1. Introduction
Mortality and morbidity from pneumonia-induced sepsis remains high despite advances in critical care, understanding of the pathophysiology, and in treatment strategy [
1,
2,
3,
4,
5]. Early recognition and risk stratification are necessary to improve the outcomes in patients with pneumonia-induced sepsis [
6,
7]. However, definitive and accurate prognostic indicators for pneumonia-induced sepsis have not been found.
Increased percentages of immature granulocytes in systemic circulation are regarded as indicators of increased myeloid cell production and are associated with infection of systemic inflammation[
8,
9]. However, the measurement is difficult to obtain in clinical practice because manual measurement is neither accurate nor reproducible[
10]. Nahm
et al. suggested that the delta neutrophil index (DNI), which represents the differences in leukocyte subfractions assessed by an automated blood cell analyzer, may be useful [
11]. The DNI reflects the fraction of circulating immature granulocytes based on the differences between the leukocyte differentials measured in the myeloperoxidase (MPO) reactions and the nuclear lobularity of white blood cells. Recent studies showed a strong correlation of DNI with the manual immature granulocyte count, in addition to a strong correlation with disseminated intravascular coagulation scores, positive blood culture rates, and mortality in patients with suspected sepsis [
11,
12,
13,
14,
15,
16,
17].
However, little is known about the clinical usefulness of DNI in assessing the prognosis of patients with pneumonia-induced sepsis in the ICU. In this study, we evaluated the clinical utility of DNI in ICU patients with pneumonia-induced sepsis as an indicator of 28 day-mortality.
2. Materials AND Method
2.1. Patients
This retrospective study included patients admitted to the medical ICU of Kangdong Sacred Heart Hospital between July 2022 and March 2024. Pneumonia patients with sepsis or septic shock were included. Patients were excluded if they were younger than 18 years or stayed in the ICU for less than 24 hours. Permission was obtained from the Institutional Review Board of the Kangdong Sacred Heart Hospital to review.
2.2. Data Collection
Epidemiological and clinical data available at the time of ICU admission were collected from patients’ medical records. Data included age, sex, comorbid conditions, severity of illness score, laboratory values, and therapeutic interventions performed during the stay in the ICU, such as vasopressor use, renal replacement therapy, or tracheostomy. Further, 28-day mortality and cause of death were evaluated.
Blood samples for the analyses of DNI and other laboratory parameters were obtained within the first 24 hours, 48 hours, and 72 hours after ICU admission. Blood samples were analyzed at the time of ICU admission, and an automatic cell analyzer (ADIVA 2120 Hematology System, Siemens Healthcare Diagnostics, Forchheim, Germany) was used to calculate DNI. This hematologic analyzer is flow cytometry-based and analyzes WBCs by MPO and lobularity/nuclear density channels. After red blood cell lysis, the tungsten-halogen-based optical system of the MPO channel measured cell size and stain intensity in order to count and differentiate granulocytes, lymphocytes, and monocytes based on size and MPO content. Next, the laser diode-based optical system of the lobularity/nuclear density channel countered and classified the cells according to size, lobularity, and nuclear density. The resulting data were inserted in the following formula to determine DNI:
DNI = leukocyte subfraction assayed in the MPO channel by cytochemical reaction minus the leukocyte subfraction counted in the nuclear lobularity channel by reflected light beam[
11].
2.3. Definitions
We defined sepsis as "life-threatening organ dysfunction caused by a dysregulated host response to infection," and septic shock as "a subset of sepsis characterized by particularly profound circulatory, cellular, and metabolic abnormalities, clinically confirmed by the requirement of vasopressors to maintain a mean arterial pressure of 65 mm Hg or greater in the absence of hypovolemia and a serum lactate level greater than 2 mmol/L (>18 mg/dL)[
3,
18]. Patient severity can be identified as Acute Physiologic Assessment and Chronic Health Evaluation (APACHE) II Scoring System[
19]. We defined DNI 1 as DNI at 24 hours of ICU admission, DNI 2 as DNI at 48 hours of ICU admission, and DNI 3 as DNI at 72 hours of ICU admission.
