Submitted:
29 January 2025
Posted:
29 January 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Bioelectric Membrane Potential in Breast Cancer
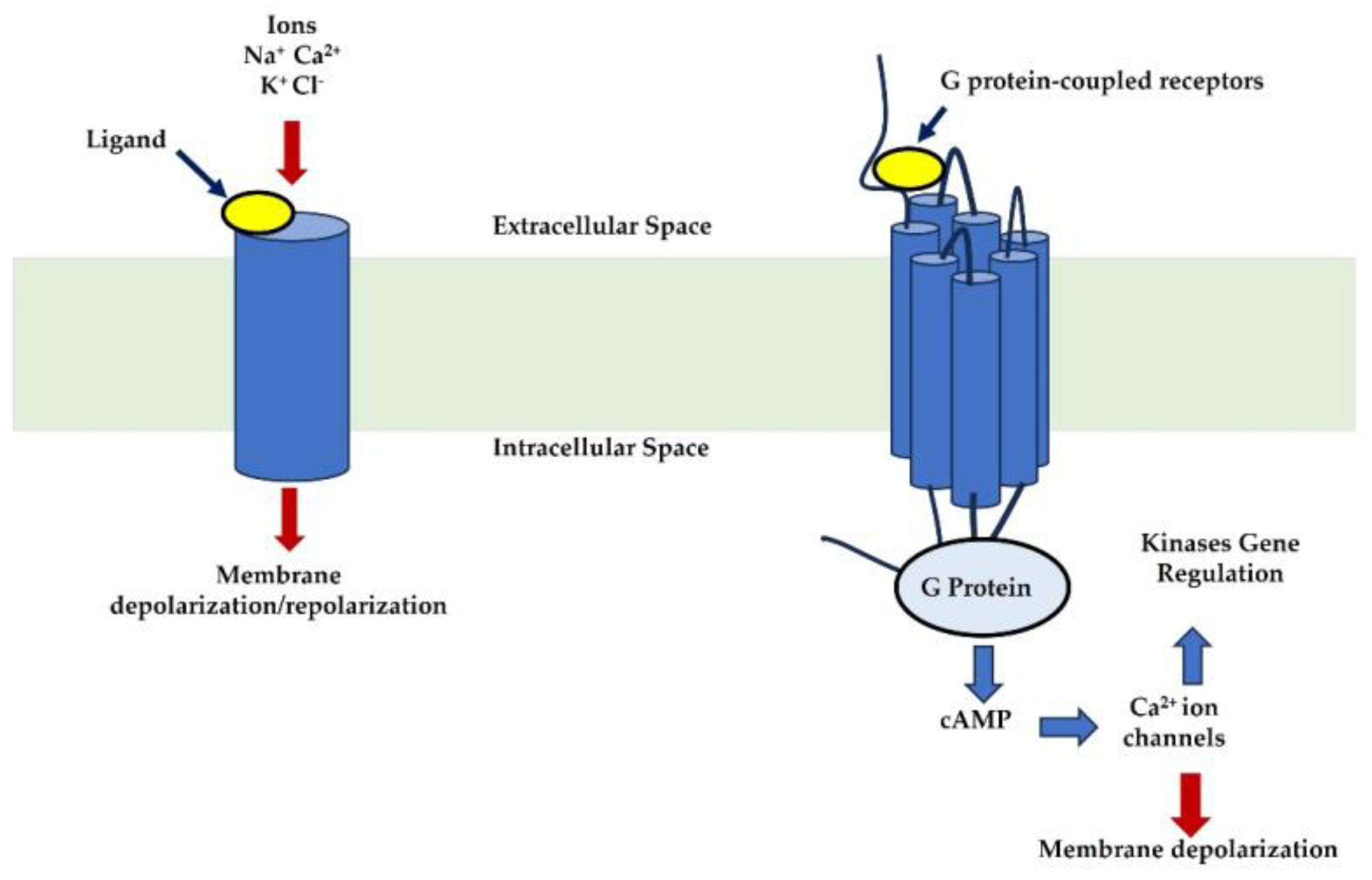
3. Neuroreceptors, Neurotransmitters, Membrane Potential, and Breast Cancer
4. Neuroreceptor Pharmacology and Therapeutic Advances
| Neuropharmacological Identifier | Associated Neurotransmitters | Membrane Potential |
|---|---|---|
| NCT03108937, NCT04454515, NCT03109990, NCT02013492 | Norepinephrine and epinephrine | Depolarization |
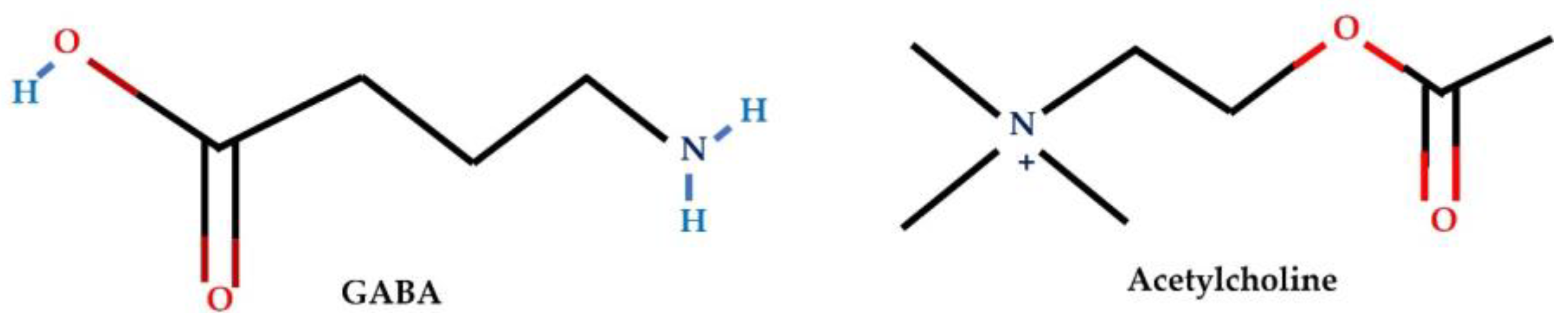
| NCT01530373, NCT02312934 | Acetylcholine | Depolarization or Repolarization |
| NCT02312934 NCT03122444, NCT00198250 | Serotonin | Depolarization |
| NCT01730729, NCT02312934, NCT02861859 | Dopamine | Depolarization |
5. Future Directions and Challenges
- Identifying bioelectric signatures for different breast cancer subtypes: Identifying bioelectric signatures in breast cancer subtypes can be improved using voltage-sensitive dyes for membrane potential monitoring, patch-clamp techniques for ion channel activity, and single-cell transcriptomics to link gene expression with bioelectric signaling. Combining these with imaging tools like fluorescence microscopy or optogenetics helps reveal bioelectricity's role in tumor behavior, offering insights into new therapeutic targets for personalized treatment strategies, and ultimately supporting personalized treatment strategies for different breast cancer subtypes.
- Developing ion channel modulators targeting cancer cells while minimizing off-target effects: Ion channel modulators show promise as breast cancer therapies by regulating cell functions such as proliferation and migration. For instance, Nav1.7 sodium channels contribute to metastasis, with inhibitors like tetrodotoxin being studied to limit cancer spread. TRPM7, involved in calcium and magnesium influx, is linked to poor prognosis, and inhibitors like NS8593 may slow tumor growth. Additionally, TPCs (TPC1 and TPC2) regulate calcium signaling and tumor progression, with modulators potentially reducing cell proliferation. While preclinical results are promising, further research is needed to evaluate their clinical effectiveness.
- Exploring the combination of bioelectric therapies with conventional treatments in clinical trials: Combining bioelectric therapies with conventional treatments like chemotherapy or immunotherapy is gaining attention in clinical trials. This approach aims to enhance treatment efficacy, overcome drug resistance, and minimize side effects. For example, ion channel modulators could sensitize cancer cells to chemotherapy, while bioelectric stimulation may boost immune response during immunotherapy. Ongoing trials are exploring how these combinations could provide synergistic benefits, leading to more effective and personalized cancer treatments.
