Submitted:
29 January 2025
Posted:
31 January 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Data Source and Search Strategy
2.3. Bibliometric Analyses
3. Results
3.1. Overview
3.2. Scholarly Journal Influence and Collaboration Networks
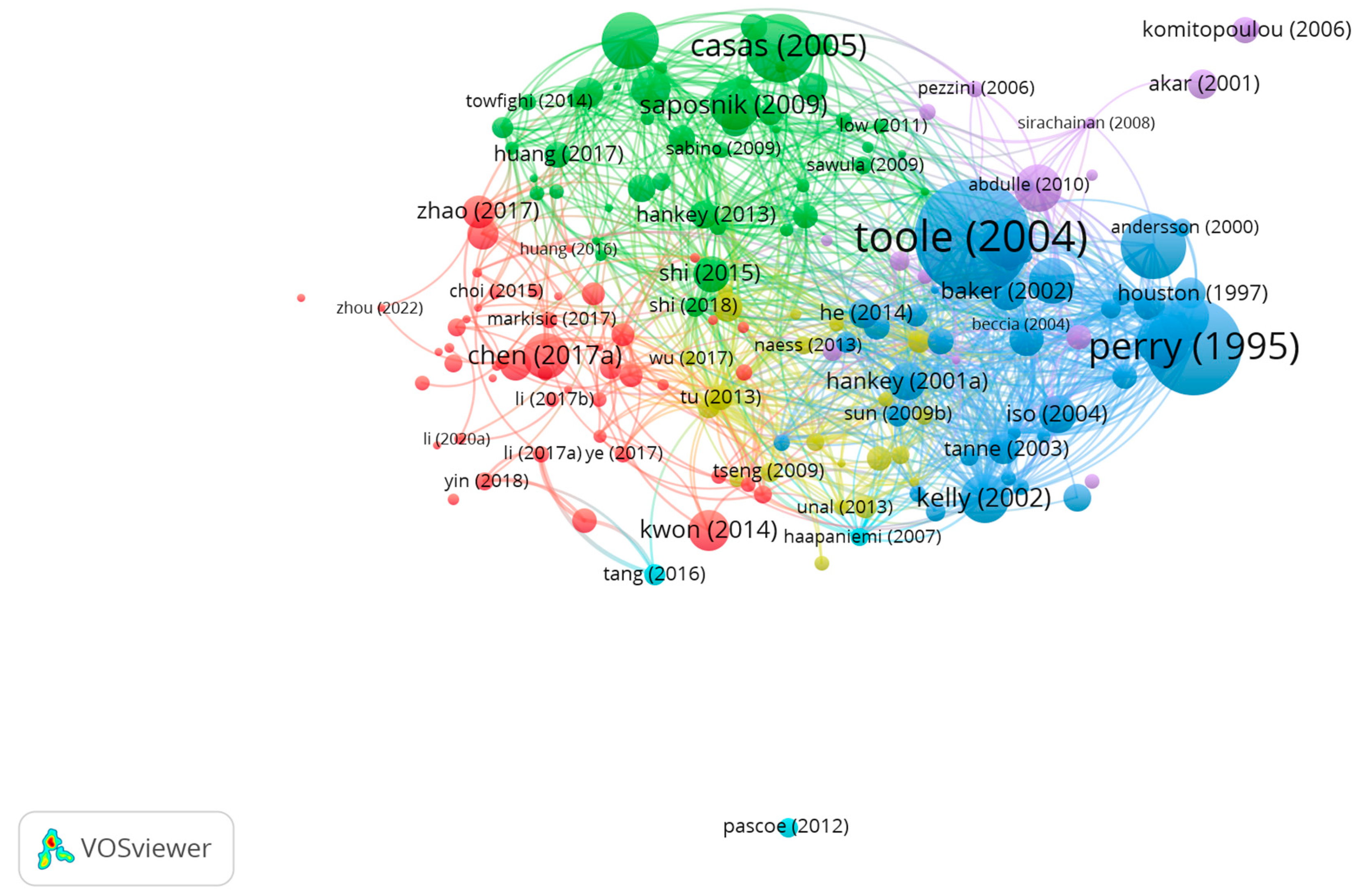
3.3. Bibliographic Coupling
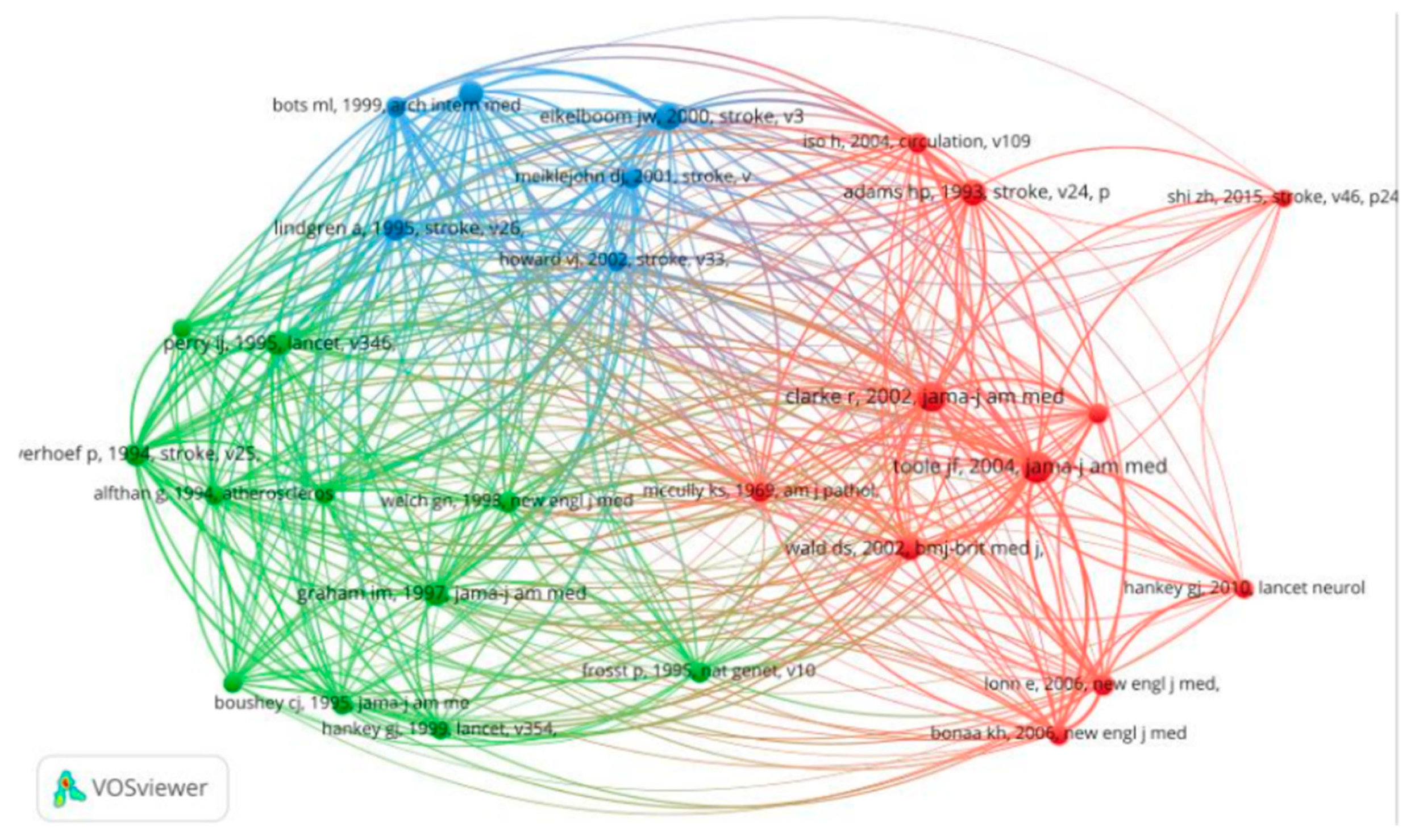
3.4. Co-Cited References
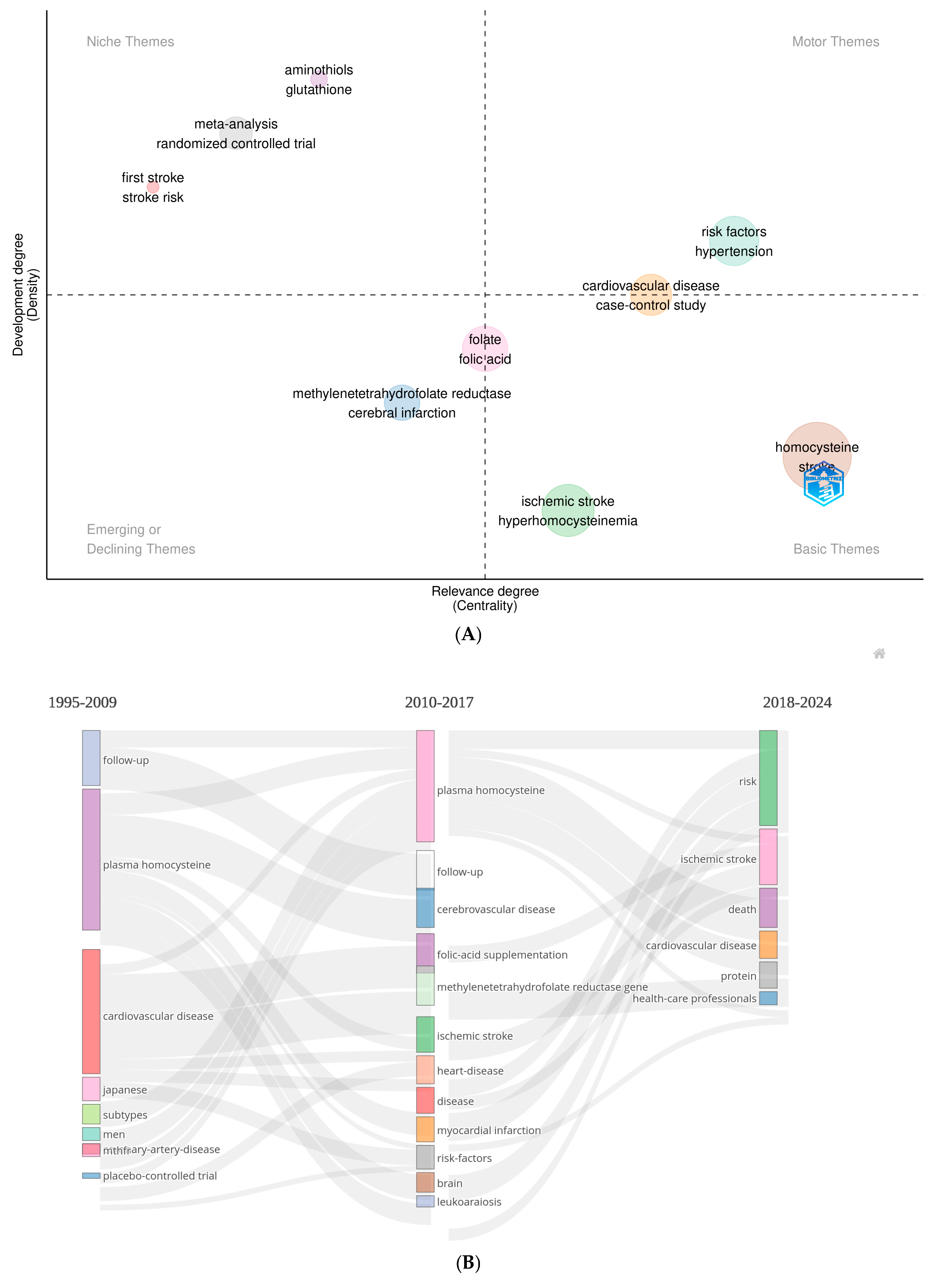
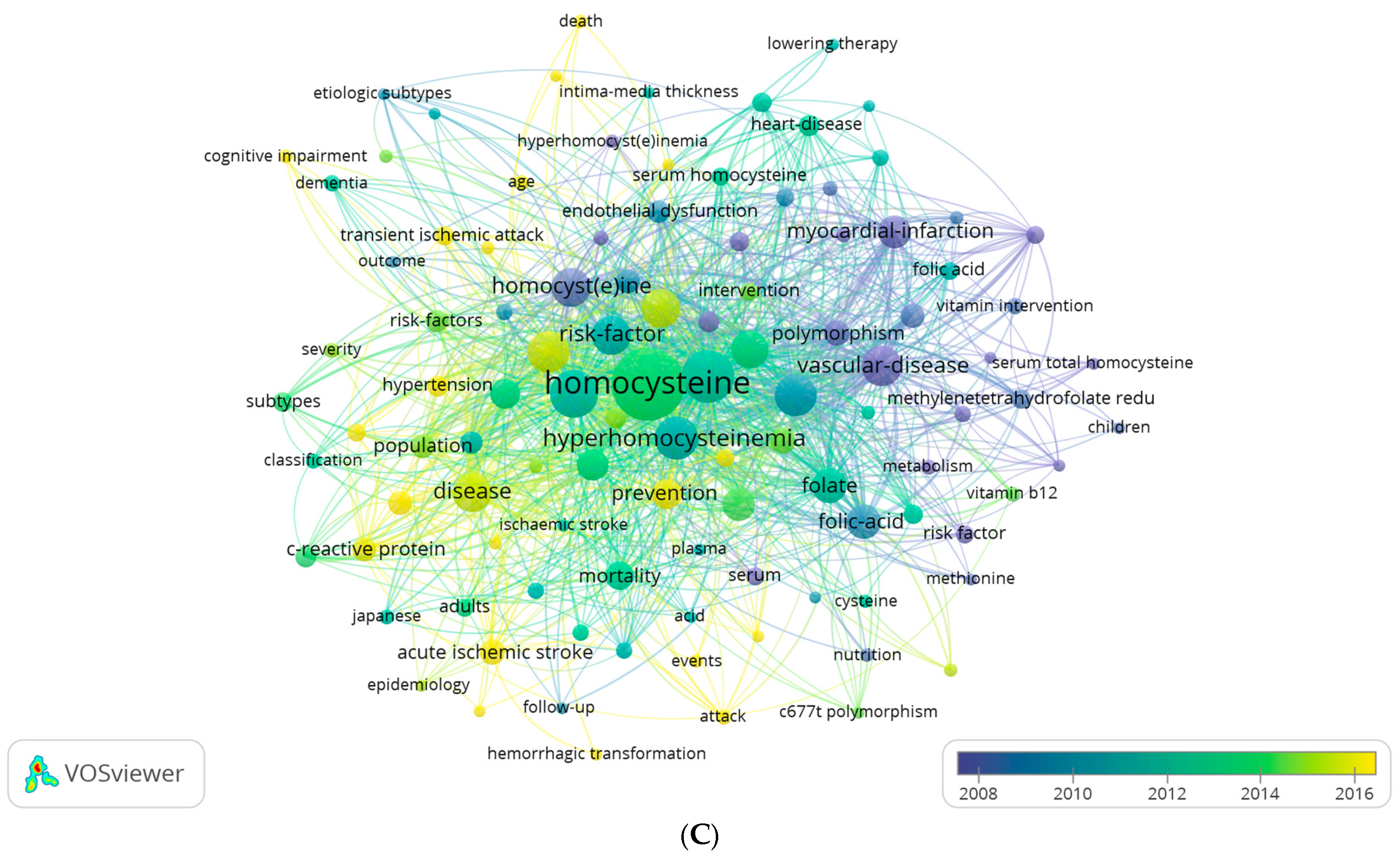
3.5. Thematic Structure, Thematic Evolution, Co-Words and Burst Analysis
4. Discussion
4.1. Overview of Research Landscape
4.2. Research Trend and Research Hotspots
4.2.1. Homocysteine and Vascular Disease
4.2.2. Emerging Focus on Ischemic Stroke and Risk Factors
4.2.3. Folic Acid and B-Vitamin Supplementation: Implications for Stroke
4.2.4. Acute Stroke Phases and Homocysteine Dynamics
4.3. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Data Availability Statement
Conflicts of Interest
References
- G.J. Hankey, J.W. Eikelboom, Homocysteine and stroke, Curr. Opin. Neurol. 14 (2001) 95–102. [CrossRef]
- L.K. Wei, A. Au, S. Menon, S.H. Gan, L.R. Griffiths, Clinical relevance of MTHFR, eNOS, ACE, and ApoE gene polymorphisms and serum vitamin profile among Malay patients with ischemic stroke, J. Stroke Cerebrovasc. Dis. 24 (2015) 2017–2025. [CrossRef]
- C. Zhang, F.L. Chi, T.H. Xie, Y.H. Zhou, Effect of B-vitamin supplementation on stroke: A meta-analysis of randomized controlled trials, PLoS ONE 8 (2013) e81577. [CrossRef]
