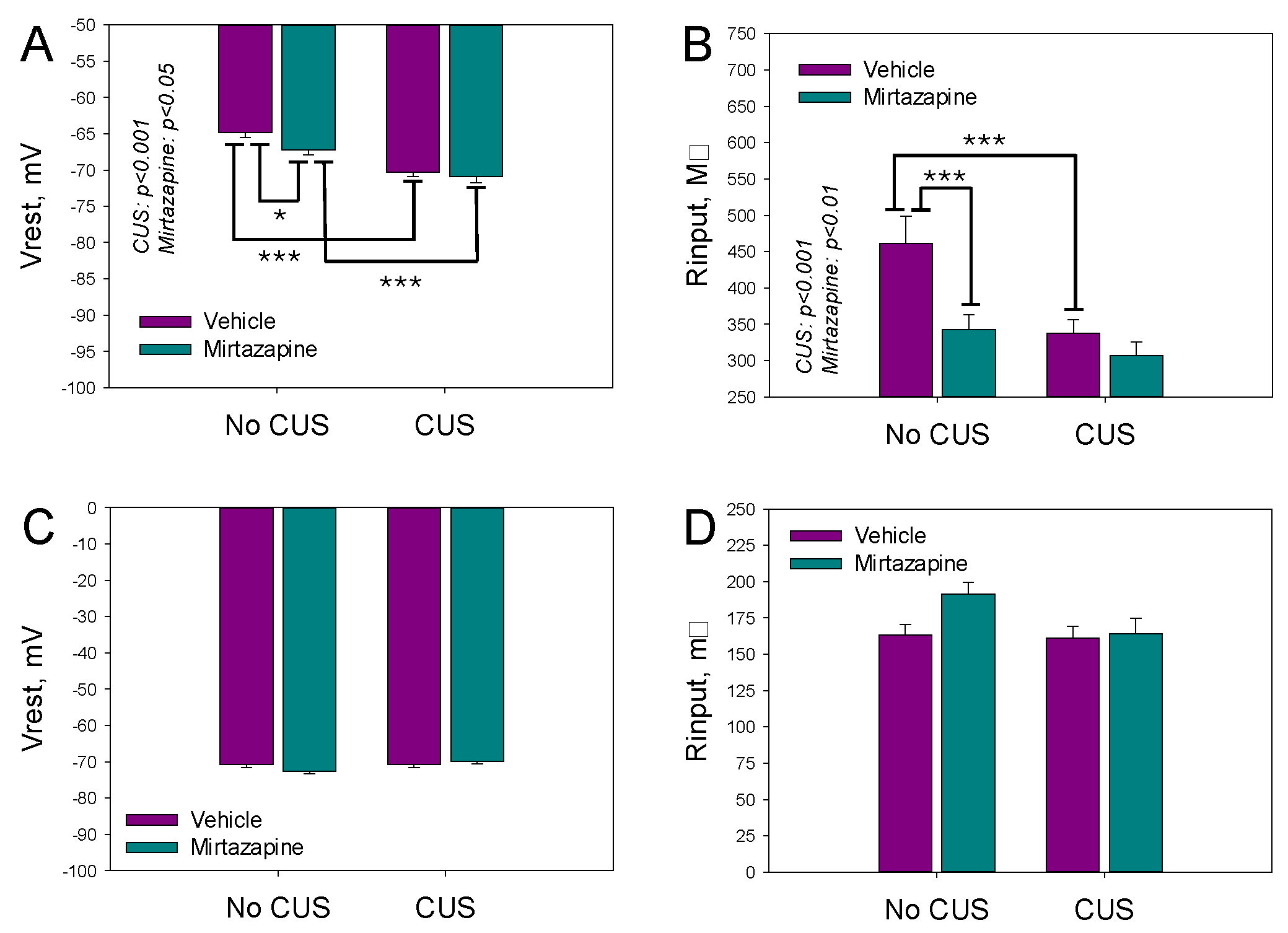1. Introduction
Major depression is a common disease that limits psychosocial functioning and quality of life. According to the WHO, 3.8% of the population suffers from depression, and depression is approximately 50% more common among women than among men. The higher depression incidence in women may be related to ovarian and other hormone fluctuations [
1]. The WHO ranks depressive disorder as the third most common disease worldwide, and by 2030, it is predicted to rank first [
2].
Maternal depression is a general term referring to a spectrum of depressive conditions that typically occur during pregnancy and up to 12 months postpartum [
3]. It is also a critical period of fetal and child development, and maternal disorders can have far-reaching impacts on offspring [
4,
5]. Maternal depression is a known risk factor for preterm birth, low birth weight, and intrauterine growth restriction [
6,
7]. These complications are reported to be the leading cause of death in children under five years of age [
8,
9].
Many studies have examined the effects of prenatal maternal stress, anxiety, and depression on child development, impacting cognitive, behavioral, social, mood, language, and attachment outcomes [
10,
11,
12]. Children of depressed mothers also face higher rates of cognitive, social, and mood disorders later in life [
13,
14]. Distinguishing the effects of untreated prenatal depression from those of other factors is challenging. Animal models, with their controlled environments, help us better understand the adverse effects of maternal stress and depression on offspring development.
The most frequently used antidepressant drugs are the second-generation antidepressants such as selective serotonin (5-HT) reuptake inhibitors (SSRIs) [
15,
16]. However, concerns have been raised about their use during pregnancy and the postpartum period because SSRIs easily cross the placenta and are present in breast milk, and fetuses and infants may have difficulty metabolizing SSRIs efficiently [
17,
18,
19]. SSRIs may inadvertently affect serotonin signaling in offspring, leading to doubts about whether prescribing SSRIs to pregnant and postpartum women is safe for offspring. Several studies suggest that prenatal SSRI exposure has developmental consequences (enhancement of anxiety states) in both rats and human offspring [
20,
21].
Mirtazapine, a third-generation antidepressant, according to a recent meta-study, belongs to a small group of the most effective antidepressants [
22] and is recommended for use during pregnancy. Mirtazapine is an antagonist of α
2-adrenergic inhibitory, 5-HT
2, and 5-HT
3 receptors [
23]. However, insufficient data are available to support its safe use during pregnancy [
24].
In this study, we focused on the possible effects of maternal depression and its pharmacological treatment during gestation on the neuronal development and behavior of offspring in an early stage of postnatal development (up to 2 weeks). As it is involved in learning memory, cognitive function, and emotion regulation, the hippocampus is among the key brain regions affected by the pathogenesis of depression [
25,
26,
27]. Therefore, we focused on possible alterations in hippocampal excitability.
As an animal model of maternal depression, we have used pregestational exposure to chronic unpredictable stress (CUS) [
19,
28,
29]. We had previously reported that the CUS dams showed behavioral features resembling the symptoms of depression, as well as impaired hippocampal neurogenesis. Furthermore, we have shown that the behavioral and neuroanatomical abnormalities in the CUS dams were reversed by chronic antidepressant treatment [
30]. CUS dams therefore possess constructive and predictive validity as a model of prenatal depression. In this work we tested whether the previously observed alterations are reflected in altered hippocampal excitability. We compared the hippocampal excitability of offspring from 4 experimental groups: i) dams not exposed to CUS and treated with vehicle; ii) dams not exposed to CUS and treated with mirtazapine; iii) dams exposed to CUS and treated with vehicle; and iv) dams exposed to CUS and treated with mirtazapine.
2. Results
2.1. Effects of Pregestational Stress and Prenatal Mirtazapine on the Electrophysiological Characteristics of Cultivated Pyramidal Neurons Isolated from Newborn Offspring Hippocampi
The resting membrane potentials (Vrest) and input resistance (Rinp) reflect the expression of voltsge gated ion channels in respective cells. Vrest and Rinp of the cultivated neurons isolated from the hippocampi of the newborn offspring of the dams exposed to pregestational CUS, prenatal mirtazapine treatment, or their combination are shown in Figures 1A. Two-way ANOVA revealed a significant effect of CUS and perinatal mirtazapine on Vrest (F1,280 = 38.85, p < 0.001, and F1,280 = 4.04, p = 0.045, respectively) and Rinp (F1,279 = 11.57, p < 0.001, and F1,279 = 10.02, p = 0.002, respectively). The interaction between these factors for Vrest and Rinp was not statistically significant.