- Advanced Imaging: New fluorescent probes and imaging techniques are needed to measure membrane potential and ion channel activity in real-time within live cells and tissues [94].
- Integrative Multi-Omics: Combining genomics, proteomics, and electrophysiology can provide comprehensive models of membrane potential modulation by neuroreceptors and ion channels [95].
- Personalized Medicine: Understanding individual responses to agonists and antagonists can help design personalized therapeutic strategies [96].
- Animal Models and Clinical Trials: Translating laboratory findings into animal models and clinical trials is essential for testing the efficacy of bioelectric-based therapies [97].
- Digital Twin and Computational Electrophysiology: Digital twin models, which replicate patients or biological systems, offer promising tools to address drug resistance in cancer by integrating clinical and multi-omics data to simulate disease progression and treatment outcomes. Initiatives like the PRIMUS project and NIH-funded INCEPTION have utilized digital twins to predict resistance mechanisms in cancers such as pancreatic, breast, and lung cancer. Combining these models with CRISPR screening enables genome-wide identification of resistance-related genes, like ABC transporters or MYC, and simulates their impact on tumor behavior. This approach refines therapeutic strategies, suggests combination therapies, and accelerates drug development by reducing preclinical costs while exploring immune escape mechanisms, paving the way for personalized cancer treatments. Digital twin models offer customized virtual representations of patients, enabling predictive modeling to optimize treatment strategies [98].
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Siegel, Rebecca L., Angela N. Giaquinto, and Ahmedin Jemal. "Cancer statistics, 2024." CA: a cancer journal for clinicians 74, no. 1 (2024): 12-49. [CrossRef]
- Neri, Dario, and Paul M. Sondel. "Immunocytokines for cancer treatment: past, present and future." Current opinion in immunology 40 (2016): 96-102. [CrossRef]
- Kumar, Pankaj, and Rupali Aggarwal. "An overview of triple-negative breast cancer." Archives of gynecology and obstetrics 293 (2016): 247-269. [CrossRef]
- Mir, Manzoor Ahmad, and Hina Qayoom. "Introduction to breast cancer." In Therapeutic potential of cell cycle kinases in breast cancer, pp. 1-22. Singapore: Springer Nature Singapore, 2023.
