Submitted:
03 January 2025
Posted:
04 January 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Cells in Established Atherosclerotic Plaques
2.1. Endothelial Cells (ECs)
2.2. Vascular Smooth Muscle Cells (VSMCs)
2.3. Neutrophils
2.4. Macrophages
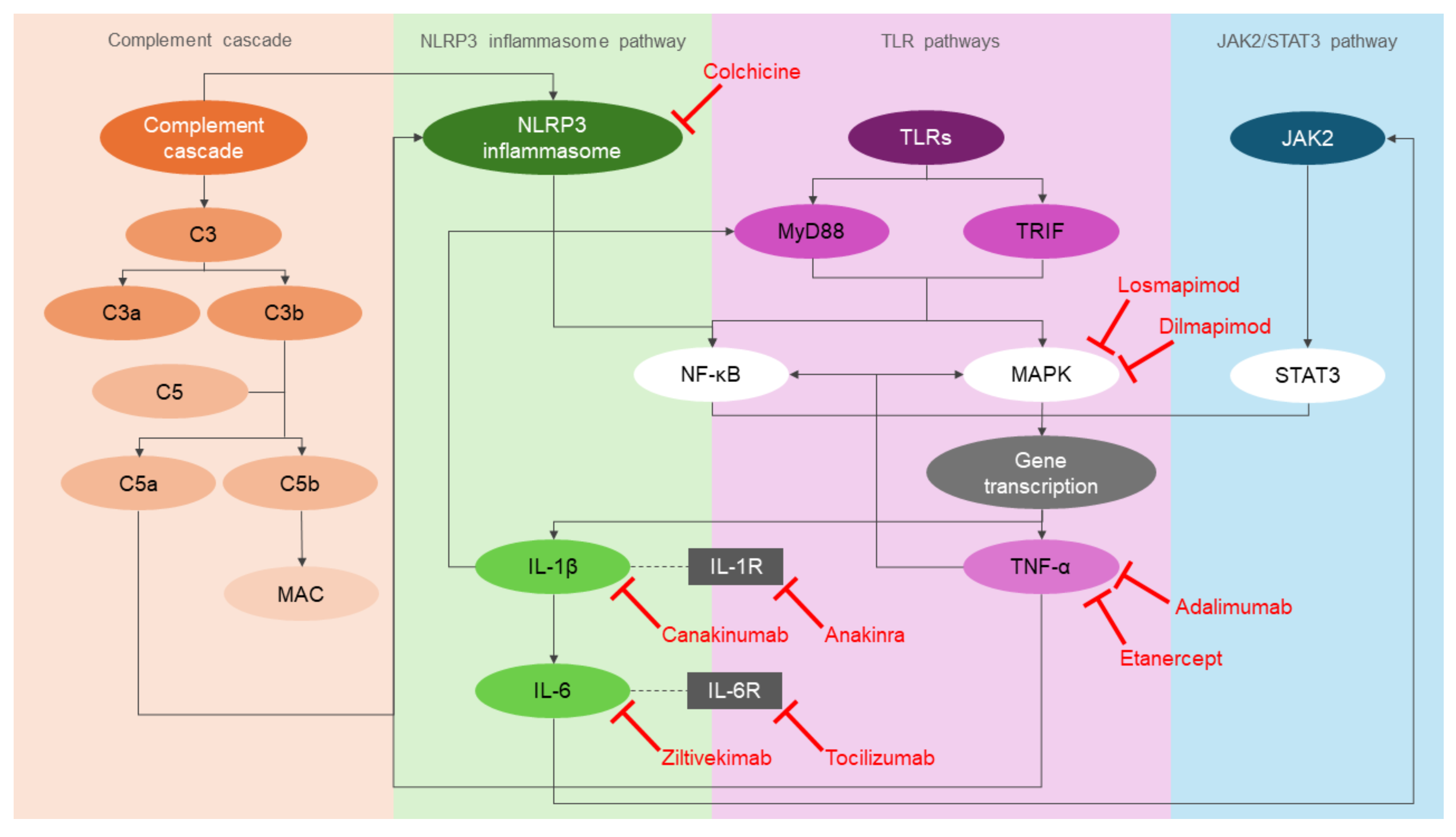
3. Inflammatory Pathways in Atherogenesis
3.1. NOD-, LRR- and Pyrin Domain-Containing Protein 3 (NLRP3) Inflammasome Pathway
3.2. Toll-Like Receptor (TLR) Pathways
3.3. Complement Cascade
3.4. Janus Kinase 2/Signal Transducer and Activator of Transcription 3 (JAK2/STAT3) Pathway
4. Clinical Trials Using Anti-Inflammatory Drugs for Secondary Prevention of CAD
4.1. IL-1 Signalling Pathway Inhibitors: Canakinumab and Anakinra
4.2. IL-6 Signalling Pathway Inhibitors: Tocilizumab and Ziltivekimab
4.3. TNF-α Signalling Pathway Inhibitors: Etanercept and Adalimumab
4.4. p38 MAPK Signalling Pathway Inhibitors: Losmapimod and Dilmapimod
4.5. Colchicine
5. Limitations of Previous Studies and Future Directions
5.1. Cell Culture Models
5.2. Mouse Models
5.3. Human Biospecimens
5.4. Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- British Heart Foundation. (2024, January). Global Heart & Circulatory Diseases Factsheet. https://www.bhf.org.uk/-/media/files/for-professionals/research/heart-statistics/bhf-cvd-statistics-global-factsheet.pdf.
- Mir, M. A., Dar, M. A., & Qadir, A. (2024). Exploring the Landscape of Coronary Artery Disease: A Comprehensive Review. American Journal of Biomedicine and Pharmacy, 1(1), 9-22.
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of Plaque Formation and Rupture. Circ. Res. 2014, 114, 1852–1866. [CrossRef]
- Virmani, R., Kolodgie, F. D., Burke, A. P., Farb, A., & Schwartz, S. M. (2000). Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arteriosclerosis, thrombosis, and vascular biology, 20(5), 1262-1275. [CrossRef]
- Otsuka, F.; Yasuda, S.; Noguchi, T.; Ishibashi-Ueda, H. Pathology of coronary atherosclerosis and thrombosis. Cardiovasc. Diagn. Ther. 2016, 6, 396–408. [CrossRef]
- Luo, X.; Lv, Y.; Bai, X.; Qi, J.; Weng, X.; Liu, S.; Bao, X.; Jia, H.; Yu, B. Plaque Erosion: A Distinctive Pathological Mechanism of Acute Coronary Syndrome. Front. Cardiovasc. Med. 2021, 8. [CrossRef]
- Stark, B.; Johnson, C.; Roth, G.A. GLOBAL PREVALENCE OF CORONARY ARTERY DISEASE: AN UPDATE FROM THE GLOBAL BURDEN OF DISEASE STUDY. Circ. 2024, 83, 2320. [CrossRef]
- Byrne, P., Demasi, M., Jones, M., Smith, S. M., O’Brien, K. K., & DuBroff, R. (2022). Evaluating the association between low-density lipoprotein cholesterol reduction and relative and absolute effects of statin treatment: a systematic review and meta-analysis. JAMA internal medicine, 182(5), 474-481. [CrossRef]
- Nidorf, S.M.; Eikelboom, J.W.; Budgeon, C.A.; Thompson, P.L. Low-Dose Colchicine for Secondary Prevention of Cardiovascular Disease. Circ. 2013, 61, 404–410. [CrossRef]
- O’Donoghue, M. L., Glaser, R., Cavender, M. A., Aylward, P. E., Bonaca, M. P., Budaj, A., ... & LATITUDE-TIMI 60 Investigators. (2016). Effect of losmapimod on cardiovascular outcomes in patients hospitalized with acute myocardial infarction: a randomized clinical trial. Jama, 315(15), 1591-1599. [CrossRef]
- Rymer, J.A.; Newby, L.K. Failure to Launch: Targeting Inflammation in Acute Coronary Syndromes. 2017, 2, 484–497. [CrossRef]
- Vallejo, J., Cochain, C., Zernecke, A., & Ley, K. (2021). Heterogeneity of immune cells in human atherosclerosis revealed by scRNA-Seq. Cardiovascular research, 117(13), 2537-2543. [CrossRef]
- de Winther, M.P.J.; Bäck, M.; Evans, P.; Gomez, D.; Goncalves, I.; Jørgensen, H.F.; Koenen, R.R.; Lutgens, E.; Norata, G.D.; Osto, E.; et al. Translational opportunities of single-cell biology in atherosclerosis. Eur. Hear. J. 2022, 44, 1216–1230. [CrossRef]
- Milutinović, A.; Šuput, D.; Zorc-Pleskovič, R. Pathogenesis of atherosclerosis in the tunica intima, media, and adventitia of coronary arteries: An updated review. Bosn. J. Basic Med Sci. 2019, 20, 21–30. [CrossRef]
- Tricot, O.; Mallat, Z.; Heymes, C.; Belmin, J.; Lesèche, G.; Tedgui, A. Relation Between Endothelial Cell Apoptosis and Blood Flow Direction in Human Atherosclerotic Plaques. Circulation 2000, 101, 2450–2453. [CrossRef]
- Zhang, Y.; Xie, Y.; You, S.; Han, Q.; Cao, Y.; Zhang, X.; Xiao, G.; Chen, R.; Liu, C. Autophagy and Apoptosis in the Response of Human Vascular Endothelial Cells to Oxidized Low-Density Lipoprotein. Cardiology 2015, 132, 27–33. [CrossRef]
- Lee, C.N.; Cheng, W.F.; Chang, M.C.; Su, Y.N.; Chen, C.A.; Hsieh, F.J. Hypoxia-induced apoptosis in endothelial cells and embryonic stem cells. Apoptosis 2005, 10, 887–894. [CrossRef]
- Rastogi, S.; Rizwani, W.; Joshi, B.; Kunigal, S.; Chellappan, S.P. TNF-α response of vascular endothelial and vascular smooth muscle cells involve differential utilization of ASK1 kinase and p73. Cell Death Differ. 2011, 19, 274–283. [CrossRef]
- Zhang, J.; Wang, Z.; Zuo, G.; Li, B.; Zhang, J.; Tian, N.; Chen, S. Low shear stress induces human vascular endothelial cell apoptosis by activating Akt signal and increasing reactive oxygen species.. 2013, 33, 313–7.
