Submitted:
08 February 2025
Posted:
10 February 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
| Position | 1Ha | 1Hb | 13Ca | 13Cb |
| 1a | 2.05 m | 1.13 m |
39.8 |
39.8 |
| 1b | 1.25 m | 1,74 m | ||
| 2a | 2.48 td (14.3, 5.7) | 2.21 ddd (13.6, 3.9, 2.6) | 38.3 | 38.3 |
| 2b | 2.30 m | 2.09 td (13.6, 5.6) | ||
| 3 | 213.4 | 210.5 | ||
| 4 | 2.13 m | 1.88 m | 45.8 | 45.9 |
| 5 | 1.44 m | 1.73 m | 50.5 | 50.5 |
| 6a | 2.15 m | 1.92 m | 28.1 | 28.5 |
| 6b | 1.90 m | 1.54 m | ||
| 7 | 5.21 brs | 5.16 br dd (5.6, 2.0) | 117.4 | 118.4 |
| 8 | 139.4 | 139.3 | ||
| 9 | 1.75 m | 1.48 m | 49.5 | 49.8 |
| 10 | 35.4 | 35.5 | ||
| 11a | 1.61 m | 1.38 (2H, m) | 22.1 | 22.1 |
| 11b | 1.55 m | |||
| 12a | 2.16 m | 1.99 dt (12.5, 3.7) | 40.1 | 40.2 |
| 12b | 1.48 m | 1.13 m | ||
| 13 | 43.6 | 43.9 | ||
| 14 | 1.80 m | 1.73 m | 55.1 | 55.5 |
| 15a | 1.53 m | 1.55 m | 23.7 | 23.7 |
| 15b | 1.42 m | 1.43 m | ||
| 16a | 1.79 m | 1.90 m | 29.8 | 28.6 |
| 16b | 1.27 m | 1.28 m | ||
| 17 | 1.25 m | 1.20 m | 56.1 | 56.6 |
| 18 | 0.56 m | 0.59 s | 12.07 | 12.4 |
| 19 | 1.08 s | 0.80 s | 13.9 | 13.8 |
| 20 | 1.33 m | 1.33 m | 36.8 | 37.5 |
| 21 | 0.92 d (6.4) | 0.99 d (6.5) | 19.2 | 19.6 |
| 22a | 1.24 m | 1.33 m | 30.6 | 31.3 |
