Submitted:
27 December 2024
Posted:
30 December 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
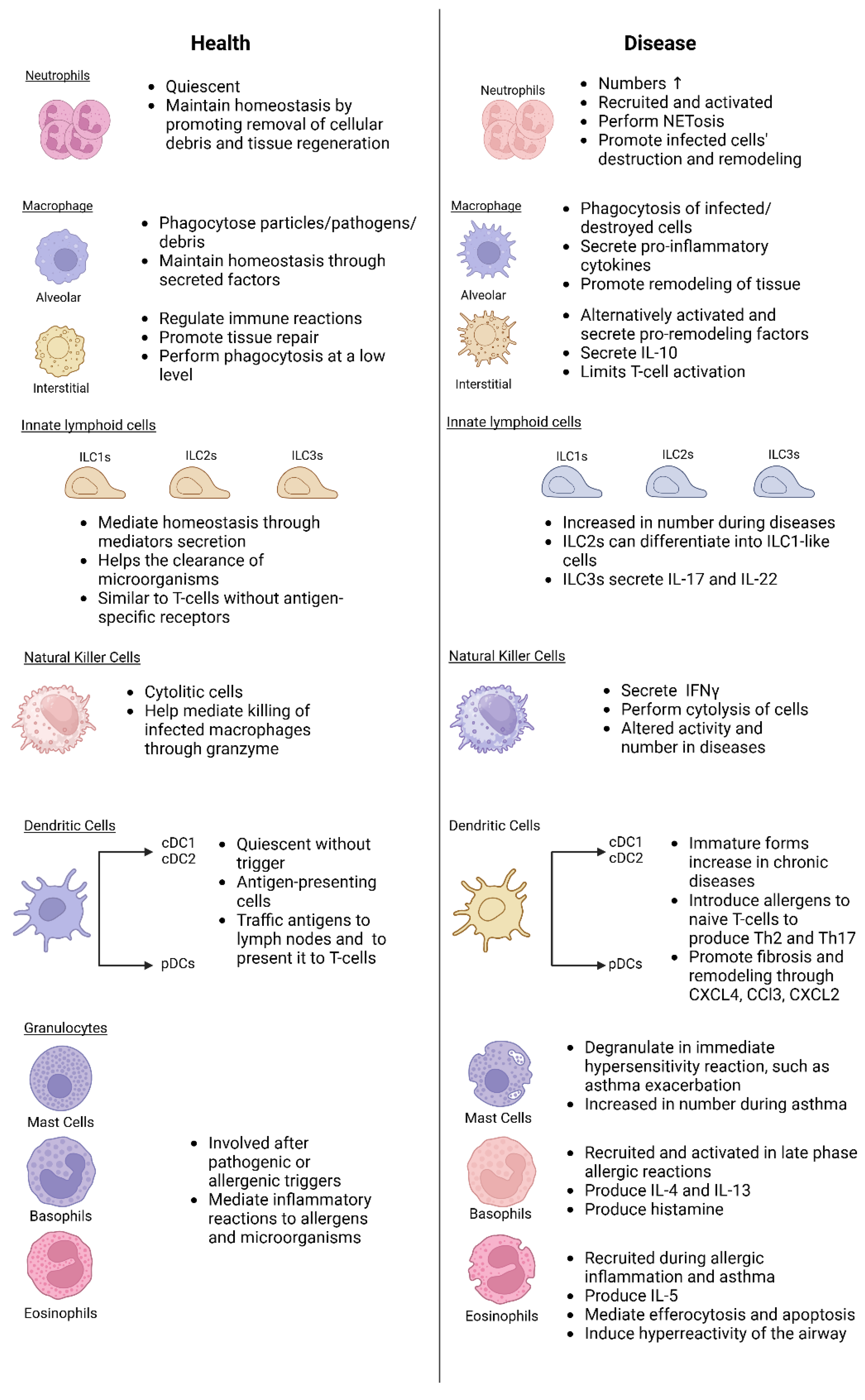
2. Components of Lung Innate Immunity
3. The role of innate immunity during lung diseases
3.1. Lung Infections
3.2. COPD
3.3. Asthma
3.4. Pulmonary Fibrosis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murgia, N.; Gambelunghe, A. Occupational COPD —The most under-recognized occupational lung disease? Respirology 2022, 27, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Iversen, I.B.; Vestergaard, J.M.; Basinas, I.; Ohlander, J.; Peters, S.; Bendstrup, E.; Bonde, J.P.E.; Schlünssen, V.; Rasmussen, F.; Stokholm, Z.A.; et al. Risk of hypersensitivity pneumonitis and other interstitial lung diseases following organic dust exposure. Thorax 2024, 79, 853–860. [Google Scholar] [CrossRef]
- Harari, S.; Raghu, G.; Caminati, A.; Cruciani, M.; Franchini, M.; Mannucci, P. Fibrotic interstitial lung diseases and air pollution: a systematic literature review. Eur. Respir. Rev. 2020, 29, 200093. [Google Scholar] [CrossRef]
- Janeway, C.A.; Medzhitov, R. I NNATE I MMUNE R ECOGNITION. Annu. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Greene, C.M.; Hiemstra, P.S. Innate Immunity of the Lung. J. Innate Immun. 2020, 12, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.R. Innate Immunity in the Lungs. Proc. Am. Thorac. Soc. 2005, 2, 403–411. [Google Scholar] [CrossRef]
- Zhang, H.; He, F.; Li, P.; Hardwidge, P.R.; Li, N.; Peng, Y. The Role of Innate Immunity in Pulmonary Infections. Biomed Res. Int. 2021, 2021, 11–13. [Google Scholar] [CrossRef]
- Korkmaz, F.T.; Traber, K.E. Innate immune responses in pneumonia. Pneumonia 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Deckers, J.; Branco Madeira, F.; Hammad, H. Innate immune cells in asthma. Trends Immunol. 2013, 34, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V. Pulmonary Innate Immune Response Determines the Outcome of Inflammation During Pneumonia and Sepsis-Associated Acute Lung Injury. Front. Immunol. 2020, 11, 1722. [Google Scholar] [CrossRef] [PubMed]
- Ardain, A.; Marakalala, M.J.; Leslie, A. Tissue-resident innate immunity in the lung. Immunology 2020, 159, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Aegerter, H.; Lambrecht, B.N.; Jakubzick, C. V. Biology of lung macrophages in health and disease. Immunity 2022, 55, 1564–1580. [Google Scholar] [CrossRef] [PubMed]
- Hussell, T.; Bell, T.J. Alveolar macrophages: plasticity in a tissue-specific context. Nat. Rev. Immunol. 2014, 14, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Malainou, C.; Abdin, S.M.; Lachmann, N.; Matt, U.; Herold, S. Alveolar macrophages in tissue homeostasis, inflammation, and infection: evolving concepts of therapeutic targeting. J. Clin. Invest. 2023, 133. [Google Scholar] [CrossRef]
