1. Introduction
Ostreoidea appeared in the Triassic and comprise a superfamily with a long paleontological record [
1,
2,
3]. Ostreoidea evolved into ecologically very successful marine organisms and have today a global distribution across a wide range of salinities and habitats [
4,
5]. Ostreoidea colonize intertidal to subtidal, marine to brackish environments [
3,
6]; nonetheless, some species (
Neopycnodonte zibrowii Gofas, Salas and Taviani 2009) extend into deep water, up to about 2000 m water depths [
6,
7].
The shells of Ostreoidea are composites of biopolymers and minerals (e.g. [
3,
8,
9,
10,
11,
12]). The biopolymer component is present in the shell (i) as a network between the crystallites, (ii) as thick membranes encasing the columns, (iii) as an organic glue surrounding the cementation granules and, only for the
Ostrea sp., (iv) as an organic layer incorporating an assembly of rhombohedral crystals [
13].
The superfamily of Ostreoidea comprises the families Gryphaeidae (foam oysters) and Ostreidae (true oysters) [
12,
14,
15]. Species of both families form their shell of low-Mg calcite and myostracal and ligament aragonite [
16,
17,
18]. In this regard, the Ostreoidea are specific among bivalves. While species of many bivalve families secrete for shell formation aragonite or superimposed layers of aragonite and calcite, Ostreoidea use calcite for shell generation, disregarding the myostracal and ligament aragonite [
3,
19].
Below an outermost thin organic periostracum [
17], Ostreoidea secrete six Ca-carbonate microstructures. The bulk of the Ostreoidea shell consists of foliated calcite (e.g. [
16,
17,
18,
19,
20,
21,
22]), complemented, however, along the external surface of both valves, though particularly the right one, with a seam of columnar calcite [
16,
23,
24], and, along sections of the proximal surface of the lower valve, with a cementation layer comprising granular calcite [
4,
25,
26,
27]. The muscle attachment sites, myostraca, are formed of prismatic aragonite. The columnar, foliated, granular calcite and myostracal, prismatic, aragonite microstructures generate compact shell layers. However, Ostreoidea incorporate voids into their shell; a further structural characteristic that is specific for these bivalves. Ostreidae incorporate large volumes of a meshwork of wavy calcite blades and laths into the foliated shell, forming the chalk lenses [
17,
19,
28]. Gryphaeidae incorporate lenses of submicrometer- to micrometer-sized pores within the foliated shell [
13].
The Ostreoidea have life spans of about 10 years, up to a maximum of 20 years. Nonetheless, they form thick shells within a few years [
29]. This can be achieved by incorporating large volumes of cavities into the structural material of the shell; a unique strategy developed, among bivalves, only by the Ostreoidea for producing in short time thick and robust shells [
30]. The chalk and the vesicular lenses have irregular extensions and varying thicknesses within the shells. In shell cross-sections, the growth lines are widely spaced between the foliated layer and the chalk or between the foliated layer and the vesicular microstructure. This indicates a higher thickening speed for the cavity-rich microstructures, relative to the speed of thickening of the foliated material [
12,
22]. In addition, it is also demonstrated that the chalk assists with its cavity-rich structural plasticity the attachment of the oyster shell to uneven-surfaced substrates (e.g. [
31] and references therein).
In the study presented here we summarize and review crystallographic aspects for all types of crystals and crystal units that we observe, so far, for Ostreoidea shells. The latter are ideal for understanding the morphological, crystallographic and organizational diversity of biologically secreted Ca-carbonate crystals, as the compact Ostreoidea shell layers comprise not only the five main microstructures (columnar, foliated, granular, prismatic (the myostraca) and rhombohedral (the latter the Ostreidae only)), but also the cavity-rich assemblies of the chalk blades and laths in the Ostreidae and the fractal-shaped dendritic polyhedral crystals, surrounding the vesicular pores in the Gryphaeidae. In addition, due to this huge diversity in crystal shapes, types and organizational patterns, Ostreoidea shells are best suited for examining the crystallographic nature at the changeover from one microstructure into the other. We find five transitions between adjacent crystal assembly patterns. Disregarding the aragonite of the myostraca, all Ostreoidea shell microstructures are formed of only one Ca-carbonate polymorph, calcite, hence, there is no delimitation between adjacent microstructures in form of, e.g. a biopolymer membrane, as it is often the case when the shell is formed of superimposed layers of calcite and aragonite [
32]. Accordingly, the changeover from one microstructure into the other is, to a large degree, controlled for Ostreoidea shells by crystallographic aspects of the relevant crystals and microstructures.
The foliated shell layer with its specific microstructure and outstanding texture is topologically related to all other shell microstructures. Accordingly, for understanding the shell as a structural entity, one major aim of this study, it is essential to assess the crystallographic-structural attributes of all types of shell-forming crystals and crystal units as well as of the microstructures these generate. Crystallographic characteristics of crystals and their assemblies are best characterized with diffraction methods, X-ray diffraction or electron backscatter diffraction (EBSD) carried out with a scanning electron microscope (SEM) or a transmission electron microscope (TEM). The advantage of X-ray diffraction analysis for structure determination is that the measurement covers a large section of the sample [
21,
24,
32,
33], however, it determines the texture of the material and does not render its microstructural characteristics. Electron backscatter diffraction measures and visualizes both the microstructure and the texture of the material in question, nonetheless, high-resolution individual EBSD scans cover small sample portions, relative to sample sections that can be examined with XRD measurements. However, the advantage of EBD measurements is that, in addition to texture determination, many crystallographic attributes, e.g. the mode and degree of misorientation between crystals, crystal morphology, degree of interlinkage and many more are obtained. These can be related to the observed texture patterns and, even though, in general, smaller sample sections are scanned, the mode of crystal organization can be better assessed and understood. In addition, EBSD measurements render very local, very small-scaled, crystallographic-structural attributes as well, in comparison to what is obtained with XRD measurements.
When EBSD is carried out conventionally with an SEM-EBSD system, an acceleration voltage of 20 kV is used. This gives a minimal space resolution between 300 and 400 nm. The foliated calcite of Ostreoidea shells consists of parallel arrays of 200 to 400 nm thick, 2-4 µm wide and up to 20 µm long folia (e.g. this study and [
20,
21,
28]). The columnar, granular, blade/laths-shaped, fractal-dendritic and prismatic crystals are topologically related to the foliated shell. Hence, for high-quality EBSD measurements, as it is required for the crystallographic characterization of the foliated, granular and chalk structures, EBSD data acquisition must be conducted with a space resolution of 100 to 200 nm. A space resolution of 300-400 nm is not fine scaled enough for deciphering on the nm range crystallographic and structural aspects of the crystals.
We performed for this study low kV electron backscatter diffraction (EBSD) measurements on adult Ostreidae and Gryphaeidae shells. Measurements were conducted with an acceleration voltage below 20 kV, down to very few kV’s, as described for biological carbonates [
34]. This renders a space resolution, depending on the used kV, of 100 to 200 nm. We performed for this study EBSD measurements at 15, 12, even at 8 kV and investigated shell crystal microstructure and texture for: the Ostreidae
Magallana gigas (Thunberg, 1793),
Ostrea stentina Payraudeau, 1826,
Ostrea edulis Linnaeus, 1758 and the Gryphaeidae
Hyotissa hyotis (Linnaeus, 1758),
Hyotissa mcgintyi (H. W. Harry, 1985) and
Neopycnodonte cochlear (Poli, 1795). EBSD results were complemented with FE-SEM micrographs, taken on fractured surfaces. As our study is conducted on very small-scale levels, we wanted to ensure that the structural information is not distorted by etching artefacts, hence, we did not image etched shell surfaces.
The following questions led to the present study:
Do we find a comparable diversity in crystal texture in Ostreidae shells, as observed for the microstructures?
How are crystallographic axes of crystals organized at the transition between adjacent microstructures? Is there continuity or discontinuity in crystallographic axes orientation between adjacent microstructures?
How are the round surfaces of the pores accomplished within the shells?
How is a convex and concave shell surface generated with foliated crystal units?
How can we address the nature of the foliated shell layer texture?
Accordingly, subsequently, we characterize the nanometer scale internal structure of the shell forming crystals and crystal units, address their microstructure, address their texture and describe the degree and mode of misorientation between the crystals and the crystal units. Based on results gained from the latter we demonstrate (i) crystallographic axes discontinuity and some continuity between adjacent microstructures, (ii) the mode of crystallographic axes organization at the change from one microstructure into the other and (iii) discuss possible determinants for the changeover from one shell structure into the other, such as crystal twinning, gradation in crystal orientation or oriented nucleation. (v) We address the very specific texture of the foliated shell layer and discuss for the latter possible texture modes, such as: an axial texture, a turbostratic texture or a spherulitic texture. (v) We illustrate, from a structural point of view, the accomplishment of curvature within the shells as well as at shell inner surface.
Sample Preparation for FE-SEM Imaging and EBSD Measurements
The shells were cut transversely. Shell pieces were embedded into epoxy resin and several mechanical grinding and polishing steps were performed on them. The final polishing, consisting of etch-polishing with colloidal alumina, with ∼0.06 μm particle size, was performed using a vibratory polisher. EBSD measurements required 4-6 nm of carbon coating, while for FE-SEM imaging samples were coated with 5 nm Pt/Pd.
EBSD Measurements and Data Analysis
A Hitachi SU5000 field emission SEM, equipped with an Oxford Instruments Nordlys II EBSD detector, was used to perform the measurements. The SEM operation during measurements was mainly at 12 and 15 kV, for some measurements at 8 kV. We discuss six Ostreoidea species. In order to understand the structure of the shell well, we performed 12 to 14 EBSD measurements per species.
For Kikuchi indexing, the Oxford Instruments CHANNEL 5 and AZTec Crystal software were used. Most measurements were done with increments of 100 to 200 nm, for none of the measurements was the step size higher than 300 nm. This ensured very high indexing rates, over 95%. The latter is necessary for understanding the foliated calcite arrangements and the nature of the transitions from one microstructure into the other.
Terminology
This study uses the terms: crystallites, crystals, crystal units, mesocrystals, assemblies of crystallites and assemblies of crystals.
The idealized concept of a crystal or of a crystallite refers to a structure in which matter is arranged in a perfectly regular, periodically repeating spatial pattern. The latter is the crystal lattice, which extends coherently in all directions over the space occupied by the crystal.
For the crystals discussed in this study, the crystal lattice is 3-dimensional.
Real crystals develop during their growth, imperfections, e.g. incorporate impurities, adopt dislocations. Due to these
real crystals implement
small-angle grain boundaries, e.g. at arrays of dislocations and at these boundaries the crystal lattice takes a slightly different orientation [
35]. Accordingly,
real crystals consist of subunits and these are tilted to each other by a small angle, for which an upper limit of 10° is often used [
35]. These subunits are termed mosaic blocks or mosaic domains.
We regard the foliated units to be
mesocrystals. A mesocrystal is defined as a mesoscopically structured crystal, consisting of submicrometer-sized crystallites and organized within the mesocrystal with a good crystallographic register. Nonetheless, the crystallites in mesocrystals are separated from each other by voids and/or impurities [
36,
37,
38].
We use in this study the term crystal unit for morphological mosaic- or mesocrystal entities such as columns, prisms, blades, laths, rhombohedra, polyhedra or granules. These crystal units assemble in the shell of the investigated species to microstructures. These have different modes of preferred crystallographic orientation or crystal textures.
Shell microstructures are presented in this study with EBSD band contrast measurement and crystal orientation maps. EBSD band contrast is shown grey-scaled, crystal orientation in the maps are shown color-coded. We use the inverse pole figure (IPF) and all-Euler colouring codes. The relevant coloring-code is either given in the figure or indicated in the figure caption.
Crystal texture or the crystallographic preferred orientation of crystals or crystal units is shown with pole figures. These display either crystal orientation data or their density distribution. The density distributions that we show in this study are calculated with a half-width of 5° and a cluster size of 3°. The half-width gives the spread of the poles over the surface of the projection sphere. The cluster with a chosen size gives information on the amount of poles that have the same orientation.
We discuss in this contribution different carbonate crystal textures.
A single-crystal-like crystal textures is present when we observe in the pole figure one cluster for c-axes and clusters as well for either the three a*-axes of the calcite or the a- and b-axes of the aragonite.
A single-crystal-like texture with a graded distribution of calcite c- and a*-axes is given when we find in the pole figures individual clusters for the crystallographic axes orientations, however, the clusters display a longish appearance, due to the recurrent gradual tilt of crystallographic axes distribution for each cluster.
An axial crystal textures is given when we observe clustering in c-axes orientation, however, scattering of the corresponding a*-axes (calcite) or a- and b-axes (aragonite) orientations on a great circle perpendicular to the texture axis, here the c-axis, direction.
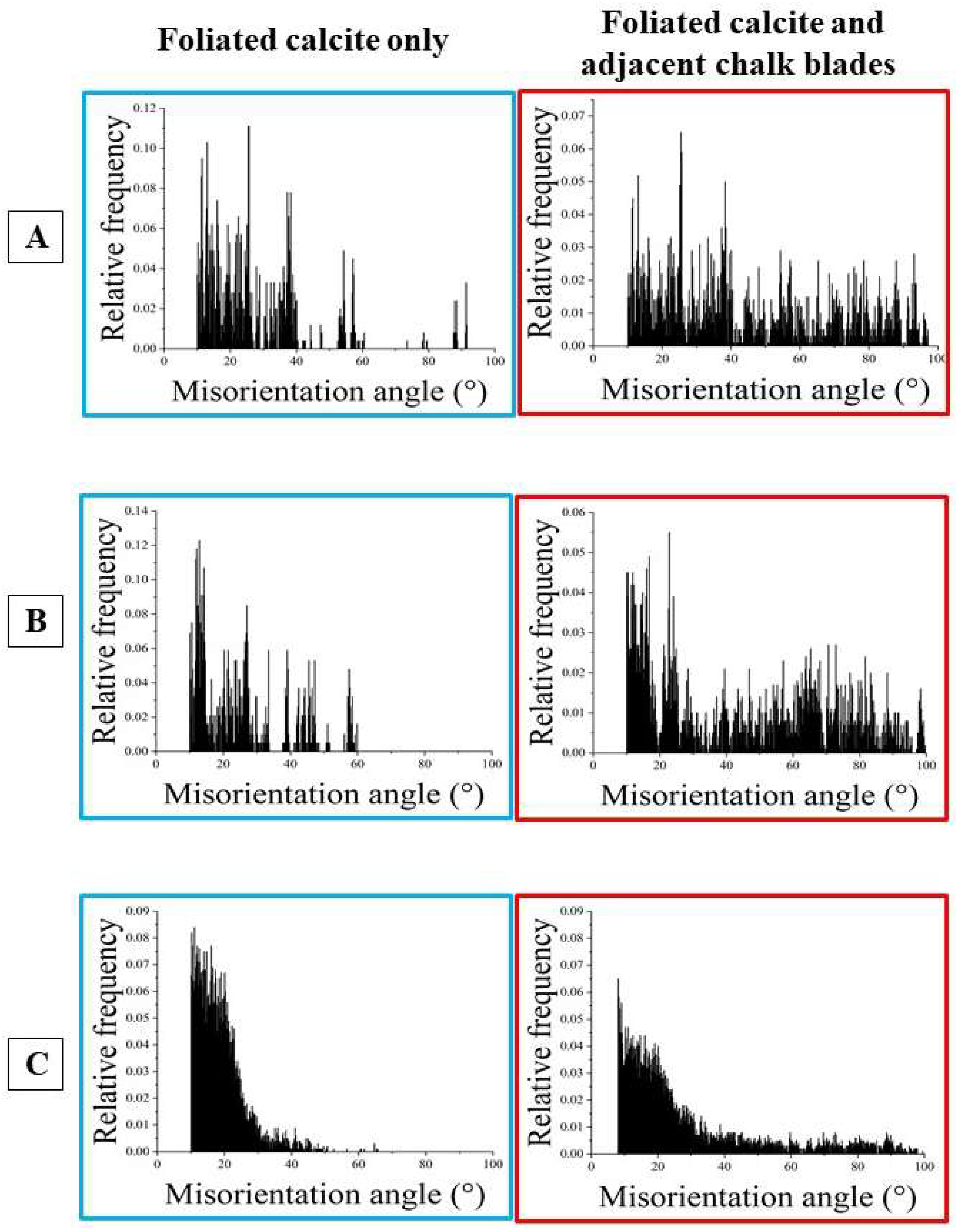
Crystal co-orientation strength is calculated from the density distribution of EBSD orientation data and is given either for entire EBSD scans or the subsets of these. Crystal co-orientation strength is presented with MUD (multiple of uniform (random) distribution) values. A high MUD indicates a high crystal co-orientation strength, a low MUD indicates low or no crystal co-orientation strength. If, at a half-width of 5° and a cluster size of 3°, the MUD value is below 3, the orientation data have a random distribution or lack preferred crystal orientation. If, at a half-width of 5° and a cluster size of 3°, the MUD values are higher than 700, this indicates a very high, single-crystal-like, crystal co-orientation.