| 22b | 0.88 m | 0.98 m | ||
| 23a | 1.40 m | 1.45 m | 37.5 | 38.0 |
| 23b | 1.20 m | 1.23 m | ||
| 24 | 28.9 | 39.3 | ||
| 25 | 152.5 | 152.4 | ||
| 26a | 4.73 brs | 4.88 brs | 109.5 | 110.6 |
| 26b | 4.66 brs | 4.85brs | ||
| 27 | 1.69 brs | 1.71 brs | 19.6 | 20.0 |
| 28 | 1.01c | 1.06 d (6.5) | 11.6 | 12.5 |
| 29 | 1.01 s | 1.08 s | 27.7 | 27.8 |
| 30 | 1.02 s | 1.08 s | 27.4 | 28.1 |
| a CDCl3, 600, 150 MHz. b C6D6, 600, 150 MHz. c Partially hidden. | ||||
| δH (J values are given in Hz) δC | ||||
| atom/position | (2) | (3) | (2) | (3) |
| H-1a | 1.87 m | 1.71 m |
40.1 |
38.7 |
| H-1b | 0.95 m | 1.06 m | ||
| H-2a | 2.27 m | 1.64 m |
34.4 |
23.9 |
| H-2b | 2.23 m | 1.64 m | ||
| H-3 | C-3 | 4.49 d d (10.7, 5.5) | 215.5 | 80.9 |
| 47.6 | 38.1 | |||
| H-5 | 1.10 m | 0.85 m | 55.6 | 56.3 |
| H-6a | 1.31 m | 1.51 m |
20.1 |
18.1 |
| H-6b | 1.22 m | 1.46 t d (12.8, 3.0) | ||
| H-7a | 1.40 m | 1.59 m |
35.3 |
35.4 |
| H-7b | 1.14 m | 1.28 m | ||
| 40.7 | 40.5 | |||
| H-9 | 1.15 m | 1.33 dd (12.3, 3.0) | 50.7 | 50.9 |
| 37.2 | 37.1 | |||
| H-11a | 1.17 m | 1.52 m |
22.35/22.33a |
21.3 |
| H-11a | 1.05 m | 1.20 m | ||
| H-12a | 1.56 (2H, m) | 1.58 m |
25.6 |
25.0 |
| H-12b | 1.56 (2H, m) | 1.08 m | ||
