1. Introduction
Parkinson’s Disease (PD) is a progressive multi-system neurodegenerative disease affecting people, mainly in the later years of life. The nonmotor symptoms of PD can be categorized as cognitive and psychiatric disturbances, sleep disturbances, sensory symptoms, and autonomic dysfunction. Autonomic dysfunction, which has emerged as an important aspect of nonmotor dysfunction and is present in both the early and later stages of PD, covers a broad spectrum that includes gastrointestinal, urological, sexual, thermoregulatory, and cardiovascular [
1].
Cognitive and psychiatric disturbances are among the most common non-motor features of PD, potentially emerging at any stage of the disease—even preceding motor symptoms. Depression is particularly prevalent, often co-occurring with anxiety and apathy, the latter frequently overlapping with depressive symptoms, cognitive impairment, and psychosis. Clinically significant depression and anxiety affect approximately 30–35% of PD patients [
2]. Psychosis is reported in 25–40% of cases, with visual hallucinations occurring in 15–30%, non-visual hallucinations (including auditory, tactile, and olfactory) in up to 35%, and delusions in about 4% of patients [
2]. The effect of medications should also be mentioned, as virtually all anti-parkinsonian medications have been reported to induce hallucinations and psychosis. Cognitive deterioration and dementia are common in PD and can manifest at any stage of the disease, either early or late in its course. Early cognitive changes often include deficits in executive function, visuospatial dysfunction, impaired speech fluency, and memory impairment, which may be subtle but progressively impact daily functioning [
3].
Sleep disorders are also commonly observed in patients with PD. The neuropathology of PD is known to affect anatomical structures and central neurotransmitters involved in modulating the physiological sleep cycle. Patients with PD also tend to have more shallow sleep and frequent awakenings in the night due to other symptoms, such as difficulties turning around in bed, frequent nocturia, and nocturnal tremors. Depression may also lead to fractionated sleep [
3,
4].
Apart from the nonmotor symptoms, autonomic dysfunction can present before the diagnosis becomes apparent with disease progression or be induced by medications. It is believed that the involvement of both the central and peripheral postganglionic autonomic nervous systems is the cause of these symptoms [
5]. Gastrointestinal symptoms are prevalent, such as constipation, which is conceded to be the most common complaint. Sialorrhea occurs in over 50% of early PD patients [
6], and bedside diurnal drooling causes embarrassment and increases the risk of aspiration pneumonia, postprandial fullness, and gastric retention. Difficulty in rectal evacuation because of rectal sphincter dysfunction can also be observed [
7].
Along with gastroparesis and dysphagia, which occur in 11-81% of PD patients [
8]. Almost half of PD patients show weight loss during disease progression, which may be related to these gastrointestinal symptoms or medications such as levodopa [
9]. Urinary symptoms and erectile dysfunction are common in males [
10], with control disturbances including urinary frequency, urgency, and incontinence [
5]. Frequent nocturia is reported by 60% of patients due to detrusor overactivity, which results in a significant impact on the individual's quality of life, so most patients should undergo a full urodynamic investigation, including cytometry, flowmetry, and ultrasonography before treatment is initiated [
11].
Cardiovascular autonomic dysfunction in PD can involve both pre-ganglionic and post-ganglionic lesions, and the interplay between these contributes to the development of classical orthostatic hypotension (OH) and neurogenic orthostatic hypotension (nOH). This dysfunction is often accompanied by additional cardiovascular abnormalities, including supine hypertension and postprandial hypotension, further complicating blood pressure regulation in PD patients.
OH is a reduction in systolic blood pressure (SBP) of at least 20 mmHg or diastolic blood pressure (DBP) of at least 10 mmHg within 3 minutes of an upright posture. As one of the autonomic disorders, OH can be thought of as being either primary or secondary, which may occur in association with any condition of autonomic neurocirculatory dysfunction that causes a significant decline in blood or extracellular fluid volume, making a person bedridden or immobile for an extended period [
12]. When performing the tilt table test, some centers consider a systolic drop of ≥30 mmHg as significant, while others use a ≥15 mmHg cutoff; the test aims to eliminate the leg muscle pump effect, which is usually active during standing [
13]. In PD, nOH is typically present, with inadequate neurocirculatory responses to postural changes such as baroreflex failure and impaired release of norepinephrine [
14]. At the same time, post-prandial hypotension (PPH) and exercise-induced hypotension can also occur in patients with PD. PPH occurs after consuming large, carbohydrate-heavy meals and may develop within 15 minutes after eating and persist for up to 3 hours. Elderly PD patients may be most susceptible to this phenomenon, which can be avoided by reducing carbohydrate intake and eating smaller, more frequent meals [
15].
On the other hand, supine hypertension occurs in approximately 50% of individuals with nOH. It is defined as a SBP ≥ 140 mmHg and DBP ≥ 90 mmHg after at least 5 minutes of rest in the supine position [
16]. The decision of whether to treat supine hypertension or not in an individual with PD is challenging, as the consequences of nOH, such as falling and syncope, are more immediate and acutely dangerous than the delayed potential complications of supine hypertension. Nondipping is the loss of any decrease in nocturnal BP. Although limited, studies on non-dipping in PD report that up to 88% of patients exhibit a non-dipping blood pressure pattern [
17]. Also, it was found to be more prevalent in PD patients with OH than in those without.
This review offers a comprehensive analysis of the relationship between OH and nOH in patients with PD, focusing on the epidemiology, diagnostic criteria, and clinical manifestations. It further explores the underlying pathophysiological mechanisms and critically evaluates current and emerging management strategies. Emphasizing the clinical relevance, the review highlights the impact of OH on the quality of life of patients living with PD.
2. Epidemiology
2.1. Prevalence and Incidence of OH
Although the prevalence of nOH in PD is relatively high, not all patients have symptoms of organ hypoperfusion, and only a third of patients (approximately 16%) have symptomatic nOH [
18]. Between 5.7% and 64.9% of patients with PD have OH, and the prevalence of OH in PD increases with age and disease duration (
Table 1).
In individual studies that defined nOH by BP reduction criteria, the prevalence of nOH in patients with PD ranges from 10 to 65% [
18,
33]. A meta-analysis of 25 studies identified an estimated point prevalence of 30% [
34]. The prevalence estimates of symptomatic nOH in patients with PD are also varied, with reported rates ranging from 16 to 89% in individual investigations [
18,
28,
29,
33]. A total of 907 PD patients with baseline orthostatic vitals were included in the most recent study. Using the Heart rate (HR)/SBP ratio to determine the prevalence of nOH and non-nOH in the Parkinson’s Progression Markers Initiative (PPMI), although the prevalence of nOH and non-nOH increased yearly (P = 0.012, chi-square), the increase was modest (baseline: 5.6% [95% CI: 4.3–7.3%]; month 48: 8.6% [6.4–11.5%]). In comparison to PD patients without OH, nOH patients were older, and nOH was associated with more significant impairment of motor and independent functioning than the non-nOH/OH categories. Cognitive function and typical OH symptoms were generally worse in PD+OH [
32].
In another study, OH—whether spontaneous (SOH) or levodopa-induced (LOH)—was associated with older age, motor fluctuations, probable REM sleep behavior disorder, and greater autonomic, cardiovascular, and digestive dysfunction compared to non-OH patients. Both SOH and LOH were linked to increased disease severity and poorer quality of life, supporting the hypothesis that OH may serve as a clinical marker for the body-first subtype of PD [
35]. Interestingly, the Hoehn & Yahr stage (OR 4.950) emerged as a strong predictor of OH risk, while the PDQ-39 score reflected a significant decline in quality of life, particularly in motor function, cognition, physical comfort, and daily living. The Hoehn & Yahr stage alone predicted OH with moderate accuracy (AUC = 0.679); however, predictive accuracy improved when combined with levodopa dosage and MDS-UPDRS Part III scores [
36].
Other original studies, as summarized in
Table 1, have investigated the prevalence of OH in PD. A retrospective cohort study by Wüllner et al. reported a prevalence of 10.6% in a large sample of 3414 patients enrolled in the German Network on PD, while a cross-sectional study by Adhiyaman et al. found a prevalence of 46.2% in 13 patients [
20]. Another cross-sectional study by Allcock et al. reported a prevalence of 49.7% in 175 patients [
19], and a cross-sectional study by Matinolli et al. found a prevalence of 52.5% in 120 patients [
24]. Finally, Haensch et al. found a prevalence of 48.3% in 58 patients [
22], while Jamnadas-Khoda et al. reported a prevalence of 22% in 50 patients [
23]. Goldstein et al. found a prevalence of 64.9% in 77 patients [
21], while Shibata et al. reported a prevalence of 18.1% in 72 patients [
26]. Finally, Schmidt et al. found a prevalence of 38% in 26 patients [
25]. It is important to note that the definitions of OH used in these studies varied, and the sample sizes were relatively small, making it difficult to draw firm conclusions about the prevalence of OH in PD.
3. Diagnosis
3.1. Clinical Manifestations
OH is commonly referred to as a sudden decrease in BP upon standing and is associated with different neurological symptoms in patients suffering from PD [
13,
37]. The pathophysiology of the neurological symptoms presented due to OH in PD primarily pertains to a reduction in blood flow toward the brain, occurring as an aftereffect of the inability of the body to maintain BP due to postural changes in the patient [
13,
38]. One of the most common symptoms is lightheadedness, often described as dizziness accompanied by a sensation of unsteadiness and feeling faint or about to lose consciousness [
39,
40]. Lightheadedness may severely impair an individual's ability to maintain an upright position while standing or walking for extended periods, making everyday activities particularly challenging [
39,
41,
42].
Another common clinical feature of OH in PD is visual disruption [
28,
39]. These are often manifested as blurred or dimmed vision, whereby individuals cannot clearly distinguish between objects or the environment around them and find everyday tasks that require sharp visual acuity challenging [
39,
43]. This blurred vision tends to come suddenly when one stands and generally clears up when resuming a seated or lying position, immediately linking it to postural changes [
44]. Diminished blood flow to the visual cortex in the brain is thought to be a primary contributor to these visual symptoms [
45,
46].
Many subjects with OH in PD complain of a sensation of cognitive slowing or “brain fog” when standing, which might conceivably engender significant functional impairment [
42,
47]. Mental clouding includes concentration problems, an inability to remember things, and generally slowed thought processes that eliminate spontaneity from daily life [
47,
48]. Concentrating on conversations and making decisions can become especially difficult, leading to frustration and inadequacy [
47,
49]. Notably, these cognitive deficits are often unnoticed by the affected individual but can be readily apparent to others [
39,
49,
50].
In a study analyzing data from 453 PD patients in the Cincinnati Cohort Biomarkers Program, OH was not independently linked to excessive daytime sleepiness (EDS), as measured by the Epworth Sleepiness Scale. However, in cognitively impaired patients, OH was associated with depression, which in turn was strongly linked to EDS, suggesting an indirect pathway [
51]. In another cross-sectional study of 82 patients with PD, nOH was independently associated with impaired gait and balance, including reduced gait speed, shorter stride length, longer postural transition times, and increased postural sway. These effects were more pronounced in patients with hemodynamically relevant nOH, with higher postural sway linked to a 7.9-fold increased fall risk [
52].
This array of neurological symptoms combined with OH in PD testifies to the extent of inadequate blood supply to the brain, manifested by the impairment across various cognitive domains [
46,
53]. Although symptoms like dizziness and visual disturbances may be temporary and improve when lying down, they can significantly impact quality of life and increase fall risk [
54,
55]. Early detection of OH and timely management are crucial for reducing symptoms, enhancing quality of life, and promoting independence in people with PD [
37,
56].
3.2. Clinical Evaluation
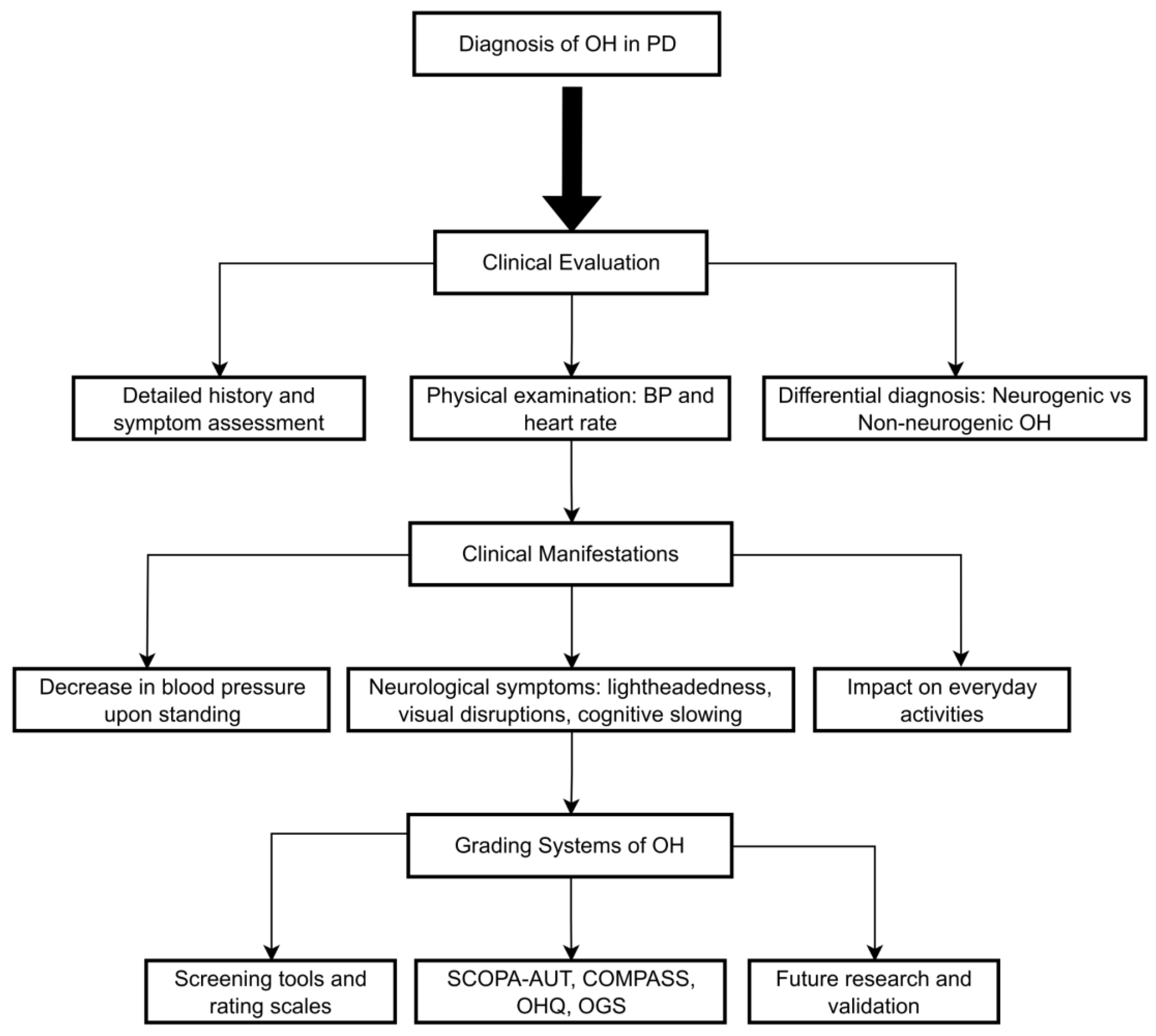
The clinical assessment of suspected OH should be based on a good history, including the onset and duration, specific characteristics of symptoms on standing, or change of posture (
Figure 1) [
13,
37]. It is also important to remember that the prevalence of OH increases with age [
57]. Symptoms can range from dizziness and lightheadedness to visual disturbances (such as blurring or dimming), weakness, fatigue, and cognitive impairment, often referred to as “brain fog [
37,
39].” OH is reported among patients with PD. It has also been connected to faster motor and cognitive decline, a higher fall rate, and increased hospitalizations [
58]. Clinicians should ascertain whether these symptoms occur exclusively during upright postures and subside when the patient lies down, thus confirming the postural nature of their presentation [
39,
59]. Discussing potential trigger events is essential, as prolonged standing, warm environments, large meals, alcohol, and certain medications often provoke orthostatic symptoms [
42,
56,
60]. For example, the early morning hours are typically the most symptomatic due to overnight fluid shifts [
61]. The physical examination should include meticulous BP and HR measurements in the supine and standing positions—ahead of 3 minutes of standing—which will allow quantitation of the BP drop and identification of any concomitant supine hypertension [
41]. A neurological examination is also paramount in ruling out other conditions, such as ataxia, parkinsonism, or peripheral neuropathy [
62].
An essential point in diagnosing OH is the differential diagnosis between neurogenic and non-neurogenic forms (
Table 2). nOH is due to a deficiency of norepinephrine release due to damage to sympathetic nerves, often occurring in synucleinopathies such as PD, multiple system atrophy, and Pure Autonomic Failure (PAF) [
39,
63]. Evidence has singled out nOH as an early manifestation of synucleinopathies, possibly many years before the manifestation of motor symptoms [
64,
65]. In contrast, non-nOH has been attributed to a wide variety of causes, essentially anything from volume depletion, heart problems such as heart failure and arrhythmias, medications, and other medical diseases [
66,
67,
68]. Recognizing this distinction has implications for choosing effective treatment strategies since therapies for nOH typically focus on enhancing norepinephrine signaling or counteracting its deficiency, as noted by Kaufmann [
69]. Several clinical clues, outlined in
Table 2, can assist clinicians in differentiating between these two forms. Notably, HR response to standing is a key indicator: minimal or absent HR increase in the presence of a significant BP drop strongly suggests nOH [
40,
70]. nOH can be effectively ruled out if the HR increases by at least 0.5 beats per minute for each millimeter of mercury reduction in SBP following three minutes of head-up tilt, making this a reliable marker in clinical assessment [
59].