- Lanyi, Marton. "Malignant and Benign Lobular and Ductal Lesions with Perifocal Reactions." In Mammography: Diagnosis and Pathological Analysis, pp. 145-212. Berlin, Heidelberg: Springer Berlin Heidelberg, 2003. [CrossRef]
- Feng, Yixiao, Mia Spezia, Shifeng Huang, Chengfu Yuan, Zongyue Zeng, Linghuan Zhang, Xiaojuan Ji et al. "Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis." Genes & diseases 5, no. 2 (2018): 77-106. [CrossRef]
- Mahapatra, Chitaranjan, and Ravinder Kumar. "Biophysical Mechanisms of Vaginal Smooth Muscle Contraction: The Role of the Membrane Potential and Ion Channels." Pathophysiology 31, no. 2 (2024): 225-243. [CrossRef]
- An, Qimin, Gengyu Yue, Xiaoxu Yang, Jun Lou, Weixi Shan, Jianhong Ding, Zhe Jin et al. "Pathophysiological role of purinergic P2X receptors in digestive system diseases." Frontiers in Physiology 12 (2021): 781069. [CrossRef]
- Sundelacruz, Sarah, Michael Levin, and David L. Kaplan. "Role of membrane potential in the regulation of cell proliferation and differentiation." Stem cell reviews and reports 5 (2009): 231-246. [CrossRef]
- Déliot, Nadine, and Bruno Constantin. "Plasma membrane calcium channels in cancer: Alterations and consequences for cell proliferation and migration." Biochimica et Biophysica Acta (BBA)-Biomembranes 1848, no. 10 (2015): 2512-2522. [CrossRef]
- Rao, Vidhya R., Mathew Perez-Neut, Simon Kaja, and Saverio Gentile. "Voltage-gated ion channels in cancer cell proliferation." Cancers 7, no. 2 (2015): 849-875. [CrossRef]
- Kunzelmann, Karl. "Ion channels and cancer." The Journal of membrane biology 205 (2005): 159-173. [CrossRef]
- Prevarskaya, Natalia, Roman Skryma, and Yaroslav Shuba. "Ion channels in cancer: are cancer hallmarks oncochannelopathies?." Physiological reviews 98, no. 2 (2018): 559-621. [CrossRef]
- Lauder, Jean M. "Neurotransmitters as growth regulatory signals: role of receptors and second messengers." Trends in neurosciences 16, no. 6 (1993): 233-240. [CrossRef]
- Hnasko, Thomas S., and Robert H. Edwards. "Neurotransmitter corelease: mechanism and physiological role." Annual review of physiology 74, no. 1 (2012): 225-243. [CrossRef]
- Ständer, Sonja, and Thomas A. Luger. "Neuroreceptors and neuromediators." Pruritus (2010): 7-15. [CrossRef]
- Rahmann, Hinrich. "Calcium-ganglioside interactions and modulation of neuronal functions." Current Aspects of the Neurosciences: Volume 4 (1992): 87-125. [CrossRef]
- Brown, A. G., and A. G. Brown. "The Postsynaptic Neuron I: Actions of Neurotransmitters." Nerve Cells and Nervous Systems: An Introduction to Neuroscience (2001): 87-100. [CrossRef]
- Mahaut-Smith, Martyn P., Kirk A. Taylor, and Richard J. Evans. "Calcium Signalling through ligand-gated ion channels such as P2X1 receptors in the platelet and other non-excitable cells." Calcium Entry Pathways in Non-excitable Cells (2016): 305-329. [CrossRef]
- Moreddu, Rosalia. "Nanotechnology and Cancer Bioelectricity: Bridging the Gap Between Biology and Translational Medicine." Advanced Science 11, no. 1 (2024): 2304110. [CrossRef]
- Marino, Andrew A., Ilko G. Iliev, Michael A. Schwalke, Enrique Gonzalez, Kevin C. Marler, and Carol A. Flanagan. "Association between cell membrane potential and breast cancer." Tumor Biology 15, no. 2 (1994): 82-89. [CrossRef]
- Yang, Ming, and William J. Brackenbury. "Membrane potential and cancer progression." Frontiers in physiology 4 (2013): 185. [CrossRef]
- Dai, Jiapei. "The Continuous Relative Deficiency of Intracellular Potassium Is a Core Mechanism for the Occurrence and Metastasis of Tumor Cancer Cells." Natural Science 14, no. 11 (2022): 492-496. [CrossRef]
