Submitted:
13 February 2025
Posted:
14 February 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
- Cell banks for bacterial culture and plasmid amplification (where applicable), characterisation of plasmid DNA template for mRNA synthesis, bulk purified RNA and mRNA encapsulation into LNPs
- Identity testing of the sequence and expressed products
- Potency assurance of mRNA products during manufacture and at release
- Confirmatory stability data for the drug substance and the drug product
2. Considerations for mRNA Product Design – the Beginning of the Lifecycle
3. Clinical Trials of mRNA Products
4. CMC Life Cycle Considerations in mRNA Product Manufacturing and Deployment
5. Regulatory, Lifecycle and Deployment Considerations of mRNA Therapeutics
Rare genetic Diseases
New Approaches Will Be Needed to Keep Pace with Therapeutics Development for the Large Number of Rare Diseases
Life Cycle Considerations for mRNA Rare Disease Therapies
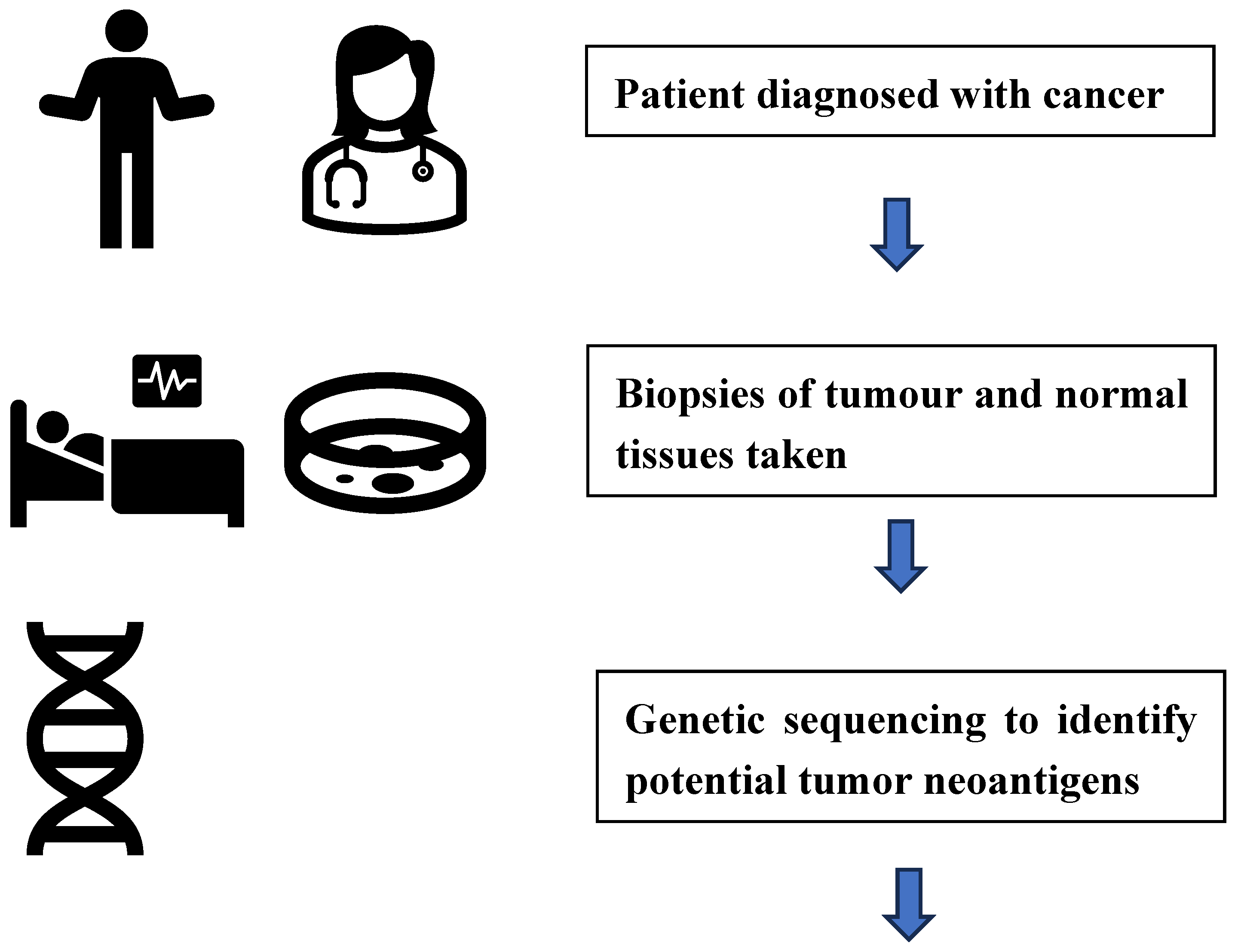
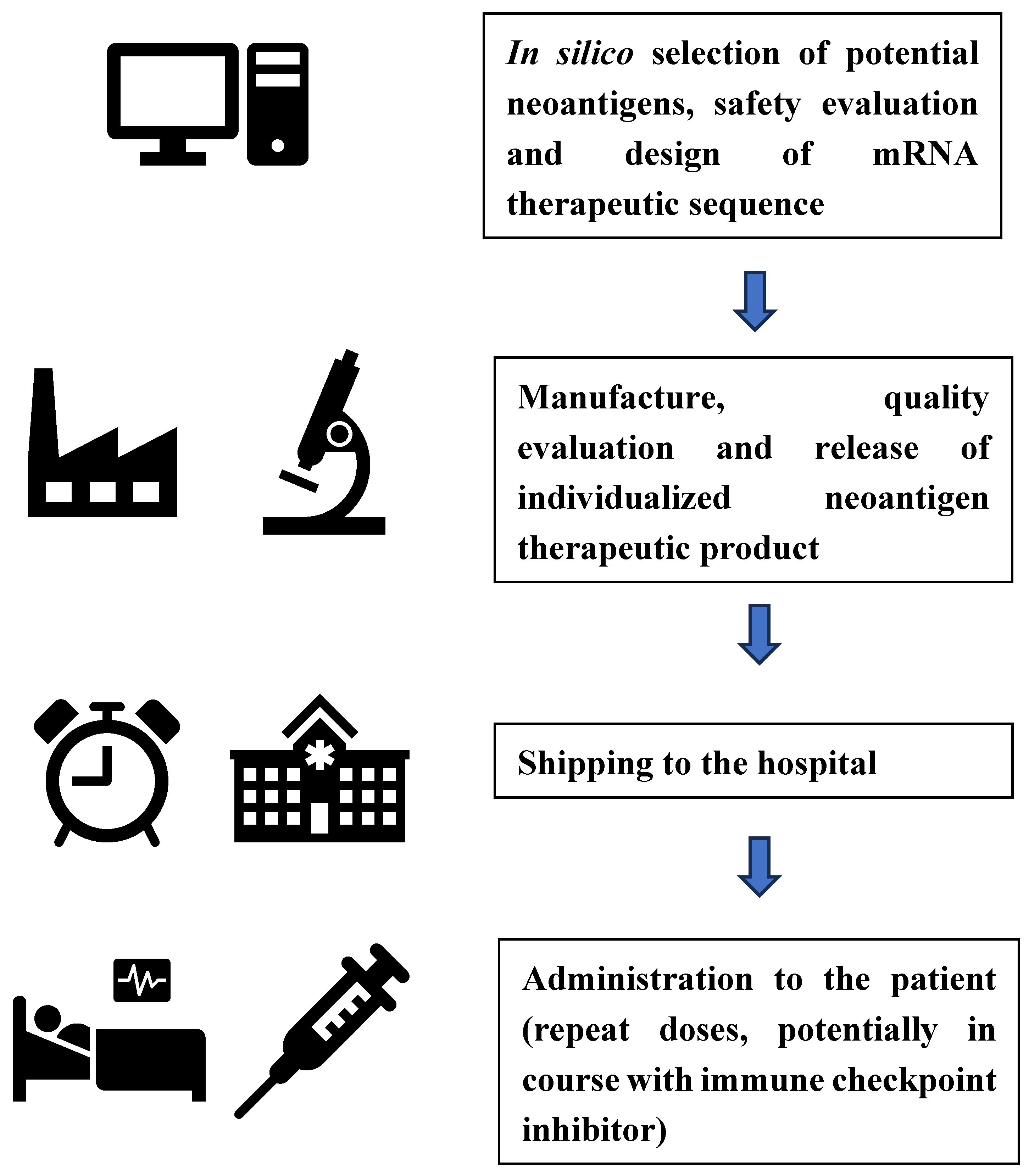
Oncology - Individualized Neoantigen Therapies (INT)
- simultaneous delivery of multiple tumor antigens, reducing risk of resistances or to antigen loss or change
- full-length antigens can be encoded if required, enabling multiple epitopes to be presented
- induction of a broad T-cell response
- manufacture is rapid and scalable compared with some of the other approaches
- Ensuring that the biopsy of the tumor tissue taken for sequencing is representative
- Justification of the algorithms used to select neoantigen peptides
- Assessment of controls over automated parallel manufacturing processes
- Establishing the consistency of a test product across multiple batches based on agreed representative quality attributes
- Using pooled stability data from multiple batches
6. Emerging Regulatory Trends and Issues
Need for Greater International Alignment in Regulatory Pathways
Differing International Approaches to the Regulatory Application of Platform Technology
- Starting materials: including DNA plasmids, enzymes, cell banks for expression systems