Observed differences were diminished during 13-13 days of postnatal development
; as shown in
Figure 1C and 1D. According to the two-way ANOVA results, maternal stress and mirtazapine treatment did not significantly affect Vrest or Rinp. The interactions between these factors were also not statistically significant
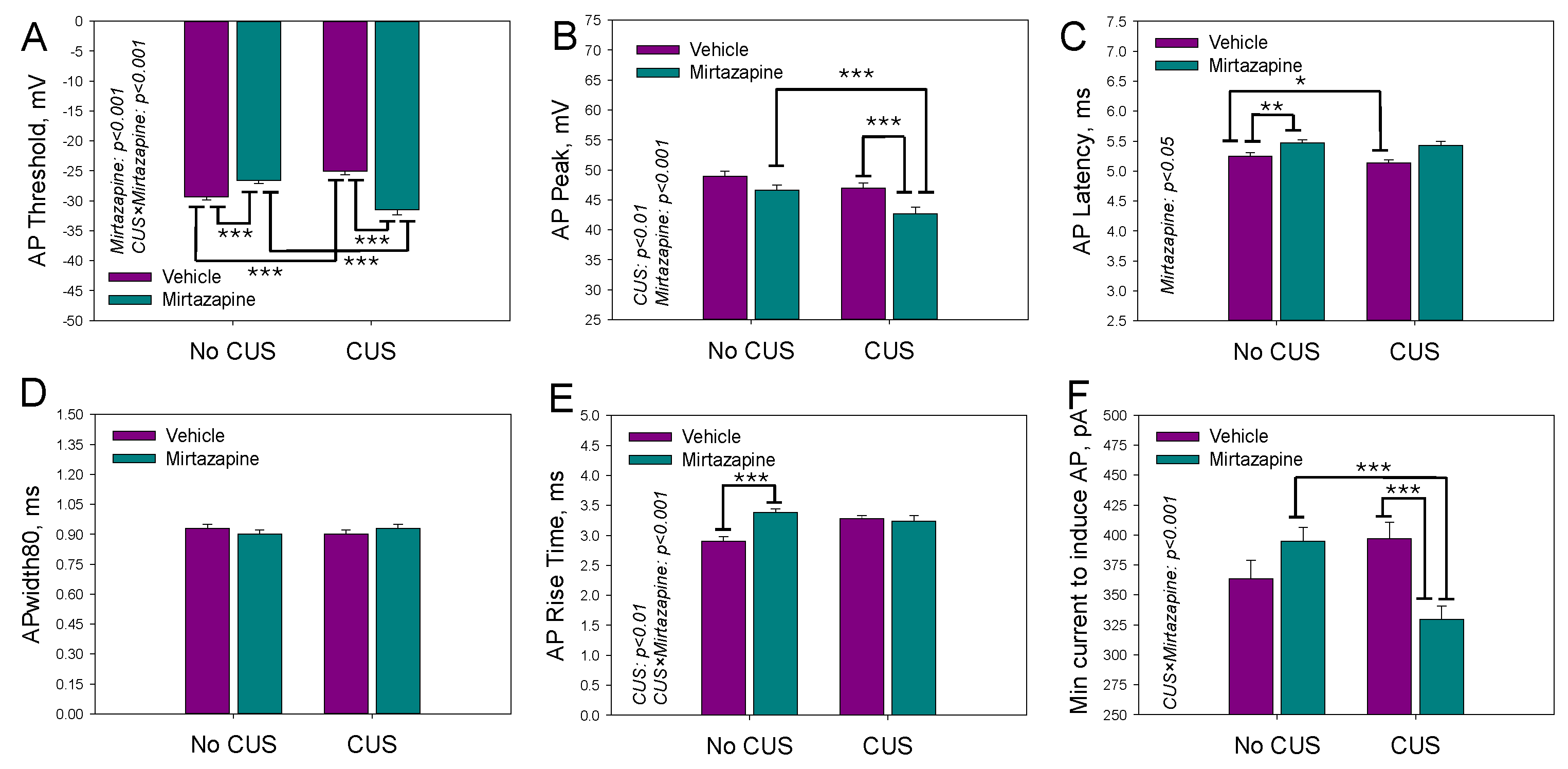
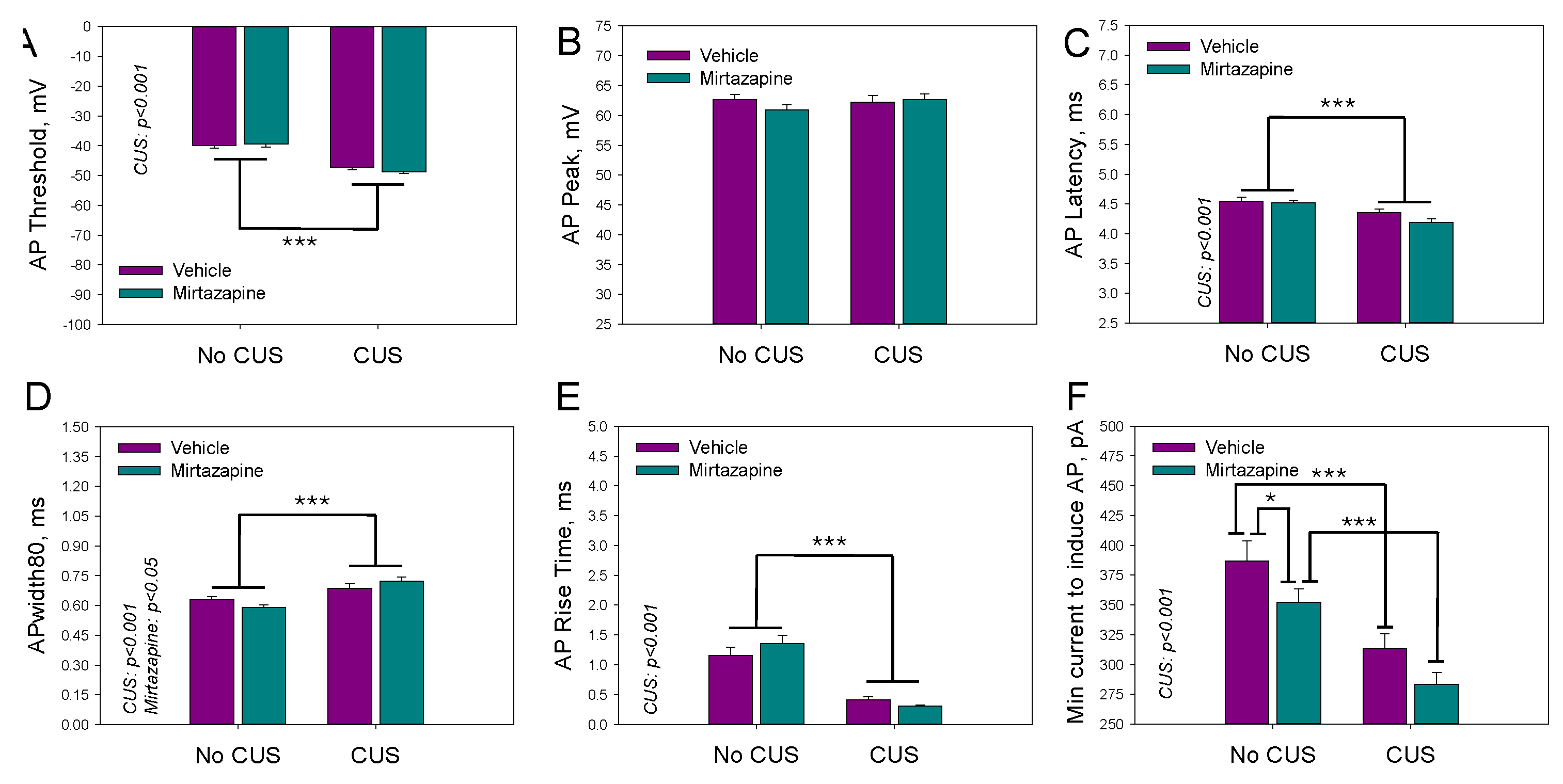
The waveform of a single action potential (AP) activated by a brief depolarizing current pulse was characterized by the threshold (
Figure 2A) and peak (
Figure 2B) voltages, latency time (
Figure 2C), width of action potential (
Figure 2D), rise time (
Figure 2E), and minimal current needed to evoke a single action potential (
Figure 2F). CUS depressed excitability by increasing voltage threshold for AP generation. Treatmen by mirtazapine facilitated it by decreasing both voltage threshold and minimal current amplitude needed for an activation of an AP.
For the monitored parameters, a significant effect of CUS on the peak voltage of the AP (F1,278 = 9.35 p = 0.002; two-way ANOVA) and its rise time (F1,278 = 9.67, p < 0.002; two-way ANOVA) was detected; a significant effect of maternal mirtazapine treatment on the threshold (F1,278 = 7.99, p = 0.005; two-way ANOVA) and peak (F1,278 = 11.28, p < 0.001; two-way ANOVA) voltages and latency times (F1,278 = 4.13, p = 0.04; two-way ANOVA); and a significant interaction between these factors concerning the voltage threshold (F1,278 = 49.59, p < 0.001; two-way ANOVA), rise time (F1,278 = 13.27, p < 0.001; two-way ANOVA), and the minimal current needed to induce a single action potential (F1,278 = 14.23, p < 0.001; two-way ANOVA).
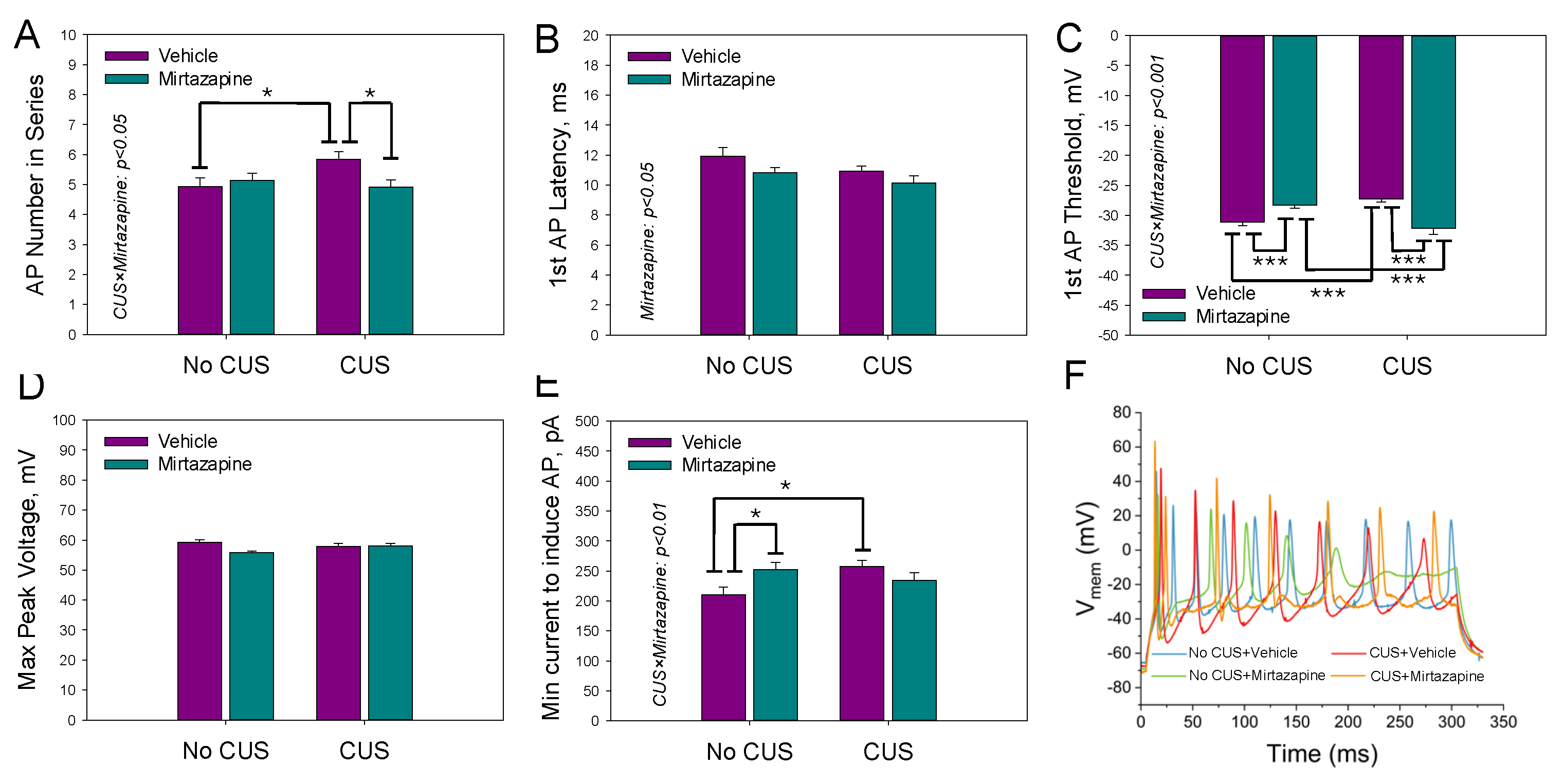
The parameters characterizing the AP series (3 or more APs generated during a depolarizing current pulse) activated by the second suprathreshold current pulse were analyzed in the same experimental groups are shown in
Figure 3.