2.4. Statistical Analysis
Continuous variables are expressed as the mean ± standard deviation. Categorical variables were expressed as numbers and percentages. Student’s t-test or the Mann–Whitney U test was used to compare continuous variables, whereas the chi-square test or Fisher’s exact test was used to compare categorical variables. Prognostic factors for 28-day mortality were evaluated. Variables with p < 0.1 in the univariate analysis were considered in the multivariable analyses to include potential variables with clinical significance. Multivariate logistic regression analysis results were reported as odds ratios and 95% confidence intervals (CIs). Receiver operating characteristic curves (ROC) were constructed, and the area under the curve (AUC) was evaluated. We also evaluated cut off value of prognostic factor. Our study made an effort to predict the optimum cut point based on time-to-event through using the technique of Contal and O’Quigley. All tests were two-sided, and a P value of less than 0.05 was considered statistically significant. Data were analyzed using the PASW statics software version 22 (SPSS Inc. Chicago, IL)
3. Results
3.1. Patient Characteristics
During the study period, 227 patients who met the inclusion criteria were included in the analysis. The main demographic and clinical characteristics are summarized in
Table 1. The mean age was 74 ± 13.7 years. Sixty six patients were male. The main underlying diseases were Diabetes Mellitus (124, 54.6 %), Hypertension (79, 34.8%), and Chronic Obstructive Pulmonary Disease (36, 15.9%). One hundred twenty six (55.5%) patients were diagnosed with septic shock. One hundred twenty nine patients received mechanical ventilation and twenty five patients received renal replacement therapy. The median duration of mechanical ventilation was 4.5 (2–80) days. The median duration of ICU stay was 7 (1–90) days. Causative pathogens were identified in 87 patients (39.3%), with
Acinetobacter baumannii being the most common pathogen in patients discharged from hospitals (8.6%), while
Streptococcus pneumoniae was the predominant pathogen in community-acquired pneumonia cases (5.2%).
3.2. Factors Associated with 28-Day Mortality
The 28-day mortality was 20.3% (46/227). The clinical and laboratory values of survivors and non-survivors were compared in
Table 2. In univariate analysis, age (
p=0.05), DNI 1 (
p =0.01), DNI 2 (
p=0.00), DNI 3 (
p=0.00), combined DNI (
p=0.00). lactic acid (
p =0.00) were significantly associated with 28-day mortality (
Table 2). However, in multivariate analysis, lactic acid (adj. OR. 0.86, 95% CI: 0.78-0.95,
p = 0.00), and DNI 3 (adj. OR. 0.94, 95% CI: 0.89-0.99,
p = 0.04) were the risk factors for 28-day mortality (
Table 3). In the higher DNI 3 group (≥ 2.6), 67.4% of the patients died within 28 days, whereas in the lower DNI 3 group (< 2.6), 32.6 % of patients died during the first 28 days (
p = 0.00). Using a cutoff value of 2.6%, the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the DNI 3 in sepsis were found to be 69%, 73.9%, 77.9%, and 64.1%, respectively (Figure 1). DNI 3 were more specific predictor for 28days mortality. The area under the curve of DNI 3 was 0.781 (95% CI 0.694-0.868), whereas DNI 1 (0.647, 95 % CI 0.558-0.736), DNI 2 (0.721, 95% CI 0.63-0.81), lactic acid (0.655, 95% CI 0.553-0.756) (Figure 2).
