1. Introduction
Stress is a normal response to a difficult situation in an individual’s life; however, prolonged and excessive mental or physical stress can be detrimental. Chronic stress affects cognition and results in decreased functioning of multiple domains affecting daily activities which in turn can give rise to several conditions, such as depression, hypertension, and metabolic disorders [
1,
2]. Furthermore, individuals suffering from chronic stress are vulnerable to negative emotions, such as compulsion, hostility, paranoia, anxiety, and depression, in addition to interpersonal sensitivity, sleep disruption, and altered eating patterns [
3,
4]. The high global prevalence (~25%) and detrimental effects of stress underscore the importance of effective stress management to safeguard individuals' mental, emotional, and physical health [
5]. Additionally, there exists a gap in the available approaches for effective stress management in standard medical practice. These factors together underline the requirement for effective stress management options.
Adaptogens are substances derived from certain plants that can improve an individual’s ability to cope with stress and aid homeostasis or physiological balance [
1]. They also have neuroprotective, anti-fatigue, anti-depressant, anxiolytic, nootropic, and central nervous system stimulating properties [
6]. Ashwagandha,
Withania somnifera (known as Indian ginseng or winter cherry), is an Indian herb which has been used in Ayurveda, the traditional Indian medicine, as an adaptogen since approximately 3000 BC [
7]. Ashwagandha root extract has been demonstrated to augment the immune system, promote healthy aging, rejuvenate the body systems, boost resistance to adverse environmental factors, and promote a sense of well-being [
8]. The pharmacologically active phytochemical constituents of Ashwagandha root are steroidal lactones and their glycosides, known as withanolides [
9].
Clinical studies have demonstrated the efficacy of withanolides in reducing chronic stress, anxiety, and cortisol levels, suggesting their potential role in the management of stress [
1,
9]. Ashwagandha root powder is required to be administered in grams divided over 2-3 servings daily, and standard immediate release Ashwagandha formulations are commonly prescribed in clinical practice in a twice or thrice daily dose. Ashwagandha root extract sustained-release (SR) capsule 300 mg was developed to provide convenience of administration and improve compliance [
10]. The SR formulation showed superior bioavailability of withanolides and a longer elimination half-life indicating a sustained-release profile after single daily administration compared to the reference product (marketed Ashwagandha root extract capsule) [
10,
11]. The efficacy of Ashwagandha root extract SR capsules (AshwaSR; Prolanza
TM/ashwanova
TM, Nutriventia Limited, Mumbai, Maharashtra, India, and Laila Nutraceuticals, Vijayawada, Andhra Pradesh, India) was demonstrated in preclinical and clinical studies [
11,
12]. In an in vitro study, dose-dependent, anti-neuroinflammatory activity of AshwaSR was shown in terms of inhibition of tumor necrosis factor alpha (TNF-α), interleukin-1 beta (IL-1β), and superoxide production. Additionally, in an in vivo assessment of the chronic unpredictable stress model, AshwaSR demonstrated anxiolytic and stress-relieving effects [
11]. Further, a randomized, double-blind, placebo-controlled clinical study demonstrated improvement in cognitive function, psychological well-being, sleep quality, and stress level in healthy adult, stressed subjects after treatment for 90 days with AshwaSR 300 mg in a single daily dose compared to placebo [
12].
There is a paucity of evidence assessing efficacy and safety of low-dose formulations of Ashwagandha in stress management. This study aimed to assess whether a low-dose formulation (150 mg) of AshwaSR capsules could effectively reduce stress, compared to its standard-dose SR formulation (300 mg) or placebo. We evaluated the efficacy and safety of AshwaSR capsules at doses of 150 mg and 300 mg in reducing stress and associated symptoms in healthy adults experiencing stress, over a period of 60 days of administration. Additionally, we evaluated the effect of both doses of AshwaSR on mood, sleep quality, impaired eating habits owing to stress, and serum cortisol levels.
2. Materials and Methods
2.1. Study Design
This was a 60-day randomized, double-blind, three-arm, parallel-group, placebo-controlled prospective clinical trial conducted from June 2023 to October 2023 at two sites (Bangalore Neuro Centre, Bangalore and Santhosh Hospital, Bangalore) in India.
2.2. Ethical Considerations
The study was conducted in accordance with Good Clinical Practice and the Helsinki Declaration Standards, in addition to The New Drug and Clinical Trial Rules 2019 and the Ethical Guidelines for Biomedical Research on Human Subjects, issued by the Indian Council of Medical Research. The study was registered on Clinical Trials Registry-India (CTRI) on 08/06/2023 (CTRI Reg no: CTRI/2023/06/053662). The study protocol, informed consent form and other relevant study documents were reviewed and approved by an independent, registered Ethics Committee of the Santhosh hospital (Reg. no. ECR/1062/Inst/KA/2018/RR-21) before initiating the study recruitment. A written informed consent form was obtained from each subject prior to study commencement.
2.3. Key Eligibility Criteria of Subjects
The study included healthy male or female subjects, aged 20-55 years, with BMI of 18-30 kg/m2 and PSS score between 14 and 26. Female subjects of childbearing potential were required to use a medically acceptable form of birth control other than contraceptives and those of non-childbearing potential needed to be amenorrheic for ≥ 1 year or have undergone hysterectomy and/or bilateral oophorectomy.