3.3. Diagnostic Tests
Diagnosing OH hinges on identifying a persistent reduction in BP upon transitioning to an upright position [
39]. This process encompasses a detailed medical history, a thorough physical examination, and, if necessary, a selection of specialized diagnostic tests [
69,
76]. Obtaining an appropriate history is key, including investigating the patient's symptom experience: the time of onset, duration, character, and any identifiable triggers or exacerbating factors [
37,
42]. OH presents with traditional symptoms, including lightheadedness or dizziness, feeling faint, weakness, fatigue, visual blurring, and cognitive difficulties [
39].
The active standing test is a fundamental diagnostic tool that accurately measures the HR and BP response in both supine and standing positions [
77,
78]. OH will be diagnosed if the SBP falls by at least 20 mm Hg or the mean DBP drops by at least 10 mm Hg within 3 minutes of standing or head-up tilt. Notably, some patients experience delayed OH where this decrease in BP occurs beyond 3 minutes, thus necessitating longer monitoring time [
41,
62]. In a study of 132 newly diagnosed PD patients, 14% exhibited delayed (DOH), while 42% had classical OH (COH). Both DOH and COH were associated with more severe hyposmia compared to patients without OH, suggesting a link between olfactory dysfunction and autonomic impairment. While norepinephrine (NE) levels were reduced in COH, they remained relatively preserved in DOH, indicating less peripheral sympathetic dysfunction. In contrast, vasopressin (ADH) levels were elevated in DOH, possibly reflecting a compensatory mechanism [
79].
In addition to the active standing test, various diagnostic tools can help identify the underlying causes of OH and guide effective management strategies [
80]. Such multifactorial assessment is often carried out with the use of specialized tools. ABPM recordings provide a continuous record of changes in BP over the 24 hours, outlining overall patterns of changes in BP during the day and night and may point out cases of nocturnal hypertension and OH episodes that would not be picked up by one office visit [
81,
82,
83]. More importantly, ABPM can identify symptomatic OH and pressor agent administration times and needs [
84].
Abnormal autonomic function testing provides an exact transition of the autonomic nervous system, especially how appropriately the sympathetic and parasympathetic branches respond to certain stimuli. Therefore, as we mentioned before, it is essential to differentiate neurogenic from non-neurogenic causes [
85]. This testing also follows standardized protocols or techniques, such as the valsalva maneuver, HR variability assessments, and the tilt-table test, providing specific insights into autonomic nervous system functioning [
86]. Additional autonomic function testing may include sudomotor testing, a widely available and reliable method to determine postganglionic sympathetic cholinergic function, mainly examining sweating integrity. Quantitative sensory testing evaluates small-fiber neuropathy commonly encountered in disorders with autonomic features [
80].
Functional near-infrared spectroscopy (fNIRS) combined with convolutional neural networks (CNN) offers a promising approach for evaluating OH in PD. This method effectively analyzes resting-state fNIRS data, achieving over 85% accuracy, which increases to over 90% with the inclusion of correlation matrix inputs [
87]. CNN-based fNIRS analysis outperforms traditional machine learning methods, providing a non-invasive and accurate diagnostic tool that reduces patient discomfort and risks linked to conventional tests like the head-up tilt test.
A large cohort study involving 318 PD patients compared the diagnostic accuracy of sit-to-stand versus supine-to-stand BP tests. The study found OH in 35.8% of patients using the supine-to-stand method, significantly outperforming the sit-to-stand test, which had a low sensitivity of 0.39. OH was associated with older age, lower body mass index, longer disease duration, more severe motor and cognitive symptoms, overactive bladder symptoms, functional disabilities, and reduced fluid intake. Notably, three-quarters of OH cases were neurogenic, with many also exhibiting supine hypertension. Continuous BP monitoring further identified OH in 25% of patients missed by clinic-based tests [
88].
OH has been increasingly recognized as a key non-motor feature linked to neuronal injury in early-stage PD. Marques et al. demonstrated that serum neurofilament light chain (NfL) effectively distinguishes atypical parkinsonism disorders (APDs) from PD, with significantly higher NfL levels in APDs, achieving 91% diagnostic accuracy [
89]. Park et al. revealed that elevated plasma NfL levels, a neuroaxonal damage biomarker, have been associated with OH in PD, suggesting more extensive neurodegeneration in affected patients [
90].
Blood tests can identify anemia, diabetes, or thyroid dysfunction contributing to the problem [
66]. Measuring plasma NE levels can help to differentiate the source of nOH, showing postganglionic damage due to decreased norepinephrine versus a preganglionic origin where blood norepinephrine levels are normal or high [
64]. Electrocardiography is also utilized to discover occult disturbances in heart rhythm, such as atrial fibrillation, which may contribute to or even mimic symptoms of OH; thus, it plays an essential role in diagnosis and treatment [
62,
64]. In the tilt-table test, a patient is strapped to a motor-driven table passively tilted upright while monitoring BP and HR under controlled conditions [
49]. This controlled approach makes it a valuable tool for identifying delayed OH and orthostatic intolerance in general, helping clinicians distinguish between types of syncope that present with OH (
Table 3).
3.4. Grading Systems of Orthostatic Hypotension in Parkinson’s Disease
OH is a common non-motor symptom in PD and is estimated to affect a significant proportion of patients [
92]. It is a debilitating condition characterized by a drop in BP on standing and may be accompanied by symptoms such as dizziness and lightheadedness, sometimes resulting in syncope [
93]. Several grading systems have been developed to improve the understanding and management of OH in PD, broadly categorized into two types: screening tools designed to detect the presence of OH, as previously discussed, and rating scales aimed at quantifying the severity and frequency of associated symptoms [
94].
Scales for OH-related symptoms give interesting information about their severity as well. The SCOPA-AUT (Autonomic Scale for Outcomes in PD) and the COMPASS (Composite Autonomic Symptom Scale) are recommended but with limitations. SCOPA-AUT is a heavily used questionnaire of 25 self-administrated items, covering most autonomic dysfunctions: cardiovascular, gastrointestinal, urinary, thermoregulatory, pupillomotor, and sexual [
95,
96]. OH symptoms are assessed using three items, which provide a good assessment of their presence and frequency [
95]. However, despite demonstrating excellent clinometric properties in recent validation studies involving 387 PD patients, its sensitivity for detecting milder orthostatic symptoms is considered limited [
94,
97]. Also, the COMPASS questionnaire is much more extended, with 73 items covering nine autonomic domains [
98,
99]. Their nine-item orthostatic symptom subscale correlated with objective CASS scores [
99,
100]. However, the length and complexity of the instrument serve as a barrier to its broad utilization in clinical practice [
98].
Beyond the abovementioned instruments recommended for OH assessment, the following scales are frequently used, providing information about the condition. The OH Questionnaire (OHQ) assesses symptom burden and severity using ten items widely validated in different patient populations. It is subdivided into the OH Symptom Assessment and OH Daily Activity Scale [
101,
102]. It is useful specifically for OH in PD.
The Orthostatic Grading Scale (OGS) consists of five items that measure symptom frequency and severity and the effects of OH on daily life activities [
103]. The OGS has the advantage of having a good correlation with the CASS, providing a reliable assessment of autonomic function and orthostatic symptoms [
103]. It is also the case that some scales perform single-item screening for OH without rating severity. For example, two relevant questions are part of the assessment of 30-item non-motor symptom burden in PD by NMS Quest [
104,
105]. In this respect, it would represent a beneficial initial screening for identifying potential OH and further and more detailed investigations where necessary [
94]. Moreover, the original Unified Parkinson’s Disease Rating Scale (UPDRS) included only the assessment of orthostatic dizziness. Its revised version, the Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), maintains this approach. While both scales capture a key symptom, they were not validated as stand-alone instruments for evaluating the severity of OH. Therefore, they serve primarily as screening tools rather than comprehensive assessment measures [
94,
106].
Beyond these scales, there is another scale clinicians commonly use for assessing autonomic dysfunction, including orthostatic symptoms explicitly related to PD: the Scale for Outcome in PD—Autonomic [
95]. This tool is predominantly presented as a general assessment of autonomic involvement in PD. It has not been explored for its potential to delineate the severity of OH. Another tool of interest is the Novel Non-Motor Symptoms Scale for PD, which provides a somewhat different perspective on non-motor symptoms and seems to focus on orthostatic symptoms related to syncope and fainting [
107]. While this scale has not had much broader application, its presence underlines the expansion of interest in these symptoms, reflecting a significant part of the patient experience [
107].
Grading systems for OH in PD will evolve further, providing clinicians with a wide array of assessment tools, including comprehensive scales and concise screening instruments [
67,
108]. However, further research and validation are needed to enhance their sensitivity and applicability across diverse PD populations. This will aim to standardize and reliably manage OH and eventually enhance patient well-being.
4. Management
4.1. Clinical Versus Nonclinical Significance
Asymptomatic OH patients should be followed up on and treated; a study found that asymptomatic OH was linked to impairments in activities of daily living (ADLs), instrumental IADLs, and ambulatory capacity measures (ACM) comparable to those of symptomatic OH. Regardless of postural lightheadedness, those findings suggest screening for OH in patients with PD [
30].
The clinical significance of asymptomatic OH remains uncertain [
30]. Orthostatic symptoms may not directly relate to the exact BP measurement or the extent of the BP decrease [
109]. Moreover, relying exclusively on patient-reported symptoms when standing to determine whether to treat OH may overlook those who have undetected orthostatic cognitive fluctuations, which can happen without apparent symptoms [
47,
110]. While not many studies directly compare asymptomatic and symptomatic OH in PD, studies indicate that both groups have similar levels of ambulatory and functional capacity, falls, and healthcare consumption [
30,
33]. Therefore, failing to address recurrent instances of asymptomatic cerebral hypoperfusion may lead to a gradual decline in cognitive function [
111]. Multiple longitudinal studies examining the correlation between OH and cognition in people with PD have discovered that OH is linked to a deterioration in cognitive function [
92,
112].
OH is frequently observed in PD, with studies reporting a prevalence of 30–50%, though many patients remain asymptomatic [
34]. One study of 250 PD patients found measured OH in 30% and subjective orthostatic symptoms in 44%, with the latter linked to older age, dementia, and L-dopa use. Interestingly, measured OH did not correlate with clinical features or specific medications [
113]. While lowering both sitting and standing systolic blood pressure, Dopaminergic therapy did not increase OH incidence, likely due to reduced motor tone rather than direct vascular effects [
13]. Seasonal variations had no impact on blood pressure measurements [
113]. Other studies similarly highlight the disconnect between objective OH measurements and reported symptoms, emphasizing the complexity of autonomic dysfunction in PD.
Multiple reasons can contribute to the failure to diagnose nOH. One factor is that symptoms of lightheadedness may be absent. In addition, individuals may have more modest symptoms, such as cognitive deceleration [
114]. Furthermore, a patient may exhibit no symptoms due to the expansion of the autoregulated range [
45]. Several studies have indicated a correlation between OH and higher mortality rates. Two meta-analyses demonstrated an increased risk of all-cause mortality (p < 0.001) in subjects with OH compared with those without this condition [
115,
116].
4.2. Nonpharmacological
Depending on the severity of OH symptoms, non-pharmacological and pharmacological interventions should be implemented gradually if no reversible cause of OH can be found or if symptoms of OH remain after potentially aggravating variables have been eliminated [
117].
Patients should refrain from alcohol consumption, extended standing, heat exposure, large meals high in carbohydrates, and valsalva-like motions during bowel or micturition. Particularly after spending much time lying supine, patients should rise slowly. In this case, they may remain seated before standing up. Daily routine adjustments may be required, such as sitting while peeing or taking a shower in a chair for male patients; if dizziness prevents you from sitting or lying down, try BP-raising techniques by bending forward, crossing your legs, tensely flexing your gluteal and abdominal muscles, or clenching your fists. A training session under continuous BP monitoring may help teach the patient the proper form for these motions and assist them in selecting the most beneficial one [
118]. Sleep with the entire body tilted 10 to 20 degrees [
119] to promote antidiuretic hormone synthesis throughout the night.
One of the most critical non-pharmacological ways to treat OH is to increase the daily intake of salt (6–10 g) and water (up to 2.5 l) [
120]. 500 ml of water consumed as a bolus causes a 30 to 90-minute rise in BP [
121]. Patients with established heart, renal, or liver failure should take caution while supplementing with salt and water. Additional solutions that have been suggested are predicated on data about distinct elements, such as eating fewer carbohydrates regularly, which may reduce the postprandial hypotension component [
122]. Food has been proposed to reduce BP in autonomic failure by insulin secretion [
123] and vasodilatation [
122]. It has been proposed to abstain from alcohol during the day [
124,
125].
Abdominal binders also alleviate OH by decreasing splanchnic venous pooling [
16,
126]. In contrast, compression stockings did not show any benefit [
120,
127] and could be challenging for the elderly.
Medications that cause vasodilatation, decrease intravascular volume, or inhibit norepinephrine release or activity at the neurovascular junction aggravate OH and exacerbate symptoms. Often prescribed for benign prostatic hypertrophy, α-blockers, nitrates, phosphodiesterase-5 inhibitors (sildenafil for erectile dysfunction), centrally acting α2-agonists (clonidine), calcium-channel blockers, beta-blockers, and tricyclic antidepressants are among the common offenders. A personalized risk-benefit analysis may lead to considering a dose change for L-dopa and dopamine agonists, which may also lower BP. On the other hand, there is rarely a correlation between angiotensin-converting enzyme inhibitors and higher BP [
57,
128,
129,
130].
Anemia from chronic diseases is more common in patients with non-OH [
131]. Because anemia lowers blood viscosity and oxygen-carrying capacity, it exacerbates OH. Notably, hemoglobin scavenges nitric oxide, a potent vasodilator [
132]. It is likely, though still unproven, that nitric oxide-mediated processes exacerbate vasodilation in anemic patients with nOH, just as they do in other illnesses [
133]. Anemia needs to be appropriately diagnosed and treated. Recombinant erythropoietin therapy has been shown in small case series to reduce BP while standing up [
134]. OH can also be brought on or made worse by a vitamin B12 deficiency (<250 pg/mL with high methylmalonic acid levels), which must be treated [
135].
Acupuncture at the stellate ganglion appears to be an effective adjunctive therapy for OH in PD, offering benefits beyond standard pharmacological treatment. In a randomized study of 68 PD patients with OH, those receiving a combination of acupuncture and midodrine hydrochloride showed more significant improvements in orthostatic SBP and DBP, alongside substantial reductions in Orthostatic Hypotension Questionnaire scores, Traditional Chinese Medicine syndrome scores, and UPDRS scores compared to those receiving medication alone. Additionally, serum NE levels increased significantly in the combination group, suggesting enhanced sympathetic activation as a potential mechanism. The total effective rate was notably higher in the combination group (87.1%) than in the medication-only group (63.6%), supporting stellate ganglion acupuncture's potential role in improving hemodynamic stability and clinical symptoms in PD-related OH [
136].
The effects of deep brain stimulation (DBS) on the cardiovascular system of patients with resistant neurological disorders are still unknown [
137]. In a study of 20 PD patients, cardiovascular reflex tests showed no significant differences in BP control before and six months after subthalamic nucleus (STN)-DBS in the medication-off state. However, after levodopa intake, the drop in BP during the head-up tilt test was more pronounced both pre- and post-DBS. Notably, levodopa-induced OH was observed in 25% of patients before DBS, decreasing to 5% post-DBS [
138]. These findings suggest that while STN-DBS does not directly impact autonomic cardiovascular control, it may reduce the frequency of levodopa-induced OH, improving blood pressure stability in PD patients.
4.3. Pharmacological
Pharmacological therapy should be used if non-pharmacological therapies are unable to control OH symptoms adequately (
Table 4). Given the uncertainty surrounding its long-term safety, the pharmacological treatment of nOH is complicated and should be cautiously administered [
139]. It should be considered for pharmacological treatment, which consists of pressor medications and intravascular volume expansion [
13,
140]. Fludrocortisone, a mineralocorticoid agonist, initially causes the extracellular fluid volume to expand, raising BP [
141]. Compared to mineralocorticoid agonists, pressor medications, such as the synthetic norepinephrine precursor droxidopa and the α-adrenergic receptor agonist midodrine, have a shorter half-life and are safer [
142]. On the other hand, when peripheral sympathetic neurons are functioning, like in the case of individuals with preganglionic or premotor sympathetic lesions, such as multiple system atrophy, noradrenaline-reuptake inhibitors, like atomoxetine, have a pressor effect [
142]. When taken alone, the acetylcholinesterase inhibitor pyridostigmine has only mild vasopressor effects [
143,
144]. The α-glucosidase inhibitors acarbose [
13,
140] and caffeine [
145] can be used to treat postprandial hypotension.
Fludrocortisone is a typical synthetic mineralocorticoid used to treat hypotension. It increases water retention and sodium reabsorption rates. Research has demonstrated that in individuals with nOH brought on by diabetic neuropathy or PD, fludrocortisone raises SBP, alleviates symptoms, and reduces orthostatic tachycardia [
146,
154]. Headaches, peripheral edema, and supine hypertension are the most frequent side effects [
154]. Fludrocortisone is contraindicated in treating patients with hypertension, hyperalbuminemia, and systemic fungal infections.
Pyridostigmine, an acetylcholinesterase inhibitor, increases cholinergic neurotransmission at the sympathetic ganglia, and it is used off-label to treat nOH. Under orthostatic stress, this elevated cholinergic tone may increase norepinephrine release [
147,
155]. In a comparative experiment, fludrocortisone appeared more successful than pyridostigmine bromide in increasing peripheral systolic supine BP and mean arterial BP [
146]. Pyridostigmine also caused supine SBP, but not as much as midodrine did. A combination trial of midodrine and pyridostigmine improved the DBP and SBP, making it the most effective treatment for hypotension [
147,
156]. Researchers observed adverse effects that included headaches, vertigo, gastrointestinal distress, tremors in the limbs, and possibly even sadness, apathy, and insomnia [
156]. Similarly, pyridostigmine alone was not as effective as pyridostigmine in combination with other drugs, such as atomoxetine and propranolol or bisoprolol [
157,
158].
