitis [
4]. Prevention of agglomeration, desired morphology and uniform size of the nanoparticles are very challenging aspects for nanoparticles based antimicrobial treatment of textiles. Meanwhile selection of proper polymer matrices is very crucial for the development of polymer nanocomposites coatings for textiles.
Among various natural products proteolytic enzymes are found to be very interesting class of antimicrobial agents. They can destruct the microorganism structures and chemically degrade the molecules that could favour the adsorption of the microbes on textiles. Application of enzymes is an effective alternative process to impart antimicrobial property to textiles [
5]. As they are natural materials enzymes are environmentally safe to use as antimicrobial agent although microorganisms show diverse procedures to adhere to a substrate. Researchers have shown that common proteases reduce the adhering strength of the microorganisms to a surface and also to textiles [
6,
7,
8,
9].
When virus SARS-CoV-2pandemic happens causing the Corona virus Disease 2019 (COVID-19), there was no specific treatment and gains an unexpected but predictable importance. Recycling non- reusable PPEs with antimicrobial properties has been an interesting area of research, particularly after the market shortage [
10,
11,
12].
Antimicrobial textiles are essential to guard health workers from possible infections. Using silver nanoparticles (AgNPs) on polymeric fiber mats is a easy way to provide antimicrobial properties to textiles [
13,
14], while the antibacterial tests for AgNPs treated PPEs are generally carried out in solution, the performance of treated PPEs in a gaseous environment is uncertain. Organic macromolecules having nitrogen-halogen generally show intense oxidative properties thropugh oxidative halonium ions release in an aqueous environment [
15] and could be used onto fiber mats to impart antimicrobial properties [
16,
17]. N-halamine is one of the most commonly used macromolecule in spite of its weak intrinsic toxicity [
15,
18]. Unlike silver nanoparticles, ion release using N-halamine may take place in humid environment, which can be used for inhalation protection applications. In order to make the antimicrobial textiles bio safe and environment friendly, botanic extracts were used for textile functionalization [
19,
20].
There is no universal alternative for sterilizing textiles used for medical purpose. There are four methods of reprocessing exist namely, thermal, chemical, radiation and energetic [
21,
22]. Thermal treatments are commonly used for virus deactivation since it induces changes in virus protein structure [112].These types of treatments may face scalability problems and deform fabrics [
12,
21].
During this period of pandemic diseases, a contamination-safe PPEs can be designed with the support of nanotechnology. Effective antimicrobial property can be achieved using copper, silver, and zinc nanostructures which have a controlled and long-lasting ionic release. However, frequently in the case of virus, nanoparticles are not able to completely destabilize the protein capsid of the virion. In this situation, the use of proteolytic enzymes which are able to digest the capsid proteins before the virus enters the target cell or to destabilize membrane proteins in bacteria have been successfully tested [
22]. Nanoparticles are also used as carriers for enzyme immobilization and they were successfully employed in redox enzymes immobilization, stabilization, catalytic enhancement for bioelectrochemical sensor devices and biomedical applications [
23,
24]. There are basically two main concepts of antimicrobial surfaces involving nanotechnology: (i) Passive super hydrophobic microscopically rough surfaces that mimic the well-known lotus leaves effect repelling water and microorganisms; (ii) Active surfaces that can actively destabilize or destroy microorganism by metal or metal-oxide nanoparticles or enzymes as nanocatalyst. However, rarely the both strategy are used at the same time and usually nanoparticles and enzymes are not present in the same nanocoatings. Moreover, enzymes incorporation or grafting into and onto metal NPs and textiles to prevent microbial colonization remains an area of experimental need. Using polymer matrices as dispersion medium for these nanoparticles not only imparts hydrophobicity and antiviral properties but also permanently bind these nanoparticles with textile surfaces.
Development of these multifunctional super hydrophobic and in-situ self-cleaning, antimicrobial and antiviral textiles with nanoparticles immobilized with proteolytic enzymes is a step ahead of the current state-of-the-art. The interdisciplinary research area combining nanotechnology, biotechnology and textile science is still in its initial stages. To our knowledge the approach proposed in this paper is unique and there is need to study the properties and performance of such bionanocomposites.
2. Materials and Methods
The metal oxide precursor, zinc acetate dihydrate (Zn (C2H3O2)2.2H2O), was purchased from Sigma-Aldrich, a-Chymotrypsin (from bovine pancreas), was obtained from Sigma Chemical Co. Poly (methyl methacrylate)(PMMA) (MW 996 kDa), was obtained from Aldrich. Commercial grade reagents and solvents were used.
Green Synthesis of ZnO Nanoparticles
To prepare neem leave extract 20gm dried neem leaves were crushed and added to 50 ml deionised water. The mixture was stirred in a magnetic stirrer for 1 hour at 60 °C. When the mixture became yellow colour, it was filtered. ZnO nanoparticles were prepared by using this extract. Further 21.94 gm of Zn(CH3COO)2.2H2O was added to 50 ml deionised water and kept under stirring for 20 minutes at 35⁰ C. 4 gm of sodium hydroxide was mixed with 50 ml deionised water and kept under stirring for 20 minutes at 35 ⁰C separately. Both the solutions were stirred well in a stirrer. The neem leaf extract was added drop by drop, while stirring. The product obtained was filtered and dried at 80 ⁰C for 4h and then calcined at 250⁰ C for 4h. After calcination, the product was ground well to get ZnO nanoparticles.
Amino-Functionalization of ZnO Nanoparticles
Hydrothermal Method: Amine-functionalized zinc oxide nanoparticles were prepared by hydrothermal method. 200 mg ZnO NPs were mixed in 50 mL dimethylformamide (DMF) and then sonicated for 30 minutes for complete dispersion of ZnO NPs. Subsequently, 1 g urea was mixed in the solutions and sonicated for another 10 minutes. Further, the mixed solutions were transferred in a Teflon-lined stainless-steel autoclave. The urea was used to functionalize the amine group on the ZnO NPs surface. Afterward, the solution was heated at 200 °C for 24 h. When the reaction was completed, they were cooled at room temperature and washed three times using milli-Q water and dried at 25 °C. Subsequently, the washed NPs were equilibrated with 0.1 M phosphate buffered (pH 7.4) and incubated with 1 mL of 25% glutaraldehyde for 2 hours at room temperature with continuous stirring. After the incubation, the NPs were washed several times with milli-Q water and dried at 60 °C for 12 h.
Chemical Method: Amine-functionalization of ZnO nanoparticles was carried out by a chemical method. 200 mg ZnO NPs were mixed in 25 mL dimethylformamide (DMF) and then sonicated for 30 minutes for complete dispersion of ZnO NPs. Subsequently, 1 g urea was mixed in the solutions and sonicated for another 10 minutes. The urea was used to functionalize the amine group on the ZnO NPs surface. Afterwards, the solution was stirred at 200 °C for 24 h on a magnetic stirrer. When the reaction was completed, it was cooled to room temperature, then washed three times using milli-Q water and dried at 25 °C. Subsequently, the washed NPs were equilibrated with 0.1 M phosphate buffered (pH 7.4) and incubated with 1 mL of 25% glutaraldehyde for 2 hours at room temperature with continuous stirring. After the incubation, the nanoparticles were washed with milli-Q water and dried at 60 °C for 12 h.