| H-13 | 1.79 m | 1.67 m | 46.26/46.07a | 45.6 |
| 49.9 | 49.5 | |||
| H15a | 1.60 m | 1.60 m |
31.96/31.94a |
31.3 |
| H-15b | 1.08 m | 1.11 m | ||
| H-16a | 1.99 m | 1.93 m |
29.9/29.8a |
29.2 |
| H16b | 1.57 m | 1.41 m | ||
| H-17 | 2.31 m | 2.23 t d (10.6, 6.8) | 48.58/48.47a | 48.06 |
| Me 18 | 0.80 brs | 0.98 s | 16.3 | 15.82 |
| Me-19 | 0.70 s | 0.87 s | 16.4 | 16.3 |
| 153.86/153.81a | 153.7 | |||
| H-21a | 4.97 brs | 4.76 brs |
108.12/108.05a |
107.61 |
| H-21b | 4.93 m | 4.74 brs | ||
| H-22a | 2.23 m | 2.12 m |
29.56/29.55a |
34.1 |
| H-22b | 2.07 m | 2.12 m | ||
| H-23a | 1.77 (2H, m) | 2.18 m |
39.7/40.0a |
30.3 |
| H-23b | 1.77 (2H, m) | 2.18 m | ||
| 75.38/75.32a | 158.3 | |||
| 150.9 | 36.3 | |||
| H-26a | 5.12 brd (1.3) | 1.08 s |
110.4 |
29.5 |
| H-26b | 4.86 dd (2.7, 1.3) | |||
| Me-27 | 1.651/1.639 brs | 1.08 s | 19.95 | 29.5 |
| Me-28 | 1.16 s | 0.86 s | 28.71/28.68a | 28.2 |
| Me-29 | 0.99 s | 0.85 s | 21.5 | 16.5 |
| Me-30 | 1.09 s | 0.87 s | 27.1 | 15.9 |
| Me-31 | 1.16 s | 4.88 br s H31a | 28.7/28.68a |
106.14 |
| 4.71 brs H31b | ||||
| Me-32 | 1.08 s | 29.5 | ||
| Ac | 2.05 (3H, s) | 21.39, 171.12 | ||
3. Experimental
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation of the Constituents