- L.K. Wei, H.S. Heidi, L.R. Griffiths, Epigenetics and ischemic stroke: A bibliometric analysis from 2014 to 2024, Epigenetics Insights (In press).
- J.F. Toole, M.R. Malinow, L.E. Chambless, J.D. Spence, L.C. Pettigrew, V.J. Howard,... G. Howard, Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: The Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial, JAMA 291 (2004) 565–575. [CrossRef]
- I.J. Perry, R.W. Morris, S.B. Ebrahim, A.G. Shaper, H. Refsum, P.M. Ueland, Prospective study of serum total homocysteine concentration and risk of stroke in middle-aged British men, Lancet 346 (1995) 1395–1398. [CrossRef]
- Brattström, B. Norrving, B. Hultberg, A. Andersson, B.B. Johansson, Plasma homocysteine in the acute and convalescent phases after stroke, Stroke 26 (1995) 795–800. [CrossRef]
- A.G. Bostom, I.H. Rosenberg, H. Silbershatz, P.F. Jacques, J. Selhub, R.B. D’Agostino,... P.W. Wilson, Nonfasting plasma total homocysteine levels and stroke incidence in elderly persons: The Framingham Study, Ann. Intern. Med. 131 (1999) 352–355. [CrossRef]
- D.J. Meiklejohn, M.A. Vickers, R. Dijkhuisen, M. Greaves, Plasma homocysteine concentrations in the acute and convalescent periods of atherothrombotic stroke, Stroke 32 (2001) 57–62. [CrossRef]
- H. Iso, Y. Moriyama, S. Sato, A. Kitamura, T. Tanigawa, K. Yamagishi,... M. Konishi, Serum total homocysteine concentrations and risk of stroke and its subtypes in Japanese, Circulation 109 (2004) 2766–2772. [CrossRef]