The degree of tilt or misorientation between crystallites and crystals is obtained from EBSD measurements. We show misorientation between crystals with (i) relative frequency-misorientation angle or (ii) misorientation angle-distance diagrams. The misorientation-distance diagrams are given for trajectories a to b, c to d. The chosen trajectories run either parallel to the length of a foliated unit or perpendicular to the length of a foliated unit. For the misorientation angle-distance diagrams we give either the cumulative misorientation angle, thus, the cumulative increase in misorientation relative to the first point on the trajectory, or we give the local misorientation, misorientation from point to point along the trajectory, hence, misorientation from crystallite to crystallite along the trajectory.
2. Results
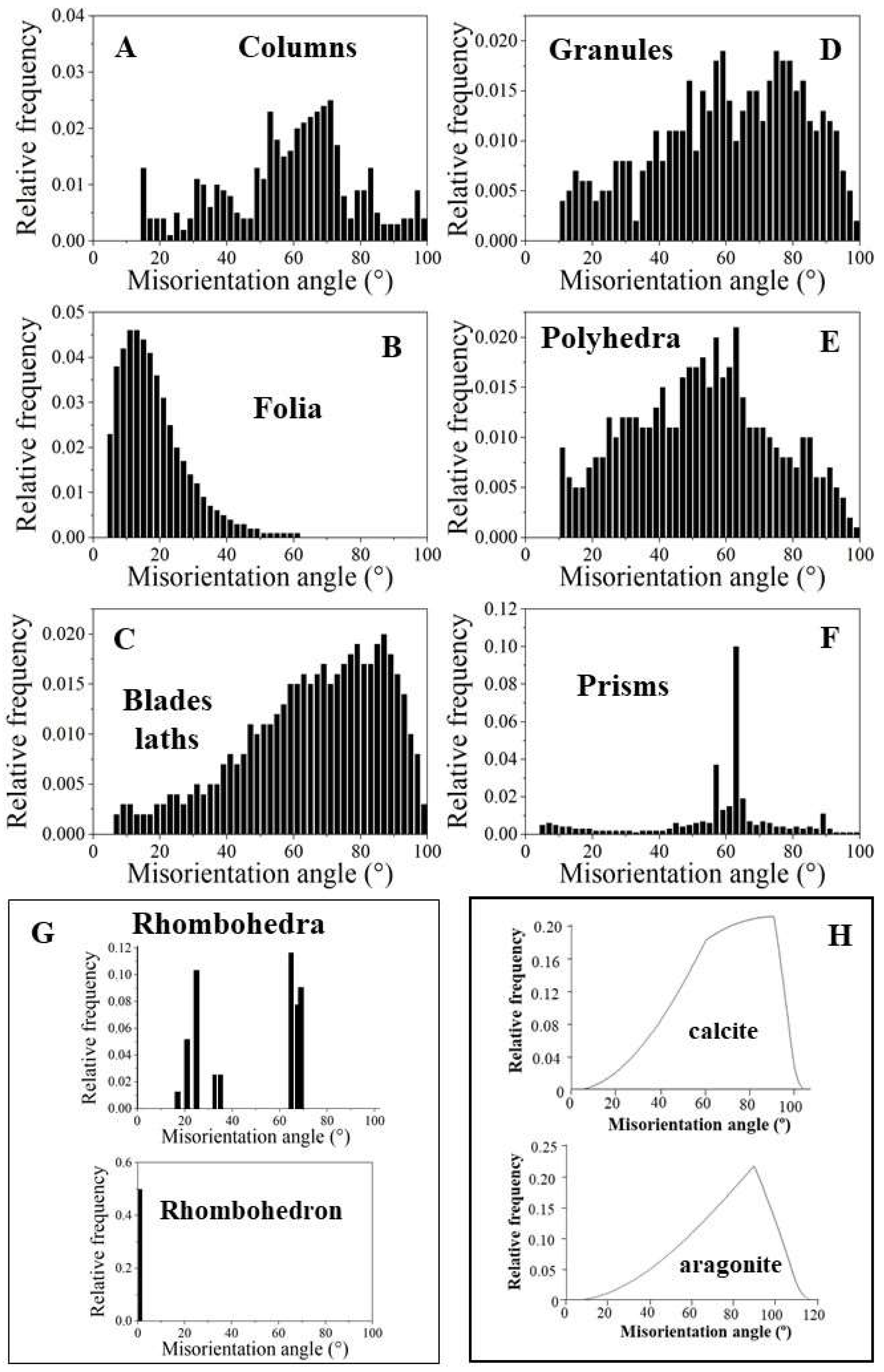
EBSD band contrast measurement maps of
Figure 1 highlight the different microstructures that were observed for the investigated Ostreoidea shells. As the crystal assembly patterns are not only characterized by crystal or/and crystal unit shape, size, structure and organization, but also by the degree and mode of misorientation between the constituting crystals, we highlight for the different modes of crystal organization the corresponding pattern of misorientation between the crystals (
Figure 1 and
Figure 2). For the investigated Ostreoidea species we find an ordered as well as a disordered pattern of misorientation between the comprising crystals (
Figure 2). For discrimination between ordered and disordered misorientation patterns, we show the MacKenzie curves for calcite and aragonite. These give the distribution of misorientation angles for randomly textured carbonate polycrystals (
Figure 2H, [
39]).
We observe significant structural differences for the crystals that comprise the microstructures of the investigated Ostreoidea shells. Columnar prisms are graded in size towards the outer shell surface (Figuer 1A and Figure S1). Individual columns are markedly structured (
Figure 1A and Figure S1); however, the comparison of the band contrast maps and the misorientation angle patterns shows that the structuring within the columns is not resembling the internal structuring of the foliated units (
Figure 1A, B and Figure S1). For the latter, well visible from the band contrast map is the, more or less, parallel arrangement of folia (
Figure 1B). This is not observed for the internal architecture of the columns. The layer that cements the outer surface of the lower valve comprises a conglomerate of granules and small prism-shaped crystals (
Figure 1C). The microstructure of the chalk is a meshwork of interlaced, in part, connected calcite blades and laths, where the latter often have curved/undulated morphologies (
Figure 1D). The occlusion of vesicular pores into the shell is accomplished with the generation of a shell layer microstructure consisting of a tight interlinkage of variously sized, strongly fractal-shaped, polycrystals (
Figure 1E). These form a dense microstructure and encase the round-shaped pores (
Figure 1E). The microstructure of adductor and pallial myostraca (the muscle attachment sites) results from an interconnection of aragonite prisms (
Figure 1F). The prisms are graded in size and increase in dimension towards the inner shell surface (
Figure 1F). Most striking and only observed for the Ostreidae is the formation of crystals with strictly rhombohedral morphologies (
Figure 1G). The morphology of these crystals is distinct to that of other biologically secreted Ca-carbonate crystals and resemble inorganic rhombohedra precipitated from solution [
41,
42]. A detailed description of the rhombohedron-shaped crystals is given by Sancho Vaquer et al. (2025) [
13]. Accordingly, we not only find significant differences in morphologic-structural attributes of crystal, crystal unit and shell layer microstructures, but also in the corresponding misorientations between the microstructures comprising crystals (
Figure 2). For the columns (
Figure 2A), the granules (
Figure 2D), the dendritic crystals (
Figure 2E) and the chalk blades/laths we observe a wide range in misorientation angle, up to 100°, and a misorientation angle distribution that equals random distribution (see the MacKenzie curves in
Figure 2H). Random distribution of misorientation angles is not observed for the assembly of folia (
Figure 2B), for the rhombohedral crystals (
Figure 2G and [
13]) and for the assembly of myostracal prisms (
Figure 2F). The misorientation angle distribution of myostracal prisms is specific. We find a large range in misorientation angle distribution (
Figure 2F), however, the myostracal crystal misorientation diagram is dominated by a marked peak at 64° misorientation (
Figure 2F). Note also the difference in the degree of misorientation angle between the assembly of foliated crystals (
Figure 2B) and the assembly of chalk blades/laths (
Figure 2C). For the foliated crystals we see a large peak at low-angle misorientations, while for the chalk blades, we see a large peak mainly at large-angle misorientations.
Subsequently, we present in more detail the structural and crystallographic attributes of the crystals and crystal units that form the different shell layers of the investigated Ostreoidea species (
Figure 3,
Figure 4,
Figure 5,
Figure 6,
Figure 7,
Figure 8,
Figure 9,
Figure 10,
Figure 11,
Figure 12,
Figure 13 and
Figure 14, Figures S1–S5).
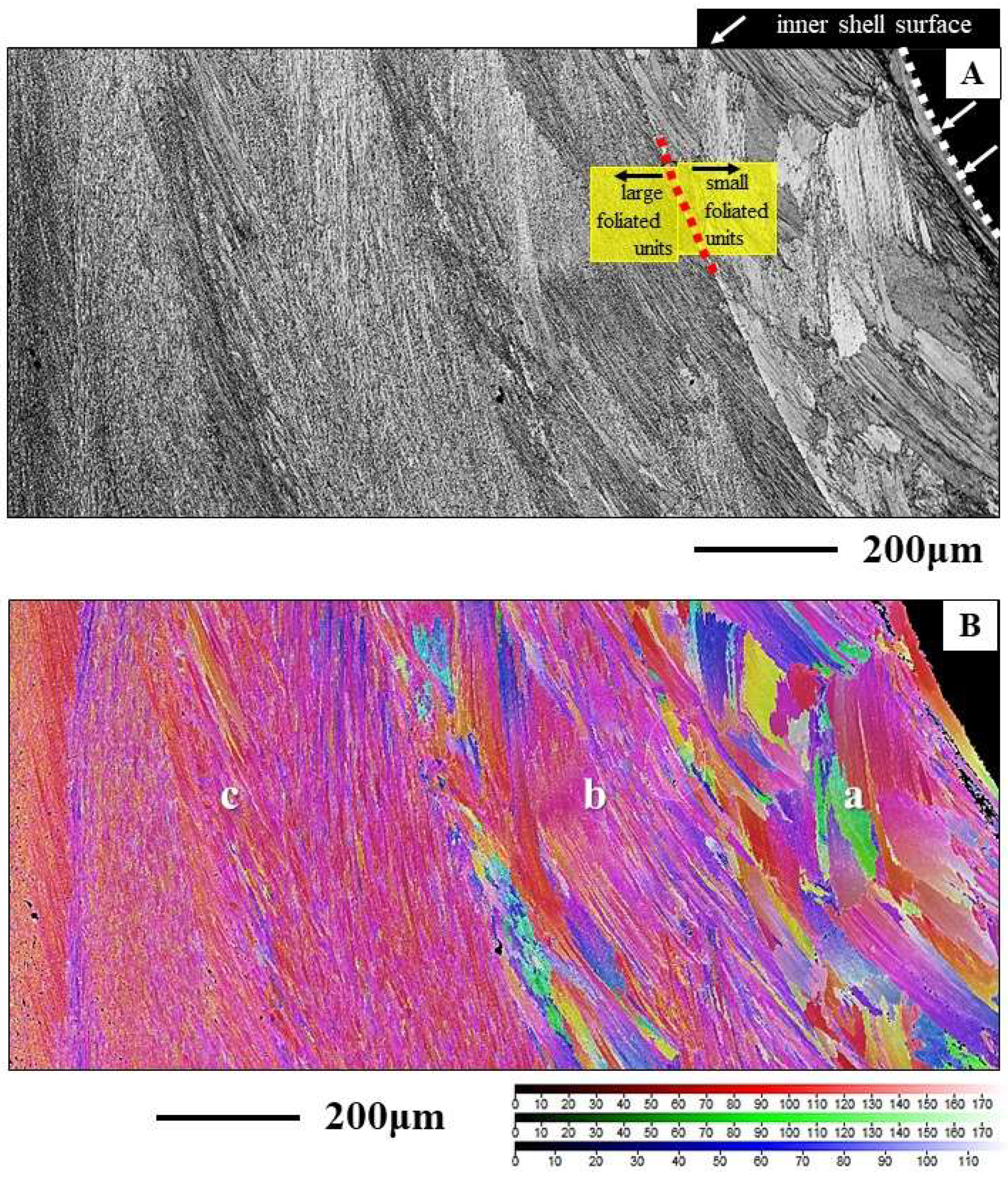
Calcite columns with dimensions of 8-10 mm x 4-5 mm x 40-50 mm (e.g., for
M. gigas) seam large sections of the distal, outer, surface of the two valves (
Figure 3A). BSE images show an internal layering of individual columns, consisting of equally sized dark and light bands (
Figure 3B, C). These reflect differences in organic content (light layers are more mineralized, the dark bands are more organic-rich) and indicate different stages of column growth. The internal structure of the columns is specific (
Figure 3D, E, F, Figures S2 and S3). We observe in the band contrast measurement images that the columns consist of subunits or domains (
Figure 3E, F and Figure S3). These are formed of platy crystals (Figure S3C), nonetheless, the width, length and morphology of these platy crystals do not resemble at all those of the folia which form the foliated units of the foliated shell (compare images of Figure S3A to C with images shown in
Figure 4A to C). The columnar subunits (domains) consist of thin, platy, irregularly shaped crystallites, resembling flakes. Nonetheless, as it is the case for the folia in foliated units, the crystallites within a columnar subunit are strongly co-oriented; misorientation within the columnar subunits/domains is low, it scatters up to about 5°. Even though the calcite of individual columns is well co-oriented, columnar calcite cannot be addressed as being single crystalline (
Figure 3J, K). Contrasting to the latter, the calcite of arrays of columns is very little co-oriented (see MUD value in
Figure 3I), a characteristic that resembles the arrangement of folia in foliated crystals and that of foliated crystals in the foliated shell layer (see MUD value in
Figure 3H). We find for the investigated Ostreoidea shells that within the individual crystals or crystal units (a column or a foliated unit) the crystallites are very well co-oriented, however, for the assembly of these crystals/crystal units we find very low crystal co-orientation strength. The texture pattern of individual columns is single-crystal-like (pole figure in
Figure 3J), while the texture pattern of an assembly of columns is axial (pole figure in
Figure 3I).
Structural properties and crystallographic attributes of
units forming the foliated shell layer are presented with
Figure 4,
Figure 5,
Figure 6,
Figure 7,
Figure 8 and
Figure 9 and Figure S4. BSE images of
Figure 4A, C visualize the thickness and width of individual folia, while
Figure 4B shows the shape and size of crystals that comprise a folium. A folium is formed of, more or less, parallel arrays of lath-shaped crystals with arrowhead endings (
Figure 4B and [
20]). The calcite of individual folia is very co-oriented (MUD values 591, 626,
Figure 4D). It can be addressed to be single crystal like, as EBSD measurements show that individual folia have a single-crystal-like long-range crystallographic order (see pole figures in
Figure 4D).
When investigating calcite c- and a*-axes orientation of a stack of folia, generating a foliated unit (e.g.,
Figure 4E, F), we observe an elongated appearance of the c- and a*-axes orientation data points in the pole figure. This is an expression of the gradation in calcite c- and a*-axes orientation (see pole figures in
Figure 4E, F); it is the result of a graded assembly of folia in the foliated unit (
Figure 5). Gradation of one of the crystallographic axes of calcite or aragonite crystals is observed for biological carbonates, however, gradation in all crystallographic axes of carbonate crystals is outstanding. For biologically secreted calcite, this study is the second report so far that demonstrates the gradation of both the c- as well as the a*-axis of the calcite in a crystal unit.