Acarbose functions as an inhibitor of α-glucosidase. Due to its ability to slow down the small intestine's enzymatic breakdown of carbs, it is mainly utilized in treating type 2 diabetes. Due to its efficaciousness in reducing the dip in BP that follows meals, acarbose could also be used to manage PPH in individuals with chronic autonomic failure. Lower plasma glucose levels brought on by acarbose treatment may decrease plasma insulin levels. Lowering the plasma levels of insulin, a known vasodilator, lowers PPH [
148]. Occasional cases of reversible elevations in liver transaminase levels have been reported [
159].
The US Food and Drug Administration (FDA) has approved midodrine as a medication for the treatment of OH. Midodrine is an α1 agonist that causes increased peripheral vascular resistance and vasoconstriction, leading to increased BP [
160]. According to reports, midodrine raises standing SBP, lessens sensations of dizziness, and enhances investigators' and patients' overall symptom alleviation scores [
161]. Furthermore, it has been observed that midodrine reduces intradialytic hypotension symptoms [
160]. Pilomotor responses, urine retention, and supine hypertension are the most frequent side effects reported by midodrine users [
161]. Patients with spinal cord injuries who were previously hypotensive and were given 10 mg of midodrine experienced a considerable increase in BP and a decrease in hypotensive episodes [
162].
The FDA has approved atomoxetine, a selective norepinephrine transporter blocker, to treat attention deficit hyperactivity disorder (ADHD) [
62]. It has a well-established track record of long-term effectiveness and safety in treating ADHD patients [
163]. Orthostatic BP drop and OH-related symptoms in patients with non-OH have been successfully treated with atomoxetine at a daily dose of 18 mg [
157]. Due to its potential to improve norepinephrine availability in the synaptic cleft, it has been used off-label to control nOH [
62].
A prodrug of norepinephrine is droxidopa [
164]. The administration of droxidopa, a synthetic amino acid that the enzyme L-aromatic amino acid decarboxylase converts to norepinephrine, raises standing BP, reduces OH symptoms, and enhances a patient's capacity to adjust posture in individuals with non-OH conditions caused by degenerative autonomic disorders such as PD [
165]. Droxidopa has only incidental effects. Headache, lightheadedness, nausea, and infrequently neuroleptic malignant syndrome are typical adverse effects of this medication [
164].
4.4. Current Clinical Trials
It is important to emphasize that the initial line of treatment for OH is always nonpharmacologic. The effect of drinking water on BP in patients with nOH was initially investigated in a study with 28 patients with multiple system atrophy (MSA), 19 patients with PAF, and 19 healthy individuals as controls. This study was conducted in an open-label manner. Following 30 minutes of consuming 500 ml of tap water, the seated SBP and DBP increased by an average of 33 ± 5/16 ± 3 mmHg in patients diagnosed with MSA and by 37 ± 7/14 ± 3 mmHg in patients with PAF (p < 0.0001 compared to the initial measurements in both illnesses). The rise in SBP lasted for more than 60 minutes [
166,
167].
In another clinical trial investigating nonpharmacologic interventions, a total of 15 patients diagnosed with PD and OH were randomly assigned to either use a compressive abdominal binder (20 mmHg of abdominal pressure) or a sham abdominal binder (3 mmHg of abdominal pressure) during a head-up tilt procedure [
16]. The main objective was to measure the average BP following 3 minutes of head-up tilt. The compression abdominal binder resulted in a rise of 7.7 mmHg, while the sham abdominal binder caused a decrease of -2.7 mmHg. Subsequently, there was a 4-week period where all patients used the compression abdominal binder. This led to a reduction of orthostatic symptoms, as indicated by a drop of 2.2 points on the OH Questionnaire (OHQ) compared to the initial measurement (p=0.003) [
16].
Many patients, though, will require pharmacological treatment. Even though the pharmacologic treatment of OH may appear overwhelming, knowing the underlying pathophysiology and the basic principles of clinical pharmacology can help us find the most personalized therapy. Individuals with PAF, PD, degeneration of peripheral noradrenergic neurons, and low plasma norepinephrine seem to respond better to "norepinephrine replacers" (midodrine, droxidopa), and individuals with multiple system atrophy and normal or slightly decreased plasma norepinephrine (relatively sparing sympathetic reserve) typically react better to "norepinephrine enhancers" (pyridostigmine, atomoxetine). Fludrocortisone acetate is an oral synthetic corticosteroid with potent mineralocorticoid effects. A recent study on the effectiveness of fludrocortisone in 13 PD with OH found that taking 0.2 mg of fludrocortisone daily helped lower the orthostatic fall in DBP and raise the mean BP while standing. Acarbose may attenuate the decrease in postprandial BP and prevent hypotension.
An open-label clinical study was conducted recently in which 87 patients with OH were randomly assigned to one of three therapy groups: midodrine 2.5 mg only, pyridostigmine 30 mg solo, or a combination of midodrine 2.5 mg and pyridostigmine 30 mg [
156]. The primary outcome measure was the change in BP (ΔBP) within 3 minutes of standing, assessed three months after treatment. The secondary objectives included assessing the ΔBP after 3 minutes of standing after one month and the OH Questionnaire (OHQ). The authors observed that the SBP and DBP changes were less severe following therapy, independent of the specific medication used. This experiment had a significant bias in multiple aspects: it lacked blinding; despite the authors' claim of exclusively including patients with nOH, no confirmatory tests were conducted, allowing for the potential enrolment of patients with OH from other causes.
The study was a randomized, single-masked, crossover trial involving 65 individuals with nOH. The trial compared the effects of a single atomoxetine dosage (18 mg) with midodrine (5–10 mg) [
168]. The primary measure of interest was the SBP while standing, 1 minute and 1 hour after administering the medication. The secondary endpoints assessed in the study were posttreatment seated SBP and DBP, standing DBP, and HR, as well as scores for the OH questionnaire (OHQ) and OHQ item 1. Atomoxetine caused a substantial increase in standing SBP by 20 mmHg and DBP by 11 mmHg compared to the placebo. This difference was statistically significant, with a p-value of less than 0.001 in both cases. In addition, midodrine caused a considerable increase in standing SBP of 12 mmHg and standing DBP of 7 mmHg compared to the placebo (p < 0.001 for both). Atomoxetine demonstrated a more significant improvement in standing SBP than midodrine, with a mean difference of 7.5 mmHg (95% CI, 0.6 to 14.5; p = 0.03).
Droxidopa was authorized for the treatment of nOH based on three randomized clinical trials that showed improvement in OH symptoms [
81,
169,
170]. Supine hypertension, which refers to high BP while lying down, is the most frequent adverse effect of droxidopa. However, the occurrence of this side effect is relatively low, with a rate of ≤7.9% compared to ≤4.6% for a placebo [
171]. It is worth noting that droxidopa is believed to be safer than midodrine in terms of causing supine hypertension. A recent meta-analysis found that midodrine, but not droxidopa, significantly increases the risk of supine hypertension [
172].
4.5. Approach in Patients with Parkinson’s Disease and Orthostatic Hypotension
OH is a common condition in which BP drops significantly when standing, possibly resulting in dizziness, lightheadedness, and even syncope [
39]. Treatment for OH, particularly with autonomic dysfunction nOH, requires a multifaceted approach [
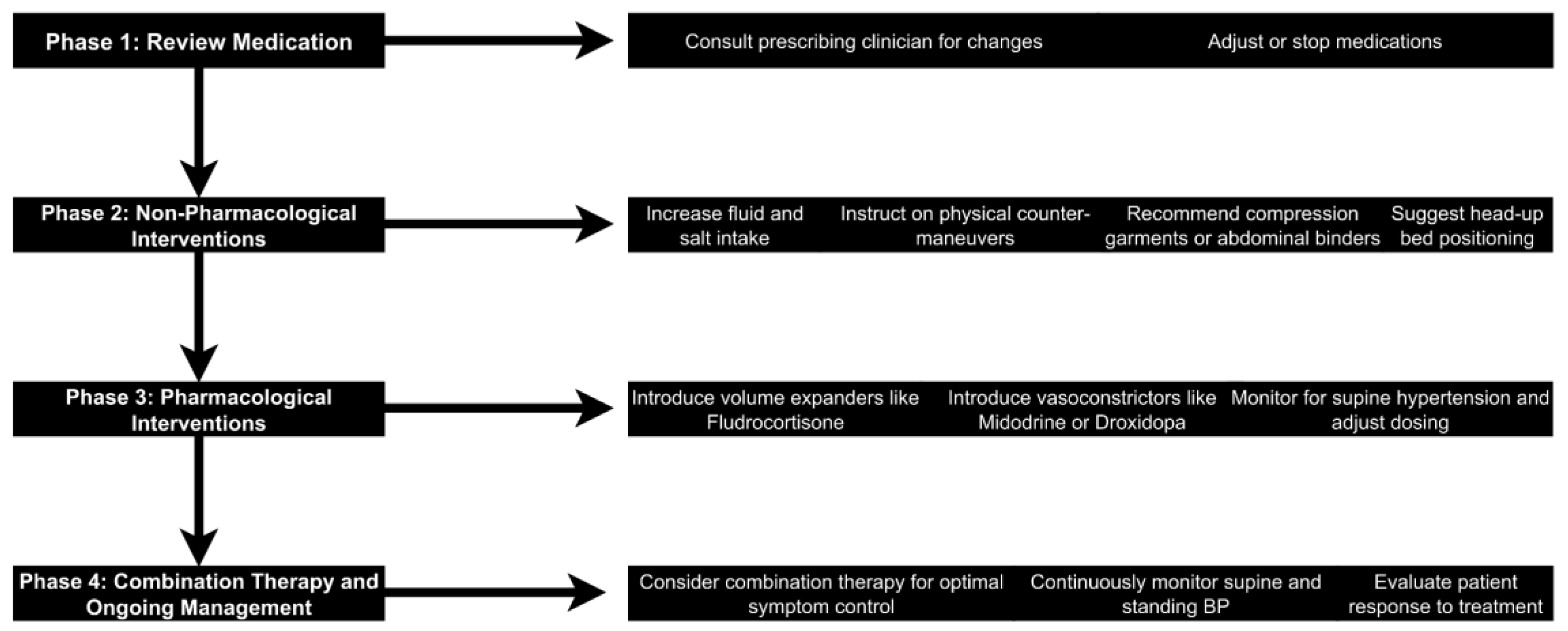
117]. This summary reviews an algorithmic approach to OH management: medication review, non-pharmacological interventions, pharmacological interventions, and combination therapy.
4.5.1. Review and Adjust or Stop Offending Medication
Identifying and correcting any medication potentially contributing to or exacerbating OH are essential first steps in its management [
77]. Many medications have hypotensive effects, including some very commonly used ones for conditions like hypertension, benign prostatic hyperplasia, or even PD [
130]. The drug history, particularly about vasodilators, diuretics, alpha-blockers, and drugs with negative chronotropic actions, must be carefully reviewed by the clinician [
93]. In some patients, withdrawal or reduction in the dose of these medications is adequate to relieve symptoms [
161]. The prescribing clinician must participate in planned medication changes [
117].
4.5.2. Non-Pharmacological Interventions
Non-pharmacological interventions are vital in managing OH and are usually employed as a first line of treatment, particularly during the management of milder cases [
173]. The main aims of such interventions are to increase intravascular volume and minimize venous pooling [
39].
The patient should be encouraged to have an adequate fluid intake of 2 to 2.5 liters of water daily. Likewise, salt intake should be liberalized by adding 1 to 2 teaspoons of salt per day to their diet [
56]. Simple maneuvers like crossing legs on standing, standing on tiptoes, and clenching the buttocks demonstrate increased venous return and orthostatic tolerance [
174]. The patient should be instructed about the benefits of slow positional changes and avoiding standing in one spot [
39].
Waist-high compression stockings may reduce the venous pooling of blood in the legs [
175]. Compliance is often a critical concern, particularly in elderly patients [
153]. Elastic abdominal binders warrant an exemplary alternative with promising results [
16]. Elevating the head of the bed by 6–9 inches can, to a certain extent, reduce supine hypertension and nocturnal diuresis, improving orthostatic tolerance in the morning [
119].
4.5.3. Pharmacological Interventions
Pharmacological treatment is indicated if non-pharmacological measures prove inadequate to control symptoms [
77]. Pharmacological treatment mainly consists of two categories of medications: volume expanders and vasoconstrictors [
93].
Fludrocortisone is a synthetic mineralocorticoid that increases renal sodium and water reabsorption, thereby expanding intravascular volume [
176]. It is frequently used to treat OH but is not FDA-approved for this indication [
39]. Its use is associated with long-term complications, such as supine hypertension, hypokalemia, and edema [
177]. Midodrine is an FDA-approved drug for OH. The α1-adrenergic receptor agonist raises vascular resistance [
161]. Another FDA-approved agent is droxidopa, a norepinephrine pro-drug that directly increases neurotransmitter levels [
81]. Both can cause supine hypertension and need careful dosing [
169].
Combination therapy and ongoing management may include using combination therapies in some cases to achieve optimal symptom control [
77]. Although great caution is required, combining agents that increase BP could worsen supine hypertension [
178]. Regular monitoring is essential for successful treatment. It would be best to guide treatment adjustments by continuously monitoring supine and standing blood pressure [
82]. The assessment of the patient's response to therapy should be multifaceted, concerning improvement in symptoms, tolerance of standing, and impact on daily activities (
Figure 2) [
117].
5. Pathophysiology
5.1. Neuropathology
The sympathetic nervous system is essential for preserving stable BP (
Figure 3). A crucial component of this system is the release of norepinephrine, a neurotransmitter that instructs blood vessels to contract, by postganglionic efferent sympathetic neurons. The degeneration of these particular neurons results in inadequate release of norepinephrine in diseases such as PD. Consequently, this impedes the essential vasoconstriction upon standing, leading to a decrease in BP called OH [
179].
Alpha-synuclein accumulation in Lewy bodies, the pathological hallmark of PD, disrupts autonomic function, particularly in brain regions governing sympathetic activity. This disruption leads to OH, a common and debilitating symptom characterized by a significant drop in BP upon standing. Additionally, alpha-synuclein accumulation triggers chronic inflammation in the brain and can spread to other regions, contributing to the progressive nature of PD and its diverse clinical manifestations [
180].
Neuroinflammation, a hallmark of PD, particularly in the brainstem, is implicated in the development of OH. This complication arises from the detrimental effects of activated microglia and pro-inflammatory cytokines on the nucleus tractus solitarius, a critical BP regulatory center. Further investigations are warranted to elucidate the intricate relationship between neuroinflammation and OH in PD, paving the way for novel therapeutic interventions aimed at preventing or mitigating this debilitating condition [
181].
5.2. Genetic Factors
Several genes are associated with an elevated risk of developing OH in PD. These genes are implicated in various aspects of BP regulation, including the production of norepinephrine, a crucial neurotransmitter responsible for maintaining vascular tone [
64]. α-synuclein is a protein known to be associated with PD, and it is also found in Lewy bodies, which are abnormal protein aggregates characteristic of the disease. Goldstein et al. found that individuals with PD and OH had cardiac sympathetic denervation and a loss of functional sympathetic nerves [
182]. This suggests that α-synuclein may play a role in developing OH in these patients, as it is involved in the loss of sympathetic nerves. While the α-synuclein gene has been the focus of much research regarding its potential role in OH, other genes may also play a role. Interestingly, patients with GBA gene variants (GBA-PD) to 313 idiopathic PD revealed a more significant drop in systolic blood pressure during postural changes in the GBA-PD group. In contrast, the groups' DBP and HR remained similar [
183].
The CYP17A1 gene encodes the enzyme 17α-hydroxylase, which synthesizes cortisol [
184]. Cortisol is a hormone that helps regulate BP, and low cortisol levels can contribute to OH. NPR-C encodes the natriuretic peptide receptor C, which regulates BP by promoting vasodilation and renal sodium and water excretion [
182]. Mutations in this gene have been associated with OH.
In a cross-sectional study of 304 PD patients, specific single-nucleotide polymorphisms (SNPs) were found to influence the risk of nOH, which was present in 11.5% of the cohort. SNPs in genes such as SCN3A, MIR4696, LZTS3/DDRGK1, FAM47E-STBD1, TMEM175, SH3GL2, CCDC62, and SNCA were associated with a higher risk of nOH, while variants in SIPA1L2, ITGA8, NDUFAF2, and IP6K2 were linked to a lower risk. These genetic variants were found to affect the expression of genes within key autonomic nervous system structures and influence metabolic and signaling pathways, notably IP3/Ca²⁺ signaling, the PKA-CREB pathway, and fatty acid metabolism [
185].
5.3. Environmental Factors
Dehydration lowers blood volume, making it more difficult for the heart to pump blood at the proper pressure. Heat causes vasodilation, which widens blood vessels and lowers BP. Sweating also causes the body to lose more fluids, intensifying the consequences of heat. Lastly, several drugs, including antidepressants, diuretics, and vasodilators, can disrupt the body's normal BP regulation, raising the possibility of OH [
186].
Critical preventative measures for this illness include drinking enough water, avoiding heated places, and talking to your doctor about changing your prescription [
187]. Several environmental factors can exacerbate OH in individuals with PD. These include dehydration, exposure to high temperatures, prolonged standing, and sudden changes in body position. Dehydration reduces blood volume, leading to decreased venous return and a subsequent drop in BP. High temperatures cause vasodilation, further decreasing BP. Prolonged standing allows blood to pool in the lower extremities, reducing venous return and BP. Sudden changes in body position, such as standing up quickly, can overwhelm the body's compensatory mechanisms, leading to a transient drop in BP [
188].
5.4. Mechanisms
OH, in PD, is a complex phenomenon with multiple contributing mechanisms. These mechanisms can be broadly categorized into five main areas.
5.4.1. Central Autonomic Dysfunction
Central autonomic dysfunction (CAD), a hallmark of neurodegenerative diseases, plays a critical role in OH by impairing the baroreflex, leading to inadequate BP adjustments upon standing. This dysfunction also manifests as supine hypertension and loss of diurnal BP variation, highlighting the complex autonomic control impairments in these conditions. Understanding the mechanisms of central autonomic dysfunction in OH is crucial for developing effective treatment strategies, including addressing baroreflex impairment, managing associated symptoms like supine hypertension, potentially exploring interventions that target the underlying neurodegenerative processes, and implementing lifestyle modifications to minimize orthostatic stress [
189].