- mRNAs that encode antigens of interest
- mRNA-LNP control and testing of LNP size and mRNA encapsulation
- Unit operations throughout the manufacturing process
- Analytical techniques throughout the manufacturing process – identity, quantity, purity, integrity, characterization, potency and safety (contamination)
- Approaches to the validation of processes and methods used for manufacture and analysis
- Understanding of the degradation pathways and metabolism of mRNA and LNP components in consideration of non-clinical assessment and determination of shelf life
- Clinical experience justifying specification limits for certain shared attributes between products, such as particle size and product-related impurities
mRNA Platform Master Files
mRNA Vaccine Laboratory Lot or Batch Release
- Bell’s palsy (temporary one-sided facial drooping)
- swelling of the face
- severe allergic reaction
- extensive swelling of the vaccinated limb
- swelling of the face in patients who have had facial dermatological fillers
- a skin reaction (erythema multiforme)
- unusual (paraesthesia) or decreased sensation in the skin (hypoaesthesia)
- heavy menstrual bleeding
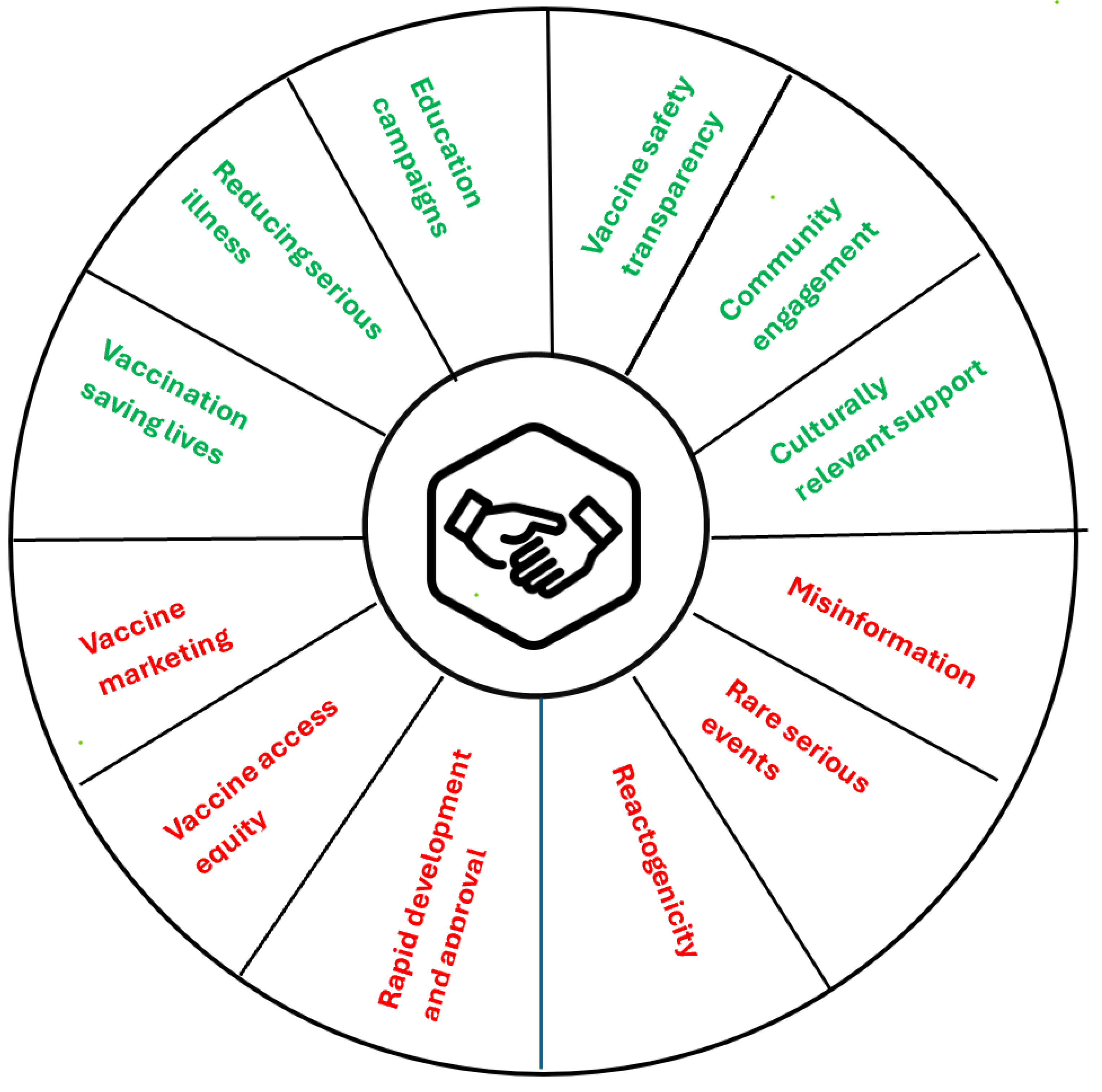
8. Social License to Operate for mRNA Products
9. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skerritt, J.H.; Tucek-Szabo, C.; Sutton, B.; Nolan, T. The Platform Technology Approach to mRNA Product Development and Regulation. Vaccines 2024, 12, 528–556. [Google Scholar] [CrossRef] [PubMed]
- Webb, C.; Ip, S.; Bathula, N.V.; Popova, P.; Soriano, S.K.V.; Ly, H.H.; Eryilmaz, B.; Huu, V.A.N.; Broadhead, R.; Rabel, M.; et al. Current Status and Future Perspectives on MRNA Drug Manufacturing. Mol. Pharm. 2022, 19, 1047–1058. [Google Scholar] [CrossRef]
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). Harmonised guideline technical and regulatory considerations for pharmaceutical product lifecycle management (Q12) November 2019. Available online: https://database.ich.org/sites/default/files/Q12_Guideline_Step4_2019_1119.pdf (accessed on 10 January 2025).