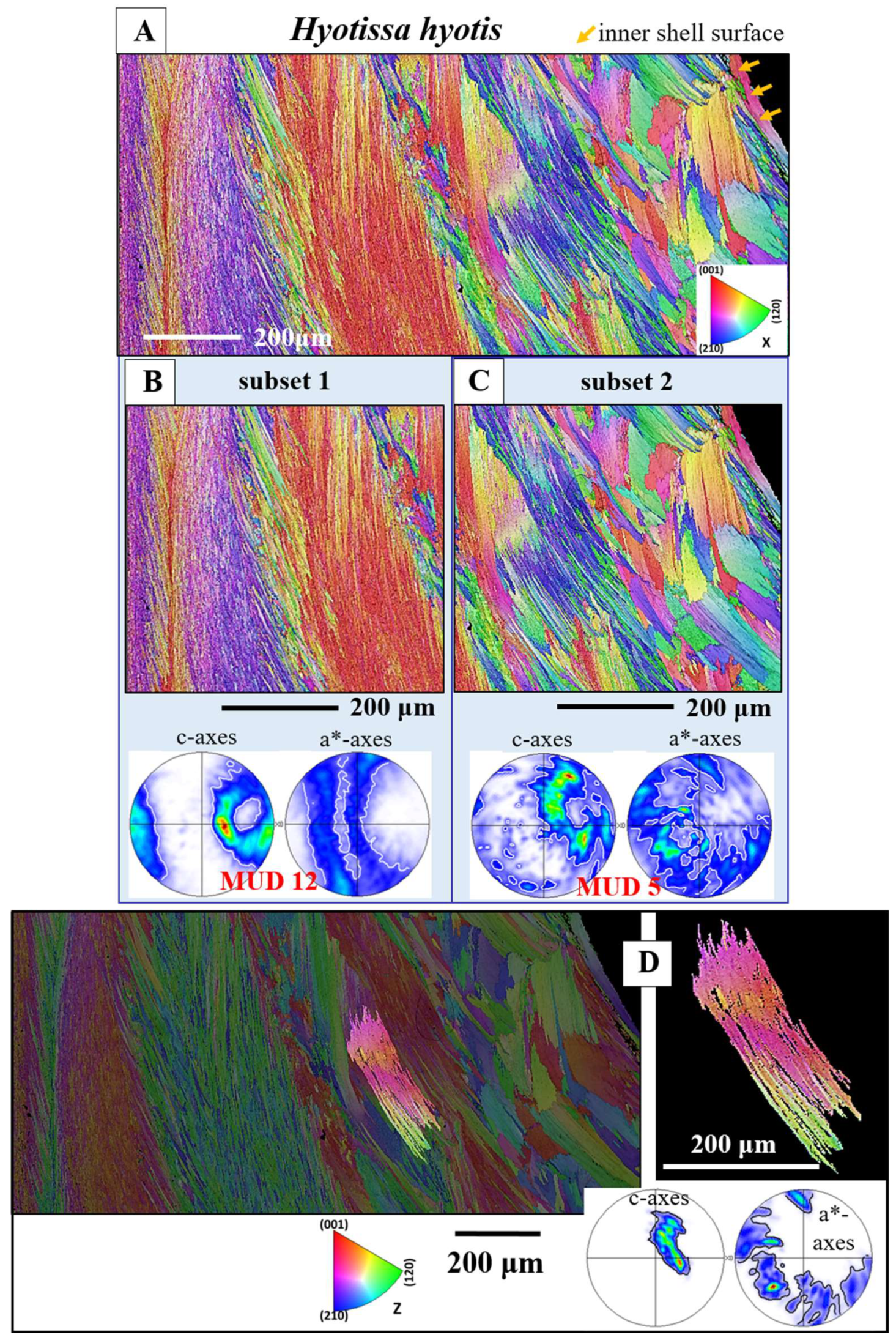
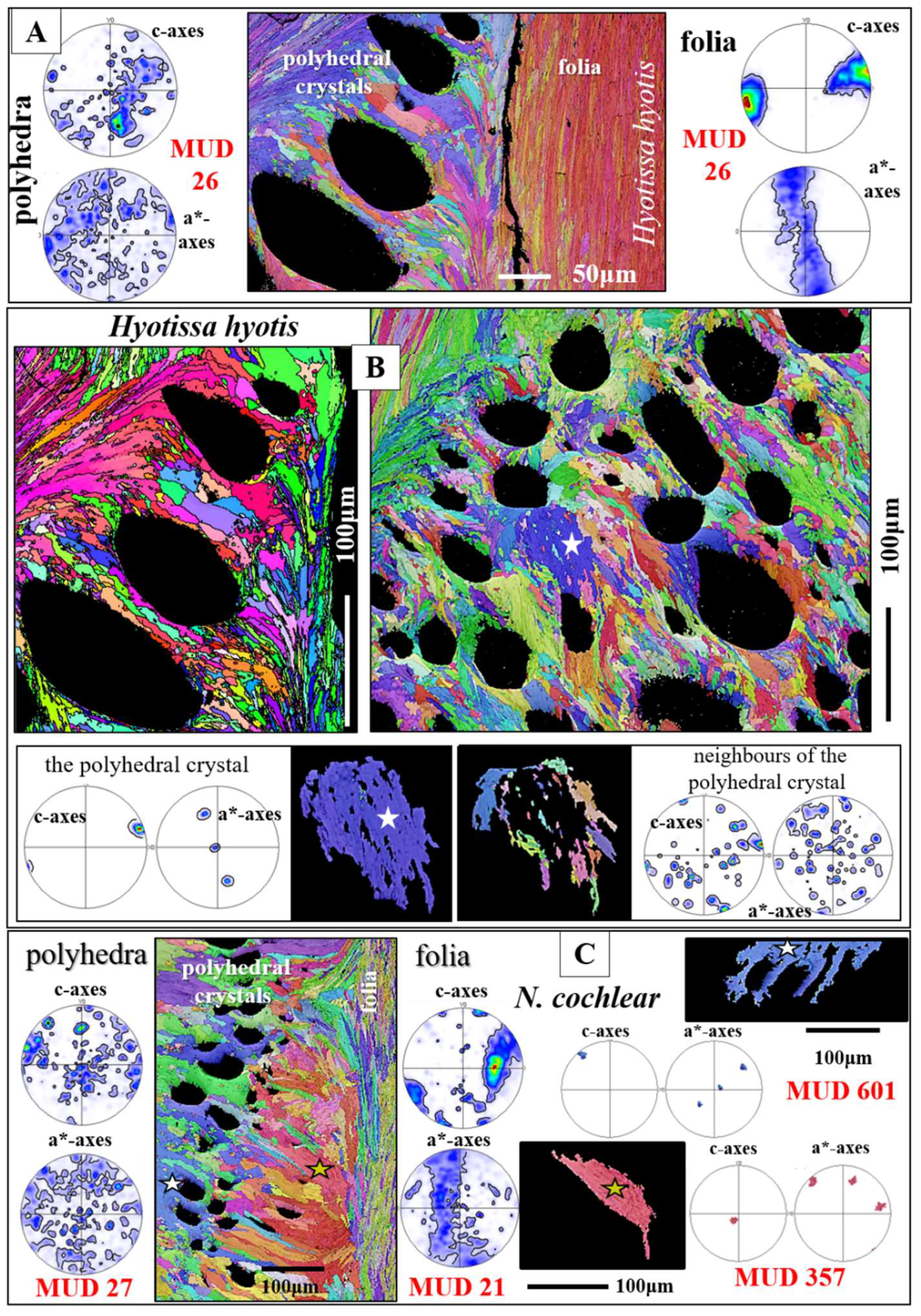
Figure 5A gives for the foliated shell layer of
H. hyotis a large EBSD scan which extends from the inner surface of the shell well into the foliated section of the shell. We find a marked increase in crystal size when moving away and inward from the inner surface of the shell. The consequence of the latter is that, while the smaller foliated units are scanned fully with the chosen size of the EBSD scan, the large foliated crystal units are scanned only in part; the EBSD measurement covers only a portion of the large foliated units. The red, yellow and white stars in the map shown in
Figure 5A point to foliated units that will subsequently be described in more detail. These three foliated units were deliberately taken from different parts of the EBSD map and were selected for their difference in size as well as position in the foliated shell layer. We show, for the selected foliated units, with sketched crystals and pole figures the orientation pattern of calcite c- and a*-axes. For the foliated units given in
Figure 5B, C we see clearly the gradual rotation of calcite c- and a*-axes with the length of the foliated unit and, to a lesser degree, the rotation of crystallographic axes perpendicular to the length of the foliated unit (see the corresponding pole figures and sketched crystals).
Figure 5 gives misorientation angle distributions for individual foliated units along trajectories from a to b and c to d. Trajectories a to b (indicated with white arrows on the foliated units) run parallel to the length of a foliated unit, trajectories c to d (indicated with red arrows on the foliated units) run orthogonal to the length of a foliated unit.
We show two types of misorientation angle distributions with the lengths of the foliated units:
- (i)
with the misorientation angle relative to the first point on the trajectory the overall, cumulative, misorientation angle is given for a foliated unit, while
- (ii)
with the misorientation angle from point to point on the trajectory the local, internal, misorientation angle is given for a foliated unit; hence, the misorientation angle from crystallite to crystallite or/and from folium to folium.
- (iii)
In addition, we give for each misorientation angle diagram the corresponding misorientation angle gradient (the value in the misorientation angle diagram).
- (iv)
We observe the following main features from the misorientation angle diagrams (
Figure 5):
- (v)
Irrespective of the orientation of the trajectory, we find for both cumulative and local misorientation, an increase in misorientation angle with the increasing length of the trajectory.
- (vi)
The latter is lowest for the foliated unit shown in
Figure 5B (the unit that is closest to the inner surface of the shell), is slightly higher for the foliated unit shown in
Figure 5C and is highest for the foliated unit shown in
Figure 5D (the unit that is most far away from the inner surface of the shell).
- (vii)
The increase in misorientation angle is smoother when the trajectory runs parallel to the length of the foliated unit, relative to a trajectory that runs orthogonal to the length of the foliated unit.
- (viii)
When the trajectory is orthogonal to the length of the foliated unit and spans across many folia in the foliated unit, then the increase in cumulative misorientation angle is rather irregular and not smooth.
- (ix)
For all investigated foliated units, we find, irrespective of the direction of the trajectory, similar low misorientation angle gradients.
The crystal shown in
Figure 5D is, in the chosen EBSD scan field (
Figure 5A), most far away from the inner surface of the foliated shell and is, relative to the other crystals (given in
Figure 5B, C), largest in size. It should be kept in mind that we cover only a part of the crystal with the size of the conducted EBSD measurement. For the crystal portion shown in
Figure 5D, we find a different internal structuring, as visualized with the corresponding sketched crystals, pole figures and misorientations. For that crystal portion (
Figure 5D and
Figure 6B) we do not see any more a rotation from folium to folium, from calcite c- and a*-axis to calcite c- and a*-axis, as it is the case for the crystals shown in
Figure 5B, C, but mainly the tilting between two crystal orientations (see the sketched crystals, the poles and misorientation diagrams of
Figure 5D). For the crystal portion shown in
Figure 5D we observe two interdigitating substructures, however, these closely related in orientation to each other (see pole figures in
Figure 5D). In the corresponding misorientation diagrams (
Figure 5D) we find, for both trajectories (a to b, c to d), an increase in cumulative misorientation with distance away from the first point on the trajectory, however, the course of the increase in misorientation is strongly jagged (not smooth, as it is the case for the crystals shown in
Figure 5B, C) and we observe formation of a larger degree of misorientation from point to point, between the adjacent crystallites (see misorientation diagrams in
Figure 5D). The following explanation might explain the structural difference for the crystals shown in (C) and (D) on the one hand side, and (E) on the other. The foliated units in
Figure 5B, C are individual blocks/units of co-oriented folia, the crystal portion of the foliated unit in
Figure 5D is a zoom-in into a foliated unit and we observe the interdigitation of two adjacent subunits, calcite c-axes of these subunits being tilted to each other by about 30°.
The texture of the foliated crystal units, irrespective if these are small or large, is very specific (see pole figures in
Figure 6,
Figure 7 and Figure S4). We observe for an EBSD scan, comprising various foliated crystal units a ring in c-axis as well as a ring in a*-axes orientation (black arrows in pole figures shown in
Figure 6C, D). With
Figure 6 we attempt to visualize how the ring in c- and a*-axes orientation is generated for an assembly of adjacent foliated units. When reduced to the core crystal of a large foliated unit (
Figure 6B), we see in the pole figure a cluster in c-axis and three clusters in a*-axes orientation. Nonetheless, for the clusters in the pole figure and in the corresponding EBSD map we observe that the core crystal of the foliated unit consists of two, differently oriented, subunits. When two to three adjacent foliated units are regarded, we find that each of these is structured by interdigitating subunits and that, in turn, each foliated unit adds in the pole figure a cluster in c- and three clusters in a*-orientation. Hence, for a 3D assembly of adjacent foliated units the c- and a*-axes clusters of the different foliated units merge and generate a ring-shaped c- and a*-axes orientation distribution. Although not as well observed as for the large foliated units, the small foliated units are also interdigitations of adjacent, substructured foliated units and for these we see as well the generation of c-axes and a*-axes rings in the pole figure (see pole figures in
Figure 7B, C, D). Nonetheless, as the EBSD scan covers many, to each other strongly misoriented, small foliated units, the ring-shaped c- and a*-axes orientation distribution for small foliated units is rather noisy, not as clear-cut as it is the case for the large foliated units (compare the pole figure shown in
Figure 7B with that shown in
Figure 7C).
As demonstrated in
Figure 4E, F, we find for the foliated units a gradation in calcite c- and a*-axes. This points to a mesocrystalline nature of the foliated units. A mesocrystal is defined as a mesoscopically structured crystal consisting of submicrometer-sized crystallites, with a crystallographic register [
35,
36,
40]. The distinction of the individual nanocrystals is not clearcut because the crystal lattice is continuous across them [
40]. The length, width, and thickness of crystallites that form a mesocrystal do not have to be similar in size [
40], as it is the case for biologically-formed mesocrystals, e.g., the foliated units of Ostreoidea shells. For the generation of a mesocrystal, the change in crystallographic axes orientation needs to have a, in 3D, controlled tilt [
40], and this we find for the foliated units of the investigated Ostreoidea shells. For individual foliated units, we see in the relative frequency - misorientation angle diagrams only low-angle (up to 10°) misorientations and a very small range in misorientation angle (
Figure 4E, F).
Figure 8 shows an EBSD scan that covers many foliated crystals and extends from the inner shell surface well into the foliated shell layer. We see in this measurement how inner shell surface curvature is generated with foliated units. It is formed with a decrease in foliated unit size towards the curved surface thus, at the curved surface, we find an accumulation of small foliated units.
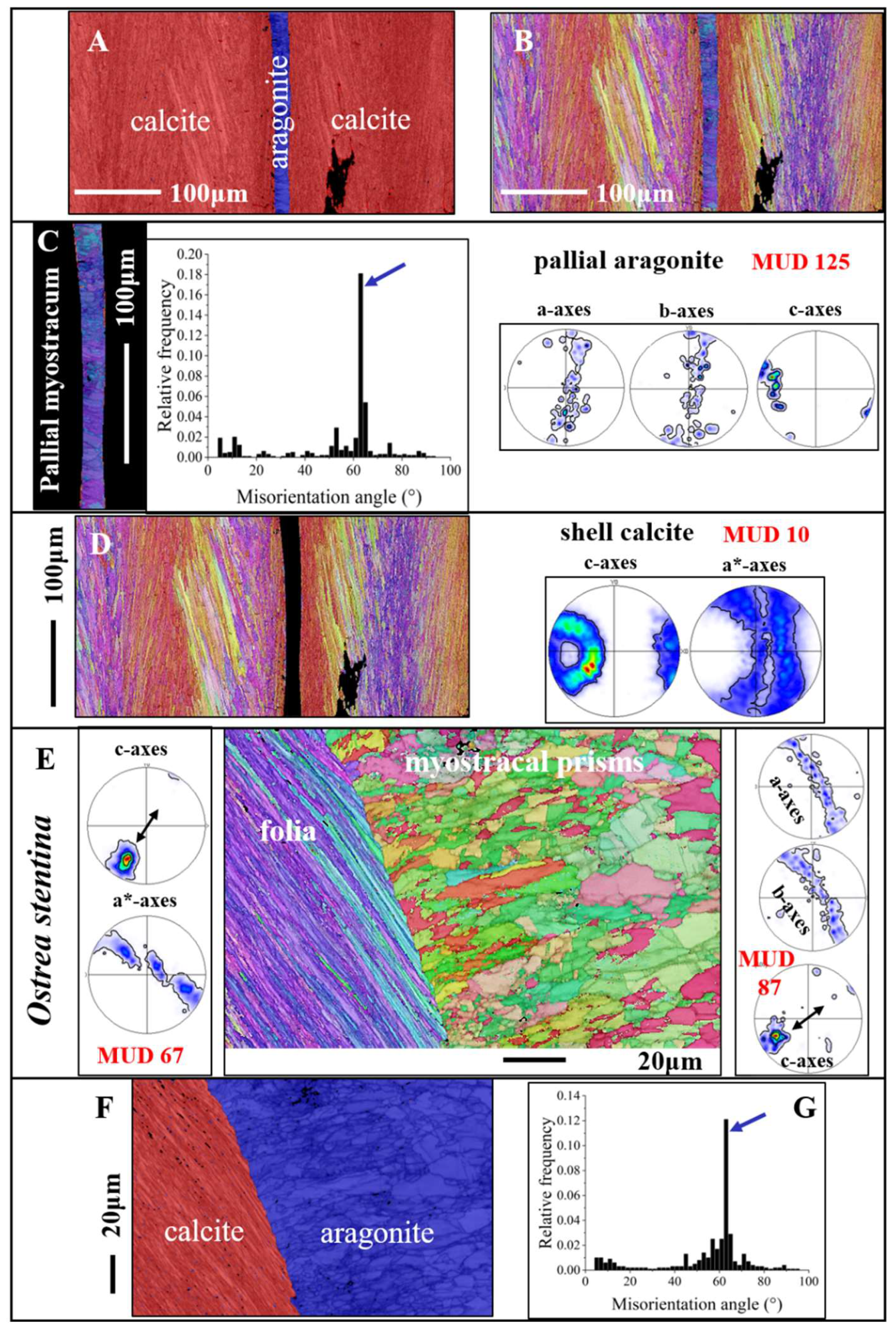
Figure 9 gives calcium carbonate phase, microstructure, texture and crystal co-orientation strength of granular to prismatic crystals that comprise the shell at the attachment sites,
myostraca, of pallial and adductor muscles. We observe for these aragonitic shell sections a different Ca-carbonate phase, as that of the calcitic rest of the shell (
Figure 9A, F), a specific microstructure (
Figure 9C, E), which is distinct from that of the rest of the shell, an axial texture (see pole figures for aragonite in
Figure 9C, E), a higher crystal co-orientation strength, as observed for all other shell portions (see MUD values in
Figure 9C, E) and a marked peak at 64° misorientation in the misorientation angle diagram (black arrows in
Figure 9C, G). The crystallographic-structural difference between myostraca and the other valve sections is the result of different calcium carbonate hard tissue forming determinants (e.g., [
37,
38]).