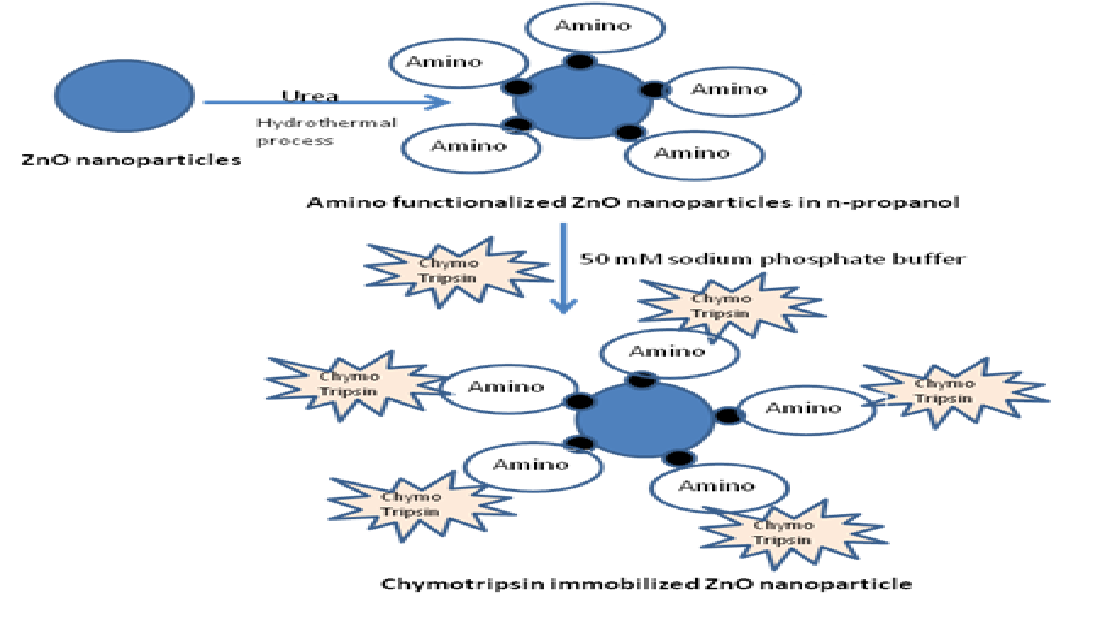
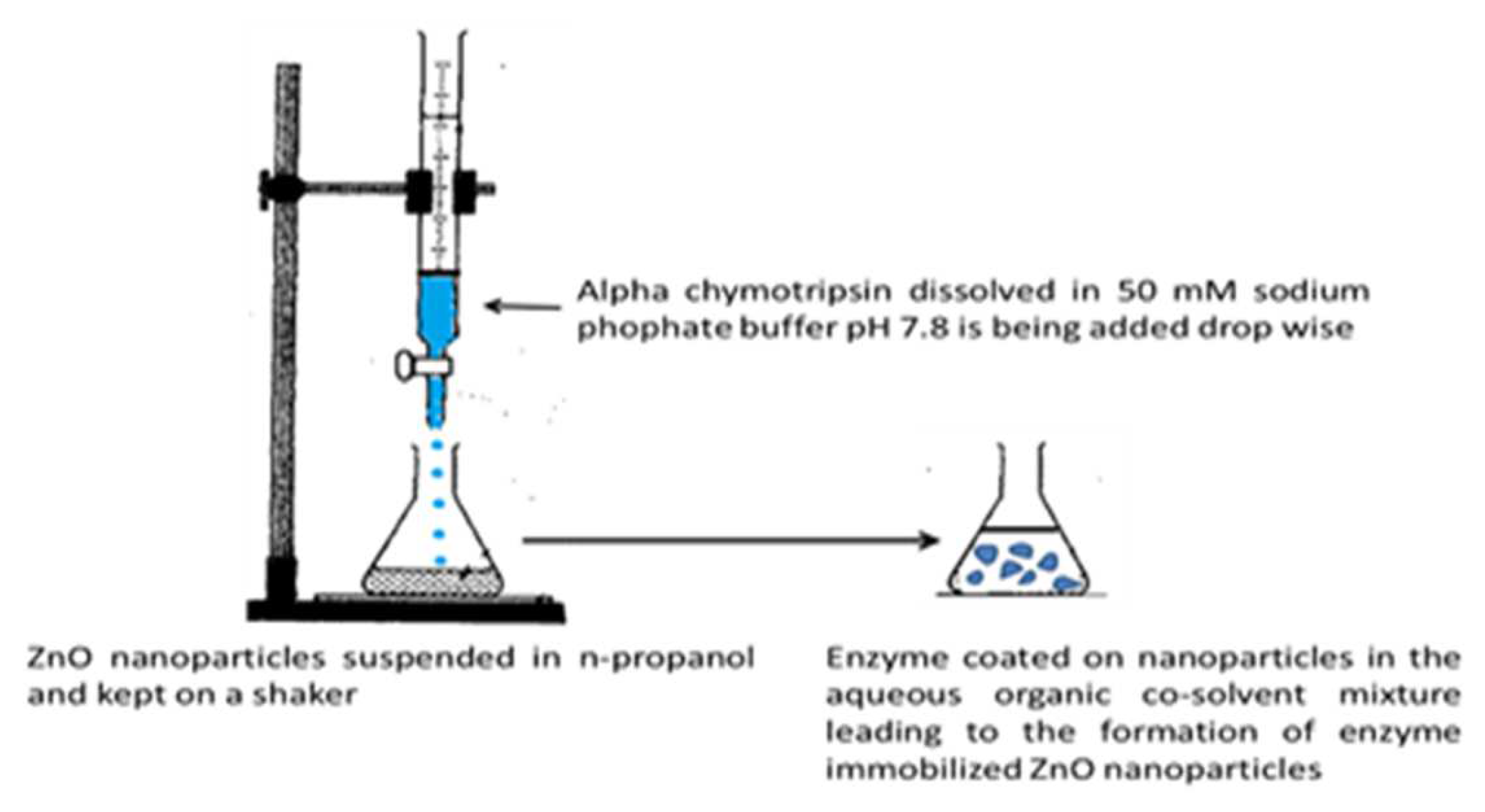
Immobilization of alpha chymotripsin on ZnO nanoparticles: Precipitation of alpha chymotripsin over ZnO nanoparticles was carried out by mixing with an organic solvent by Dyshlyuk et.al
25. The precipitation method is outlined in
Figure 1. This processes minimizes enzyme inactivation by non covalent approaches. The reasons for selecting Alpha chymotrypsin are: (1) It has been widely used for diversified application in organic media like, peptide synthesis. (2) It has antimicrobial properties. To prepare an uniform suspension of enzyme immobilized nanoparticles, A weighed amount of ZnO nanoparticles was taken in avial containing a fixed volume of n-propanol and placed a shaker. The alpha chymotrypsin solution was prepared with buffer and added to the above suspension (
Figure 1). The mixing was done by adding of aqueous buffer to the organic solvent. This mode favours retention of original structure of alpha chymotrypsin.
26 This method helps is to incorporate enzyme uniformly over the nanoparticles. Thsese enzyme immobilised ZnO nanoparticles were filtereds and used for making polymer nanocomposites with polymethyl methacrylate(PMMA).