- Androsavich, J.R. Frameworks for the transformational breakthroughs in RNA-based medicines. Nature Rev. Drug Discov. 2024, 23, 412–444. [Google Scholar] [CrossRef] [PubMed]
- Niu, D.; Wu, Y.; Lian, J. Circular RNA vaccine in disease prevention and treatment. Sig Transduct. Target Ther, 2023, 8, 341. [Google Scholar] [CrossRef]
- Su, K.; Shi, L.; Sheng, T.; Yan, X.; Lin, L.; Meng, C.; Wu, S.; Chen, Y.; Zhang, Y.; Wang, C.; et al. Reformulating lipid nanoparticles for organ-targeted mRNA accumulation and translation. Nature Comm. 2024, 15, 5659. [Google Scholar] [CrossRef]
- Kon, E.; Ad-El, N.; Hazan-Halevy, I.; Stosky-Oterin, L.; Peer, D. Targeting cancer with mRNA-lipid nanoparticles: key considerations and future prospects. Nat Rev. Clin. Oncol. 2023, 20, 739–0754. [Google Scholar] [CrossRef]
- Cullis, P.R.; Feigner, P.L. The 60-year evolution of lipid nanoparticles for nucleic acid delivery. Nat. Rev. Drug Discov. 2024, 23, 709–722. [Google Scholar] [CrossRef]
- Kang, D.D.; Li, H.; Dong, Y. Advancements of in vitro transcribed mRNA (IVT mRNA) to enable translation into the clinics. Adv. Drug Deliv. Rev. 2023, 199, 114961. [Google Scholar] [CrossRef] [PubMed]
- Metkar, M.; Pepin, C.S.; Moore, M.J. Tailor made: the art of therapeutic mRNA design. Nature Rev. Drug Discov. 2024, 23, 67–83. [Google Scholar] [CrossRef]
- Wei, H.-H.; Zheng, L.; Wang, Z. mRNA therapeutics: new vaccination and beyond. Fundamental Res. 2023, 3, 749–759. [Google Scholar] [CrossRef]
- Brisse, M.; Vrba, S.M.; Kirk, N.; Liang, Y.; Ly, H. Emerging Concepts and Technologies in Vaccine Development. Front. Immunol. 2020, 11, 583077. [Google Scholar] [CrossRef] [PubMed]
- Roseman, D.S.; Khan, T.; Rajas, F.; Jun, L.S.; Asrani, K.H.; Isaacs, C.; Farelli, J.D.; Subramanian, R.R. G6PC mRNA therapy positively regulates fasting blood glucose and decreases liver abnormalities in a mouse model of glycogen storage disease 1a. Mol. Ther. 2018, 26, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Bicknell, A.A.; Reid, D.W.; Licata, M.C.; Jones, A.K.; Cheng, Y.M.; Li, M.; Hsiao, C.J.; Pepin, C.S.; Metkar, M.; Levdansky, Y.; et al. Attenuating ribosome load improves protein output from mRNA by limiting translation-dependent mRNA decay. Cell Reports. 2024, 43, 114098. [Google Scholar] [CrossRef]
- Vostrosablin, N.; Lim, S.; Gopal, P.; Brazdilova, K.; Parajuli, S.; Wei, X.; Gromek, A.; Prihoda, D.; Spale, M.; Muzdal, A.; et al. mRNAid, an open-source platform for therapeutic mRNA design and optimization strategies. NAR Genom. Bioinformat. 2024, 6, Iqae028. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.; Lin, A.; Xu. C.; Li. Z.; Liu, K.; Liu, B.; Ma, X.; Zhao, F.; Jiang, H. et.al. Algorithm for optimized mRNA design improves stability and immunogenicity. Nature 2023, 621, 396–403. [CrossRef] [PubMed]
- Lv, H.; Shi, l.; Berkenpas, J.W.; Dao, F-Y.; Zulfiqar, H.; Ding, H.; Zhang, Y.; Yang, L. Cao, R. Application of artificial intelligence and machine learning for COVID-19 drug discovery and vaccine design. Brief Bioinform. 2021, 22, bbab320.
- Leppek, K.; Byeon, G.W.; Kladwang, W.; Wayment-Steele, H.K.; Kerr, C.H.; Xu, A.F.; Kim, D.S.; Topkar, V.V.; Choe, C.; Rothschild, D.; et al. Combinatorial optimization of mRNA structure, stability, and translation for RNA-based therapeutics. Nature Commun. 2022, 13, 1536–1557. [Google Scholar] [CrossRef]
- McCaffrey, P. Artificial intelligence for vaccine design. Methods Mol. Biol. 2022, 2412, 3–13. [Google Scholar]
- Thomas, S.; Abraham, A.; Baldwin, J.; Piplani, S.; Petrovsky, N. Artificial intelligence in vaccine and drug design. Methods Mol. Biol. 2022, 2410, 131–146. [Google Scholar]
- Dolgin, E. Personalized cancer vaccines pass first major clinical test. Nat. Rev. Drug Discov. 2023, 22, 607–609. [Google Scholar] [CrossRef]
- Kim, Y-A.; Mousavi, K.; Yazdi, A.; Zwierzyna, M.; Cardinali, M.; Fox, D.; Peel, T.; Coller, J.; Aggarwal, K.; Maruggi, G. Computational design of mRNA vaccines. Vaccine, 2024, 42, 1831–1840.
- Xu, Z.; Wang, X.; Zeng, S.; Ren, X.; Yan, Y.; Gong, Z. Applying artificial intelligence for cancer immunotherapy. Acta Pharm. Sin. B, 2021, 11, 3393–3405. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.C.; Chai, S.; Washington, A.R.; et al. Machine-learning prediction of tumor antigen immunogenicity in the selection of therapeutic epitopes. Cancer Immunol. Res. 2019, 7, 1591–1604. [Google Scholar] [CrossRef]
- Shao, X.M.; Bhattacharya, R.; Huang, J.; Sivakumar, I.K.A.; Tolkheim, C.; Zheng, L.; Hirsch, D.; Kaminow, B.; Omdahl, A.; Bonsack, M.; et al. High-throughput prediction of MHC class I and II neoantigens with MHCnuggets. Cancer Immunol. Res. 2020, 8, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A. , Dixit, S., Srinivasan, K.M. D.; Vincent, P.M.D.R. Personalized cancer vaccine design using AI-powered technologies. Front. Immunol. 2024, 15, 1357217. [Google Scholar] [CrossRef]
- Lewis, M.M., Beck, T.J; Ghosh, D. Applying machine learning to identify ionizable lipids for nanoparticle-mediated delivery of mRNA. 2023, Preprint. [CrossRef]
- Xu, Y.; Ma, S.; Cui, H.; Chen, J.; Xu, S.; Gong, F.; Golubovic, A.; Zhou, M.; Wang, K.C.; Varley, A.; et al. AGILE platform: a deep learning powered approach to accelerate LNP development for mRNA delivery. Nature Comms. 2024, 15, 6305. [Google Scholar] [CrossRef]
- Van der Meel, R.; Grisoni, F.; Mulder, W.J.M. Lipid discovery for mRNA delivery guided by machine learning. Nature Materials, 2024, 23, 880–881. [Google Scholar] [CrossRef]
- Our World in Data, “COVID-19, vaccinations”. Available online: https://ourworldindata.org/grapher/cumulative-covid-vaccinations (accessed on December 22, 2024).
- Sparrow, E.; Hasso-Agopsowicz, M.; Kaslow, D.C.; Singh, K.; Rao, R.; Chibi, M.; Makubalo, L.E.; Reeder, J.C.; Kang, G. Karron, R.A.; et al. Leveraging mRNA Platform Technology to Accelerate Development of Vaccines for Some Emerging and Neglected Tropical Diseases Through Local Vaccine Production. Front. Trop. Dis., 2022, 3, 844039. [Google Scholar] [CrossRef]
- Van Tilbeurgh, M.; Lemdani, K.; Beignon, A.-S.; Chapon, C.; Tchitchek, N.; Cheraitia, l.; Lopez, E.M.; Pascal, Q.; Le Grand, R.; Maisonnasse, P.; et al. Predictive Markers of Immunogenicity and Efficacy for Human Vaccines. Vaccines 2021, 9, 579–615. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Expedited Program for Serious Conditions — Accelerated Approval of Drugs and Biologics Guidance for Industry. Draft Guidance December 2024. Available online: https://www.fda.gov/media/184120/download (accessed on 8 January 2025).