Two-way ANOVA revealed a statistically significant effect of maternal mirtazapine on the first AP latency time (F
1,225 = 4.93, p = 0.03;
Figure 3B) and a significant interaction between maternal stress and mirtazapine with respect to the number of APs within the series (F
1,225 = 4.46, p = 0.04;
Figure 3A), first AP threshold voltage (F
1,225 = 33.65, p < 0.001;
Figure 3C), and minimal current required to induce the series (F
1,225 = 7.52, p = 0.007;
Figure 3E). Again, CUS suppressed excitability by increasing both voltage threshold for generation of the first AP and minimal current amplitude needed for the generation of the first Ap series. Mirtazapine rectified both changes.
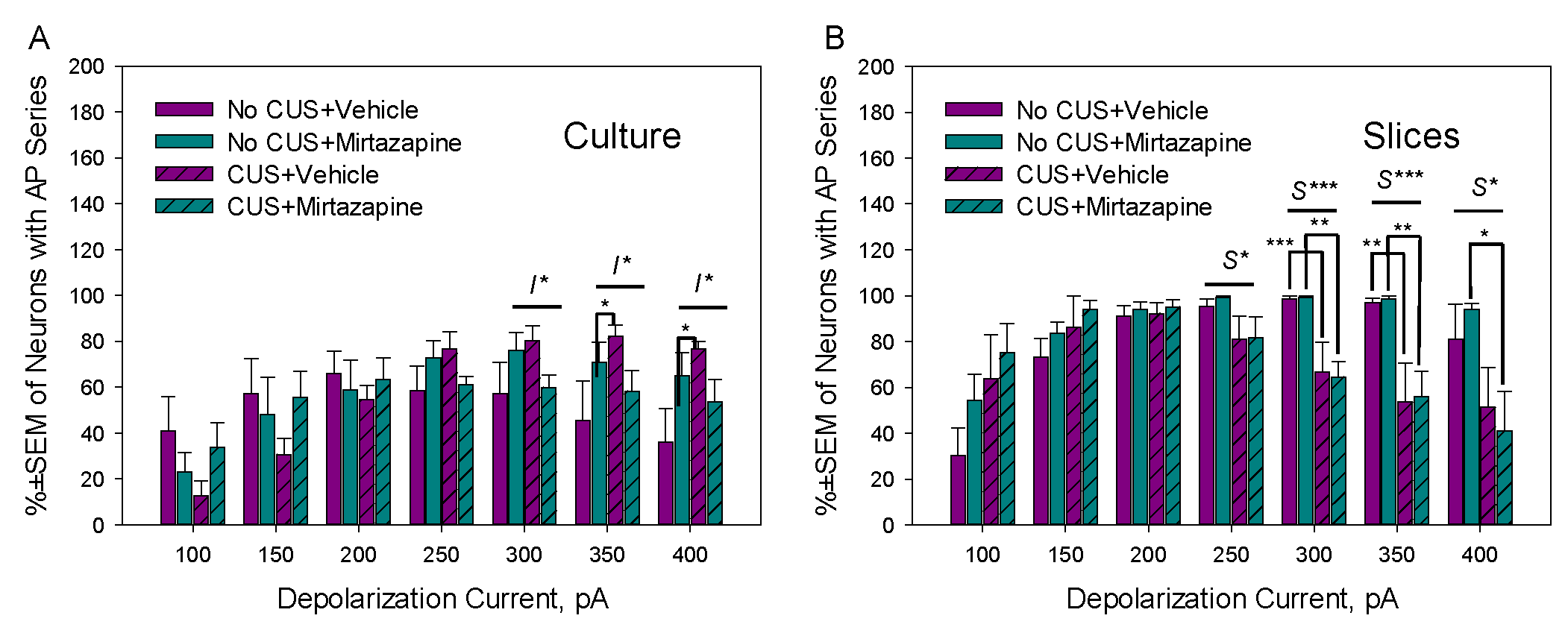
Figure 4 illustrates the effects of maternal stress, mirtazapine, and their combination on the percentage of neurons in primary cultures with AP series observed during depolarizing current pulses with amplitudes between 100 and 400 pA. A significant interaction effect between maternal stress and mirtazapine was detected during depolarizing pulses of 300 (F
1,19 = 4.77, p = 0.04), 350 (F
1,19 = 5.14, p = 0.04), and 400 pA (F
1,19 = 6.44, p = 0.02). CUS increased excitability at higher depolarizing pulses and mirtazapine treatment suppressed this effect.
2.3. Effects of Pregestational Stress and Prenatal Mirtazapine on the Electrophysiological Characteristics of Pyramidal Neurons in Hippocampal Slices Isolated from 11–13-Day-Old Offspring
Individual AP parameters of the pyramidal neurons in the slices isolated from the hippocampi of 11–13-day-old offspring of the dams that experienced pregestational stress, prenatal mirtazapine treatment, or their combination are shown in
Figure 5.
Two-way ANOVA revealed a significant effect of maternal stress on the threshold voltage (F1,189 = 89.92, p < 0.001), latency time (F1,189 = 17.75, p < 0.001), width of action potential (F1,189 = 28.15, p < 0.001), rise time (F1,189 = 59.21, p < 0.001) and minimal current needed to induce a series of action potentials (F1,189 = 28.05, p < 0.001). With respect to the width of the action potential, there was also a significant interaction effect between maternal stress and mirtazapine treatment (F1,189 = 4.53, p = 0.04).
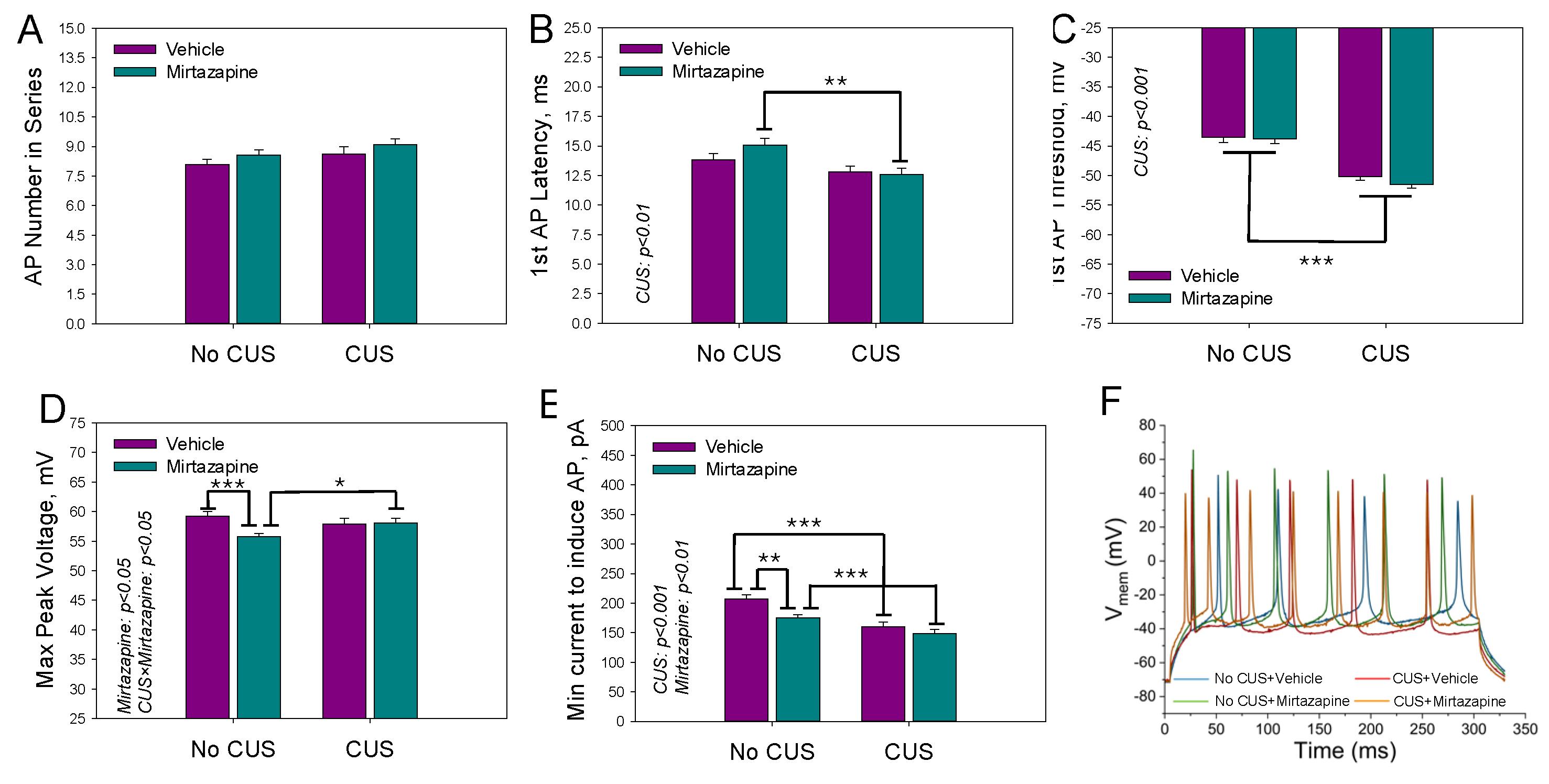
Figure 6 shows the mean number of action potentials within the depolarization current-induced series (A), latency time (B) and threshold voltage (C) of the first AP, maximal peak voltage within the series of APs (D), and minimal current needed to induce the series (E).