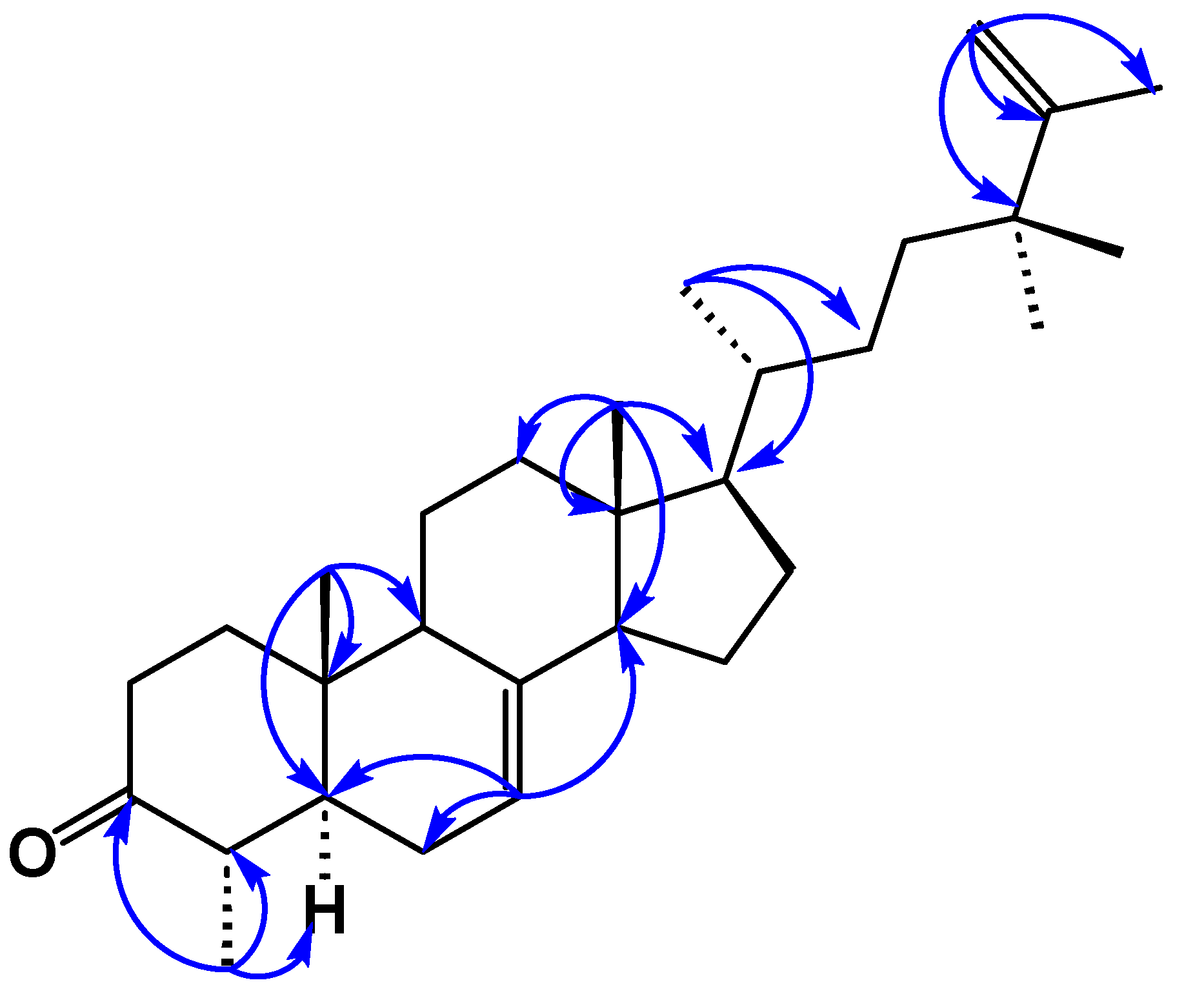
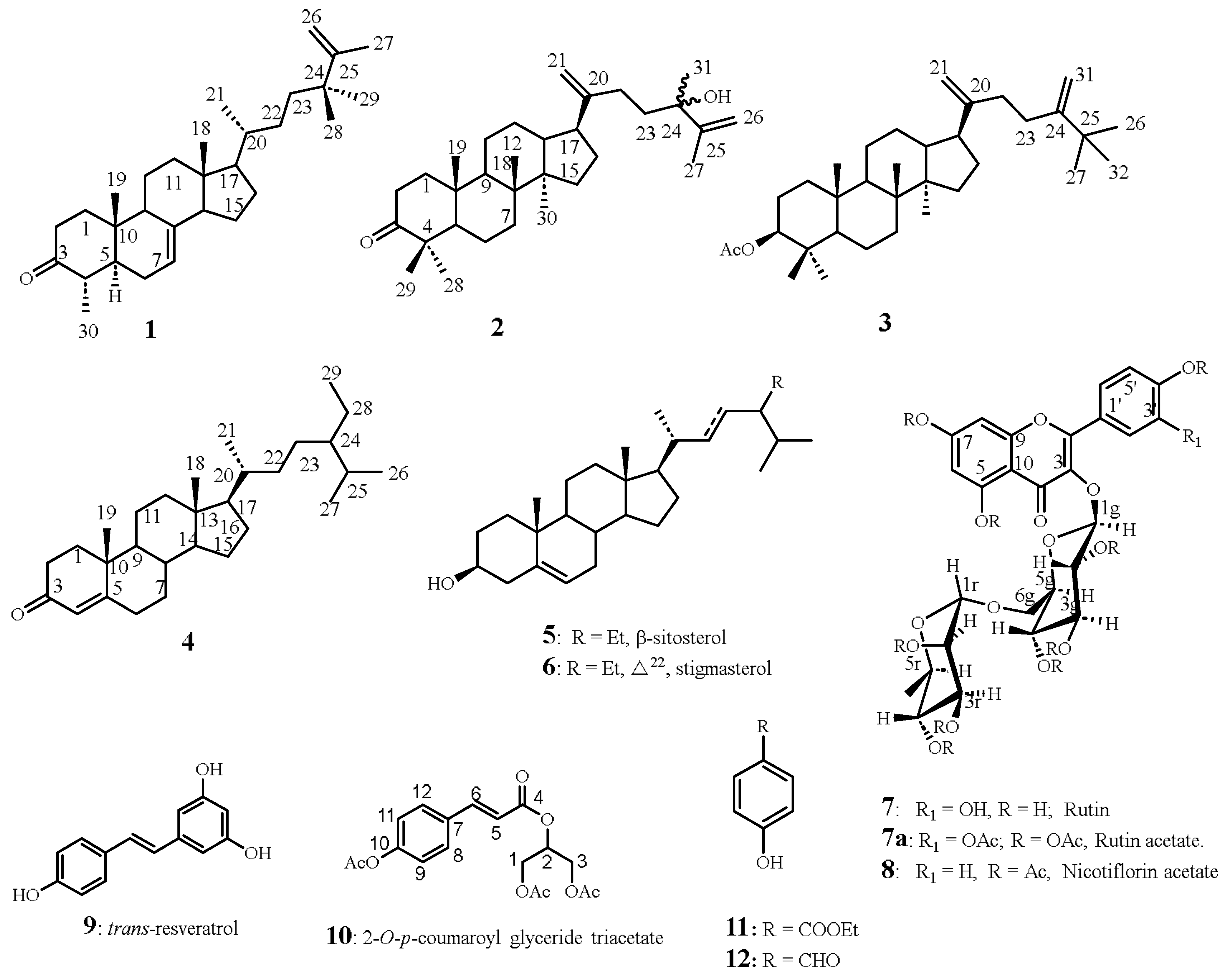
3.3.1. 24,24-Dimethyl-5α-cholesta-7,25-dien-3-one (1)
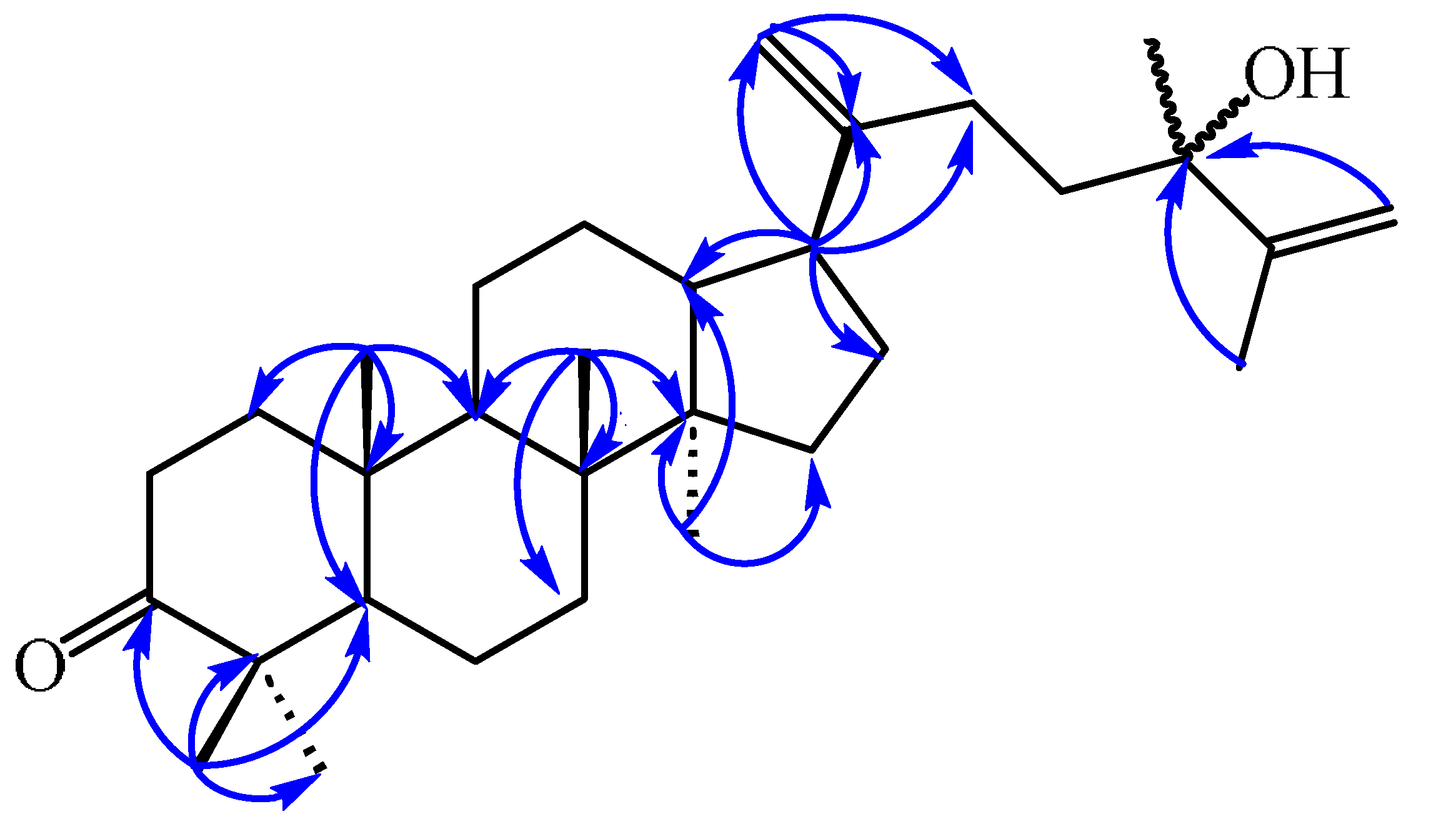
3.3.2. 24-Hydroxy-24-methyl-dammara-20,25-dien-3-one (2)
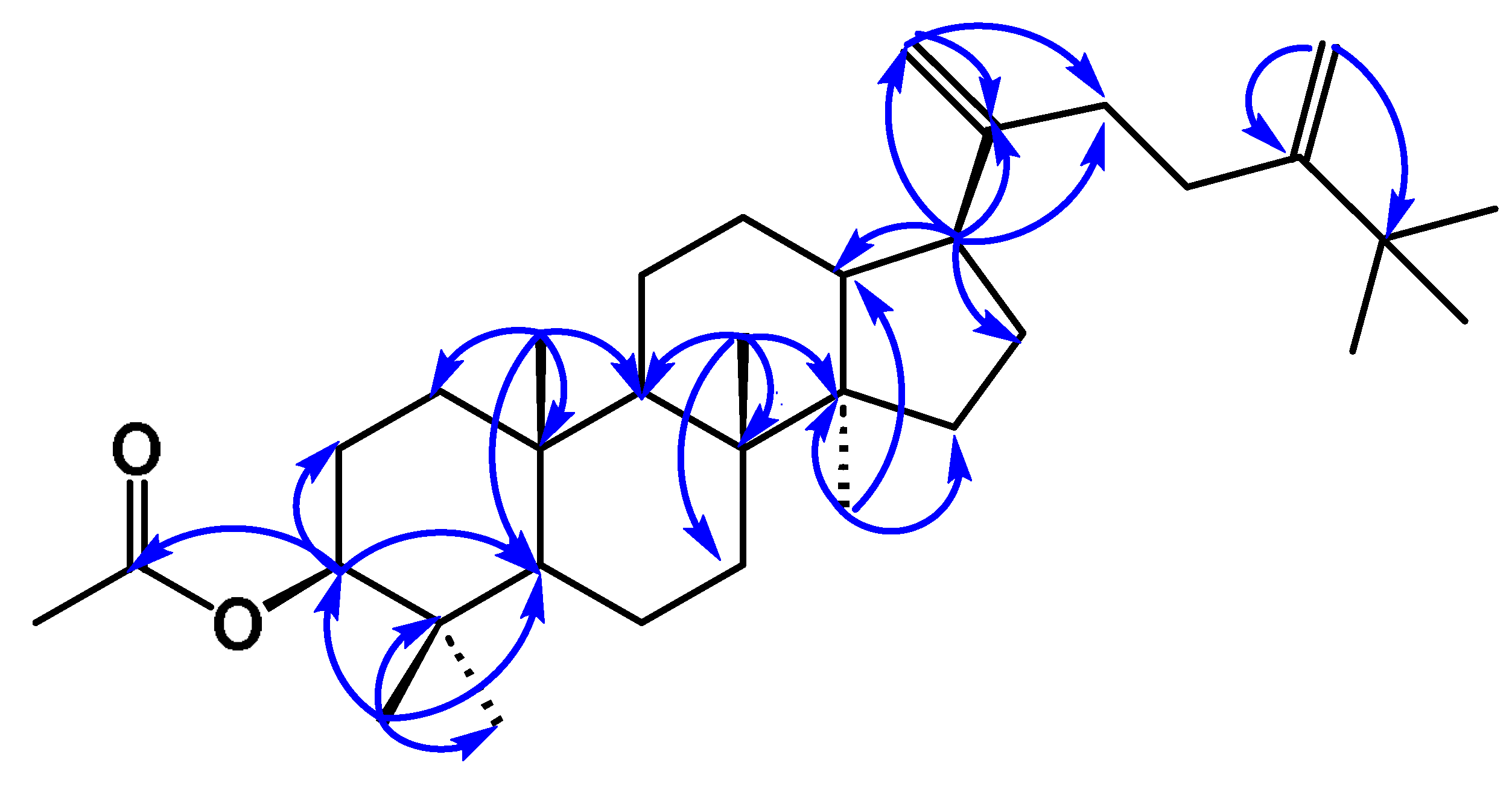
3.3.4. Stigmast-4-en-3-one (4)
3.3.5. Quercetin-3-O-rutinoside, Rutin (7)
3.3.6. Kaempherol-3-O-rutinosidenonaacetate, nicotiflorin acetate (8).
3.3.7. Trans-resveratrol (3,4′,5-trihydroxystilbene) (9).
3.3.8. 2-O-p-Coumaroylglycerol triacetate (Juncusyl ester B triacetate) (10).
3.3.9. Ethyl p-hydroxybenzoate (11).
3.3.10. p-Hydroxybenzaldehyde (12).
References
- Shao, B., Guo, H.Z., Cui, Y.J., Ye, M., Han, J., Guo, D.A. Steroidal saponins from Smilax China and their anti-inflammatory activities. Phytochemistry, 2007, 68, 623–630. [CrossRef]
- Abdala, S., Martin-Herrera, D., Benjumea, D., Pérez-Paz, P. Diuretic activity of Smilax canariensis, an endemic Canary Island species. Journal of Ethnopharmacology, 2008, 119, 12–16. [CrossRef]
- Toshihiro Itoh, Tsutomu Tamura, Masakazu Sagawa, Toshitake Tamura, Taro Matsumoto, 1980. 4α-Methyl-5α-cholest-8(14)-en-3β-ol from the seeds of Capsicum annuum. Phytochemistry, 1983, 22, 11, 2621−2622. [CrossRef]