Subjects with psychiatric illnesses as per the Diagnostic and Statistical Manual of Mental Disorders, 5th edition criteria; with uncontrolled systemic illnesses; who had concurrent use of supplements for stress, anxiety, mood, and sleep or other indications; who had concurrent use of β-blockers or contraceptive or psychotropic medications; who were treated for infertility or sexual function enhancement within 6 months prior to study enrolment; who used body composition enhancing agents; who demonstrated hypersensitivity to any of the ingredients in the study products; who demonstrated alcohol dependence or had established substance abuse concerns, were excluded.
2.4. Randomization and Treatment Allocation
After screening, eligible subjects were randomized (1:1:1) to receive AshwaSR 150 mg capsules (group A) or 300 mg capsules (group B) or placebo capsules (group C), according to a software-generated randomization schedule (block size of 6). The randomization schedule was generated by the study statistician using SAS®, version 9.4 (SAS Institute Inc., Cary, NC, USA). Subjects were assigned a unique randomization code by an unblinded pharmacist at each study site. Since the study was planned as a double-blind, treatment assignment was not disclosed to subjects or investigators. In addition, batch numbers of all investigational product containers were masked and given sequential numbers.
AshwaSR (ProlanzaTM/ashwanovaTM, Nutriventia Limited, Mumbai, Maharashtra, India, and Laila Nutraceuticals, Vijayawada, Andhra Pradesh, India) is an SR capsule containing Withania somnifera root extract 150 mg or 300 mg and permitted excipients. Placebo capsules were manufactured identical to the test capsules in color, size, and shape. The subjects were instructed to consume one capsule orally with water, daily after breakfast in the morning for a period of 60 days.
2.5. Study Procedure
The study included a total of eight visits: screening (visit 1, day −7 to day 0), baseline/randomization (visit 2, day 1), interim visits (visits 3 to 7, days 3 [±1], 7 [±2], 15 [±2], 30 [±2], and 45 [±3]), and end of treatment (visit 8, day 60 [±3]). During the screening visit, subjects were assessed for eligibility criteria. At the baseline/randomization visit, eligible subjects were randomized and allocated to the treatment. Subjects were assessed for study outcomes at baseline, interim, and end of treatment visits. Subjects’ diaries were collected and reviewed for treatment compliance during all follow-up visits.
2.6. Study Endpoints
The primary efficacy endpoint was to evaluate the change in PSS score from screening to visit 8 (day 60) in group A and group B as compared to group C.
Secondary efficacy endpoints included comparison of change in PSS score from screening to days 3, 7, 15, 30, 45, and 60 between all the groups; comparison of Pittsburgh Sleep Quality Index (PSQI); Oxford Happiness Questionnaire-8 (OHQ-8) score from baseline to days 3, 7, 15, 30, 45, and 60; Three-Factor Eating Questionnaire - Revised 18 (TFEQ-R18) score; and serum cortisol level from baseline to days 7, 30, and 60 between all three groups. In addition, subjects’ and physicians’ global assessment of therapy at the end of treatment was assessed as a secondary efficacy endpoint.
Treatment-emergent adverse events (AEs) were reported under safety assessment.
2.7. Study Assessment
The PSS score [
13] was assessed using a 10-item subjective questionnaire, which is the most widely used psychological instrument for measuring perception of stress, in terms of the degree to which situations in an individual’s life are appraised as stressful. Items were designed to determine how unpredictable, uncontrollable, and overloaded subjects found their lives, with response scores ranging between 0 and 40 (scores 0-13, low; 14-26, moderate; 27-40, high perceived stress) [
14].
The PSQI [
15] is an effective instrument used to measure the quality and patterns of sleep. It differentiates “poor” from “good” sleep by measuring seven domains: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction. Responses to each of the seven domains were measured on the Likert Scale 0-3, giving a global PSQI score of 0 to 21. A global PSQI score of more than 5 indicated poor sleep relative to clinical and laboratory measures [
16].
The TFEQ-R18 [
17,
18] consists of 18 items on a 4-point Likert scale (1 = definitely true, 2 = mostly true, 3 = mostly false, 4 = definitely false). Responses to each of the 18 items are summated into scale scores for cognitive restraint (6-item), uncontrolled eating (9-item), and emotional eating (3-item). Higher scores in the respective scales are indicative of greater cognitive restraint, and uncontrolled or emotional eating.
The OHQ-8 [
19] consists of eight items with a series of statements to which respondents indicate their agreement or disagreement on a scale ranging from 1 = strongly disagree, 2 = moderately disagree, 3 = slightly disagree, 4 = slightly agree, 5 = moderately agree, to 6 = strongly agree. It is a widely used psychological assessment tool designed to measure an individual’s subjective happiness level. A score below 4 is considered as the unhappy category and 4 or above, as the happy category [
20].
For serum cortisol estimation, subjects’ blood samples were collected in the morning (9-11 am).
2.8. Statistical Analysis
Sample size was calculated based on the previous double-blind, randomized, parallel-group, placebo-controlled study [
12] where the true difference between test and placebo in terms of PSS was 5.7 (13.0 and 18.7 for test and placebo products, respectively, at end of treatment) and the expected population standard deviation (SD) was assumed to be 8.2. To establish superiority between test and placebo at 80% power at 5% level of significance (i.e., α = 0.05) with equal allocation (i.e., k = 1) and approximately 15% drop out and non-compliance, the sample size of 45 subjects per group was obtained. Therefore, a total of 135 subjects were enrolled and were equally randomized to test and placebo arms, to achieve evaluable subjects totaling 38 per arm (total 114 subjects to complete the trial).