The underlying mechanisms of CAD-induced OH in PD are complex and multifactorial. Degeneration of noradrenergic neurons in the brainstem, particularly within the locus coeruleus (LC), plays a pivotal role. This degeneration disrupts the sympathetic nervous system's ability to effectively increase HR and peripheral vasoconstriction upon standing, leading to inadequate BP compensation [
190]. However, Palermo et al. found no direct link between LC integrity and OH in PD. While PD patients showed reduced LC signal intensity compared to healthy controls, no significant differences existed between those with and without OH. Moreover, the extent of LC damage did not correlate with orthostatic BP drops or the severity of autonomic symptoms, suggesting that additional mechanisms beyond LC degeneration may contribute to OH in PD [
191].
nOH leads to significant orthostatic reductions in cerebral blood flow velocity (CBFv), even in the absence of overt symptoms, potentially contributing to poor neurological outcomes due to subclinical cerebral hypoperfusion. In this context, individuals with nOH experience more significant CBFv declines than healthy controls (Hedge g = -0.64, p < 0.001). However, CBFv reductions did not significantly differ between nOH patients and disease-matched controls without nOH. Importantly, symptomatic nOH patients exhibited more significant CBFv drops than asymptomatic patients (Hedge g = 0.84, p = 0.009), suggesting that symptom severity may reflect more significant cerebral hypoperfusion [
192]. Moreover, in PD patients with OH, near-infrared spectroscopy revealed more substantial and prolonged reductions in oxygenated hemoglobin in the left hemisphere during the head-up tilt test and delayed peak time during the squat-to-stand test, despite no significant differences in transcranial Doppler-measured mean flow velocity [
193]. However, considerable variability exists in the literature regarding fNIRS findings, with 58% of studies reporting a positive correlation between brain oxygenation changes and blood pressure variations, 3% showing a negative correlation, and 39% finding no correlation [
194].
5.4.2. Peripheral Autonomic Dysfunction
Peripheral autonomic dysfunction, characterized by impaired sympathetic nerve activity and reduced vasoconstriction, plays a significant role in the development of OH. This dysfunction manifests as inadequate adjustments in vascular tone upon postural changes, leading to a substantial drop in BP and associated symptoms like dizziness, lightheadedness, and fainting. Beyond its role in OH, peripheral autonomic dysfunction also contributes to supine hypertension. This seemingly paradoxical finding underscores the complex interplay between central and peripheral mechanisms in maintaining BP homeostasis. Understanding the mechanisms of peripheral autonomic dysfunction is paramount for developing effective treatment strategies that target both autonomic dysfunction’s central and peripheral components. Such strategies promise to improve BP control and alleviate the debilitating symptoms of OH in affected individuals [
195].
Peripheral autonomic dysfunction, frequently associated with conditions such as diabetes mellitus and PD, emerges as a significant mechanism underlying OH. This dysfunction compromises the body's ability to effectively regulate BP and HR upon standing, resulting in a substantial drop in BP and symptoms such as dizziness and lightheadedness [
196].
5.4.3. Alpha-Synuclein And Other Toxicities
Alpha-synuclein pathology, a hallmark of PD, extends beyond the central nervous system (CNS), affecting the peripheral autonomic nervous system and potentially contributing to OH, a common and debilitating non-motor symptom in PD. The aggregation of alpha-synuclein in sympathetic and parasympathetic nerves may impair norepinephrine release and baroreceptor reflex function, leading to an inability to maintain adequate BP upon standing. Additionally, alpha-synuclein pathology in the enteric nervous system could contribute to OH through impaired fluid and electrolyte absorption. Understanding the link between alpha-synuclein and OH could pave the way for novel therapeutic strategies to prevent or manage this debilitating symptom in PD patients [
197].
In PD, mitochondrial dysfunction can lead to impaired autonomic nervous system function, resulting in an inability to adjust BP and HR effectively upon standing, which causes OH. Additionally, mitochondrial dysfunction can increase oxidative stress and damage neurons in the autonomic nervous system, further exacerbating OH symptoms [
187].
5.4.4. Cardiac Dysfunction
Cardiac dysfunction stands as a critical factor in the development of hypotension upon standing, a condition marked by a significant drop in BP upon transitioning from a lying to a standing position. This phenomenon arises from the heart's inability to adequately pump blood to meet the body's increased demand upon standing, leading to decreased blood flow to the brain. This insufficient perfusion manifests as dizziness, lightheadedness, and even syncope [
189]. Cardiac dysfunction plays a pivotal role in the pathogenesis of OH. Impaired myocardial contractility and reduced stroke volume, hallmarks of heart failure, directly contribute to an inadequate BP response upon standing. This diminishes the ability to maintain adequate tissue perfusion during orthostasis, leading to the characteristic symptoms of OH [
198]. A review of 54 studies involving 3,114 Parkinson’s disease patients found that ¹²³I-MIBG scintigraphy has high sensitivity (0.81–0.83) and specificity (0.80–0.86) for detecting cardiac sympathetic denervation [
199].
5.4.5. Medication-Associated Hypotension in Parkinson’s Disease
Several medications used to treat PD can contribute to OH. Dopaminergic medications like levodopa can increase dopamine levels in the brain but can cause vasodilation and OH. Also, some anticholinergic drugs, such as benztropine and trihexyphenidyl, may block acetylcholine, affecting BP regulation and potentially leading to OH. Other medications that can also contribute to OH in PD are antidepressants, antipsychotics, and diuretics. The mechanism by which these medications cause OH varies. Dopaminergic drugs can directly cause vasodilation, while anticholinergics can interfere with the autonomic nervous system's ability to regulate BP. Other medications may indirectly affect BP regulation [
200].
While hydrochlorothiazide's vasodilatory effect contributes to its antihypertensive action, it can also lead to OH, a condition characterized by a sudden drop in BP upon standing. This hypotensive response can manifest as dizziness, lightheadedness, and even fainting, posing a significant risk for falls and injuries, especially in the elderly population [
201].
6. Expert Recommendations and Future Studies
Experts recommend careful titration and monitoring of PD medications to reduce the risk of OH. This approach includes identifying drugs that may exacerbate OH, adjusting dosages, or substituting them with alternatives that carry a lower risk. Continuous BP monitoring is essential to evaluate these adjustments' effectiveness and detect potential OH episodes.
The severity and response to treatment for OH vary widely among PD patients, highlighting the need for personalized treatment plans. These plans should consider medication regimens, comorbidities, and lifestyle habits. Developing effective customized treatment plans remains challenging, requiring further research to identify reliable predictors of OH risk and response to different interventions.
Patient education regarding OH is crucial to empower individuals with PD to recognize and manage their symptoms effectively. This education should include information about the causes and symptoms of OH, strategies for preventing OH episodes, and appropriate actions to take if OH occurs. Educational materials and resources should be tailored to the individual's needs and cognitive abilities to ensure practical understanding and implementation.
Cardiovascular autonomic dysfunction has emerged as a potential differentiating factor between tremor-dominant PD (TDPD) and essential tremor (ET), conditions that often present overlapping motor symptoms, complicating diagnosis. Recent studies have demonstrated that heart rate variability (HRV) analysis and OH assessment can aid in distinguishing these disorders. In particular, TDPD patients exhibit more frequent OH and a more significant SBP drop during tilt tests compared to ET patients and healthy controls. Additionally, HRV parameters, such as reduced low-frequency power and decreased variability in RR intervals, are commonly impaired in TDPD but remain unaffected in ET. Diagnostic measures like SBP drop and specific HRV metrics have shown strong discriminative power, with area under the curve (AUC) values exceeding 0.8 [
202].
Future research on OH in PD should focus on developing and evaluating interventions to improve the quality of life for affected individuals. Potential assessment areas include non-pharmacological interventions like physical therapy, compression garments, dietary modifications, and novel pharmacological agents. Physical therapy programs can improve balance, gait, and muscle strength, while compression garments can improve blood flow and reduce OH symptoms. Dietary changes, such as increasing fluid intake and salt consumption, can also impact OH symptoms and overall well-being. Novel pharmacological agents target specific mechanisms underlying OH in PD, such as agents that improve autonomic nervous system function or enhance vascular tone.
An investigation of combining pharmacological and non-pharmacological interventions to address multiple underlying causes of OH in PD could provide more comprehensive and effective management strategies.
7. Conclusions
OH is a common and frequently debilitating non-motor symptom in PD that impacts considerably on the day-to-day lives and general well-being of many patients struggling with this complex neurodegenerative disorder. This review has an overview of the heavy burden of OH in PD, which explains its complex pathophysiology, including central and peripheral autonomic dysfunction, alpha-synuclein accumulation, and the medications for PD. We have underscored the extreme importance of an early and accurate diagnosis, achieved through a detailed clinical evaluation involving thorough history-taking and physical examination, supplemented by specialized tests such as an active standing and autonomic function tests. This review has further underlined the necessity of individualized management strategies that underscore the importance of non-pharmacological interventions, including lifestyle modifications, fluid and salt intake manipulations, and physical counter-maneuvers, while reserving pharmacological interventions, such as volume expanders and vasoconstrictors, for when the former measures are insufficient. It also opens up promising avenues through ongoing clinical trials of novel OH therapies in PD, including new pharmacological agents. This review finally requires a collaborative effort linking clinicians, researchers, and patients together towards increasing our understanding of OH in PD, acting for more effective and tailored treatment strategies, and empowering people living with OH to lead fuller, engaged, and empowered lives.
Author Contributions
J.P.R., I.K., K.I., A.D., A.S.A.S., and A.L.F.C. conceived and designed the methodology of the literature review. J.P.R., I.K., K.I., A.D., and A.S.A.S. extracted and collected the relevant information and drafted the manuscript. A.L.F.C. supervised the article selection and reviewed and edited the manuscript. J.P.R. and A.L.F.C. reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ABM |
Ambulatory blood pressure monitoring |
| ACM |
Ambulatory capacity measure |
| ADL |
Activities of daily living |
| BP |
Blood pressure |
| CNS |
Central nervous system |
| CYP17A1 |
Cytochrome P450 17A1 |
| DBP |
Diastolic blood pressure |
| FDA |
Food and drug administration |
| HR |
Heart rate |
| IADL |
Instrumental activities of daily living |
| MDS |
Movement disorder society |
| MSA |
Multiple system atrophy |
| NPR-C |
Natriuretic peptide receptor C |
| nOH |
Neurogenic orthostatic hypotension |
| OGS |
Orthostatic grading scale |
| OH |
Orthostatic hypotension |
| OHQ |
Orthostatic grading scale |
| PAF |
Pure autonomic failure |
| PD |
Parkinson’s disease |
| PPH |
Post-prandial hypotension |
| PPMI |
Parkinson’s progression markers initiative |
| SBP |
Systolic blood pressure |
| SCOPA-AUT |
Autonomic scale for outcomes in Parkinson's disease |
| UPDRS |
Unified Parkinson's disease rating scale |
References
- Pfeiffer, R.F. Autonomic Dysfunction in Parkinson’s Disease. Neurotherapeutics 2020, 17, 1464–1479. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, D.; Aarsland, D.; Chaudhuri, K.R.; Dobkin, R.D.; Leentjens, A.F.; Rodriguez-Violante, M.; Schrag, A. The Neuropsychiatry of Parkinson’s Disease: Advances and Challenges. Lancet Neurol 2022, 21, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Sveinbjornsdottir, S. The Clinical Symptoms of Parkinson’s Disease. J Neurochem 2016, 139 Suppl 1, 318–324. [Google Scholar] [CrossRef]
- Yong, M.-H.; Fook-Chong, S.; Pavanni, R.; Lim, L.-L.; Tan, E.-K. Case Control Polysomnographic Studies of Sleep Disorders in Parkinson’s Disease. PLoS One 2011, 6, e22511. [Google Scholar] [CrossRef]
- Jost, W.H. Autonomic Dysfunctions in Idiopathic Parkinson’s Disease. J Neurol 2003, 250 Suppl 1, I28–30. [Google Scholar] [CrossRef]
- Malek, N.; Lawton, M.A.; Grosset, K.A.; Bajaj, N.; Barker, R.A.; Burn, D.J.; Foltynie, T.; Hardy, J.; Morris, H.R.; Williams, N.M.; et al. Autonomic Dysfunction in Early Parkinson’s Disease: Results from the United Kingdom Tracking Parkinson’s Study. Mov Disord Clin Pract 2017, 4, 509–516. [Google Scholar] [CrossRef]
- Jost, W.H. Gastrointestinal Dysfunction in Parkinson’s Disease. J Neurol Sci 2010, 289, 69–73. [Google Scholar] [CrossRef]
- Takizawa, C.; Gemmell, E.; Kenworthy, J.; Speyer, R. A Systematic Review of the Prevalence of Oropharyngeal Dysphagia in Stroke, Parkinson’s Disease, Alzheimer’s Disease, Head Injury, and Pneumonia. Dysphagia 2016, 31, 434–441. [Google Scholar] [CrossRef]
- Cersosimo, M.G.; Raina, G.B.; Pellene, L.A.; Micheli, F.E.; Calandra, C.R.; Maiola, R. Weight Loss in Parkinson’s Disease: The Relationship with Motor Symptoms and Disease Progression. Biomed Res Int 2018, 2018, 9642524. [Google Scholar] [CrossRef]
- Sakakibara, R.; Kishi, M.; Ogawa, E.; Tateno, F.; Uchiyama, T.; Yamamoto, T.; Yamanishi, T. Bladder, Bowel, and Sexual Dysfunction in Parkinson’s Disease. Parkinsons Dis 2011, 2011, 924605. [Google Scholar] [CrossRef]
- Yeo, L.; Singh, R.; Gundeti, M.; Barua, J.M.; Masood, J. Urinary Tract Dysfunction in Parkinson’s Disease: A Review. Int Urol Nephrol 2012, 44, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Kanjwal, K.; George, A.; Figueredo, V.M.; Grubb, B.P. Orthostatic Hypotension: Definition, Diagnosis and Management. J Cardiovasc Med (Hagerstown) 2015, 16, 75–81. [Google Scholar] [CrossRef]