- Robinson, Andie J., Akhil Jain, Harry G. Sherman, Richard JM Hague, Ruman Rahman, Paola Sanjuan-Alberte, and Frankie J. Rawson. "Toward hijacking bioelectricity in cancer to develop new bioelectronic medicine." Advanced Therapeutics 4, no. 3 (2021): 2000248. [CrossRef]
- Sheth, Maulee, and Leyla Esfandiari. "Bioelectric dysregulation in cancer initiation, promotion, and progression." Frontiers in Oncology 12 (2022): 846917. [CrossRef]
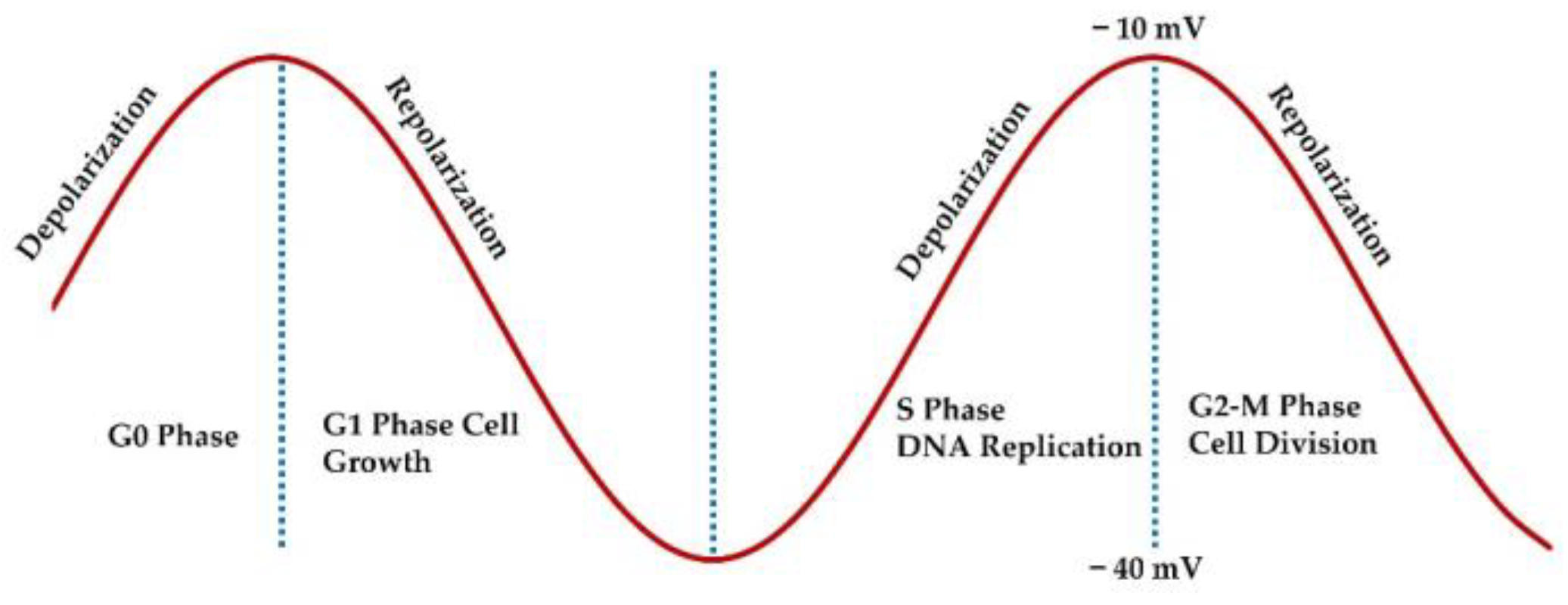
- Wang, Zhixiang. "Cell cycle progression and synchronization: an overview." Cell-Cycle Synchronization: Methods and Protocols (2022): 3-23. [CrossRef]
- Wang, Shiyi, Zaroui Melkoumian, Karen A. Woodfork, Carrie Cather, Ann G. Davidson, William F. Wonderlin, and Jeannine S. Strobl. "Evidence for an early G1 ionic event necessary for cell cycle progression and survival in the MCF-7 human breast carcinoma cell line." Journal of cellular physiology 176, no. 3 (1998): 456-464. [CrossRef]
- Guan, Xiangming. "Cancer metastases: challenges and opportunities." Acta pharmaceutica sinica B 5, no. 5 (2015): 402-418. [CrossRef]
- Fnu, Gulimirerouzi, and Georg F. Weber. "Alterations of ion homeostasis in cancer metastasis: Implications for treatment." Frontiers in Oncology 11 (2021): 765329. [CrossRef]
- Garbern, Jessica C., and Richard T. Lee. "Mitochondria and metabolic transitions in cardiomyocytes: lessons from development for stem cell-derived cardiomyocytes." Stem Cell Research & Therapy 12 (2021): 1-25. [CrossRef]
- Iorio, Jessica, Giulia Petroni, Claudia Duranti, and Elena Lastraioli. "Potassium and sodium channels and the Warburg effect: Biophysical regulation of cancer metabolism." Bioelectricity 1, no. 3 (2019): 188-200. [CrossRef]
- Wang, L., P. Zhou, R. W. Craig, and L. Lu. "Protection from cell death by mcl-1 is mediated by membrane hyperpolarization induced by K+ channel activation." The Journal of membrane biology 172 (1999): 113-120. [CrossRef]
- Tajbakhsh, Amir, Alireza Pasdar, Mehdi Rezaee, Mostafa Fazeli, Saman Soleimanpour, Seyed Mahdi Hassanian, Zahra FarshchiyanYazdi, Tayebe Younesi Rad, Gordon A. Ferns, and Amir Avan. "The current status and perspectives regarding the clinical implication of intracellular calcium in breast cancer." Journal of Cellular Physiology 233, no. 8 (2018): 5623-5641. [CrossRef]
- Quicke, Peter, Yilin Sun, Mar Arias-Garcia, Melina Beykou, Corey D. Acker, Mustafa BA Djamgoz, Chris Bakal, and Amanda J. Foust. "Voltage imaging reveals the dynamic electrical signatures of human breast cancer cells." Communications Biology 5, no. 1 (2022): 1178. [CrossRef]
- Berzingi, Seher, Mackenzie Newman, and Han-Gang Yu. "Altering bioelectricity on inhibition of human breast cancer cells." Cancer cell international 16 (2016): 1-9. [CrossRef]
- Stevens, Edward B., and Gary J. Stephens. "Ion channels as targets in drug discovery: outlook and perspectives." In Ion Channels as Targets in Drug Discovery, pp. 1-34. Cham: Springer International Publishing, 2024. [CrossRef]
- Dugas, Hermann, Christopher Penney, Hermann Dugas, and Christopher Penney. "Bioorganic chemistry of the amino acids." Bioorganic Chemistry: A Chemical Approach to Enzyme Action (1981): 13-92. [CrossRef]
- Akyuz, Enes, Ayse Kristina Polat, Ece Eroglu, Irem Kullu, Efthalia Angelopoulou, and Yam Nath Paudel. "Revisiting the role of neurotransmitters in epilepsy: An updated review." Life sciences 265 (2021): 118826. ttps://doi.org/10.1016/j.lfs.2020.118826.