- Dong, G.; Yang, S.; Cao, X.; Yu, N.; Yu, J.; Qu, X. Low shear stress-induced autophagy alleviates cell apoptosis in HUVECs. Mol. Med. Rep. 2017, 15, 3076–3082. [CrossRef]
- Hu, Y.; Hur, S.S.; Lei, L.; Wang, Y.; Chien, S. Shear Stress Induces Apoptosis via Cytochrome C Release from Dynamic Mitochondria in Endothelial Cells. FASEB J. 2017, 31. [CrossRef]
- Jimenez, J.J.; Jy, W.; Mauro, L.M.; Soderland, C.; Horstman, L.L.; Ahn, Y.S. Endothelial cells release phenotypically and quantitatively distinct microparticles in activation and apoptosis. Thromb. Res. 2003, 109, 175–180. [CrossRef]
- Hussein, M.A.; Böing, A.; Biró, É.; Hoek, F.; Vogel, G.; Meuleman, D.; Sturk, A.; Nieuwland, R. Phospholipid composition of in vitro endothelial microparticles and their in vivo thrombogenic properties. Thromb. Res. 2008, 121, 865–871. [CrossRef]
- Mallat, Z.; Benamer, H.; Hugel, B.; Benessiano, J.; Steg, P.G.; Freyssinet, J.-M.; Tedgui, A. Elevated Levels of Shed Membrane Microparticles With Procoagulant Potential in the Peripheral Circulating Blood of Patients With Acute Coronary Syndromes. Circulation 2000, 101, 841–843. [CrossRef]
- Bernal-Mizrachi, L.; Jy, W.; Jimenez, J.J.; Pastor, J.; Mauro, L.M.; Horstman, L.L.; de Marchena, E.; Ahn, Y.S. High levels of circulating endothelial microparticles in patients with acute coronary syndromes. Am. Hear. J. 2003, 145, 962–970. [CrossRef]
- Alvandi, Z.; Bischoff, J. Endothelial-Mesenchymal Transition in Cardiovascular Disease. Arter. Thromb. Vasc. Biol. 2021, 41, 2357–2369. [CrossRef]
- Evrard, S.M.; Lecce, L.; Michelis, K.C.; Nomura-Kitabayashi, A.; Pandey, G.; Purushothaman, K.-R.; D’escamard, V.; Li, J.R.; Hadri, L.; Fujitani, K.; et al. Endothelial to mesenchymal transition is common in atherosclerotic lesions and is associated with plaque instability. Nat. Commun. 2016, 7, 11853. [CrossRef]
- Newman, A.A.C.; Serbulea, V.; Baylis, R.A.; Shankman, L.S.; Bradley, X.; Alencar, G.F.; Owsiany, K.; Deaton, R.A.; Karnewar, S.; Shamsuzzaman, S.; et al. Multiple cell types contribute to the atherosclerotic lesion fibrous cap by PDGFRβ and bioenergetic mechanisms. Nat. Metab. 2021, 3, 166–181. [CrossRef]
- Kovacic, J.C.; Dimmeler, S.; Harvey, R.P.; Finkel, T.; Aikawa, E.; Krenning, G.; Baker, A.H. Endothelial to Mesenchymal Transition in Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 190–209. [CrossRef]
- Harman, J.L.; Jørgensen, H.F. The role of smooth muscle cells in plaque stability: Therapeutic targeting potential. Br. J. Pharmacol. 2019, 176, 3741–3753. [CrossRef]
- Burke, A.P.; Farb, A.; Malcom, G.T.; Liang, Y.-H.; Smialek, J.; Virmani, R. Coronary Risk Factors and Plaque Morphology in Men with Coronary Disease Who Died Suddenly. N. Engl. J. Med. 1997, 336, 1276–1282. [CrossRef]
- Mocci, G.; Sukhavasi, K.; Örd, T.; Bankier, S.; Singha, P.; Arasu, U.T.; Agbabiaje, O.O.; Mäkinen, P.; Ma, L.; Hodonsky, C.J.; et al. Single-Cell Gene-Regulatory Networks of Advanced Symptomatic Atherosclerosis. Circ. Res. 2024, 134, 1405–1423. [CrossRef]
- Basatemur, G. L., Jørgensen, H. F., Clarke, M. C., Bennett, M. R., & Mallat, Z. (2019). Vascular smooth muscle cells in atherosclerosis. Nature reviews cardiology, 16(12), 727-744. [CrossRef]
- Geng, Y.J.; Libby, P. Evidence for apoptosis in advanced human atheroma. Colocalization with interleukin-1 beta-converting enzyme.. 1995, 147, 251–66.