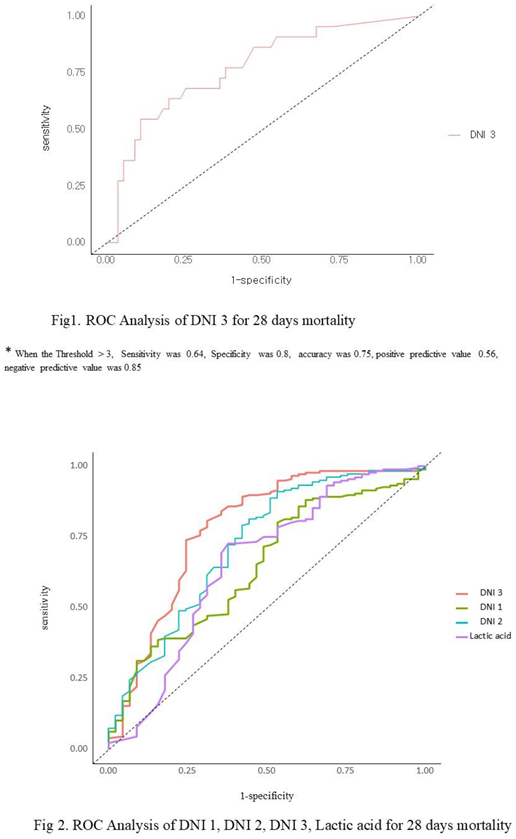
3.3. The Subgroup Analysis of the Delta Neutrophil Index (DNI) as Predictor of 28 Days Mortality.
Patients were classified into subgroups according to age (≥70 or < 70). Interestingly, age ≥70 group did not show statistically significant difference of DNI 1 values between survivor and non- survivor group, in univariate analysis. Age < 70 group showed statistically significant difference DNI 1values between survivor and non- survivor group. DNI 2 and DNI 3 showed statistically significant difference DNI 1values between survivor and non- survivor group in all age (
Table 4).
Table 1.
Baseline characteristics of the patients with pneumonia sepsis.
Table 1.
Baseline characteristics of the patients with pneumonia sepsis.
| Variables |
Values (n=227) |
| Age, mean (SD) |
74 ± 13.7 |
| Sex (Male) (N, %) |
150 (66.1%) |
| Septic shock (N, %) |
116 (51.1%) |
| Cormobidities(N, %) |
|
| DM |
124(54.6 %) |
| HTN |
79 (34.8%) |
| Heart Disease |
32 (14.1%) |
| Stroke |
14 (6.2%) |
| COPD |
36(15.9%) |
| IPF* |
12(4.8%) |
| Dementia |
24(10.6%) |
| Chronic Liver Disease |
27 (11.9%) |
| Solid cancer |
32 (14.1%) |
| Severity on ICU admission |
|
| APACHE II (SD) |
22.0 ± 5.9 |
| Treatment in ICU(N, %) |
|
| Mechanical ventilation |
129 (56.8%) |
| Tracheostomy |
87 (38.3%) |
| CRRT |
25 (11.0 %) |
| ECMO |
5 (2.2%) |
| ILA |
4(1.7%) |
| Laboratory findings (SD) |
Values |
| WBC(103/ul) |
13227.8 ± 7864.3 |
| Hb (g/dl) |
11.8 ± 3.1 |
| Platelet (103/ul) |
244.6 ± 133.9 |
| Neutrophil(%) |
79.1 ± 16.5 |
| DNI 1 (%) |
6.9 ± 12.9 |
| DNI 2 (%) |
5.9 ± 12.0 |
DNI 3 (%)
Combined DNI(%) |
4.9 ± 12.5
17.6 ± 33.5
|
| Na (mEq/L) |
137.2 ± 10.0 |
| K(mEq/L) |
4.2 ± 0.8 |
| BUN (mg/dl) |
28.4 ± 21.0 |
| Cr(mg/dl) |
1.4 ± 2.2 |
| AST(IU/L) |
67.4 ± 213.9 |
| ALT( IU/L) |
41.5 ± 123.1 |
| BNP (pg/ml) |
371.7 ± 564.5 |
| CRP(mg/dl) |
125.4 ± 108.6 |
| Procalcitonin(ng/ml) |
5.2 ± 16.9 |
| Lactic acid (mmol/L) |
3.4 ± 3.8 |
Table 2.
Univariable analysis in Survivor vs Nonsurvivor in 28 days mortality.
Table 2.
Univariable analysis in Survivor vs Nonsurvivor in 28 days mortality.