Treatment compliance of ≥ 80% was considered acceptable for efficacy analysis. Subjects who completed the study treatment without any major protocol deviations with treatment compliance of at least ≥ 80% were included in per protocol population analysis.
Statistical analysis was performed using SAS® version 9.4. Descriptive statistics was used for qualitative data (presented as number and percentage) and quantitative data (presented as mean and SD). Homogeneity of demographic data pertaining to each treatment and baseline values of each outcome measure were verified using analysis of variance (ANOVA). To evaluate variance among all study treatment arms, ANOVA for independent means was used. It was followed by post-hoc Tukey honest significant difference (HSD) to evaluate the HSD among paired comparisons of study treatment arms. Covariate adjustment with baseline values as covariate was carried out for all parameters to nullify influence of baseline differences among parameters. In addition, Student’s t-test for independent measures was used as applicable to evaluate differences between active study treatment arms. A p-value of less than 0.05 was considered statistically significant.
3. Results
3.1. Subject Disposition and Characteristics
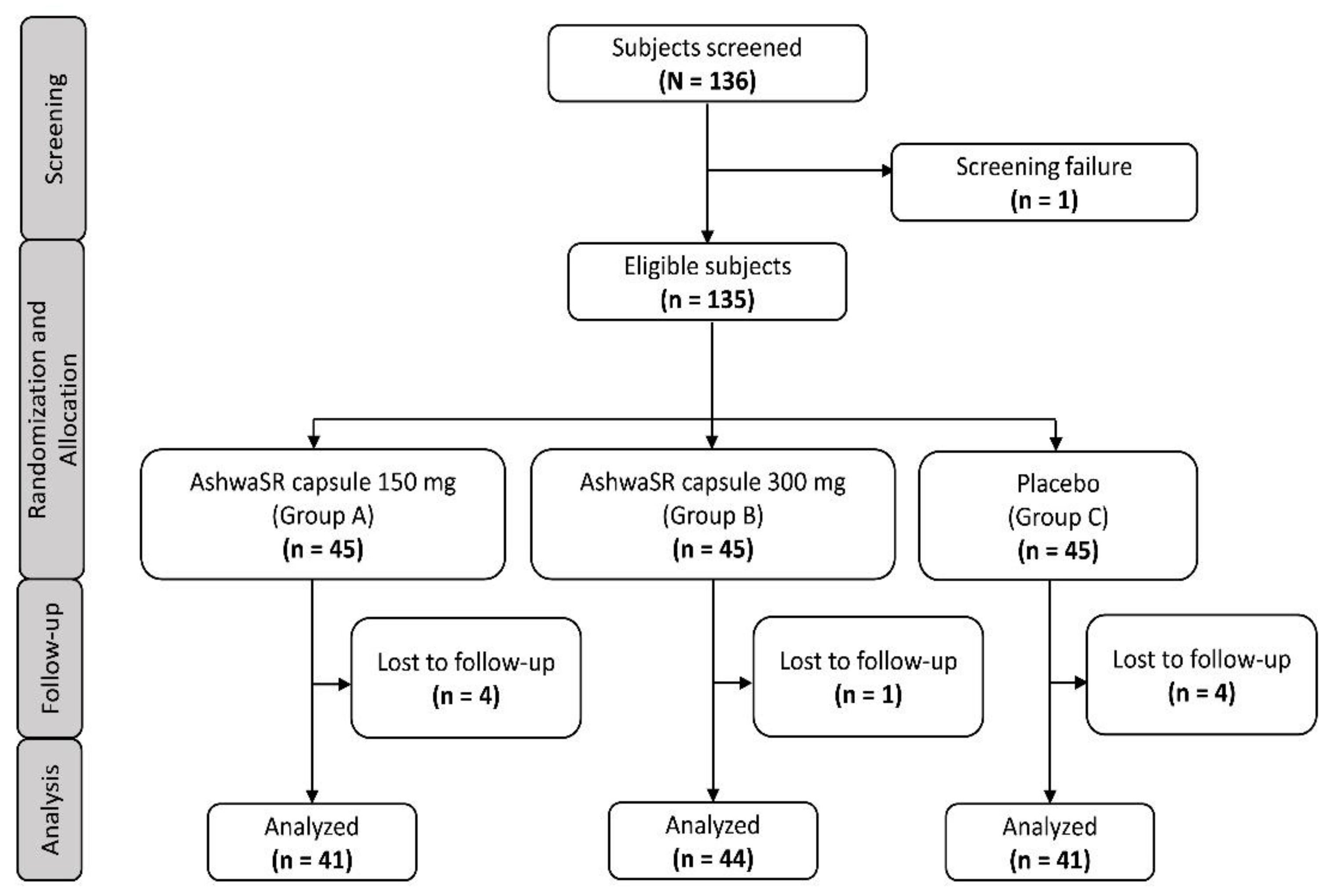
Of the total 136 subjects screened, 135 were randomized (screen failure, n = 1) to test group A, group B, or group C. Of these, nine subjects were lost to follow-up (group A [n=04]; group B [n=01]; and group C [n=04]) by the end of the study. Overall, 126 subjects completed the study, with treatment compliance of ≥ 80%, and were included in the analysis (group A, n = 41; group B, n = 44; group C, n = 41) (
Figure 1). At baseline, age (mean ± SD) of subjects was 34.79 ± 8.16 years, and 53.17% were male. Demographics and baseline characteristics of subjects are summarized in
Table 1. Subjects did not differ in demographics or baseline characteristics across arms (p > 0.05).