- Lutgens, E.; de Muinck, E.D.; Kitslaar, P.J.; Tordoir, J.H.; Wellens, H.J.; Daemen, M.J. Biphasic pattern of cell turnover characterizes the progression from fatty streaks to ruptured human atherosclerotic plaques. Cardiovasc. Res. 1999, 41, 473–479. [CrossRef]
- Kockx, M.M.; De Meyer, G.R.Y.; Muhring, J.; Jacob, W.; Bult, H.; Herman, A.G. Apoptosis and Related Proteins in Different Stages of Human Atherosclerotic Plaques. Circulation 1998, 97, 2307–2315. [CrossRef]
- Clarke, M.C.H.; Figg, N.; Maguire, J.J.; Davenport, A.P.; Goddard, M.; Littlewood, T.D.; Bennett, M.R. Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat. Med. 2006, 12, 1075–1080. [CrossRef]
- Clarke, M.C.; Littlewood, T.D.; Figg, N.; Maguire, J.J.; Davenport, A.P.; Goddard, M.; Bennett, M.R. Chronic Apoptosis of Vascular Smooth Muscle Cells Accelerates Atherosclerosis and Promotes Calcification and Medial Degeneration. Circ. Res. 2008, 102, 1529–1538. [CrossRef]
- Bauriedel, G.; Hutter, R.; Welsch, U.; Bach, R.; Sievert, H.; Lüderitz, B. Role of smooth muscle cell death in advanced coronary primary lesions: implications for plaque instability. Cardiovasc. Res. 1999, 41, 480–488. [CrossRef]
- Schrijvers, D.M.; De Meyer, G.R.; Kockx, M.M.; Herman, A.G.; Martinet, W. Phagocytosis of Apoptotic Cells by Macrophages Is Impaired in Atherosclerosis. Arter. Thromb. Vasc. Biol. 2005, 25, 1256–1261. [CrossRef]
- Allahverdian, S.; Chehroudi, A.C.; McManus, B.M.; Abraham, T.; Francis, G.A. Contribution of Intimal Smooth Muscle Cells to Cholesterol Accumulation and Macrophage-Like Cells in Human Atherosclerosis. Circulation 2014, 129, 1551–1559. [CrossRef]
- Allahverdian, S.; Pannu, P.S.; Francis, G.A. Contribution of monocyte-derived macrophages and smooth muscle cells to arterial foam cell formation. Cardiovasc. Res. 2012, 95, 165–172. [CrossRef]
- Yancey, P.G.; Blakemore, J.; Ding, L.; Fan, D.; Overton, C.D.; Zhang, Y.; Linton, M.F.; Fazio, S. Macrophage LRP-1 Controls Plaque Cellularity by Regulating Efferocytosis and Akt Activation. Arter. Thromb. Vasc. Biol. 2010, 30, 787–795. [CrossRef]
- Clarke, M. C., Talib, S., Figg, N. L., & Bennett, M. R. (2010). Vascular smooth muscle cell apoptosis induces interleukin-1–directed inflammation: effects of hyperlipidemia-mediated inhibition of phagocytosis. Circulation research, 106(2), 363-372. [CrossRef]
- Matthews, C., Gorenne, I., Scott, S., Figg, N., Kirkpatrick, P., Ritchie, A., ... & Bennett, M. (2006). Vascular smooth muscle cells undergo telomere-based senescence in human atherosclerosis: effects of telomerase and oxidative stress. Circulation research, 99(2), 156-164. [CrossRef]
- Bennett, M. R., Sinha, S., & Owens, G. K. (2016). Vascular smooth muscle cells in atherosclerosis. Circulation research, 118(4), 692-702. [CrossRef]
- Wang, J.; Uryga, A.K.; Reinhold, J.; Figg, N.; Baker, L.; Finigan, A.; Gray, K.; Kumar, S.; Clarke, M.; Bennett, M. Vascular Smooth Muscle Cell Senescence Promotes Atherosclerosis and Features of Plaque Vulnerability. Circulation 2015, 132, 1909–1919. [CrossRef]
- Zhang, X.; Kang, Z.; Yin, D.; Gao, J. Role of neutrophils in different stages of atherosclerosis. J. Endotoxin Res. 2023, 29, 97–109. [CrossRef]
- Naruko, T.; Ueda, M.; Haze, K.; van der Wal, A.C.; van der Loos, C.M.; Itoh, A.; Komatsu, R.; Ikura, Y.; Ogami, M.; Shimada, Y.; et al. Neutrophil Infiltration of Culprit Lesions in Acute Coronary Syndromes. Circulation 2002, 106, 2894–2900. [CrossRef]
- Soehnlein, O. Multiple Roles for Neutrophils in Atherosclerosis. Circ. Res. 2012, 110, 875–888. [CrossRef]
- Quinn, K.L.; Henriques, M.; Tabuchi, A.; Han, B.; Yang, H.; Cheng, W.-E.; Tole, S.; Yu, H.; Luo, A.; Charbonney, E.; et al. Human Neutrophil Peptides Mediate Endothelial-Monocyte Interaction, Foam Cell Formation, and Platelet Activation. Arter. Thromb. Vasc. Biol. 2011, 31, 2070–2079. [CrossRef]
- Grechowa, I.; Horke, S.; Wallrath, A.; Vahl, C.; Dorweiler, B. Human neutrophil elastase induces endothelial cell apoptosis by activating the PERK-CHOP branch of the unfolded protein response. FASEB J. 2017, 31, 3868–3881. [CrossRef]
- Moilanen, M.; Sorsa, T.; Stenman, M.; Nyberg, P.; Lindy, O.; Vesterinen, J.; Paju, A.; Konttinen, Y.T.; Stenman, U.-H.; Salo, T. Tumor-Associated Trypsinogen-2 (Trypsinogen-2) Activates Procollagenases (MMP-1, -8, -13) and Stromelysin-1 (MMP-3) and Degrades Type I Collagen. Biochemistry 2003, 42, 5414–5420. [CrossRef]
- Momiyama, Y.; Ohmori, R.; Tanaka, N.; Kato, R.; Taniguchi, H.; Adachi, T.; Nakamura, H.; Ohsuzu, F. High plasma levels of matrix metalloproteinase-8 in patients with unstable angina. Atherosclerosis 2009, 209, 206–210. [CrossRef]
- Herman, M. P., Sukhova, G. K., Libby, P., Gerdes, N., Tang, N., Horton, D. B., ... & Schönbeck, U. (2001). Expression of neutrophil collagenase (matrix metalloproteinase-8) in human atheroma: a novel collagenolytic pathway suggested by transcriptional profiling. Circulation, 104(16), 1899-1904. [CrossRef]
- Weiss, S.J.; Peppin, G.; Ortiz, X.; Ragsdale, C.; Test, S.T. Oxidative Autoactivation of Latent Collagenase by Human Neutrophils. Science 1985, 227, 747–749. [CrossRef]
- Dollery, C. M., Owen, C. A., Sukhova, G. K., Krettek, A., Shapiro, S. D., & Libby, P. (2003). Neutrophil elastase in human atherosclerotic plaques: production by macrophages. Circulation, 107(22), 2829-2836. [CrossRef]
- Mokry, M.; Boltjes, A.; Slenders, L.; Bel-Bordes, G.; Cui, K.; Brouwer, E.; Mekke, J.M.; Depuydt, M.A.C.; Timmerman, N.; Waissi, F.; et al. Transcriptomic-based clustering of human atherosclerotic plaques identifies subgroups with different underlying biology and clinical presentation. Nat. Cardiovasc. Res. 2022, 1, 1140–1155. [CrossRef]
- Gierlikowska, B., Stachura, A., Gierlikowski, W., & Demkow, U. (2021). Phagocytosis, degranulation and extracellular traps release by neutrophils—the current knowledge, pharmacological modulation and future prospects. Frontiers in Pharmacology, 12, 666732. [CrossRef]
- Pertiwi, K. R., van der Wal, A. C., Pabittei, D. R., Mackaaij, C., van Leeuwen, M. B., Li, X., & de Boer, O. J. (2018). Neutrophil extracellular traps participate in all different types of thrombotic and haemorrhagic complications of coronary atherosclerosis. Thrombosis and haemostasis, 118(06), 1078-1087. [CrossRef]