- Toshihiro Itoh, Tsutomu Tamura, Masakazu Sagawa, Toshitake Tamura, Taro Matsumoto. 4(R)-ethyllophenol from Solanum melongena seeds. Phytochemistry, 1980, 19, 11, 2491−2492. [CrossRef]
- Toshihiro Akihisa, Parthasarathi Ghosh, Swapnadip Thakur, Hiroshi Nagata, Toshitake Tamura, Taro Matsumoto. 24,24-Dimethyl-25-dehydrolophenol, a 4α-methylsterol from Clerodendrum inerme. Phytochemistry, 1990, 29, 5, 1639−1641. [CrossRef]
- Buana C. de Almeida, Bruno Q. Araújo, Elcio D. S. Barros,a Sâmya D. L. Freitas, Dayany S. A. Maciel,b Ari J. S. Ferreira, Rafael C. Guadagnin, Gerardo M. Vieira Júnior, João H. G. Lagob, and Mariana H. Chaves. Dammarane Triterpenoids from Carnauba, Copernicia prunifera (Miller) H. E. Moore (Arecaceae), Wax. Journal of the Brazilian Chemical Society, 2017, 28, 8, 1371−1376. [CrossRef]
- Thanika Pathomwichaiwat, Pannee Ochareon, Noppamas Soonthomchareonnon, Zulfiqar Ali, Ikhlas A. Khan, Sompop Prathanturarug. Alkaline phosphatase activity-guided isolation of active compounds and new dammarane-type triterpenes from Cissus quadrangularis hexane extract. Journal of Ethnopharmacology, 2015, 160, 52−60. [CrossRef]
- V. Anjaneyulu, G. Sambasiva Rao, J. D. Connolly. Occurrence of 24-epimers of cycloart-25-ene-3-β, 24-diols in the stems of Euphorbia trigona. Phytochemistry, 1985, 24, 1610−1612. [CrossRef]