3.2. Primary Outcomes
3.2.1. Change in PSS Score
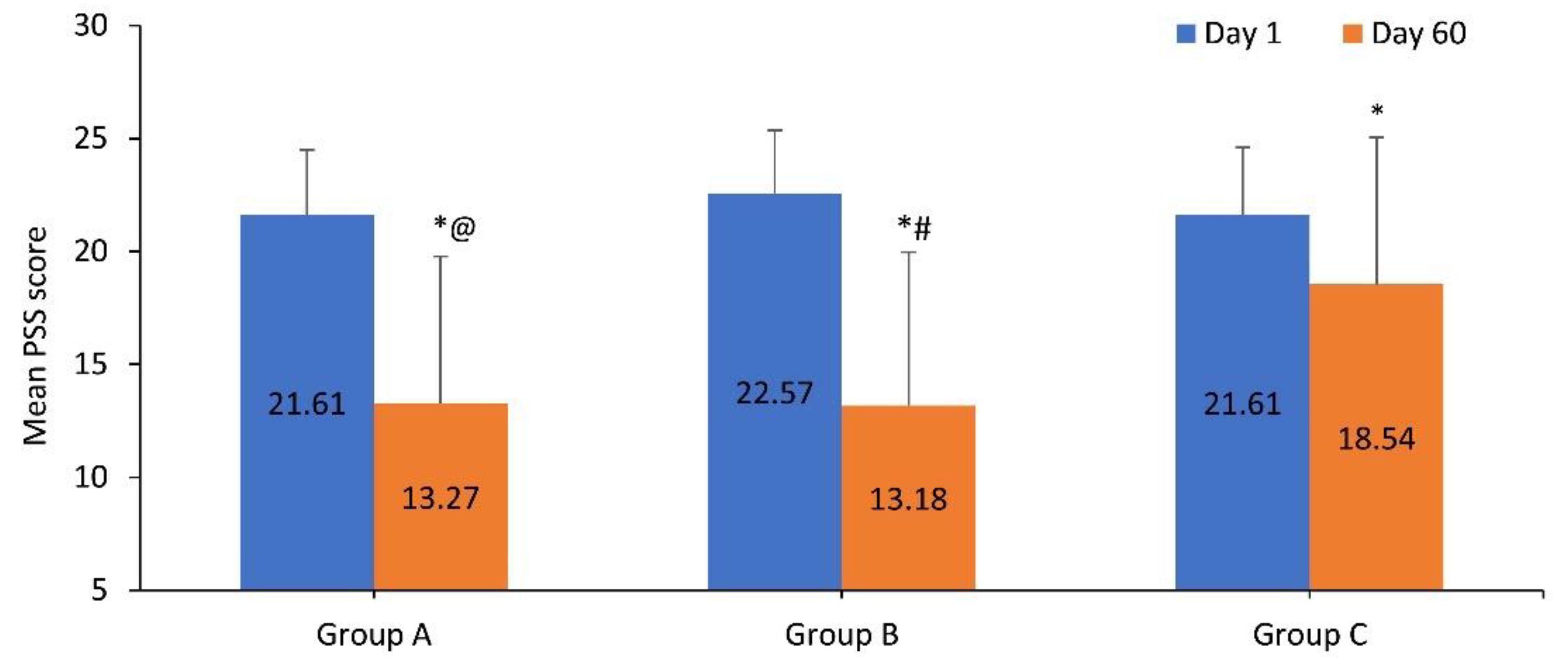
The PSS score (mean ± SD) in group A, group B, and group C was 21.61 ± 2.89, 22.57 ± 2.79, and 21.61 ± 2.99 at baseline, respectively. The mean PSS score at baseline was not significantly different between study groups (p > 0.05). The PSS score for group A, group B, and group C significantly reduced from baseline to 13.27 ± 6.51, 13.18 ± 6.79, and 18.54 ± 6.51 at day 60, respectively (p < 0.05). Mean change in PSS score (%) from baseline to day 60 was higher in group B (41.6%), followed by group A (38.6%) and group C (14.2%) (
Figure 2).
Compared to baseline, mean PSS score significantly reduced as early as day 15 in group A and group B, and reduction was sustained up to day 60 (all p < 0.05). Compared to group C, mean PSS score significantly reduced as early as day 15 in group B and day 30 in group A. The reduction was sustained up to day 60 (all p < 0.05). Mean PSS score was comparable between group A and group B at all visits (
Table 2).
3.3. Secondary Outcomes
3.3.1. Change in PSQI Global Score
At baseline, PSQI global score (mean ± SD) in group A, group B, and group C was 9.44 ± 3.35, 9.32 ± 3.34, and 8.17 ± 3.26, respectively, and was comparable (p > 0.05). At day 60, the PSQI global score (mean ± SD) significantly reduced from baseline to 5.54 ± 3.02 in group A and 4.52 ± 2.90 in group B (p < 0.05), with a higher mean change (%) from baseline observed in group B (51.5%), followed by group A (41.3%). Compared to baseline, the PSQI global score significantly reduced as early as day 3 in group B and day 7 in group A (p < 0.05), and the reduction was sustained up to day 60; the PSQI global score in group C was 7.59 ± 3.46 at day 60 (mean change (%) from baseline 6.6%, p = 0.1783). Moreover, PSQI score was not significantly different from baseline until day 60 in group C. Compared to group C, PSQI global score significantly reduced as early as day 7 in group A and group B (
Table 2).