We observed a significant effect of maternal stress on the latency time (F1,195 = 9.73, p < 0.002; two-way ANOVA) and threshold voltage (F1,195 = 79.40, p < 0.001; two-way ANOVA) of the first AP and the minimal current needed to induce the series (F1,195 = 25.42, p < 0.001; two-way ANOVA), a significant effect of maternal mirtazapine on the peak voltage within the series of APs (F1,195 = 4.49, p < 0.04; two-way ANOVA) and the minimal current needed to induce the series (F1,195 = 9.06, p < 0.003; two-way ANOVA), and significant interactions between these factors with respect to the peak voltage (F1,195 = 5.47, p < 0.02; two-way ANOVA).
The effects of maternal stress, mirtazapine, and their combination on the percentage of neurons in hippocampal slices with AP series observed during depolarizing current pulses with amplitudes between 100 and 400 pA are shown above on the
Figure 4B. A significant effect of maternal stress was detected during depolarizing pulses of 250 (F
1,18 = 5.79, p = 0.03), 300 (F
1,18 = 26.30, p < 0.001), 350 (F
1,18 = 21.86, p < 0.001), and 400 pA (F
1,18 = 8.53, p = 0.01).
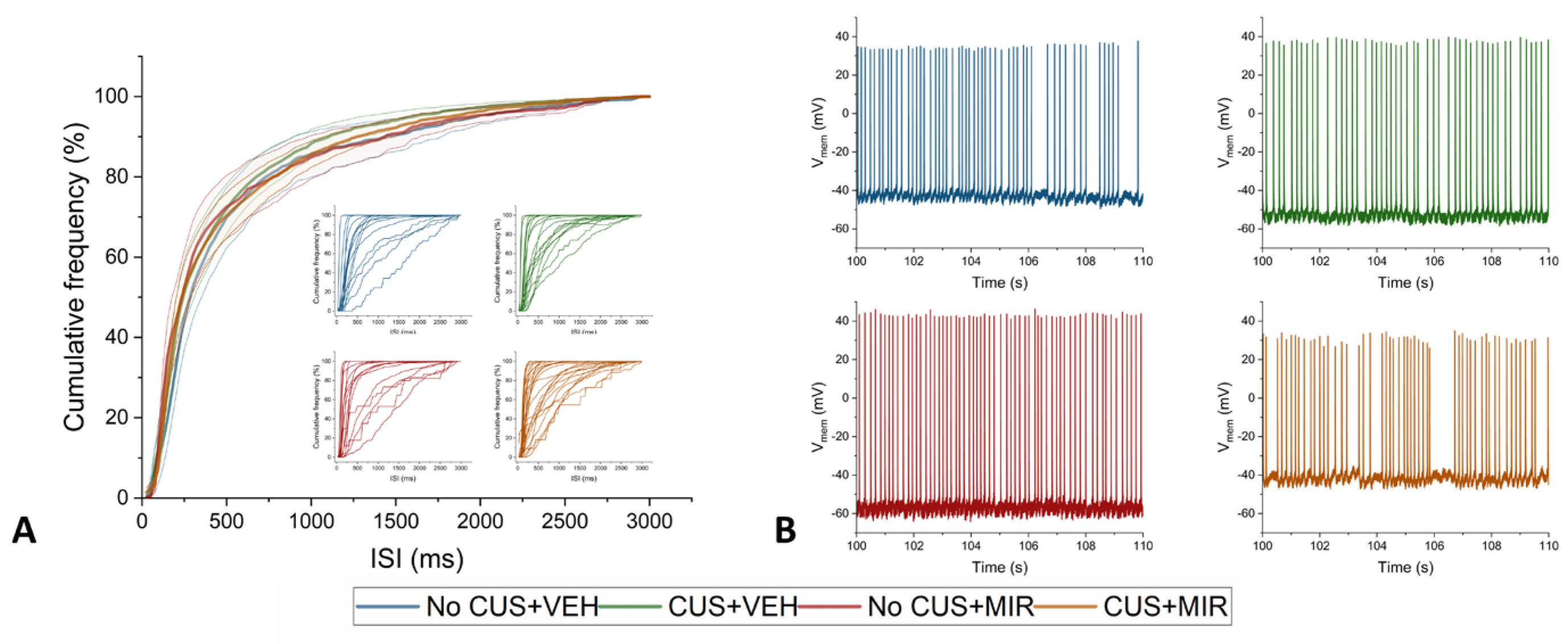
2.4. Spontaneous Activity of Pyramidal Neurons in Hippocampal Slices Isolated from 11–13-Day-Old Offspring
The spontaneous activity of hippocampal neurons was measured only in the slices. On PNDs 11-13, of all the measured cells, 25% of the neurons in the offspring of nonstressed vehicle-treated dams, 22% of the neurons in the nonstressed mirtazapine-treated group, 35% of the neurons in the CUS-exposed vehicle-treated group, and up to 54% of the neurons in the CUS-exposed mirtazapine-treated group manifested spontaneous activity. Cumulative histograms of the frequency of interspike intervals were compiled from the obtained data, but no significant changes between individual groups were detected (
Figure 7). The parameters of spontaneous activity, i.e., the average number of fired action potentials, the mean instantaneous frequency, and the firing frequency of action potentials, were evaluated, and no significant differences were found.
3. Discussion
The effects of depression on offspring development and how antidepressant treatment during pregnancy influences such changes are still open questions. Animal models offer the possibility to precisely control the pregestational and gestational states of the mother and pharmacotherapy. The offspring of treated mothers can be monitored under defined conditions for a longer period of time. Moreover, it is possible to combine behavioral, molecular, and electrophysiological analyses in the same settings [
29].
In this study, we used a well-established pregestational chronic unpredictable stress (CUS) rat model of maternal depression [
23,
31]. As a tested antidepressant, a nonselective alpha 2-adrenoceptor antagonist, mirtazapine, which has antidepressant activity in major depression, was used. In this model, we recently reported behavioral changes in offspring at juvenile and adolescent ages [
23]. Here, we analyzed possible alterations in early postnatal behavior together with possible changes in hippocampal excitability.
CUS and/or mirtazapine significantly affected several parameters of hippocampal excitability in cultured primary neurons, whereas another set of parameters was not changed. CUS suppresses the excitability, as manifested in hyperpolarized resting membrane potential, increased the voltage threshold for an action potential (AP), and increased the minimal depolarizing current pulse amplitude for activation of both single AP and AP series. Similar effects were caused by mirtazapine when it was administered to dams not exposed to CUS: the resting membrane potential was hyperpolarized, the AP threshold and latency were increased, the minimal depolarizing current pulse amplitude for the activation of a single AP was increased, and the AP rise time was prolonged. Mirtazapine administered to CUS-exposed dams had a positive effect on excitability in selected CUS-affected parameters: it lowered the voltage threshold for the generation of a single AP and AP series and lowered the minimal depolarizing current pulse amplitude for the activation of a single AP. Taken together, the administration of mirtazapine to control dams had minor effects on the hippocampal excitability of offspring. Pregestational CUS, however, suppressed the hippocampal excitability of offspring to a moderate extent. Treatment of CUS-exposed dams with mirtazapine partly compensated for the observed changes in hippocampal excitability.
Approximately two weeks of maturation of the hippocampus under native conditions resulted in a different pattern of excitability alterations observed in acute slices. CUS mostly facilitates excitability by lowering both the threshold for AP activation and the minimal current amplitude needed to activate a single AP and AP series, shortening the AP latency and accelerating the AP rise time. The negative effects of mirtazapine administration to healthy control offspring almost disappeared during first weeks of postnatal development. It was restricted to lowering the minimal current amplitude needed to activate a single AP and AP series (facilitation of excitability) and lowering the AP peak voltage in the AP series (negative effect). Mirtazapine administered to CUS-exposed dams facilitated the excitability of offspring in a majority of the analyzed parameters. The effect of CUS was opposite to that observed in primary cultures but remained minor to moderate. Mirtazapine treatment did not compensate for these changes.
While numerous studies have analyzed the behavioral and/or metabolic effects of maternal stress on offspring, alterations in neuronal excitability measured at the level of individual neurons are less frequently investigated. Grigoryan and Segal [
35] analyzed the excitability of hippocampal neurons in a primary culture established from offspring of dams exposed to stress during gestation on the day of birth. They reported no differences in the resting membrane potential, input resistance, or AP threshold voltage between the stressed and nonstressed groups. Nevertheless, they reported reduced GABAergic inhibition, which resulted in a greater frequency of network activity in cultured neurons [
35]. In the prenatally stressed group, a reduction in inhibitory tone was also confirmed in hippocampal slices from 2–3-week-old rats from the same cohort. These findings support our findings that maternal stress may facilitate the hippocampal excitability of offspring in early life, whereas they contrast with our findings in primary cultures. This difference may arise from a different stress paradigm and the time of its application. The stress paradigm applied during gestation might have had a stronger effect at the time of birth. Another study in primary hippocampal culture [
29] reported a negative effect of pregestational stress on AP generation, which aligns with the findings of this study. Furthermore, maternal CUS decreased the expression of 5-HT and serotonin transporters. Moreover, it increased the 5-HT level in the hippocampi of 20-day-old embryos [
36], which is also in line with the decreased excitability of hippocampal neurons just after birth observed in this work.
Experiments on acute slices reflect not only two weeks of neuronal development in a native environment but also altered developmental interactions with mothers and siblings. Indeed, the results we obtained from the slices differ from those obtained from the primary cultures. The effect of the administration of mirtazapine to nonstressed dams was markedly reduced in slices and restricted to only two parameters. The significant change in the resting membrane potential observed in cultures disappeared, and several parameters of excitability were increased in offspring from CUS-exposed mothers. These results parallel those of several other studies in which a pregestational maternal stress model was used. The antidepressant Venlafaxine decreased the number of mature hippocampal neurons in juvenile (31 days) offspring of control but not CUS dams, and this effect disappeared in adolescent (47 days) and adult (75 days) offspring [
37]. In adult male rats, pregestational maternal stress reduces neurogenesis in hippocampal granule cells, and perinatal treatment with another antidepressant, fluoxetine, reverses this effect [
38].