- Seongki Kim, Toshihiroakihisa, Toshitaketamura, Taro Matsumoto,Takaoyokota, Nobutaka. 24-Methylene-25-methylcholesterol in Phaseolus vulgaris seed: structural relation to brassinosteroids. Phytochemistry, 1988, 27, 2, 629−631. [CrossRef]
- Jesús G. Díaz, Chemical composition of Hypericum Coadunatum Chr. from the Canary Islands. Journal of Molecular Structure, 2022, 1248, 15, 131447. [CrossRef]
- P.C. Kiprono, F. Kaberia, J.M. Keriko, J.N. Karanja. The in vitro anti-fungal and antibacterial activities of beta-sitosterol from Senecio lyratus (Asteraceae). Zeitschrift für Naturforschung, 2000, 55c, 485−488.
- Fathaiya Jamaluddin, Sohaila Mohamed and Md Nordin Lajisb. Hypoglycemic effect of Stigmast-4-en-3-one, from Parkia speciosa empty pods. Food Chemistry, 1995, 54, 1, 9−13. [CrossRef]
- Kohei Kazuma, Naonobu Noda, Masahiko Suzuki. Malonylated flavonol glycosides from the petals of Clitoria ternatea. Phytochemistry, 2003, 62, 229–237. [CrossRef]
- De Alcantara Pinto, Douglas Chaves; Pitasse-Santos, Paulo; de Souza, Gabriela Alves; Castro, Rosane Nora; Freire de Lima, Marco Edilson. Peracetylation of polyphenols under rapid and mild reaction conditions. Natural Product Research, 2023, 37, 2279−2284. [CrossRef]