3.3.2. Change in OHQ-8 Global Score
The OHQ-8 score was 3.14 ± 0.52 in group A, 2.93 ± 0.6 in group B, and 3.47 ± 0.48 in group C at baseline. At day 60, the OHQ-8 score was increased significantly to 3.75 ± 0.60 in group A, 4.00 ± 0.65 in group B, and 3.73 ± 0.60 in group C (p < 0.05), with a higher mean change from baseline observed in group B (36.3%), followed by group A (19.2%), and group C (7.6%). Compared to baseline, OHQ-8 score was significantly improved as early as day 7 in group A and group B (p < 0.05), and the improvement was sustained up to day 60. Compared to group C, mean OHQ-8 score was significantly improved as early as day 7 until day 60 in group B, whereas the OHQ-8 score was significantly improved as early as day 15 in group A vs group C. The improvement was sustained until day 60; however, it was not significant over group C. Furthermore, the mean OHQ-8 score was comparable between group A and group B at all visits except day 60 (
Table 2).
3.3.3. Change in TFEQ-R18 Global Score
The TFEQ-R18 score for cognitive restraint, uncontrolled eating, and emotional eating is summarized in
Table 3. Compared to baseline, the TFEQ-R18 score for cognitive restraint significantly reduced to 10.59 ± 2.77 in group A and 10.93 ± 3.41 in group B at day 60 (p < 0.05), with a slightly higher mean change (%) of 22.78% observed in group A followed by 21.53% in group B. Similarly, TFEQ-R18 score for uncontrolled eating and emotional eating significantly reduced in group A (14.95 ± 3.71 and 4.95 ± 1.53) and group B (14.25 ± 3.50 and 4.73 ± 1.34), respectively, at day 60 (p < 0.05), with a higher mean change (%) in TFEQ-R18 score for uncontrolled eating and emotional eating observed in group B (23.91% and 24.64%), followed by group A (22.11% and 22.22%), respectively. Compared to baseline, the TFEQ-R18 scores for cognitive restraint and uncontrolled eating were significantly reduced at day 7 up to day 60, and the score for emotional eating was significantly reduced at days 30 and 60 in group A and group B (all p < 0.05). There was no significant reduction in scores from baseline to day 60 in group C. Compared to group C, TFEQ-R18 score for cognitive restraint was significantly reduced at days 30 and 60 in group A and group B (p < 0.05), while the TFEQ-R18 score for uncontrolled eating was significantly reduced as early as day 7 up to day 60 in group A and group B. Furthermore, TFEQ-R18 scores for emotional eating were significantly reduced at day 60 in group A and group B compared to group C. The TFEQ-R18 score for cognitive restraint, uncontrolled eating, and emotional eating was comparable between group A and group B at all visits.
3.3.4. Serum Cortisol Level
Serum cortisol (mg/dL) level was 7.96 ± 2.88, 7.83 ± 2.61, and 7.05 ± 2.74 in group A, group B, and group C, respectively, at baseline. Compared to baseline, serum cortisol remained unchanged at days 7, 30, and 60 in group A and group C whereas, in group B, it significantly reduced to 6.33 ± 2.01 at day 60 (p < 0.05).
3.3.5. Subjects’ and Physicians’ Global Assessment of Therapy
The majority of subjects reported treatments as “Good/Very Good” (group A, 95.12%; group B, 84.09%; group C, 90.24%). Likewise, most physicians reported treatments as “Good/Very Good” (group A, 97.56%; group B, 97.73%; group C, 92.68%).
3.3.6. Safety
Overall, five subjects reported self-limiting, mild, and transient AEs such as pyrexia, myalgia, emesis, pruritus, and gastroesophageal reflux during the study period (group A, n = 3; group B, n = 1; group C, n = 1). Duration of AEs was limited to 4-5 days, and as per the causality assessment, the AEs were unrelated to the study treatment and completely resolved without any complications, allowing all subjects to continue their participation in the study. The AEs were comparable in terms of duration, severity, and seriousness in group A and group B to those observed in group C.
4. Discussion
This study assessed the efficacy and safety of AshwaSR 150 mg and 300 mg capsules in a single daily dose in healthy adult, stressed subjects. The results showed that AshwaSR 150 mg and 300 mg capsules improved perceived stress level, sleep quality, eating behavior, and overall well-being in subjects over 60 days of treatment. Improvements in outcomes (from baseline to day 60) were comparable between 150 mg and 300 mg SR capsules; however, a quicker and higher percentage of improvement was observed with AshwaSR 300 mg capsules. Furthermore, AshwaSR 300 mg capsules demonstrated reduction in perceived stress level as early as day 15 and improvement in happiness and uncontrolled eating as early as day 7 after administration, which lasted over 60 days of treatment.