Figure 10,
Figure 11,
Figure 12,
Figure 13 and Figure S6 visualize the structural arrangement of calcite crystals when
voids, and
pores are incorporated into Ostreoidea shells. Ostreidae incorporate a meshwork of calcite in between stacks of folia, Gryphaeidae occlude lenses of vesicular pores within the stacks of folia. When based on SEM images, the crystal meshwork of the chalk appears to be entirely unstructured (
Figure 10A to C). This is the case to a large degree, as indicated by the large-angle misorientations in the relative frequency-misorientation angle diagrams (
Figure 10E, F). However, we also observe some structuring of the chalk by larger-sized blades/struts (see EBSD measurements in
Figure 11D). We often find low MUD values for the chalk (e.g. MUD 4;
Figure 10F), but also an increased MUD (e.g. MUD 45,
Figure 11D; MUD 24,
Figure 11E). Thus, the crystallites that comprise the chalk are not at all entirely random in orientation. High-resolution EBSD measurements demonstrate the presence, when based on structure, of a transitional zone between the foliated crystals and the calcite meshwork of chalk (white stars in
Figure 10D,
Figure 10G,
Figure 11B). The structure of this transitional zone is chaotic, we do not find in the latter the typical structure of either foliated crystals, or of the calcite meshwork of the chalk (e.g., 11A to C). Regarding crystal organization for the topologically closely-related foliated, transitional and chalk crystal arrangements (
Figure 11A to C), we observe a rotation in c-axis orientation from the folia (
Figure 11C) to the crystals at the transition from the folia to the chalk blades/laths (
Figure 11B). While c-axis orientation of calcite folia is rather within the plane of view (green to yellow data points in the pole figure in
Figure 11C), it becomes gradually tilted out of the plane of view and being oriented perpendicular to the plane of view when approaching the transitional section to the chalk (yellow to red data points in the pole figure in
Figure 11C). Calcite c-axis orientation of the transitional zone between the folia and chalk is mainly perpendicular to the plane of view (red data points in the pole figure in
Figure 11B).
Our study shows that calcite with a chalk-like microstructure is not only present in lenses within the foliated shell. We observe for
O. stentina, formation of a layer with a chalk-like microstructure, texture, and misorientation angle distribution also at outer shell portions (
Figure 11E), however, always in topological relation to the foliated shell layer (EBSD map in
Figure 11E).
Figure 12 and
Figure 13 show for
H. mcgintyi,
H. hyotis, and
N. cochlear the mode of crystal organization around voids and pores (e.g.,
Figure 12B, 13B, C). The calcite microstructure that surrounds the pores consists of an assembly of dendritic crystals (
Figure 12 and
Figure 13). These are very diverse in size (e.g.,
Figure 13B) and, in particular, have highly fractal morphologies (e.g.,
Figure 12B, D and
Figure 13B). Adjacent crystals interlock strongly in 3D with neighboring fractal crystals (Figures 12D and 13B). The calcite of individual dendritic crystals is well co-oriented (
Figure 13C), hence, we find for individual dendritic crystals only low-angle misorientation (
Figure 10E). Even though crystal co-orientation strength within an individual crystal is high, due to the, more or less, random interconnection of crystals, crystal co-orientation strength of the whole microstructure is low (Figuer 12A), nonetheless, it is comparable to that of the adjacent foliated shell (
Figure 13A). The texture of the dendritic granular microstructure has a very low preferred orientation, thus, a very weak texture (pole figures in
Figure 13A, C). Nonetheless, in rare cases we see for the assembly of dendritic crystals the development of a weak axial texture (pole figure in
Figure 12A). Thus, for the latter example, we see a directed assembly of dendritic crystals, however, irrespective of which texture is developed, with a very decreased crystal co-orientation strength (see the low MUD values for the measurements in
Figure 12A and 13A, C).
Calcite crystals within the
cementation layer are mainly granular (
Figure 14A); when slightly larger-sized (
Figure 14B), the morphology of the cementation layer crystals tends to be prismatic (
Figure 14B). Crystal co-orientation strength for the cementation layer crystals is low (see MUD values in
Figure 14A, B). Crystal texture can be very vaguely axial (pole figure in
Figure 14B). However, the axial texture can also be developed with some directed c- and a*-axes orientation (pole figure for the granules in
Figure 14A).
Crystal organization of the
rhombohedra (
Figure 1G) is not discussed in this contribution, but in detail in Sancho Vaquer et al. 2025 [
13].
Figure 1.
Ostreoidea shell crystal morphology, shell layer internal structuring and relative frequency-misorientation angle distributions for the different crystal assembly patterns. A to G: EBSD band contrast measurement images. The inserts give relative frequency versus misorientation angle diagrams for the shown EBSD scans and, thus, microstructures. A: Sequence of columns seaming outer valve surfaces. B: Array of folia forming the foliated units. C: String of granules intercalated into the organic matrix of the cementation layer, the layer that attaches the lower valve external surface to the substrate. D: Meshwork of blades and laths forming the cavity-rich structure of the chalk. E: Conglomeration of variously sized dendritic, polyhedral crystals facilitating the occlusion of pores into the Gryphaeidae shells. F: The prisms of the myostraca, the shell section where the muscles attach to. G: Accumulation of rhombohedral crystals within organic substance in Ostreidae shells.
Figure 1.
Ostreoidea shell crystal morphology, shell layer internal structuring and relative frequency-misorientation angle distributions for the different crystal assembly patterns. A to G: EBSD band contrast measurement images. The inserts give relative frequency versus misorientation angle diagrams for the shown EBSD scans and, thus, microstructures. A: Sequence of columns seaming outer valve surfaces. B: Array of folia forming the foliated units. C: String of granules intercalated into the organic matrix of the cementation layer, the layer that attaches the lower valve external surface to the substrate. D: Meshwork of blades and laths forming the cavity-rich structure of the chalk. E: Conglomeration of variously sized dendritic, polyhedral crystals facilitating the occlusion of pores into the Gryphaeidae shells. F: The prisms of the myostraca, the shell section where the muscles attach to. G: Accumulation of rhombohedral crystals within organic substance in Ostreidae shells.
Figure 2.
Diversity in relative frequency-misorientation angle patterns for the microstructures that comprise Ostreoidea shells (A to G). H: MacKenzie curves for random misorientation angle distribution [
40], given for calcite and aragonite. A, C, D, E: Random distribution of misorientation angles. B, F, G: Structured distribution of misorientation angles, note the structured distribution of misorientation angles for folia (B). We find for (A) and (E) a comparable pattern for the distribution of misorientation angles, alike for (C) and (D). For the latter, we find a gradual change and increasing development to the formation of large misorientations. Note the difference in magnitude of misorientation angle between the assembly of folia (B) and the assembly of chalk blades/laths (C).
Figure 2.
Diversity in relative frequency-misorientation angle patterns for the microstructures that comprise Ostreoidea shells (A to G). H: MacKenzie curves for random misorientation angle distribution [
40], given for calcite and aragonite. A, C, D, E: Random distribution of misorientation angles. B, F, G: Structured distribution of misorientation angles, note the structured distribution of misorientation angles for folia (B). We find for (A) and (E) a comparable pattern for the distribution of misorientation angles, alike for (C) and (D). For the latter, we find a gradual change and increasing development to the formation of large misorientations. Note the difference in magnitude of misorientation angle between the assembly of folia (B) and the assembly of chalk blades/laths (C).
Figure 3.
Internal structure, microstructure, crystal texture and crystal co-orientation strength of the array of columns that seam the outermost valve layer of
Magallana gigas shells. A to D, F: BSE micrographs. E: EBSD band contrast measurement image. F, G, I, J, K: Color-coded crystal orientation (EBSD maps), crystal texture (pole figures) and crystal co-orientation strength (MUD value) of individual columns (J, K, L) and of arrays of columns (G, I). H: The foliated shell adjacent to a sequence of columns (I). Note the difference in texture but similarity in crystal co-orientation strength for the columns and for the adjacent sequence of foliated units (H, I). Individual columns are internally structured and are formed of subunits (domains) consisting of well co-oriented calcite crystallites (F, J) (Figure S3B). The internal structure of the columns as well as of their subunits is different to that of the foliated units, nonetheless, we find only low angle misorientations within the columns (see the relative frequency-misorientation angle diagrams for individual columns in (K)), as it is also the case for the assembly of folia and foliated units (see the misorientation angle diagram in
Figure 1B). Note the layering within the columns (A to C), visualizing different growth stages. Black arrows in the pole figures in (H) and (I) indicate the overall direction of c-axis orientation and show an about 30° misorientation in c-axis orientation between an array of columns and the adjacent foliated shell (H, I). At EBSD data acquisition and EBSD data evaluation, the three Euler angles were kept at 0°; φ1=0°, Φ=0°, φ2=0°.
Figure 3.
Internal structure, microstructure, crystal texture and crystal co-orientation strength of the array of columns that seam the outermost valve layer of
Magallana gigas shells. A to D, F: BSE micrographs. E: EBSD band contrast measurement image. F, G, I, J, K: Color-coded crystal orientation (EBSD maps), crystal texture (pole figures) and crystal co-orientation strength (MUD value) of individual columns (J, K, L) and of arrays of columns (G, I). H: The foliated shell adjacent to a sequence of columns (I). Note the difference in texture but similarity in crystal co-orientation strength for the columns and for the adjacent sequence of foliated units (H, I). Individual columns are internally structured and are formed of subunits (domains) consisting of well co-oriented calcite crystallites (F, J) (Figure S3B). The internal structure of the columns as well as of their subunits is different to that of the foliated units, nonetheless, we find only low angle misorientations within the columns (see the relative frequency-misorientation angle diagrams for individual columns in (K)), as it is also the case for the assembly of folia and foliated units (see the misorientation angle diagram in
Figure 1B). Note the layering within the columns (A to C), visualizing different growth stages. Black arrows in the pole figures in (H) and (I) indicate the overall direction of c-axis orientation and show an about 30° misorientation in c-axis orientation between an array of columns and the adjacent foliated shell (H, I). At EBSD data acquisition and EBSD data evaluation, the three Euler angles were kept at 0°; φ1=0°, Φ=0°, φ2=0°.
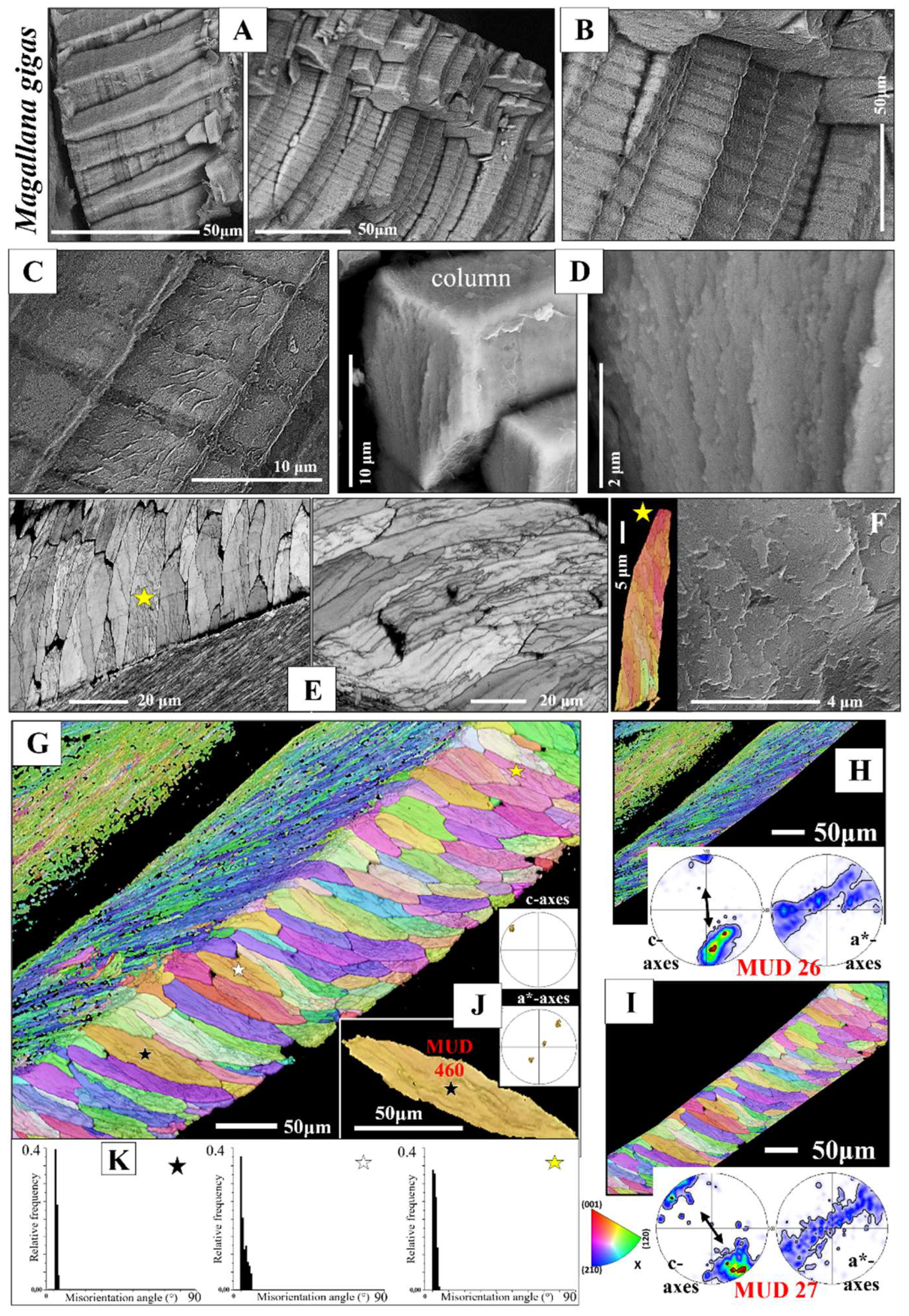
Figure 4.
The folia and assemblies of folia that generate ostreoidean foliated units, e.g., as those shown in E and F. A to C: BSE micrographs highlighting the morphology and dimension (width and thickness) of lath-shaped, arrowhead ended crystals and of individual folia. D: Crystal orientation, crystal texture and crystal co-orientation strength of two individual folia. The calcite of a folium is strongly co-oriented (see MUD values in D). E, F: EBSD scans of foliated units, consisting of stacks of folia. Note the mode of c- and a*-axes orientation distribution in the pole figures (E, F). The gradual change in color (EBSD map and pole figures), as well as the gradual change in tilt of both crystallographic axes (see the pole figures), demonstrates that the foliated crystal units are entities with a graded calcite c- and a*-axes orientation. We observe a marked gradedness in orientation for both, calcite c- as well as a*-axes. E, F: Relative frequency-misorientation angle diagrams are given for the crystal shown in the figure; we observe very low misorientation angles between adjacent folia. Euler angles were kept at zero for EBSD data acquisition and analysis for the measurements shown in D, E, F.
Figure 4.
The folia and assemblies of folia that generate ostreoidean foliated units, e.g., as those shown in E and F. A to C: BSE micrographs highlighting the morphology and dimension (width and thickness) of lath-shaped, arrowhead ended crystals and of individual folia. D: Crystal orientation, crystal texture and crystal co-orientation strength of two individual folia. The calcite of a folium is strongly co-oriented (see MUD values in D). E, F: EBSD scans of foliated units, consisting of stacks of folia. Note the mode of c- and a*-axes orientation distribution in the pole figures (E, F). The gradual change in color (EBSD map and pole figures), as well as the gradual change in tilt of both crystallographic axes (see the pole figures), demonstrates that the foliated crystal units are entities with a graded calcite c- and a*-axes orientation. We observe a marked gradedness in orientation for both, calcite c- as well as a*-axes. E, F: Relative frequency-misorientation angle diagrams are given for the crystal shown in the figure; we observe very low misorientation angles between adjacent folia. Euler angles were kept at zero for EBSD data acquisition and analysis for the measurements shown in D, E, F.
