Submitted:
03 March 2025
Posted:
04 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
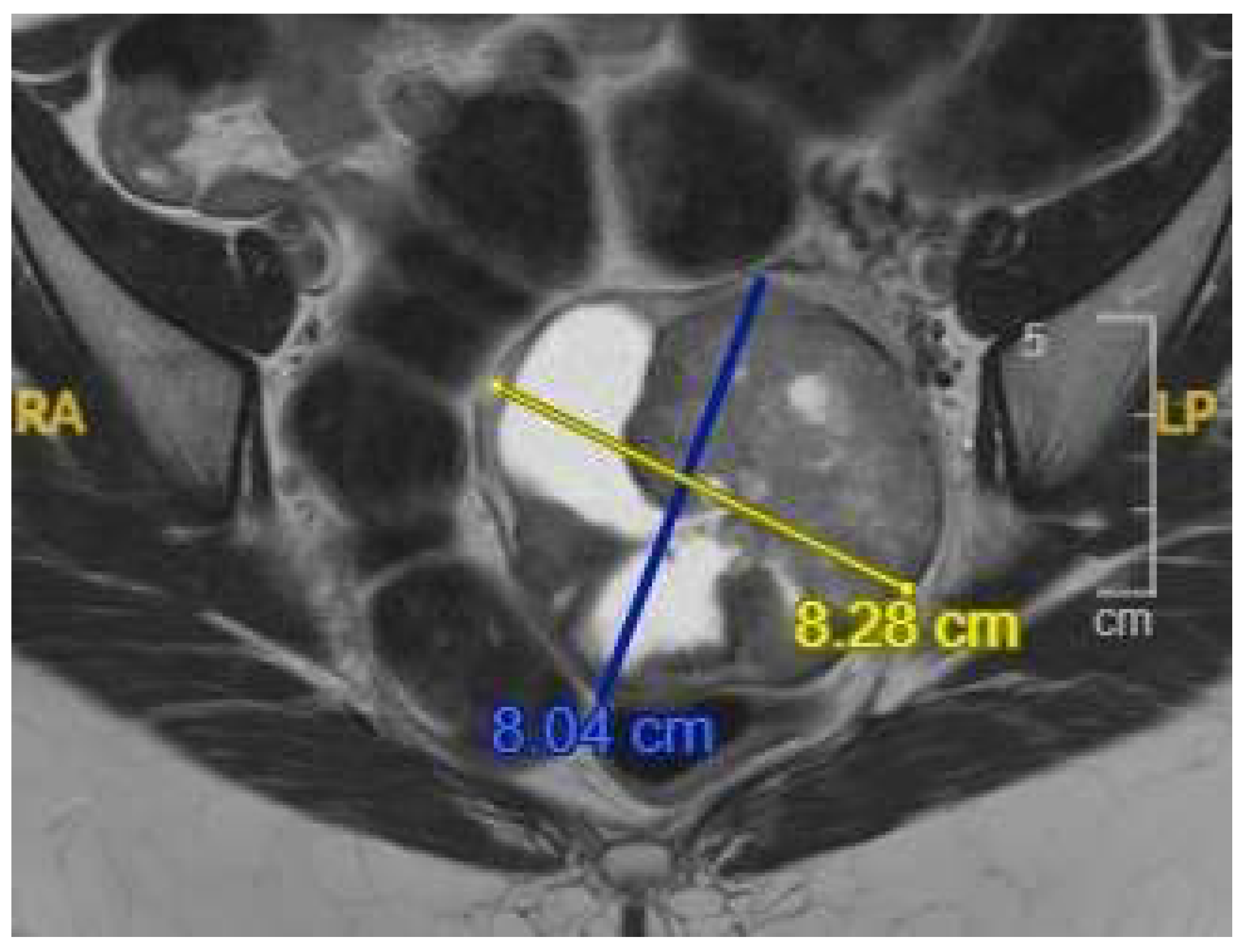
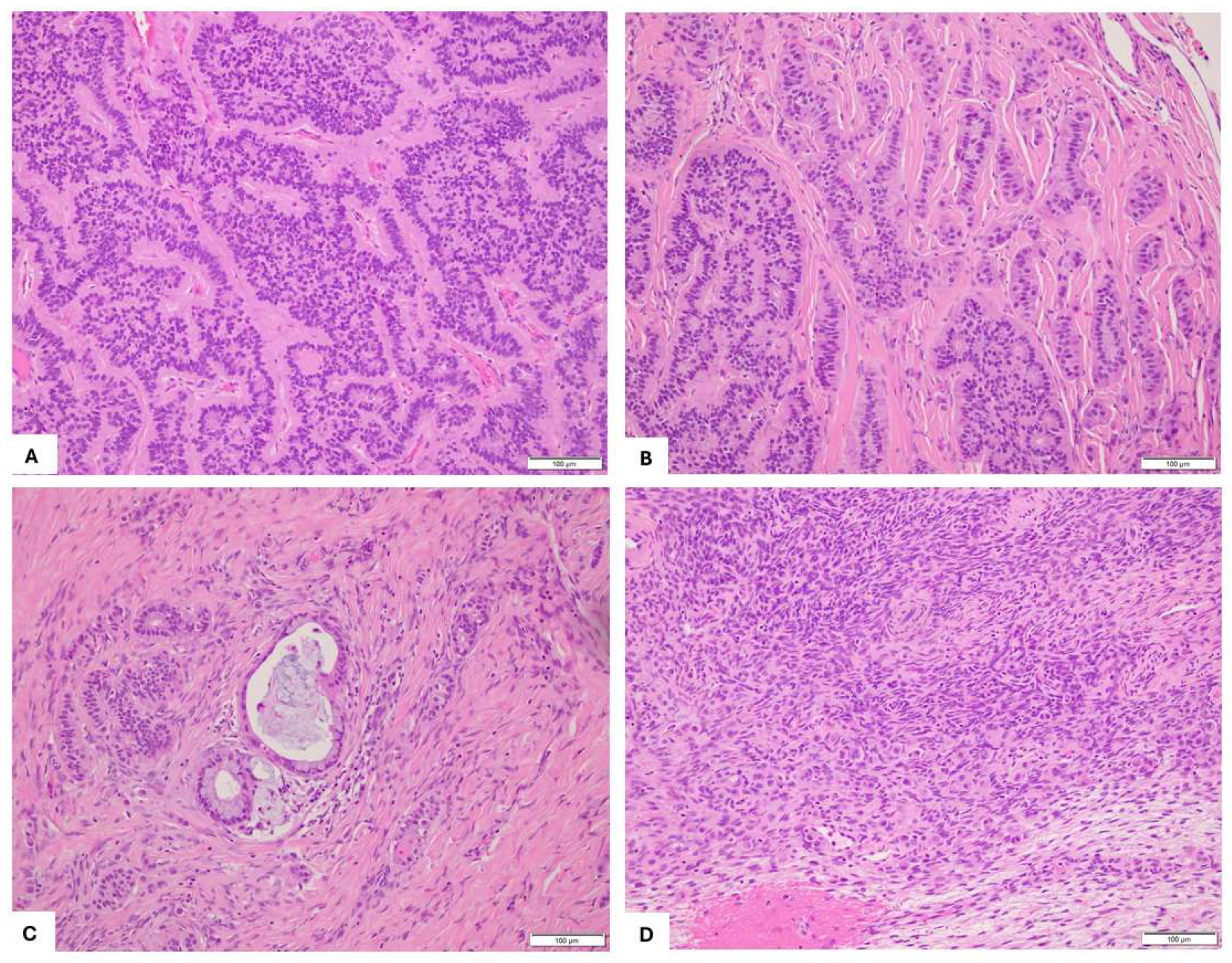
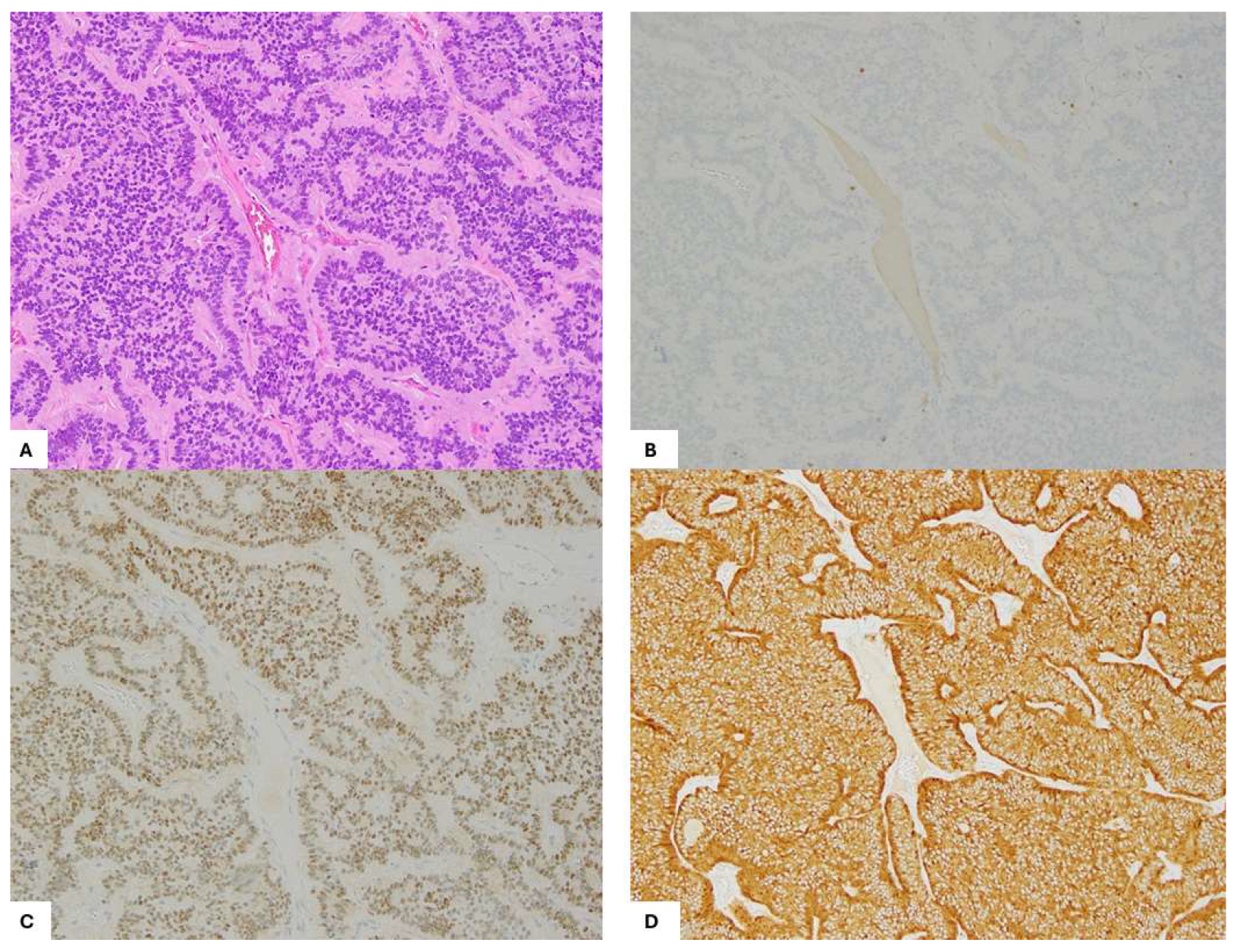
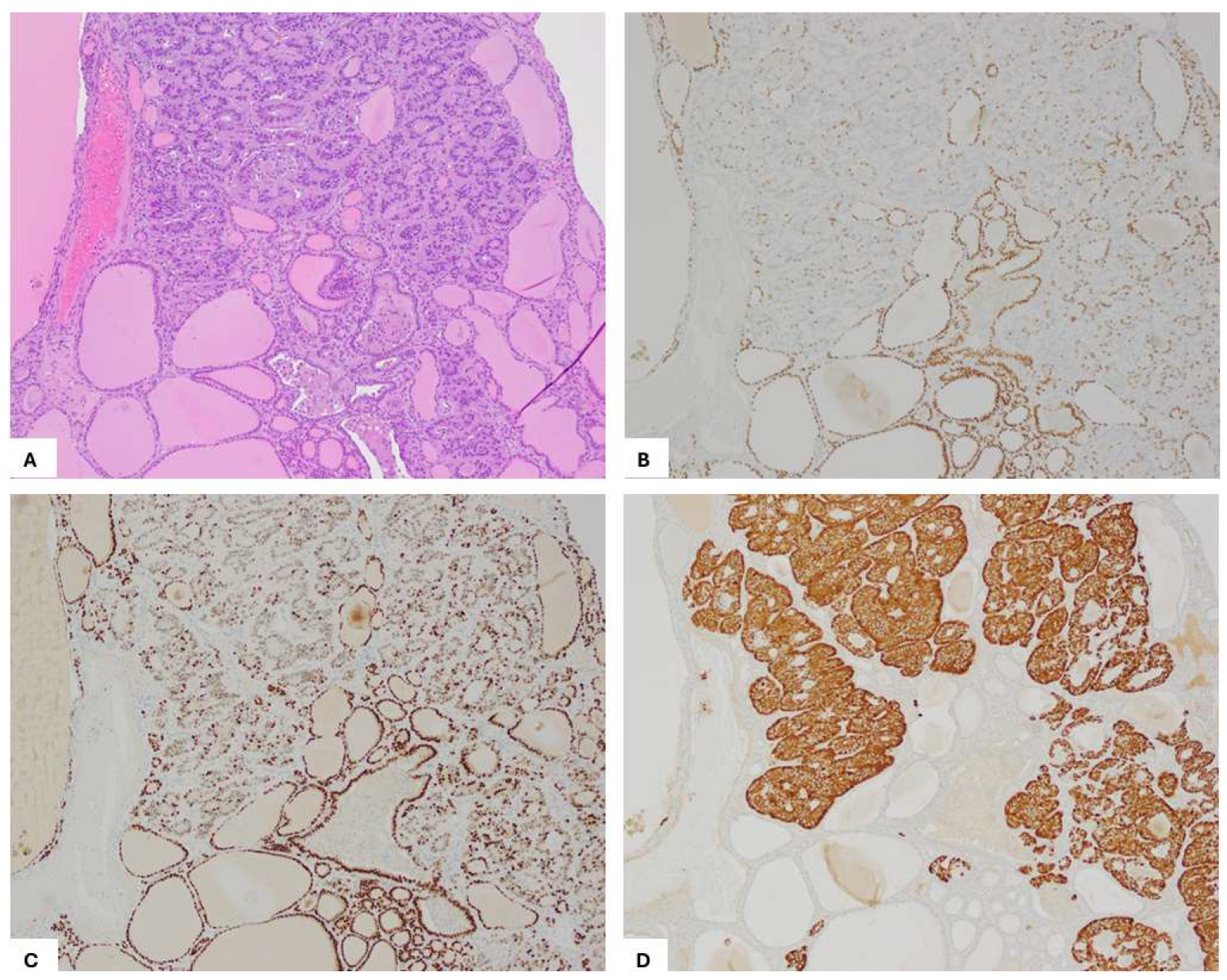
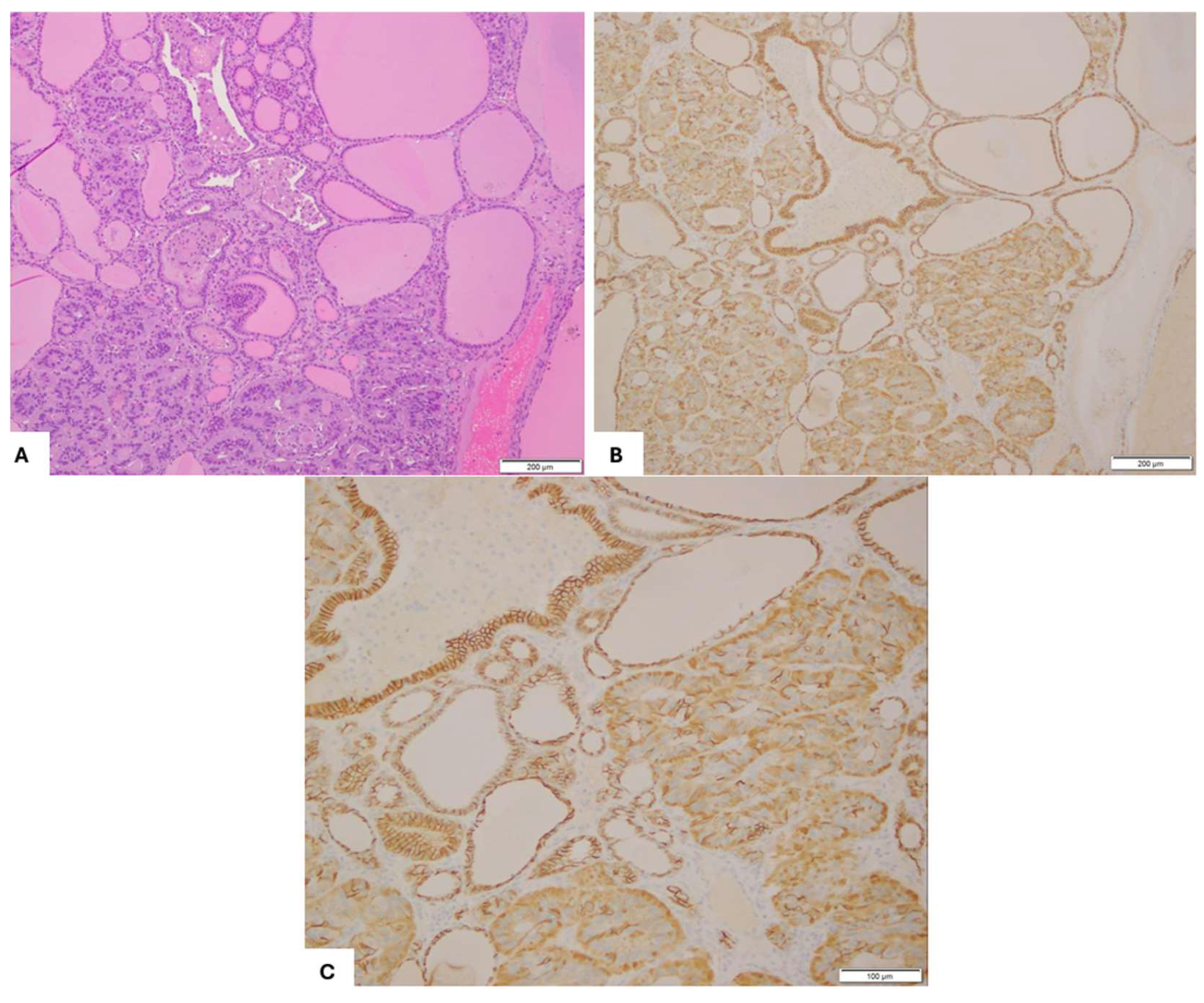
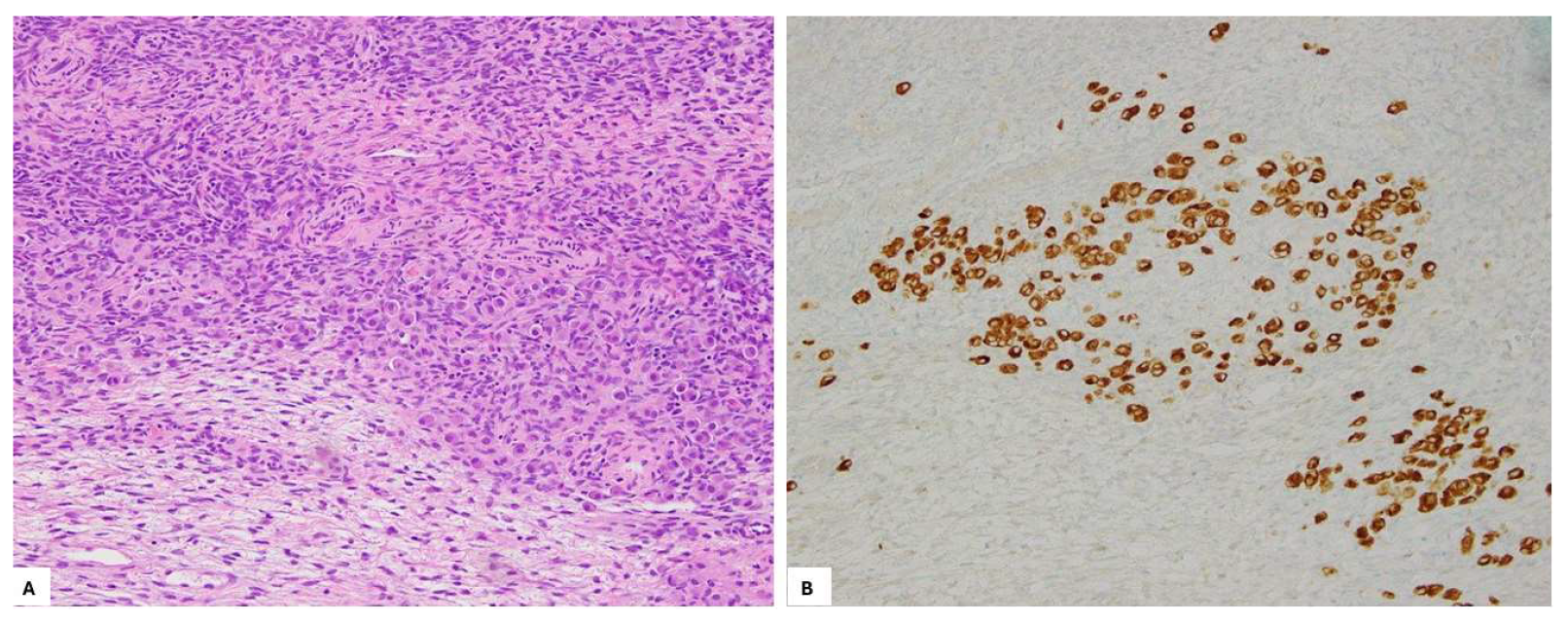
2. Case Presentation
3. Discussion and Literature Review
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Blaustein, A. Calcitonin secreting struma-carcinoid tumor of the ovary. Hum. Pathol. 1979, 10, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Apostol, D.G.C.; Buţureanu, T.A.; Socolov, D.G.; Scripcaru, D.C.; Rosin, O.L.; Lozneanu, L. Ovarian strumal carcinoid - case report. Rom J Morphol Embryol 2017, 58, 1035–1040. [Google Scholar]