| Variables |
Survivors (N=181) |
Non survivors (N=46) |
p-value |
| Age |
73.1 ± 13.8 |
77.6 ± 13.1 |
0.05 |
| Sex (Male, %) |
115 (63.5) |
35 (76.1) |
0.15 |
| Septic shock |
98 (54.1) |
28 (60.9) |
0.09 |
| Severity scores |
|
|
|
| APACHE |
21.7 ± 5.9 |
23.3 ± 6.1 |
0.10 |
| Treatment in ICU |
|
|
|
| MV |
97 (53.6) |
32 (69.6) |
0.07 |
| CRRT |
19 (10.5) |
22 (47.8) |
0.82 |
| ECMO |
2 (1.1) |
2 (4.3) |
0.57 |
| ILA |
3 (1.6) |
2 (4.3) |
0.64 |
| Tracheostomy |
69 (38.1) |
18 (39.1) |
0.98 |
| Laboratory findings |
|
|
|
| WBC(103/ul) |
12998.0 ± 7834.8 |
14132.0 ± 8001.2 |
0.39 |
| Neutrophil (%) |
80.5 ± 14.5 |
73.6 ± 22.0 |
0.05 |
| DNI 1(%) |
5.8 ± 12.4 |
11.4 ± 13.8 |
0.01 |
| DNI 2(%) |
3.8 ± 9.2 |
14.5 ± 17.1 |
0.00 |
| DNI 3(%) |
2.3 ± 8.6 |
14.9 ± 19.0 |
0.00 |
| Combined DNI (%) |
4.6 (0.6-12.3) |
21.5 (5.7-67.0) |
0.00 |
| Hb (g/dl) |
11.8 ± 3.2 |
11.5 ± 2.5 |
0.48 |
| Platelet(103/ul) |
248.3 ± 138.0 |
229.9 ± 116.8 |
0.36 |
| Na (mEq/L) |
136.9 ± 9.9 |
138.4 ± 10.4 |
0.39 |
| K(mEq/L) |
4.6 ± 0.9 |
4.1 ± 0.8 |
0.06 |
| BUN (mg/dl) |
36.8 ± 25.0 |
26.3 ± 19.3 |
0.07 |
| Cr(mg/dl) |
1.5 ± 1.3 |
1.4 ± 2.4 |
0.53 |
| AST(IU/L) |
140.5 ± 460.2 |
48.8 ± 52.8 |
0.18 |
| ALT(IU/L) |
68.0 ± 226.5 |
34.8 ± 77.3 |
0.33 |
| BNP (pg/ml) |
128.5 ± 106.5 |
124.7 ± 109.4 |
0.83 |
| CRP(mg/dl) |
6.0 ± 14.6 |
5.0 ± 17.5 |
0.69 |
| Procalcitonin(ng/ml) |
446.8 ± 546.4 |
352.3 ± 569.0 |
0.32 |
| Lactic acid (mmol/L) |
5.5 ± 5.6 |
2.8 ± 2.9 |
0.00 |
Table 3.
Multivariable analysis of predictive factors for 28-day mortality.
Table 3.
Multivariable analysis of predictive factors for 28-day mortality.
| Variables |
Adj Odds ratio ( 95% CI) |
p-value |
| Age |
0.97 (0.95, 1.1) |
0.05 |
| Sex |
2.07 (0.86, 5.03) |
0.11 |
| DNI 1 |
1.04 (0.98, 1.1) |
0.17 |
| DNI 2 |
0.98 (0.9, 1.06) |
0.60 |
| DNI 3 |
0.94 (0.89, 0.99) |
0.04 |
| Lactic acid |
0.86 (0.78, 0.95) |
0.00 |
Table 4.
Age -subgroup analysis of DNI for 28 days mortality.
Table 4.
Age -subgroup analysis of DNI for 28 days mortality.
| |
Coeff.(95%CI) |
P value |
| DNI 1 |
|
|
| ≥70 |
-4.23 (-9.2,0.73) |
0.097 |
| <70 |
-11.67 (-19.6,-3.74) |
0.005 |
| DNI 2 |
|
|
| ≥70 |
-8.93 (-13.43,-4.43) |
< 0.001 |
| <70 |
-19.38 (-25.83,-12.94) |
<0.001 |
| DNI 3 |
|
|
| ≥70 |
-10.47 (-14.74,-6.2) |
< 0.001 |
| <70 |
-23.76 (-31.67,-15.86) |
< 0.001 |
4. Discussion
This study showed that DNI, which reflects the number of circulating granulocyte precursors in the blood, at 72 hours after ICU admission, can be a useful prognostic factor for 28-day mortality in patients with pneumonia-induced sepsis. In our study, DNI was higher in the non-survivor group than in the survivor group throughout the treatment period, although statistical significance was confirmed only at 72 hours from ICU admission. In our study, in multivariate analysis, lactic acid (adj. OR. 0.86, 95% CI: 0.78-0.95, p = 0.00), and DNI 3 (adj. OR. 0.94, 95% CI: 0.89-0.99, p = 0.04) were the risk factors for 28-day mortality. Using a cutoff value of 2.6%, the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the DNI 3 in sepsis were found to be 69%, 73.9%, 77.9%, and 64.1%. Interestingly, age ≥70 group did not show statistically significant difference of DNI 1 values between survivor and non- survivor group, in univariate analysis.