- Lo, M.K.; Spengler, J.R.; Welch, S.R.; Harmon, J.R.; Coleman-McCray, J.D.; Scholte, F.E.M.; Shrivastava-Ranjan, P.; Montgomery, J.M.; Nichol, S.T.; Weissman, D. et.al. Evaluation of a Single-Dose Nucleoside-Modified Messenger RNA Vaccine Encoding Hendra Virus-Soluble Glycoprotein Against Lethal Nipah virus Challenge in Syrian Hamsters. J Infect Dis. 2019, 221 (Suppl 4), S493–S498. [Google Scholar] [CrossRef]
- Loomis, R.J.; DiPiazza, A.T.; Falcone, S.; Ruckwardt, T.J.; Morabito, K.M.; Abiona, O.M.; Chang, L.A.; Caringal, R.T.; Presnyak, V.; Narayanan, E.; et al. Chimeric Fusion (F) and Attachment (G) Glycoprotein Antigen Delivery by mRNA as a Candidate Nipah Vaccine. Front. Immunol. 2021, 12, 772864. [Google Scholar] [CrossRef] [PubMed]
- Geisbert, T.W.; Bobb, K.; Borisevich, V.; Geisbert, J.B.; Agans, K.N.; Cross, R.W.; Prasad, A.N.; Fenton, K.A.; Yu, H. Fouts, T.R.; et al. A single dose investigational subunit vaccine for human use against Nipah virus and Hendra virus. Npj Vaccines, 2021, 6, 23. [Google Scholar] [CrossRef]
- Meyer, M.; Huang, E.; Yuzakov, O.; Ramanathan, P.; Ciaramella, G.; Bukreyev, A. Modified mRNA-based vaccines elicit robust immune responses and protect guinea pigs from Ebola virus disease. J. Infect. Dis. 2018, 217, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Alberer, M. Gand-Vogt, U.; Hong, H.S.; Mehr, K.T.; Backert, L.; Finak, G.; Gottardo, R.; Bica, M.A. Garofino, A.; Koch, S.D.; et al. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: an open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. Lancet 2017, 390, 1511–1520. [Google Scholar] [PubMed]
- Richner, J. M.; Himansu, S.; Dowd, K.A.; Butler, S.L.; Salazar, V.; Fox, J.M.; Julander, J.G.; Tang, W.W.; Shresta, S.; Pierson, T.C.; et al. Modified mRNA vaccines protect against Zika virus infection. Cell 2017, 168, 1114–1125. [Google Scholar] [CrossRef]
- Mucker, E.M.; Freyn, A.W.; Bixler, S.L.; Cizmeci, D.; Atyeo, C.; Earl, P.L.; Natarajan, H.; Santos, G.; Frey, T.R.; Levin, R.H.; et al. Comparison of protection against mpox following mRNA or modified vaccinia Ankara vaccination in nonhuman primates. Cell 2024, 187, 5540–5553. [Google Scholar] [CrossRef]
- August, A. , Attarwala, H.Z., Himansu, S.; et al. A phase 1 trial of lipid-encapsulated mRNA encoding a monoclonal antibody with neutralizing activity against Chikungunya virus. Nat Med 2021, 27, 2224–2233. [Google Scholar] [CrossRef]
- US Food and Drug Administration. New drug and biological drug products: evidence needed to demonstrate effectiveness of new drug when human efficacy studies are not ethical or feasible, USFDA final rule, May 2002. Fed Register. 2002, 67, 37988–37998. [Google Scholar]
- US Food and Drug Administration. Product Development Under the Animal Rule Guidance for Industry, October 2015. Available online: www.fda.gov/media/88625/download. (accessed on 3 August 2024).
- European Medicines Agency. Guideline on procedures for the granting of a marketing authorisation under exceptional circumstances, pursuant to article 14 (8) of Regulation (EC) No 726/2004. Available online: www.ema.europa.eu/en/documents/regulatory-procedural-guideline/guideline-procedures-granting-marketing-authorisation-under-exceptional-circumstances-pursuant/2004_en.pdf (accessed on 3 August 2024).
- Mukonzo, J.K.; Ndagije, H.B.; Sabbiah, G.T.; Mathenge, W.; Price, D.A.; Grasela, T.H. Expanding regulatory science: Regulatory complementarity and reliance. Clin. Trans. Sci. 2024, 17, e13683. [Google Scholar] [CrossRef]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J.A. mRNA-lipid nanoparticle COVID-19 vaccines: structure and stability. Int J. Pharmaceutics. 2021, 601, 120586. [Google Scholar] [CrossRef]
- Demongeot, J.; Fougere, C. mRNA COVID-19 Vaccines—Facts and hypotheses on fragmentation and encapsulation. Vaccines 2023, 11, 40–65. [Google Scholar] [CrossRef]
- Cheng, F.; Wang, Y.; Bai, Y.; Liang, Z.; Mao, Q.; Liu, D.; Wu, X.; Xu, M. ; Research advances on the stability of mRNA vaccines. Viruses 2023, 15, 668–683. [Google Scholar] [CrossRef] [PubMed]
- Chheda, U.; Pradeepan, S.; Esposito, E.; Strezsak, S.; Fernadez-Delgado, O.; Kranz, J. Factors affecting stability of RNA – Temperature, length, concentration, pH and buffering species. J. Pharm. Sci. 2024, 113, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Binzel, D.W. , Li X., Burns N., Khan E., Lee W., Chen L.-C., Ellipilli S., Miles W., Soon Ho Y., Guo P. Thermostability, tunability and tenacity of RNA as Rubbery Anionic Polymeric Materials in nanotechnology and nanomedicine—Specific cancer targeting with undetectable toxicity. Chem. Rev. 2021, 121, 1322–1335. [Google Scholar]
- Oude Blenke, E.; Ornskov, E.; Schoneich, C.; Nilsson, G.A.; Volkin, D.B.; Mastrobattista, E.; Almarsson, O.; Crommelin, D.J.A. The Storage and In-Use Stability of mRNA Vaccines and Therapeutics: Not A Cold Case. Pharm. Sci. 2023, 112, 386–403. [Google Scholar] [CrossRef]
- Wouters, O.J.; Shadlen, K.C.; Salcher-Konrad, M.; Pollard, A.J.; Larson, H.J.; Teerawattananon, Y.; Jit, M. Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment. Lancet. 2021, 397, 1023–1034. [Google Scholar] [CrossRef]
- Ai, L.; Li, Y.; Zhou, L.; Yao, W.; Zhang, H.; Hu, Z.; Han, J.; Wang, W.; Wu, J.; Xu. P.; et al. Lyophilized mRNA-lipid nanoparticle vaccines with long-term stability and high antigenicity against SARS-CoV-2. Cell Discovery 2023, 9, 9–24. [Google Scholar] [CrossRef]
- Muramatsu, H.; Lam, K.; Bajusz, C.; Laczko, D.; Kariko, K.; Screiner, P.; Martin, A.; Lutwyche, P.; Heyes, J.; Pardi, N. Lyophilization provides long-term stability for a lipid nanoparticle-formulated nucleoside modified mRNA vaccine. Mol. Ther. 2022, 30, 1941–1962. [Google Scholar] [CrossRef]
- Zhao, P.; Hou, X.; Yan, J.; Du, S.; Xue, Y.; Li, W.; Xiang, G.; Dong, Y. Long-term storage of lipid-like nanoparticles for mRNA delivery. Bioact. Mater. 2020, 5, 358–363. [Google Scholar] [CrossRef]
- Panther, L.; Basnet, S.; Fierro, C.; Brune, D.; Legett, D.; Paterson, J.; Pickrell, P.; Lin, J.; Wu, K.; Lee, H.; et al. 2892. Safety and immunogenicity of mRNA-1647, an mRNA-based cytomegalovirus vaccine in healthy adults: Results of a phase 2, randomized, observer-blind, placebo-controlled, dose-finding trial. Open Forum Infect. Dis. 2023, 10 (suppl 2). [Google Scholar] [CrossRef]
- Zhai, J.; Cote, T.; Chen, Y. Challenges and advances in the stability of mRNA delivery therapeutics. Nucl. Acids Insights 2024, 1, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Stewart-Jones, G.B.E.; Elbashir, S.M.; Wu, K.; Lee, D.; Renzi, I.; Ying, B.; Kock, M.; Sein, C.E.; Choi, A.; Whitener, B.; et al. Domain-based mRNA vaccines encoding spike protein N-terminal and receptor binding domains confer protection against SARS-CoV-2. Sci. Transl. Med. 2023, 15, eadf4100. [Google Scholar] [CrossRef]
- Blin, O.; Lefebvre, N.; Rascol, O.; Micallef, J. Orphan drug clinical development. Therapies. 2020, 75, 141–147. [Google Scholar] [CrossRef]
- Berraondo, P.; Martini, P.G.V.; Avila, M.A.; Fontanellas, A. Messenger RNA therapy for rare genetic metabolic diseases. Gut 2019, 68, 1323–1330. [Google Scholar] [CrossRef]
- Córdoba, K.M.; Jericó, D.; Sampedro, A.; Jiang, L.; Iraburu, M.J.; Martini, P.G.V.; Berraondo, V.P.; Avila, M.A.; Fontanellas, A. Messenger RNA as a personalized therapy: The moment of truth for rare metabolic diseases. Int. Rev. Cell Mol. Biol. 2022, 372, 55–96. [Google Scholar] [PubMed]
- Martini, P.G.V.; Guey, L.T. A new era for rare genetic diseases: Messenger mRNA therapy. Human Gene Therapy, 2019, 30, 1180–1188. [Google Scholar] [CrossRef]
- An, D.; Schneller, D.L.; Frassetto, A.; Liang, S.; Zhu, X.; Park, J.S.; Theisen, M.; Hong, S.J.; Zhou, J.; Rajendran, R.; et al. Systemic messenger RNA therapy as a treatment for methylmalonic acidemia. Cell Rep. 2017, 21, 3548–3558. [Google Scholar] [CrossRef] [PubMed]
- An, D.; Frassetto, A.; Jacquinet, E.; Eybye, M.; Milano, J.; DeAntonis, C.; Nguyen, V.; Laureano, R.; Milton, J.; Sabnis, S.; Lukacs, C.M.; Guey, L.T. Long-term efficacy and safety of mRNA therapy in two murine models of methylmalonic acidemia. EBioMedicine 2019, 45, 519–528. [Google Scholar] [CrossRef]