- Pervizaj-Oruqaj, L.; Ferrero, M.R.; Matt, U.; Herold, S. The guardians of pulmonary harmony: alveolar macrophages orchestrating the symphony of lung inflammation and tissue homeostasis. Eur. Respir. Rev. 2024, 33, 230263. [Google Scholar] [CrossRef] [PubMed]
- Suber, T.; Camiolo, M.J.; Ray, A. A no-Wnt situation for alveolar macrophage self-renewal. Immunity 2021, 54, 1099–1101. [Google Scholar] [CrossRef] [PubMed]
- McCubbrey, A.L.; Barthel, L.; Mohning, M.P.; Redente, E.F.; Mould, K.J.; Thomas, S.M.; Leach, S.M.; Danhorn, T.; Gibbings, S.L.; Jakubzick, C. V.; et al. Deletion of c-FLIP from CD11b hi Macrophages Prevents Development of Bleomycin-induced Lung Fibrosis. Am. J. Respir. Cell Mol. Biol. 2018, 58, 66–78. [Google Scholar] [CrossRef] [PubMed]
- García, J.E.L.; Rodríguez, F.M.; de Cabo, M.R.M.; Salgado, M.J.G.; Losada, J.P.; Villarón, L.G.; López, A.J.; Arellano, J.L.P. Evaluation of Inflammatory Cytokine Secretion by Human Alveolar Macrophages. Mediators Inflamm. 1999, 8, 43–51. [Google Scholar] [CrossRef]
- Shi, T.; Denney, L.; An, H.; Ho, L.-P.; Zheng, Y. Alveolar and lung interstitial macrophages: Definitions, functions, and roles in lung fibrosis. J. Leukoc. Biol. 2021, 110, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.; Watanabe, S.; Verma, R.; Jablonski, R.P.; Chen, C.-I.; Cheresh, P.; Markov, N.S.; Reyfman, P.A.; McQuattie-Pimentel, A.C.; Sichizya, L.; et al. A spatially restricted fibrotic niche in pulmonary fibrosis is sustained by M-CSF/M-CSFR signalling in monocyte-derived alveolar macrophages. Eur. Respir. J. 2020, 55, 1900646. [Google Scholar] [CrossRef] [PubMed]
- Kawano, H.; Kayama, H.; Nakama, T.; Hashimoto, T.; Umemoto, E.; Takeda, K. IL-10-producing lung interstitial macrophages prevent neutrophilic asthma. Int. Immunol. 2016, 28, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Legrand, C.; Vanneste, D.; Hego, A.; Sabatel, C.; Mollers, K.; Schyns, J.; Maréchal, P.; Abinet, J.; Tytgat, A.; Liégeois, M.; et al. Lung Interstitial Macrophages Can Present Soluble Antigens and Induce Foxp3 + Regulatory T Cells. Am. J. Respir. Cell Mol. Biol. 2024, 70, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Bae, G.H.; Kim, Y.S.; Park, J.Y.; Lee, M.; Lee, S.K.; Kim, J.C.; Kim, J.G.; Shin, Y.J.; Lee, H.; Kim, S.Y.; et al. Unique characteristics of lung-resident neutrophils are maintained by PGE2/PKA/Tgm2-mediated signaling. Blood 2022, 140, 889–899. [Google Scholar] [CrossRef]
- Giacalone, V.D.; Margaroli, C.; Mall, M.A.; Tirouvanziam, R. Neutrophil Adaptations upon Recruitment to the Lung: New Concepts and Implications for Homeostasis and Disease. Int. J. Mol. Sci. 2020, 21, 851. [Google Scholar] [CrossRef] [PubMed]