- Quillard, T.; Araújo, H.A.; Franck, G.; Shvartz, E.; Sukhova, G.; Libby, P. TLR2 and neutrophils potentiate endothelial stress, apoptosis and detachment: implications for superficial erosion. Eur. Hear. J. 2015, 36, 1394–1404. [CrossRef]
- Saffarzadeh, M.; Juenemann, C.; Queisser, M.A.; Lochnit, G.; Barreto, G.; Galuska, S.P.; Lohmeyer, J.; Preissner, K.T. Neutrophil Extracellular Traps Directly Induce Epithelial and Endothelial Cell Death: A Predominant Role of Histones. PLOS ONE 2012, 7, e32366. [CrossRef]
- Silvestre-Roig, C.; Braster, Q.; Wichapong, K.; Lee, E.Y.; Teulon, J.M.; Berrebeh, N.; Winter, J.; Adrover, J.M.; Santos, G.S.; Froese, A.; et al. Externalized histone H4 orchestrates chronic inflammation by inducing lytic cell death. Nature 2019, 569, 236–240. [CrossRef]
- Kolodgie, F.D.; Narula, J.; Burke, A.P.; Haider, N.; Farb, A.; Hui-Liang, Y.; Smialek, J.; Virmani, R. Localization of Apoptotic Macrophages at the Site of Plaque Rupture in Sudden Coronary Death. Am. J. Pathol. 2000, 157, 1259–1268. [CrossRef]
- Popa-Fotea, N.-M.; Ferdoschi, C.-E.; Micheu, M.-M. Molecular and cellular mechanisms of inflammation in atherosclerosis. Front. Cardiovasc. Med. 2023, 10, 1200341. [CrossRef]
- Gordon, S. (2003). Alternative activation of macrophages. Nature reviews immunology, 3(1), 23-35. [CrossRef]
- Mallat, Z., Hugel, B., Ohan, J., Leseche, G., Freyssinet, J. M., & Tedgui, A. (1999). Shed membrane microparticles with procoagulant potential in human atherosclerotic plaques: a role for apoptosis in plaque thrombogenicity. Circulation, 99(3), 348-353. [CrossRef]
- Gonzalez, L.; Trigatti, B.L. Macrophage Apoptosis and Necrotic Core Development in Atherosclerosis: A Rapidly Advancing Field with Clinical Relevance to Imaging and Therapy. Can. J. Cardiol. 2017, 33, 303–312. [CrossRef]
- Thorp, E.; Tabas, I. Mechanisms and consequences of efferocytosis in advanced atherosclerosis. J. Leukoc. Biol. 2009, 86, 1089–1095. [CrossRef]
- Otsuka, F.; Kramer, M.C.; Woudstra, P.; Yahagi, K.; Ladich, E.; Finn, A.V.; de Winter, R.J.; Kolodgie, F.D.; Wight, T.N.; Davis, H.R.; et al. Natural progression of atherosclerosis from pathologic intimal thickening to late fibroatheroma in human coronary arteries: A pathology study. Atherosclerosis 2015, 241, 772–782. [CrossRef]
- Björkegren, J.L.; Lusis, A.J. Atherosclerosis: Recent developments. Cell 2022, 185, 1630–1645. [CrossRef]
- Huang, W. C., Sala-Newby, G. B., Susana, A., Johnson, J. L., & Newby, A. C. (2012). Classical macrophage activation up-regulates several matrix metalloproteinases through mitogen activated protein kinases and nuclear factor-κB. [CrossRef]
- Lenglet, S.; Mach, F.; Montecucco, F. Role of Matrix Metalloproteinase-8 in Atherosclerosis. Mediat. Inflamm. 2013, 2013, 1–6. [CrossRef]
- Fligiel, S.E.; Varani, J.; Datta, S.C.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Collagen Degradation in Aged/Photodamaged Skin In Vivo and After Exposure to Matrix Metalloproteinase-1 In Vitro. J. Investig. Dermatol. 2003, 120, 842–848. [CrossRef]
- Sukhova, G.K.; Schönbeck, U.; Rabkin, E.; Schoen, F.J.; Poole, A.R.; Billinghurst, R.C.; Libby, P. Evidence for Increased Collagenolysis by Interstitial Collagenases-1 and -3 in Vulnerable Human Atheromatous Plaques. Circulation 1999, 99, 2503–2509. [CrossRef]
- Shah, P.K.; Falk, E.; Badimon, J.J.; Fernandez-Ortiz, A.; Mailhac, A.; Villareal-Levy, G.; Fallon, J.T.; Regnstrom, J.; Fuster, V. Human monocyte-derived macrophages induce collagen breakdown in fibrous caps of atherosclerotic plaques. Potential role of matrix-degrading metalloproteinases and implications for plaque rupture.. 1995, 92, 1565–9.
- Shi, X.; Xie, W.-L.; Kong, W.-W.; Chen, D.; Qu, P. Expression of the NLRP3 Inflammasome in Carotid Atherosclerosis. J. Stroke Cerebrovasc. Dis. 2015, 24, 2455–2466. [CrossRef]
- Silvis, M.J.M.; Demkes, E.J.; Fiolet, A.T.L.; Dekker, M.; Bosch, L.; van Hout, G.P.J.; Timmers, L.; de Kleijn, D.P.V. Immunomodulation of the NLRP3 Inflammasome in Atherosclerosis, Coronary Artery Disease, and Acute Myocardial Infarction. J. Cardiovasc. Transl. Res. 2020, 14, 23–34. [CrossRef]
- Kong, P.; Cui, Z.-Y.; Huang, X.-F.; Zhang, D.-D.; Guo, R.-J.; Han, M. Inflammation and atherosclerosis: signaling pathways and therapeutic intervention. Signal Transduct. Target. Ther. 2022, 7, 131. [CrossRef]
- Misawa, T.; Takahama, M.; Kozaki, T.; Lee, H.; Zou, J.; Saitoh, T.; Akira, S. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat. Immunol. 2013, 14, 454–460. [CrossRef]
- Varghese, G.P.; Folkersen, L.; Strawbridge, R.J.; Halvorsen, B.; Yndestad, A.; Ranheim, T.; Krohg-Sørensen, K.; Skjelland, M.; Espevik, T.; Aukrust, P.; et al. NLRP3 Inflammasome Expression and Activation in Human Atherosclerosis. J. Am. Hear. Assoc. 2016, 5. [CrossRef]
- Jiang, C.; Jiang, L.; Li, Q.; Liu, X.; Zhang, T.; Dong, L.; Liu, T.; Liu, L.; Hu, G.; Sun, X.; et al. Acrolein induces NLRP3 inflammasome-mediated pyroptosis and suppresses migration via ROS-dependent autophagy in vascular endothelial cells. Toxicology 2018, 410, 26–40. [CrossRef]
- Burger, F.; Baptista, D.; Roth, A.; da Silva, R.F.; Montecucco, F.; Mach, F.; Brandt, K.J.; Miteva, K. NLRP3 Inflammasome Activation Controls Vascular Smooth Muscle Cells Phenotypic Switch in Atherosclerosis. Int. J. Mol. Sci. 2021, 23, 340. [CrossRef]
- Münzer, P.; Negro, R.; Fukui, S.; di Meglio, L.; Aymonnier, K.; Chu, L.; Cherpokova, D.; Gutch, S.; Sorvillo, N.; Shi, L.; et al. NLRP3 Inflammasome Assembly in Neutrophils Is Supported by PAD4 and Promotes NETosis Under Sterile Conditions. Front. Immunol. 2021, 12, 683803. [CrossRef]
- Zheng, F.; Xing, S.; Gong, Z.; Mu, W.; Xing, Q. Silence of NLRP3 Suppresses Atherosclerosis and Stabilizes Plaques in Apolipoprotein E-Deficient Mice. Mediat. Inflamm. 2014, 2014, 1–8. [CrossRef]
- Jin, M.; Fang, J.; Wang, J.-J.; Shao, X.; Xu, S.-W.; Liu, P.-Q.; Ye, W.-C.; Liu, Z.-P. Regulation of toll-like receptor (TLR) signaling pathways in atherosclerosis: from mechanisms to targeted therapeutics. Acta Pharmacol. Sin. 2023, 44, 2358–2375. [CrossRef]
- Edfeldt, K.; Swedenborg, J.; Hansson, G.K.; Yan, Z.-Q. Expression of toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation.. Circulation 2002, 105, 1158–1161. [CrossRef]
- Monaco, C.; Gregan, S.M.; Navin, T.J.; Foxwell, B.M.; Davies, A.H.; Feldmann, M. Toll-Like Receptor-2 Mediates Inflammation and Matrix Degradation in Human Atherosclerosis. Circulation 2009, 120, 2462–2469. [CrossRef]