- Greco, M.A.; Livolsi, V.A.; Pertschuk, L.P.; Bigelow, B. Strumal carcinoid of the ovary.An analysis of its components. Cancer 1979, 43, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Hamazaki, S.; Okino, T.; Tsukayama, C.; Okada, S. Expression of thyroid transcription factor-1 in strumal carcinoid and struma ovarii: An immunohistochemical study. Pathol. Int. 2002, 52, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Hart, W.R.; Regezi, J.A. Strumal Carcinoid of the Ovary: Ultrastructural Observations and Long-term Follow-up Study. Am. J. Clin. Pathol. 1978, 69, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Jukić, Z.; Limani, R.; Luci, L.G.; Nikić, V.; Mijić, A.; Tomas, D.; Krušlin, B. hGH and GHR expression in large cell neuroendocrine carcinoma of the colon and rectum. Anticancer Res. 2012, 32, 3377–81. [Google Scholar] [PubMed]
- Kimura, N.; Sasano, N.; Namiki, T. Evidence of Hybrid Cell of Thyroid Follicular Cell and Carcinoid Cell in Strumal Carcinoid. Int. J. Gynecol. Pathol. 1986, 5, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, X.; Sui, X.; Zhang, X.; Yin, M.; Yang, J. Clinical characteristics and survival outcomes in patients with ovarian strumal carcinoid. BMC Cancer 2022, 22, 1–9. [Google Scholar] [CrossRef]
- Macháleková, K.; Kolníková, G.; Redecha, M.; Žúbor, P.; Kajo, K. Strumal carcinoid of the ovary - report of two cases and review of literature. Ceska Gynekol. 2018, 83, 452–457. [Google Scholar] [PubMed]