- Palma, J.-A.; Kaufmann, H. Orthostatic Hypotension in Parkinson Disease. Clin Geriatr Med 2020, 36, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Cutsforth-Gregory, J.K.; Low, P.A. Neurogenic Orthostatic Hypotension in Parkinson Disease: A Primer. Neurol Ther 2019, 8, 307–324. [Google Scholar] [CrossRef]
- Umehara, T.; Matsuno, H.; Toyoda, C.; Oka, H. Clinical Characteristics of Supine Hypertension in de Novo Parkinson Disease. Clin Auton Res 2016, 26, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Fanciulli, A.; Goebel, G.; Metzler, B.; Sprenger, F.; Poewe, W.; Wenning, G.K.; Seppi, K. Elastic Abdominal Binders Attenuate Orthostatic Hypotension in Parkinson’s Disease. Mov Disord Clin Pract 2016, 3, 156–160. [Google Scholar] [CrossRef]
- Sommer, S.; Aral-Becher, B.; Jost, W. Nondipping in Parkinson’s Disease. Parkinsons Dis 2011, 2011, 897586. [Google Scholar] [CrossRef]
- Palma, J.-A.; Gomez-Esteban, J.C.; Norcliffe-Kaufmann, L.; Martinez, J.; Tijero, B.; Berganzo, K.; Kaufmann, H. Orthostatic Hypotension in Parkinson Disease: How Much You Fall or How Low You Go? Mov Disord 2015, 30, 639–645. [Google Scholar] [CrossRef]
- Allcock, L.M.; Kenny, R.A.; Mosimann, U.P.; Tordoff, S.; Wesnes, K.A.; Hildreth, A.J.; Burn, D.J. Orthostatic Hypotension in Parkinson’s Disease: Association with Cognitive Decline? Int J Geriatr Psychiatry 2006, 21, 778–783. [Google Scholar] [CrossRef]
- Adhiyaman, V.; Hobson, P.; Meara, R.J. Central and Peripheral Autonomic Integrity in Parkinson’s Disease. Age Ageing 2008, 37, 578–581. [Google Scholar] [CrossRef]
- Goldstein, D.S.; Holmes, C.; Bentho, O.; Sato, T.; Moak, J.; Sharabi, Y.; Imrich, R.; Conant, S.; Eldadah, B.A. Biomarkers to Detect Central Dopamine Deficiency and Distinguish Parkinson Disease from Multiple System Atrophy. Parkinsonism Relat Disord 2008, 14, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Haensch, C.-A.; Lerch, H.; Jörg, J.; Isenmann, S. Cardiac Denervation Occurs Independent of Orthostatic Hypotension and Impaired Heart Rate Variability in Parkinson’s Disease. Parkinsonism Relat Disord 2009, 15, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Jamnadas-Khoda, J.; Koshy, S.; Mathias, C.J.; Muthane, U.B.; Ragothaman, M.; Dodaballapur, S.K. Are Current Recommendations to Diagnose Orthostatic Hypotension in Parkinson’s Disease Satisfactory? Mov Disord 2009, 24, 1747–1751. [Google Scholar] [CrossRef]
- Matinolli, M.; Korpelainen, J.T.; Korpelainen, R.; Sotaniemi, K.A.; Myllylä, V.V. Orthostatic Hypotension, Balance and Falls in Parkinson’s Disease. Mov Disord 2009, 24, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Herting, B.; Prieur, S.; Junghanns, S.; Schweitzer, K.; Globas, C.; Schöls, L.; Reichmann, H.; Berg, D.; Ziemssen, T. Valsalva Manoeuvre in Patients with Different Parkinsonian Disorders. J Neural Transm (Vienna) 2009, 116, 875–880. [Google Scholar] [CrossRef]
- Shibata, M.; Morita, Y.; Shimizu, T.; Takahashi, K.; Suzuki, N. Cardiac Parasympathetic Dysfunction Concurrent with Cardiac Sympathetic Denervation in Parkinson’s Disease. J Neurol Sci 2009, 276, 79–83. [Google Scholar] [CrossRef]
- Wüllner, U.; Schmitz-Hübsch, T.; Antony, G.; Fimmers, R.; Spottke, A.; Oertel, W.H.; Deuschl, G.; Klockgether, T.; Eggert, K. Autonomic Dysfunction in 3414 Parkinson’s Disease Patients Enrolled in the German Network on Parkinson’s Disease (KNP e.V.): The Effect of Ageing. Eur J Neurol 2007, 14, 1405–1408. [Google Scholar] [CrossRef]
- Senard, J.M.; Raï, S.; Lapeyre-Mestre, M.; Brefel, C.; Rascol, O.; Rascol, A.; Montastruc, J.L. Prevalence of Orthostatic Hypotension in Parkinson’s Disease. J Neurol Neurosurg Psychiatry 1997, 63, 584–589. [Google Scholar] [CrossRef]
- Ha, A.D.; Brown, C.H.; York, M.K.; Jankovic, J. The Prevalence of Symptomatic Orthostatic Hypotension in Patients with Parkinson’s Disease and Atypical Parkinsonism. Parkinsonism Relat Disord 2011, 17, 625–628. [Google Scholar] [CrossRef]
- Merola, A.; Romagnolo, A.; Rosso, M.; Lopez-Castellanos, J.R.; Wissel, B.D.; Larkin, S.; Bernardini, A.; Zibetti, M.; Maule, S.; Lopiano, L.; et al. Orthostatic Hypotension in Parkinson’s Disease: Does It Matter If Asymptomatic? Parkinsonism Relat Disord 2016, 33, 65–71. [Google Scholar] [CrossRef]
- Klanbut, S.; Phattanarudee, S.; Wongwiwatthananukit, S.; Suthisisang, C.; Bhidayasiri, R. Symptomatic Orthostatic Hypotension in Parkinson’s Disease Patients: Prevalence, Associated Factors and Its Impact on Balance Confidence. J Neurol Sci 2018, 385, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Beach, P.; McKay, J.L. Longitudinal Prevalence of Neurogenic Orthostatic Hypotension in the Idiopathic Parkinson Progression Marker Initiative (PPMI) Cohort. Auton Neurosci 2024, 253, 103173. [Google Scholar] [CrossRef] [PubMed]
- Merola, A.; Romagnolo, A.; Rosso, M.; Suri, R.; Berndt, Z.; Maule, S.; Lopiano, L.; Espay, A.J. Autonomic Dysfunction in Parkinson’s Disease: A Prospective Cohort Study. Mov Disord 2018, 33, 391–397. [Google Scholar] [CrossRef]
- Velseboer, D.C.; de Haan, R.J.; Wieling, W.; Goldstein, D.S.; de Bie, R.M.A. Prevalence of Orthostatic Hypotension in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Parkinsonism Relat Disord 2011, 17, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Mei, S.; Wang, X.; Mao, W.; Liu, Y.; Tian, Z.; Han, C.; Chan, P. Orthostatic Hypotension: A Clinical Marker for the Body-First Subtype of Patients with Parkinson’s Disease. NPJ Parkinsons Dis 2024, 10, 173. [Google Scholar] [CrossRef]
- Meng, Y.; Tang, T.; Wang, J.; Yu, K. The Correlation of Orthostatic Hypotension in Parkinson Disease with the Disease Course and Severity and Its Impact on Quality of Life. Medicine (Baltimore) 2024, 103, e38169. [Google Scholar] [CrossRef]
- Fanciulli, A.; Leys, F.; Falup-Pecurariu, C.; Thijs, R.; Wenning, G.K. Management of Orthostatic Hypotension in Parkinson’s Disease. J Parkinsons Dis 2020, 10, S57–S64. [Google Scholar] [CrossRef]
- Goldstein, D.S.; Sharabi, Y. Neurogenic Orthostatic Hypotension: A Pathophysiological Approach. Circulation 2009, 119, 139–146. [Google Scholar] [CrossRef]
- Freeman, R. Clinical Practice. Neurogenic Orthostatic Hypotension. N Engl J Med 2008, 358, 615–624. [Google Scholar] [CrossRef]
- Mathias, C.J. To Stand on One’s Own Legs. Clin Med (Lond) 2002, 2, 237–245. [Google Scholar] [CrossRef]
- Gibbons, C.H.; Freeman, R. Clinical Implications of Delayed Orthostatic Hypotension: A 10-Year Follow-up Study. Neurology 2015, 85, 1362–1367. [Google Scholar] [CrossRef] [PubMed]
- Fedorowski, A.; Ricci, F.; Hamrefors, V.; Sandau, K.E.; Hwan Chung, T.; Muldowney, J.A.S.; Gopinathannair, R.; Olshansky, B. Orthostatic Hypotension: Management of a Complex, But Common, Medical Problem. Circ Arrhythm Electrophysiol 2022, 15, e010573. [Google Scholar] [CrossRef]
- Torabi, P.; Ricci, F.; Hamrefors, V.; Sutton, R.; Fedorowski, A. Classical and Delayed Orthostatic Hypotension in Patients With Unexplained Syncope and Severe Orthostatic Intolerance. Front Cardiovasc Med 2020, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- van Wijnen, V.K.; Harms, M.P.M.; Go-Schön, I.K.; Westerhof, B.E.; Krediet, C.T.P.; Stewart, J.; Wieling, W. Initial Orthostatic Hypotension in Teenagers and Young Adults. Clin Auton Res 2016, 26, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Novak, V.; Novak, P.; Spies, J.M.; Low, P.A. Autoregulation of Cerebral Blood Flow in Orthostatic Hypotension. Stroke 1998, 29, 104–111. [Google Scholar] [CrossRef]
- Ohashi, N.; Yasumura, S.; Nakagawa, H.; Shojaku, H.; Mizukoshi, K. Cerebral Autoregulation in Patients with Orthostatic Hypotension. Ann Otol Rhinol Laryngol 1991, 100, 841–844. [Google Scholar] [CrossRef]
- Centi, J.; Freeman, R.; Gibbons, C.H.; Neargarder, S.; Canova, A.O.; Cronin-Golomb, A. Effects of Orthostatic Hypotension on Cognition in Parkinson Disease. Neurology 2017, 88, 17–24. [Google Scholar] [CrossRef]
- Mol, A.; Bui Hoang, P.T.S.; Sharmin, S.; Reijnierse, E.M.; van Wezel, R.J.A.; Meskers, C.G.M.; Maier, A.B. Orthostatic Hypotension and Falls in Older Adults: A Systematic Review and Meta-Analysis. J Am Med Dir Assoc 2019, 20, 589–597.e5. [Google Scholar] [CrossRef]
- Thijs, R.D.; Brignole, M.; Falup-Pecurariu, C.; Fanciulli, A.; Freeman, R.; Guaraldi, P.; Jordan, J.; Habek, M.; Hilz, M.; Traon, A.P.-L.; et al. Recommendations for Tilt Table Testing and Other Provocative Cardiovascular Autonomic Tests in Conditions That May Cause Transient Loss of Consciousness : Consensus Statement of the European Federation of Autonomic Societies (EFAS) Endorsed by the American Autonomic Society (AAS) and the European Academy of Neurology (EAN). Clin Auton Res 2021, 31, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Tipton, P.W.; Cheshire, W.P. Mechanisms Underlying Unawareness of Neurogenic Orthostatic Hypotension. Clin Auton Res 2020, 30, 279–281. [Google Scholar] [CrossRef]
- Mahajan, A.; Duque, K.R.; Dwivedi, A.K.; Abanto, J.; Marsili, L.; Hill, E.J.; Saraf, A.; McDonald, K.J.; Arowosegbe, A.; Deraz, H.A.; et al. Exploring the Intersection between Orthostatic Hypotension and Daytime Sleepiness in Parkinson’s Disease. J Neurol Sci 2025, 468, 123366. [Google Scholar] [CrossRef] [PubMed]
- Imbalzano, G.; Ledda, C.; Tangari, M.M.; Artusi, C.A.; Montanaro, E.; Rizzone, M.G.; Zibetti, M.; Lopiano, L.; Romagnolo, A. Unraveling the Stride: Exploring the Influence of Neurogenic Orthostatic Hypotension on Gait and Balance in Parkinson’s Disease. Clin Auton Res 2024, 34, 593–601. [Google Scholar] [CrossRef]
- Lagi, A.; Bacalli, S.; Cencetti, S.; Paggetti, C.; Colzi, L. Cerebral Autoregulation in Orthostatic Hypotension. A Transcranial Doppler Study. Stroke 1994, 25, 1771–1775. [Google Scholar] [CrossRef]
- Allcock, L.M.; Ullyart, K.; Kenny, R.A.; Burn, D.J. Frequency of Orthostatic Hypotension in a Community Based Cohort of Patients with Parkinson’s Disease. J Neurol Neurosurg Psychiatry 2004, 75, 1470–1471. [Google Scholar] [CrossRef] [PubMed]
- Finucane, C.; O’Connell, M.D.L.; Donoghue, O.; Richardson, K.; Savva, G.M.; Kenny, R.A. Impaired Orthostatic Blood Pressure Recovery Is Associated with Unexplained and Injurious Falls. J Am Geriatr Soc 2017, 65, 474–482. [Google Scholar] [CrossRef]
- Schroeder, C.; Jordan, J.; Kaufmann, H. Management of Neurogenic Orthostatic Hypotension in Patients with Autonomic Failure. Drugs 2013, 73, 1267–1279. [Google Scholar] [CrossRef]
- Rose, K.M.; Eigenbrodt, M.L.; Biga, R.L.; Couper, D.J.; Light, K.C.; Sharrett, A.R.; Heiss, G. Orthostatic Hypotension Predicts Mortality in Middle-Aged Adults: The Atherosclerosis Risk In Communities (ARIC) Study. Circulation 2006, 114, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Barrio, I.; Miki, Y.; Jaunmuktane, Z.T.; Warner, T.; De Pablo-Fernandez, E. Association Between Orthostatic Hypotension and Dementia in Patients With Parkinson Disease and Multiple System Atrophy. Neurology 2023, 100, e998–e1008. [Google Scholar] [CrossRef]
- Fanciulli, A.; Kerer, K.; Leys, F.; Seppi, K.; Kaufmann, H.; Norcliffe-Kaufmann, L.; Wenning, G.K. Validation of the Neurogenic Orthostatic Hypotension Ratio with Active Standing. Ann Neurol 2020, 88, 643–645. [Google Scholar] [CrossRef]
- van Twist, D.J.L.; Harms, M.P.M.; van Wijnen, V.K.; Claydon, V.E.; Freeman, R.; Cheshire, W.P.; Wieling, W. Diagnostic Criteria for Initial Orthostatic Hypotension: A Narrative Review. Clin Auton Res 2021, 31, 685–698. [Google Scholar] [CrossRef]
- Omboni, S.; Smit, A.A.; van Lieshout, J.J.; Settels, J.J.; Langewouters, G.J.; Wieling, W. Mechanisms Underlying the Impairment in Orthostatic Tolerance after Nocturnal Recumbency in Patients with Autonomic Failure. Clin Sci (Lond) 2001, 101, 609–618. [Google Scholar] [PubMed]
- Cheshire, W.P. Chemical Pharmacotherapy for the Treatment of Orthostatic Hypotension. Expert Opin Pharmacother 2019, 20, 187–199. [Google Scholar] [CrossRef]
- Wieling, W.; Kaufmann, H.; Claydon, V.E.; van Wijnen, V.K.; Harms, M.P.M.; Juraschek, S.P.; Thijs, R.D. Diagnosis and Treatment of Orthostatic Hypotension. Lancet Neurol 2022, 21, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.S. Cardiac Denervation in Patients with Parkinson Disease. Cleve Clin J Med 2007, 74 Suppl 1, S91–94. [Google Scholar] [CrossRef]
- Goldstein, D.S.; Sewell, L.; Sharabi, Y. Autonomic Dysfunction in PD: A Window to Early Detection? J Neurol Sci 2011, 310, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D.; Robertson, R.M. Causes of Chronic Orthostatic Hypotension. Arch Intern Med 1994, 154, 1620–1624. [Google Scholar]
- Low, P.A.; Singer, W. Management of Neurogenic Orthostatic Hypotension: An Update. Lancet Neurol 2008, 7, 451–458. [Google Scholar] [CrossRef]
- Cooper, V.L.; Hainsworth, R. Effects of Head-up Tilting on Baroreceptor Control in Subjects with Different Tolerances to Orthostatic Stress. Clin Sci (Lond) 2002, 103, 221–226. [Google Scholar] [CrossRef]
- Kaufmann, H.; Norcliffe-Kaufmann, L.; Palma, J.-A. Baroreflex Dysfunction. N Engl J Med 2020, 382, 163–178. [Google Scholar] [CrossRef]
- Goldstein, D.S.; Holmes, C.S.; Dendi, R.; Bruce, S.R.; Li, S.-T. Orthostatic Hypotension from Sympathetic Denervation in Parkinson’s Disease. Neurology 2002, 58, 1247–1255. [Google Scholar] [CrossRef]
- Goldstein, D.S.; Holmes, C.; Sharabi, Y.; Wu, T. Survival in Synucleinopathies: A Prospective Cohort Study. Neurology 2015, 85, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- Masaki, K.H.; Schatz, I.J.; Burchfiel, C.M.; Sharp, D.S.; Chiu, D.; Foley, D.; Curb, J.D. Orthostatic Hypotension Predicts Mortality in Elderly Men: The Honolulu Heart Program. Circulation 1998, 98, 2290–2295. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, H.; Norcliffe-Kaufmann, L.; Palma, J.-A.; Biaggioni, I.; Low, P.A.; Singer, W.; Goldstein, D.S.; Peltier, A.C.; Shibao, C.A.; Gibbons, C.H.; et al. Natural History of Pure Autonomic Failure: A United States Prospective Cohort. Ann Neurol 2017, 81, 287–297. [Google Scholar] [CrossRef]
- Smit, A.A.; Halliwill, J.R.; Low, P.A.; Wieling, W. Pathophysiological Basis of Orthostatic Hypotension in Autonomic Failure. J Physiol 1999, 519 Pt 1, 1–10. [Google Scholar] [CrossRef]
- Ziemssen, T.; Reichmann, H. Cardiovascular Autonomic Dysfunction in Parkinson’s Disease. J Neurol Sci 2010, 289, 74–80. [Google Scholar] [CrossRef]
- Palma, J.-A.; Kaufmann, H. Epidemiology, Diagnosis, and Management of Neurogenic Orthostatic Hypotension. Mov Disord Clin Pract 2017, 4, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Shibao, C.; Okamoto, L.; Biaggioni, I. Pharmacotherapy of Autonomic Failure. Pharmacol Ther 2012, 134, 279–286. [Google Scholar] [CrossRef]
- Okamoto, L.E.; Shibao, C.; Gamboa, A.; Choi, L.; Diedrich, A.; Raj, S.R.; Black, B.K.; Robertson, D.; Biaggioni, I. Synergistic Effect of Norepinephrine Transporter Blockade and α-2 Antagonism on Blood Pressure in Autonomic Failure. Hypertension 2012, 59, 650–656. [Google Scholar] [CrossRef]
- Shiraishi, T.; Umehara, T.; Oka, H.; Nakahara, A.; Sato, T.; Matsuno, H.; Komatsu, T.; Omoto, S.; Murakami, H.; Iguchi, Y. Clinical and Neuroendocrinological Characteristics of Delayed Orthostatic Hypotension in Parkinson’s Disease. Clin Auton Res 2021, 31, 425–431. [Google Scholar] [CrossRef]
- Low, P.A.; Tomalia, V.A.; Park, K.-J. Autonomic Function Tests: Some Clinical Applications. J Clin Neurol 2013, 9, 1–8. [Google Scholar] [CrossRef]