- Yang, K.; Zhang, X.J.; Cao, L.J.; Liu, X.H.; Liu, Z.H.; Wang, X.Q.; Chen, Q.J.; Lu, L.; Shen, W.F.; Liu, Y. Toll-Like Receptor 4 Mediates Inflammatory Cytokine Secretion in Smooth Muscle Cells Induced by Oxidized Low-Density Lipoprotein. PLOS ONE 2014, 9, e95935. [CrossRef]
- Koushki, K.; Shahbaz, S.K.; Mashayekhi, K.; Sadeghi, M.; Zayeri, Z.D.; Taba, M.Y.; Banach, M.; Al-Rasadi, K.; Johnston, T.P.; Sahebkar, A. Anti-inflammatory Action of Statins in Cardiovascular Disease: the Role of Inflammasome and Toll-Like Receptor Pathways. Clin. Rev. Allergy Immunol. 2020, 60, 175–199. [CrossRef]
- Wang, L.; Peng, Y.; Song, L.; Xia, D.; Li, C.; Li, Z.; Li, Q.; Yu, A.; Lu, C.; Wang, Y. Colchicine-Containing Nanoparticles Attenuates Acute Myocardial Infarction Injury by Inhibiting Inflammation. Cardiovasc. Drugs Ther. 2021, 36, 1075–1089. [CrossRef]
- Kiss, M.G.; Binder, C.J. The multifaceted impact of complement on atherosclerosis. Atherosclerosis 2022, 351, 29–40. [CrossRef]
- Martínez-López, D.; Roldan-Montero, R.; García-Marqués, F.; Nuñez, E.; Jorge, I.; Camafeita, E.; Minguez, P.; de Cordoba, S.R.; López-Melgar, B.; Lara-Pezzi, E.; et al. Complement C5 Protein as a Marker of Subclinical Atherosclerosis. Circ. 2020, 75, 1926–1941. [CrossRef]
- Garcia-Arguinzonis, M.; Diaz-Riera, E.; Peña, E.; Escate, R.; Juan-Babot, O.; Mata, P.; Badimon, L.; Padro, T. Alternative C3 Complement System: Lipids and Atherosclerosis. Int. J. Mol. Sci. 2021, 22, 5122. [CrossRef]
- Oksjoki, R.; Laine, P.; Helske, S.; Vehmaan-Kreula, P.; Mäyränpää, M.I.; Gasque, P.; Kovanen, P.T.; Pentikäinen, M.O. Receptors for the anaphylatoxins C3a and C5a are expressed in human atherosclerotic coronary plaques. Atherosclerosis 2007, 195, 90–99. [CrossRef]
- Speidl, W.S.; Kasd, S.P.; Hutter, R.; Katsaros, K.M.; Kaun, C.; Bauriedel, G.; Maurer, G.; Huber, K.; Badimon, J.J.; Wojta, J. The complement component C5a is present in human coronary lesions in vivo and induces the expression of MMP-1 and MMP-9 in human macrophages in vitro. FASEB J. 2010, 25, 35–44. [CrossRef]
- Speidl, W.S.; Exner, M.; Amighi, J.; Kastl, S.P.; Zorn, G.; Maurer, G.; Wagner, O.; Huber, K.; Minar, E.; Wojta, J.; et al. Complement component C5a predicts future cardiovascular events in patients with advanced atherosclerosis. Eur. Hear. J. 2005, 26, 2294–2299. [CrossRef]
- Oksjoki, R., Kovanen, P. T., Mäyränpää, M. I., Laine, P., Blom, A. M., Meri, S., & Pentikäinen, M. O. (2007). Complement regulation in human atherosclerotic coronary lesions: immunohistochemical evidence that C4b-binding protein negatively regulates the classical complement pathway, and that C5b-9 is formed via the alternative complement pathway. Atherosclerosis, 192(1), 40-48. [CrossRef]
- Si, W.; He, P.; Wang, Y.; Fu, Y.; Li, X.; Lin, X.; Chen, F.; Cao, G.; Zhang, H. Complement Complex C5b-9 Levels Are Associated with the Clinical Outcomes of Acute Ischemic Stroke and Carotid Plaque Stability. Transl. Stroke Res. 2018, 10, 279–286. [CrossRef]
- Lindberg, S.; Pedersen, S.H.; Mogelvang, R.; Galatius, S.; Flyvbjerg, A.; Jensen, J.S.; Bjerre, M. Soluble form of membrane attack complex independently predicts mortality and cardiovascular events in patients with ST-elevation myocardial infarction treated with primary percutaneous coronary intervention. Am. Hear. J. 2012, 164, 786–792. [CrossRef]
- Monsinjon, T.; Gasque, P.; Chan, P.; Ischenko, A.; Brady, J.J.; Fontaine, M. Regulation by complement C3a and C5a anaphylatoxins of cytokine production in human umbilical vein endothelial cells. FASEB J. 2003, 17, 1003–1014. [CrossRef]
- Samstad, E.O.; Niyonzima, N.; Nymo, S.; Aune, M.H.; Ryan, L.; Bakke, S.S.; Lappegård, K.T.; Brekke, O.-L.; Lambris, J.D.; Damås, J.K.; et al. Cholesterol Crystals Induce Complement-Dependent Inflammasome Activation and Cytokine Release. J. Immunol. 2014, 192, 2837–2845. [CrossRef]
- Niyonzima, N.; Bakke, S.S.; Gregersen, I.; Holm, S.; Sandanger, Ø.; Orrem, H.L.; Sporsheim, B.; Ryan, L.; Kong, X.Y.; Dahl, T.B.; et al. Cholesterol crystals use complement to increase NLRP3 signaling pathways in coronary and carotid atherosclerosis. EBioMedicine 2020, 60. [CrossRef]
- Ikeda, K.; Nagasawa, K.; Horiuchi, T.; Tsuru, T.; Nishizaka, H.; Niho, Y. C5a Induces Tissue Factor Activity on Endothelial Cells. Thromb. Haemost. 1997, 77, 394–398. [CrossRef]
- Li, B.; Xu, Y.-J.; Chu, X.-M.; Gao, M.-H.; Wang, X.-H.; Nie, S.-M.; Yang, F.; Lv, C.-Y. Molecular mechanism of inhibitory effects of CD59 gene on atherosclerosis in ApoE (−/−) mice. Immunol. Lett. 2013, 156, 68–81. [CrossRef]
- Fosbrink, M.; Niculescu, F.; Rus, H. The Role of C5b-9 Terminal Complement Complex in Activation of the Cell Cycle and Transcription. Immunol. Res. 2005, 31, 37–46. [CrossRef]
- Pulanco, M.C.; Cosman, J.; Ho, M.-M.; Huynh, J.; Fing, K.; Turcu, J.; Fraser, D.A. Complement Protein C1q Enhances Macrophage Foam Cell Survival and Efferocytosis. J. Immunol. 2017, 198, 472–480. [CrossRef]
- Wei, L.-L.; Ma, N.; Wu, K.-Y.; Wang, J.-X.; Diao, T.-Y.; Zhao, S.-J.; Bai, L.; Liu, E.; Li, Z.-F.; Zhou, W.; et al. Protective Role of C3aR (C3a Anaphylatoxin Receptor) Against Atherosclerosis in Atherosclerosis-Prone Mice. Arter. Thromb. Vasc. Biol. 2020, 40, 2070–2083. [CrossRef]
- Pang, Q.; You, L.; Meng, X.; Li, Y.; Deng, T.; Li, D.; Zhu, B. Regulation of the JAK/STAT signaling pathway: The promising targets for cardiovascular disease. Biochem. Pharmacol. 2023, 213, 115587. [CrossRef]
- Chen, Q.; Lv, J.; Yang, W.; Xu, B.; Wang, Z.; Yu, Z.; Wu, J.; Yang, Y.; Han, Y. Targeted inhibition of STAT3 as a potential treatment strategy for atherosclerosis. Theranostics 2019, 9, 6424–6442. [CrossRef]
- Zhang, X.; Chen, S.; Yin, G.; Liang, P.; Feng, Y.; Yu, W.; Meng, D.; Liu, H.; Zhang, F. The Role of JAK/STAT Signaling Pathway and Its Downstream Influencing Factors in the Treatment of Atherosclerosis. J. Cardiovasc. Pharmacol. Ther. 2024, 29. [CrossRef]
- Mazière, C.; Conte, M.-A.; Mazière, J.-C. Activation of JAK2 by the oxidative stress generated with oxidized low-density lipoprotein. Free. Radic. Biol. Med. 2001, 31, 1334–1340. [CrossRef]
- Taleb, S.; Romain, M.; Ramkhelawon, B.; Uyttenhove, C.; Pasterkamp, G.; Herbin, O.; Esposito, B.; Perez, N.; Yasukawa, H.; Van Snick, J.; et al. Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J. Exp. Med. 2009, 206, 2067–2077. [CrossRef]