- Paris, A.J.; Liu, Y.; Mei, J.; Dai, N.; Guo, L.; Spruce, L.A.; Hudock, K.M.; Brenner, J.S.; Zacharias, W.J.; Mei, H.D.; et al. Neutrophils promote alveolar epithelial regeneration by enhancing type II pneumocyte proliferation in a model of acid-induced acute lung injury. Am. J. Physiol. Cell. Mol. Physiol. 2016, 311, L1062–L1075. [Google Scholar] [CrossRef]
- Barlow, J.L.; McKenzie, A.N.J. Innate Lymphoid Cells of the Lung. Annu. Rev. Physiol. 2019, 81, 429–452. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Lim, M.; Kim, J.; Kim, H.Y. Versatile roles of innate lymphoid cells at the mucosal barrier: from homeostasis to pathological inflammation. Exp. Mol. Med. 2023, 55, 1845–1857. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Lee, J.; Koga, S.; Ricardo-Gonzalez, R.R.; Nussbaum, J.C.; Smith, L.K.; Villeda, S.A.; Liang, H.-E.; Locksley, R.M. Tissue-Resident Group 2 Innate Lymphoid Cells Differentiate by Layered Ontogeny and In Situ Perinatal Priming. Immunity 2019, 50, 1425–1438.e5. [Google Scholar] [CrossRef] [PubMed]
- Weizman, O.-E.; Adams, N.M.; Schuster, I.S.; Krishna, C.; Pritykin, Y.; Lau, C.; Degli-Esposti, M.A.; Leslie, C.S.; Sun, J.C.; O’Sullivan, T.E. ILC1 Confer Early Host Protection at Initial Sites of Viral Infection. Cell 2017, 171, 795–808.e12. [Google Scholar] [CrossRef] [PubMed]
- Halim, T.Y.F.; Steer, C.A.; Mathä, L.; Gold, M.J.; Martinez-Gonzalez, I.; McNagny, K.M.; McKenzie, A.N.J.; Takei, F. Group 2 Innate Lymphoid Cells Are Critical for the Initiation of Adaptive T Helper 2 Cell-Mediated Allergic Lung Inflammation. Immunity 2014, 40, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Darby, M.; Roberts, L.B.; Mackowiak, C.; Chetty, A.; Tinelli, S.; Schnoeller, C.; Quesniaux, V.; Berrard, S.; Togbe, D.; Selkirk, M.E.; et al. ILC3-derived acetylcholine promotes protease-driven allergic lung pathology. J. Allergy Clin. Immunol. 2021, 147, 1513–1516.e4. [Google Scholar] [CrossRef] [PubMed]
- Ardain, A.; Porterfield, J.Z.; Kløverpris, H.N.; Leslie, A. Type 3 ILCs in Lung Disease. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Cong, J.; Wei, H. Natural Killer Cells in the Lungs. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Marquardt, N.; Kekäläinen, E.; Chen, P.; Kvedaraite, E.; Wilson, J.N.; Ivarsson, M.A.; Mjösberg, J.; Berglin, L.; Säfholm, J.; Manson, M.L.; et al. Human lung natural killer cells are predominantly comprised of highly differentiated hypofunctional CD69 − CD56 dim cells. J. Allergy Clin. Immunol. 2017, 139, 1321–1330.e4. [Google Scholar] [CrossRef]
- Du, X.; Zhu, H.; Jiao, D.; Nian, Z.; Zhang, J.; Zhou, Y.; Zheng, X.; Tong, X.; Wei, H.; Fu, B. Human-Induced CD49a+ NK Cells Promote Fetal Growth. Front. Immunol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Kopf, M.; Schneider, C.; Nobs, S.P. The development and function of lung-resident macrophages and dendritic cells. Nat. Immunol. 2015, 16, 36–44. [Google Scholar] [CrossRef]