- Z. Li, L. Sun, H. Zhang, Y. Liao, D. Wang, B. Zhao, et al., Elevated plasma homocysteine was associated with hemorrhagic and ischemic stroke, but methylenetetrahydrofolate reductase gene C677T polymorphism was a risk factor for thrombotic stroke, Stroke 34 (2003) 2085–2090. [CrossRef]
- M.L. Bots, L.J. Launer, J. Lindemans, A.W. Hoes, A. Hofman, J.C.M. Witteman, et al., Homocysteine and short-term risk of myocardial infarction and stroke in the elderly, Arch. Intern. Med. 159 (1999) 38–44. [CrossRef]
- Z. Shi, Y. Guan, Y.R. Huo, S. Liu, M. Zhang, H. Lu, et al., Elevated total homocysteine levels in acute ischemic stroke are associated with long-term mortality, Stroke 46 (2015) 2419–2425. [CrossRef]
- G. Saposnik, J.G. Ray, P. Sheridan, M. McQueen, E. Lonn, Homocysteine-lowering therapy and stroke risk, severity, and disability, Stroke 40 (2009) 1365–1372. [CrossRef]
- J.P. Casas, L.E. Bautista, L. Smeeth, P. Sharma, A.D. Hingorani, Homocysteine and stroke: evidence on a causal link from mendelian randomisation, Lancet 365 (2005) 224–232. [CrossRef]
- Homocysteine Studies Collaboration, Homocysteine and risk of ischemic heart disease and stroke, JAMA 288 (2002) 2015–2022. [CrossRef]
- J.W. Eikelboom, G.J. Hankey, S.S. Anand, E. Lofthouse, N. Staples, R.I. Baker, Association between high homocyst(e)ine and ischemic stroke due to large- and small-artery disease but not other etiologic subtypes of ischemic stroke, Stroke 31 (2000) 1069–1075. [CrossRef]
- H.P. Adams, B.H. Bendixen, L.J. Kappelle, J. Biller, B.B. Love, D.L. Gordon, et al., Classification of subtype of acute ischemic stroke: Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment, Stroke 24 (1993) 35–41. [CrossRef]