- Matsunami, K.; Takagi, H.; Ichigo, S.; Murase, T.; Ikeda, T.; Imai, A. Peptide YY producing strumal carcinoid tumor of the ovary. Eur J Gynaecol Oncol. 2011, 32, 201–2. [Google Scholar] [PubMed]
- Motoyama, T.; Kafayama, Y.; Watanabe, H.; Okazaki, E.; Shibuya, H. Functioning ovarian carcinoids induce severe constipation. Cancer 1992, 70, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.K.; Kwon, B.S.; Kim, Y.H.; Lee, N.K.; Choi, K.U.; Suh, D.S.; Lee, D.H.; Kim, K.H. Peptide YY producing strumal carcinoid tumor of the ovary in a postmenopausal woman: a rare cause of chronic constipation. Obstet. Gynecol. Sci. 2017, 60, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Robboy, S.J.; Scully, R.E. Strumal carcinoid of the ovary: An analysis of 50 cases of a distinctive tumor composed of thyroid tissue and carcinoid. Cancer 1980, 46, 2019–2034. [Google Scholar] [CrossRef] [PubMed]
- Sarcević, B.; Separović, V.; Sćukanec-Spoljar, M.; Pejsa, V. [Strumal carcinoid tumor of the ovary--histologic and electron microscopy characteristics of the tumor]. Lijec Vjesn. 1990, 112, 381–393. [Google Scholar] [PubMed]
- Scully, R.E. Recent progress in ovarian cancer. Hum. Pathol. 1970, 1, 73–98. [Google Scholar] [CrossRef] [PubMed]
- Senterman, M.K.; Cassidy, P.N.; Fenoglio, C.M.; Ferenczy, A. Histology, ultrastructure, and immunohistochemistry of strumal carcinoid: A case report. Int J Gynecol Pathol 1984, 3, 232–240. [Google Scholar] [PubMed]