- Kaufmann, H.; Freeman, R.; Biaggioni, I.; Low, P.; Pedder, S.; Hewitt, L.A.; Mauney, J.; Feirtag, M.; Mathias, C.J. Droxidopa for Neurogenic Orthostatic Hypotension: A Randomized, Placebo-Controlled, Phase 3 Trial. Neurology 2014, 83, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Norcliffe-Kaufmann, L.; Kaufmann, H. Is Ambulatory Blood Pressure Monitoring Useful in Patients with Chronic Autonomic Failure? Clin Auton Res 2014, 24, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, S.H.; Dashtipour, K.; Mehdirad, A.A.; Peltier, A.C. Management Strategies for Comorbid Supine Hypertension in Patients with Neurogenic Orthostatic Hypotension. Curr Neurol Neurosci Rep 2021, 21, 18. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; De Backer, G.; Dominiczak, A.; Cifkova, R.; Fagard, R.; Germano, G.; Grassi, G.; Heagerty, A.M.; Kjeldsen, S.E.; Laurent, S.; et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2007, 28, 1462–1536. [Google Scholar] [CrossRef]
- Freeman, R. Diabetic Autonomic Neuropathy. Handb Clin Neurol 2014, 126, 63–79. [Google Scholar] [CrossRef]
- Cheshire, W.P.; Freeman, R.; Gibbons, C.H.; Cortelli, P.; Wenning, G.K.; Hilz, M.J.; Spies, J.M.; Lipp, A.; Sandroni, P.; Wada, N.; et al. Electrodiagnostic Assessment of the Autonomic Nervous System: A Consensus Statement Endorsed by the American Autonomic Society, American Academy of Neurology, and the International Federation of Clinical Neurophysiology. Clin Neurophysiol 2021, 132, 666–682. [Google Scholar] [CrossRef]
- Lee, S.H.; Paik, S.-H.; Kang, S.-Y.; Phillips, Z. 5th; Kim, J.B.; Kim, B.-J.; Kim, B.-M. Convolutional Neural Networks Can Detect Orthostatic Hypotension in Parkinson’s Disease Using Resting-State Functional near-Infrared Spectroscopy Data. J Biophotonics 2024, 17, e202400138. [Google Scholar] [CrossRef]
- Lim, K.B.; Lim, S.-Y.; Hor, J.W.; Krishnan, H.; Mortadza, F.; Lim, J.L.; Chinna, K.; Saedon, N.I.; Tan, A.H. Orthostatic Hypotension in Parkinson’s Disease: Sit-to-Stand vs. Supine-to-Stand Protocol and Clinical Correlates. Parkinsonism Relat Disord 2024, 123, 106980. [Google Scholar] [CrossRef]
- Marques, T.M.; van Rumund, A.; Oeckl, P.; Kuiperij, H.B.; Esselink, R.A.J.; Bloem, B.R.; Otto, M.; Verbeek, M.M. Serum NFL Discriminates Parkinson Disease from Atypical Parkinsonisms. Neurology 2019, 92, e1479–e1486. [Google Scholar] [CrossRef]
- Park, D.G.; Kim, J.W.; An, Y.-S.; Chang, J.; Yoon, J.H. Plasma Neurofilament Light Chain Level and Orthostatic Hypotension in Early Parkinson’s Disease. J Neural Transm (Vienna) 2021, 128, 1853–1861. [Google Scholar] [CrossRef]
- Sarasin, F.P.; Louis-Simonet, M.; Carballo, D.; Slama, S.; Junod, A.-F.; Unger, P.-F. Prevalence of Orthostatic Hypotension among Patients Presenting with Syncope in the ED. Am J Emerg Med 2002, 20, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Hiorth, Y.H.; Pedersen, K.F.; Dalen, I.; Tysnes, O.-B.; Alves, G. Orthostatic Hypotension in Parkinson Disease: A 7-Year Prospective Population-Based Study. Neurology 2019, 93, e1526–e1534. [Google Scholar] [CrossRef] [PubMed]
- Freeman, R.; Wieling, W.; Axelrod, F.B.; Benditt, D.G.; Benarroch, E.; Biaggioni, I.; Cheshire, W.P.; Chelimsky, T.; Cortelli, P.; Gibbons, C.H.; et al. Consensus Statement on the Definition of Orthostatic Hypotension, Neurally Mediated Syncope and the Postural Tachycardia Syndrome. Auton Neurosci 2011, 161, 46–48. [Google Scholar] [CrossRef] [PubMed]
- Pavy-Le Traon, A.; Amarenco, G.; Duerr, S.; Kaufmann, H.; Lahrmann, H.; Shaftman, S.R.; Tison, F.; Wenning, G.K.; Goetz, C.G.; Poewe, W.; et al. The Movement Disorders Task Force Review of Dysautonomia Rating Scales in Parkinson’s Disease with Regard to Symptoms of Orthostatic Hypotension. Mov Disord 2011, 26, 1985–1992. [Google Scholar] [CrossRef]
- Visser, M.; Marinus, J.; Stiggelbout, A.M.; Van Hilten, J.J. Assessment of Autonomic Dysfunction in Parkinson’s Disease: The SCOPA-AUT. Mov Disord 2004, 19, 1306–1312. [Google Scholar] [CrossRef] [PubMed]
- Papapetropoulos, S.; Paschalis, C.; Athanassiadou, A.; Papadimitriou, A.; Ellul, J.; Polymeropoulos, M.H.; Papapetropoulos, T. Clinical Phenotype in Patients with Alpha-Synuclein Parkinson’s Disease Living in Greece in Comparison with Patients with Sporadic Parkinson’s Disease. J Neurol Neurosurg Psychiatry 2001, 70, 662–665. [Google Scholar] [CrossRef]
- Rodriguez-Blazquez, C.; Forjaz, M.J.; Frades-Payo, B.; de Pedro-Cuesta, J.; Martinez-Martin, P. Independent Validation of the Scales for Outcomes in Parkinson’s Disease-Autonomic (SCOPA-AUT). Eur J Neurol 2010, 17, 194–201. [Google Scholar] [CrossRef]
- Suarez, G.A.; Opfer-Gehrking, T.L.; Offord, K.P.; Atkinson, E.J.; O’Brien, P.C.; Low, P.A. The Autonomic Symptom Profile: A New Instrument to Assess Autonomic Symptoms. Neurology 1999, 52, 523–528. [Google Scholar] [CrossRef]
- Schoffer, K.L.; Henderson, R.D.; O’Maley, K.; O’Sullivan, J.D. Nonpharmacological Treatment, Fludrocortisone, and Domperidone for Orthostatic Hypotension in Parkinson’s Disease. Mov Disord 2007, 22, 1543–1549. [Google Scholar] [CrossRef]
- Low, P.A. Composite Autonomic Scoring Scale for Laboratory Quantification of Generalized Autonomic Failure. Mayo Clin Proc 1993, 68, 748–752. [Google Scholar] [CrossRef]
- Kaufmann, H.; Malamut, R.; Norcliffe-Kaufmann, L.; Rosa, K.; Freeman, R. The Orthostatic Hypotension Questionnaire (OHQ): Validation of a Novel Symptom Assessment Scale. Clin Auton Res 2012, 22, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Frith, J.; Newton, J.L. Validation of a Questionnaire for Orthostatic Hypotension for Routine Clinical Use. Geriatr Gerontol Int 2016, 16, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Schrezenmaier, C.; Gehrking, J.A.; Hines, S.M.; Low, P.A.; Benrud-Larson, L.M.; Sandroni, P. Evaluation of Orthostatic Hypotension: Relationship of a New Self-Report Instrument to Laboratory-Based Measures. Mayo Clin Proc 2005, 80, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, K.R.; Martinez-Martin, P.; Schapira, A.H.V.; Stocchi, F.; Sethi, K.; Odin, P.; Brown, R.G.; Koller, W.; Barone, P.; MacPhee, G.; et al. International Multicenter Pilot Study of the First Comprehensive Self-Completed Nonmotor Symptoms Questionnaire for Parkinson’s Disease: The NMSQuest Study. Mov Disord 2006, 21, 916–923. [Google Scholar] [CrossRef]
- Martinez-Martin, P.; Schapira, A.H.V.; Stocchi, F.; Sethi, K.; Odin, P.; MacPhee, G.; Brown, R.G.; Naidu, Y.; Clayton, L.; Abe, K.; et al. Prevalence of Nonmotor Symptoms in Parkinson’s Disease in an International Setting; Study Using Nonmotor Symptoms Questionnaire in 545 Patients. Mov Disord 2007, 22, 1623–1629. [Google Scholar] [CrossRef]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale Presentation and Clinimetric Testing Results. Mov Disord 2008, 23, 2129–2170. [Google Scholar] [CrossRef]
- Chaudhuri, K.R.; Martinez-Martin, P.; Brown, R.G.; Sethi, K.; Stocchi, F.; Odin, P.; Ondo, W.; Abe, K.; Macphee, G.; Macmahon, D.; et al. The Metric Properties of a Novel Non-Motor Symptoms Scale for Parkinson’s Disease: Results from an International Pilot Study. Mov Disord 2007, 22, 1901–1911. [Google Scholar] [CrossRef]
- Hobart, J.C.; Cano, S.J.; Zajicek, J.P.; Thompson, A.J. Rating Scales as Outcome Measures for Clinical Trials in Neurology: Problems, Solutions, and Recommendations. Lancet Neurol 2007, 6, 1094–1105. [Google Scholar] [CrossRef]
- Freeman, R.; Illigens, B.M.W.; Lapusca, R.; Campagnolo, M.; Abuzinadah, A.R.; Bonyhay, I.; Sinn, D.-I.; Miglis, M.; White, J.; Gibbons, C.H. Symptom Recognition Is Impaired in Patients With Orthostatic Hypotension. Hypertension 2020, 75, 1325–1332. [Google Scholar] [CrossRef]
- Sforza, M.; Assogna, F.; Rinaldi, D.; Sette, G.; Tagliente, S.; Pontieri, F.E. Orthostatic Hypotension Acutely Impairs Executive Functions in Parkinson’s Disease. Neurol Sci 2018, 39, 1459–1462. [Google Scholar] [CrossRef]
- Khalil, I.; Sayad, R.; Kedwany, A.M.; Sayed, H.H.; Caprara, A.L.F.; Rissardo, J.P. Cardiovascular Dysautonomia and Cognitive Impairment in Parkinson’s Disease (Review). Med Int (Lond) 2024, 4, 70. [Google Scholar] [CrossRef]
- Anang, J.B.M.; Nomura, T.; Romenets, S.R.; Nakashima, K.; Gagnon, J.-F.; Postuma, R.B. Dementia Predictors in Parkinson Disease: A Validation Study. J Parkinsons Dis 2017, 7, 159–162. [Google Scholar] [CrossRef]
- Heisler, J.M.; Toledo-Atucha, J.; Lin, C.-C.; Patel, H.N.; Ondo, W.G. Orthostatic Hypotension and Subjective Symptomatic Orthostasis in Parkinson’s Disease: Associations and Correlations. Clin Park Relat Disord 2024, 11, 100262. [Google Scholar] [CrossRef] [PubMed]
- Low, P.A.; Opfer-Gehrking, T.L.; McPhee, B.R.; Fealey, R.D.; Benarroch, E.E.; Willner, C.L.; Suarez, G.A.; Proper, C.J.; Felten, J.A.; Huck, C.A. Prospective Evaluation of Clinical Characteristics of Orthostatic Hypotension. Mayo Clin Proc 1995, 70, 617–622. [Google Scholar] [CrossRef]
- Angelousi, A.; Girerd, N.; Benetos, A.; Frimat, L.; Gautier, S.; Weryha, G.; Boivin, J.-M. Association between Orthostatic Hypotension and Cardiovascular Risk, Cerebrovascular Risk, Cognitive Decline and Falls as Well as Overall Mortality: A Systematic Review and Meta-Analysis. J Hypertens 2014, 32, 1562–1571. [Google Scholar] [CrossRef] [PubMed]
- Xin, W.; Lin, Z.; Mi, S. Orthostatic Hypotension and Mortality Risk: A Meta-Analysis of Cohort Studies. Heart 2014, 100, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, C.H.; Schmidt, P.; Biaggioni, I.; Frazier-Mills, C.; Freeman, R.; Isaacson, S.; Karabin, B.; Kuritzky, L.; Lew, M.; Low, P.; et al. The Recommendations of a Consensus Panel for the Screening, Diagnosis, and Treatment of Neurogenic Orthostatic Hypotension and Associated Supine Hypertension. J Neurol 2017, 264, 1567–1582. [Google Scholar] [CrossRef] [PubMed]
- Wieling, W.; van Dijk, N.; Thijs, R.D.; de Lange, F.J.; Krediet, C.T.P.; Halliwill, J.R. Physical Countermeasures to Increase Orthostatic Tolerance. J Intern Med 2015, 277, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Ten Harkel, A.D.; Van Lieshout, J.J.; Wieling, W. Treatment of Orthostatic Hypotension with Sleeping in the Head-up Tilt Position, Alone and in Combination with Fludrocortisone. J Intern Med 1992, 232, 139–145. [Google Scholar] [CrossRef]
- Eschlböck, S.; Wenning, G.; Fanciulli, A. Evidence-Based Treatment of Neurogenic Orthostatic Hypotension and Related Symptoms. J Neural Transm (Vienna) 2017, 124, 1567–1605. [Google Scholar] [CrossRef]
- Schroeder, C.; Bush, V.E.; Norcliffe, L.J.; Luft, F.C.; Tank, J.; Jordan, J.; Hainsworth, R. Water Drinking Acutely Improves Orthostatic Tolerance in Healthy Subjects. Circulation 2002, 106, 2806–2811. [Google Scholar] [CrossRef]
- Thomaides, T.; Bleasdale-Barr, K.; Chaudhuri, K.R.; Pavitt, D.; Marsden, C.D.; Mathias, C.J. Cardiovascular and Hormonal Responses to Liquid Food Challenge in Idiopathic Parkinson’s Disease, Multiple System Atrophy, and Pure Autonomic Failure. Neurology 1993, 43, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, S.; Kang, J.; Miyai, I.; Matsumura, T. [Postprandial hypotension in Parkinson’s disease--the incidence and risk factor]. Rinsho Shinkeigaku 1993, 33, 1135–1139. [Google Scholar] [PubMed]
- Chaudhuri, K.R.; Thomaides, T.; Watson, L.; Mathias, C.J. Octreotide Reduces Alcohol-Induced Hypotension and Orthostatic Symptoms in Primary Autonomic Failure. QJM 1995, 88, 719–725. [Google Scholar]
- Narkiewicz, K.; Cooley, R.L.; Somers, V.K. Alcohol Potentiates Orthostatic Hypotension : Implications for Alcohol-Related Syncope. Circulation 2000, 101, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, L.E.; Diedrich, A.; Baudenbacher, F.J.; Harder, R.; Whitfield, J.S.; Iqbal, F.; Gamboa, A.; Shibao, C.A.; Black, B.K.; Raj, S.R.; et al. Efficacy of Servo-Controlled Splanchnic Venous Compression in the Treatment of Orthostatic Hypotension: A Randomized Comparison With Midodrine. Hypertension 2016, 68, 418–426. [Google Scholar] [CrossRef]
- Newton, J.L.; Frith, J. The Efficacy of Nonpharmacologic Intervention for Orthostatic Hypotension Associated with Aging. Neurology 2018, 91, e652–e656. [Google Scholar] [CrossRef]
- Rose, K.M.; Tyroler, H.A.; Nardo, C.J.; Arnett, D.K.; Light, K.C.; Rosamond, W.; Sharrett, A.R.; Szklo, M. Orthostatic Hypotension and the Incidence of Coronary Heart Disease: The Atherosclerosis Risk in Communities Study. Am J Hypertens 2000, 13, 571–578. [Google Scholar] [CrossRef]
- Fotherby, M.D.; Potter, J.F. Orthostatic Hypotension and Anti-Hypertensive Therapy in the Elderly. Postgrad Med J 1994, 70, 878–881. [Google Scholar] [CrossRef]
- Kamaruzzaman, S.; Watt, H.; Carson, C.; Ebrahim, S. The Association between Orthostatic Hypotension and Medication Use in the British Women’s Heart and Health Study. Age Ageing 2010, 39, 51–56. [Google Scholar] [CrossRef]
- Biaggioni, I.; Robertson, D.; Krantz, S.; Jones, M.; Haile, V. The Anemia of Primary Autonomic Failure and Its Reversal with Recombinant Erythropoietin. Ann Intern Med 1994, 121, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Azarov, I.; Huang, K.T.; Basu, S.; Gladwin, M.T.; Hogg, N.; Kim-Shapiro, D.B. Nitric Oxide Scavenging by Red Blood Cells as a Function of Hematocrit and Oxygenation. J Biol Chem 2005, 280, 39024–39032. [Google Scholar] [CrossRef] [PubMed]
- Allen, B.W.; Piantadosi, C.A. How Do Red Blood Cells Cause Hypoxic Vasodilation? The SNO-Hemoglobin Paradigm. Am J Physiol Heart Circ Physiol 2006, 291, H1507–1512. [Google Scholar] [CrossRef] [PubMed]
- Perera, R.; Isola, L.; Kaufmann, H. Effect of Recombinant Erythropoietin on Anemia and Orthostatic Hypotension in Primary Autonomic Failure. Clin Auton Res 1995, 5, 211–213. [Google Scholar] [CrossRef]
- Toru, S.; Yokota, T.; Inaba, A.; Yamawaki, M.; Yamada, M.; Mizusawa, H.; Hayashi, M. Autonomic Dysfunction and Orthostatic Hypotention Caused by Vitamin B12 Deficiency. J Neurol Neurosurg Psychiatry 1999, 66, 804–805. [Google Scholar] [CrossRef]
- Li, M.; Zhou, L.; Ma, J. [Acupuncture at stellate ganglion combined with western medication for orthostatic hypotension in Parkinson’s disease: a randomized controlled trial]. Zhongguo Zhen Jiu 2024, 44, 1119–1124. [Google Scholar] [CrossRef]
- Rissardo, J.P.; Vora, N.M.; Tariq, I.; Mujtaba, A.; Caprara, A.L.F. Deep Brain Stimulation for the Management of Refractory Neurological Disorders: A Comprehensive Review. Medicina (Kaunas) 2023, 59, 1991. [Google Scholar] [CrossRef]
- Cani, I.; Giannini, G.; Guaraldi, P.; Barletta, G.; Sambati, L.; Baldelli, L.; Cortelli, P.; Calandra-Buonaura, G. Exploring Cardiovascular Autonomic Function before and after Chronic Deep Brain Stimulation in Parkinson’s Disease. Mov Disord Clin Pract 2024, 11, 698–703. [Google Scholar] [CrossRef]
- Pavy-Le Traon, A.; Foubert-Samier, A.; Ory-Magne, F.; Fabbri, M.; Senard, J.-M.; Meissner, W.G.; Rascol, O.; Amar, J. Ambulatory Blood Pressure and Drug Treatment for Orthostatic Hypotension as Predictors of Mortality in Patients with Multiple System Atrophy. Eur J Neurol 2022, 29, 1025–1034. [Google Scholar] [CrossRef]
- Shibao, C.A.; Biaggioni, I. Management of Orthostatic Hypotension, Postprandial Hypotension, and Supine Hypertension. Semin Neurol 2020, 40, 515–522. [Google Scholar] [CrossRef]