- I.M. Graham, L.E. Daly, H.M. Refsum, K. Robinson, L.E. Brattström, P.M. Ueland, et al., Plasma homocysteine as a risk factor for vascular disease, JAMA 277 (1997) 1775–1781. [CrossRef]
- D.S. Wald, Homocysteine and cardiovascular disease: Evidence on causality from a meta-analysis, BMJ 325 (2002) 1202–1206. [CrossRef]
- D.J. Meiklejohn, M.A. Vickers, R. Dijkhuisen, M. Greaves, Plasma homocysteine concentrations in the acute and convalescent periods of atherothrombotic stroke, Stroke 32 (2001) 57–62. [CrossRef]
- A.D. Smith, H. Refsum, Homocysteine – from disease biomarker to disease prevention, J. Intern. Med. 290 (2021) 826–854. [CrossRef]
- Y. Ma, Y. Chen, T. Yang, X. He, Y. Yang, J. Chen, et al., Blood biomarkers for post-stroke cognitive impairment: a systematic review and meta-analysis, J. Stroke Cerebrovasc. Dis. (2024) 107632. [CrossRef]
- M.Y. Maksimova, A.V. Ivanov, E.D. Virus, K.A. Nikiforova, F.R. Ochtova, E.T. Suanova, et al., Impact of glutathione on acute ischemic stroke severity and outcome: possible role of aminothiols redox status, Redox Rep. 26 (2021) 117–123. [CrossRef]
- L.K. Wei, H. Sutherland, A. Au, E. Camilleri, L.M. Haupt, S.H. Gan, et al., A potential epigenetic marker mediating serum folate and vitamin B12 levels contributes to the risk of ischemic stroke, Biomed. Res. Int. 2015 (2015) 1–4. [CrossRef]