Pregestational stress decreased the metabolism of the inhibitory neurotransmitter GABA in the hippocampus of 30-day-old offspring, as documented by MRI scans [
39], in line with a model used by Grigoryan and Segal [
35] and in line with the increased excitability observed in our model in slices. The same results were obtained in our model by Makova et al. [
40].
The effects of mirtazapine reported in this study may also be partly explained by its action as an antagonist of 5-HT
2 and 5-HT
3 receptors. While the activation of 5-HT
2, 5-HT
3, or 5-HT
4 receptors results in depolarization of the membrane potential and increased neuronal excitability [
41,
42,
43], the activation of 5-HT
1A receptors causes hyperpolarization and decreased excitability [
41,
44,
45].
Spontaneous activity in developing neural circuits is a crucial source of signals for neuron and development of neuronal networks. These mechanisms may involve various factors, including the depolarizing action of GABA (γ-aminobutyric acid), transient synaptic connections, extrasynaptic transmission, gap junction coupling, and the presence of pacemaker-like neurons [
46]. In our study, we observed spontaneous activity in hippocampal neurons from all four offspring groups, with no significant differences in quantitative parameters of AP firing between the groups. However, percentage of spontaneously active neurons was increased in CUS group. Research has demonstrated that maternal stress can affect the spontaneous activity of offspring neurons. In earlier work [
29], we reported increased levels of spontaneous activity in neurons from primary hippocampal cultures in the stressed group. Our findings can be interpreted in terms of altered GABAergic and glutamatergic systems which enhance spontaneous action potential firing [
10]. This finding is consistent with other studies that reported alterations in GABA and glutamate signaling in rats exposed to stress [
47,
48], as well as increased expression of glutamate transporters in the hippocampi of prenatally stressed rats [
39,
49]. Notably, these studies investigated neuronal excitability in vitro or at different developmental stages than our research did.
4. Materials and Methods
4.1. Animals
Female and male Wistar rats (initial weight 200–220 g; 2–3 months old) were obtained from the Department of Toxicology and Laboratory Animals Breeding, Centre of Experimental Medicine of the Slovak Academy of Sciences, Dobra Voda, Slovak Republic. All the animals were housed (38 × 59 × 25 cm large cages) under standard laboratory conditions (temperature: 22 ± 2°C, humidity: 55 ± 10%) with a 12 h light/12 h dark cycle (lights on at 7.00 a.m.). Pelleted food and tap water were available ad libitum. The State Veterinary and Food Administration of the Slovak Republic approved all the experimental procedures. The rats were handled according to the Guide for the Care and Use of Laboratory Animals (N.R.C., 1996) and the European Communities Council Directive of September 22, 2010 (2010/63/EU, 74).
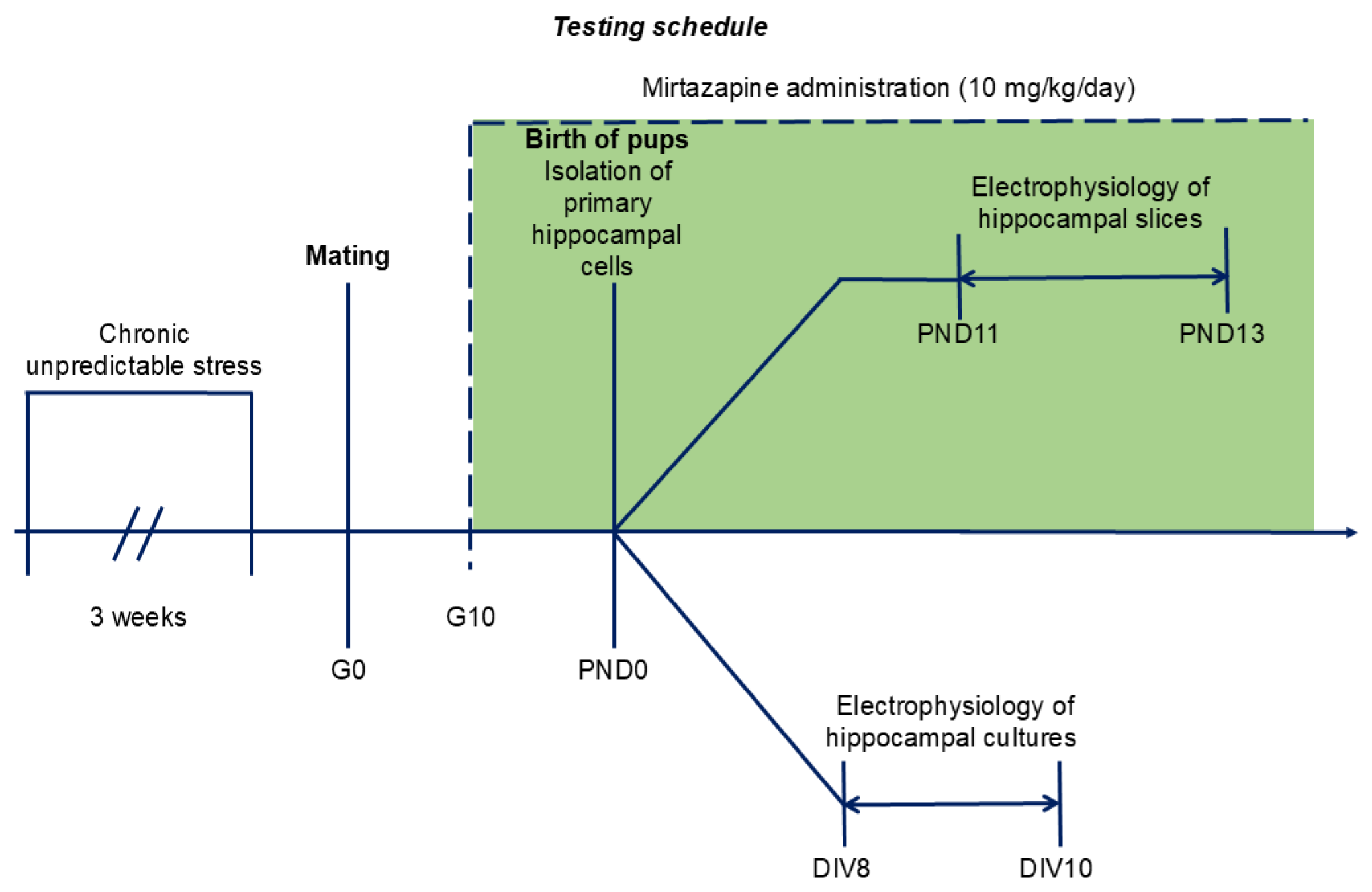
4.2. Experimental Design
The timing of the experiments is shown on the
Figure 8.
4.3. Chronic Unpredictable Stress Schedule
After one week of adaptation, the female rats were randomly allocated to one of the following four groups: no chronic unpredictable stress + vehicle (NO CUS+VEH), no chronic unpredictable stress + mirtazapine (NO CUS+MIR), chronic unpredictable stress +vehicle (CUS+VEH), and chronic unpredictable stress + mirtazapine (CUS+MIR). Animals from the stressed group were randomly exposed to 6 distinct stressors for three consecutive weeks: 1) exposure to damp bedding (12 h); 2) food deprivation (24 h); 3) predator stress–clothing with cat odor (10 h); 4) water deprivation (12 h); 5) cage decline at a 45° angle (6 h); and 6) Strobe light flicker lights (12 h). After the stress procedure, female rats from all groups were mated with males (3:1). At gestational day 0 (G0), each pregnant rat was housed separately. The G0 day was detected by presence of sperms in vaginal smears.
4.4. Mirtazapine Treatment
Mirtazapine (Mikrochem Trade s.r.o.; M = 265.36 g/mol) was dissolved in citric acid and administered orally via a wafer biscuit (size of 1 cm
3) to pregnant rats at a clinically relevant dose of 10 mg/kg/day (
Figure 8). The dose used in this experiment was chosen on the basis of previous results [
23]. The female rats in the control groups received a 1 cm
3 wafer biscuit once daily, which was filled with vehicle.
4.5. Primary Hippocampal Cell Culture
Primary hippocampal cultures were prepared from PND 0 pups according to the methods of Reichova et al. [
50]. The animals were euthanized by cutting off the head using a special guillotine designed for killing laboratory rodents. The brains (n = 4–5 pups/groups) were dissected in ice-cold Hank's balanced salt solution (HBSS; Gibco, USA) supplemented with 1% antibiotic-antimycotic (Sigma–Aldrich, Germany) and 0.3 M HEPES (Sigma–Aldrich, Germany) under a stereomicroscope (Optika Microscopes, Italy). Hippocampal tissues were dissociated for 20 min at 37 °C with an enzymatic solution containing HBSS, 0.1% trypsin (Gibco, USA), and 0.1 mg/ml DNAse I (Roche, Germany). The cells were subsequently resuspended and plated on 24-well plates containing coverslips (Sarstedt, Germany) coated with 10 µg/ml poly-D-lysine (Sigma‒Aldrich, Germany) at a density of 0.8-1x10
5 cells/ml in RPMI medium (Gibco, USA) containing 10% fetal bovine serum (Sigma‒Aldrich, Germany). After 3 hours of cell culture at 37°C in a 5% CO
2 incubator, the medium was replaced with selective growth medium (Neurobasal A; Gibco, USA) supplemented with 1% antibiotic-antimycotic (Sigma‒Aldrich, Germany), 1% glutamine (Sigma‒Aldrich, Germany) and 2% B27 supplement (Gibco, USA). This was assigned as day 1 in vitro (DIV 1). At DIV5, 50% of the fresh growth medium was replaced. Cultured hippocampal neurons with pyramidal-like morphology were used for electrophysiology experiments at DIV 8--10.