- Jiang, L.; Berraondo, P.; Jerico, D.; Guey, L.T.; Sampedro, A.; Frassetto, A.; Benenato, K.E.; Burke, K.; Santamaria, E.; Alegre, M.; et al. Systemic messenger RNA as an etiological treatment for acute intermittent porphyria. Nat. Med. 2018, 24, 1899–1909. [Google Scholar] [CrossRef]
- Yu, H.; Brewer, E.; Shields, M.; Crowder, M.; Sacchetti, C.; Soontornniyomkij, B.; Dou, D.; Clemente, B.; Sablad, M.; Chivukula, P.; et al. Restoring ornithine transcarbamylase (OTC) activity in an OTC-deficient mouse model using LUNAR-OTC mRNA. Clin. Transl. Disc. 2022, 2, e33. [Google Scholar] [CrossRef]
- Jiang, L.; Park, J.-S. , Yin, L.; Laureano, R.; Jacquinet, E.; Yang, J.; Liang, S.; Frassetto, A.; Zhuo, J.; Yan, X.; et al. Dual mRNA therapy restores metabolic function in long-term studies in mice with propionic aciduria. Nat. Commun. 2020, 11, 5339–5348. [Google Scholar] [CrossRef]
- Truong, B. Allegri, G.; Liu, X.B.; Burke, K.E.; Zhu, X.; Cederbaum, S.D.; Häberle, J.; Martini, P.G.V.; Lipshutz, G.S.; et al. Lipid nanoparticle-targeted mRNA therapy as a treatment for the inherited metabolic liver disorder arginase deficiency. Proc. Natl. Acad. Sci. USA. 2019, 116, 21150–21159. [Google Scholar] [CrossRef]
- Khoja, S.; Liu, X.B.; Truong, B.; Nitzahn, M.; Lambert, J.; Eliav, A.; Nasser, E.; Randolph, E.; Burke, K.E.; White, R. et. al. Intermittent lipid nanoparticle mRNA administration prevents cortical dysmyelination associated with arginase deficiency. Mol. Ther. Nucleic Acids 2022, 28, 859–874. [Google Scholar] [CrossRef] [PubMed]
- Gurung, S.; Timmermand, O.V.; Perocheau, D.; Gil-Martinez, A.L.; Minnion, M.; Touramanidou, l.; Fang, S.; Messina, M.; Khalil, Y.; Spiewak, J.; et al. RNA therapy corrects defective glutathione metabolism and restores ureagenesis in preclinical argininosuccinic aciduria. Sci. Transl. Med. 2024, 16, 729. [Google Scholar] [CrossRef] [PubMed]
- Koeberl, D.; Schulze, A.; Sondheimer, N.; Lipschultz, G.S.; Geberhiwot, T.; Li, R.; Saini, R.; Luo, J.; Sikirica, V.; Jin, L.; et al. Interim analysis of a first in human phase 1/2 mRNA trial for propionic aciduria. Nature 2024, 628, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Haendel, M.; Vasilevsky, N.; Unni, D.; Bologa, C.; Harris, N.; Rehm, H.; Hamosh, A.; Baynam, G.; Groza, T.; McMurry, J.; et al. How many rare diseases are there? Nat Rev Drug Discov. 2020, 19, 77–78. [Google Scholar] [CrossRef]
- Fermaglich, L.J.; Miller, K.L. A comprehensive study of the rare diseases and conditions targeted by orphan drug designations and approvals over the forty years of the Orphan Drug Act. Orphanet J. Rare Dis. 2023, 18, 163–170. [Google Scholar] [CrossRef]
- Zanello, G.; Garrido-Estepa, M.; Crespo, A.; O’Connor, D.; Nabbout, R.; Waters, C.; Hall, A.; Taglialatela, M.; Chan, C.-H.; Pearce, D.A.; et al. Targeting shared molecular etiologies to accelerate drug development for rare diseases. EMBO Mol Med 2023, 1, e17159. [Google Scholar] [CrossRef]
- Muller, A.R.; Brands, M.M.M.G.; van de Ven, P.M.; Roes, K.C.B.; Cornel, M.C.; van Karnebeek, C.D.M.; Wijburg, F.A.; Daams, J.G.; Boot, E.; van Eeghen, A.M. Systematic Review of N-of-1 Studies in Rare Genetic Neurodevelopmental Disorders: The Power of 1. Neurology, 2021, 96, 529–540. [Google Scholar] [CrossRef]
- Zablin, M.A.; Novack, G. N-of-1 Clinical Trials: A Scientific Approach to Personalized Medicine for Patients with Rare Retinal Diseases Such as Retinitis Pigmentosa. J Ocul Pharmacol Ther. 2021, 37, 495–501. [Google Scholar]
- Lee, C.E.; Singleton, K.S.; Wallin, M.; Faundez, V. Rare Genetic Diseases: Nature’s Experiments on Human Development. iScience. 2020, 23, 101123. [Google Scholar] [CrossRef] [PubMed]
- Iyer, V.R.; Praveen, P.; Kaduskar, B.D.; Moharir, S.C.; Mishra, R.K. mRNA biotherapeutics landscape for rare genetic disorders. J. Biosci. 2024, 49, 33–60. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Liu, J.; Yang, H.; Xie, N.; Yang, Y. ; mRNA therapies: pioneering a new era in rare genetic disease treatment. J. Control. Rel. 2024, 369, 696–721. [Google Scholar] [CrossRef]
- Brooks, P.J.; Ottinger, E.A.; Portero, D.; Lomash, R.M.; Alimardanov, A.; Terse, P.; Xu, X.; Chandler, R.J.; Hauserman, J.G.; Esposito, E.; et al. The Platform Vector Gene Therapies Project: Increasing the Efficiency of Adeno-Associated Virus Gene Therapy Clinical Trial Startup. Hum. Gene Ther. 2020, 31, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Vervaeke, P.; Borgos, S.E.; Sanders, N.N.; Combes, F. ; Regulatory guidelines and preclinical tools to study the biodistribution of RNA therapeutics. Adv. Drug Deliv. Res. 2022, 184, 114236. [Google Scholar] [CrossRef]
- Qin, S.; Tang, X.; Chen, Y.; Chen, K.; Fan, N.; Xiao, W.; Zheng, Q.; Li, G.; Teng, Y.; Wu, M.; et al. mRNA-based therapeutics: Powerful and versatile tools to combat diseases. Signal Transduct. Target. Ther. 2022, 7, 166. [Google Scholar] [CrossRef]
- Rohner, E.; Yang, R.; Foo, K.S.; Goedel, A.; Chien, K.R. ; Unlocking the promise of mRNA therapeutics. Nat. Biotech 2022, 40, 1586–1600. [Google Scholar] [CrossRef]
- Parhiz, H.; Atochina-Vasserman, E.N.; Weissman, D. ; mRNA-based therapeutics: Looking beyond COVID-19 vaccines. Lancet 2024, 403, 1192–1204. [Google Scholar] [CrossRef]
- Blass, E.; Ott, P.A. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat Rev. Clin. Oncol. 2021, 18, 215–229. [Google Scholar] [CrossRef]
- Xie, N.; Shen, G.; Gao, W.; Huang, Z.; Huang, C.; Fu, Li. Neoantigens: promising targets for cancer therapy. Signal Transduct. Targeted Ther. 2023, 8, 9–46. [Google Scholar] [CrossRef]
- Trivedi, V.; Yang, C.; Klippel, K.; Yegorov, O.; von Roemeling, C.; Hoang-Minh, L.; Fenton, G.; Ogando-Rivas, E.; Castillo, P.; Moore, G.; et al. mRNA-based precision targeting of neoantigens and tumor-associated antigens in malignant brain tumors. Genome Med, 2024, 16, 17. [Google Scholar] [CrossRef] [PubMed]
- Wu, D-W. ; Jia, S-P.; Xing, S-J.; Ma, H-I.; Wang, X.; Tang Q-Y.; Li, Z-W.; Wu, Q.; Bai, M.; Zhang, X-Y.; et al. Personalized neoantigen cancer vaccines: current progression, challenges and a bright future. Clin. Exp. Med. 2024, 24, 229–239. [Google Scholar] [CrossRef]
- Cafri, G.; Gartner, J.J.; Zaks, T.; Hopson, K.; Levin, N.; Paria, B.C.; Parkhurst, M.R.; Yossef, R.; Lowery, F.J. Jafferji, M.S.; et al. mRNA vaccine–induced neoantigen-specific T cell immunity in patients with gastrointestinal cancer. J. Clin. Invest. 2020, 130, 5976–5988. [Google Scholar] [CrossRef]
- Weber, J.S.; Carlino, M.S.; Khattak, A.; Meniawy, T.; Ansstas, G.; Taylor, M.H.; Kim, K.B.; McKean, M.; Long, G.V.; Sullivan, R.J.; et al. Individualised neoantigen therapy mRNA-4157 (V940) plus pembrolizumab versus pembrolizumab monotherapy in resected melanoma (KEYNOTE-942): A randomized, phase 2b study. Lancet 2024, 403, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Schrors, B.; Lower, M.; Tureci, O.; Sahin, U. Identification of neoantigens for individualized therapeutic cancer vaccines. Nature Rev. Drug Discov. 2022, 21, 261–282. [Google Scholar] [CrossRef] [PubMed]
- Rojas, L.A.; Sethna, Z.; Soares, K.C.; Olcese, C.; Pang, N.; Patterson, E.; Lihm, J.; Ceglia, N.; Guasp, P.; Chu, A.; et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature 2023, 618, 144–150. [Google Scholar] [CrossRef]
- May, M. How mRNA is powering a personalized vaccine revolution. Nature Med. 2024, 30, 2097–2098. [Google Scholar] [CrossRef]
- Jonker, A.H.; Taturu, E-A. ; Graessner, H.; Dimmock, D.; Jaffe, A.; Bayman, G.; Davies, J.; Mitkus, S.; Ilianch, O.; Horgan, R.; et al. The state-of-the-art of N-of-1 therapies and the IRDiRC N-of-1 development roadmap. Nat. Rev. Drug Discov. 2025, 24, 40–56. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Guidance for Industry: Clinical Considerations for Therapeutic Cancer Vaccines, October 201. Available online: www.fda.gov/media/82312/download. (accessed on August 3, 2024).