- M. Sikora, E. Bretes, J. Perła-Kaján, O. Utyro, K. Borowczyk, J. Piechocka, et al., Homocysteine thiolactone and other sulfur-containing amino acid metabolites are associated with fibrin clot properties and the risk of ischemic stroke, Sci. Rep. 14 (2024). [CrossRef]
- A.R. Mathew, G. Di Matteo, P. La Rosa, S.A. Barbati, L. Mannina, S. Moreno, et al., Vitamin B12 deficiency and the nervous system: Beyond metabolic decompensation—Comparing biological models and gaining new insights into molecular and cellular mechanisms, Int. J. Mol. Sci. 25 (2024) 590. [CrossRef]
- Z.J. Yang, S.Y. Huang, K.Y. Zhong, W.G. Huang, Z.H. Huang, T.T. He, et al., Betaine alleviates cognitive impairment induced by homocysteine through attenuating NLRP3-mediated microglial pyroptosis in an m6A-YTHDF2-dependent manner, Redox Biol. 69 (2024) 103026. [CrossRef]
- N. Zhang, Z. Zhou, X. Chi, F. Fan, S. Li, Y. Song, et al., Folic acid supplementation for stroke prevention: a systematic review and meta-analysis of 21 randomized clinical trials worldwide, Clin. Nutr. 43 (2024) 1706–1716. [CrossRef]
- Q. Zhou, Z. Xu, Y. Duan, H. Tang, H. Zhang, H. Liu, MTHFR C677T, hyperhomocysteinemia, and their interactions with traditional risk factors in early neurological deterioration in Chinese patients with ischemic stroke, Heliyon 10 (2024) e31003. [CrossRef]
- M. Holmen, A.M. Hvas, J.F.H. Arendt, Hyperhomocysteinemia and ischemic stroke: A potential dose-response association—A systematic review and meta-analysis, TH Open 5 (2021) e420–437. [CrossRef]
- Z.H. Lu, J. Li, X.L. Li, M. Ding, C.J. Mao, X.Y. Zhu, et al., Hypertension with hyperhomocysteinemia increases the risk of early cognitive impairment after first-ever ischemic stroke, Eur. Neurol. 82 (2019) 75–85. [CrossRef]