- Malinak, David, Jan Korabecny, Ondrej Soukup, Lukas Gorecki, Eugenie Nepovimova, Miroslav Psotka, Rafael Dolezal et al. "A review of the synthesis of quaternary acetylcholinesterase reactivators." Current Organic Chemistry 22, no. 16 (2018): 1619-1648. [CrossRef]
- Berntson, Gary G., John T. Cacioppo, and Karen S. Quigley. "Respiratory sinus arrhythmia: Autonomic origins, physiological mechanisms, and psychophysiological implications." Psychophysiology 30, no. 2 (1993): 183-196. [CrossRef]
- Nantel, Francois, and Michel Bouvier. "Receptor regulation." In New Comprehensive Biochemistry, vol. 24, pp. 99-109. Elsevier, 1993.
- Lemoine, Damien, Ruotian Jiang, Antoine Taly, Thierry Chataigneau, Alexandre Specht, and Thomas Grutter. "Ligand-gated ion channels: new insights into neurological disorders and ligand recognition." Chemical reviews 112, no. 12 (2012): 6285-6318. [CrossRef]
- Mahapatra, Chitaranjan, Keith L. Brain, and Rohit Manchanda. "A biophysically constrained computational model of the action potential of mouse urinary bladder smooth muscle." PloS one 13, no. 7 (2018): e0200712. [CrossRef]
- Insel, Paul A., and Rennolds S. Ostrom. "Forskolin as a tool for examining adenylyl cyclase expression, regulation, and G protein signaling." Cellular and molecular neurobiology 23 (2003): 305-314. [CrossRef]
- Striessnig, Jörg, and Nadine J. Ortner. "Ca2+ channel blockers." In Encyclopedia of Molecular Pharmacology, pp. 375-383. Cham: Springer International Publishing, 2022. [CrossRef]
- Di Resta, Chiara, and Andrea Becchetti. "Introduction to ion channels." Integrins and Ion Channels: Molecular Complexes and Signaling (2010): 9-21.
- Ubuka, Takayoshi. "Noradrenaline/adrenaline." In Handbook of Hormones, pp. 1041-1044. Academic Press, 2021.
- Silva, Dany, Clara Quintas, Jorge Gonçalves, and Paula Fresco. "Contribution of adrenergic mechanisms for the stress-induced breast cancer carcinogenesis." Journal of Cellular Physiology 237, no. 4 (2022): 2107-2127. [CrossRef]
- Eng, Jason W-L., Chelsey B. Reed, Kathleen M. Kokolus, Rosemarie Pitoniak, Adam Utley, Mark J. Bucsek, Wen Wee Ma, Elizabeth A. Repasky, and Bonnie L. Hylander. "Housing temperature-induced stress drives therapeutic resistance in murine tumour models through β2-adrenergic receptor activation." Nature communications 6, no. 1 (2015): 6426. [CrossRef]
- quốc Lu’o’ng, Khanh vinh, and Lan Thi Hoàng Nguyễn. "The roles of beta-adrenergic receptors in tumorigenesis and the possible use of beta-adrenergic blockers for cancer treatment: possible genetic and cell-signaling mechanisms." Cancer management and research (2012): 431-445. [CrossRef]
- Strous, Ger J., and Julia A. Schantl. "β-arrestin and Mdm2, unsuspected partners in signaling from the cell surface." Science's STKE 2001, no. 110 (2001): pe41-pe41. [CrossRef]
- Yan, Man, Minying Zheng, Rui Niu, Xiaohui Yang, Shifeng Tian, Linlin Fan, Yuwei Li, and Shiwu Zhang. "Roles of tumor-associated neutrophils in tumor metastasis and its clinical applications." Frontiers in Cell and Developmental Biology 10 (2022): 938289. [CrossRef]
- Conceição, Francisco, Daniela M. Sousa, Joana Paredes, and Meriem Lamghari. "Sympathetic activity in breast cancer and metastasis: partners in crime." Bone research 9, no. 1 (2021): 9. [CrossRef]
- Alicia Luthy, Isabel, Ariana Bruzzone, and Cecilia Perez Pinero. "Adrenergic action in breast cancer." Current Cancer Therapy Reviews 8, no. 2 (2012): 90-99. [CrossRef]
- Jayachandran, Priya, Francesca Battaglin, Carly Strelez, Annika Lenz, Sandra Algaze, Shivani Soni, Jae Ho Lo et al. "Breast cancer and neurotransmitters: emerging insights on mechanisms and therapeutic directions." Oncogene 42, no. 9 (2023): 627-637. [CrossRef]
- Johnston, Stephen RD. "The role of chemotherapy and targeted agents in patients with metastatic breast cancer." European journal of cancer 47 (2011): S38-S47. [CrossRef]
- Pantziarka, Pan, Gauthier Bouche, Vidula Sukhatme, Lydie Meheus, Ilse Rooman, and Vikas P. Sukhatme. "Repurposing Drugs in Oncology (ReDO)—Propranolol as an anti-cancer agent." ecancermedicalscience 10 (2016).