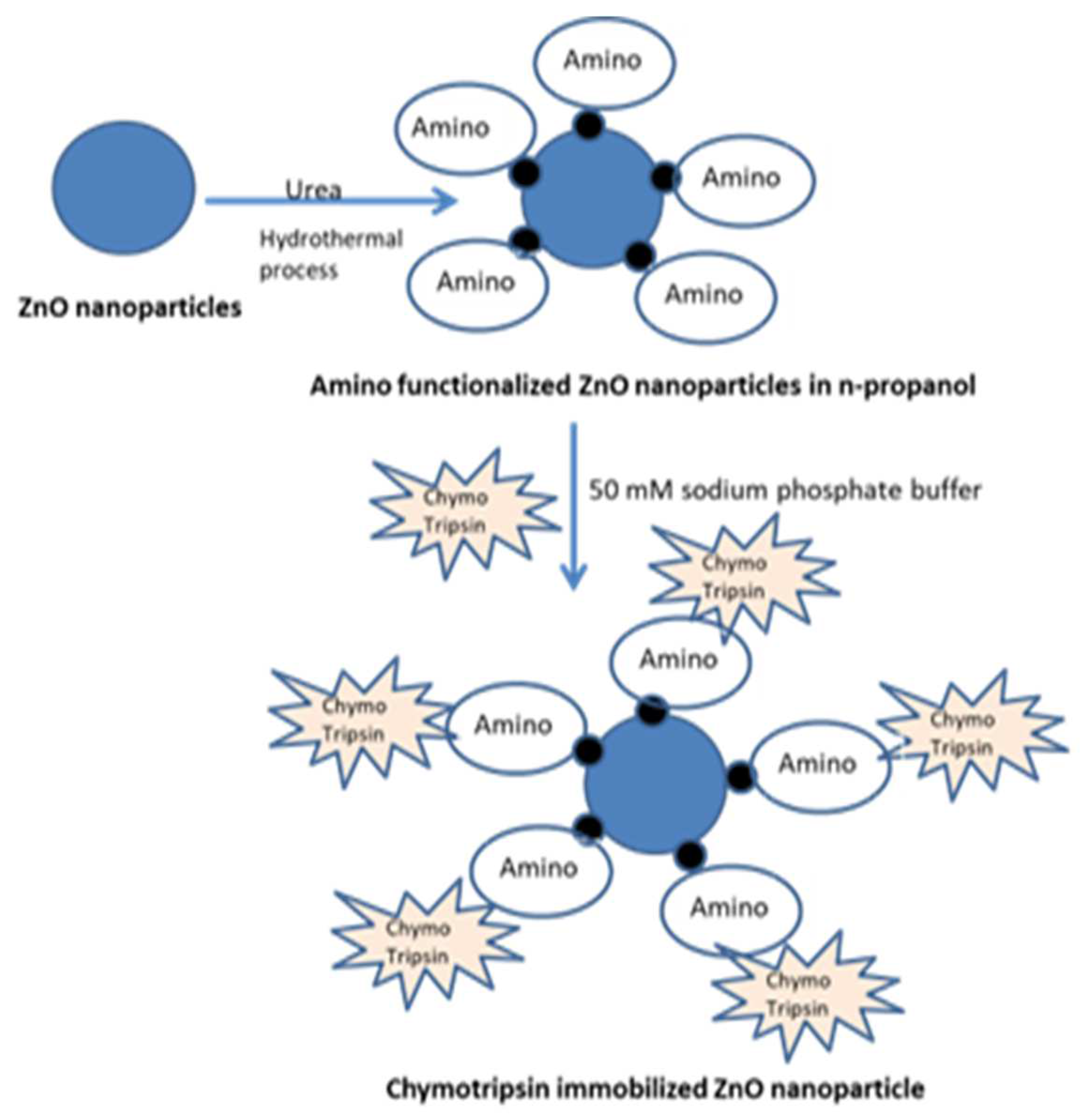
Follwing
Figure 2 shows the schematic representation of enzyme immobilasation on amonio modified ZnO naoparticles
Preparation of enzyme-ZnO/PMMA polymer nanocomposites coated cotton fabric: cotton fabric was cleaned by washing the fabric with detergent and drying in the air before use.
Coating the cotton fabric with enzyme- ZnO–PMMA
5% stock solution of PMMA was prepared by dissolving (PMMA) in toluene. Zinc oxide nanoparticles of different percentages (0.1, 0.5, and 1) were mixed with PMMA. A homogeneous solution of the mixture was obtain by stirring it well in a stirrer. The fabric was cut into size of 30 X 30 cm and dipped in nano ZnO solutions with different concentrations of ZnO and kept under ultrasonic vibrations for 15 min and air dried. Then the fabric was rinsed with deionised water and air dried again.
Testing for Antimicrobial Properties of Treated Textiles.
The treated cotton fabric with ZnO/ PMMA polymer nanocomposite was evaluated for antibacterial activity at BTRA, Mumbai as per JIS L 1902-2015 Method against Staphylococcus aureus by ATCC 6538 and against Klebsiella pneumoniae by ATCC 4352.
Antibacterial Activity of Fabrics Assessment of Textile Materials–Parallel Streak Method- AATCC 147
To assess antimicrobial activity of the coated fabrics by Parallel Streak Method- AATCC 147; the coated fabrics was cut in to samples of size 25mmX 50mm. The test was conducted in Nutrient Agar media. The test organisms were Staphylococcus aureus ATCC 6538 and Klebsiella pneumoniae (ATCC 4352). The incubation of the textile samples was carried out at 37 ⁰C for 24 Hrs and antimicrobial activity was assessed.
Antimicrobial Testing by Plate Count Method:
For quantitative measurement the samples were weighed to 0.4± 0.05 g. Tryptone Soya Agar was used as medium. The test organisms were Staphylococcus aureus ATCC 6538 and Klebsiella pneumoniae ATCC 4352. The incubation conditions were37°C for 24-48 hrs. The samples were sterilised by autoclaving at 120⁰C for 15 minutes. Physiological saline was used as diluent. The culrure density was 1-3x105 CFU/ml.
Characterization of ZnO Nanoparticles Enzyme-ZnO/PMMA Coated Cotton Fabric
FT-IR anaalysis:
FT-IR spectroscopic analysis was carried out with Nicolet Avatar 360 spectrophotometer for the chemical analysis of the prepared nanoparticles ZnO and ZnO–PMMA.
A high-resolution transmission electron microscope (HR-TEM) (Make: JEOL, Model: JEM-F200) was used for morphological analysis. The size and morphology of microscopy images of ZnO, amino functionalized ZnO were observed. HR-TEM samples were prepared by drop-casting 6 μl of nanoparticles solution onto carbon-coated copper grids. The developed film was dried at room temperature.
X- ray difractometer Analysis:
X-ray diffractometer (Model: Bruker D8 Discover) was used for XRD pattern analysis of nanoparticles using Cu Ka radiation at wavelength k = 0.15406 nm. The scan range was 2h = 20–90, at scanning rate = 0.02 deg/s (applied voltage 40 kV, current 20 mA).
Contact angle measurement:
Contact angle measurements were carried out in in Automatic Contact Angle Meter DMs-400, Kyowa Interface Science Co-Ltd, Japan using distilled water. Water droplets (5 µl) were delivered to different points of the textile specimen and at a height close to the textile surface. The height of the needle was adjusted in a such a way the needle remain in contact with the water drop. After withdrawing the needle, the image of the drop was captured to measure the static contact angle.
3. Results
X- Ray Diffraction Analysis of ZnO Nanoparticles
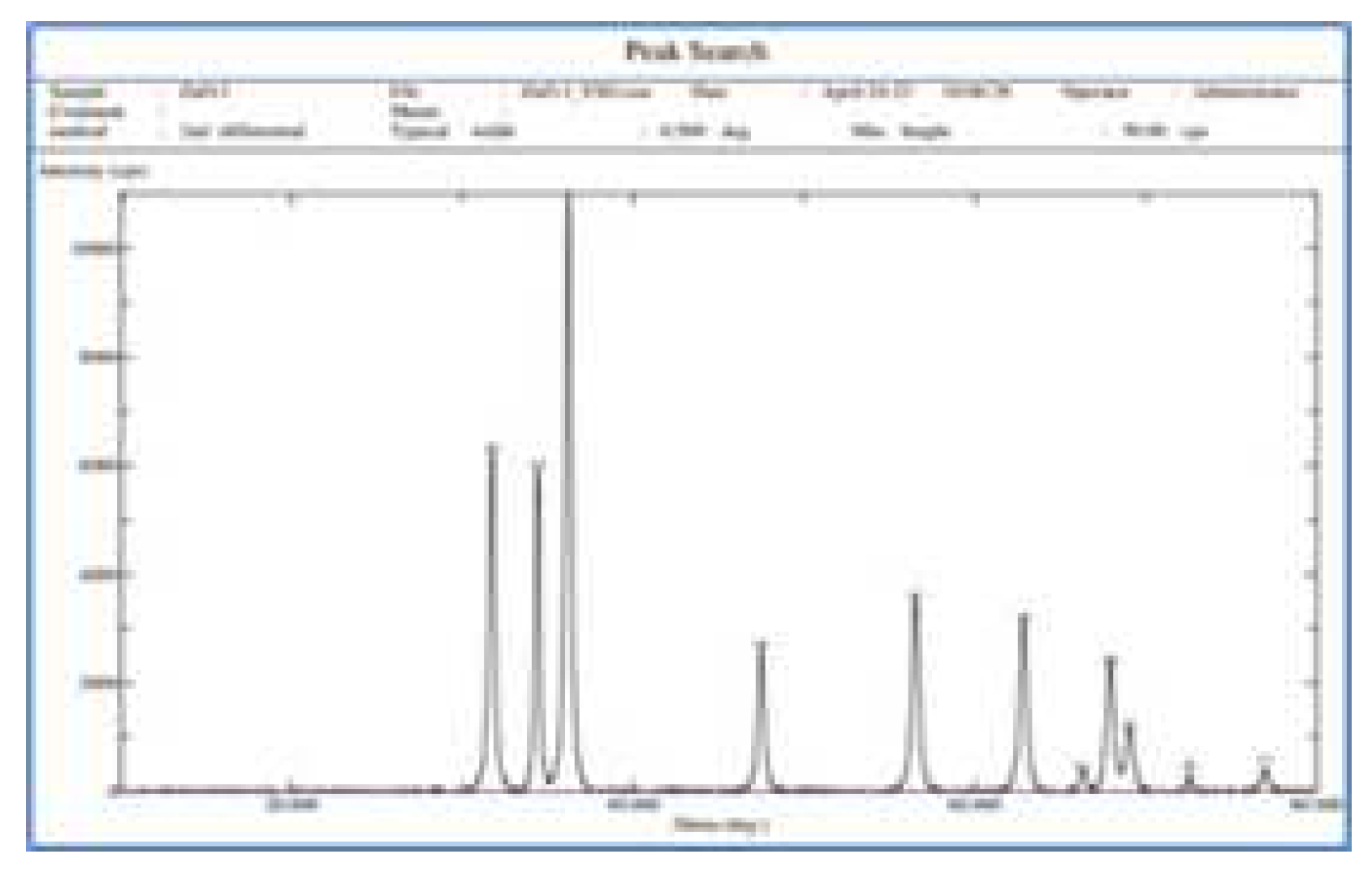
XRD spectrum of the ZnO nanoparticles (
Figure 3) showed typical peaks of Zincite in ZnO having crystal structure. The diffractogram of ZnO nanoparticles have shown characteristic peaks of crystalline zinc oxide nanoparticles with 2θ° values: 31.8, 33.9, 36.8, 47.9, 57.9, 62.8, 66.4, 67.9, 68. 72.9, and 79.9.