- S. Huang, J. Cai, Y. Tian, The prognostic value of homocysteine in acute ischemic stroke patients: A systematic review and meta-analysis, Front. Syst. Neurosci. 14 (2021). [CrossRef]
- T. Zhang, Y. Jiang, S. Zhang, T. Tie, Y. Cheng, X. Su, et al., The association between homocysteine and ischemic stroke subtypes in Chinese, Med. 99 (2020) e19467. [CrossRef]
- H. Li, L. Shu, Q. Dai, T. Wu, Association between plasma total homocysteine (tHcy) and strokes: A meta-analysis, Pteridines 33 (2022) 58–68. [CrossRef]
- Y. Ji, S. Tan, Y. Xu, A. Chandra, C. Shi, B. Song, et al., Vitamin B supplementation, homocysteine levels, and the risk of cerebrovascular disease, Neurol. 81 (2013) 1298–1307. [CrossRef]
- S. Tripathi, M. Nath, S. Misra, P. Kumar, From A to E: Uniting vitamins against stroke risk—A systematic review and network meta‐analysis, Eur. J. Clin. Invest. 54 (2024). [CrossRef]
- N. Kataria, P. Yadav, R. Kumar, N. Kumar, M. Singh, R. Kant, et al., Effect of vitamin B6, B9, and B12 supplementation on homocysteine level and cardiovascular outcomes in stroke patients: A meta-analysis of randomized controlled trials, Cureus (2021). [CrossRef]
- N. Zhang, Z. Wu, X. Bai, Y. Song, P. Li, X. Lu, et al., Dosage exploration of combined B-vitamin supplementation in stroke prevention: A meta-analysis and systematic review, Am. J. Clin. Nutr. 119 (2024) 821–828. [CrossRef]