- Ministry of Food and Drug Safety, South Korea. Considerations on the Development of Personalized Neoantigen-Targeted Therapy Products (Guidance for Industry), September 2024. Available online: www.mfds.go.kr/eng/brd/m_1127/list.do. (accessed on 12 November 2024).
- Medicines and Health products Regulatory Agency. Draft guideline on individualised mRNA cancer immunotherapies, February 2025. Available online: https://assets.publishing.service.gov.uk/media/6799ef4d9a6dc0352ab34225/Individualised_mRNA_cancer_immunotherapies_0.6.5.pdf (accessed on 10 February 2025).
- Guerriaud, M.; Kohli, E. RNA-based drugs and regulation: Toward a necessary evolution of the definitions issued from the European union legislation. Front. Med. 2022, 9, 1012497. [Google Scholar] [CrossRef]
- von Fritschen, M.; Haber, C.; Straus, W.; Schneider, C.K.; Janosz, E.; Jägle, U.; Mendila, M.; Blume, C. Defining Gene Therapy Medicinal Products in the EU: Scientific and Regulatory Perspectives. DIA Global Forum, March 2024. Available online: https://globalforum.diaglobal.org/issue/march-2024/defining-gene-therapy-medicinal-products-in-the-eu/ (accessed on 8 January 2025).
- US Food and Drug Administration. Frequently Asked Questions — Developing Potential Cellular and Gene Therapy Products Draft Guidance for Industry. Nov 2024. Available online: www.fda.gov/media/183631/download (accessed on 8 January 2025).
- Aust, A. Regulatory strategies for developing and manufacturing RNA-LNPs. Nucl. Acids Insights. 2024, 1, 139–145. [Google Scholar] [CrossRef]
- Djonova, J. Swiss regulatory aspects and evaluation considerations for ATMPs and other nucleic acid-based products such as mRNA vaccines. Vaccine Insights. 2024, 3, 91–95. [Google Scholar] [CrossRef]
- Wan, K-W. ; Galway, F. MHRA regulatory considerations in the quality of mRNA products. Nucl. Acids Insights, 2024, 1, 149–154.
- International Conference On Harmonisation Of Technical Requirements For Registration Of Pharmaceuticals For Human Use. Development and manufacture of drug substances (chemical entities and biotechnological/biological entities). Q11 guideline, May 2012. Available online: https://database.ich.org/sites/default/files/Q11%20Guideline.pdf (accessed on 14 November 2024).
- International Council For Harmonisation Of Technical Requirements For Pharmaceuticals For Human Use. ICH harmonised guideline analytical procedure development Q14 guideline, March 2022. Available online: https://database.ich.org/sites/default/files/ICH_Q14_Document_Step2_Guideline_2022_0324.pdf (accessed on 14 November 2024).
- European Medicines Agency. 20 April 1873.
- US Food and Drug Administration (Draft) Guidance Document: Platform Technology Designation Program for Drug Development, May 2024. Available online at: www.fda.gov/regulatory-information/search-fda-guidance-documents/platform-technology-designation-program-drug-development (accessed ). 3 August.
- World Health Organization. Evaluation of the Quality, Safety and Efficacy of Messenger RNA Vaccines for the Prevention of Infectious Diseases: Regulatory Considerations. World Health Organization Expert Committee on Biological Standardization 74th Report, 2022, Annex 3.Available online at: www.who.int/publications/m/item/evaluation-of-the-quality-safety-and-efficacy-of-messenger-rna-vaccines-for-the-prevention-of-infectious-diseases-regulatory-considerations. (accessed on 11 November 2024).
- European Medicines Agency. Concept Paper on the Development of a Guideline on the 5 Quality Aspects of mRNA Vaccines, May 2023. Available online at: www.ema.europa.eu/en/documents/scientific-guideline/concept-paper-development-guideline-quality-aspects-mrna-vaccines_en.pdf. (accessed on 26 March 2024).
- European Directorate for the Quality of Medicines and Healthcare. European Pharmacopoeia Commission adopts first three general texts on mRNA vaccines, January 2025. Available online at: www.edqm.eu/en/-/european-pharmacopoeia-commission-adopts-first-three-general-texts-on-mrna-vaccines. (accessed on 31 January 2025).
- European Medicines Agency. Procedural advice for vaccine platform technology master 5 file (vPTMF) certification. EMA Guidance EMA/CVMP/IWP/286631/2021, May 2022. Available online at: www.ema.europa.eu/en/documents/scientific-guideline/draft-procedural-advice-vaccine-platform-technology-master-file-vptmf-certification_en.pdf. (accessed on 3 August 2024).
- European Directorate for the Quality of Medicines and HealthCare, Human OCABR Guidelines, Available online at: www.edqm.eu/en/omcl/human-ocabr-guidelines (accessed ). 8 January.