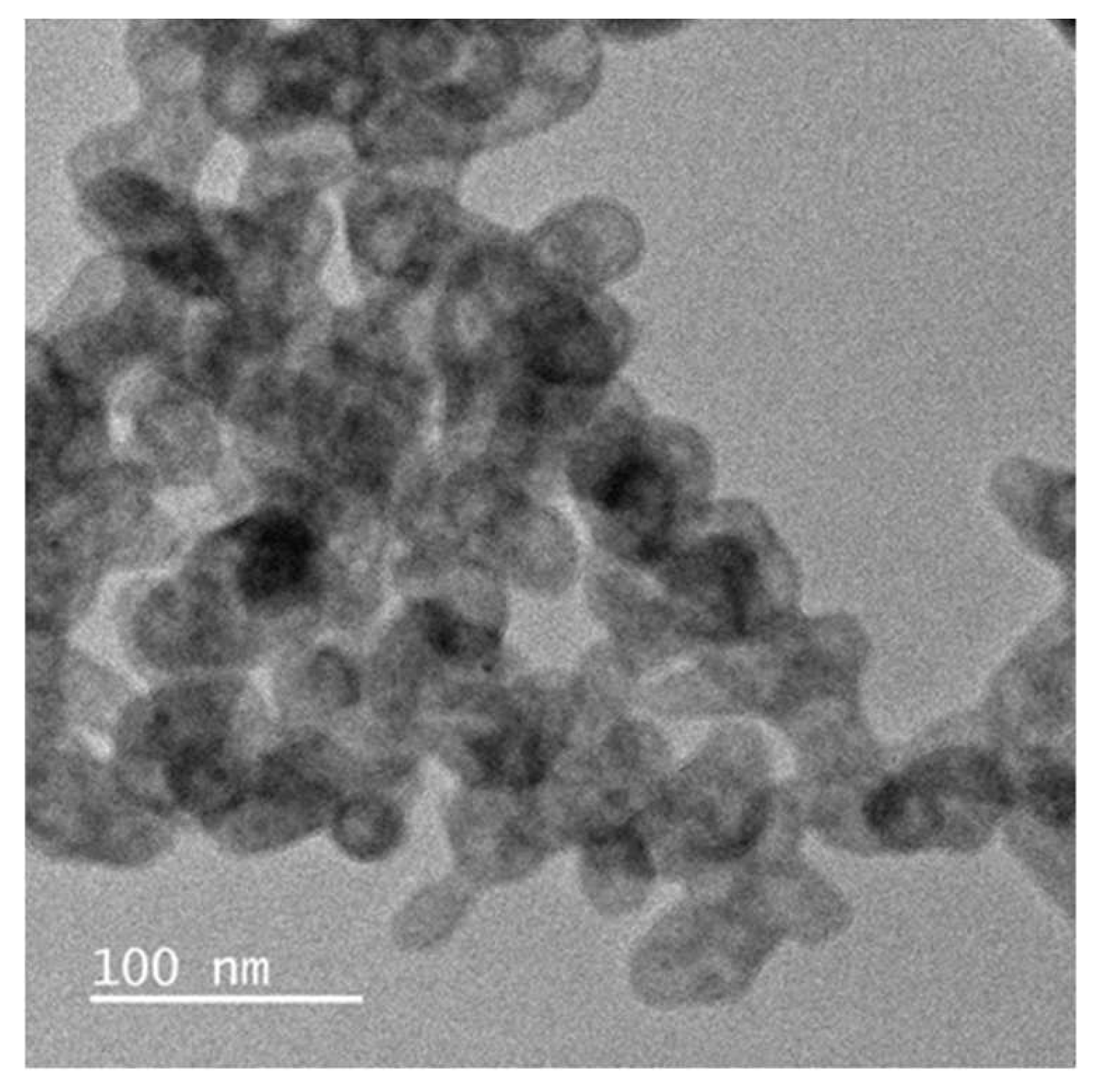
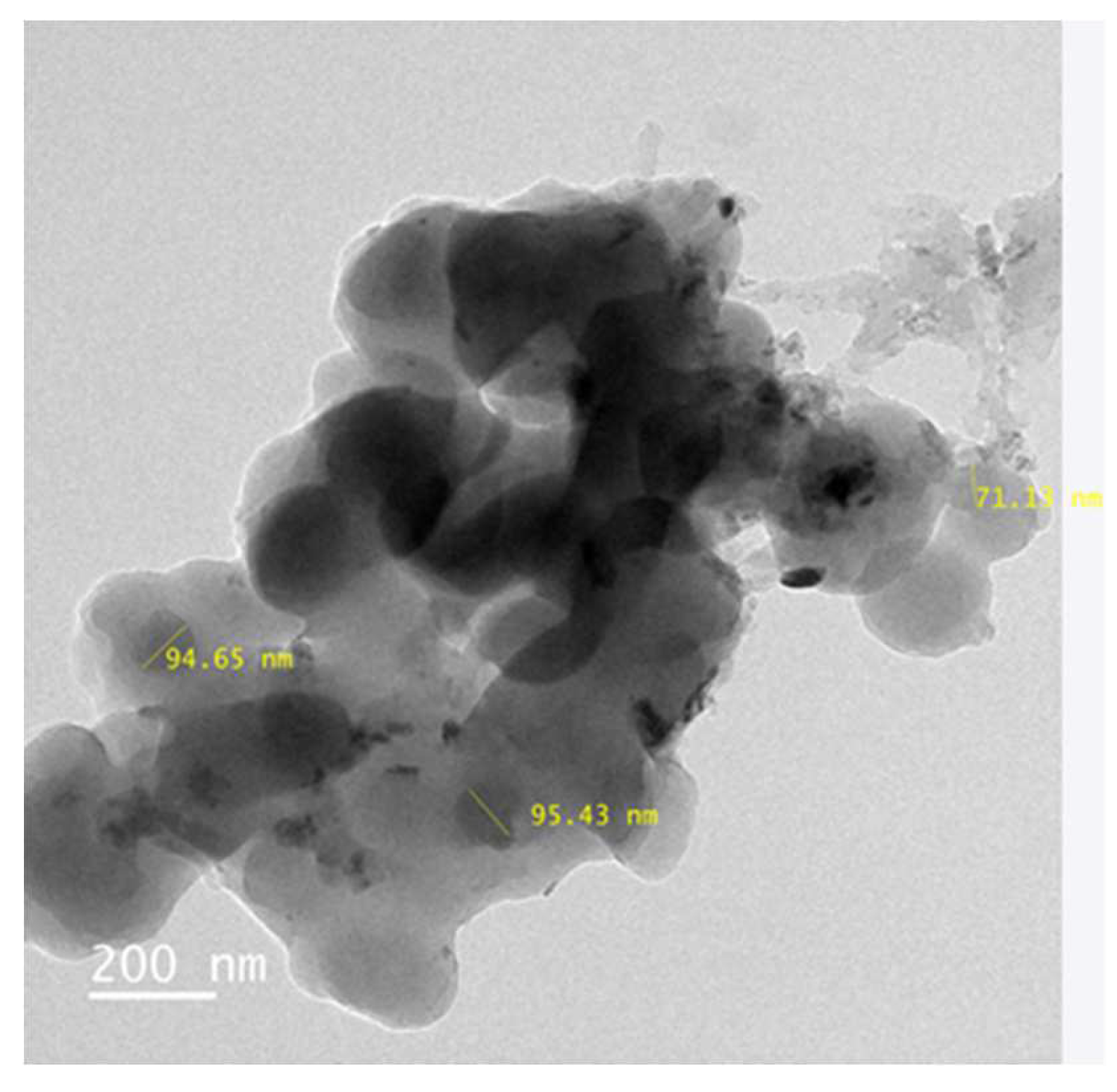
The TEM micrographs (Figure.4) showed the Zinc Oxide nanoparticles were in network form. The TEM results are supported by XRD analysis. It has been observed that the synthesized Zinc Oxide nanoparticles were in spherical shape with 40 to 65 nanometers size.