- Gharavi, N.M.; Alva, J.A.; Mouillesseaux, K.P.; Lai, C.; Yeh, M.; Yeung, W.; Johnson, J.; Szeto, W.L.; Hong, L.; Fishbein, M.; et al. Role of the JAK/STAT Pathway in the Regulation of Interleukin-8 Transcription by Oxidized Phospholipids in Vitro and in Atherosclerosis in Vivo. J. Biol. Chem. 2007, 282, 31460–31468. [CrossRef]
- Manea, A.; Tanase, L.I.; Raicu, M.; Simionescu, M. JAK/STAT Signaling Pathway Regulates Nox1 and Nox4-Based NADPH Oxidase in Human Aortic Smooth Muscle Cells. Arter. Thromb. Vasc. Biol. 2010, 30, 105–112. [CrossRef]
- Yeh, M.; Gharavi, N.M.; Choi, J.; Hsieh, X.; Reed, E.; Mouillesseaux, K.P.; Cole, A.L.; Reddy, S.T.; Berliner, J.A. Oxidized Phospholipids Increase Interleukin 8 (IL-8) Synthesis by Activation of the c-src/Signal Transducers and Activators of Transcription (STAT)3 Pathway. J. Biol. Chem. 2004, 279, 30175–30181. [CrossRef]
- Alanazi, A. Z., & Clark, M. A. (2019). Angiotensin III induces JAK2/STAT3 leading to IL-6 production in rat vascular smooth muscle cells. International Journal of Molecular Sciences, 20(22), 5551. [CrossRef]
- Khan, J.A.; Cao, M.; Kang, B.-Y.; Liu, Y.; Mehta, J.L.; Hermonat, P.L. AAV/hSTAT3-gene delivery lowers aortic inflammatory cell infiltration in LDLR KO mice on high cholesterol. Atherosclerosis 2010, 213, 59–66. [CrossRef]
- Wang, K., Li, B., Xie, Y., Xia, N., Li, M., & Gao, G. (2020). Statin rosuvastatin inhibits apoptosis of human coronary artery endothelial cells through upregulation of the JAK2/STAT3 signaling pathway. Molecular Medicine Reports, 22(3), 2052-2062. [CrossRef]
- Dutzmann, J.; Daniel, J.-M.; Bauersachs, J.; Hilfiker-Kleiner, D.; Sedding, D.G. Emerging translational approaches to target STAT3 signalling and its impact on vascular disease. Cardiovasc. Res. 2015, 106, 365–374. [CrossRef]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2017, 281, 8–27. [CrossRef]
- Thompson, P.L.; Nidorf, S.M. Anti-inflammatory therapy with canakinumab for atherosclerotic disease: lessons from the CANTOS trial. J. Thorac. Dis. 2018, 10, 695–698. [CrossRef]
- Abbate, A.; Toldo, S.; Marchetti, C.; Kron, J.; Van Tassell, B.W.; Dinarello, C.A. Interleukin-1 and the Inflammasome as Therapeutic Targets in Cardiovascular Disease. Circ. Res. 2020, 126, 1260–1280. [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [CrossRef]
- Morton, A.C.; Rothman, A.M.K.; Greenwood, J.P.; Gunn, J.; Chase, A.; Clarke, B.; Hall, A.S.; Fox, K.; Foley, C.; Banya, W.; et al. The effect of interleukin-1 receptor antagonist therapy on markers of inflammation in non-ST elevation acute coronary syndromes: the MRC-ILA Heart Study. Eur. Hear. J. 2014, 36, 377–384. [CrossRef]
- Abbate, A.; Trankle, C.R.; Buckley, L.F.; Lipinski, M.J.; Appleton, D.; Kadariya, D.; Canada, J.M.; Carbone, S.; Roberts, C.S.; Abouzaki, N.; et al. Interleukin-1 Blockade Inhibits the Acute Inflammatory Response in Patients With ST-Segment–Elevation Myocardial Infarction. J. Am. Hear. Assoc. 2020, 9, e014941. [CrossRef]
- Van Tassell, B.W.; Toldo, S.; Mezzaroma, E.; Abbate, A. Targeting Interleukin-1 in Heart Disease. Circulation 2013, 128, 1910–1923. [CrossRef]
- Interleukin-1 Blockade in Acute Myocardial Infarction to Prevent Heart Failure (VA-ART4). Clinicaltrials.gov. (2024, May 03). https://clinicaltrials.gov/study/NCT05177822.
- Anderson, D.R.; Poterucha, J.T.; Mikuls, T.R.; Duryee, M.J.; Garvin, R.P.; Klassen, L.W.; Shurmur, S.W.; Thiele, G.M. IL-6 and its receptors in coronary artery disease and acute myocardial infarction. Cytokine 2013, 62, 395–400. [CrossRef]
- Peeters, W., Hellings, W. E., De Kleijn, D. P. V., De Vries, J. P. P. M., Moll, F. L., Vink, A., & Pasterkamp, G. (2009). Carotid atherosclerotic plaques stabilize after stroke: insights into the natural process of atherosclerotic plaque stabilization. Arteriosclerosis, thrombosis, and vascular biology, 29(1), 128-133. [CrossRef]
- Reiss, A. B., Siegart, N. M., & De Leon, J. (2017). Interleukin-6 in atherosclerosis: atherogenic or atheroprotective?. Clinical Lipidology, 12(1), 14-23. [CrossRef]
- Garbers, C., Aparicio-Siegmund, S., & Rose-John, S. (2015). The IL-6/gp130/STAT3 signaling axis: recent advances towards specific inhibition. Current opinion in immunology, 34, 75-82. [CrossRef]
- Rakocevic, J.; Dobric, M.; Borovic, M.L.; Milutinovic, K.; Milenkovic, S.; Tomasevic, M. Anti-Inflammatory Therapy in Coronary Artery Disease: Where Do We Stand?. Rev. Cardiovasc. Med. 2023, 24, 10. [CrossRef]
- Kleveland, O.; Kunszt, G.; Bratlie, M.; Ueland, T.; Broch, K.; Holte, E.; Michelsen, A.E.; Bendz, B.; Amundsen, B.H.; Espevik, T.; et al. Effect of a single dose of the interleukin-6 receptor antagonist tocilizumab on inflammation and troponin T release in patients with non-ST-elevation myocardial infarction: a double-blind, randomized, placebo-controlled phase 2 trial. Eur. Hear. J. 2016, 37, 2406–2413. [CrossRef]
- Broch, K.; Anstensrud, A.K.; Woxholt, S.; Sharma, K.; Tøllefsen, I.M.; Bendz, B.; Aakhus, S.; Ueland, T.; Amundsen, B.H.; Damås, J.K.; et al. Randomized Trial of Interleukin-6 Receptor Inhibition in Patients With Acute ST-Segment Elevation Myocardial Infarction. Circ. 2021, 77, 1845–1855. [CrossRef]
- Kunkel, J.B.; Frydland, M.; Holle, S.D.; Kjaergaard, J.; Pecini, R.; Bang, L.E.; Palm, P.; Wiberg, S.; Holmvang, L.; Engstroem, T.; et al. Low-dose dobutamine infusion and single-dose tocilizumab in acute myocardial infarction patients with high risk of cardiogenic shock development - rationale and design of the DOBERMANN trial. Eur. Hear. Journal. Acute Cardiovasc. Care 2023, 12. [CrossRef]
- Kawashiri, S.-Y.; Kawakami, A.; Yamasaki, S.; Imazato, T.; Iwamoto, N.; Fujikawa, K.; Aramaki, T.; Tamai, M.; Nakamura, H.; Ida, H.; et al. Effects of the anti-interleukin-6 receptor antibody, tocilizumab, on serum lipid levels in patients with rheumatoid arthritis. Rheumatol. Int. 2009, 31, 451–456. [CrossRef]
- Ridker, P.M. From RESCUE to ZEUS: will interleukin-6 inhibition with ziltivekimab prove effective for cardiovascular event reduction?. Cardiovasc. Res. 2021, 117, e138–e140. [CrossRef]
- Specifying the Anti-inflammatory Effects of Ziltivekimab (SPIDER). Clinicaltrials.gov. (2024, March 25). https://clinicaltrials.gov/study/NCT06263244.
- ARTEMIS - A Research Study to Look at How Ziltivekimab Works Compared to Placebo in People With a Heart Attack (ARTEMIS). Clinicaltrials.gov. (2024, November 15). https://clinicaltrials.gov/study/NCT06118281.