- Liu, D., Z. Yang, T. Wang, H. Chen, Y. Hu, C. Hu, L. Guo et al. "β2-AR signaling controls trastuzumab resistance-dependent pathway." Oncogene 35, no. 1 (2016): 47-58. [CrossRef]
- Xia, M., N. N. Ji, M. L. Duan, J. H. Tong, J. G. Xu, Y. M. Zhang, and S. H. Wang. "Dexmedetomidine regulate the malignancy of breast cancer cells by activating α2-adrenoceptor/ERK signaling pathway." Eur Rev Med Pharmacol Sci 20, no. 16 (2016): 3500-3506.
- Kim, Myoung H., Ju E. Oh, Seho Park, Joo H. Kim, Ki Y. Lee, Sun J. Bai, Hyunjik Song, Hye J. Hwang, Dong W. Kim, and Young C. Yoo. "Tramadol use is associated with enhanced postoperative outcomes in breast cancer patients: a retrospective clinical study with in vitro confirmation." British journal of anaesthesia 123, no. 6 (2019): 865-876. [CrossRef]
- Resende, Rodrigo R., and Avishek Adhikari. "Cholinergic receptor pathways involved in apoptosis, cell proliferation and neuronal differentiation." Cell Communication and Signaling 7 (2009): 1-20. [CrossRef]
- Bertrand, Daniel, and Tanya L. Wallace. "A review of the cholinergic system and therapeutic approaches to treat brain disorders." Behavioral Pharmacology of the Cholinergic System (2020): 1-28. [CrossRef]
- Khodabandeh, Zhila, Mohammad Valilo, Kobra Velaei, and Abbas Pirpour Tazehkand. "The potential role of nicotine in breast cancer initiation, development, angiogenesis, invasion, metastasis, and resistance to therapy." Breast Cancer 29, no. 5 (2022): 778-789.
- Ochirbat, Sonjid, Tzu-Chun Kan, Chun-Chun Hsu, Tzu-Hsuan Huang, Kuo-Hsiang Chuang, Michael Chen, Chun-Chia Cheng, Chun-Chao Chang, Sri Rahayu, and Jungshan Chang. "The angiogenic role of the alpha 9-nicotinic acetylcholine receptor in triple-negative breast cancers." Angiogenesis 27, no. 4 (2024): 827-843. [CrossRef]
- Español, Alejandro, Agustina Salem, Yamila Sanchez, and María Elena Sales. "Breast cancer: Muscarinic receptors as new targets for tumor therapy." World Journal of Clinical Oncology 12, no. 6 (2021): 404. [CrossRef]
- Stull, Malinda A., Vaibhav Pai, Archie J. Vomachka, Aaron M. Marshall, George A. Jacob, and Nelson D. Horseman. "Mammary gland homeostasis employs serotonergic regulation of epithelial tight junctions." Proceedings of the National Academy of Sciences 104, no. 42 (2007): 16708-16713. [CrossRef]
- Olfati, Zahra, Garshasb Rigi, Hajar Vaseghi, Zahra Zamanzadeh, Mojtaba Sohrabi, and Seyed Hesamaldin Hejazi. "Evaluation of serotonin receptors (5HTR2A and 5HTR3A) mRNA expression changes in tumor of breast cancer patients." Medical Journal of the Islamic Republic of Iran 34 (2020): 99. [CrossRef]
- Sarrouilhe, Denis, Jonathan Clarhaut, Norah Defamie, and Marc Mesnil. "Serotonin and cancer: what is the link?." Current molecular medicine 15, no. 1 (2015): 62-77. [CrossRef]
- Bala Bhaskar, S., and M. Manjuladevi. "Drugs, Fluids and Cancer." Textbook of Onco-Anesthesiology (2021): 103-116.
- Chen, Lulu, Shuting Huang, Xiaoxue Wu, Weiling He, and Mei Song. "Serotonin signalling in cancer: Emerging mechanisms and therapeutic opportunities." Clinical and Translational Medicine 14, no. 7 (2024): e1750. [CrossRef]
- Antoszczak, Michał, Anna Markowska, Janina Markowska, and Adam Huczyński. "Antidepressants and antipsychotic agents as repurposable oncological drug candidates." Current medicinal chemistry 28, no. 11 (2021): 2137-2174. [CrossRef]
- Liby, Karen, Bonnie Neltner, Lisa Mohamet, Lindsey Menchen, and Nira Ben-Jonathan. "Prolactin overexpression by MDA-MB-435 human breast cancer cells accelerates tumor growth." Breast cancer research and treatment 79 (2003): 241-252. [CrossRef]
- Ben-Jonathan, Nira, and Robert Hnasko. "Dopamine as a prolactin (PRL) inhibitor." Endocrine reviews 22, no. 6 (2001): 724-763. [CrossRef]
- Jiang, Shu-Heng, Li-Peng Hu, Xu Wang, Jun Li, and Zhi-Gang Zhang. "Neurotransmitters: emerging targets in cancer." Oncogene 39, no. 3 (2020): 503-515. [CrossRef]
- Souteiro, P., and N. Karavitaki. "Dopamine agonist resistant prolactinomas: any alternative medical treatment?." Pituitary 23, no. 1 (2020): 27-37. [CrossRef]
- Feng, Zhanzhan, Yong Xia, Tiantao Gao, Fuyan Xu, Qian Lei, Cuiting Peng, Yufei Yang et al. "The antipsychotic agent trifluoperazine hydrochloride suppresses triple-negative breast cancer tumor growth and brain metastasis by inducing G0/G1 arrest and apoptosis." Cell death & disease 9, no. 10 (2018): 1006.