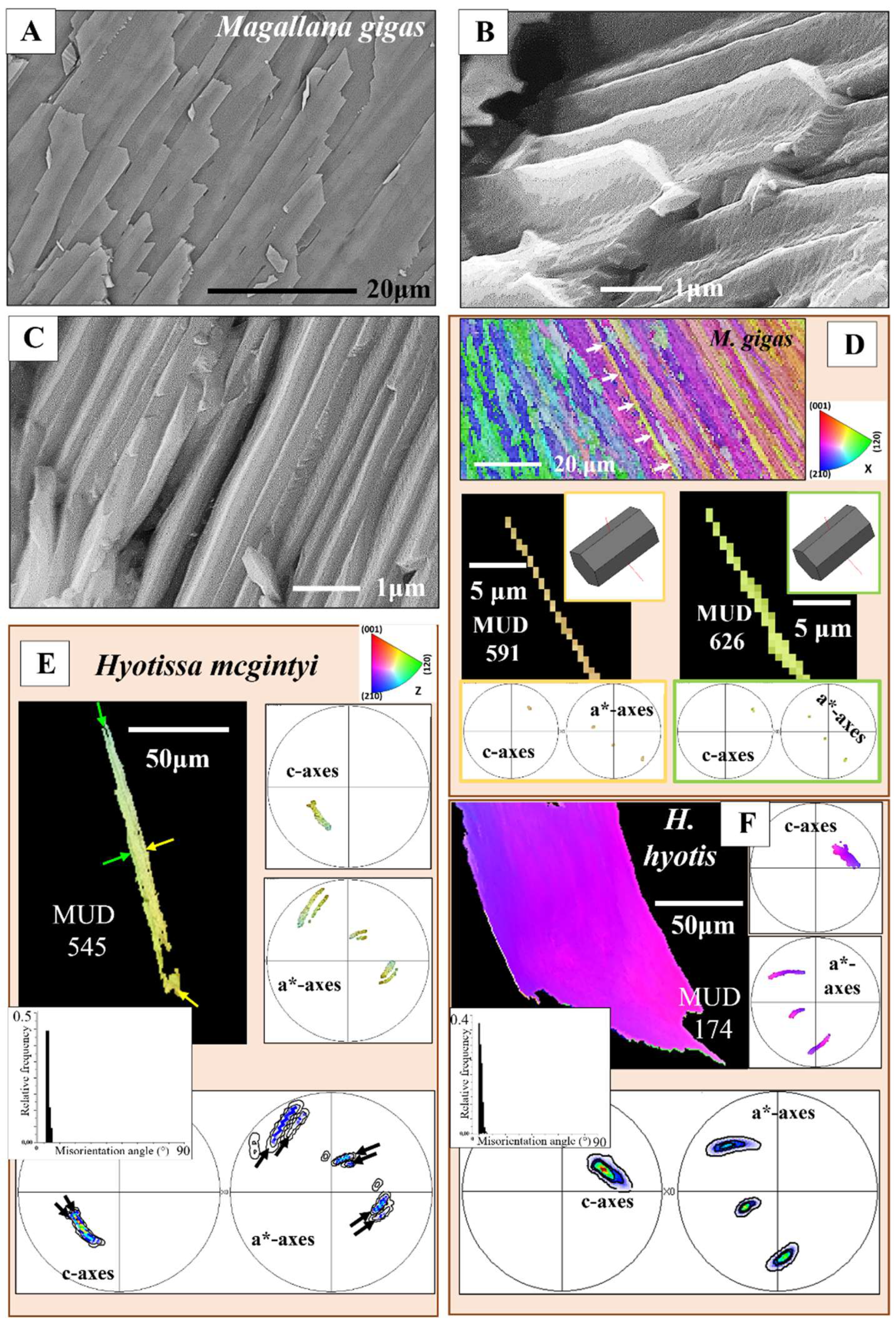
Figure 5.
A: EBSD crystal orientation map of an assembly of foliated crystals for the shell of Hyotissa hyotis, visualizing their size, internal structure and interlinkage. B to D: Individual foliated units selected from different parts of the foliated shell (indicated with a red, yellow or white star in the EBSD map in (A)). The organization of crystallographic c- and a*-axes of/in the foliated units is indicated with sketched crystals and corresponding misorientation angle - distance diagrams. These show misorientation between crystallites along trajectories a to b and c to d (B to D). We give (i) the cumulative misorientation angle, the increase in misorientation angle, relative to the first point on the trajectory and (ii) the misorientation angle from point to point along the trajectory, thus the local misorientation angle, misorientation between adjacent crystals. For the foliated units shown in (B) and (C), we observe a gradual rotation of crystallographic c- and a*-axes orientation, in both directions, along the length as well as perpendicular to the length of the foliated unit, see sketched crystals in (B) and (C). The result is that we find a gradual increase in misorientation angle, relative to the first point on the trajectory and a very low degree of misorientation from point to point (B, C). The crystals in (B) and (C) are small, foliated units, while the crystal shown in (D) is a large foliated unit. For the latter crystal (D) we do not find for the crystal portion that is scanned with EBSD such a clear-cut gradual rotation in crystallographic axes orientation, but rather, for adjacent crystallites (possibly folia) an abrupt tilt between two crystallographic axes orientations (see the sketched crystals in (D)). This is also observable from the misorientation angle-distance diagrams (E). Even though an increase in the degree of misorientation angle, relative to the first point on the trajectory, is still present in the misorientation angle - distance diagrams (D), the gradual increase in cumulative misorientation angle, with distance away from the first point on the trajectory, is not as smooth, as it is for the foliated units shown in (B) and (C). In addition, we find, for the crystal in (D) a large degree of misorientation from crystallite to crystallite, from folium to folium. Note as well that the degree of misorientation, relative to the first point as well as from point to point is different for the crystals given in (B), (C), (D) thus, different for the different parts of the foliated shell. The degree of misorientation between adjacent crystallites increases significantly with distance away from the inner shell surface.
Figure 5.
A: EBSD crystal orientation map of an assembly of foliated crystals for the shell of Hyotissa hyotis, visualizing their size, internal structure and interlinkage. B to D: Individual foliated units selected from different parts of the foliated shell (indicated with a red, yellow or white star in the EBSD map in (A)). The organization of crystallographic c- and a*-axes of/in the foliated units is indicated with sketched crystals and corresponding misorientation angle - distance diagrams. These show misorientation between crystallites along trajectories a to b and c to d (B to D). We give (i) the cumulative misorientation angle, the increase in misorientation angle, relative to the first point on the trajectory and (ii) the misorientation angle from point to point along the trajectory, thus the local misorientation angle, misorientation between adjacent crystals. For the foliated units shown in (B) and (C), we observe a gradual rotation of crystallographic c- and a*-axes orientation, in both directions, along the length as well as perpendicular to the length of the foliated unit, see sketched crystals in (B) and (C). The result is that we find a gradual increase in misorientation angle, relative to the first point on the trajectory and a very low degree of misorientation from point to point (B, C). The crystals in (B) and (C) are small, foliated units, while the crystal shown in (D) is a large foliated unit. For the latter crystal (D) we do not find for the crystal portion that is scanned with EBSD such a clear-cut gradual rotation in crystallographic axes orientation, but rather, for adjacent crystallites (possibly folia) an abrupt tilt between two crystallographic axes orientations (see the sketched crystals in (D)). This is also observable from the misorientation angle-distance diagrams (E). Even though an increase in the degree of misorientation angle, relative to the first point on the trajectory, is still present in the misorientation angle - distance diagrams (D), the gradual increase in cumulative misorientation angle, with distance away from the first point on the trajectory, is not as smooth, as it is for the foliated units shown in (B) and (C). In addition, we find, for the crystal in (D) a large degree of misorientation from crystallite to crystallite, from folium to folium. Note as well that the degree of misorientation, relative to the first point as well as from point to point is different for the crystals given in (B), (C), (D) thus, different for the different parts of the foliated shell. The degree of misorientation between adjacent crystallites increases significantly with distance away from the inner shell surface.
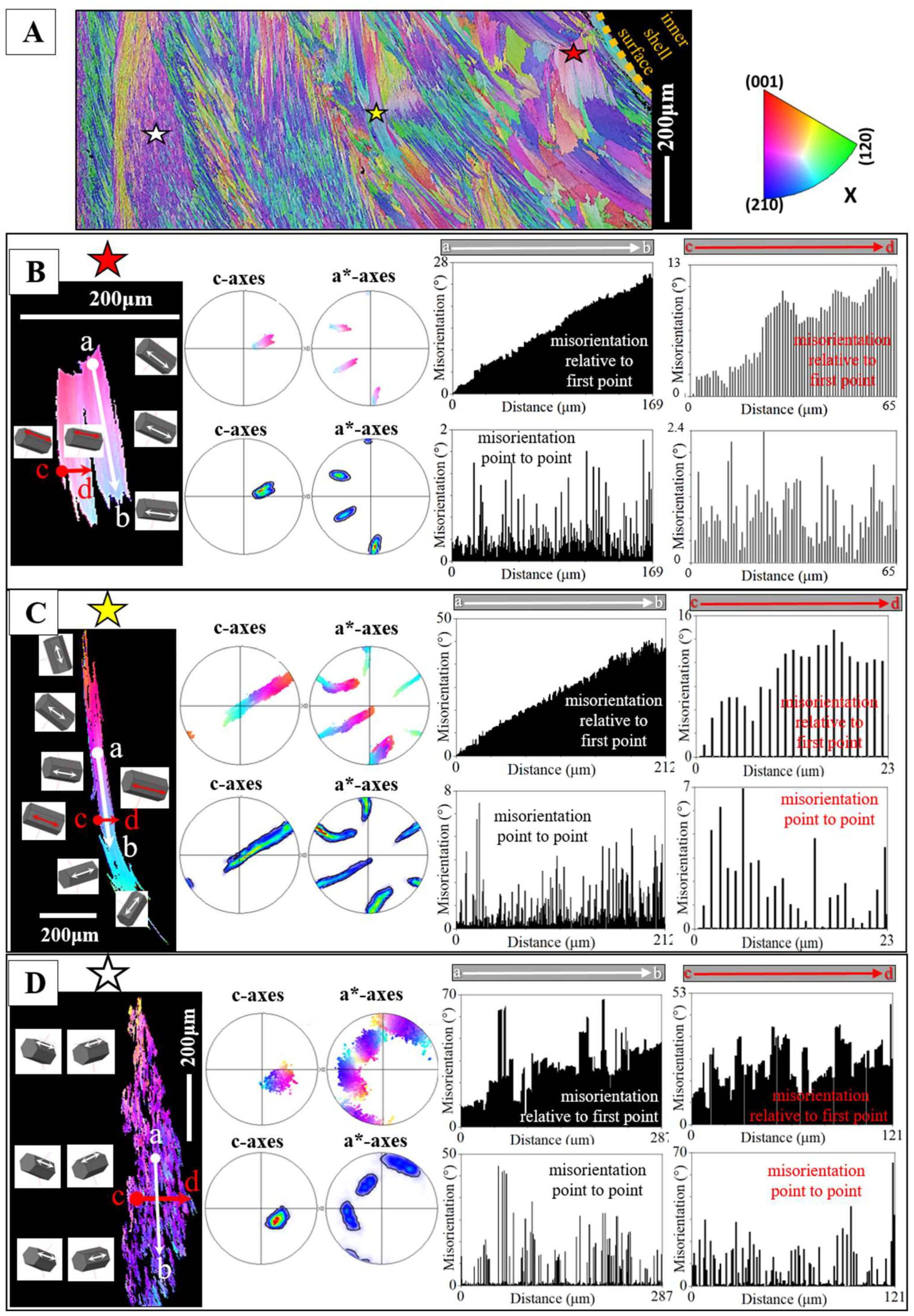
Figure 6.
The microstructure of large foliated units (B to D) and the nature of their texture (B to D). Formation and development of c- and a*-axes orientation rings for the texture pattern of the foliated units (B to D). The colors in
Figure 6 code for crystal orientation. The core crystal entity (e.g. as that shown in (B)) of a large foliated unit is a structured arrangement of crystallites and consists of an interwoven meshwork of, mainly, two subunits having slightly different orientations (see blue and green colors in the map and pole figure in (B), see the sketched crystals in (B). Nonetheless, we find in the corresponding pole figure (B) a cluster for c-axes and three clusters for the a*-axes orientations. However, we observe well in the pole figures (B) that the c- and a*-axes clusters are substructured. Selecting a larger section of a foliated unit (C), e.g. the core crystal entity and adjacent foliated crystals (C), in the pole figures we find that additional c- and a*-axes clusters are added to the c- and a*-axes clusters of the core crystal entity, in such a way that the c- and a*-axes orientations of a few adjacent large foliated units form a ring in the c- and a*-axes pole figures (black arrows in C, D). This is a very specific texture pattern and very different to the texture of fibrous, columnar and nacreous biocalcified microstructures, where the texture pattern of the latter microstructures is axial. A: The entire EBSD scan, with the colors indicating crystal orientation. The dashed yellow, red and black rectangles in (A) depict the EBSD scan portions that are shown in (B), (C) and (D).
Figure 6.
The microstructure of large foliated units (B to D) and the nature of their texture (B to D). Formation and development of c- and a*-axes orientation rings for the texture pattern of the foliated units (B to D). The colors in
Figure 6 code for crystal orientation. The core crystal entity (e.g. as that shown in (B)) of a large foliated unit is a structured arrangement of crystallites and consists of an interwoven meshwork of, mainly, two subunits having slightly different orientations (see blue and green colors in the map and pole figure in (B), see the sketched crystals in (B). Nonetheless, we find in the corresponding pole figure (B) a cluster for c-axes and three clusters for the a*-axes orientations. However, we observe well in the pole figures (B) that the c- and a*-axes clusters are substructured. Selecting a larger section of a foliated unit (C), e.g. the core crystal entity and adjacent foliated crystals (C), in the pole figures we find that additional c- and a*-axes clusters are added to the c- and a*-axes clusters of the core crystal entity, in such a way that the c- and a*-axes orientations of a few adjacent large foliated units form a ring in the c- and a*-axes pole figures (black arrows in C, D). This is a very specific texture pattern and very different to the texture of fibrous, columnar and nacreous biocalcified microstructures, where the texture pattern of the latter microstructures is axial. A: The entire EBSD scan, with the colors indicating crystal orientation. The dashed yellow, red and black rectangles in (A) depict the EBSD scan portions that are shown in (B), (C) and (D).
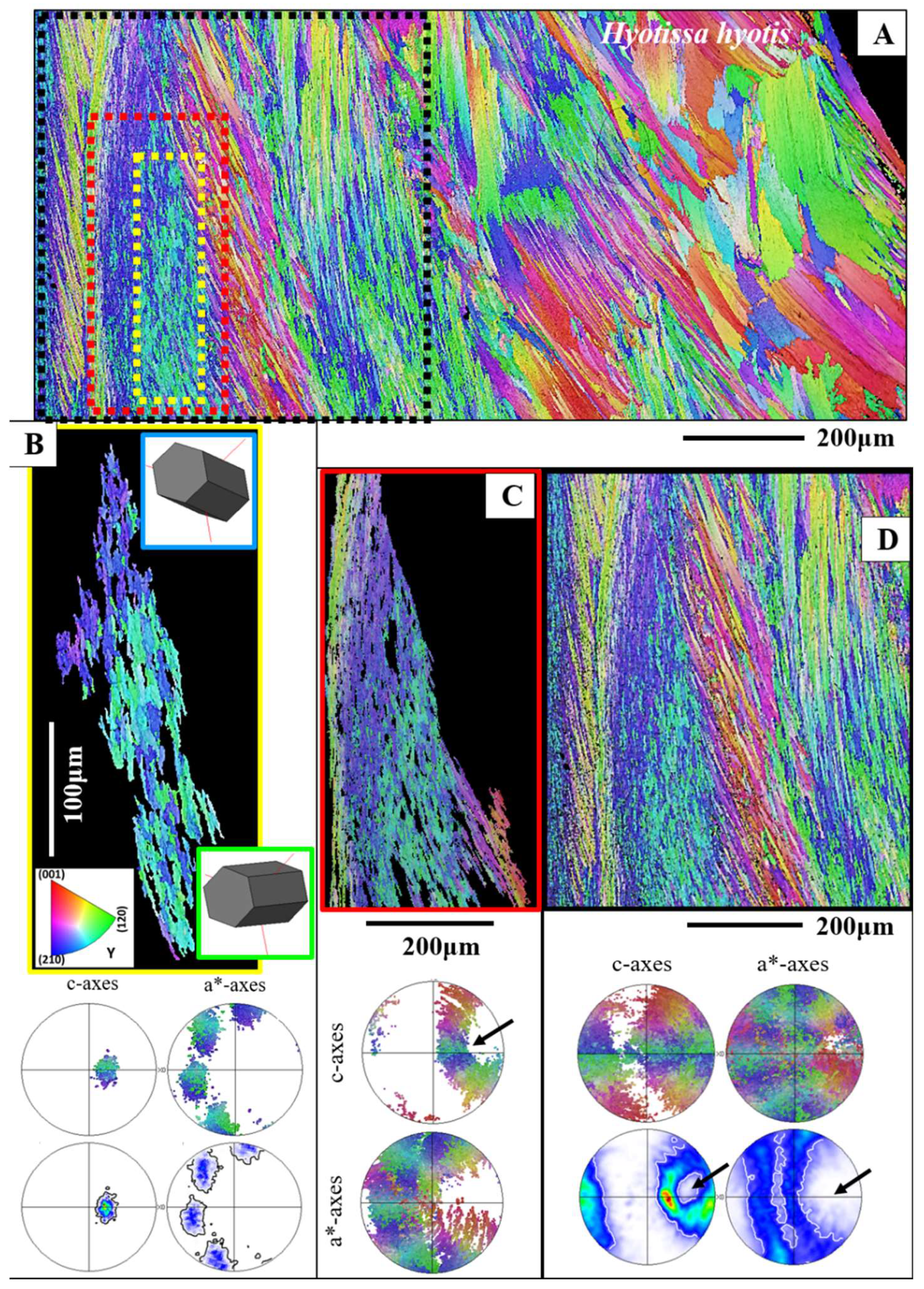
Figure 7.