- Kojima, Y.; Volkmer, J.-P.; McKenna, K.; Civelek, M.; Lusis, A.J.; Miller, C.L.; Direnzo, D.; Nanda, V.; Ye, J.; Connolly, A.J.; et al. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature 2016, 536, 86–90. [CrossRef]
- Rolski, F.; Błyszczuk, P. Complexity of TNF-α Signaling in Heart Disease. J. Clin. Med. 2020, 9, 3267. [CrossRef]
- Brånén, L.; Hovgaard, L.; Nitulescu, M.; Bengtsson, E.; Nilsson, J.; Jovinge, S. Inhibition of Tumor Necrosis Factor-α Reduces Atherosclerosis in Apolipoprotein E Knockout Mice. Arter. Thromb. Vasc. Biol. 2004, 24, 2137–2142. [CrossRef]
- Chistiakov, D.A.; Melnichenko, A.A.; Grechko, A.V.; Myasoedova, V.A.; Orekhov, A.N. Potential of anti-inflammatory agents for treatment of atherosclerosis. Exp. Mol. Pathol. 2018, 104, 114–124. [CrossRef]
- Mann, D. L., McMurray, J. J., Packer, M., Swedberg, K., Borer, J. S., Colucci, W. S., ... & Fleming, T. (2004). Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation, 109(13), 1594-1602. [CrossRef]
- Oberoi, R.; Schuett, J.; Schuett, H.; Koch, A.-K.; Luchtefeld, M.; Grote, K.; Schieffer, B. Targeting Tumor Necrosis Factor-α with Adalimumab: Effects on Endothelial Activation and Monocyte Adhesion. PLOS ONE 2016, 11, e0160145. [CrossRef]
- Gonzalez-Juanatey, C.; Vazquez-Rodriguez, T.R.; Miranda-Filloy, J.A.; Gomez-Acebo, I.; Testa, A.; Garcia-Porrua, C.; Sanchez-Andrade, A.; Llorca, J.; González-Gay, M.A. Anti-TNF-Alpha-Adalimumab Therapy Is Associated with Persistent Improvement of Endothelial Function without Progression of Carotid Intima-Media Wall Thickness in Patients with Rheumatoid Arthritis Refractory to Conventional Therapy. Mediat. Inflamm. 2012, 2012, 1–8. [CrossRef]
- Kearney, N., Chen, X., Bi, Y., Hew, K., Smith, K. M., & Kirby, B. (2024). Treatment of hidradenitis suppurativa with adalimumab in the PIONEER I and II randomized controlled trials reduced indices of systemic inflammation, recognized risk factors for cardiovascular disease. Clinical and Experimental Dermatology, llae324. [CrossRef]
- Boesten, L. S., Zadelaar, A. S. M., van Nieuwkoop, A., Gijbels, M. J., de Winther, M. P., Havekes, L. M., & van Vlijmen, B. J. (2005). Tumor necrosis factor-α promotes atherosclerotic lesion progression in APOE* 3-leiden transgenic mice. Cardiovascular research, 66(1), 179-185. [CrossRef]
- Fisk, M.; Gajendragadkar, P.R.; Mäki-Petäjä, K.M.; Wilkinson, I.B.; Cheriyan, J. Therapeutic Potential of p38 MAP Kinase Inhibition in the Management of Cardiovascular Disease. Am. J. Cardiovasc. Drugs 2014, 14, 155–165. [CrossRef]
- Elkhawad, M.; Rudd, J.H.; Sarov-Blat, L.; Cai, G.; Wells, R.; Davies, L.C.; Collier, D.J.; Marber, M.S.; Choudhury, R.P.; Fayad, Z.A.; et al. Effects of p38 Mitogen-Activated Protein Kinase Inhibition on Vascular and Systemic Inflammation in Patients With Atherosclerosis. JACC: Cardiovasc. Imaging 2012, 5, 911–922. [CrossRef]
- Sarov-Blat, L.; Morgan, J.M.; Fernandez, P.; James, R.; Fang, Z.; Hurle, M.R.; Baidoo, C.; Willette, R.N.; Lepore, J.J.; Jensen, S.E.; et al. Inhibition of p38 Mitogen-Activated Protein Kinase Reduces Inflammation After Coronary Vascular Injury in Humans. Arter. Thromb. Vasc. Biol. 2010, 30, 2256–2263. [CrossRef]
- Newby, L.K.; Marber, M.S.; Melloni, C.; Sarov-Blat, L.; Aberle, L.H.; E Aylward, P.; Cai, G.; de Winter, R.J.; Hamm, C.W.; Heitner, J.F.; et al. Losmapimod, a novel p38 mitogen-activated protein kinase inhibitor, in non-ST-segment elevation myocardial infarction: a randomised phase 2 trial. 2014, 384, 1187–1195. [CrossRef]
- Hammaker, D., & Firestein, G. S. (2010). “Go upstream, young man”: lessons learned from the p38 saga. Annals of the rheumatic diseases, 69(Suppl 1), i77-i82. [CrossRef]
- Leung, Y.Y.; Yao Hui, L.L.; Kraus, V.B. Colchicine—Update on mechanisms of action and therapeutic uses. Semin. Arthritis Rheum. 2015, 45, 341–350. [CrossRef]
- Forkosh, E.; Kenig, A.; Ilan, Y. Introducing variability in targeting the microtubules: Review of current mechanisms and future directions in colchicine therapy. Pharmacol. Res. Perspect. 2020, 8, e00616. [CrossRef]
- Takenouchi, T.; Iwamaru, Y.; Sugama, S.; Sato, M.; Hashimoto, M.; Kitani, H. Lysophospholipids and ATP Mutually Suppress Maturation and Release of IL-1β in Mouse Microglial Cells Using a Rho-Dependent Pathway. J. Immunol. 2008, 180, 7827–7839. [CrossRef]
- Vaidya, K.; Tucker, B.; Kurup, R.; Khandkar, C.; Pandzic, E.; Barraclough, J.; Machet, J.; Misra, A.; Kavurma, M.; Martinez, G.; et al. Colchicine Inhibits Neutrophil Extracellular Trap Formation in Patients With Acute Coronary Syndrome After Percutaneous Coronary Intervention. J. Am. Hear. Assoc. 2021, 10, e018993. [CrossRef]
- Nidorf, S.M.; Fiolet, A.T.L.; Mosterd, A.; Eikelboom, J.W.; Schut, A.; Opstal, T.S.J.; The, S.H.K.; Xu, X.-F.; Ireland, M.A.; Lenderink, T.; et al. Colchicine in Patients with Chronic Coronary Disease. N. Engl. J. Med. 2020, 383, 1838–1847, . [CrossRef]
- Tardif, J.-C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [CrossRef]
- Tong, D. C., Quinn, S., Nasis, A., Hiew, C., Roberts-Thomson, P., Adams, H., ... & Layland, J. (2020). Colchicine in patients with acute coronary syndrome: the Australian COPS randomized clinical trial. Circulation, 142(20), 1890-1900. [CrossRef]
- D'Entremont, M.-A.; Lee, S.F.; Mian, R.; Kedev, S.; Montalescot, G.; Cornel, J.H.; Stankovic, G.; Moreno, R.; Storey, R.F.; Henry, T.D.; et al. Design and rationale of the CLEAR SYNERGY (OASIS 9) trial: A 2x2 factorial randomized controlled trial of colchicine versus placebo and spironolactone vs placebo in patients with myocardial infarction. Am. Hear. J. 2024, 275, 173–182. [CrossRef]