- Guo, Shenghao, Yanni Gu, Jiayin Qu, and Anne Le. "Bridging the metabolic parallels between neurological diseases and cancer." The Heterogeneity of Cancer Metabolism (2021): 229.
- Gumireddy, Kiranmai, Anping Li, Andrew V. Kossenkov, Masayuki Sakurai, Jinchun Yan, Yan Li, Hua Xu et al. "The mRNA-edited form of GABRA3 suppresses GABRA3-mediated Akt activation and breast cancer metastasis." Nature communications 7, no. 1 (2016): 10715.
- Carnero, Amancio, and Jesus M. Paramio. "The PTEN/PI3K/AKT pathway in vivo, cancer mouse models." Frontiers in oncology 4 (2014): 252.
- Garib, V., K. Lang, B. Niggemann, K. S. Zänker, L. Brandt, and T. Dittmar. "Propofol-induced calcium signalling and actin reorganization within breast carcinoma cells." European journal of anaesthesiology 22, no. 8 (2005): 609-615. [CrossRef]
- Hardwick, Matthew, Djamil Fertikh, Martine Culty, Hua Li, Branislav Vidic, and Vassilios Papadopoulos. "Peripheral-type benzodiazepine receptor (PBR) in human breast cancer: correlation of breast cancer cell aggressive phenotype with PBR expression, nuclear localization, and PBR-mediated cell proliferation and nuclear transport of cholesterol." Cancer research 59, no. 4 (1999): 831-842.
- Marwah, Harneet, Janmejay Pant, Jiten Yadav, Kamal Shah, and Hitesh K. Dewangan. "Biosensor Detection of COVID-19 in Lung Cancer: Hedgehog and Mucin Signaling Insights." Current Pharmaceutical Design 29, no. 43 (2023): 3442-3457. [CrossRef]
- Liberati, Sonia, Maria Beatrice Morelli, Massimo Nabissi, Matteo Santoni, and Giorgio Santoni. "Oncogenic and anti-oncogenic effects of transient receptor potential channels." Current topics in medicinal chemistry 13, no. 3 (2013): 344-366. [CrossRef]
- Medina, Vanina, Máximo Croci, Ernesto Crescenti, Nora Mohamad, Francisca Sanchez-Jiménez, Noelia Massari, Mariel Nuñez et al. "The role of histamine in human mammary carcinogenesis: H3 and H4 receptors as potential therapeutic targets for breast cancer treatment." Cancer biology & therapy 7, no. 1 (2008): 28-35. [CrossRef]
- Sterle, Helena A., Melisa B. Nicoud, Noelia A. Massari, Monica A. Taquez Delgado, María V. Herrero Ducloux, Graciela A. Cremaschi, and Vanina A. Medina. "Immunomodulatory role of histamine H4 receptor in breast cancer." British journal of cancer 120, no. 1 (2019): 128-138. [CrossRef]
- Wang, Ling, Chao Yang, Xin-bo Liu, Li Wang, and Fu-biao Kang. "B7-H4 overexpression contributes to poor prognosis and drug-resistance in triple-negative breast cancer." Cancer Cell International 18 (2018): 1-12. [CrossRef]
- Ospital, Ignacio A., Mónica A. Táquez Delgado, Melisa B. Nicoud, Michelle F. Corrêa, Gustavo A. Borges Fernandes, Isabela W. Andrade, Paolo Lauretta et al. "Therapeutic potential of LINS01 histamine H3 receptor antagonists as antineoplastic agents for triple negative breast cancer." Biomedicine & Pharmacotherapy 174 (2024): 116527. [CrossRef]
- Souazé, Frédérique, Sandra Dupouy, Véronique Viardot-Foucault, Erik Bruyneel, Samir Attoub, Christian Gespach, Anne Gompel, and Patricia Forgez. "Expression of neurotensin and NT1 receptor in human breast cancer: a potential role in tumor progression." Cancer research 66, no. 12 (2006): 6243-6249. [CrossRef]
- Wang, Xiaoyu, Jia Xu, and Qinglin Kang. "Neuromodulation of bone: Role of different peptides and their interactions." Molecular Medicine Reports 23, no. 1 (2021): 1-1. [CrossRef]
- Pouya, F. Danesh, Y. Rasmi, and E. Roshani Asl. "Role of neurotransmitters and neuropeptides in breast cancer metastasis." Biochemistry (Moscow), Supplement Series A: Membrane and Cell Biology 14 (2020): 107-116. [CrossRef]
- Tariq, Muhammad, Jieqiong Zhang, Guikai Liang, Ling Ding, Qiaojun He, and Bo Yang. "Macrophage polarization: anti-cancer strategies to target tumor-associated macrophage in breast cancer." Journal of cellular biochemistry 118, no. 9 (2017): 2484-2501. [CrossRef]
- Arnoux, Alizée, and Luc Dupuis. "Serotonin in Amyotrophic and the Lateral 5-HT2B Sclerosis Receptor." 5-HT2B Receptors: From Molecular Biology to Clinical Applications 35 (2021): 367.