- Cook, P.C.; MacDonald, A.S. Dendritic cells in lung immunopathology. Semin. Immunopathol. 2016, 38, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Collin, M.; Bigley, V. Human dendritic cell subsets: an update. Immunology 2018, 154, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Izumi, G.; Nakano, H.; Nakano, K.; Whitehead, G.S.; Grimm, S.A.; Fessler, M.B.; Karmaus, P.W.; Cook, D.N. CD11b+ lung dendritic cells at different stages of maturation induce Th17 or Th2 differentiation. Nat. Commun. 2021, 12, 5029. [Google Scholar] [CrossRef] [PubMed]
- Stone, K.D.; Prussin, C.; Metcalfe, D.D. IgE, mast cells, basophils, and eosinophils. J. Allergy Clin. Immunol. 2010, 125, S73–S80. [Google Scholar] [CrossRef] [PubMed]
- Okayama, Y.; Okumura, S.; Tomita, H.; Katayama, H.; Yuki, K.; Kagaya, S.; Kashiwakura, J.; Saito, H. Human mast cell activation through Fc receptors and Toll-like receptors. Allergol. Int. 2004, 53, 227–233. [Google Scholar] [CrossRef]
- Nagata, Y.; Suzuki, R. FcεRI: A Master Regulator of Mast Cell Functions. Cells 2022, 11, 622. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kang, S.-J. The ontogenesis and heterogeneity of basophils. Discov. Immunol. 2024, 3. [Google Scholar] [CrossRef]
- Schuijs, M.J.; Brenis Gomez, C.M.; Bick, F.; Van Moorleghem, J.; Vanheerswynghels, M.; van Loo, G.; Beyaert, R.; Voehringer, D.; Locksley, R.M.; Hammad, H.; et al. Interleukin-33–activated basophils promote asthma by regulating Th2 cell entry into lung tissue. J. Exp. Med. 2024, 221. [Google Scholar] [CrossRef]
- Felton, J.M.; Lucas, C.D.; Rossi, A.G.; Dransfield, I. Eosinophils in the Lung – Modulating Apoptosis and Efferocytosis in Airway Inflammation. Front. Immunol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Arredouani, M.; Yang, Z.; Ning, Y.; Qin, G.; Soininen, R.; Tryggvason, K.; Kobzik, L. The Scavenger Receptor MARCO Is Required for Lung Defense against Pneumococcal Pneumonia and Inhaled Particles. J. Exp. Med. 2004, 200, 267–272. [Google Scholar] [CrossRef]
- Preston, J.A.; Bewley, M.A.; Marriott, H.M.; McGarry Houghton, A.; Mohasin, M.; Jubrail, J.; Morris, L.; Stephenson, Y.L.; Cross, S.; Greaves, D.R.; et al. Alveolar Macrophage Apoptosis–associated Bacterial Killing Helps Prevent Murine Pneumonia. Am. J. Respir. Crit. Care Med. 2019, 200, 84–97. [Google Scholar] [CrossRef]
- Wu, T.T.-H.; Travaglini, K.J.; Rustagi, A.; Xu, D.; Zhang, Y.; Andronov, L.; Jang, S.; Gillich, A.; Dehghannasiri, R.; Martínez-Colón, G.J.; et al. Interstitial macrophages are a focus of viral takeover and inflammation in COVID-19 initiation in human lung. J. Exp. Med. 2024, 221. [Google Scholar] [CrossRef] [PubMed]