FTIR Analysis of ZnO Nanoparticles
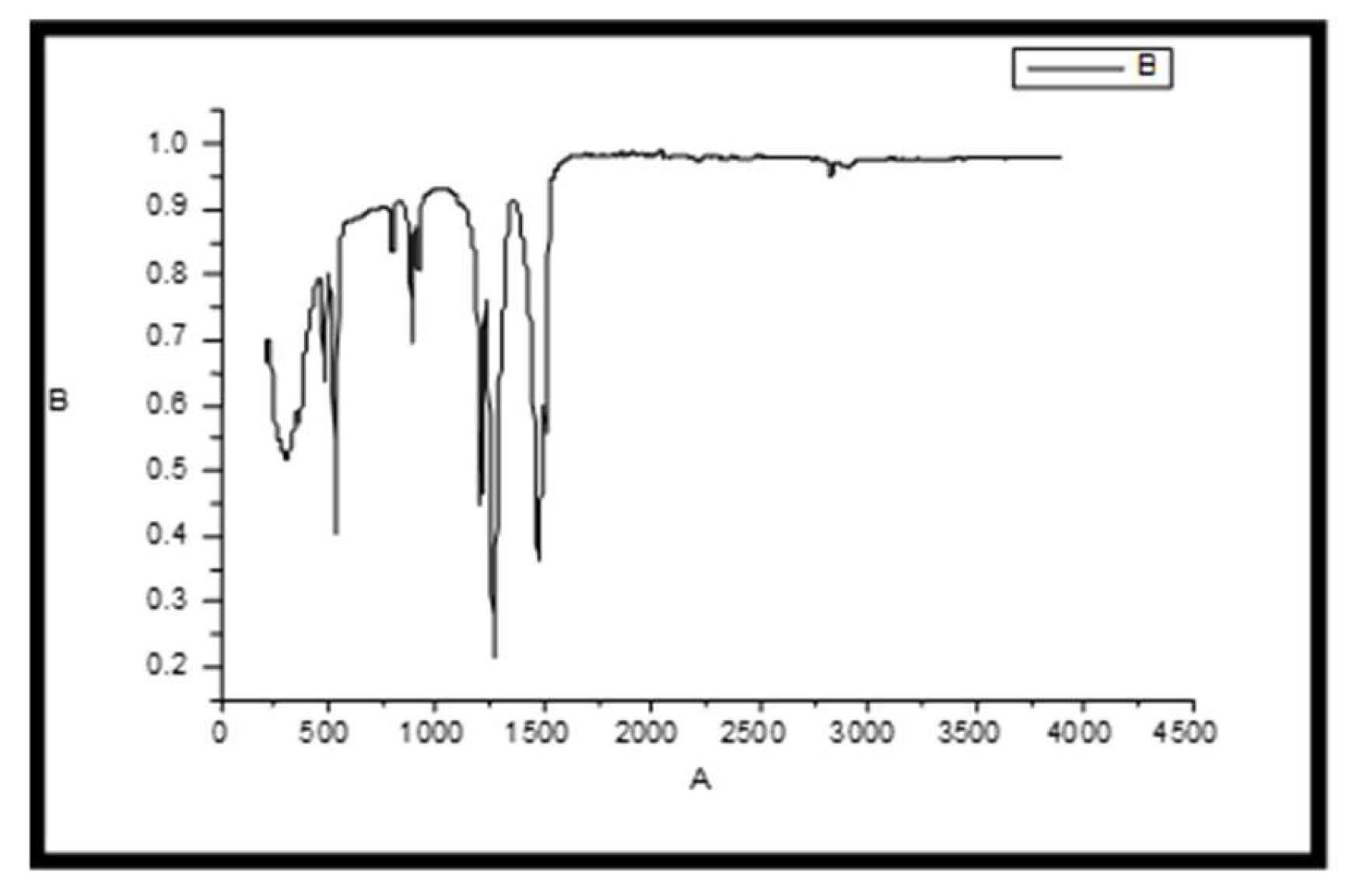
The FT-IR spectroscopy is very useful to for the structural analysis compounds. The FT-IR spectrum of the synthesized nano ZnO (
Figure 5).
Amine-functionalization of ZnO Nanoparticles has been carried out by both chemical ad hydrothermal method.
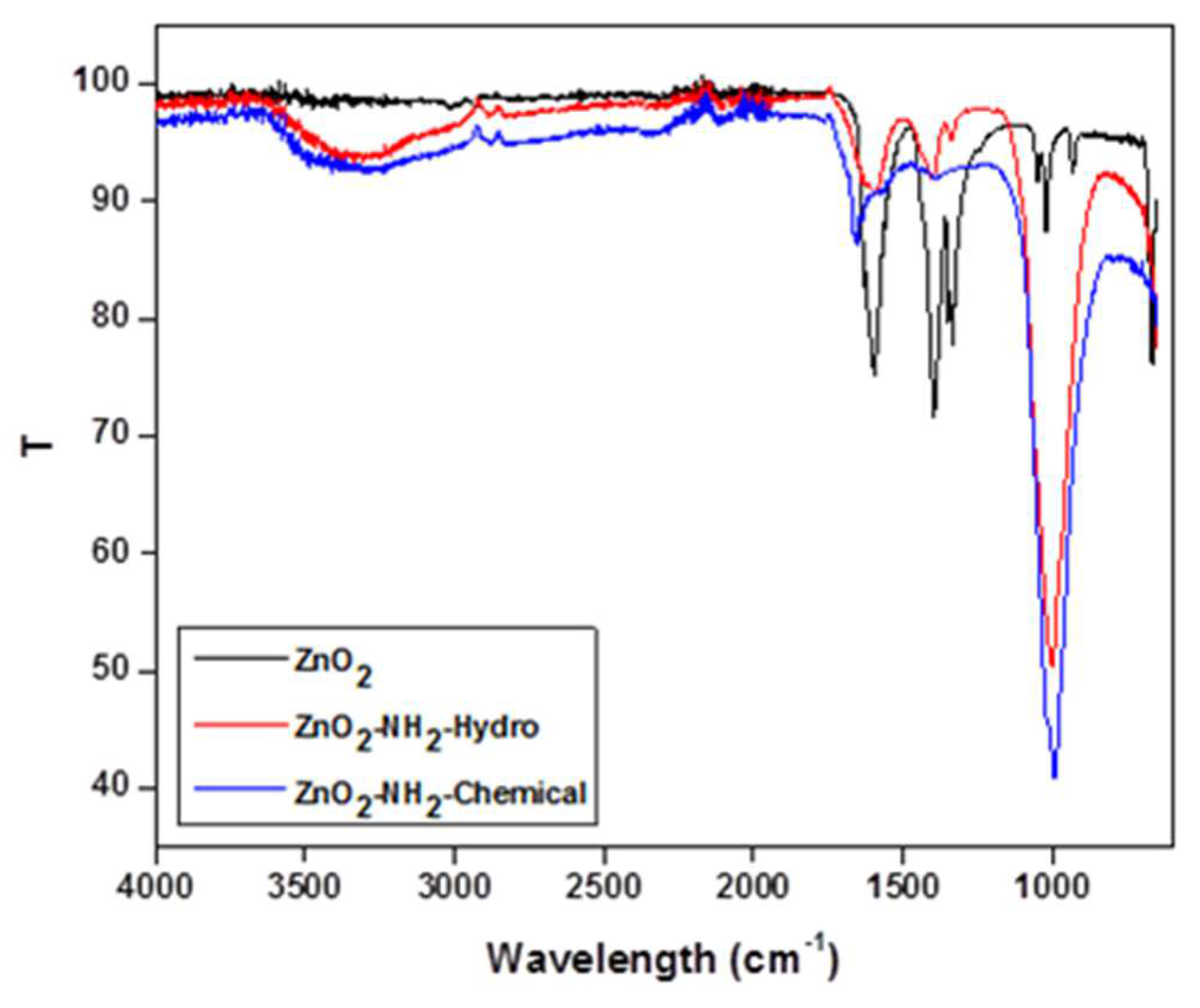
Successful Amino functionalisation of ZnO nanoparticles was confirmed by FTIR spectra as shown in
Figure 6.