Stress, whether from internal or external sources, acts as a triggering or aggravating factor for many diseases and pathological conditions. Therefore, it is crucial to prevent and manage stress effectively [
21]. In this study, PSS score was reduced from 22.57 at baseline to 13.18 at day 60 in the AshwaSR 300 mg-treated group, which was similar to reductions reported in previous clinical studies on Ashwagandha extract [
1,
12]; whereas, in the first clinical trial of AshwaSR 300 mg capsules, a study by Gopukumar et al., PSS score was reduced from 19.5 to 13.0 at day 90 with AshwaSR 300 mg [
12]. A double-blind, randomized, placebo-controlled trial showed reduction in PSS score from 22.95 to 14.15 after 8 weeks of treatment with Ashwagandha root extract 600 mg in healthy adults [
22]. In this study, PSS score was reduced by 41.6% with 300 mg and 38.6% with AshwaSR 150 mg capsules, which was close to the reductions observed after 60 days of treatment with Ashwagandha 300 mg capsules in the previous study [
1]. This could be attributed to anti-neuroinflammatory activity of Ashwagandha root extract, as demonstrated in terms of dose-dependent inhibition of TNF-α, IL-1β, and superoxide production in an in vitro study [
11].
Sleep is considered an important physiological phenomenon, with a restorative and rejuvenating influence on physiological systems [
23], which can be disrupted by elevated stress levels. Disturbed sleep and poor sleep quality compromise cognitive function, mood regulation, overall well-being, and happiness [
24]. A randomized, double-blind, placebo-controlled study in healthy subjects showed improved sleep quality after 6 weeks of treatment with Ashwagandha root extract (120 mg); in particular in subjects with frequent non-restorative sleep [
25]. Our study demonstrated a significant improvement in PSQI global score within 60 days of treatment with AshwaSR 300 mg and 150 mg capsules. Moreover, improvement in sleep quality was observed from as early as day 3 with AshwaSR 300 mg and day 7 with AshwaSR 150 mg capsules. The improvement in sleep quality may be attributed to an adaptogenic effect of Ashwagandha to regulate mood and overall well-being [
8].
Furthermore, a PSQI score < 5 indicates good sleep quality [
16] and an OHQ-8 score ≥ 4 indicates enhanced happiness in subjects [
20]. In this study, PSQI and OHQ-8 scores of 4.52 and 4.00, respectively, at day 60 demonstrated subjects experienced good sleep quality and enhanced happiness with AshwaSR 300 mg. In contrast, PSQI and OHQ-8 scores of 5.54 and 3.75, respectively, in subjects with AshwaSR 150 mg did not surpass respective thresholds at day 60. However, the trend of improvement in scores suggests that treatment with AshwaSR 150 mg for an increased time period may be required to cross cut-off values.
Perceived stress has been demonstrated to have a significantly negative impact on eating behavior [
26]. A higher cognitive restraint can lead to inhibition which is frequently interspersed with loss of control, described as hyperphagic or bulimic bouts and compulsive eating. A more rational strategy to normalize the eating behavior is probably to restore the individual’s hunger and satiety physiological regulation systems [
27]. In this study, AshwaSR 300 mg and 150 mg capsules demonstrated a restoration of positive eating behavior with an optimum balance of all the three domains of eating behavior as evaluated by the TFEQ-R18. A reduction in the cognitive restraint and uncontrolled eating scores from day 7 was observed with reduction in emotional eating score from day 30, which lasted till day 60 of the treatment.
Cortisol plays a crucial role in regulating the body’s stress response from onset to recovery. Variation in cortisol level has been observed during instances of psychological stress [
28]. Ashwagandha root extract, by acting on the hypothalamic–pituitary–adrenal axis, normalizes cortisol secretion and prevents excessive elevation of cortisol during stress [
28,
29]. Several clinical studies have reported reduction in cortisol level after treatment with Ashwagandha root extract [
1,
22,
30]. Similarly, in this study serum cortisol levels reduced from baseline to day 60 by 19.15% with 300 mg and by 11.44% with 150 mg AshwaSR, which suggests the association of the stress-reducing ability of Ashwagandha root extract with reduction in serum cortisol level.
AshwaSR 150 mg and 300 mg capsules were well tolerated, without any serious AEs reported throughout treatment. In this study, AEs were mild and did not result in subjects withdrawing from the study.
To our knowledge, this was a first-of-its-kind randomized three-arm, placebo-controlled, multicenter study that compared a low-dose formulation of AshwaSR with a standard dose (150 mg vs 300 mg) AshwaSR capsules and demonstrated multiple benefits in terms of improvement in stress, sleep quality, eating behavior, and overall well-being in healthy adult subjects with stress, over a period of 60 days of treatment. However, with a daily dose of AshwaSR 150 mg, administration for an increased time period may be required for achieving the threshold for good sleep quality score (PSQI). Moreover, AshwaSR 300 mg capsules showed a reduction in perceived stress levels that was observed as early as day 15, with improvement in happiness and eating behavior as early as day 7 of administration, and these effects were sustained until the end of treatment (60 days). In addition, the study reported the effect of AshwaSR 300 mg and 150 mg capsules on serum cortisol reduction over 60 days.
Limitations of the study should be considered. The effects of AshwaSR 300 mg and 150 mg capsules were evaluated over 60 days; however, long-term follow-up could provide further insights into safety aspects. Also, further studies in older subjects could help to provide insights into efficacy across diverse age groups.