These findings agree with some previous reports. An elevated DNI often reflects a heightened immune response due to the body's attempt to combat severe infection. Research suggests that DNI is a valuable early indicator in sepsis as it may help differentiate sepsis from other non-infectious conditions and provide a quick estimate of the severity of infection. Higher DNI levels have been associated with increased severity of sepsis, poorer patient outcomes, and even higher mortality rates. [
5,
7,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29].
Recent studies suggest that sepsis impairs innate immunity of patients[
30,
31]. Neutrophil paralysis in sepsis results in the failure of neutrophils to migrate to the site of infection and causes inappropriate neutrophil sequestration in remote organs[
32]. We proposed that neutrophil paralysis in sepsis may cause a rapid and early production of immature neutrophils to compensate for the deficiency of active neutrophil. Since higher DNI levels correlate with increased numbers of immature neutrophils, patients with higher DNI levels may have more dysregulated immune functions. Those patients who maintain a high DNI until 72 hours after start of treatment may have sustained dysregulation of immunity. Thus, patients with higher DNI levels may have worse prognosis in the pathogenesis of pneumonia sepsis. Therefore, DNI at 72 hours could be an alarming marker to check the patient’s status again and to consider other treatment strategies.
However, cut-off values of DNI for predicting mortality varied. In our study, the optimal cut-off DNI for predicting mortality was 2.6%. The higher DNI group (≥2.6), measured 72 hours after ICU admission, showed significantly higher 28-day mortality (
p = 0.00) than the lower DNI group (<2.6). Similarly, Lee at al reported that a cut-off DNI of 2% at 72 hours after onset of neonatal sepsis was associated with the 7-day mortality rate[
33]. However, a previous study by Kim
et al. reported that the optimal cut-off DNI for predicting mortality was 7.6% in patients with gram negative bacteremia[
20]. Furthermore, Park
et al. reported that a DNI >6.5% was a good predictor of severe sepsis and septic shock within 24 hours of admission to an ICU [
13]. Therefore, further evaluation of the adequate cut-off value of DNI is needed.
We demonstrated that the DNI correlated with the severity of pneumonia-induced sepsis in the ICU. DNI values were higher in the septic shock group compared to the sepsis group. Previously, Park et al. showed DNI may be used as a marker of disease severity in critically ill patients with sepsis[
13]. Given that the process of granular leukocyte differentiation starts from immature granulocyte formation, the change in DNI may have preceded the change in absolute numbers of WBCs or neutrophils, thus contributing to predicting the development of septic shock. Therefore, it is important for clinicians to identify patients who are at risk of developing septic shock before the signs of organ dysfunction or circulatory failure appear.
Interestingly, in our study, elderly patients (≥70), a DNI 1 did not show statistical significance between the survival group and the non survival group. It could be several explanations related to the immune system and its response in aging individuals: First theory is immune depression in old age group. Immunosenescence refers to the gradual deterioration of the immune system associated with aging. In older individuals, both the innate and adaptive immune responses tend to weaken, which means that their bodies may not mount the same level of response to infection as younger people do[
34]. A lower DNI in older adults could be due to a decreased production of neutrophils, particularly immature granulocytes, during infections or inflammatory processes. The bone marrow in older individuals may not respond as robustly to signals that normally stimulate neutrophil production[
35]. Second theory is older individuals often experience chronic low-grade inflammation, sometimes referred to as "inflammaging." This persistent, low-level inflammation might lead to a baseline activation of the immune system, which can mask or reduce the body's capacity to produce a surge of neutrophils in response to acute infections[
36]. This state of chronic immune activation could potentially lead to lower increases in immature neutrophils, resulting in a lower DNI when acute infection occurs. Third theory is older adults often have multiple comorbid conditions (e.g., diabetes, chronic kidney disease) that can affect their immune response and ability to produce neutrophils. Certain medications commonly used in older populations, such as immune suppressants or corticosteroids, can also blunt the body's inflammatory response and neutrophil production. These factors can contribute to a lower DNI in older patients, as their overall immune response may be suppressed or altered by both their underlying conditions and treatments.
Our study has the following strengths. Although there has been substantial research on DNI, studies specifically on pneumonia sepsis are limited. This study demonstrated that DNI values could be significant in predicting outcomes for patients with pneumonia sepsis. Notably, DNI was measured sequentially on days 1, 2, and 3, and the third-day value was shown to have predictive significance for prognosis. This suggests that continuous measurement of DNI may be practically necessary. Additionally, this study indicates that DNI interpretation may differ in elderly patients, emphasizing the importance of serial follow-up for older patients.