- Diamantis, E.; Kyriakos, G.; Quiles-Sanchez, L.V.; Farmaki, P.; Troupis, T. The Anti-Inflammatory Effects of Statins on Coronary Artery Disease: An Updated Review of the Literature. Curr. Cardiol. Rev. 2017, 13, 209–216. [CrossRef]
- Ridker, P. M., Danielson, E., Fonseca, F. A., Genest, J., Gotto Jr, A. M., Kastelein, J. J., ... & Glynn, R. J. (2008). Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. New England journal of medicine, 359(21), 2195-2207. [CrossRef]
- Stefanadi, E.; Tousoulis, D.; Antoniades, C.; Katsi, V.; Bosinakou, E.; Vavuranakis, E.; Triantafyllou, G.; Marinou, K.; Tsioufis, C.; Papageorgiou, N.; et al. Early initiation of low-dose atorvastatin treatment after an acute ST-elevated myocardial infarction, decreases inflammatory process and prevents endothelial injury and activation. Int. J. Cardiol. 2008, 133, 266–268. [CrossRef]
- Chen, J.; Zhang, X.; Millican, R.; Lynd, T.; Gangasani, M.; Malhotra, S.; Sherwood, J.; Hwang, P.T.; Cho, Y.; Brott, B.C.; et al. Recent Progress in in vitro Models for Atherosclerosis Studies. Front. Cardiovasc. Med. 2022, 8, 790529. [CrossRef]
- Zhang, Y.; Fatima, M.; Hou, S.; Bai, L.; Zhao, S.; Liu, E. Research methods for animal models of atherosclerosis (Review). Mol. Med. Rep. 2021, 24, 1–14. [CrossRef]
- Seeger, F.H.; Sedding, D.; Langheinrich, A.C.; Haendeler, J.; Zeiher, A.M.; Dimmeler, S. Inhibition of the p38 MAP kinase in vivo improves number and functional activity of vasculogenic cells and reduces atherosclerotic disease progression. Basic Res. Cardiol. 2009, 105, 389–397. [CrossRef]
- Golforoush, P.; Yellon, D.M.; Davidson, S.M. Mouse models of atherosclerosis and their suitability for the study of myocardial infarction. Basic Res. Cardiol. 2020, 115, 1–24. [CrossRef]
- Han, Y.; Gao, S.; Muegge, K.; Zhang, W.; Zhou, B. Advanced Applications of RNA Sequencing and Challenges. Bioinform. Biol. Insights 2015, 9s1, BBI.S28991–46. [CrossRef]
- Ramazi, S.; Zahiri, J. Post-translational modifications in proteins: resources, tools and prediction methods. Database 2021, 2021. [CrossRef]
- Baldini, C.; Moriconi, F.R.; Galimberti, S.; Libby, P.; De Caterina, R. The JAK–STAT pathway: an emerging target for cardiovascular disease in rheumatoid arthritis and myeloproliferative neoplasms. Eur. Hear. J. 2021, 42, 4389–4400. [CrossRef]
- Schlotter, F.; Halu, A.; Goto, S.; Blaser, M.C.; Body, S.C.; Lee, L.H.; Higashi, H.; DeLaughter, D.M.; Hutcheson, J.D.; Vyas, P.; et al. Spatiotemporal Multi-Omics Mapping Generates a Molecular Atlas of the Aortic Valve and Reveals Networks Driving Disease. Circulation 2018, 138, 377–393. [CrossRef]
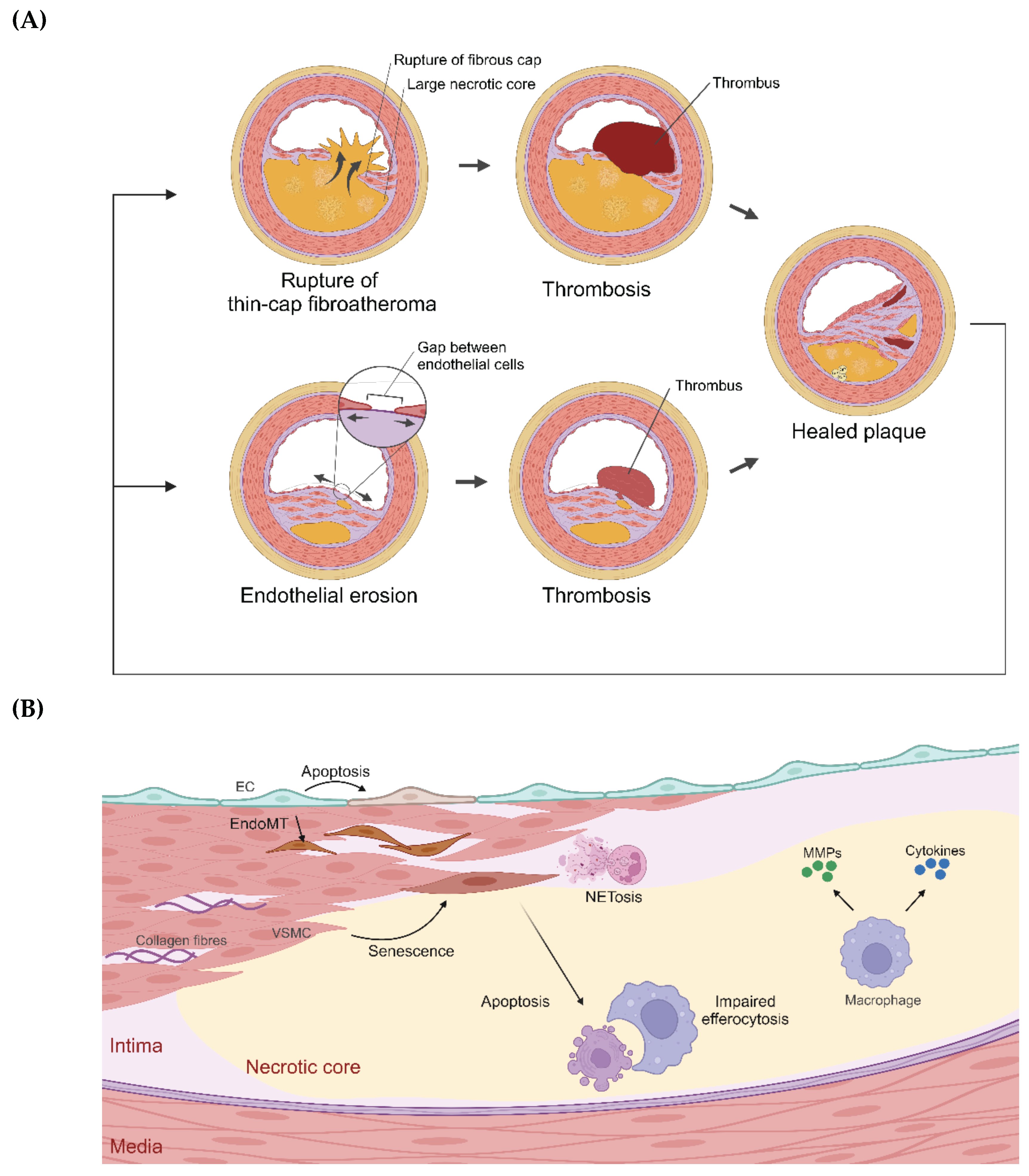
| Target | Drug/compound name | Clinical trial | Study population | Treatment outcome |
|---|---|---|---|---|
| IL-1 signalling pathway | Canakinumab (Rejected by FDA) | CANTOS [124] | Patients with a history of MI and CRP levels > 2 mg/L | Canakinumab reduced inflammation and adverse cardiovascular events, but the risk of fatal infection and the high cost of clinical application outweighed the benefits. |
| Anakinra (Phase II trial ongoing) |
MRC-ILA Heart [125] | Patients with ACS (NSTEMI) | Anakinra reduced inflammation but not the risk of future adverse cardiovascular events. | |
| VCU-ART3 [126] | Patients with ACS (STEMI) | Anakinra reduced inflammation and adverse cardiovascular events. | ||
| VA-ART4 [128] | Patients with ACS (STEMI) | Clinical trial ongoing. | ||
| IL-6 signalling pathway | Tocilizumab (Phase II trial ongoing) |
A phase II clinical trial [134] | Patients with ACS (NSTEMI) | Tocilizumab reduced inflammation and myocardial damage but not the risk of future adverse cardiovascular events. |
| ASSAIL-MI [135] | Patients with ACS (STEMI) | |||
| DOBERMANN [136] | Patients recently received PCI | Clinical trial ongoing. | ||
| Ziltivekimab (Phase III trials ongoing) |
ZEUS [138] | Chronic kidney disease patients with evidence of CAD | Clinical trial ongoing. | |
| SPIDER [139] | Patients with CAD and CRP levels > 2 mg/L | |||
| ARTEMIS [140] | Patients with ACS | |||
| TNF-α signalling pathway | Etanercept | RECOVER [145] | Heart failure patients | Etanercept did not improve clinical outcomes. |
| RENAISSANCE [145] | ||||
| Adalimumab | A subclinical trial [147] | Rheumatoid arthritis patients | Adalimumab reduced inflammation. Changes in cardiovascular events were not assessed. | |
| PIONEER [148] | Hidradenitis suppurativa patients (patients with high risk of cardiovascular disease) | |||
| p38 MAPK signalling pathway | Losmapimod | A phase II clinical trial [151] | Patients with diagnosed atherosclerotic disease | Losmapimod reduced inflammation. Cardiovascular outcomes were not assessed. |
| SOLSTICE [153] | Patients with ACS (NSTEMI) | Losmapimod reduced inflammation but not the risk of future adverse cardiovascular events. | ||
| LATITUDE-TIMI 60 [10] | Patients with ACS | |||
| Dilmapimod | A phase II clinical trial [152] | Patients with CAD | Dilmapimod reduced inflammation caused by PCI. | |
| NLRP3 inflammasome pathway [80,155,156], RhoA/ROCK pathway [157] | Colchicine (Phase III trial ongoing) |
LoDoCo [9] | Patients with stable CAD | Colchicine reduced adverse cardiovascular events. |
| COLCOT [160] | Patients with a history of MI in the previous 30 days | |||
| LoDoCo2 [159] | Patients with stable CAD | |||
| COPS [161] | Patients with ACS | No statistically significant benefits. | ||
| CLEAR SYNERGY (OASIS 9) [162] | Patients with ACS (STEMI) and received PCI | Clinical trial ongoing. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