5. Conclusions
Ashwagandha root extract SR capsules at doses of 150 mg and 300 mg showed multiple benefits, in terms of improvement in perceived stress level, improvement of sleep quality, reinforcement of positive eating behavior, and enhancement of happiness levels in healthy adult, stressed subjects. Although benefits were comparable between low dose and standard dose of AshwaSR, treatment with standard-dose AshwaSR (300 mg) capsules demonstrated a rapid and sustained improvement in stress levels, happiness, and eating behavior over 60 days of study duration. Both doses of AshwaSR (150 mg and 300 mg) capsules were well tolerated and safe over 60 days. Overall, the study demonstrated beneficial effects of AshwaSR 150 mg and 300 mg capsules in the management of stress.
Author Contributions
Conceptualization, S.T. and R.S.; formal analysis and data curation, A.V.KR., S.T., P.D. and A.B; investigation, P.D. and A.B.; writing— original draft preparation, S.T.; writing— review and editing, all authors; visualization, R.S.; supervision, S.T. and A.V.KR.; project administration, S.T. and A.V.KR.; resources, R.S. and K.B.; and funding acquisition, R.S. and K.B. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by Nutriventia Limited and Laila Nutraceuticals.
Institutional Review Board Statement
The study was conducted in accordance with Good Clinical Practice and the Helsinki Declaration Standards, in addition to The New Drug and Clinical Trial Rules 2019 and the Ethical Guidelines for Biomedical Research on Human Subjects, issued by the Indian Council of Medical Research. The study was registered on Clinical Trials Registry-India (CTRI) on 08/06/2023 (CTRI Reg no: CTRI/2023/06/053662). The study protocol, informed consent form and other relevant study documents were reviewed and approved by an independent, registered Ethics Committee of the Santhosh hospital (Reg. no. ECR/1062/Inst/KA/2018/RR-21) before initiating the study recruitment.
Informed Consent Statement
The written informed consent was obtained from each participant prior to initiation of the clinical study.
Data Availability Statement
The data generated or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We would like to thank Roshni Patel, PhD and Sonali Dalwadi, PhD, CMPP™ of IWANA Consultancy Solutions for providing medical writing support for this manuscript, which was funded by Nutriventia Ltd, Mumbai, India and Laila Nutraceuticals.
Conflicts of Interest
Dr. Shefali Thanawala and Rajat Shah are employees of Nutriventia Limited, India. Dr. Krishnaraju Venkata Alluri and Kiran Bhupathiraju are employees of Laila Nutraceuticals, India. Dr Prabakaran Desomayanandanam and Arun Bhuvenendran are employees of In vitro Research Solutions Pvt Ltd, India. The authors do not have any other conflicts of interest to declare.
Abbreviations
The following abbreviations are used in this manuscript:
| SR |
sustained-release |
| AshwaSR |
Ashwagandha root extract SR |
| PSS |
Perceived Stress Scale |
| CTRI |
Clinical Trials Registry-India |
| PSQI |
Pittsburgh Sleep Quality Index |
| OHQ-8 |
Oxford Happiness Questionnaire-8 score |
| TFEQ-R18 |
Three-Factor Eating Questionnaire- Revised 18 score |
| AEs |
Adverse events |
| SD |
Standard deviation |
| ANOVA |
Analysis of variance |
| HSD |
Honest significant difference |
References
- Chandrasekhar, K.; Kapoor, J.; Anishetty, S. A prospective, randomized double-blind, placebo-controlled study of safety and efficacy of a high-concentration full-spectrum extract of Ashwagandha root in reducing stress and anxiety in adults. Indian J. Psychol. Med. 2012, 34, 255–262. [Google Scholar] [CrossRef]
- Khan, S.I.R.; Aljammaz, G.; Alosail, L.A.; Almeshrafi, A.; Ramachandran, A.; Siddeeqh, S.; Alfadley, A. Psychological stress as a determinant of increased maximum voluntary bite force - A clinical observational study. Cureus. 2023, 15, e46106. [Google Scholar] [CrossRef]
- Ning, L.; Guan, S.; Liu, J. An investigation into psychological stress and its determinants in Xinjiang desert oil workers. Medicine 2018, 97, e0323. [Google Scholar] [CrossRef] [PubMed]
- Yau, Y.H.; Potenza, M.N. Stress and eating behaviors. Minerva Endocrinol. 2013, 38, 255–267. [Google Scholar] [PubMed]
- Mahmud, S.; Mohsin, M.; Dewan, M.N.; Muyeed, A. The global prevalence of depression, anxiety, stress, and insomnia among general population during COVID-19 pandemic: A systematic review and meta-analysis. Trends in Psychol. 2023, 31, 143–170. [Google Scholar] [CrossRef]
- Panossian, A.; Wikman, G. Effects of adaptogens on the central nervous system and the molecular mechanisms associated with their stress - Protective activity. Pharmaceuticals. 2010, 3, 188–224. [Google Scholar] [CrossRef]
- Bokelmann, J.M. Ashwagandha (Withania somnifera): Root. In J. M. Bokelmann (Ed.), Medicinal herbs in primary care. Elsevier; 2022, 195–201.