- J.D. Spence, G.J. Hankey, Problem in the recent American Heart Association guideline on secondary stroke prevention: B vitamins to lower homocysteine do prevent stroke, Stroke 53 (2022) 2702–2708. [CrossRef]
- W.C. Dong, J.L. Guo, L. Xu, X.H. Jiang, C.H. Chang, Y. Jiang, et al., Impact of homocysteine on acute ischemic stroke severity: Possible role of aminothiols redox status, BMC Neurol. 24 (2024). [CrossRef]
- Y. Wang, R. Hou, Y. Liu, Plasma homocysteine (Hcy) concentration functions as a predictive biomarker of SPECT-evaluated post-ischemic hyperperfusion in acute ischemic stroke, Pharmacogenomics Pers. Med. 16 (2023) 481–489. [CrossRef]
- Žitňanová, P. Šiarnik, B. Kollár, M. Chomová, P. Pazderová, L. Andrezálová, et al., Oxidative stress markers and their dynamic changes in patients after acute ischemic stroke, Oxid. Med. Cell Longev. 2016 (2016) 1–7. [CrossRef]
- M.Y. Maksimova, A.V. Ivanov, E.D. Virus, K.A. Nikiforova, F.R. Ochtova, E.T. Suanova, et al., Impact of glutathione on acute ischemic stroke severity and outcome: Possible role of aminothiols redox status, Redox Rep. 26 (2021) 117–123. [CrossRef]
- F.Z. Kamal, R. Lefter, H. Jaber, I.M. Balmus, A. Ciobica, A.C. Iordache, The role of potential oxidative biomarkers in the prognosis of acute ischemic stroke and the exploration of antioxidants as possible preventive and treatment options, Int. J. Mol. Sci. 24 (2023) 6389. [CrossRef]
| Rank | DOI, [Reference] | Normalized Global Citations (NGC) |
Normalized Local Citations (NLC) |
NGC/NLC Ratio |
|---|---|---|---|---|
| 1 | 10.1001/jama.291.5.565 [5] | 5.00 | 3.91 | 1.28 |