TEM Analysis of Amino modified ZnO Nanoparticles
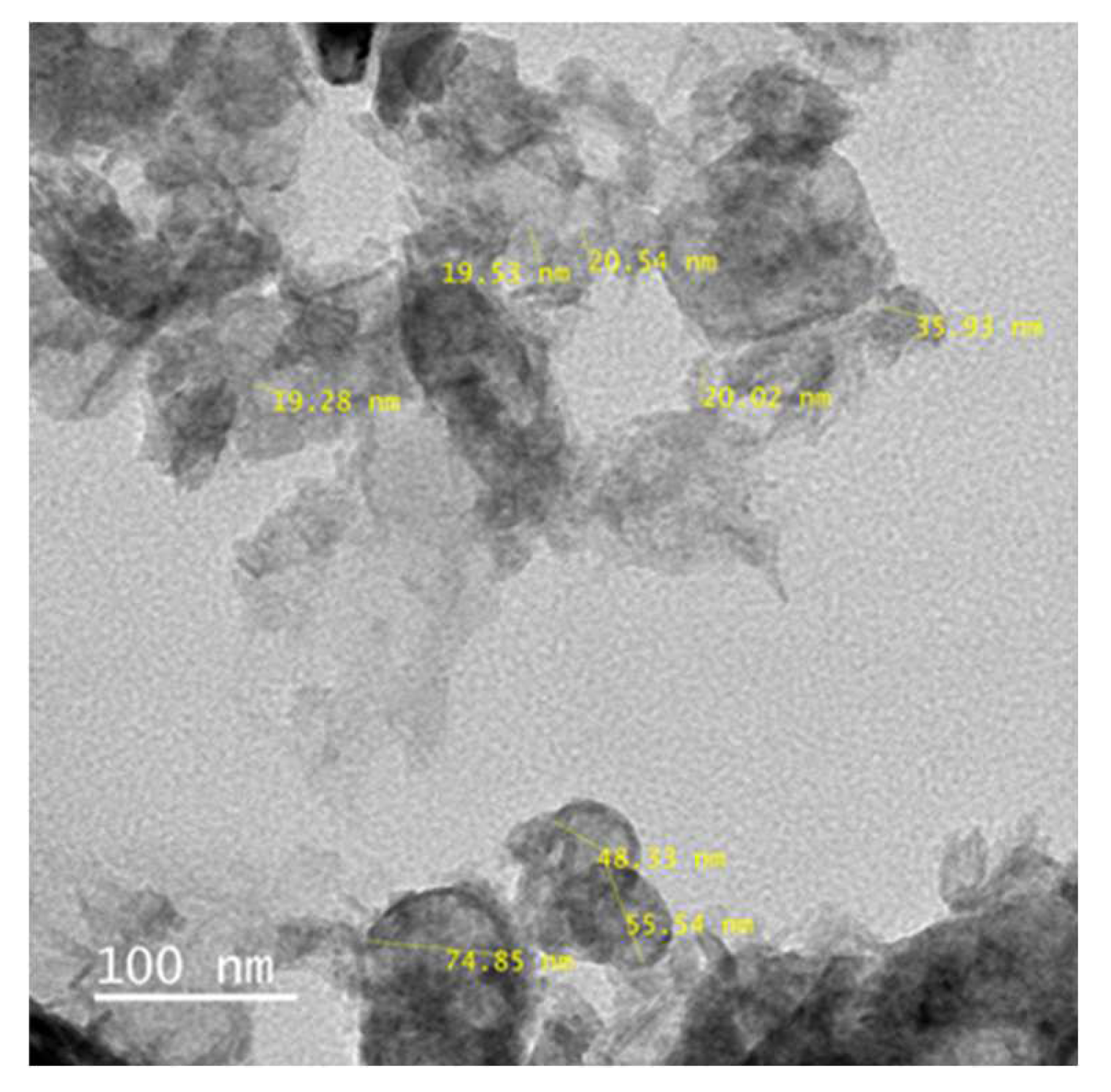
Figure 7 and
Figure 8 show the TEM analyisis of ZnO prepared by both the chemical and hydrothermal mehod. Both the products were compared for their shape and size distribution.
Antimicrobial Testing of Treated Textile Samples
Cotton fabric coated with 1% ZnO/PMMA and enzyme -ZnO/PMMA polymer nanocomposites coatings were evaluated for antibacterial activity value tested at BTRA, Mumbai against Staphylococcus aureus by ATCC 6538 and against Klebsiella pneumoniae by ATCC 4352 as per JIS L 1902-2015 Method.
The sample in contact with individual test organisms were kept under observation for 18-24 hrs and the results by Parallel Streak Method are given in
Table 1 and
Table 2. The antimicrobial activity results by Plate Count Method are given in
Table 3 and
Table 4.
P.S.
| Antibacterial Activity value : A |
Efficacy of antibacterial property |
| 2<A<3 |
Effect |
| A>3 |
Full Effect |
4. Discussions
The XRD spectrum values showed that the crystallinity of the synthesized ZnO. The broad diffraction maxima showed size of the crystallites are very small similar to the size of ZnO nanoparticles observed by the SEM. These peaks obtained were in well agreement with those reported in the literature and also very well consistent with the JCPDS file card number 01-075-0576, indicated that the synthesized nanoparticles were identical to hexagonal phase of ZnO. Particle size plays important role in peak broadening in XRD analysis.
The FT-IR spectrum of Zinc oxide showed absorption band aound 438 cm-1. Peaks at 1635 cm−1 were due to the bending vibrations of water molecules. Bandwidth at 1500–1600 cm−1 represent the fingerprint region of ZnO nanoparticles. A small peak at 1650 cm−1 was due the N-H bending and weak peaks. Symmetric and asymmetric vibration of C=O showed peak at1508 cm−1 and 1641 cm−1. The broad peak around 3400 cm−1 was assigned to - OH stretching bond vibration which may be due to adsorption of water on the surface of Zinc Oxide.
Successful Amino functionalisation of ZnO nanoparticles was confirmed by FTIR spectra. (Blue line) represents the FTIR spectra of amino functionalized Zinc oxide nanoparticles by chemical method with characteristic peaks at 3400 cm
−1, 1632 cm
−1. Bandwidth at 1500–1600 cm
−1 represent the fingerprint region of ZnO nanoparticles. Symmetric and asymmetric vibration of C=O showed peak at1508 cm
−1 and 1641 cm
−1. The weak peaks at 1400 cm
−1 and 1462 cm
−1 were due to O–H and N–H bending, showing less amount of N–H group. The red line represents FTIR spectra of amine functionalized ZnO NP, prepared by hydrothermal method. It shows the presence of O–H stretching frequency along with –N–H stretching frequency of NH
2 group at 3373 cm-1. Presence of a peak at 2930 cm−1 was due to the asymmetric C–H stretching frequency of the CH
2 group of APTES fragment used for amino functionalisation. Peaks at 1580 cm
−1 and 1470 cm
−1 were due to NH
2 scissoring of primary amine. A clear peak at 1411 cm
−1 was due to low intensity –O–H bending. A peak between 2800 and 3800 cm
-1 includes the symmetric stretching vibration of primary amino group(3250-3450 cm
-1). and 1025 cm
-1 sample showed new peak at about 2928 cm
-1. The NH
2 - ZnO peak at 1630 cm
-1 shifted to 1548 cm
-1 for bending mode of N-H bond. Amino functionalisation by hydrothermal process showed very distinct peaks when compare to the aminofunctionalised ZnO by chemical method. So in the present study amonifunctionalised ZnO by hydrothermal process was used in the further studies. While comparing TEM analysis of both the products, ZnO nano particles by both the amino modification process, the modification by hydrothernal process (
Figure 7 and
Figure 8) maintain the shape and size, when compare to the ZnO amino modified by Chemical method. In both the methods the avearage particle size is maintained within 100nm. Aminomodified ZnO by hydrothermal process was used for further studies.