- U.L.B. Jayasinghe, B.A.I.S. Balasooriya, A.G.D. Bandara & Y. Fujimoto, Glycosides from Grewia damine and Filicium decipiens. Natural Product Research: Formerly Natural Product Letters, 2004, 18, 6, 499−502. [CrossRef]
- Šmidrkal, J., Harmatha, J., Buděšínský, M., Vokáč, K., Zídek, Z., Kmoníčková, E., Merkl R., Filip, V. Modified approach for preparing (E)-stilbenes related to resveratrol and evaluating their potential immunobiological effects. Collection of Czechoslovak Chemical Communications, 2010, 75, 2, 175–186. [CrossRef]
- Fier, Patrick S.; Maloney, Kevin M. Direct conversion of haloarenes to phenols under mild, transition-metal-free conditions. Organic Letters, 2016, 18, 2244−2247.. [CrossRef]
- Kashparova, Vera P.; Klushin, Victor A.; Zhukova, Irina Yu.; Kashparov, Igor S.; Chernysheva, Daria V.; Il'chibaeva, Irina B.; Smirnova, Nina V.; Kagan, Efim Sh.; Chernyshev, Victor M. A TEMPO-like nitroxide combined with an alkyl-substituted pyridine: An efficient catalytic system for the selective oxidation of alcohols with iodine. Tetrahedron Letters, 2017, 58, 36, 3517−3521. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