4.6. Acute Hippocampal Slices
Acute hippocampal slices were prepared on PNDs 11–13. The animals were euthanized by cutting off the head using a special guillotine designed for killing laboratory rodents. The brains were cut on a vibratome (Campden Instruments, England) into 300 µm thick slices in ice-cold artificial cerebrospinal fluid (ACSF) containing the following (in mM): 125 NaCl, 3 KCl, 25 NaHCO3, 1.25 NaH2PO4, 2 CaCl2, 1 MgCl2, and 10 D-glucose (all chemicals were purchased from Sigma‒Aldrich, Germany). ACSF was oxygenated with 95% O2 and 5% CO2, pH 7.4. Before recording, the slices were incubated for 60 min at 37°C and then for 30 min at room temperature in oxygenated ACSF solution.
4.7. Electrophysiological Experiments
All electrophysiology measurements were performed via the whole-cell current clamp configuration of the patch-clamp technique. The signals were recorded via a HEKA EPC10 amplifier (HEKA Electronics, Lambrecht, Germany) controlled by Patchmaster v2x73.3 (HEKA Electronics, Lambrecht/Pfalz, Germany) software. The data were analyzed via Fitmaster v2x73.3 (HEKA Electronics, Lambrecht/Pfalz, Germany) and Origin 2022 Academic (OriginLab Co., Northampton, MA, USA) software. The parameters of an action potential (AP) series were extracted from the experimental traces with custom code via the open-source Electrophysiological Feature Extraction Library (eFEL,
https://github.com/BlueBrain/eFEL).
Patch electrodes were pulled from borosilicate glass with 3–4 MΩ resistance. The extracellular solution in primary hippocampal cultures contained (in mM): 130 NaCl; 3 KCl; 10 HEPES; 2 CaCl2; 1 MgCl2; 10 D-glucose; pH 7.4 (with NaOH), and the intracellular solution contained (in mM): 120 K-gluconate; 20 KCl; 2 MgCl2; 2 Na2ATP; 0.25 Na2GTP; 10 HEPES; pH 7.4 (with KOH) (all chemicals from Sigma‒Aldrich, Germany). The extracellular solution for acute neuronal slices was ASCF solution oxygenated with 95% O2 and 5% CO2, pH 7.4, and the intracellular solution was the same as that for the primary hippocampal cultures. The osmolarity of an intracellular solution was approximately 290–300 mOsm/L (Osmomat 030-Gonotec, Germany). The osmolarity of an extracellular solution was adjusted by adding sucrose to a final osmolarity that was 2–3 mOsm/L lower than the osmolarity of the corresponding intracellular solution.
The actual resting membrane potential (Vrest) was read immediately after establishing the whole-cell configuration and switching to current-clamp mode. During all other experiments, the resting membrane potential was held at -70 mV. The input resistance (Rinp) was measured by a series of 250 ms long hyperpolarizing current pulses with the amplitude decreasing from -70 mV with a step of -25 pA. Membrane potential at the end of each current pulse was read and the Rinp value was calculated from the fit of the voltage‒current relationship by the Ohm equation. Single APs were activated by a series of 5 ms long depolarizing current pulses with the amplitude increasing with a step of +50 pA. A series of APs were activated by a series of 300 ms long depolarizing current pulses with the amplitude increasing with a step of +50 pA. Spontaneous activity was measured for 5 minutes from each neuron's resting membrane potential.
4.8. Statistical Analyses
The statistical analyses were performed via GraphPad Prism 8.3 software. Outliers were identified and excluded via GraphPad PRISM´s ROUT method. Behavioral and electrophysiological data were analyzed by two-way analysis of variance (ANOVA) with treatment (mirtazapine) and conditions (stress vs. nonstress) as the main factors, followed by the Bonferroni post hoc test. The results are expressed as the mean ± standard error of the mean (SEM), and a value of p ≤ 0.05 was considered statistically significant.
5. Conclusions
To summarize, we have shown that in our animal model, the treatment of control nonstressed dams with mirtazapine during the pregnancy moderately affects the hippocampal excitability of offspring. Prenatal CUS results in moderately suppressed hippocampal excitability in offspring at birth, and this effect reverses to moderate facilitation during early postnatal development. Treatment with mirtazapine during pregnancy partly compensates for the effects of CUS, and this compensation becomes more prominent during postnatal development. Therefore, treatment with mirtazapine may be also beneficial during the prenatal and perinatal periods.
Author Contributions
Conceptualization, M.D. and L.L.; methodology, L.D.H., A.F., S.B., B.J.T. and K.O., Software, M.T.; validation, M.D, E.D., and L.L.; formal analysis, L.D.H., A.F., M.T. and E.D.; investigation, L.D.H., A.F., S.B., K.O., and BJT; resources, M.D, E.D., and L.L.; data curation, L.D.H., A.F., S.B., K.O.; writing—original draft preparation, L.D.H. and K.O.; writing—review and editing, L.D.H., A.F., S.B., K.O., M.T., B.J.T., M.D, E.D., and L.L..; visualization, E.D. and L.L.; supervision, M.D. and L.L.; project administration, M.D. and L.L.; funding acquisition, M.D., E.D. and L.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Slovak Research and Development Agency under Contracts No. APVV-APVV-19-0435, APVV-20-0202, and APVV-22-0061 and by the Research Grant Agency of the Slovak Academy of Sciences and Ministry of Education, Science, Research and Sport of the Slovak Republic via the grants No. VEGA 2/0057/22, VEGA 2/0081/22, VEGA 2/0133/23.
Institutional Review Board Statement
The State Veterinary and Food Administration of the Slovak Republic approved all experimental procedures with animals (No. 3334-5/2020-220).
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Acknowledgments
The authors thank Alexandra Reichová, PhD, for practical advice and recommendations on preparing primary hippocampal cultures.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
5-HT
ANOVA
AP
CUS
MIR
MDPI
Rinp |
Serotonin
Analysis of variance
Action potential
Chronic unpredictable stress
Mirtazapine
Multidisciplinary Digital Publishing Institute
Input resistance |
SSRIs
VEH |
Selective serotonin reuptake inhibitors
Vehicle |
| Vrest
|
Resting membrane potentials |
| WHO |
World Health Organization |
References
- Slavich, G. M.; Sacher, J., Stress, sex hormones, inflammation, and major depressive disorder: Extending Social Signal Transduction Theory of Depression to account for sex differences in mood disorders. Psychopharmacology (Berl) 2019, 236, (10), 3063-3079. [CrossRef]
- Malhi, G. S.; Mann, J. J., Depression. Lancet 2018, 392, (10161), 2299-2312. [CrossRef]
- Shidhaye, P.; Giri, P., Maternal depression: a hidden burden in developing countries. Ann Med Health Sci Res 2014, 4, (4), 463-5. [CrossRef]
- Barker, E. D.; Jaffee, S. R.; Uher, R.; Maughan, B., The contribution of prenatal and postnatal maternal anxiety and depression to child maladjustment. Depress Anxiety 2011, 28, (8), 696-702. [CrossRef]
- Stein, A.; Pearson, R. M.; Goodman, S. H.; Rapa, E.; Rahman, A.; McCallum, M.; Howard, L. M.; Pariante, C. M., Effects of perinatal mental disorders on the fetus and child. Lancet 2014, 384, (9956), 1800-19. [CrossRef]
- Gelaye, B.; Rondon, M. B.; Araya, R.; Williams, M. A., Epidemiology of maternal depression, risk factors, and child outcomes in low-income and middle-income countries. Lancet Psychiatry 2016, 3, (10), 973-982. [CrossRef]
- Accortt, E. E.; Cheadle, A. C.; Dunkel Schetter, C., Prenatal depression and adverse birth outcomes: an updated systematic review. Matern Child Health J 2015, 19, (6), 1306-37. [CrossRef]
- Silveira, M. F.; Victora, C. G.; Horta, B. L.; da Silva, B. G. C.; Matijasevich, A.; Barros, F. C.; Pelotas Cohorts Study, G., Low birthweight and preterm birth: trends and inequalities in four population-based birth cohorts in Pelotas, Brazil, 1982-2015. Int J Epidemiol 2019, 48, (Suppl 1), i46-i53. [CrossRef]
- Eshete, A.; Alemu, A.; Zerfu, T. A., Magnitude and Risk of Dying among Low Birth Weight Neonates in Rural Ethiopia: A Community-Based Cross-Sectional Study. Int J Pediatr 2019, 2019, 9034952. [CrossRef]
- Ahun, M. N.; Cote, S. M., Maternal depressive symptoms and early childhood cognitive development: a review of putative environmental mediators. Arch Womens Ment Health 2019, 22, (1), 15-24. [CrossRef]
- Kingston, D.; Kehler, H.; Austin, M. P.; Mughal, M. K.; Wajid, A.; Vermeyden, L.; Benzies, K.; Brown, S.; Stuart, S.; Giallo, R., Trajectories of maternal depressive symptoms during pregnancy and the first 12 months postpartum and child externalizing and internalizing behavior at three years. PLoS One 2018, 13, (4), e0195365. [CrossRef]
- Rogers, A.; Obst, S.; Teague, S. J.; Rossen, L.; Spry, E. A.; Macdonald, J. A.; Sunderland, M.; Olsson, C. A.; Youssef, G.; Hutchinson, D., Association Between Maternal Perinatal Depression and Anxiety and Child and Adolescent Development: A Meta-analysis. JAMA Pediatr 2020, 174, (11), 1082-1092. [CrossRef]