- Shia, J., L. H. Tang, M. R. Weiser, B. Brenner, N. V. Adsay, E. B. Stelow, L. B. Saltz, J. Qin, R. Landmann, G. D. Leonard, D. Dhall, L. Temple, J. G. Guillem, P. B. Paty, D. Kelsen, W. D. Wong and D. S. Klimstra (2008). "Is nonsmall cell type high-grade neuroendocrine carcinoma of the tubular gastrointestinal tract a distinct disease entity?" Am J Surg Pathol 32(5): 719-731.
- Snyder, R. R. and F. A. Tavassoli (1986). "Ovarian strumal carcinoid: immunohistochemical, ultrastructural, and clinicopathologic observations." Int J Gynecol Pathol 5(3): 187-201.
- Stagno, P. A., R. E. Petras and W. R. Hart (1987). "Strumal carcinoids of the ovary. An immunohistologic and ultrastructural study." Arch Pathol Lab Med 111(5): 440-446.
- Takubo, K., H. Yasui and K. Toi (1986). "Strumal carcinoid of the ovary. A case report." Acta Pathol Jpn 36(5): 765-771.
- Theurer, S.; Ingenwerth, M.; Herold, T.; Herrmann, K.; Schmid, K.W. Immunohistochemical Profile and 47-Gene Next-Generation Sequencing (NGS) Solid Tumor Panel Analysis of a Series of 13 Strumal Carcinoids. Endocr. Pathol. 2020, 31, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Tsubura, A. and M. Sasaki (1986). "Strumal carcinoid of the ovary. Ultrastructural and immunohistochemical study." Acta Pathol Jpn 36(9): 1383-1390.
- Turla, A., M. Zamparini, M. Milione, S. Grisanti, V. Amoroso, R. Pedersini, D. Cosentini and A. Berruti (2022). "Ovarian Strumal Carcinoid: Case Report, Systematic Literature Review and Pooled Analysis." Front Endocrinol (Lausanne) 13: 871210.