While studying the antimicrobial properties, in qualitative analysis by parallel streak method, ZnO/PMMA coated Fabric showed Antibacterial Activity against Staphylococcus aureus ATCC 6538 and Klebsiella pneumoniae ATCC 4352, when tested according to AATCC 147. Chymotrypsin immobilized ZnO / PMMA coated fabric showed Antibacterial Activity against Staphylococcus aureus ATCC 6538 and Klebsiella pneumoniae ATCC 4352, when tested according to AATCC 147. In quantitative analysis by plate count method, ZnO /PMMA coated textile showed Antibacterial Activity value of 2.98 against Klebsiella pneumoniae ATCC 4352 and that of 5.76 against Staphylococcus aureus ATCC 6538, when tested as per JIS L 1902 Method.: The sample Chymotrypsin immobilized ZnO/PMMA coated textile showed Antibacterial Activity value of 3.20 against Klebsiella pneumoniae ATCC 4352 and that of 6.52 against Staphylococcus aureus ATCC 6538, when tested as per JIS L 1902 Method. Chymotrypsin immobilized ZnO/PMMA coated textile showed full effect against Klebsiella pneumoniae ATCC 4352 and Staphylococcus aureus ATCC 6538 compared to untreated ZnO/PMMA coated textile samples.
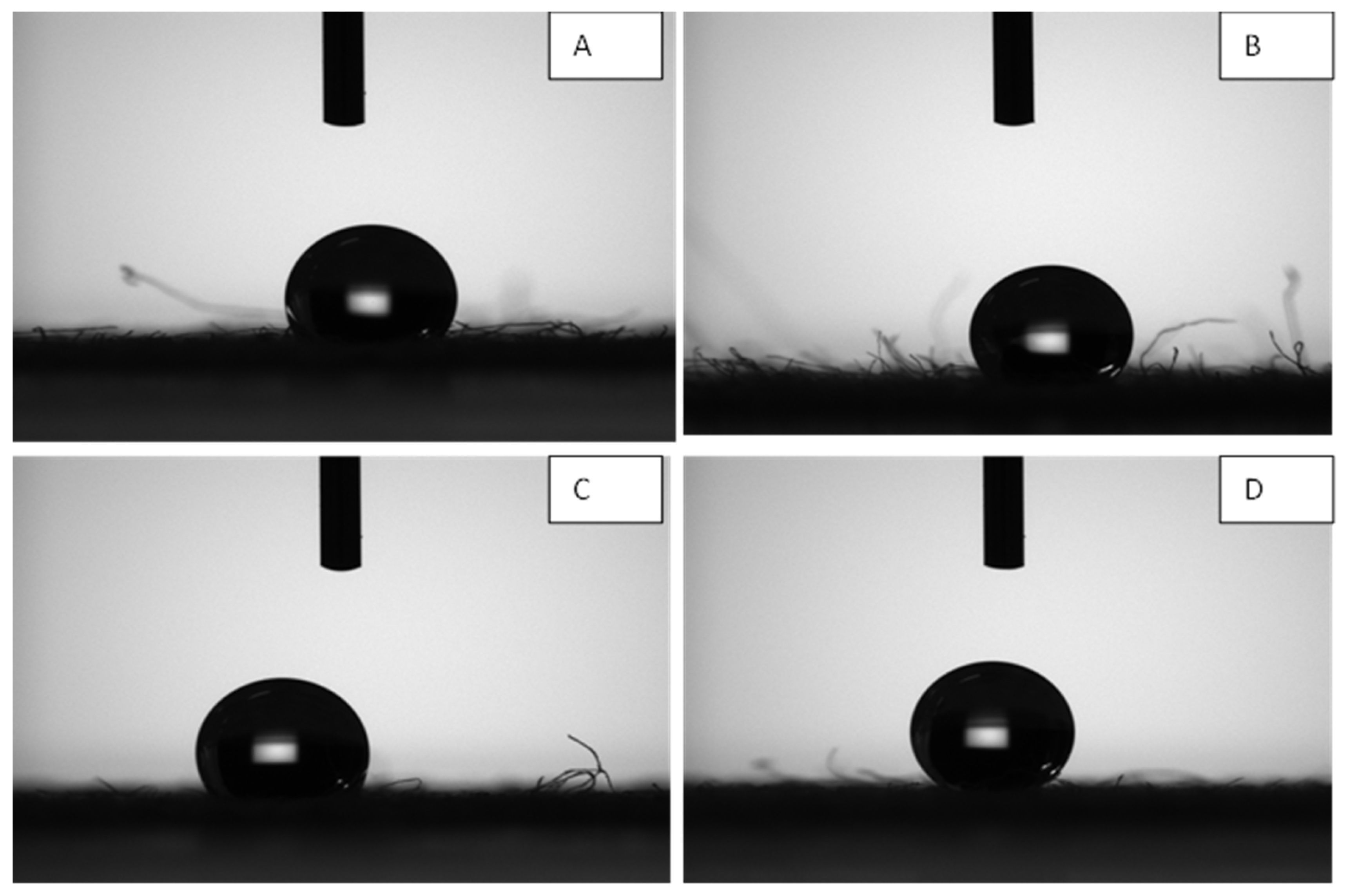
The values of contact angle for enzyme-ZnO/PMMA treated cotton fabric are 138.7, 138.6,143.8, and 144.9 for 0.2, 0.5, 0.8, and 1% of enzymes immobilized ZnO nanoparticles concentration respectively. The contact angle values of ZnO/PMMA coated textile samples are 134.1,135.6,136,136.6 for 0.2, 0.5, 0.8, and 1% (
Figure 9 and
Figure 10) ZnO nanoparticles concentration respectively The contact angle values of ZnO/PMMA coated were slightly lesser than the enzyme immobilized ZnO/PMMA coated textile samples, which may be due to hydrophobic nature of the chymotripsin, but still both the textiles samples showed significant hydrophobicity due to higher contact angle values. Both the ZnO/PMMA and enzyme immobilized ZnO/PMMA treated fabrics showed linear relationship in contact angle values.
5. Conclusions
The contact angle for water on the fabric coated with ZnO./PMMA coating and enzyme-ZnO/PMMA coatings increased with increase of the ZnO nanoparticles concentration in both the coatings and they found to be hydrophobic. The contact angle values of ZnO/PMMA coated were slightly lesser than the enzyme immobilized ZnO/PMMA coated textile samples, which may be due to the hydrophobic nature of the chymotripsin, but still both the textiles samples showed significant hydrophobicity. In qualitative analysis by parallel streak method, ZnO/PMMA coated Fabric showed Antibacterial Activity against Staphylococcus aureus. and Klebsiella pneumoniae. Chymotrypsin immobilized ZnO / PMMA coated fabric also showed Antibacterial Activity against Staphylococcus aureus and Klebsiella pneumoniae. In quantitative analysis by plate count method, ZnO /PMMA coated textile showed Antibacterial Activity value of 2.98 against Klebsiella pneumoniae and 5.76 against Staphylococcus aureus. and Chymotrypsin immobilized ZnO/PMMA coated textile showed Antibacterial Activity value of 3.20 against Klebsiella pneumoniae and 6.52 against Staphylococcus aureus. Chymotrypsin immobilized ZnO/PMMA coated textile showed full effect against Klebsiella pneumoniae and Staphylococcus aureus compared to untreated ZnO/PMMA coated textile samples. The results showed that coated textile with polymer nanocomposites with enzyme immobilized ZnO has shown good hydrophobicity and enhanced antimicrobial properties. These results offer exciting prospects for the development of health care products such as personal protective equipment (PPEs), Sanitary napkins and wound dressings.