- Braun, K.; Bock, J.; Wainstock, T.; Matas, E.; Gaisler-Salomon, I.; Fegert, J.; Ziegenhain, U.; Segal, M., Experience-induced transgenerational (re-)programming of neuronal structure and functions: Impact of stress prior and during pregnancy. Neurosci Biobehav Rev 2020, 117, 281-296. [CrossRef]
- Grundwald, N. J.; Brunton, P. J., Prenatal stress programs neuroendocrine stress responses and affective behaviors in second generation rats in a sex-dependent manner. Psychoneuroendocrinology 2015, 62, 204-16. [CrossRef]
- Benard-Laribiere, A.; Pambrun, E.; Sutter-Dallay, A. L.; Gautier, S.; Hurault-Delarue, C.; Damase-Michel, C.; Lacroix, I.; Begaud, B.; Pariente, A., Patterns of antidepressant use during pregnancy: a nationwide population-based cohort study. Br J Clin Pharmacol 2018, 84, (8), 1764-1775. [CrossRef]
- Molenaar, N. M.; Kamperman, A. M.; Boyce, P.; Bergink, V., Guidelines on treatment of perinatal depression with antidepressants: An international review. Aust N Z J Psychiatry 2018, 52, (4), 320-327. [CrossRef]
- Kim, J.; Riggs, K. W.; Misri, S.; Kent, N.; Oberlander, T. F.; Grunau, R. E.; Fitzgerald, C.; Rurak, D. W., Stereoselective disposition of fluoxetine and norfluoxetine during pregnancy and breast-feeding. Br J Clin Pharmacol 2006, 61, (2), 155-63. [CrossRef]
- Rampono, J.; Simmer, K.; Ilett, K. F.; Hackett, L. P.; Doherty, D. A.; Elliot, R.; Kok, C. H.; Coenen, A.; Forman, T., Placental transfer of SSRI and SNRI antidepressants and effects on the neonate. Pharmacopsychiatry 2009, 42, (3), 95-100. [CrossRef]
- Dubovicky, M.; Belovicova, K.; Csatlosova, K.; Bogi, E., Risks of using SSRI / SNRI antidepressants during pregnancy and lactation. Interdiscip Toxicol 2017, 10, (1), 30-34. [CrossRef]
- Gemmel, M.; Hazlett, M.; Bogi, E.; De Lacalle, S.; Hill, L. A.; Kokras, N.; Hammond, G. L.; Dalla, C.; Charlier, T. D.; Pawluski, J. L., Perinatal fluoxetine effects on social play, the HPA system, and hippocampal plasticity in pre-adolescent male and female rats: Interactions with pre-gestational maternal stress. Psychoneuroendocrinology 2017, 84, 159-171. [CrossRef]
- Millard, S. J.; Weston-Green, K.; Newell, K. A., The effects of maternal antidepressant use on offspring behaviour and brain development: Implications for risk of neurodevelopmental disorders. Neurosci Biobehav Rev 2017, 80, 743-765. [CrossRef]
- Cipriani, A.; Furukawa, T. A.; Salanti, G.; Chaimani, A.; Atkinson, L. Z.; Ogawa, Y.; Leucht, S.; Ruhe, H. G.; Turner, E. H.; Higgins, J. P. T.; Egger, M.; Takeshima, N.; Hayasaka, Y.; Imai, H.; Shinohara, K.; Tajika, A.; Ioannidis, J. P. A.; Geddes, J. R., Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet 2018, 391, (10128), 1357-1366. [CrossRef]
- Viñas-Noguera, M.; Csatlósová, K.; Šimončičová, E.; Bögi, E.; Ujházy, E.; Dubovický, M.; Belovičová, K., Sex- and age- dependent effect of pre-gestational chronic stress and mirtazapine treatment on neurobehavioral development of Wistar rat offspring. PLoS One 2022, 17, (2), e0255546. [CrossRef]
- Smit, M.; Dolman, K. M.; Honig, A., Mirtazapine in pregnancy and lactation - A systematic review. Eur Neuropsychopharmacol 2016, 26, (1), 126-135. [CrossRef]
- Idunkova, A.; Lacinova, L.; Dubiel-Hoppanova, L., Stress, depression, and hippocampus: from biochemistry to electrophysiology. Gen Physiol Biophys 2023, 42, (2), 107-122. [CrossRef]
- Castanheira, L.; Silva, C.; Cheniaux, E.; Telles-Correia, D., Neuroimaging Correlates of Depression-Implications to Clinical Practice. Front Psychiatry 2019, 10, 703. [CrossRef]
- Aleksandrova, L. R.; Wang, Y. T.; Phillips, A. G., Evaluation of the Wistar-Kyoto rat model of depression and the role of synaptic plasticity in depression and antidepressant response. Neurosci Biobehav Rev 2019, 105, 1-23. [CrossRef]
- Bogi, E.; Belovicova, K.; Csatlosova, K.; Dubovicky, M., Animal models of maternal depression for monitoring neurodevelopmental changes occurring in dams and offspring. Interdiscip Toxicol 2017, 10, (1), 35-39. [CrossRef]
- Bogi, E.; Belovicova, K.; Moravcikova, L.; Csatlosova, K.; Dremencov, E.; Lacinova, L.; Dubovicky, M., Pre-gestational stress impacts excitability of hippocampal cells in vitro and is associated with neurobehavioral alterations during adulthood. Behav Brain Res 2019, 375, 112131. [CrossRef]
- Belovicova, K.; Bogi, E.; Koprdova, R.; Ujhazy, E.; Mach, M.; Dubovicky, M., Effects of venlafaxine and chronic unpredictable stress on behavior and hippocampal neurogenesis of rat dams. Neuro Endocrinol Lett 2017, 38, (1), 19-26.
- Czarzasta, K.; Bogacki-Rychlik, W.; Segiet-Swiecicka, A.; Kruszewska, J.; Malik, J.; Skital, V.; Kasarello, K.; Wrzesien, R.; Bialy, M.; Sajdel-Sulkowska, E. M., Gender differences in short- vs. long-term impact of maternal depression following pre-gestational chronic mild stress. Exp Neurol 2022, 353, 114059.
- Malyshev, A. V.; Razumkina, E. V.; Rogozinskaia, E.; Sarkisova, K.; Dybynin, V. A., [Behavior and functional state of the dopaminergic brain system in pups of depressive WAG/Rij rats]. Zh Vyssh Nerv Deiat Im I P Pavlova 2014, 64, (3), 334-46.
- Lozano, A. F. Q.; Moura, M. S.; Tavares, B. M.; Kempinas, W. G., Exposure of pregnant rats to stress and/or sertraline: Side effects on maternal health and neurobehavioral development of male offspring. Life Sci 2021, 285, 119960. [CrossRef]
- Al-Fadel, N.; Alrwisan, A., Antidepressant Use During Pregnancy and the Potential Risks of Motor Outcomes and Intellectual Disabilities in Offspring: A Systematic Review. Drugs Real World Outcomes 2021, 8, (2), 105-123. [CrossRef]
- Grigoryan, G.; Segal, M., Prenatal stress affects network properties of rat hippocampal neurons. Biol Psychiatry 2013, 73, (11), 1095-102. [CrossRef]
- Huang, Y.; Xu, H.; Li, H.; Yang, H.; Chen, Y.; Shi, X., Pre-gestational stress reduces the ratio of 5-HIAA to 5-HT and the expression of 5-HT1A receptor and serotonin transporter in the brain of foetal rat. BMC Neurosci 2012, 13, 22. [CrossRef]
- Belovicova, K.; Simoncicova, E.; Noguera, M. V.; Dubovicky, M.; Bogi, E., Long-term effects of pre-gestational stress and perinatal venlafaxine treatment on neurobehavioral development of female offspring. Behav Brain Res 2021, 398, 112944. [CrossRef]
- Gemmel, M.; De Lacalle, S.; Mort, S. C.; Hill, L. A.; Charlier, T. D.; Pawluski, J. L., Perinatal fluoxetine has enduring sexually differentiated effects on neurobehavioral outcomes related to social behaviors. Neuropharmacology 2019, 144, 70-81. [CrossRef]
- Huang, Y.; Shen, Z.; Hu, L.; Xia, F.; Li, Y.; Zhuang, J.; Chen, P.; Huang, Q., Exposure of mother rats to chronic unpredictable stress before pregnancy alters the metabolism of gamma-aminobutyric acid and glutamate in the right hippocampus of offspring in early adolescence in a sexually dimorphic manner. Psychiatry Res 2016, 246, 236-245. [CrossRef]
- Makova, M.; Kasparova, S.; Tvrdik, T.; Noguera, M.; Belovicova, K.; Csatlosova, K.; Dubovicky, M., Mirtazapine modulates Glutamate and GABA levels in the animal model of maternal depression. MRI and (1)H MRS study in female rats. Behav Brain Res 2023, 442, 114296.
- Xiang, Z.; Prince, D. A., Heterogeneous actions of serotonin on interneurons in rat visual cortex. J Neurophysiol 2003, 89, (3), 1278-87. [CrossRef]
- McMahon, L. L.; Kauer, J. A., Hippocampal interneurons are excited via serotonin-gated ion channels. J Neurophysiol 1997, 78, (5), 2493-502. [CrossRef]
- Ropert, N.; Guy, N., Serotonin facilitates GABAergic transmission in the CA1 region of rat hippocampus in vitro. J Physiol 1991, 441, 121-36. [CrossRef]
- Beck, S. G.; Choi, K. C.; List, T. J., Comparison of 5-hydroxytryptamine1A-mediated hyperpolarization in CA1 and CA3 hippocampal pyramidal cells. J Pharmacol Exp Ther 1992, 263, (1), 350-9. [CrossRef]
- Tanaka, E.; North, R. A., Actions of 5-hydroxytryptamine on neurons of the rat cingulate cortex. J Neurophysiol 1993, 69, (5), 1749-57. [CrossRef]
- Blankenship, A. G.; Feller, M. B., Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nat Rev Neurosci 2010, 11, (1), 18-29. [CrossRef]
- Kim, Y. B.; Colwell, C. S.; Kim, Y. I., Long-term ionic plasticity of GABAergic signalling in the hypothalamus. J Neuroendocrinol 2019, 31, (8), e12753. [CrossRef]
- Tornese, P.; Sala, N.; Bonini, D.; Bonifacino, T.; La Via, L.; Milanese, M.; Treccani, G.; Seguini, M.; Ieraci, A.; Mingardi, J.; Nyengaard, J. R.; Calza, S.; Bonanno, G.; Wegener, G.; Barbon, A.; Popoli, M.; Musazzi, L., Chronic mild stress induces anhedonic behavior and changes in glutamate release, BDNF trafficking and dendrite morphology only in stress vulnerable rats. The rapid restorative action of ketamine. Neurobiol Stress 2019, 10, 100160. [CrossRef]
- Adrover, E.; Pallares, M. E.; Baier, C. J.; Monteleone, M. C.; Giuliani, F. A.; Waagepetersen, H. S.; Brocco, M. A.; Cabrera, R.; Sonnewald, U.; Schousboe, A.; Antonelli, M. C., Glutamate neurotransmission is affected in prenatally stressed offspring. Neurochem Int 2015, 88, 73-87. [CrossRef]
- Reichova, A.; Schaller, F.; Bukatova, S.; Bacova, Z.; Muscatelli, F.; Bakos, J., The impact of oxytocin on neurite outgrowth and synaptic proteins in Magel2-deficient mice. Dev Neurobiol 2021, 81, (4), 366-388. [CrossRef]
Figure 1.