- Ulbright, T.M.; Roth, L.M.; Ehrlich, C.E. Ovarian Strumal Carcinoid: An Immunocytochemical and Ultrastructural Study of Two Cases. Am. J. Clin. Pathol. 1982, 77, 622–631. [Google Scholar] [CrossRef] [PubMed]
| Reference | Number of Cases | Immunostaining/Ultrastructural Findings in strumal component | Immunostaining/Ultrastructural Findings in Carcinoid Component | Proposed Carcinoid Origin Hypothesis |
|---|---|---|---|---|
| S Robboy, et al 1980 (Robboy and Scully 1980) | 50 | -Calcium oxalate crystals present in half cases -Cells with argentaffin granules lined thyroid follicles -Positive for TG (n=2/50) |
-Argentaffin granules and electron-dense granules are present | Support unified Origin |
| Takubo K, et al 1986 (Takubo, Yasui et al. 1986) | 1 | -Neurosecretory-type granules in the cytoplasm of thyroid follicular -Positive for TG |
-Neurosecretory-type granules in the cytoplasm of carcinoid | Support unified origin |
| Tsubura A 1986 (Tsubura and Sasaki 1986) | 1 | -Positive for TG | -Carcinoid cells expanding toward the inner layer of the follicles -Negative for CAL and amyloid |
Support unified origin Not support origin from C cell |
| Stagno P, et al 1987 (Stagno, Petras et al. 1987) | 6 | -Positive for TG (6/6) -Thyroid follicular epithelium lined by non-neuroendocrine cells |
-Positive for CGA (5/6) and CAL (1/6) -Carcinoid cells progressively replace thyroid epithelial cells |
Support origin from thyroid tissue in strumal ovarii |
| S. Theurer, et al 2020 (Theurer, Ingenwerth et al. 2020) |
13 | -Positive for TG (100%), TTF-1(84.6%), CGA (76.9%), SYN (7.7%) -Negative for CDX2, CAL SSTR2a, and SSTR5 |
-Positive for TG (100%), TTF-1(84.6%), CGA (92.3%), SYN (100%), SSTR2a (92.3%), SSTR5 (46.1%) -Negative for CDX2 and CAL |
Support origin from thyroid tissue in strumal ovarii Not support origin from C cells |
| L. Greco, et al 1979 (Greco, LiVolsi et al. 1979) | 1 | -Positive for TG in thyroid follicles and transitional zone | -Negative for TG -Positive for CAL |
Support origin from thyroid tissue in strumal ovarii |
| M Senterman,et al 1984 (Senterman, Cassidy et al. 1984) | 1 | -Positive for TG in thyroid follicles and transitional zone -Negative for CAL |
-Negative for TG and CAL |
Support origin from thyroid tissue in strumal ovarii Not support origin from C cell |
| R. Snyder, et al 1986 (Snyder and Tavassoli 1986) | 13 | -Positive for TG in thyroid follicles and transitional zone (12/12) - NE granules in thyroid follicles (6/6) |
-Negative for TG and CAL (12/12) - NE granules in carcinoid (6/6) |
Support origin from thyroid tissue in strumal ovarii Not support origin from C cell |
| Kimura N, et al 1986 (Kimura, Sasano et al. 1986) | 1 | - Strong positive for TG - Intensive argyrophilia - Abundant neurosecretory granules, microfilaments, colloid-like droplets in thyroid follicles |
- Weak positive for TG - Intensive argyrophilia - Abundant neurosecretory granules, microfilaments, colloid-like droplets in carcinoid |
Support origin from hybrid cells having both thyroid and neuroendocrine features |
| S Hamazaki, et al 2002 (Hamazaki, Okino et al. 2002) | 2 | -Positive for TTF-1, TG -Negative for CGA, SYN, PAP, CAL |
-Positive for CGA, SYN, PAP -Negative for TTF-1, TG, CAL |
Divergent differentiation from a common progenitor Not support origin from C cell |
| K Macháleková 2018 (Machalekova, Kolnikova et al. 2018) | 2 | -Positive for TTF-1, TG -Negative for CAL, CEA |
-Positive for CGA, SYN -Negative for CAL, CEA |
Not support origin from C cell |
| C Apostol ,et al 2017 (Ciobanu Apostol, BuTureanu et al. 2017) | 1 | -Positive for TTF-1, TG, CD56. CK7, CK19 -Negative for CAL, HBME1, GFAP |
-Positive for SYN, CGA, CD56 -Negative for CK7, CAL, GFAP |
Not support origin from C cell |
| Blaustein, A 1979 (Blaustein 1979) | 1 | N/A | -Positive for CAL and 5-HA - Ultrastructural similarity to medullary carcinoma |
Support origin from C cell |
| Ulbright T, et al 1982(Ulbright, Roth et al. 1982) | 2 | -Granule-containing cells replaced some follicular epithelial cells |
-Positive for CAL in transitional zone and carcinoid | Support origin from C cell |
| A.Turla, et al 2022(Turla, Zamparini et al. 2022) | 117 | -Positive for TG (41/45), TTF-1(22/25) |
-Positive for CGA (39/39), SYN (31/31), CD56 (16/16), NSE (13/13), CAL (12/23) | Support origin from C cells |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