- Veazie, S.; Peterson, K.; Ansari, Y.; Chung, K.A.; Gibbons, C.H.; Raj, S.R.; Helfand, M. Fludrocortisone for Orthostatic Hypotension. Cochrane Database Syst Rev 2021, 5, CD012868. [Google Scholar] [CrossRef] [PubMed]
- Shibao, C.A.; Palma, J.-A.; Celedonio, J.E.; Martinez, J.; Kaufmann, H.; Biaggioni, I. Predictors of the Pressor Response to the Norepinephrine Transporter Inhibitor, Atomoxetine, in Neurogenic Orthostatic Hypotension. Hypertension 2021, 78, 525–531. [Google Scholar] [CrossRef]
- Shibao, C.; Okamoto, L.E.; Gamboa, A.; Yu, C.; Diedrich, A.; Raj, S.R.; Robertson, D.; Biaggioni, I. Comparative Efficacy of Yohimbine against Pyridostigmine for the Treatment of Orthostatic Hypotension in Autonomic Failure. Hypertension 2010, 56, 847–851. [Google Scholar] [CrossRef]
- Singer, W.; Opfer-Gehrking, T.L.; McPhee, B.R.; Hilz, M.J.; Bharucha, A.E.; Low, P.A. Acetylcholinesterase Inhibition: A Novel Approach in the Treatment of Neurogenic Orthostatic Hypotension. J Neurol Neurosurg Psychiatry 2003, 74, 1294–1298. [Google Scholar] [CrossRef]
- Furukawa, K.; Suzuki, T.; Ishiguro, H.; Morikawa, H.; Sonoda, K.; Iijima, K.; Ito, M.; Hanyu, O.; Sone, H. Prevention of Postprandial Hypotension-Related Syncope by Caffeine in a Patient with Long-Standing Diabetes Mellitus. Endocr J 2020, 67, 585–592. [Google Scholar] [CrossRef]
- Schreglmann, S.R.; Büchele, F.; Sommerauer, M.; Epprecht, L.; Kägi, G.; Hägele-Link, S.; Götze, O.; Zimmerli, L.; Waldvogel, D.; Baumann, C.R. Pyridostigmine Bromide versus Fludrocortisone in the Treatment of Orthostatic Hypotension in Parkinson’s Disease - a Randomized Controlled Trial. Eur J Neurol 2017, 24, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Singer, W.; Sandroni, P.; Opfer-Gehrking, T.L.; Suarez, G.A.; Klein, C.M.; Hines, S.; O’Brien, P.C.; Slezak, J.; Low, P.A. Pyridostigmine Treatment Trial in Neurogenic Orthostatic Hypotension. Arch Neurol 2006, 63, 513–518. [Google Scholar] [CrossRef]
- Shibao, C.; Gamboa, A.; Diedrich, A.; Dossett, C.; Choi, L.; Farley, G.; Biaggioni, I. Acarbose, an Alpha-Glucosidase Inhibitor, Attenuates Postprandial Hypotension in Autonomic Failure. Hypertension 2007, 50, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Parsaik, A.K.; Singh, B.; Altayar, O.; Mascarenhas, S.S.; Singh, S.K.; Erwin, P.J.; Murad, M.H. Midodrine for Orthostatic Hypotension: A Systematic Review and Meta-Analysis of Clinical Trials. J Gen Intern Med 2013, 28, 1496–1503. [Google Scholar] [CrossRef]
- Byun, J.-I.; Kim, D.-Y.; Moon, J.; Shin, H.-R.; Sunwoo, J.-S.; Lee, W.-J.; Lee, H.-S.; Park, K.-I.; Lee, S.-T.; Jung, K.-H.; et al. Efficacy of Atomoxetine versus Midodrine for Neurogenic Orthostatic Hypotension. Ann Clin Transl Neurol 2020, 7, 112–120. [Google Scholar] [CrossRef]
- Biaggioni, I.; Arthur Hewitt, L.; Rowse, G.J.; Kaufmann, H. Integrated Analysis of Droxidopa Trials for Neurogenic Orthostatic Hypotension. BMC Neurol 2017, 17, 90. [Google Scholar] [CrossRef] [PubMed]
- Bacchi, S.; Chim, I.; Kramer, P.; Postuma, R.B. Domperidone for Hypotension in Parkinson’s Disease: A Systematic Review. J Parkinsons Dis 2017, 7, 603–617. [Google Scholar] [CrossRef] [PubMed]
- Podoleanu, C.; Maggi, R.; Brignole, M.; Croci, F.; Incze, A.; Solano, A.; Puggioni, E.; Carasca, E. Lower Limb and Abdominal Compression Bandages Prevent Progressive Orthostatic Hypotension in Elderly Persons: A Randomized Single-Blind Controlled Study. J Am Coll Cardiol 2006, 48, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Campbell, I.W.; Ewing, D.J.; Clarke, B.F. Therapeutic Experience with Fludrocortisone in Diabetic Postural Hypotension. Br Med J 1976, 1, 872–874. [Google Scholar] [CrossRef]
- Jordan, J.; Fanciulli, A.; Tank, J.; Calandra-Buonaura, G.; Cheshire, W.P.; Cortelli, P.; Eschlboeck, S.; Grassi, G.; Hilz, M.J.; Kaufmann, H.; et al. Management of Supine Hypertension in Patients with Neurogenic Orthostatic Hypotension: Scientific Statement of the American Autonomic Society, European Federation of Autonomic Societies, and the European Society of Hypertension. J Hypertens 2019, 37, 1541–1546. [Google Scholar] [CrossRef]
- Byun, J.-I.; Moon, J.; Kim, D.-Y.; Shin, H.; Sunwoo, J.-S.; Lim, J.-A.; Kim, T.-J.; Lee, W.-J.; Lee, H.S.; Jun, J.-S.; et al. Efficacy of Single or Combined Midodrine and Pyridostigmine in Orthostatic Hypotension. Neurology 2017, 89, 1078–1086. [Google Scholar] [CrossRef]
- Okamoto, L.E.; Shibao, C.A.; Gamboa, A.; Diedrich, A.; Raj, S.R.; Black, B.K.; Robertson, D.; Biaggioni, I. Synergistic Pressor Effect of Atomoxetine and Pyridostigmine in Patients With Neurogenic Orthostatic Hypotension. Hypertension 2019, 73, 235–241. [Google Scholar] [CrossRef]
- Moon, J.; Kim, D.-Y.; Lee, W.-J.; Lee, H.S.; Lim, J.-A.; Kim, T.-J.; Jun, J.-S.; Park, B.; Byun, J.-I.; Sunwoo, J.-S.; et al. Efficacy of Propranolol, Bisoprolol, and Pyridostigmine for Postural Tachycardia Syndrome: A Randomized Clinical Trial. Neurotherapeutics 2018, 15, 785–795. [Google Scholar] [CrossRef]
- Rosak, C.; Mertes, G. Critical Evaluation of the Role of Acarbose in the Treatment of Diabetes: Patient Considerations. Diabetes Metab Syndr Obes 2012, 5, 357–367. [Google Scholar] [CrossRef]
- Prakash, S.; Garg, A.X.; Heidenheim, A.P.; House, A.A. Midodrine Appears to Be Safe and Effective for Dialysis-Induced Hypotension: A Systematic Review. Nephrol Dial Transplant 2004, 19, 2553–2558. [Google Scholar] [CrossRef]
- Low, P.A.; Gilden, J.L.; Freeman, R.; Sheng, K.N.; McElligott, M.A. Efficacy of Midodrine vs Placebo in Neurogenic Orthostatic Hypotension. A Randomized, Double-Blind Multicenter Study. Midodrine Study Group. JAMA 1997, 277, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Wecht, J.M.; Weir, J.P.; Katzelnick, C.G.; Dyson-Hudson, T.A.; Bauman, W.A.; Kirshblum, S.C. Clinical Trial of Home Blood Pressure Monitoring Following Midodrine Administration in Hypotensive Individuals with Spinal Cord Injury. J Spinal Cord Med 2023, 46, 531–539. [Google Scholar] [CrossRef]
- Adler, L.A.; Spencer, T.J.; Williams, D.W.; Moore, R.J.; Michelson, D. Long-Term, Open-Label Safety and Efficacy of Atomoxetine in Adults with ADHD: Final Report of a 4-Year Study. J Atten Disord 2008, 12, 248–253. [Google Scholar] [CrossRef]
- Keating, G.M. Droxidopa: A Review of Its Use in Symptomatic Neurogenic Orthostatic Hypotension. Drugs 2015, 75, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, H. L-Dihydroxyphenylserine (Droxidopa): A New Therapy for Neurogenic Orthostatic Hypotension: The US Experience. Clin Auton Res 2008, 18 Suppl 1, 19–24. [Google Scholar] [CrossRef]
- Jordan, J.; Shannon, J.R.; Black, B.K.; Ali, Y.; Farley, M.; Costa, F.; Diedrich, A.; Robertson, R.M.; Biaggioni, I.; Robertson, D. The Pressor Response to Water Drinking in Humans : A Sympathetic Reflex? Circulation 2000, 101, 504–509. [Google Scholar] [CrossRef]
- May, M.; Jordan, J. The Osmopressor Response to Water Drinking. Am J Physiol Regul Integr Comp Physiol 2011, 300, R40–46. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, C.E.; Okamoto, L.E.; Arnold, A.C.; Gamboa, A.; Diedrich, A.; Choi, L.; Raj, S.R.; Robertson, D.; Biaggioni, I.; Shibao, C.A. Efficacy of Atomoxetine versus Midodrine for the Treatment of Orthostatic Hypotension in Autonomic Failure. Hypertension 2014, 64, 1235–1240. [Google Scholar] [CrossRef]
- Biaggioni, I.; Freeman, R.; Mathias, C.J.; Low, P.; Hewitt, L.A.; Kaufmann, H. Randomized Withdrawal Study of Patients with Symptomatic Neurogenic Orthostatic Hypotension Responsive to Droxidopa. Hypertension 2015, 65, 101–107. [Google Scholar] [CrossRef]
- Hauser, R.A.; Isaacson, S.; Lisk, J.P.; Hewitt, L.A.; Rowse, G. Droxidopa for the Short-Term Treatment of Symptomatic Neurogenic Orthostatic Hypotension in Parkinson’s Disease (nOH306B). Mov Disord 2015, 30, 646–654. [Google Scholar] [CrossRef]
- Fanciulli, A.; Jordan, J.; Biaggioni, I.; Calandra-Buonaura, G.; Cheshire, W.P.; Cortelli, P.; Eschlboeck, S.; Grassi, G.; Hilz, M.J.; Kaufmann, H.; et al. Consensus Statement on the Definition of Neurogenic Supine Hypertension in Cardiovascular Autonomic Failure by the American Autonomic Society (AAS) and the European Federation of Autonomic Societies (EFAS) : Endorsed by the European Academy of Neurology (EAN) and the European Society of Hypertension (ESH). Clin Auton Res 2018, 28, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Han, Y.; Tang, J.; Portillo, I.; Hauser, R.A.; Dashtipour, K. Standing and Supine Blood Pressure Outcomes Associated With Droxidopa and Midodrine in Patients With Neurogenic Orthostatic Hypotension: A Bayesian Meta-Analysis and Mixed Treatment Comparison of Randomized Trials. Ann Pharmacother 2018, 52, 1182–1194. [Google Scholar] [CrossRef]
- Lahrmann, H.; Cortelli, P.; Hilz, M.; Mathias, C.J.; Struhal, W.; Tassinari, M. EFNS Guidelines on the Diagnosis and Management of Orthostatic Hypotension. Eur J Neurol 2006, 13, 930–936. [Google Scholar] [CrossRef]
- Krediet, C.T.P.; van Lieshout, J.J.; Bogert, L.W.J.; Immink, R.V.; Kim, Y.-S.; Wieling, W. Leg Crossing Improves Orthostatic Tolerance in Healthy Subjects: A Placebo-Controlled Crossover Study. Am J Physiol Heart Circ Physiol 2006, 291, H1768–1772. [Google Scholar] [CrossRef] [PubMed]
- Diedrich, A.; Biaggioni, I. Segmental Orthostatic Fluid Shifts. Clin Auton Res 2004, 14, 146–147. [Google Scholar] [CrossRef] [PubMed]
- Chobanian, A.V.; Volicer, L.; Tifft, C.P.; Gavras, H.; Liang, C.S.; Faxon, D. Mineralocorticoid-Induced Hypertension in Patients with Orthostatic Hypotension. N Engl J Med 1979, 301, 68–73. [Google Scholar] [CrossRef]
- Grijalva, C.G.; Biaggioni, I.; Griffin, M.R.; Shibao, C.A. Fludrocortisone Is Associated With a Higher Risk of All-Cause Hospitalizations Compared With Midodrine in Patients With Orthostatic Hypotension. J Am Heart Assoc 2017, 6. [Google Scholar] [CrossRef]
- Jordan, J.; Shannon, J.R.; Biaggioni, I.; Norman, R.; Black, B.K.; Robertson, D. Contrasting Actions of Pressor Agents in Severe Autonomic Failure. Am J Med 1998, 105, 116–124. [Google Scholar] [CrossRef]
- Kaufmann, H.; Goldstein, D.S. Autonomic Dysfunction in Parkinson Disease. Handb Clin Neurol 2013, 117, 259–278. [Google Scholar] [CrossRef]
- Marras, C.; McDermott, M.P.; Rochon, P.A.; Tanner, C.M.; Naglie, G.; Rudolph, A.; Lang, A.E. Survival in Parkinson Disease: Thirteen-Year Follow-up of the DATATOP Cohort. Neurology 2005, 64, 87–93. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K.; Rüb, U.; de Vos, R.A.I.; Jansen Steur, E.N.H.; Braak, E. Staging of Brain Pathology Related to Sporadic Parkinson’s Disease. Neurobiol Aging 2003, 24, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.S.; Tack, C. Noninvasive Detection of Sympathetic Neurocirculatory Failure. Clin Auton Res 2000, 10, 285–291. [Google Scholar] [CrossRef]
- Usnich, T.; Hanssen, H.; Lohmann, K.; Lohse, C.; Klein, C.; Kasten, M.; Brüggemann, N. Pronounced Orthostatic Hypotension in GBA-Related Parkinson’s Disease. J Parkinsons Dis 2022, 12, 1539–1544. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, M.G.; Lake, C.R.; Kopin, I.J. The Sympathetic-Nervous-System Defect in Primary Orthostatic Hypotension. N Engl J Med 1977, 296, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, G.; Udovin, L.; Otero-Losada, M.; Bordet, S.; Capani, F.; Luo, S.; Goetz, C.G.; Perez-Lloret, S. Genetics of Neurogenic Orthostatic Hypotension in Parkinson’s Disease, Results from a Cross-Sectional In Silico Study. Brain Sci 2023, 13. [Google Scholar] [CrossRef]
- Maule, S.; Papotti, G.; Naso, D.; Magnino, C.; Testa, E.; Veglio, F. Orthostatic Hypotension: Evaluation and Treatment. Cardiovasc Hematol Disord Drug Targets 2007, 7, 63–70. [Google Scholar] [CrossRef]
- Rudzińska, M.; Bukowczan, S.; Stożek, J.; Zajdel, K.; Mirek, E.; Chwała, W.; Wójcik-Pędziwiatr, M.; Banaszkiewicz, K.; Szczudlik, A. Causes and Consequences of Falls in Parkinson Disease Patients in a Prospective Study. Neurol Neurochir Pol 2013, 47, 423–430. [Google Scholar] [CrossRef]
- Lach, H.W.; Reed, A.T.; Arfken, C.L.; Miller, J.P.; Paige, G.D.; Birge, S.J.; Peck, W.A. Falls in the Elderly: Reliability of a Classification System. J Am Geriatr Soc 1991, 39, 197–202. [Google Scholar] [CrossRef]
- Low, P.A.; Tomalia, V.A. Orthostatic Hypotension: Mechanisms, Causes, Management. J Clin Neurol 2015, 11, 220–226. [Google Scholar] [CrossRef]
- Smit, A.A.J.; Wieling, W.; Fujimura, J.; Denq, J.C.; Opfer-Gehrking, T.L.; Akarriou, M.; Karemaker, J.M.; Low, P.A. Use of Lower Abdominal Compression to Combat Orthostatic Hypotension in Patients with Autonomic Dysfunction. Clin Auton Res 2004, 14, 167–175. [Google Scholar] [CrossRef]
- Palermo, G.; Galgani, A.; Bellini, G.; Lombardo, F.; Martini, N.; Morganti, R.; Paoli, D.; De Cori, S.; Frijia, F.; Siciliano, G.; et al. Neurogenic Orthostatic Hypotension in Parkinson’s Disease: Is There a Role for Locus Coeruleus Magnetic Resonance Imaging? J Neural Transm (Vienna) 2024, 131, 157–164. [Google Scholar] [CrossRef]
- Baker, J.R.; Beach, P.A.; Ranada, S.I.; Patel, A.; Gewandter, J.; Tan, C.O.; Freeman, R.; Raj, S.R. Cerebral Blood Flow Dynamics in Neurogenic Orthostatic Hypotension: A Systematic Review and Meta-Analysis. Hypertension 2025, 82, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.Y.; Yoo, D.; Kim, J.-M.; Shin, C.; Ahn, T.-B. Effect of Positional Changes on Cerebral Perfusion in Parkinson’s Disease Patients With Orthostatic Hypotension. J Mov Disord 2024, 17, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Shali, R.K.; Setarehdan, S.K.; Seifi, B. Functional Near-Infrared Spectroscopy Based Blood Pressure Variations and Hemodynamic Activity of Brain Monitoring Following Postural Changes: A Systematic Review. Physiol Behav 2024, 281, 114574. [Google Scholar] [CrossRef]
- Arnold, A.C.; Raj, S.R. Orthostatic Hypotension: A Practical Approach to Investigation and Management. Can J Cardiol 2017, 33, 1725–1728. [Google Scholar] [CrossRef] [PubMed]
- Shibao, C.; Lipsitz, L.A.; Biaggioni, I. ASH Position Paper: Evaluation and Treatment of Orthostatic Hypotension. J Clin Hypertens (Greenwich) 2013, 15, 147–153. [Google Scholar] [CrossRef]
- Chen, Z.; Li, G.; Liu, J. Autonomic Dysfunction in Parkinson’s Disease: Implications for Pathophysiology, Diagnosis, and Treatment. Neurobiol Dis 2020, 134, 104700. [Google Scholar] [CrossRef]
- Mar, P.L.; Raj, S.R. Orthostatic Hypotension for the Cardiologist. Curr Opin Cardiol 2018, 33, 66–72. [Google Scholar] [CrossRef]
- Pitton Rissardo, J.; Fornari Caprara, A.L. Cardiac 123I-Metaiodobenzylguanidine (MIBG) Scintigraphy in Parkinson’s Disease: A Comprehensive Review. Brain Sci 2023, 13. [Google Scholar] [CrossRef]
- Mosnaim, A.D.; Abiola, R.; Wolf, M.E.; Perlmuter, L.C. Etiology and Risk Factors for Developing Orthostatic Hypotension. Am J Ther 2010, 17, 86–91. [Google Scholar] [CrossRef]
- Pickkers, P.; Hughes, A.D.; Russel, F.G.; Thien, T.; Smits, P. Thiazide-Induced Vasodilation in Humans Is Mediated by Potassium Channel Activation. Hypertension 1998, 32, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Malkiewicz, J.J.; Siuda, J. Evaluation of Cardiovascular Autonomic Nervous System in Essential Tremor and Tremor Dominant Parkinson’s Disease. Brain Sci 2024, 14. [Google Scholar] [CrossRef]
Figure 1.