The texture pattern of assemblies of large and small foliated units (A to C) and the texture of an individual small foliated units (D). We observe for all foliated units, small or large, the same texture pattern, namely, formation of c- and a*-axes orientation rings in the corresponding pole figures. The outline of the c- and a*-axes orientation rings is very irregular for small foliated units (C), due to their high abundance and almost random orientation organization (MUD of 5 (C)). We find also formation of c- and a*-axes rings for an individual small foliated unit.
Figure 7.
The texture pattern of assemblies of large and small foliated units (A to C) and the texture of an individual small foliated units (D). We observe for all foliated units, small or large, the same texture pattern, namely, formation of c- and a*-axes orientation rings in the corresponding pole figures. The outline of the c- and a*-axes orientation rings is very irregular for small foliated units (C), due to their high abundance and almost random orientation organization (MUD of 5 (C)). We find also formation of c- and a*-axes rings for an individual small foliated unit.
Figure 8.
The Hyotissa hyotis distribution pattern of small and large foliated units (A, B) and generation of a curved inner shell surface with foliated units (A, B). A: EBSD band contrast measurement image; B: for calcite orientation color-coded EBSD scan. For the scan shown in (B), we use the Euler angle coloring code. It is well observable that towards the inner shell surface the size of foliated units decreases gradually, hence, a curved surface is formed with an assembly of small foliated units, in contrast to the size of foliated units that are present in more outward sections of the foliated shell layer. We appear to find layers formed of small (a), larger (b) and very large (c) foliated units (B).
Figure 8.
The Hyotissa hyotis distribution pattern of small and large foliated units (A, B) and generation of a curved inner shell surface with foliated units (A, B). A: EBSD band contrast measurement image; B: for calcite orientation color-coded EBSD scan. For the scan shown in (B), we use the Euler angle coloring code. It is well observable that towards the inner shell surface the size of foliated units decreases gradually, hence, a curved surface is formed with an assembly of small foliated units, in contrast to the size of foliated units that are present in more outward sections of the foliated shell layer. We appear to find layers formed of small (a), larger (b) and very large (c) foliated units (B).
Figure 9.
The microstructure, texture, and carbonate phase of ostreoidean adductor and pallial myostraca (A to G). A, B:
Ostrea stentina. The myostraca are formed of aragonite prisms (A, F). These have a characteristic microstructure derived from their growth process [
39,
40], form an axial texture (see pole figures for aragonite in C and E), and are characterized by a marked peak at about 63° to 64° misorientation in the relative frequency-misorientation angle diagram (black arrow in C, G). The latter indicates the twinning of the aragonite on the {110} plane. In comparison to shell calcite, myostracal aragonite crystals are more co-oriented (see the difference in MUD value between myostracal aragonite (C, E) and shell calcite (D). Black arrows in the pole figures shown in (E) indicate the direction of c-axis orientation for foliated calcite and myostracal aragonite. We find for the two microstructures some correspondence in c-axes orientation.
Figure 9.
The microstructure, texture, and carbonate phase of ostreoidean adductor and pallial myostraca (A to G). A, B:
Ostrea stentina. The myostraca are formed of aragonite prisms (A, F). These have a characteristic microstructure derived from their growth process [
39,
40], form an axial texture (see pole figures for aragonite in C and E), and are characterized by a marked peak at about 63° to 64° misorientation in the relative frequency-misorientation angle diagram (black arrow in C, G). The latter indicates the twinning of the aragonite on the {110} plane. In comparison to shell calcite, myostracal aragonite crystals are more co-oriented (see the difference in MUD value between myostracal aragonite (C, E) and shell calcite (D). Black arrows in the pole figures shown in (E) indicate the direction of c-axis orientation for foliated calcite and myostracal aragonite. We find for the two microstructures some correspondence in c-axes orientation.
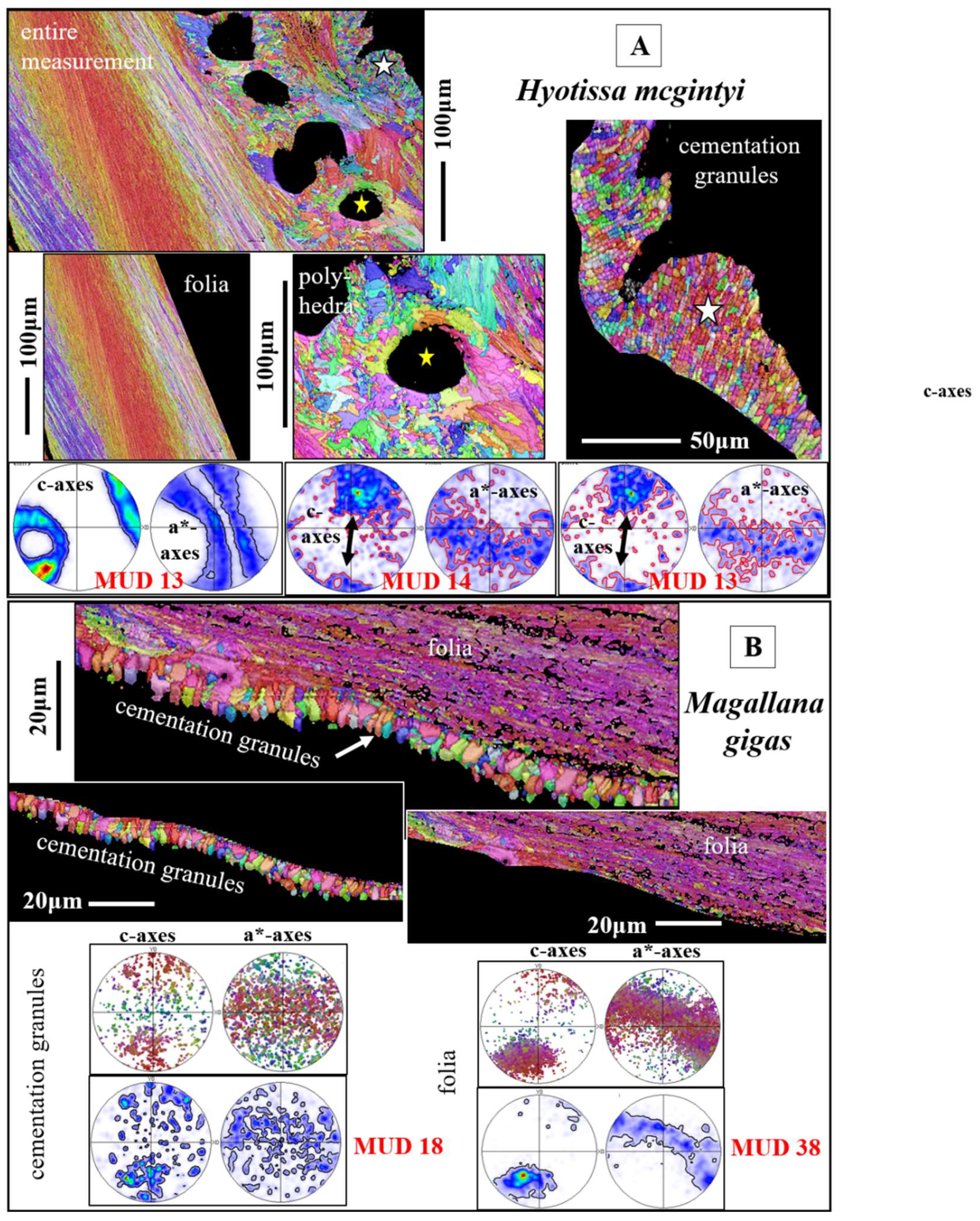
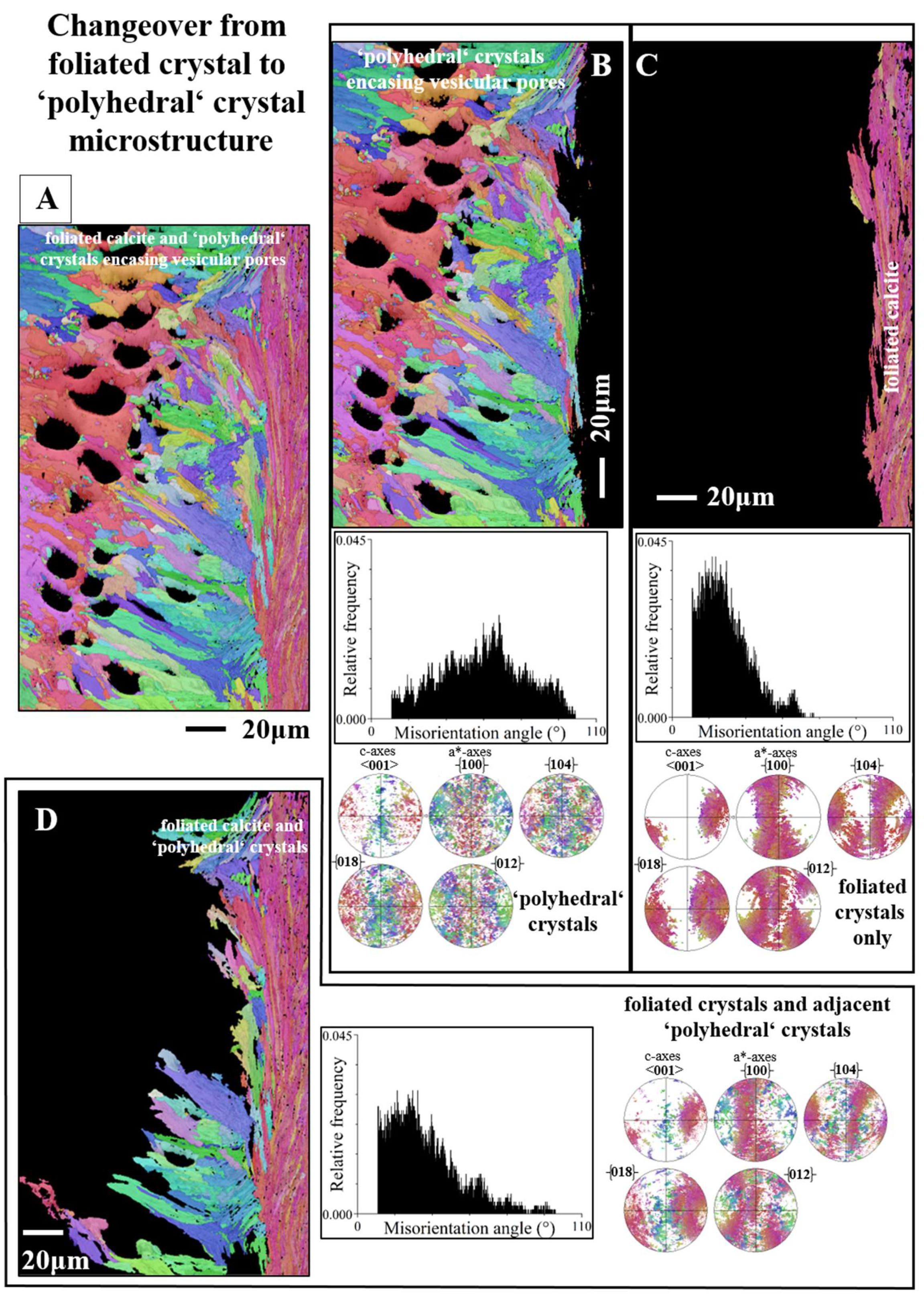
Figure 10.
The meshwork of crystals, and their microstructure and texture, that form chalk lenses in Ostreidae shells (A to C) and the crossover from the foliated to the chalk-related assembly of calcite (B to G). A to F:
Magallana gigas. A to C: BSE micrographs; D to G: EBSD maps, color-coded for crystal orientation; corresponding pole figures, showing orientation data (F) or their density distribution (E). For the microstructures and textures, we show the corresponding relative frequency-misorientation angle diagrams. Crystal co-orientation strength is given with MUD values (D, E). For the EBSD map shown in (F), the IPF coloring code was used, for the map given in (G), the all-Euler coloring code was selected. Although both microstructures are formed of blade/lath-shaped crystals, we find significant differences in crystal texture, magnitude of misorientation angle, and mode of misorientation angle distribution (D, E). Even though the assembly of folia is more co-oriented than the assembly of crystals that form the chalk, crystal co-orientation strength is low for both microstructures (see MUD values). We find between the stacks of folia and the agglomeration of chalk blades/laths a transitional zone (G, white stars in D and
Figure 11B), marked by a chaotic microstructure, not resembling in structure either the arrays of folia, or the aggregation of chalk blades/laths. At EBSD data acquisition and EBSD data evaluation the three Euler angles were kept at 0°; φ1=0°, Φ=0°, φ2=0°.
Figure 10.
The meshwork of crystals, and their microstructure and texture, that form chalk lenses in Ostreidae shells (A to C) and the crossover from the foliated to the chalk-related assembly of calcite (B to G). A to F:
Magallana gigas. A to C: BSE micrographs; D to G: EBSD maps, color-coded for crystal orientation; corresponding pole figures, showing orientation data (F) or their density distribution (E). For the microstructures and textures, we show the corresponding relative frequency-misorientation angle diagrams. Crystal co-orientation strength is given with MUD values (D, E). For the EBSD map shown in (F), the IPF coloring code was used, for the map given in (G), the all-Euler coloring code was selected. Although both microstructures are formed of blade/lath-shaped crystals, we find significant differences in crystal texture, magnitude of misorientation angle, and mode of misorientation angle distribution (D, E). Even though the assembly of folia is more co-oriented than the assembly of crystals that form the chalk, crystal co-orientation strength is low for both microstructures (see MUD values). We find between the stacks of folia and the agglomeration of chalk blades/laths a transitional zone (G, white stars in D and
Figure 11B), marked by a chaotic microstructure, not resembling in structure either the arrays of folia, or the aggregation of chalk blades/laths. At EBSD data acquisition and EBSD data evaluation the three Euler angles were kept at 0°; φ1=0°, Φ=0°, φ2=0°.
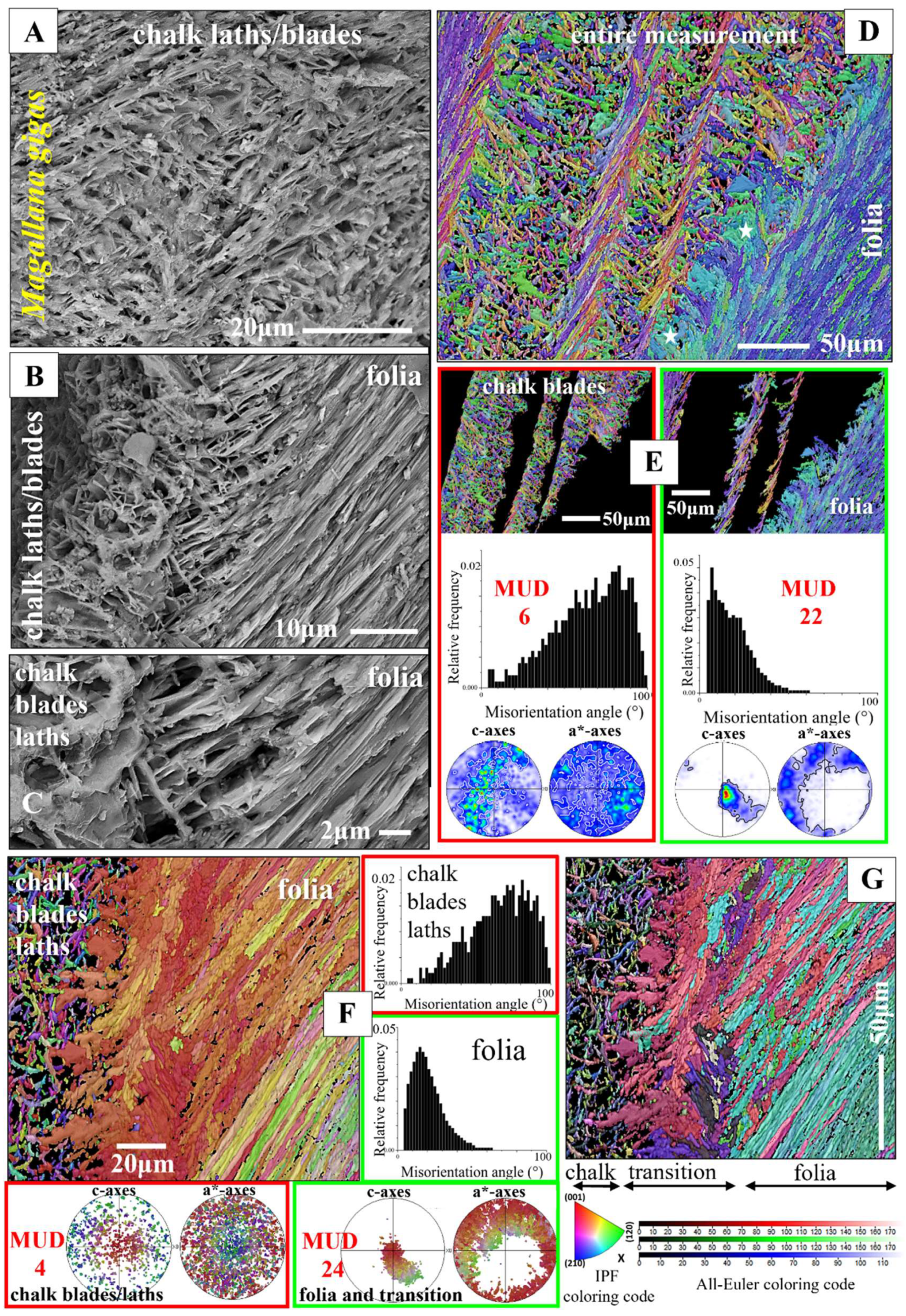
Figure 11.