- Zuttion, M.S.S.R.; Parimon, T.; Yao, C.; Stripp, B.R.; Wang, Y.; Soto, C.M.; Ortega, Z.; Li, X.; Janssen, W.J.; Chen, P. Interstitial Macrophages Mediate Efferocytosis of Alveolar Epithelium during Influenza Infection. Am. J. Respir. Cell Mol. Biol. 2024, 70, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Gaffney, E.; Murphy, D.; Walsh, A.; Connolly, S.; Basdeo, S.A.; Keane, J.; Phelan, J.J. Defining the role of neutrophils in the lung during infection: Implications for tuberculosis disease. Front. Immunol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Bordon, J.; Aliberti, S.; Fernandez-Botran, R.; Uriarte, S.M.; Rane, M.J.; Duvvuri, P.; Peyrani, P.; Morlacchi, L.C.; Blasi, F.; Ramirez, J.A. Understanding the roles of cytokines and neutrophil activity and neutrophil apoptosis in the protective versus deleterious inflammatory response in pneumonia. Int. J. Infect. Dis. 2013, 17, e76–e83. [Google Scholar] [CrossRef]
- Vashist, N.; Trittel, S.; Ebensen, T.; Chambers, B.J.; Guzmán, C.A.; Riese, P. Influenza-Activated ILC1s Contribute to Antiviral Immunity Partially Influenced by Differential GITR Expression. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Wallrapp, A.; Riesenfeld, S.J.; Burkett, P.R.; Kuchroo, V.K. Type 2 innate lymphoid cells in the induction and resolution of tissue inflammation. Immunol. Rev. 2018, 286, 53–73. [Google Scholar] [CrossRef] [PubMed]
- Khader, S.A.; Cooper, A.M. IL-23 and IL-17 in tuberculosis. Cytokine 2008, 41, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Van Maele, L.; Carnoy, C.; Cayet, D.; Ivanov, S.; Porte, R.; Deruy, E.; Chabalgoity, J.A.; Renauld, J.-C.; Eberl, G.; Benecke, A.G.; et al. Activation of Type 3 Innate Lymphoid Cells and Interleukin 22 Secretion in the Lungs During Streptococcus pneumoniae Infection. J. Infect. Dis. 2014, 210, 493–503. [Google Scholar] [CrossRef]
- Björkström, N.K.; Strunz, B.; Ljunggren, H.-G. Natural killer cells in antiviral immunity. Nat. Rev. Immunol. 2022, 22, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Paidipally, P.; Tripathi, D.; Van, A.; Radhakrishnan, R.K.; Dhiman, R.; Venkatasubramanian, S.; Devalraju, K.P.; Tvinnereim, A.R.; Valluri, V.L.; Vankayalapati, R. Interleukin-21 Regulates Natural Killer Cell Responses During Mycobacterium tuberculosis Infection. J. Infect. Dis. 2018, 217, 1323–1333. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, J.; Zhou, X.; Pan, Q.; Zhao, W.; Yang, X.; Wang, H. Natural Killer Cells Regulate Pulmonary Macrophages Polarization in Host Defense Against Chlamydial Respiratory Infection. Front. Cell. Infect. Microbiol. 2022, 11. [Google Scholar] [CrossRef]
- Jenkins, M.M.; Bachus, H.; Botta, D.; Schultz, M.D.; Rosenberg, A.F.; León, B.; Ballesteros-Tato, A. Lung dendritic cells migrate to the spleen to prime long-lived TCF1 hi memory CD8 + T cell precursors after influenza infection. Sci. Immunol. 2021, 6. [Google Scholar] [CrossRef] [PubMed]