The diagram outlines the key steps in diagnosing orthostatic hypotension (OH) in Parkinson's disease (PD), starting with the clinical evaluation. This step includes taking a detailed history, assessing symptoms, conducting a physical examination focusing on blood pressure (BP) and heart rate, and performing a differential diagnosis to distinguish between neurogenic and non-neurogenic OH. Following this, the clinical manifestations of OH are considered, which include a decrease in BP upon standing and neurological symptoms such as lightheadedness, visual disruptions, and cognitive slowing, all of which significantly impact daily activities. The final step involves grading the severity of OH using scales and screening tools like SCOPA-AUT, COMPASS, OHQ, and OGS, focusing on the need for future research and validation to improve these assessment tools.
Figure 1.
The diagram outlines the key steps in diagnosing orthostatic hypotension (OH) in Parkinson's disease (PD), starting with the clinical evaluation. This step includes taking a detailed history, assessing symptoms, conducting a physical examination focusing on blood pressure (BP) and heart rate, and performing a differential diagnosis to distinguish between neurogenic and non-neurogenic OH. Following this, the clinical manifestations of OH are considered, which include a decrease in BP upon standing and neurological symptoms such as lightheadedness, visual disruptions, and cognitive slowing, all of which significantly impact daily activities. The final step involves grading the severity of OH using scales and screening tools like SCOPA-AUT, COMPASS, OHQ, and OGS, focusing on the need for future research and validation to improve these assessment tools.
Figure 2.
The diagram presents a four-phase treatment strategy: Phase 1 involves reviewing and adjusting medications. Phase 2 focuses on non-pharmacological interventions like lifestyle changes and physical maneuvers; Phase 3 introduces medications while monitoring side effects. Phase 4 emphasizes ongoing management through combination therapy and regular monitoring to optimize patient outcomes.
Figure 2.
The diagram presents a four-phase treatment strategy: Phase 1 involves reviewing and adjusting medications. Phase 2 focuses on non-pharmacological interventions like lifestyle changes and physical maneuvers; Phase 3 introduces medications while monitoring side effects. Phase 4 emphasizes ongoing management through combination therapy and regular monitoring to optimize patient outcomes.
Figure 3.
This figure illustrates the complex factors underpinning orthostatic hypotension in Parkinson's disease within a circular framework, hopefully putting complete multifaceted neuropathological, genetic, and environmental interactions and mechanisms into view. In the second place, it definitely represents that at the very bottom of this condition lies degeneration of the sympathetic nervous system, further increased by the pathologic accumulation of alpha-synuclein and chronic neuroinflammation. Genetic predispositions, especially mutations in genes such as alpha-synuclein, CYP17A1, and NPR-C, complicate BP regulation even more, enhancing the chance of OH. Environmental stress, dehydration, heat, and adverse side effects of some medications provide other extra layers of vulnerability that uninterruptedly interfere with the body's already prejudiced autonomic functions. Thus, the main mechanisms underlying OH are deeply rooted in central and peripheral autonomic dysfunctions, cardiac insufficiencies, or the toxic influence of alpha-synuclein, all working together to undermine the body's ability to maintain stable BP.
Figure 3.
This figure illustrates the complex factors underpinning orthostatic hypotension in Parkinson's disease within a circular framework, hopefully putting complete multifaceted neuropathological, genetic, and environmental interactions and mechanisms into view. In the second place, it definitely represents that at the very bottom of this condition lies degeneration of the sympathetic nervous system, further increased by the pathologic accumulation of alpha-synuclein and chronic neuroinflammation. Genetic predispositions, especially mutations in genes such as alpha-synuclein, CYP17A1, and NPR-C, complicate BP regulation even more, enhancing the chance of OH. Environmental stress, dehydration, heat, and adverse side effects of some medications provide other extra layers of vulnerability that uninterruptedly interfere with the body's already prejudiced autonomic functions. Thus, the main mechanisms underlying OH are deeply rooted in central and peripheral autonomic dysfunctions, cardiac insufficiencies, or the toxic influence of alpha-synuclein, all working together to undermine the body's ability to maintain stable BP.
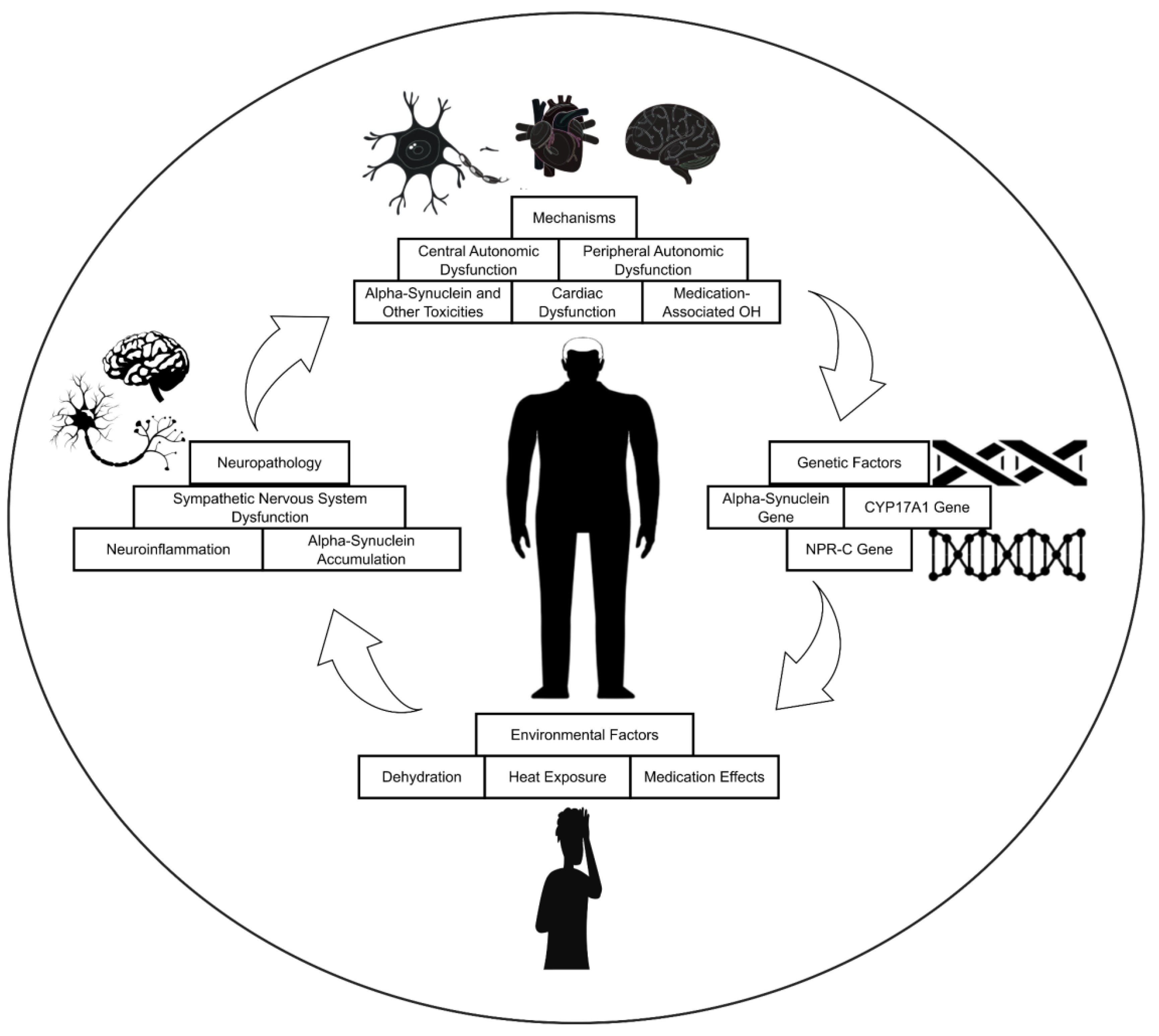
Table 1.
Epidemiological Studies On The Incidence And Prevalence Of Orthostatic Hypotension In Parkinson's Disease.
Table 1.
Epidemiological Studies On The Incidence And Prevalence Of Orthostatic Hypotension In Parkinson's Disease.
| References |
Prevalence of OH in PD (%)a
|
Population Size |
Notes |
| Senard et al. (1997) [28] |
58.2 |
65 |
OH was asymptomatic in 38.5% and symptomatic in 19.8% of patients. Symptomatic OH correlated with disease duration, severity, and higher doses of levodopa and bromocriptine. |
| Allcock et al. (2006) [19] |
49.7 |
175 |
OH was associated with cognitive decline and increased risk of falls. |
| Goldstein et al. (2008) [21] |
64.9 |
77 |
Cardiac 6-[18F]fluorodopamine-derived radioactivity can be used to diagnosis OH. |
| Haensch et al. (2009) [22] |
48.3 |
58 |
MIBG scintigraphy is a sensitive tool for detecting early cardiac sympathetic denervation in PD, independent of OH and baroreflex failure. |
| Jamnadas-Khoda et al. (2009) [23] |
42.0 |
50 |
OH frequently appears after tilting rather than standing and is often delayed (after 3 minutes) |
| Matinolli et al. (2009) [24] |
52.5 |
120 |
Patients with OH showed greater postural sway while standing compared to those without OH, though mobility and walking speed remained unaffected. |
| Schmidt et al. (2009) [25] |
22.0 |
32 |
During the valsalva maneuver, the absence of the expected BP rise in phase IV, more frequent than in phase II, was common in PD, though its correlation with OH during tilt-table testing was only moderate. |
| Shibata et al. (2009) [26] |
18.1 |
72 |
The coefficient of variation of RR intervals, an index of cardiac parasympathetic activity, predicts orthostatic hypotension risk. |
| Ha et al. (2011) [29] |
18.0 |
1125 |
Symptomatic OH was associated with older age, advanced disease stage, and longer disease duration. |
| Palma et al. (2015) [18] |
50.0 |
210 |
Despite common OH in PD, symptoms vary; standing BP <75 mmHg guides treatment decisions against supine hypertension risk. |
| Merola et al. (2016) [30] |
30.6 |
121 |
Among OH patients, 62.2% were symptomatic, and 37.8% were asymptomatic. Both groups had similar impairments in activities of daily living, worse than patients without OH. |
| Klanbut et al. (2018) [31] |
22.0 |
100 |
Symptomatic OH was associated with older age and a history of hypertension. It was observed in 18% of patients, while asymptomatic OH was present in 4%. |
| Beah et al. (2024) [32] |
5.7 |
907 |
In the PPMI early PD cohort, nOH was more prevalent than non-nOH, with a slight increase over time. |
Table 2.
Key Distinguishing Features Of Neurogenic Versus Non-Neurogenic OH.
Table 2.
Key Distinguishing Features Of Neurogenic Versus Non-Neurogenic OH.
| Feature |
Neurogenic OH |
Non-Neurogenic OH |
References |
| Epidemiology |
Prevalence increases with age and is more frequent in those with PD, MSA, or PAF. |
Common in older individuals, particularly those with comorbidities. |
Velseboer et al. (2011) [34]
Goldstein et al. (2015) [71]
Masaki et al. (1998) [72] |
| Pathophysiology |
ANS |
Central or peripheral autonomic failure; baroreflex failure, |
ANS intact; baroreceptor reflex functional |
Kaufmann et al. (2017) [73] |
| Hormonal |
Decreased plasma NE, impaired RAAS, and abnormal ADH, |
Plasma NE usually rises upon standing, and RAAS and ADH respond appropriately to hypotension. |
Goldstein et al. (2015) [71] |
| Vascular reactivity |
Impared vasoconstriction |
Preserved vasoconstriction |
Kaufmann et al. (2017) [73] |
| Cardiac autonomic function |
Cardiac sympathetic denervation (cardiac scintigraphy) |
Normal cardiac sympathetic innervation |
Goldstein et al. (2015) [71] |
| Heart rate response |
Blunted or no increase in standing despite the drop in BP (< 0.5 bpm per mmHg SBP drop). |
Marked increase when standing as a compensatory mechanism (> 0.5 bpm per mmHg SBP drop). |
Norcliffe-Kaufmann et al. (2017) [73]
Smit et al. (1999) [74] |
| Timing of BP Drop |
Usually develops gradually. Often within the first 3 minutes of standing or tilt. |
Variable; may be immediate, delayed, or situational. |
Kaufmann et al. (2017) [73] |
| Causes |
Damage to sympathetic nerves (Parkinson’s, Multiple System Atrophy, Pure Autonomic Failure). |
Dehydration, heart conditions, medications, and anemia. |
Freeman et al. (2008) [39]
Robertson et al. (1994) [66] |
| Other autonomic symptoms |
Other autonomic dysfunctions often co-exist with constipation, urinary issues, erectile dysfunction, impaired thermoregulation, etc. |
Generally not present, symptoms tend to focus on the single underlying cause (e.g., dehydration). |
Ziemssen et al. (2010) [75]
Low et al. (2008) [67] |
Table 3.
Diagnostic Tests To Evaluate Orthostatic Hypotension.
Table 3.
Diagnostic Tests To Evaluate Orthostatic Hypotension.
| Diagnostic Test |
Description |
Use in OH |
References |
| Active Standing Test |
Measuring BP and heart rate in supine and standing positions |
Essential for diagnosis; confirms a drop in BP upon standing |
Shibao et al. (2012) [77] |
| Ambulatory BP Monitoring (ABPM) |
Continuous BP monitoring over 24 hours |
Provides a comprehensive picture of BP fluctuations, detects nocturnal hypertension, identifies OH in daily life, and guides treatment decisions. |
Kaufmann et al. (2014) [81] |
| Autonomic function tests |
Assess different aspects of ANS function, including heart rate variability, Valsalva response, tilt table test, sudomotor testing, and QST. |
It helps distinguish neurogenic from non-nOH and reveals ANS dysfunction. Provides subtype-specific information |
Freeman et al. (2014) [85]
Cheshire et al. (2021) [86]
Low et al. (2013) [80] |
| Blood Tests |
Evaluate contributing factors (anemia, diabetes, thyroid) and measure norepinephrine |
Reveals potential causes of OH; norepinephrine levels can pinpoint the source of nOH. |
Robertson et al. (1994) [66]
Goldstein et al. (2007) [64] |
| Electrocardiogram (ECG) |
Records the electrical activity of the heart |
Detects cardiac arrhythmias that might contribute to or mimic OH |
Sarasin et al. (2002) [91]
Cheshire et al. (2021) [86] |
| Tilt-table Test |
Gradual tilt to the upright position while monitoring BP and HR under controlled conditions |
Evaluates orthostatic intolerance; helps diagnose delayed OH, initial OH, neurally mediated syncope, and postural orthostatic tachycardia syndrome |
Thijs et al. (2021) [49] |
Table 4.
Management Of Orthostatic Hypotension In Parkinson’s Disease.
Table 4.
Management Of Orthostatic Hypotension In Parkinson’s Disease.
| Drug |
Recommendations |
Results |
n |
Reference |
| Fludrocortisone |
14 days 3 × 60 mg/day pyridostigmine bromide or 1 × 0.2 mg/day fludrocortisone |
Significant improvement in both diastolic bp drop on the orthostatic challenge (−37%) and MBP standing (+15%) by 0.2 mg/day FC in PD |
13 |
Schreglmann et al. (2017) [146] |
| Pyridostigmine |
A single 60-mg dose of pyridostigmine bromide, alone or in combination with a subthreshold (2.5 mg) or suprathreshold (5 mg) dose of midodrine hydrochloride |
Pyridostigmine significantly improves standing BP in patients with OH without worsening supine hypertension. The greatest effect is on diastolic BP, suggesting that the improvement is due to increased total peripheral resistance. |
58 |
Singer et al. (2006) [147] |
| Acarbose |
100 mg of acarbose taken 20 minutes before meals |
Acarbose reduced the postprandial fall in SBP and DBP by 17 mm Hg and nine mm Hg, respectively, compared with the placebo. |
13 |
Shibao et al. (2007) [148] |
| Midodrine |
2.5, 10, 20 mg of midodrine |
Midodrine may improve standing SBP and global symptoms but has no significant benefit on supine to standing SBP and MBP and causes a higher incidence of adverse events. |
325 |
Parsaik et al. (2013) [149] |
| Atomoxetine |
The patients received either atomoxetine (18 mg daily) or midodrine (5 mg twice daily). |
Atomoxetine improved the standing SBP and DBP drop after one month of treatment, which was comparable to that achieved with midodrine therapy. |
50 |
Byun et al. (2019) [150] |
| Droxidopa |
The mean droxidopa dose during the double-blind administration period was 429 ± 163 mg, with 38% of patients (92/244) taking the maximum dosage of 600 mg 3 times daily. |
Treatment with droxidopa significantly increased upright SBP compared with placebo (11.5 ± 20.5 vs. 4.8 ± 21.0 mmHg; P < 0.001). A significant increase in upright DBP was also noted for droxidopa versus placebo (8.0 ± 15.5 vs. 1.8 ± 17.3 mmHg; P < 0.001). |
460 |
Biaggioni et al. (2017) [151] |
| Domperidone |
10, 20, and 50 mg TID |
Although not statistically significant, several studies have identified a trend toward symptom improvement when apomorphine is used as adjunctive therapy with domperidone |
NA |
Bacchi et al. (2017) [152] |
| Devices |
| Compression bandage of legs and abdomen |
The patient received a 10-minute leg bandage (compression pressure of 40 to 60 mm Hg), followed by an additional 10-minute abdominal bandage (20 to 30 mm Hg). |
Lower limb compression bandages effectively prevent orthostatic systolic BP decrease and reduce symptoms in elderly patients with progressive orthostatic hypotension. |
21 |
Podoleanu et al. (2006) [153] |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).