A to C: Subsets of the EBSD measurement shown in
Figure 10F highlight the difference in crystal orientation between the blades, laths of the chalk (A), the crystals that form the transitional section between the chalk and the stacks of folia (B) and for the stacks of folia (C). In the shell, these microstructures are adjacent to each other. Sketched crystals visualize calcite crystallographic axes orientation of the folia (C) and of the crystals that form the transitional section between the folia and the chalk blades (B). Calcite c-axes of the latter are perpendicular to the plane of view (B), while calcite c-axes of the folia are largely within the plane of view (C). D, E: Meshwork of chalk blades and laths within the foliated shell layer (D) as well as adjacent to the foliated layers at outer shell sections (E). The co-orientation strength of calcite crystallites that comprise the chalk is low but not random (see MUD values for the chalk in D, E). Thus, there is some structuring of the chalk, e.g. by struts (see EBSD band contrast and crystal orientation maps in D). E: A chalk-type microstructure at outer shell layers (white stars in E), developed with an axial texture (pole figure for the chalk in E), a slightly increased crystal co-orientation strength (MUD value for the chalk in E) and the typical relative frequency-misorientation angle pattern that is also observed for the chalk within the lenses that are incorporated into the foliated shell. This is the first report of a chalk-type microstructure at outer shell layers. A to C, inserts in D, E give EBSD maps, coded for crystal orientation. The micrographs in (E) and the left-hand side, grey-scaled, image in (D) are BSE images. The right-hand side, grey-scaled, image in (D) is an EBSD band contrast measurement image. At EBSD data acquisition and EBSD data evaluation, the three Euler angles were kept at 0°; φ1=0°, Φ=0°, φ2=0°.
Figure 11.
A to C: Subsets of the EBSD measurement shown in
Figure 10F highlight the difference in crystal orientation between the blades, laths of the chalk (A), the crystals that form the transitional section between the chalk and the stacks of folia (B) and for the stacks of folia (C). In the shell, these microstructures are adjacent to each other. Sketched crystals visualize calcite crystallographic axes orientation of the folia (C) and of the crystals that form the transitional section between the folia and the chalk blades (B). Calcite c-axes of the latter are perpendicular to the plane of view (B), while calcite c-axes of the folia are largely within the plane of view (C). D, E: Meshwork of chalk blades and laths within the foliated shell layer (D) as well as adjacent to the foliated layers at outer shell sections (E). The co-orientation strength of calcite crystallites that comprise the chalk is low but not random (see MUD values for the chalk in D, E). Thus, there is some structuring of the chalk, e.g. by struts (see EBSD band contrast and crystal orientation maps in D). E: A chalk-type microstructure at outer shell layers (white stars in E), developed with an axial texture (pole figure for the chalk in E), a slightly increased crystal co-orientation strength (MUD value for the chalk in E) and the typical relative frequency-misorientation angle pattern that is also observed for the chalk within the lenses that are incorporated into the foliated shell. This is the first report of a chalk-type microstructure at outer shell layers. A to C, inserts in D, E give EBSD maps, coded for crystal orientation. The micrographs in (E) and the left-hand side, grey-scaled, image in (D) are BSE images. The right-hand side, grey-scaled, image in (D) is an EBSD band contrast measurement image. At EBSD data acquisition and EBSD data evaluation, the three Euler angles were kept at 0°; φ1=0°, Φ=0°, φ2=0°.
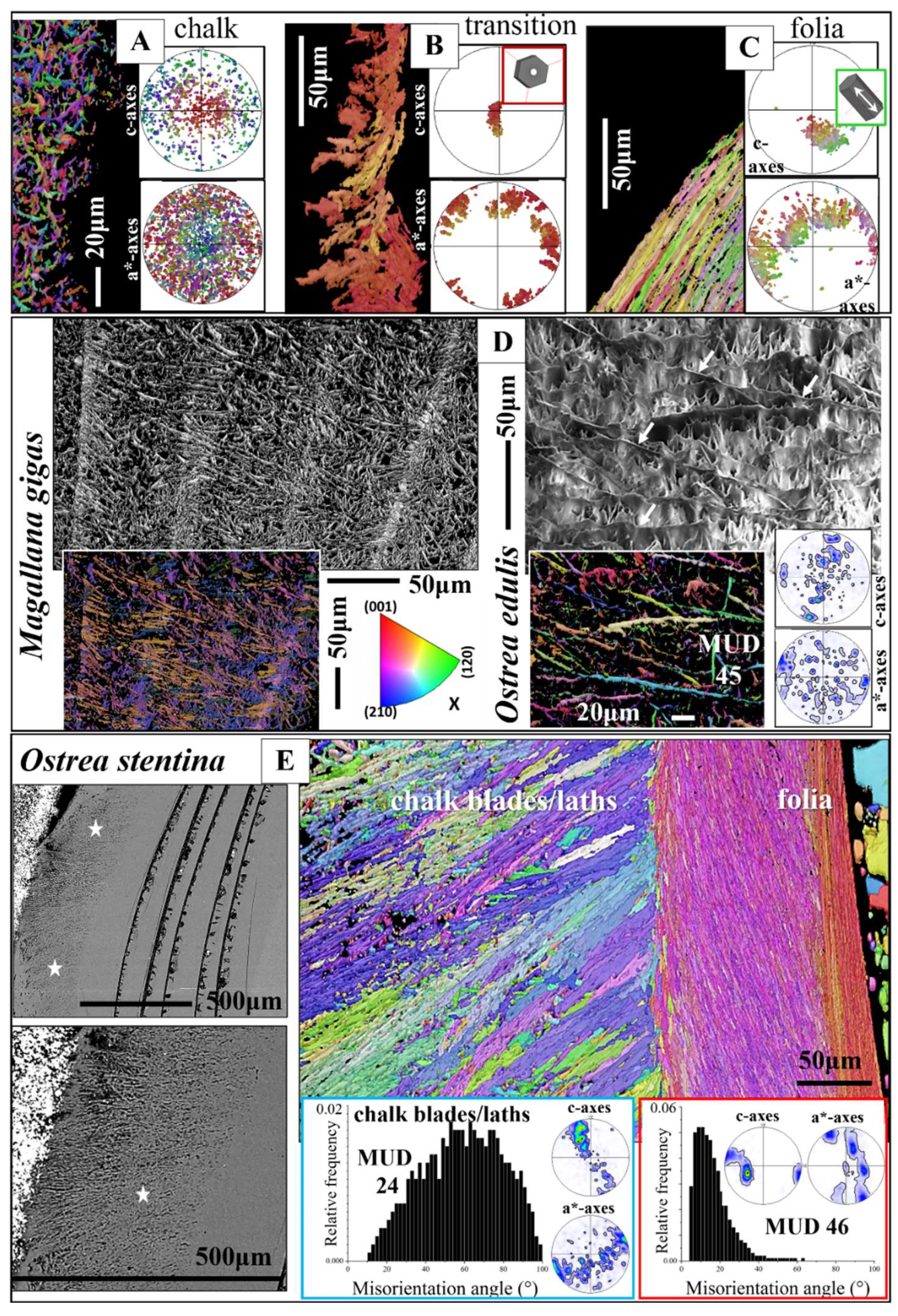
Figure 12.
The crystals, their size, morphology, microstructure and texture that encase the vesicular pores, incorporated into the shell of the gryphaeid species
Hyotissa mcgintyi (A, B, E). Although crystal co-orientation strength is very comparable between the foliated and the dendritic, polyhedral crystal, shell portion (A), we find, for the two shell layers, significant differences in microstructure, texture and misorientation angle distribution (A). The transition from folia to dendritic crystals is abrupt (A). Note the axial texture for the dendritic, polyhedral crystal, shell portion. Dendritic crystals have highly irregular, fractal morphologies (B, D). Adjacent dendritic crystals interdigitate strongly (D). The three white arrows in the EBSD map in (B) point to the crystals shown in (D). (C): The white lines are the traces of dendritic grain boundaries for the grains shown in (B) and highlight the tight interlinkage of neighboring grains as well. E: Relative frequency-misorientation angle diagrams for selected dendritic crystals. Note the low degree of misorientation angle between calcite crystallites in a dendritic crystal and the similarity of misorientation angle distribution pattern to the misorientation angle distribution pattern of a foliated crystal (compare to
Figure 4E, F). Red arrows in the pole figures in (A) indicate the overall direction of c-axis orientation. Even though being at an angle of a few degrees, there is some correspondence in c-axis orientation between the foliated shell and the adjacent dendritic crystal shell layer. At EBSD data acquisition and EBSD data evaluation, the three Euler angles were kept at 0°; φ1=0°, Φ=0°, φ2=0°.
Figure 12.
The crystals, their size, morphology, microstructure and texture that encase the vesicular pores, incorporated into the shell of the gryphaeid species
Hyotissa mcgintyi (A, B, E). Although crystal co-orientation strength is very comparable between the foliated and the dendritic, polyhedral crystal, shell portion (A), we find, for the two shell layers, significant differences in microstructure, texture and misorientation angle distribution (A). The transition from folia to dendritic crystals is abrupt (A). Note the axial texture for the dendritic, polyhedral crystal, shell portion. Dendritic crystals have highly irregular, fractal morphologies (B, D). Adjacent dendritic crystals interdigitate strongly (D). The three white arrows in the EBSD map in (B) point to the crystals shown in (D). (C): The white lines are the traces of dendritic grain boundaries for the grains shown in (B) and highlight the tight interlinkage of neighboring grains as well. E: Relative frequency-misorientation angle diagrams for selected dendritic crystals. Note the low degree of misorientation angle between calcite crystallites in a dendritic crystal and the similarity of misorientation angle distribution pattern to the misorientation angle distribution pattern of a foliated crystal (compare to
Figure 4E, F). Red arrows in the pole figures in (A) indicate the overall direction of c-axis orientation. Even though being at an angle of a few degrees, there is some correspondence in c-axis orientation between the foliated shell and the adjacent dendritic crystal shell layer. At EBSD data acquisition and EBSD data evaluation, the three Euler angles were kept at 0°; φ1=0°, Φ=0°, φ2=0°.
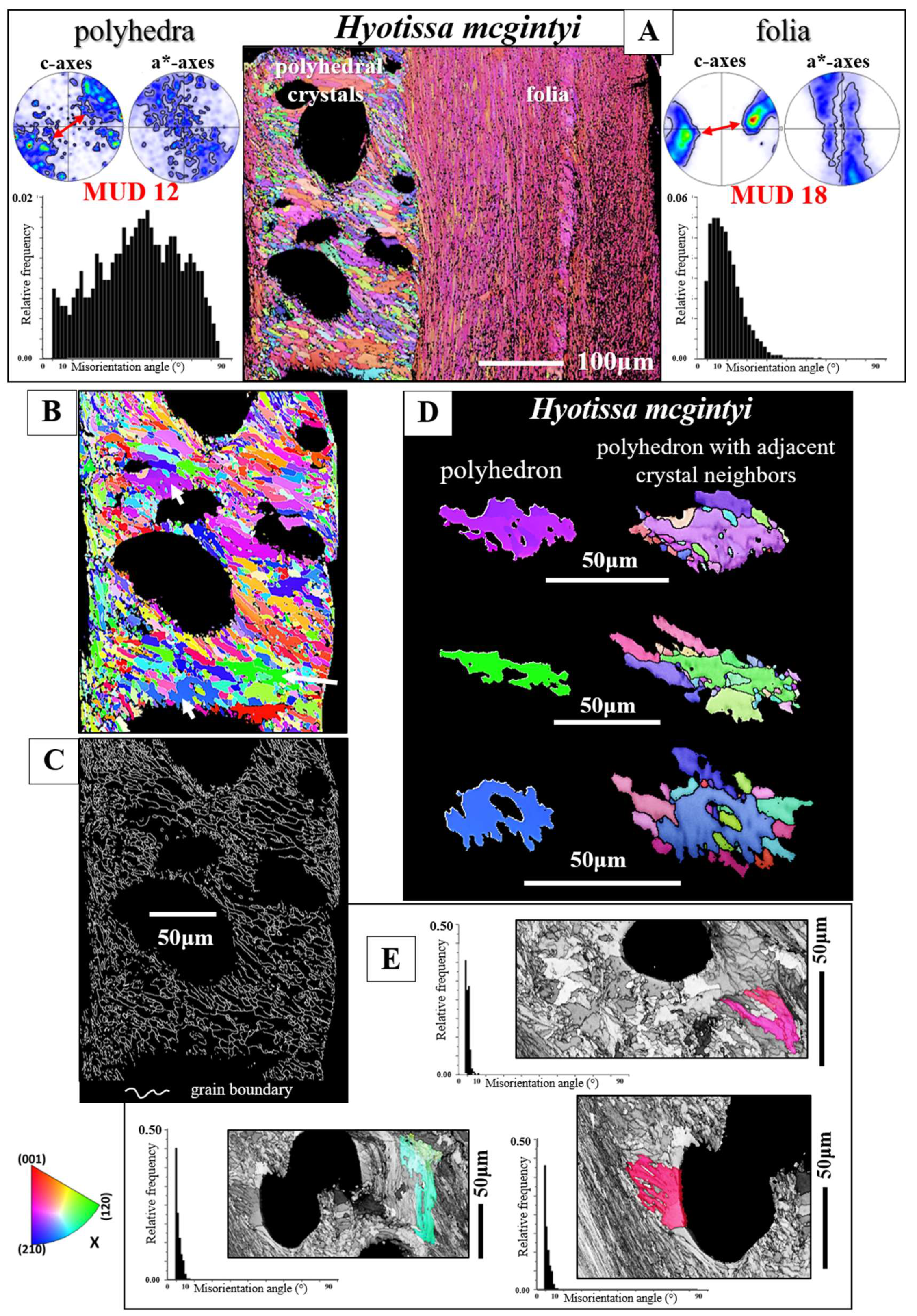
Figure 13.
Assembly of fractal-shaped (dendritic) crystals that surround the vesicular pores in Hyotissa hyotis and Neopycnodonte cochlear (Gryphaeidae) shells (A to C). Dendritic grains vary strongly in size, shape and orientation. The dendritic, polyhedral, crystal texture pattern is distinct from that of the foliated shell (see pole figures in A). B: Strong interlinkage of dendritic crystals, well visible from the EBSD maps as well as from individual crystals. For the latter see the crystal marked with a white star in (B) and its adjacent neighbors. C: The calcite of individual grains (indicated with a yellow and a white star in C) is well co-oriented. EBSD map in (C): The assembly of folia lashes out into the adjacent dendritic granular layer, encasing the vesicular pores. At EBSD data acquisition and EBSD data evaluation the three Euler angles were kept at 0°; φ1=0°, Φ=0°, φ2=0°.
Figure 13.
Assembly of fractal-shaped (dendritic) crystals that surround the vesicular pores in Hyotissa hyotis and Neopycnodonte cochlear (Gryphaeidae) shells (A to C). Dendritic grains vary strongly in size, shape and orientation. The dendritic, polyhedral, crystal texture pattern is distinct from that of the foliated shell (see pole figures in A). B: Strong interlinkage of dendritic crystals, well visible from the EBSD maps as well as from individual crystals. For the latter see the crystal marked with a white star in (B) and its adjacent neighbors. C: The calcite of individual grains (indicated with a yellow and a white star in C) is well co-oriented. EBSD map in (C): The assembly of folia lashes out into the adjacent dendritic granular layer, encasing the vesicular pores. At EBSD data acquisition and EBSD data evaluation the three Euler angles were kept at 0°; φ1=0°, Φ=0°, φ2=0°.
Figure 14.
A, B: Morphology, microstructure, texture and co-orientation strength of crystals that reinforce the biopolymer glue of Hyotissa mcgintyi (Gryphaeidae) and Magallana gigas (Ostreidae) which cements the shell to the substrate. White star in (A): The granular nature of the cementation layer and the arrangement/assembly of granules into chains/strings. We observe from the pole figures some oriented arrangement of the cementation granules as well as for the adjacent assembly of dendritic crystals (A). Black arrows in the pole figures in (A) indicate the overall direction of c-axis orientation of the granular (the cementation granules) and of the dendritic granular calcite (the calcite encasing the pores). At EBSD data acquisition and EBSD data evaluation, the three Euler angles were kept at 0°; φ1=0°, Φ=0°, φ2=0°.