Although our study suggests the prognostic value of DNI in pneumonia-induced sepsis patients, several limitations exist. First, this study was conducted retrospectively in a single center; therefore, the possibility of selection bias remains. Secondly, the elevation of immature granulocytes is not specific for infection and may be observed in various other conditions such as myeloproliferative disorder, chronic inflammatory disorders, tissue damage, acute hemorrhage, and neoplasia. Thirdly, because we evaluated only short-term mortality, it is still questionable whether DNI 3 can predict long-term outcome in patients with pneumonia-induced sepsis. Therefore, more studies with large number of patients are required to validate the clinical usefulness of DNI as a severity and prediction marker of pneumonia –induced sepsis.
5. Conclusion
These data shed new light on the role of the DNI in pneumonia-induced sepsis. DNI measured 72 hours after ICU admission may serve as a useful prognostic marker for 28-day mortality in patients with pneumonia-induced sepsis in the ICU. Especially, patients with higher DNI 3 (≥ 2.6) showed higher 28-day mortality than patients with lower DNI 3 (< 2.6) group. There was not a study on the clinical usefulness of DNI in assessing the prognosis of patients with pneumonia-induced sepsis in the ICU. Early detection and treatment initiation is essential for improving the treatment outcome in pneumonia-induced sepsis. Therefore, the identification of reliable biomarkers for diagnosis and guidance of treatment in sepsis patients is required. Our data show the usefulness of DNI at 72 hours as a prognostic marker in patients with pneumonia-induced sepsis in the ICU. Based on these results, we suggest that increased DNI value should alert clinicians to apply more aggressive therapy.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Table S1. Baseline characteristics of the patients with pneumonia sepsis. Figure S1. ROC analysis of DNI 3 for 28 days mortality.
Author Contributions
Concept and design: S-Y P. Acquisition, analysis, or interpretation of data: S-Y M Drafting of the manuscript: S-Y M, Critical revision of the manuscript for important intellectual content: Y- B P, Y-S K, C-W H, S-H P, J-W L, S-Y P, Statistical analysis: S-Y M , S-Y P, Administrative, technical, or material support: none. Supervision: S-Y M, Y-B P, Y-S K, C-W H, S-H P, J-W L, S-Y P , All authors have read and agreed to the published version of the manuscript.
Funding
Supported by a grant no. 2022-08 from the Kangdong Sacred Heart Hospital Fund.
Institutional Review Board Statement
The Institutional Review Board of each institution approved the study protocol. Informed consent was obtained before data collection. The study was therefore performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. This is a retrospective study conducted from a de-identified database, and informed consent of participation is waived by the Institutional Review Board of Kangdong Sacred Heart Hospital, Republic of Korea (IRB No. 2022-03-015-006).
Informed Consent Statement
The need for obtaining informed consent was waived because of the retrospective nature of the study. .
Data Availability Statement
The datasets are available from corresponding author on reasonable request.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Restrepo, M.I.; Mortensen, E.M.; Velez, J.A.; Frei, C.; Anzueto, A. A comparative study of community-acquired pneumonia patients admitted to the ward and the ICU. Chest 2008, 133, 610-7. [CrossRef]
- Yu, H.; Rubin, J.; Dunning, S.; Li, S.; Sato, R. Clinical and economic burden of community-acquired pneumonia in the Medicare fee-for-service population. J Am Geriatr Soc 2012, 60, 2137-43. [CrossRef]
- Watkins, R.R.; Lemonovich, T.L. Diagnosis and management of community-acquired pneumonia in adults. Am Fam Physician 2011, 83, 1299-306.
- Aliberti, S.; Amir, A.; Peyrani, P.; Mirsaeidi, M.; Allen, M.; Moffett, B.K.; Myers, J.; Shaib, F.; Cirino, M.; Bordon, J.; et al. Incidence, etiology, timing, and risk factors for clinical failure in hospitalized patients with community-acquired pneumonia. Chest 2008, 134, 955-62. [CrossRef]
- |, B.B.O.D.B.T.A.G.A.; Erdem3, A.A.S.E.Y.D. Efficacy of the delta neutrophil index in predicting 30-day mortality in COVID-19 patients requiring intensive care. The international journal of clinical practice 2021, 75, 213970.