Funding
This research was funded by the Department of Science and Technology (DST), India and The Japan Society for the Promotion of Science (JSPS), Japan, grant number DST/INT/JSPS/P-370/2023(G).
References
- Shahid-Ul-Islam, S.-U.; Butola, B.S.; Mohammad, F. Silver nanomaterials as future colorants and potential antimicrobial agents for natural and synthetic textile materials. RSC Adv. 2016, 6, 44232–44247. [Google Scholar] [CrossRef]
- Jeremiah, S.S.; Miyakawa, K.; Morita, T. Potent antiviral effect of silver nanoparticles on SARS-CoV-2. Biochem Biophys Res Commun. 2020, 533, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Román, L.E.; Gomez, E.D.; Solís, J.L.; Gómez, M.M. Antibacterial Cotton Fabric Functionalized with Copper Oxide Nanoparticles. Molecules 2020, 25, 5802. [Google Scholar] [CrossRef] [PubMed]
- Sadalage, P.S.; Nimbalkar, M.S.; Sharma, K.K.K.; Patil, P.S.; Pawar, K.D. Sustainable approach to almond skin mediated synthesis of tunable selenium microstructures for coating cotton fabric to impart specific antibacterial activity. J. Colloid Interface Sci. 2020, 569, 346–357. [Google Scholar] [CrossRef]
- Olsen, S.M.; Pedersen, L.T.; Laursen, M.H.; Kiil, S.; Dam-Johansen, K. Enzyme-based antifouling coat-ings: a review. Biofouling. 2023, 5, 369–383. [Google Scholar]
- Pettitt, M.E.; Henry, S.L.; Callow, M.E.; Callow, J.A.; Clare, A.S. Activity of commercial enzymes on settlement and adhesion of Cypris larvae of the barnacle Balanus amphitrite, spores of the green alga Ulva linza, and the Diatom Navicula perminuta, Biofouling. 2020,6, 299-311.
- Aldred, N.; Phang, I.Y.; Conlan, S. L.; Clare, A.S. The effects of a serine protease, Alcalase, on the ad-hesives of barnacle cyprids (Balanus amphitrite), Biofouling. 2024,2, 97-107.
- Caro, A.; Humblot, V.; Méthivier, C.; Minier, M.; Salmain, M.; Pradier, C.-M. Grafting of Lysozyme and/or Poly(ethylene glycol) to Prevent Biofilm Growth on Stainless Steel Surfaces. J. Phys. Chem. B 2009, 113, 2101–2109. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Gouda, M.; El-Shafei, A.M.; Abdel-Fatah, O.M. Antimicrobial activity of cotton fabrics containing immobilized enzymes. J. Appl. Polym. Sci. 2007, 104, 1754–1761. [Google Scholar] [CrossRef]
- Cadnum, J. L.; Li, D.; Redmond, S. N.; John, A. R.; Pearlmutter, B.; Donskey, C. Effectiveness of Ultravi-olet-C Light and a High-Level Disinfection Cabinet for Decontamination of N95 Respirators, Pathogens and Immunity. 2020, 20,5,1,52-67.
- Rowan, N.J.; Laffey, J.G. Challenges and solutions for addressing critical shortage of supply chain for personal and protective equipment (PPE) arising from Coronavirus disease (COVID19) pandemic – Case study from the Republic of Ireland. Sci. Total. Environ. 2020, 725, 138532–138532. [Google Scholar] [CrossRef]
- Derraik, J. G. B.; Anderson, W. A.; Connelly, E. A.; Anderson, Y. C. Rapid evidence summary on SARS-CoV-2 survivorship and disinfection, and a reusable PPE protocol using a double-hit process, Med Rxiv. 2020, 2004, 2002, 20051409. [Google Scholar]
- Hiragond, C.B.; Kshirsagar, A.S.; Dhapte, V.V.; Khanna, T.; Joshi, P.; More, P.V. Enhanced anti-microbial response of commercial face mask using colloidal silver nanoparticles. 2018, 156, 475–482. [Google Scholar] [CrossRef]
- Mokhena,T.C.; Luyt,A. Electrospun alginate nanofibres impregnated with silver nanoparticles: Prepa-ration, morphology and antibacterial properties, Carbohydr. Polym. 2017, 165, pp. 304–312.
- Hui, F. ; Debiemme-Chouvy,C. Antimicrobial N-Halamine Polymers and Coatings: A Review of Their Synthesis, Characterization, and Applications, Biomacromolecules. 2013, 14, pp. 585–601. [Google Scholar]
- Choi, J.; Moon, D.S.; Ryu, S.G.; Lee, B.; Bin Ying, W.; Lee, K.J. N-chloro hydantoin functionalized polyurethane fibers toward protective cloth against chemical warfare agents. Polymer 2018, 138, 146–155. [Google Scholar] [CrossRef]
- Tang, P.; Zhang,M.; Ji,B.; Yong,T.; Sun,G. Hierarchical Nucleophilic Nanofibrous Membranes for Fast, Durable, and Bare-Eye Visible Detoxification of Carcinogenic Alkylating Toxicants, Adv. Funct. Mater. 2019, 29, p. 1905990.
- Demir, B.; Cerkez, I.; Worley, S.D.; Broughton, R.M.; Huang, T.-S. N-Halamine-Modified Antimicrobial Polypropylene Nonwoven Fabrics for Use against Airborne Bacteria. ACS Appl. Mater. Interfaces 2014, 7, 1752–1757. [Google Scholar] [CrossRef] [PubMed]
- Ghayempour, S.; Montazer, M. Micro/nanoencapsulation of essential oils and fragrances: Focus on perfumed, antimicrobial, mosquito-repellent and medical textiles. J. Microencapsul. 2016, 33, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Kamrudi, N.; Akbari, S.; Kish, M.H. Enhanced control release of thyme essential oils from electrospun nanofiber/polyamidoamine dendritic polymer for antibacterial platforms. Polym. Adv. Technol. 2020, 31, 1719–1731. [Google Scholar] [CrossRef]
- Kenney, P.; Chan, B. K.; Kortright, K.; Cintron, M.; Havill, N.; Russi, M.; Epright, J.; Lee, L.; Balcezak, T.; Martinello, R. Hydrogen Peroxide Vapor sterilization of N95 respirators for reuse, MedRxiv. 2020, 2003, 2024, 20041087. [Google Scholar]
- Song, P.; Zhang, X.; Wang, S.; Xu, W.; Wang, F.; Fu, R.; Wei, F. Microbial proteases and their applications, Frontiers in Microbiology. 2023, 14.
- Doaa,S.R.K,; Ghazala M.; Abdullrahman E.; Mohammad A.;Mohd F.;Salma A.; Batool A. A.; Mohamed G. R. Green nanobiocatalysts: enhancing enzyme immobilization for industrial and biomedical applications, Biochem-istry, Biophysics And Molecular Biology. 2024.
- Khafaga, D.S.R.; Radwan, M.G.; Muteeb, G.; Aatif, M.; Farhan, M. Green Synthesis of Biocatalysts Based on Nanocarriers Promises an Effective Role in Pharmaceutical and Biomedical Fields. Catalysts 2023, 13, 1448. [Google Scholar] [CrossRef]
- Dyshlyuk, L.S.; Novoselova, M.V.; Rozalenok, T.A. Immobilization of chymotrypsin on magnetic Fe3O4 nanoparticles. Foods and Raw Materials. 2013, 1(2), 85–88. [Google Scholar] [CrossRef]
- Mukherjee, J.; Gupta, M.N. Alpha chymotrypsin coated clusters of Fe3O4nanoparticles for biocatalysis in low water media. BMC Chem. 2012, 6, 133–133. [Google Scholar] [CrossRef]
- Kajbafvala, A.; Zanganeh, S.; Kajbafvala, E.; Zargar, H.; Bayati, M.; Sadrnezhaad, S. Microwave-assisted synthesis of narcis-like zinc oxide nanostructures. J. Alloy. Compd. 2010, 497, 325–329. [Google Scholar] [CrossRef]
- Zhang, B.; Kong, T.; Xu, W.; Su, R.; Gao, Y.; Cheng, G. Surface functionalization of zinc oxide by car-boxyalkylphosphonic acid self-assembled monolayers. Langmuir. 2010, 26(6), 4514–4522. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).