- Joffe, Hadine, Ann Partridge, Anita Giobbie-Hurder, Xiaochun Li, Karleen Habin, Paul Goss, Eric Winer, and Judy Garber. "Augmentation of venlafaxine and selective serotonin reuptake inhibitors with zolpidem improves sleep and quality of life in breast cancer patients with hot flashes: a randomized, double-blind, placebo-controlled trial." Menopause 17, no. 5 (2010): 908-916.
- Przybylo, Magda, Tomasz Borowik, and Marek Langner. "Fluorescence techniques for determination of the membrane potentials in high throughput screening." Journal of fluorescence 20, no. 6 (2010): 1139-1157. [CrossRef]
- Tetzlaff, Svenja K., Ekin Reyhan, Nikolas Layer, C. Peter Bengtson, Alina Heuer, Julian Schroers, Anton J. Faymonville et al. "Characterizing and targeting glioblastoma neuron-tumor networks with retrograde tracing." Cell (2024).
- Yin, Ting, Jingsi Duan, Dong Xu, Mengying Huang, and Deling Yin. "Precision individualized medication strategies and challenges for cardiovascular diseases." Precision Medication 1, no. 1 (2024): 7-15. [CrossRef]
- Velikic, Gordana, Dusan M. Maric, Dusica L. Maric, Gordana Supic, Miljan Puletic, Oliver Dulic, and Danilo Vojvodic. "Harnessing the stem cell niche in regenerative medicine: innovative avenue to combat neurodegenerative diseases." International journal of molecular sciences 25, no. 2 (2024): 993. [CrossRef]
- de Oliveira El-Warrak, Leonardo, and Claudio Miceli de Farias. "Could digital twins be the next revolution in healthcare?." European Journal of Public Health (2024): ckae191. [CrossRef]
- Tilan, Jason, and Joanna Kitlinska. "Sympathetic neurotransmitters and tumor angiogenesis—link between stress and cancer progression." Journal of oncology 2010, no. 1 (2010): 539706. [CrossRef]
- Kuol, Nyanbol, Lily Stojanovska, Vasso Apostolopoulos, and Kulmira Nurgali. "Role of the nervous system in cancer metastasis." Journal of Experimental & Clinical Cancer Research 37 (2018): 1-12.
- Li, Ruo Qi, Xiao Hong Zhao, Qin Zhu, Tao Liu, Hubert Hondermarck, Rick F. Thorne, Xu Dong Zhang, and Jin Nan Gao. "Exploring neurotransmitters and their receptors for breast cancer prevention and treatment." Theranostics 13, no. 3 (2023): 1109. [CrossRef]
- Singh, Tashvinder, Kangan Sharma, Laxmipriya Jena, Prabhsimran Kaur, Sandeep Singh, and Anjana Munshi. "Mitochondrial bioenergetics of breast cancer." Mitochondrion (2024): 101951. [CrossRef]
| Neuroreceptor | Associated Neurotransmitters | Expression in Breast Cancer | Functional Role in Breast Cancer | Impact on Membrane Potential | References |
|---|---|---|---|---|---|
| ß-adrenergic receptors | Norepinephrine and epinephrine | Overexpressed in breast cancer tissues | Promotes proliferation, migration, invasion, angiogenesis, and anti-apoptosis | Depolarization | [48,49,50] |
| Nicotinic (nAChRs) and Muscarinic (mAChRs) receptors | Acetylcholine | α9-nAChR and α7-nAChR are highly expressed in triple-negative and advanced breast tumors mAChRs are upregulated in breast tumors but absent in normal breast tissues |
nAChRs enhance epithelial-to-mesenchymal transition, invasion, migration, and stemness mAChRs inhibit tumor growth and promote anti-proliferative effects |
Depolarization or Repolarization | [63,64,65] |
| 5-HT receptors | Serotonin | 5HTR2A and 5HTR3A are overexpressed in breast cancer tissues | Facilitates angiogenesis, proliferation, invasion, and autophagy | Depolarization | [67,68] |
| Dopamine receptors | Dopamine | Variable expression; some subtypes linked to tumor suppression while others promote progression | Modulates proliferation, invasion, and angiogenesis; influences prolactin secretion |
Depolarization | [74,76] |
| GABA receptors | GABA | GABAA receptor α3 is overexpressed in breast cancer, particularly in invasive and metastatic cases | Promotes proliferation, migration, invasion, and activation of the AKT pathway | Repolarization | [78,79] |
| Histamine H4 Receptor | Histamine | High expression correlates with better prognosis in triple-negative breast cancer | Reduces tumor growth, enhances apoptosis, and improves survival | Depolarization or Repolarization | [84,85] |
| Neurotensin Receptor (NTS-1) | Neurotensin | Overexpressed in approximately one-third of primary breast cancers | Promotes proliferation, invasion, migration, and resistance to apoptosis | Depolarization | [88,90] |
| Neuropeptide Y (NPY) Receptors | Neuropeptide Y | Overexpressed in metastatic breast cancer tissues | Stimulates angiogenesis, proliferation, and metastasis | Depolarization | [89,90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