| 2 | 10.1016/S0140-6736(95)92407-8 [6] | 1.59 | 1.03 | 1.54 |
| 3 | 10.1161/01.STR.26.5.795 [7] | 0.41 | 0.97 | 0.42 |
| 4 | 10.7326/0003-4819-131-5-199909070-00006 [8] | 2.22 | 2.18 | 1.02 |
| 5 | 10.1161/01.STR.32.1.57 [9] | 1.26 | 3.48 | 0.36 |
| 6 | 10.1161/01.CIR.0000131942.77635.2D [10] | 0.56 | 1.70 | 0.33 |
| 7 | 10.1161/01.STR.0000086753.00555.0D [11] | 2.09 | 2.45 | 0.85 |
| 8 | 10.1001/archinte.159.1.38 [12] | 1.40 | 1.54 | 0.91 |
| 9 | 10.1161/STROKEAHA.115.009136 [13] | 3.18 | 3.48 | 0.91 |
| 10 | 10.1161/STROKEAHA.108.529503 [14] | 4.81 | 5.02 | 0.96 |
| Rank | DOI, [Reference] | First Author [Country] |
Co-cited (n) |
Year | TLS |
|---|---|---|---|---|---|
| 1 | *[10.1001/jama.291.5.565] [5] |
Toole JF [USA] |
59 | 2004 | 239 |
| 2 | [10.1001/jama.288.16.2015] [16] |
Clarke R [USA] |
55 | 2002 | 246 |
| 3 | [10.1161/01.str.31.5.1069] [17] |
Eikelboom JW [Canada] |
43 | 2000 | 193 |
| 4 | [10.1161/01.str.24.1.35] [18] |
Adams HP [USA] |
42 | 1993 | 159 |
| 5 | [10.1001/jama.277.22.1775] [19] |
Graham IM [UK] |
34 | 1997 | 162 |
| 6 | *[10.1016/S0140-6736(95)92407-8] [6] | Perry IJ [UK] |
34 | 1995 | 202 |
| 7 | [10.1136/bmj.325.7374.1202] [20] |
Wald DS [UK] |
33 | 1995 | 161 |
| 8 | *[10.1161/01.STR.26.5.795] [7] |
Lindgren A [Sweden] |
32 | 2002 | 168 |
| 9 | *[10.7326/0003-4819-131-5-199909070-00006] [8] |
Bostom AG [USA] |
31 | 1995 | 174 |
| 10 | [10.1161/01.str.32.1.57] [21] |
Meiklejohn DJ [UK] |
29 | 1999 | 165 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