Figure 14.
A, B: Morphology, microstructure, texture and co-orientation strength of crystals that reinforce the biopolymer glue of Hyotissa mcgintyi (Gryphaeidae) and Magallana gigas (Ostreidae) which cements the shell to the substrate. White star in (A): The granular nature of the cementation layer and the arrangement/assembly of granules into chains/strings. We observe from the pole figures some oriented arrangement of the cementation granules as well as for the adjacent assembly of dendritic crystals (A). Black arrows in the pole figures in (A) indicate the overall direction of c-axis orientation of the granular (the cementation granules) and of the dendritic granular calcite (the calcite encasing the pores). At EBSD data acquisition and EBSD data evaluation, the three Euler angles were kept at 0°; φ1=0°, Φ=0°, φ2=0°.
Figure 15.
Calcite c- and a*-axes orientations at the changeover from columns to the stacks of folia (A to E) and from the assembly of folia to myostracal prisms (F). For investigation of a possible crystal twin-related change between adjacent microstructures, we show, in addition to the c-axis (the {001} pole figure) and a*-axes (the {110} pole figure) orientations, pole figures for {104}, {018} and {012} plane orientations. As it is well visible from the pole figures in
Figure 15A to C, the transition from the columns to the adjacent folia is not twin operation related. It is rather a gradual, crystal rotation-based, change between the crystallites of the columnar to the crystallites of the foliated shell layers (D, E). F: Changeover from foliated calcite to myostracal, prismatic, aragonite. Even though the microstructures, as well as the textures, are very different for these two shell layers, we find some correspondence in c-axes orientation (see pole figures encircled in red in F) and some correspondence in a*-axes orientations for the calcite and the aragonite (see the pole figures encircled in blue in F). At EBSD data acquisition and EBSD data evaluation, the three Euler angles were kept at 0°; φ1=0°, Φ=0°, φ2=0°. A to E:
Magallana gigas. F:
Ostrea stentina.
Figure 15.
Calcite c- and a*-axes orientations at the changeover from columns to the stacks of folia (A to E) and from the assembly of folia to myostracal prisms (F). For investigation of a possible crystal twin-related change between adjacent microstructures, we show, in addition to the c-axis (the {001} pole figure) and a*-axes (the {110} pole figure) orientations, pole figures for {104}, {018} and {012} plane orientations. As it is well visible from the pole figures in
Figure 15A to C, the transition from the columns to the adjacent folia is not twin operation related. It is rather a gradual, crystal rotation-based, change between the crystallites of the columnar to the crystallites of the foliated shell layers (D, E). F: Changeover from foliated calcite to myostracal, prismatic, aragonite. Even though the microstructures, as well as the textures, are very different for these two shell layers, we find some correspondence in c-axes orientation (see pole figures encircled in red in F) and some correspondence in a*-axes orientations for the calcite and the aragonite (see the pole figures encircled in blue in F). At EBSD data acquisition and EBSD data evaluation, the three Euler angles were kept at 0°; φ1=0°, Φ=0°, φ2=0°. A to E:
Magallana gigas. F:
Ostrea stentina.
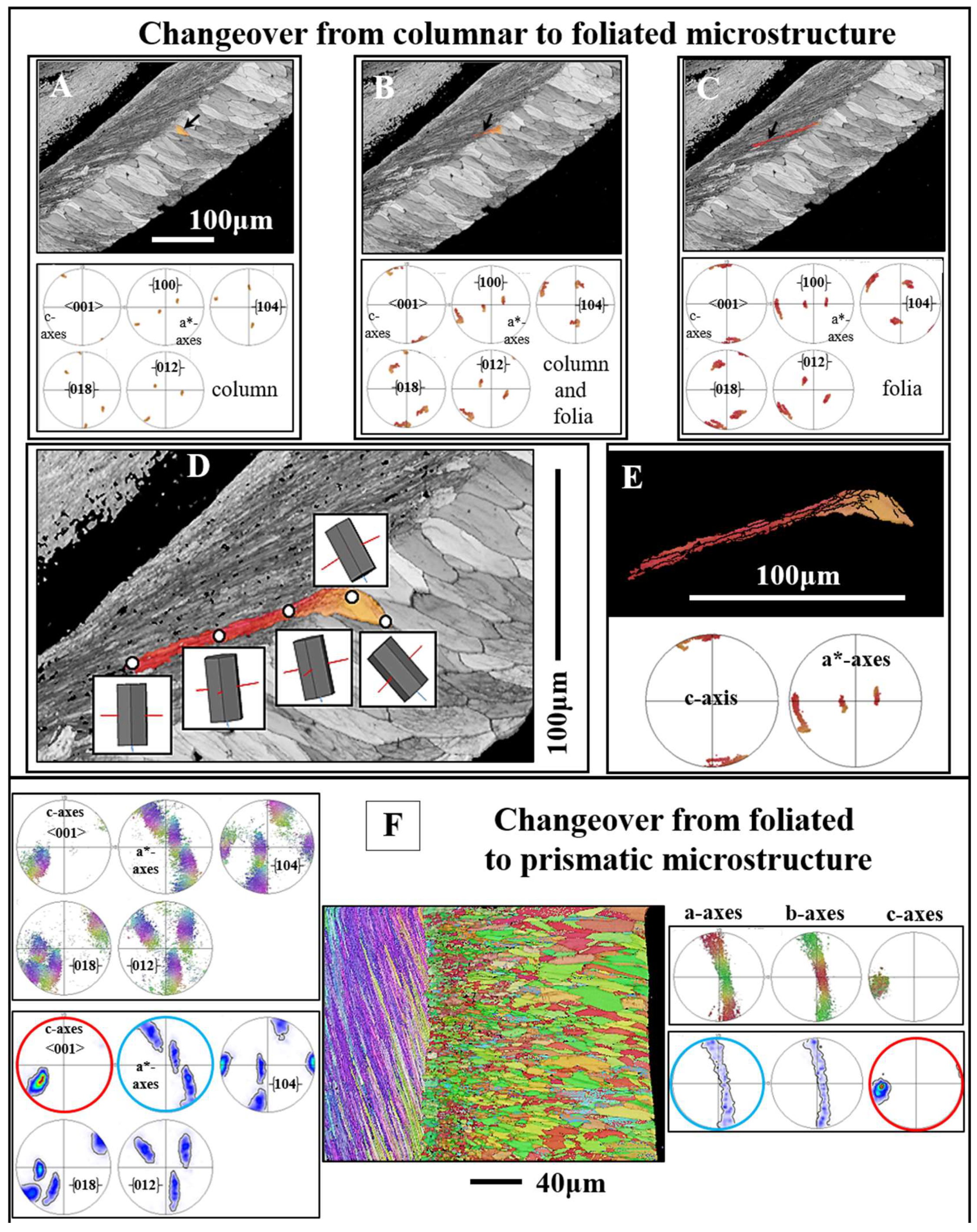
Figure 16.
EBSD scans, color-coded for crystal orientation, visualize calcite c- and a*-axes orientation at the changeover from foliated to chalky calcite (A to C). We show with pole figures c-axis ({001} pole figure) and a*-axes ({100} pole figure) as well as the {104, {018} and {012} orientations. These are shown for (i) only the foliated calcite and (ii) for the foliated calcite and the, to the calcite attaching, chalk blades. In addition, we give corresponding relative frequency-misorientation angle diagrams for the crystal orientations given in A to C. We do not find a crystal twin-related changeover from foliated to chalk calcite. If the latter was the case, we would need to observe in the misorientation angle diagrams for the folia and the directly adjacent chalk crystals a marked peak in either 60°, 78.1°, 78.8° and 104° misorientation, as defined according to the twin laws of calcite [
42,
43]. We demonstrate with the absence of a marked peak at 60°, 78°/79° and 104° misorientation and the {001}, {104, {018} and {012} pole figures that foliated and chalk calcite are not twinned and that the changeover from folia to chalk calcite is not given with a calcite twin related crystallographic operation. At EBSD data acquisition and EBSD data evaluation, the three Euler angles were kept at 0°; φ1=0°, Φ=0°, φ2=0°. A to F:
Magallana gigas.
Figure 16.
EBSD scans, color-coded for crystal orientation, visualize calcite c- and a*-axes orientation at the changeover from foliated to chalky calcite (A to C). We show with pole figures c-axis ({001} pole figure) and a*-axes ({100} pole figure) as well as the {104, {018} and {012} orientations. These are shown for (i) only the foliated calcite and (ii) for the foliated calcite and the, to the calcite attaching, chalk blades. In addition, we give corresponding relative frequency-misorientation angle diagrams for the crystal orientations given in A to C. We do not find a crystal twin-related changeover from foliated to chalk calcite. If the latter was the case, we would need to observe in the misorientation angle diagrams for the folia and the directly adjacent chalk crystals a marked peak in either 60°, 78.1°, 78.8° and 104° misorientation, as defined according to the twin laws of calcite [
42,
43]. We demonstrate with the absence of a marked peak at 60°, 78°/79° and 104° misorientation and the {001}, {104, {018} and {012} pole figures that foliated and chalk calcite are not twinned and that the changeover from folia to chalk calcite is not given with a calcite twin related crystallographic operation. At EBSD data acquisition and EBSD data evaluation, the three Euler angles were kept at 0°; φ1=0°, Φ=0°, φ2=0°. A to F:
Magallana gigas.
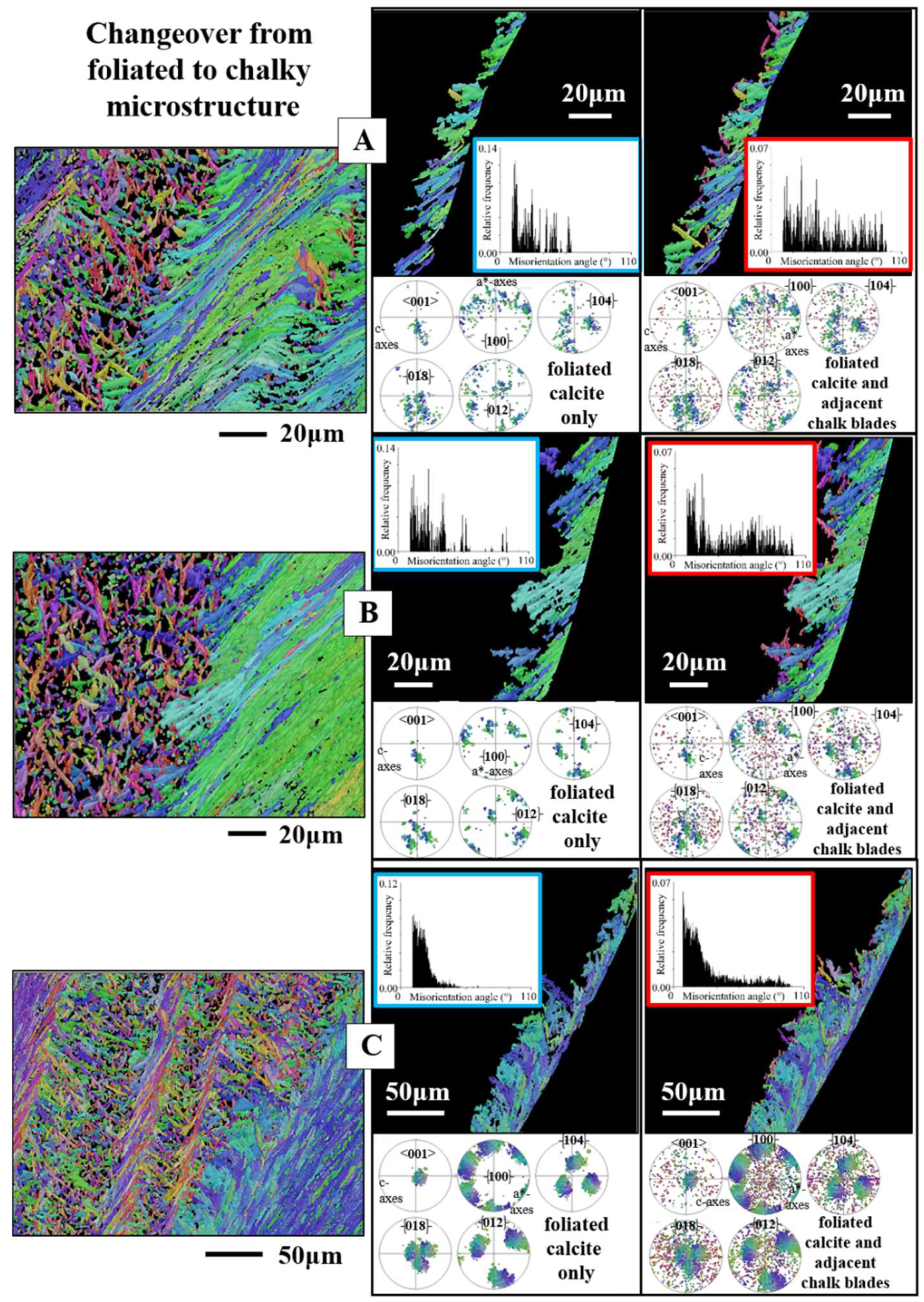
Figure 17.
Enlargement of the relative frequency-misorientation angle diagrams shown in
Figure 16. Framed in blue are misorientation angle diagrams for only the folia, framed in red are misorientation angle diagrams for the folia and the adjacent, directly touching, chalk blades. We do not observe a marked peak at 60°, 78.1, 78.8°, 104° misorientation. This demonstrates the absence of a calcite twin operation-related structural change from the foliated into the chalk microstructure. A to F:
Magallana gigas.
Figure 17.
Enlargement of the relative frequency-misorientation angle diagrams shown in
Figure 16. Framed in blue are misorientation angle diagrams for only the folia, framed in red are misorientation angle diagrams for the folia and the adjacent, directly touching, chalk blades. We do not observe a marked peak at 60°, 78.1, 78.8°, 104° misorientation. This demonstrates the absence of a calcite twin operation-related structural change from the foliated into the chalk microstructure. A to F:
Magallana gigas.
Figure 18.
The changeover from foliated to ‘dendritic’ calcite (A, D). The change from one microstructure into the other is rather abrupt. For the foliated and the adjacent dendritic, polyhedral, crystals (D) we do not find a marked peak at 60°, 78.1, 78.8°, 104° in the relative frequency-misorientation angle diagram (D), nor any indication of twin formation in the (001), (104), (018), (012) pole figures (D). This indicates that the changeover from the foliated to the ‘dendritic’ crystal shell portion is not the result of a calcite twin-related crystallographic operation. At EBSD data acquisition and EBSD data evaluation, the three Euler angles were kept at 0°; φ1=0°, Φ=0°, φ2=0°. A to D: Neopycnodonte cochlear.
Figure 18.
The changeover from foliated to ‘dendritic’ calcite (A, D). The change from one microstructure into the other is rather abrupt. For the foliated and the adjacent dendritic, polyhedral, crystals (D) we do not find a marked peak at 60°, 78.1, 78.8°, 104° in the relative frequency-misorientation angle diagram (D), nor any indication of twin formation in the (001), (104), (018), (012) pole figures (D). This indicates that the changeover from the foliated to the ‘dendritic’ crystal shell portion is not the result of a calcite twin-related crystallographic operation. At EBSD data acquisition and EBSD data evaluation, the three Euler angles were kept at 0°; φ1=0°, Φ=0°, φ2=0°. A to D: Neopycnodonte cochlear.
Table 1.
The different crystals that comprise Ostreoidea shells and the range of crystal co-orientation strength, expressed as MUD value, for individual crystals as well as for a particular microstructure. When the MUD value of a microstructure is considered, then we observe the highest crystal co-orientation strength for the myostraca and the lowest for the chalk and for the polyhedral crystals. When the MUD value of individual crystals is considered, we find the highest crystal co-orientation strength for individual rhombohedral crystals, followed by the individual prisms of the myostraca.
Table 1.
The different crystals that comprise Ostreoidea shells and the range of crystal co-orientation strength, expressed as MUD value, for individual crystals as well as for a particular microstructure. When the MUD value of a microstructure is considered, then we observe the highest crystal co-orientation strength for the myostraca and the lowest for the chalk and for the polyhedral crystals. When the MUD value of individual crystals is considered, we find the highest crystal co-orientation strength for individual rhombohedral crystals, followed by the individual prisms of the myostraca.
| |
MUD value of an
EBSD scan
covering many crystals
|
MUD value of an EBSD scan over an
individual crystal
|
| Columnar crystals |
25-30 |
250-460 |
Foliated
crystals/crystallites |
18-67 |
Folium: 591, 626
Foliated crystal:
174, 545 |
| Pallial prisms |
125-130 |
650-680 |
| Adductor prisms |
85-90 |
650-700 |
| Chalk blades/laths |
4-45 |
450-500 |
| Polyhedral crystals |
12-27 |
350-600 |
| Cementation granules |
13-18 |
600-650 |
| Rhombohedral crystals |
- |
700, >700 |