- Swiecki, M.; Colonna, M. Accumulation of plasmacytoid DC: Roles in disease pathogenesis and targets for immunotherapy. Eur. J. Immunol. 2010, 40, 2094–2098. [Google Scholar] [CrossRef]
- Hackstein, H.; Kranz, S.; Lippitsch, A.; Wachtendorf, A.; Kershaw, O.; Gruber, A.D.; Michel, G.; Lohmeyer, J.; Bein, G.; Baal, N.; et al. Modulation of respiratory dendritic cells during Klebsiella pneumonia infection. Respir. Res. 2013, 14, 91. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, M.; Wang, F.; Luo, Y.; Liu, H.; Zhu, Z.; Huang, X.; Hua, L.; Chen, H.; Wu, B.; et al. Dendritic cell targeting peptide plus Salmonella FliCd flagellin fused outer membrane protein H (OmpH) demonstrated increased efficacy against infections caused by different Pasteurella multocida serogroups in mouse models. Vaccine 2024, 42, 3075–3083. [Google Scholar] [CrossRef]
- Pouwels, S.D.; van Geffen, W.H.; Jonker, M.R.; Kerstjens, H.A.M.; Nawijn, M.C.; Heijink, I.H. Increased neutrophil expression of pattern recognition receptors during COPD exacerbations. Respirology 2017, 22, 401–404. [Google Scholar] [CrossRef]
- Kayongo, A.; Robertson, N.M.; Siddharthan, T.; Ntayi, M.L.; Ndawula, J.C.; Sande, O.J.; Bagaya, B.S.; Kirenga, B.; Mayanja-Kizza, H.; Joloba, M.L.; et al. Airway microbiome-immune crosstalk in chronic obstructive pulmonary disease. Front. Immunol. 2023, 13, 1–23. [Google Scholar] [CrossRef]
- Bu, T.; Wang, L.F.; Yin, Y.Q. How do innate immune cells contribute to airway remodeling in copd progression? Int. J. COPD 2020, 15, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Lonergan, M.; Dicker, A.J.; Crichton, M.L.; Keir, H.R.; Van Dyke, M.K.; Mullerova, H.; Miller, B.E.; Tal-Singer, R.; Chalmers, J.D. Blood neutrophil counts are associated with exacerbation frequency and mortality in COPD. Respir. Res. 2020, 21, 166. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, X.; Xiao, C.; Xiong, G.; Ye, X.; Li, L.; Fang, Y.; Chen, H.; Yang, W.; Du, X. The role of lung macrophages in chronic obstructive pulmonary disease. Respir. Med. 2022, 205, 107035. [Google Scholar] [CrossRef] [PubMed]
- Dewhurst, J.A.; Lea, S.; Hardaker, E.; Dungwa, J. V.; Ravi, A.K.; Singh, D. Characterisation of lung macrophage subpopulations in COPD patients and controls. Sci. Rep. 2017, 7, 7143. [Google Scholar] [CrossRef]
- Vlahos, R. Role of alveolar macrophages in chronic obstructive pulmonary disease. Front. Immunol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Abboud, R.T.; Wallace, A.M.; English, J.C.; Coxson, H.O.; Finley, R.J.; Shumansky, K.; Paré, P.D.; Sandford, A.J. Alveolar macrophage proteinase/antiproteinase expression in lung function and emphysema. Eur. Respir. J. 2014, 43, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Voelkel, M.A. Interstitial and Peribronchial Macrophages in Chronic Obstructive Pulmonary Disease Display an Alternatively Activated Phenotype. Proc. Am. Thorac. Soc. 2006, 3, 546b–547. [Google Scholar] [CrossRef]