- Huttunen, R.; Aittoniemi, J. New concepts in the pathogenesis, diagnosis and treatment of bacteremia and sepsis. The Journal of infection 2011, 63, 407-19. [CrossRef]
- Chiwon Ahn, W.K., Tae Ho Lim, Youngsuk Cho, Kyu-Sun Choi, Bo-Hyoung Jang. The delta neutrophil index (DNI) as a prognostic marker for mortality in adults with sepsis: a systematic review and meta-analysis. SCIENTIFIC REPORTS 2018, 26.
- Mare, T.A.e.a. The diagnostic and prognostic significance of monitoring blood levels of immature neutrophils in patients with systemic inflammation. Crit. Care 2015, 19.
- Cavallazzi, R., Bennin, C.-L., Hirani, A., Gilbert, C. & Marik, P. E. Review of A Large Clinical Series: Is the Band Count Useful in the Diagnosis of Infection? . J. Intensive Care Med 2010, 25, 353–357.
- PJ, C. Clinical utility of the band count. Clin Lab Med 2002, Mar, 101-36.
- Nahm, C.H.; Choi, J.W.; Lee, J. Delta neutrophil index in automated immature granulocyte counts for assessing disease severity of patients with sepsis. Ann Clin Lab Sci 2008, 38, 241-6.
- Seok, Y.; Choi, J.R.; Kim, J.; Kim, Y.K.; Lee, J.; Song, J.; Kim, S.J.; Lee, K.A. Delta neutrophil index: a promising diagnostic and prognostic marker for sepsis. Shock (Augusta, Ga.) 2012, 37, 242-6. [CrossRef]
- Park, B.H.; Kang, Y.A.; Park, M.S.; Jung, W.J.; Lee, S.H.; Lee, S.K.; Kim, S.Y.; Kim, S.K.; Chang, J.; Jung, J.Y.; et al. Delta neutrophil index as an early marker of disease severity in critically ill patients with sepsis. BMC Infect Dis 2011, 11, 299. [CrossRef]
- Kim, H.; Kim, Y.; Kim, K.H.; Yeo, C.D.; Kim, J.W.; Lee, H.K. Use of delta neutrophil index for differentiating low-grade community-acquired pneumonia from upper respiratory infection. Ann Lab Med 2015, 35, 647-50. [CrossRef]
- Goag, E.K.L., J. W. Roh, Y. H. Leem, A. Y. Kim, S. Y. Song, J. H. Kim, E. Y. Jung, J. Y. Park, M. S. Kim, Y. S. Kim, S. K. Chang, J. Chung, K. S. A Simplified Mortality Score Using Delta Neutrophil Index and the Thrombotic Microangiopathy Score for Prognostication in Critically Ill Patients. Shock (Augusta, Ga.) 2018, 49, 39-43. [CrossRef]
- Kim, H.; Kong, T.; Chung, S.P.; Hong, J.H.; Lee, J.W.; Joo, Y.; Ko, D.R.; You, J.S.; Park, I. Usefulness of the Delta Neutrophil Index as a Promising Prognostic Marker of Acute Cholangitis in Emergency Departments. Shock (Augusta, Ga.) 2017, 47, 303-312. [CrossRef]
- Hwang, Y.J.; Chung, S.P.; Park, Y.S.; Chung, H.S.; Lee, H.S.; Park, J.W.; Lee, J.W.; Hong, J.H.; You, J.S.; Park, I. Newly designed delta neutrophil index-to-serum albumin ratio prognosis of early mortality in severe sepsis. Am J Emerg Med 2015, 33, 1577-82. [CrossRef]
- Goncalves-Pereira, J.; Conceicao, C.; Povoa, P. Community-acquired pneumonia: identification and evaluation of nonresponders. Ther Adv Infect Dis 2013, 1, 5-17. [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive care medicine 2017, 43, 304-377. [CrossRef]
- Kim HW, Y.J., Jin SJ, Kim SB, Ku NS, Jeong SJ,. Delta neutrophil index as a prognostic marker of early mortality in gram negative bacteremia. . Infection & chemotherapy. 2014, 46, 94-102.
- Taehun Lee 1 , J.L., Dong Hoon Shin 3, Hyungdon Lee 4,*,† and Soo-Ki Kim 5,*,†. Prognostic and Diagnostic Power of Delta Neutrophil Index and Mean Platelet Component in Febrile Patients with Suspected Sepsis. Biomedicines 2023, 11, 3190.