- Ball, G.; Reblin, T.; Buchanan, J.; Hendrickson, B.A.; Lewis, E.; Schnell, P.M.; Rockhold, F.W. A framework for safety evaluation throughout the product development life-cycle. Therap. Innov. Reg. Sci. 2020, 54, 821–830. [Google Scholar] [CrossRef]
- Wilson, E.; Goswami, J.; Baqui, A.H.; Doreski, P.A.; Perez-Marc, G.; Zaman, K.; Monroy, J.; Duncan, C.J.A.; Ujiie, M.; Rämet, M.; et al. Efficacy and Safety of an mRNA-Based RSV PreF Vaccine in Older Adults. N Engl J Med. 2023, 389, 2233–2244. [Google Scholar] [CrossRef] [PubMed]
- Herve, C.; Laupeze, B.; Del Guidice, G.; Didierlaurent, A.M.; Da Silva, F.T. The how’s and what’s of vaccine reactogenicity. Npj Vaccines. 2019, 4, 39–49. [Google Scholar] [CrossRef]
- Lee, J.; Woodruff, M.C.; Kim, E.H.; Nam, J.-H. Knife’s edge: Balancing immunogenicity and reactogenicity in mRNA vaccines. Exp. Mol. Med. 2023, 55, 1305–1313. [Google Scholar] [CrossRef] [PubMed]
- Kobiyama, K.; Ishii, K. J. Making innate sense of mRNA vaccine adjuvanticity. Nat. Immunol. 2022, 23, 474–476. [Google Scholar] [CrossRef]
- Ndeupen, S,; Qin, Z. ; Jacobsen, S.; Bouteau, A.; Estanbouli, H.; Igyártó, B.Z. The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience, 2021, 24, 103479. [Google Scholar] [CrossRef]
- Alameh, M.G.; Tombacz, I.; Bettini, E.; Lederer, K.; Ndeupen, S.; Sittplangkoon, C.; Wilmore, J.R.; Gaudette, B.T.; Soliman, O.Y.; Pine, M.; et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity 2022, 55, 1136–1138. [Google Scholar] [CrossRef]
- Laurini, G.S.; Montanaro, N.; Broccoli, M.; Bonaldo, G.; Motola, D. Real-life safety profile of mRNA vaccines for COVID-19: An analysis of VAERS database. Vaccine 2023, 41, 2879–2886. [Google Scholar] [CrossRef]
- Ramos, A.S.F.; Sanchez, C.L.; Rose, T.B.; Smith, D.; Thorn, N.; Galiza, E.; Miah, T.; Pearce, J.; Hultin, C.; Cosgrove, C.; Hsia, Y.; Heath, P.T. Comparing reactogenicity of COVID-19 vaccine boosters: a systematic review and meta-analysis. Expert Rev. Vaccines. 2024, 23, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Chapin-Bardales, J.; Myers, T.; Gee, J.; Shay, D.K.; Marquez, P.; Baggs, J.; Zhang, B.; Licata, C.; Shimabukoro, T. Reactogenicity within 2 weeks after mRNA COVID-19 vaccines: Findings from the CDC v-safe surveillance system. Vaccine. 2021, 39, 7066–7073. [Google Scholar] [CrossRef] [PubMed]
- Fraiman, J.; Erviti, J.; Jones, M.; Greenland, S.; Whelan, P.; Kaplan, R.M.; Doshi, P. Serious adverse events of special interest following mRNA COVID-19 vaccination in randomized trials in adults. Vaccine 2022, 40, 5798–5805. [Google Scholar] [CrossRef]
- Graña, C.; Ghosn, L.; Evrenoglou, T.; Jarde, A.; Minozzi, S.; Bergman, H.; Buckley, B.S.; Probyn, K.; Villanueva, G.; Henschke, N.; et al. Efficacy and safety of COVID-19 vaccines. Cochrane Database Syst Rev, 0154. [Google Scholar]
- Alami, A.; Krewski, D.; Farhat, N.; Mattison, D.; Wilson, K.; Gravel, C.A.; Farrell, P.J.; Crispo, J.A.G.; Haddad, N.; Perez-Lloret, S.; et al. Risk of myocarditis and pericarditis in mRNA COVID-19 vaccinated and unvaccinated populations: a systematic review and meta-analysis. BMJ Open 2023, 13, e065687. [Google Scholar] [CrossRef]
- Faksova, K.; Walsh, D.; Jiang, Y; Griffin, J. ; Phillips, A.; Gentile, A.; Kwong, J.C.; Macartney, K.; Naus, M.; Grange, Z.; et al. Covid-19 vaccines and adverse events of special interest: A multinational global vaccine data network (GVDN) cohort study of 99 million vaccinated individuals. Vaccine, 2024, 42, 2200–2211. [Google Scholar] [CrossRef] [PubMed]
- Copland, E.; Patone, M.; Saatchi, D.; Handunnetthi, L.; Hirst, J.; Hunt, D.P.J.; Mills, N.L.; Moss, P.; Sheikh, A.; Coupland, C.A.A.; et al. Safety outcomes following COVID-19 vaccination and infection in 5.1 million children in England. Nature Comms. 2024, 15, 3822. [Google Scholar] [CrossRef]
- Xu, Y.; Li, H.; Santosa, A; Wettermark, B. ; Fall, T.; Bjork, J.; Borjesson, M.; Gisslen, M.; Nyberg, F. Cardiovascular events following coronavirus disease 2019 vaccination in adults: A nationwide Swedish study. Eur. Heart J. 2024, 30, ehae639. [Google Scholar]
- Barmada, A.; Klein, J.; Ranaswamy, A.; Brodsky, N.N.; Jaycox, J.R.; Sheikha, H.; Jones, K.M.; Habet, V.; Campbell, M.; Sumida, T.S.; et al. Cytokinopathy with aberrant cytotoxic lymphocytes and profibrotic myeloid response in SARS-CoV-2 mRNA vaccine-associated myocarditis. Sci. Immunol. 2023, 8, eadh3455. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Follmann, D.; Hachigian, G.; Strout, C.; Overcash, J.S.; Doblecki-Lewis, S.; Whitaker, J.A.; Anderson, E.J.; et al. Long-term safety and effectiveness of mRNA-1273 vaccine in adults: COVE trial open-label and booster phases. Nature Communications 2024, 15, 7469–7481. [Google Scholar] [CrossRef]
- Hulscher, N.; Hodgkinson, R.; Makis, W.; McCullough, P.A. Autopsy findings in cases of fatal COVID-19 vaccine-induced myocarditis. ESC Heart Failure 2024. [Google Scholar] [CrossRef]
- Semenzato, L; Le Vu, S; Botton, J; Bertrand, M. ; Jabagi, M-J.; Drouin, J.; Cuenot, F.; Zores, F., Dray-Spira, R.; Weill, A.; et al. Long-term prognosis of patients with myocarditis attributed to covid-19 mRNA vaccination, SARS-CoV-2 infection of conventional etiologies. JAMA 2024, 332, 1367–1377. [Google Scholar] [CrossRef]
- Heymans, S; Cooper, L. T. Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nat Rev Cardiol. 2022, 19, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Kadkhoda, K. Post RNA-based COVID vaccines myocarditis: proposed mechanisms. Vaccine. 2022, 40, 406–407. [Google Scholar] [CrossRef] [PubMed]
- Altman, N.L.; Berning, A.A.; Mann, S.C.; Quaife, R.A.; Gill, E.A.; Auerbach, S.R.; Campbell, T.B.; Bristow, M. Vaccination-Associated Myocarditis and Myocardial Injury. Circulation Res. 2023, 132, 1338–1357. [Google Scholar] [CrossRef] [PubMed]
- Buoninfante, A.; Andeweg, A.; Genov, G.; Cavaleri, M. Myocarditis associated with COVID-19 vaccination. Npj Vaccines 2024, 9, 122–129. [Google Scholar] [CrossRef]
- Ling, R.R.; Ramanathan, K.; Tan, F.L.; Tai, B.C.; Somani, J.; Fisher, D.; MacLaren, G. ; Myopericarditis following COVID-19 vaccination and non-COVID-19 vaccination: a systematic review and meta-analysis. Lancet Respir Med. 2022, 10, 679–688. [Google Scholar] [CrossRef]
- Saint-Gerons, D.M.; Ibarz, M.T.; Castro, J.L.; Fores-Martos, J.; Tabares-Seisdedsos, R. Myopericarditis associated with the Novavax COVID-19 vaccines (NVX-CoV2373): A retrospective analysis of individual case safety reports from VigiBase. Drugs- Real World Outcomes. 2023, 10, 263–270. [Google Scholar] [CrossRef]
- Ostropolets, A.; Makadia, R.; Shoaibi, A.; Rao, G.; Sena, A.G.; Martinez-Hernandez, E.; Delmestri, A.; Verhamme, K.; Rijnbeek, P.R.; Duarte-Salles, T.; Suchard, M.A.; Ryan, P.B.; Hripcsak, G.; Prieto-Alhambra, D. Characterising the background incidence rates of adverse events of special interest for covid-19 vaccines in eight countries: multinational network cohort study. BMJ. 2021, 373, n1435. [Google Scholar]
- World Health Organization. Causality assessment of an adverse event following immunization (AEFI). User manual for the revised WHO classification. Second edition WHO/HIS/EMP/SAV. Jan 2018. Available online at: https://iris.who.int/bitstream/handle/10665/259959/9789241513654-eng.pdf (accessed ). 14 November.
- European Medicines Agency (2023) Pfizer XBB 1.5 package leaflet, within Annex 1- Summary of Product Characteristics: Comirnaty, INN-tozinameran, tozinameran/famtozinameran, raxtozinameran, bretovameran. Available online at: www.ema.europa.eu/en/documents/product-information/comirnaty-epar-product-information_en.pdf (accessed ). 10 December.