Resting membrane potential (A and C) and input resistance (B and D) of cultivated neurons (A and B) and neurons in slices (C and D) isolated from the hippocampi of offspring of dams that experienced pregestational chronic unpredictable stress (CUS), prenatal mirtazapine treatment, or their combination. n (the number of individual cells/number of litters in the group) = 55/5 in NO CUS+VEH, 75/5 in NO CUS+MIR, 82/5 in CUS+VEH and 69/5 CUS+MIR in primary cultures, 54/5 in NO CUS+VEH, 63/5 in NO CUS+MIR, 38/5 in CUS+VEH and 39/5 CUS+MIR in acute slices; *p < 0.05, and ***p < 0.001, Bonferroni post hoc test.
Figure 1.
Resting membrane potential (A and C) and input resistance (B and D) of cultivated neurons (A and B) and neurons in slices (C and D) isolated from the hippocampi of offspring of dams that experienced pregestational chronic unpredictable stress (CUS), prenatal mirtazapine treatment, or their combination. n (the number of individual cells/number of litters in the group) = 55/5 in NO CUS+VEH, 75/5 in NO CUS+MIR, 82/5 in CUS+VEH and 69/5 CUS+MIR in primary cultures, 54/5 in NO CUS+VEH, 63/5 in NO CUS+MIR, 38/5 in CUS+VEH and 39/5 CUS+MIR in acute slices; *p < 0.05, and ***p < 0.001, Bonferroni post hoc test.
Figure 2.
Single action potential threshold (A) and peak (B) voltages, latency time (C), width of action potential (D), rise time (E), and the minimal current needed to induce a single action potential (F) for cultivated neurons isolated from the hippocampi of newborn offspring of dams that experienced pregestational chronic unpredictable stress (CUS), prenatal mirtazapine treatment, or their combination. n (the number of individual cells/number of litters in the group) = 55/5 in NO CUS+VEH, 75/5 in NO CUS+MIR, 81/5 in CUS+VEH, and 68/5 in CUS+MIR; *p < 0.05, **p < 0.01, and ***p < 0.001, Bonferroni post hoc test.
Figure 2.
Single action potential threshold (A) and peak (B) voltages, latency time (C), width of action potential (D), rise time (E), and the minimal current needed to induce a single action potential (F) for cultivated neurons isolated from the hippocampi of newborn offspring of dams that experienced pregestational chronic unpredictable stress (CUS), prenatal mirtazapine treatment, or their combination. n (the number of individual cells/number of litters in the group) = 55/5 in NO CUS+VEH, 75/5 in NO CUS+MIR, 81/5 in CUS+VEH, and 68/5 in CUS+MIR; *p < 0.05, **p < 0.01, and ***p < 0.001, Bonferroni post hoc test.
Figure 3.
The mean number of action potentials within the depolarization current-induced series (A), latency time (B) and threshold voltage (C) of the first action potential, peak voltage within the series of action potentials (D), and minimal current needed to induce the series (E) for cultivated neurons isolated from the hippocampi of newborn offspring of dams that experienced pregestational chronic unpredictable stress (CUS), prenatal mirtazapine treatment, or their combination. n (the number of individual cells/number of litters in the group) = 48/5 in NO CUS+VEH, 60/5 in NO CUS+MIR, 68/5 in CUS+VEH, and 50/5 in CUS+MIR; *p < 0.05, **p < 0.01, and ***p < 0.001, Bonferroni post hoc test. Panel F shows representative example of an AP recording.
Figure 3.
The mean number of action potentials within the depolarization current-induced series (A), latency time (B) and threshold voltage (C) of the first action potential, peak voltage within the series of action potentials (D), and minimal current needed to induce the series (E) for cultivated neurons isolated from the hippocampi of newborn offspring of dams that experienced pregestational chronic unpredictable stress (CUS), prenatal mirtazapine treatment, or their combination. n (the number of individual cells/number of litters in the group) = 48/5 in NO CUS+VEH, 60/5 in NO CUS+MIR, 68/5 in CUS+VEH, and 50/5 in CUS+MIR; *p < 0.05, **p < 0.01, and ***p < 0.001, Bonferroni post hoc test. Panel F shows representative example of an AP recording.
Figure 4.
Percent of neurons with AP series after depolarization pulse application in primary cultures established from the hippocampi of newborn offspring (A; = 48/5 in NO CUS+VEH, 60/5 in NO CUS+MIR, 68/5 in CUS+VEH, and 50/5 in CUS+MIR) or in slices isolated from the hippocampi of 11–13-day-old offspring (B; = 53/5 in NO CUS+VEH, 63/5 in NO CUS+MIR, 40/5 in CUS+VEH, and 40/5 in CUS+MIR) of dams that experienced pregestational chronic unpredictable stress (CUS), prenatal mirtazapine treatment, or their combination. I*, significant interaction between maternal stress and mirtazapine, p<0.05, S* and S***, significant effect of maternal stress, p<0.05 and p<0.001, respectively, two-way ANOVA; *p<0.05, **p<0.01, and ***p<0.001, between-group comparison, Bonferroni post hoc test.
Figure 4.
Percent of neurons with AP series after depolarization pulse application in primary cultures established from the hippocampi of newborn offspring (A; = 48/5 in NO CUS+VEH, 60/5 in NO CUS+MIR, 68/5 in CUS+VEH, and 50/5 in CUS+MIR) or in slices isolated from the hippocampi of 11–13-day-old offspring (B; = 53/5 in NO CUS+VEH, 63/5 in NO CUS+MIR, 40/5 in CUS+VEH, and 40/5 in CUS+MIR) of dams that experienced pregestational chronic unpredictable stress (CUS), prenatal mirtazapine treatment, or their combination. I*, significant interaction between maternal stress and mirtazapine, p<0.05, S* and S***, significant effect of maternal stress, p<0.05 and p<0.001, respectively, two-way ANOVA; *p<0.05, **p<0.01, and ***p<0.001, between-group comparison, Bonferroni post hoc test.
Figure 5.
Single action potential threshold (A) and peak (B) voltages, latency time (C), width of action potential (D), rise time (E), and the minimal current needed to induce a single action potential (F) for the neurons in the slices isolated from the hippocampi of 11–13-day-old offspring of the dams that experienced pregestational chronic unpredictable stress (CUS), prenatal mirtazapine treatment, or their combination. n (the number of individual cells/number of litters in the group) = 50/5 in NO CUS+VEH, 63/5 in NO CUS+MIR, 38/5 in CUS+VEH, and 39/5 in CUS+MIR; *p < 0.05, **p < 0.01, and ***p < 0.001, Bonferroni post hoc test.
Figure 5.
Single action potential threshold (A) and peak (B) voltages, latency time (C), width of action potential (D), rise time (E), and the minimal current needed to induce a single action potential (F) for the neurons in the slices isolated from the hippocampi of 11–13-day-old offspring of the dams that experienced pregestational chronic unpredictable stress (CUS), prenatal mirtazapine treatment, or their combination. n (the number of individual cells/number of litters in the group) = 50/5 in NO CUS+VEH, 63/5 in NO CUS+MIR, 38/5 in CUS+VEH, and 39/5 in CUS+MIR; *p < 0.05, **p < 0.01, and ***p < 0.001, Bonferroni post hoc test.
Figure 6.
The mean number of action potentials within the depolarization current-induced series (A), latency time (B) and threshold voltage (C) of the first action potential, peak voltage within the series of action potentials (D), and the minimal current needed to induce the series (E) for neurons in the slices isolated from the hippocampi of 11–13-day-old offspring of dams that experienced pregestational chronic unpredictable stress (CUS), prenatal mirtazapine treatment, or their combination. n (the number of individual cells/number of litters in the group) = 53/5 in NO CUS+VEH, 63/5 in NO CUS+MIR, 40/5 in CUS+VEH, and 40/5 in CUS+MIR; *p < 0.05, **p < 0.01, and ***p < 0.001, Bonferroni post hoc test. Panel F shows a representative example of the original AP recordings.
Figure 6.
The mean number of action potentials within the depolarization current-induced series (A), latency time (B) and threshold voltage (C) of the first action potential, peak voltage within the series of action potentials (D), and the minimal current needed to induce the series (E) for neurons in the slices isolated from the hippocampi of 11–13-day-old offspring of dams that experienced pregestational chronic unpredictable stress (CUS), prenatal mirtazapine treatment, or their combination. n (the number of individual cells/number of litters in the group) = 53/5 in NO CUS+VEH, 63/5 in NO CUS+MIR, 40/5 in CUS+VEH, and 40/5 in CUS+MIR; *p < 0.05, **p < 0.01, and ***p < 0.001, Bonferroni post hoc test. Panel F shows a representative example of the original AP recordings.
Figure 7.
Spontaneous activity of the neurons in the slices isolated from the hippocampi of 11–13-day-old offspring of dams that experienced pregestational chronic unpredictable stress (CUS), prenatal mirtazapine treatment, or their combination. A: cumulative activity; B: representative recordings from the offspring of the vehicle (VEH) or mirtazapine (MIR) treated nonstressed (No CUS) or stressed dams (CUS). ISI, interspike interval.
Figure 7.
Spontaneous activity of the neurons in the slices isolated from the hippocampi of 11–13-day-old offspring of dams that experienced pregestational chronic unpredictable stress (CUS), prenatal mirtazapine treatment, or their combination. A: cumulative activity; B: representative recordings from the offspring of the vehicle (VEH) or mirtazapine (MIR) treated nonstressed (No CUS) or stressed dams (CUS). ISI, interspike interval.
Figure 8.
Schedule of the experiment. G – gestation day; PND – postnatal day; DIV – days in vitro.
Figure 8.
Schedule of the experiment. G – gestation day; PND – postnatal day; DIV – days in vitro.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).