- ShinID, J.e.; 1, K.D.S., Hyun Jae Cha2, Jong Wook Lee3, Youn Moo Heo2,; Kwang Kyoun Kim2, T.G.K., Chan Kang4, Gi Soo Lee4, Jae Hwang SongID. Usefulness of the delta neutrophil index in predicting surgery in patients with foot and ankle infection. Plos one 2022, AUGUST.
- Jun Hyoung Kim 1 2, Y.L., Yun Suk Cho 1, Yu Jin Sohn 1, Jong Hoon Hyun 1, Sang Min Ahn 1, Woon Ji Lee 1 2, Hye Seong 1 2, Jung Ho Kim 1 2, Jin Young Ahn 1 2, Su Jin Jeong 1 2, Nam Su Ku 1 2, Jun Yong Choi 1 2, Joon-Sup Yeom 1 2, Young Goo Song 1. A Modified Simple Scoring System Using the Red Blood Cell Distribution Width, Delta Neutrophil Index, and Mean Platelet Volume-to-Platelet Count to Predict 28-Day Mortality in Patients With Sepsis. Journal of intensive care medicine 2021, 36, 873-878.
- Birkan Birben , G.A., Tezcan Akın , Aziz A. Surel , Mesut Tez. Predictive Efficacy of Delta Neutrophil Index in Diagnosis of Acute and Complicated Appendicitis. cureus 2021, 13, e14748.
- Park1, S.Y.; , J.S.L., Jihyu Oh1 and Ji-Young Park2. Delta neutrophil index as a predictive and prognostic factor for Candidemia patients: a matched case-control study. BMC Infectious Diseases 2020, 20.
- Lim2, J.S.S.S.W. Delta neutrophil index as a prognostic marker in emergent abdominal surgery. J Clin Lab Anal. 2019, 33, e22895.
- Ji Hoon Kim, Y.S.P., * Chang-Yun Yoon,† Hye Sun Lee,‡; Sinae Kim, J.W.L., § Taeyoung Kong,*jj Je Sung You,*; Jong Woo Park, a.S.P.C. DELTA NEUTROPHIL INDEX FOR THE PREDICTION OF THE DEVELOPMENT OF SEPSIS-INDUCED ACUTE KIDNEY INJURY IN THE EMERGENCY DEPARTMENT. Shock (Augusta, Ga.) 2019, 52, 414-422.
- Byung Woo Jhun 1, Yun Su Sim1, Tae Rim Shin1 & Dong-Gyu Kim1. Use of Delta Neutrophil Index for Differentiating Low-Grade Community-Acquired Pneumonia From Upper Respiratory Infection. SCIENTIFIC REPORTS 2018, 8, 12348.
- Ahn, C.; Kim, W.; Lim, T.H.; Cho, Y.; Choi, K.-S.; Jang, B.-H. The delta neutrophil index (DNI) as a prognostic marker for mortality in adults with sepsis: a systematic review and meta-analysis. Scientific Reports 2018, 8, 6621. [CrossRef]
- Hotchkiss RS, M.G., Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. The lancet infectious disease 2013, 13, 260-268.
- Willem Joost Wiersinga, S.J.L., Duncan R Cranendonk, and Tom van der Poll. Host innate immune responses to sepsis. Virulence 2014, Jan, 36-44.
- Alves-Filho, J.C.; Spiller, F.; Cunha, F.Q. Neutrophil paralysis in sepsis. Shock (Augusta, Ga.) 2010, 34 Suppl 1, 15-21. [CrossRef]
- Lee SM, E.H., Namgung R, Park MS, Park KI, Lee C. Usefulness of the delta neutrophil index for assessing neonatal sepsis. Acta paediatrica 2013, 102, e13-16.
- Kelci Straka, M.-L.T., Summer Millwood, James Swanson and; Kuhlman, K.R. Aging as a Context for the Role of Inflammation in Depressive Symptoms. frontier in psychiatry 2021, 11, 1-13.
- Ferreyro, B.L.; De Jong, A.; Grieco, D.L. Correction: How to use facemask noninvasive ventilation. Intensive Care Medicine 2024. [CrossRef]
- Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nature Reviews Endocrinology 2018, 14, 576-590.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).