- European Medicines Agency (2023) Moderna XBB 1.5 package leaflet, within Annex 1 – Summary of Product Characteristics: Spikevax, INN-elasomeran, elasomeran/imelasomeran, elasomeran/davesomeran, andusomeran. Available online at: - www.ema.europa.eu/en/documents/product-information/spikevax-epar-product-information_en.pdf (accessed ). 10 December.
- Morgan. H.J.; Clothier, H.J.; Kattan, G.S. Boyd, J.H., Buttery, J.P. Acute disseminated encephalomyelitis and transverse myelitis following COVID-18 vaccination – a self-controlled case series analysis. Vaccine, 2024, 42, 2212–2219. [Google Scholar] [CrossRef]
- Kammerer, U.; Schulz, V.; Steger, K. BioNtech RNA-based COVID-19 injections contain large amounts of residual DNA including an SV40 promoter/enhancer sequence. Sci Pub Health Policy Law. 2024, 5, 2019–2024. [Google Scholar]
- König, B.; Kirchner, J.O. Methodological Considerations Regarding the Quantification of DNA Impurities in the COVID-19 mRNA Vaccine Comirnaty®. Methods Protoc. 2024, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Therapeutic Goods Administration, Australia. Addressing misinformation about excessive DNA in the mRNA vaccines, Media Release . Available online at: www.tga.gov.au/news/media-releases/addressing-misinformation-about-excessive-dna-mrna-vaccines (accessed 14 November 2024). 18 October.
- Global vaccine data network. Debunking the DNA contamination claims in mRNA vaccines. Available online at: www.globalvaccinedatanetwork.org/news/plasmid-gate_debunking_the_DNA_contamination_claims_in_mRNA_vaccines (accessed ). 14 November.
- Therapeutic Goods Administration, Australia. Summary report of residual DNA and endotoxin on CoVID-19 mRNA vaccines conducted by TGA Laboratories, . Available online at: www.tga.gov.au/resources/publication/tga-laboratory-testing-reports/summary-report-residual-dna-and-endotoxin-covid-19-mrna-vaccines-conducted-tga-laboratories (accessed 14 November 2024). 11 November.
- Watson, O.J.; Barnsley, G.; Toor, J.; Hogan, A.B.; Winskill, P.; Ghani, A.C. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect. Dis. 2022, 22, 1293–1302. [Google Scholar] [CrossRef]
- Larson, H.J. Understanding vaccine hesitancy: a call for more social science in RNA vaccine research. Vaccine insights.
- Leong, C.; Jin, L.; Kim, D.; Kim, J.; Teo, Y.Y.; Ho, T.-H. Assessing the impact of novelty and conformity on hesitancy towards COVID-19 vaccines using mRNA technology. Comms. Med, 2022, 2, 61. [Google Scholar] [CrossRef]
- European Medicines Agency. International regulators and WHO address need to boost COVID-19 vaccine confidence, June 2021. Available online at: www.ema.europa.eu/en/news/international-regulators-and-who-address-need-boost-covid-19-vaccine-confidence. (accessed ). 14 November.
- Peretti-Watel, P.; Verger, P.; Ward, J.K. To understand mRNA vaccine hesitancy, stop calling the public anti-science. Nature Med. 2024, 30, 923–924. [Google Scholar] [CrossRef] [PubMed]
- Attwell, K.; Rizzi, M.; McKenzie, L.; Carlson, S.J.; Roberts, L.; Tomkinson, S.; Blyth, C.C. COVID-19 vaccine Mandates: An Australian attitudinal study. Vaccine, 2021, 40, 7360–7369. [Google Scholar] [CrossRef]
- Medicines Patent Pool. mRNA Technology transfer programme. Available online at: https://medicinespatentpool. 14 November.
- Bussink-Voorend, D.; Hautvast, J.L.A.; Vandenberg, L.; Visser, O.; Hulscher, M.E.J.L. A systematic literature review to clarify the concept of vaccine hesitancy. Nat. Hum. Behav. 2022, 6, 1634–1648. [Google Scholar] [CrossRef] [PubMed]
- Attwell, K.; Hannah, A.; Leask, J. COVID-19: talk of ’vaccine hesitancy’ lets governments off the hook. Nature, 2022, 602, 574–577. [Google Scholar] [CrossRef]
- de Miguel-Arribas, A.; Aleta, A.; Moreno, Y. Impact of vaccine hesitancy on secondary COVID-19 outbreaks in the US: an age-structured SIR model. BMC Infect Dis. 2022, 22, 511. [Google Scholar] [CrossRef]
- Lazarus, J.V.; Wyka, K.; White, T.M.; Picchio, C.A.; Rabin, K.; Ratzan, S.C.; Leigh, J.P.; Hu, J.; El-Mohandes, A. Revisiting COVID-19 vaccine hesitancy around the world using data from 23 countries in 2021. Nat. Commun. 2022, 12, 3801. [Google Scholar] [CrossRef]
- Romer, D.; Winneg, K.M.; Jamieson, P.E.; Brensinger, C.; Jamieson, K.H. Misinformation about vaccine safety and uptake of COVID-19 vaccines among adults and 5-11 year olds in the United States. Vaccine, 2022, 40, 6463–6470. [Google Scholar] [CrossRef]
- Jamieson, K.H.; Winneg, K.; Patterson, S.Jr.; Gibson, L.A.; Jamieson, P.E. Annenberg Science and Public Health Monitor – Summer 2024. University of Pennsylvania, Philadelphia, PA USA (https://cdn.annenbergpublicpolicycenter.org/wp-content/uploads/2024/08/asaph-report-summer-2024.
- Ashfield, S.; Donelle, L.; Uppal, G.; Bauer, M.A.; Kothari, A. Community organization perspectives on COVID-19 vaccine hesitancy and how they increased COVID-19 vaccine confidence: a Canadian Immunization Research Network, social sciences and humanities network study. Front. Publ. Health. 2023, 11, 1258742. [Google Scholar] [CrossRef]
- Shiman, L.J.; Diallo, F.; Nieves, C.I.; Brooks, B.; Dannefer, R.; Dorvil, S.; Lejano, M.; Pierre, J. “Be honest and gain trust”: a population health study to understand the factors associated with building trust in three historically disinvested neighbourhoods in New York City. Front. Publ. Health. 2023, 11, 1285152. [Google Scholar] [CrossRef]
- Iqbal, S.M.; Rosen, A.M.; Edwards, D.; Bolio, A.; Larson, H.J.; Servin, M.; Rudowitz, M.; Carfi, A.; Ceddia, F. Opportunities and challenges to implementing mRNA-based vaccines and medicines: lessons from COVOD-19. Front Publ. Health. 2024, 12, 1429265. [Google Scholar] [CrossRef] [PubMed]
- Van der Linden, S. Misinformation: susceptibility, spread, and interventions to immunize the public. Nature Med. 2022, 28, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Roozenbeek. J.; van der Linden, S.; Goldberg, B.; Rathje, S.; Lewandowsky, S.. Psychological inoculation improves resilience against misinformation on social media, Science Advances 2024, 8.
- Moffat, K.; Lacey, J.; Zhang, A.; Leipold, S. The social licence to operate: a critical review. Forestry: Int. J. Forest Res. 2016, 89, 477–488. [Google Scholar] [CrossRef]
- Kamenopoulos, S.; Agioutantis, Z. The importance of the social license to operate at the investment and operations stage of coal mining projects: application using a decision support system. Extr. Ind. Soc. 2020, 8, 100740. [Google Scholar] [CrossRef]
- Muller SHA; Kalkman, S. ; van Thiel, G.J.M.W.; Mostert, M.; van Delden, J.J.M. The social licence for data-intensive health research: towards co-creation, public value and trust. BMC Med Ethics, 2021, 22, 110–118. [Google Scholar] [CrossRef]
- Churchill, B.F.; Henkhaus, L.E.; Lawler, E.C. Effect of vaccine recommendations on consumer and firm behavior, J Policy Analysis Management, 2024, 44, 125–150. 44.
- Brewer, N.T. What Works to Increase Vaccination Uptake. Acta Pediatr. 2021, 21(4S), s9–s16. [Google Scholar] [CrossRef]
- Sergi, C.M.; Leung, A.K.C. Vaccination: a question of social responsibility. J Prev Med Hyg. 2021, 62, E46–E47. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