- Verma, N.; Gupta, S.K.; Tiwari, S.; Mishra, A.K. Safety of Ashwagandha root extract: A randomized, placebo-controlled, study in healthy volunteers. Complement. Ther. Med. 2021, 57, 102642. [Google Scholar] [CrossRef]
- Majeed, M.; Nagabhushanam, K.; Mundkur, L. A standardized Ashwagandha root extract alleviates stress, anxiety, and improves quality of life in healthy adults by modulating stress hormones: Results from a randomized, double-blind, placebo-controlled study. Med. 2023, 102, E35521. [Google Scholar] [CrossRef]
- Alluri, V.K.R.; Thanawala, S.; Upadhyay, V. A comparative pharmacokinetics study of Ashwagandha (Withania somnifera) Root Extract sustained-release capsules: An open-label, randomized, two treatment, two-sequence, two period, single-dose crossover clinical study. Int. J. Basic Clin. Pharmacol. 2021, 11, 26–34. [Google Scholar] [CrossRef]
- Krishnaraju, A.V.; Somepalli, V.; Thanawala, S.; Shah, R. . Efficacy and anti-inflammatory activity of Ashwagandha sustained-release formulation on depression and anxiety induced by chronic unpredictable stress: In vivo and in vitro Studies. J. Exp. Pharmacol. 2023, 15, 291–305. [Google Scholar] [CrossRef]
- Gopukumar, K.; Thanawala, S.; Somepalli, V.; Rao, T.S.S.; Thamatam, V.B.; Chauhan, S. Efficacy and safety of Ashwagandha root extract on cognitive functions in healthy, stressed adults: A randomized, double-blind, placebo-controlled study. Evidence-based Complement. Altern. Med. 2021, 2021, 8254344. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. J. Health. Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Rijal, D.; Paudel, K.; Adhikari, T.B.; Bhurtyal, A. Stress and coping strategies among higher secondary and undergraduate students during COVID-19 pandemic in Nepal. PLOS Glob. public Heal. 2023, 3, e0001533. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds, C.F. , 3rd, Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Park, B.K. The Pittsburg Sleep Quality Index (PSQI) and associated factors in middle-school Students: A Cross-sectional Study. Child Heal. Nurs. Res. 2020, 26, 55–63. [Google Scholar] [CrossRef]
- Karlsson, J.; Persson, L.O.; Sjöström, L.; Sullivan, M. Psychometric properties and factor structure of the Three-Factor Eating Questionnaire (TFEQ) in obese men and women. Results from the Swedish Obese Subjects (SOS) study. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 1715–1725. [Google Scholar] [CrossRef]
- Banna, J.C.; Panizza, C.E.; Boushey, C.J.; Delp, E.J.; Lim, E. Association between cognitive restraint, uncontrolled eating, emotional eating and BMI and the amount of food wasted in early adolescent girls. Nutrients. 2018, 10, 1279. [Google Scholar] [CrossRef]
- Hills, P.; Argyle, M. The Oxford Happiness Questionnaire: A compact scale for the measurement of psychological well-being. Pers. Individ. Dif. 2002, 33, 1071–1082. [Google Scholar] [CrossRef]
- Rao, R.; Naik, B.N.; Shekhar, S.; Nirala, S.K.; Singh, C.M.; Verma, M.; Ramalingam, A. Level of happiness among medical students in Bihar-An online survey. J. Educ. Health Promot. 2023, 12, 305. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Panahi, Y.; Sahraei, H.; Johnston, T.P.; Sahebkar, A. The impact of stress on body function: A review. EXCLI J. 2017, 16, 1057–1072. [Google Scholar] [CrossRef]
- Salve, J.; Pate, S.; Debnath, K.; Langade, D. Adaptogenic and anxiolytic effects of ashwagandha root extract in healthy adults: A double-blind, randomized, placebo-controlled clinical study. Cureus. 2019, 11, e6466. [Google Scholar] [CrossRef] [PubMed]
- Eugene, A.R.; Masiak, J. The neuroprotective aspects of sleep. MEDtube. Sci. 2015, 3, 35–40. [Google Scholar] [PubMed]
- Vandekerckhove, M.; Wang, Y.L. Emotion, emotion regulation and sleep: An intimate relationship. AIMS Neurosci. 2018, 5, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, A.; Irani, N.; Balkrishnan, R.; Benny, I.R. A randomized, double blind, placebo-controlled study to evaluate the effects of ashwagandha (Withania somnifera) extract on sleep quality in healthy adults. Sleep Med. 2020, 72, 28–36. [Google Scholar] [CrossRef]
- Hirotsu, C.; Tufik, S.; Andersen, M.L. Interactions between sleep, stress, and metabolism: From physiological to pathological conditions. Sleep Sci. 2015, 8, 143–152. [Google Scholar] [CrossRef]
- Apfeldorfer, G.; Zermati, J.P. Cognitive restraint in obesity. History of ideas, clinical description. Press medicale. 2001, 30, 1575–1580. [Google Scholar]
- Dziurkowska, E.; Wesolowski, M. Cortisol as a biomarker of mental disorder severity. J. Clin. Med. 2021, 10, 5204. [Google Scholar] [CrossRef]
- Elgar, K. Ashwagandha: A review of clinical use and efficacy. Nutr. Med. J. 2021, 1, 68–78. [Google Scholar]
- Remenapp, A.; Coyle, K.; Orange, T.; Lynch, T.; Hooper, D.; Hooper, S.; Conway, K.; Hausenblas, H.A. Efficacy of Withania somnifera supplementation on adult’s cognition and mood. J. Ayurveda Integr. Med. 2022, 13, 100510. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).