- Cavagnero, K.J.; Badrani, J.H.; Naji, L.H.; Amadeo, M.B.; Leng, A.S.; Lacasa, L.D.; Strohm, A.N.; Renusch, S.R.; Gasparian, S.S.; Doherty, T.A. Cyclic-di-GMP Induces STING-Dependent ILC2 to ILC1 Shift During Innate Type 2 Lung Inflammation. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Silver, J.S.; Kearley, J.; Copenhaver, A.M.; Sanden, C.; Mori, M.; Yu, L.; Pritchard, G.H.; Berlin, A.A.; Hunter, C.A.; Bowler, R.; et al. Inflammatory triggers associated with exacerbations of COPD orchestrate plasticity of group 2 innate lymphoid cells in the lungs. Nat. Immunol. 2016, 17, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Gurczynski, S.J.; Moore, B.B. IL-17 in the lung: the good, the bad, and the ugly. Am. J. Physiol. Cell. Mol. Physiol. 2018, 314, L6–L16. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Le, Y.; Xiong, J.; Pei, Y.; Sun, Y. NK Cells in the Pathogenesis of Chronic Obstructive Pulmonary Disease. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Mengistu, D.T.; Freeman, C.M. Welcome to the Neighborhood: Tissue-Resident Lung Natural Killer Cells in Chronic Obstructive Pulmonary Disease and Viral Infections. Am. J. Respir. Crit. Care Med. 2023, 207, 500–502. [Google Scholar] [CrossRef]
- Osterburg, A.R.; Lach, L.; Panos, R.J.; Borchers, M.T. Unique natural killer cell subpopulations are associated with exacerbation risk in chronic obstructive pulmonary disease. Sci. Rep. 2020, 10, 1238. [Google Scholar] [CrossRef] [PubMed]
- Urbanowicz, R.A.; Lamb, J.R.; Todd, I.; Corne, J.M.; Fairclough, L.C. Altered effector function of peripheral cytotoxic cells in COPD. Respir. Res. 2009, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, X.; Wang, M.; Zou, Q.; Zhao, S.; Sun, B.; Xu, L.; Jiang, Y. Increased Numbers of NK Cells, NKT-Like Cells, and NK Inhibitory Receptors in Peripheral Blood of Patients with Chronic Obstructive Pulmonary Disease. Clin. Dev. Immunol. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Van Pottelberge, G.R.; Bracke, K.R.; Joos, G.F.; Brusselle, G.G. The Role of Dendritic Cells in the Pathogenesis of COPD: Liaison Officers in the Front Line. COPD J. Chronic Obstr. Pulm. Dis. 2009, 6, 284–290. [Google Scholar] [CrossRef]
- Zanini, A.; Spanevello, A.; Baraldo, S.; Majori, M.; Della Patrona, S.; Gumiero, F.; Aiello, M.; Olivieri, D.; Saetta, M.; Chetta, A. Decreased Maturation of Dendritic Cells in the Central Airways of COPD Patients Is Associated with VEGF, TGF-β and Vascularity. Respiration 2014, 87, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Michel, S.; Liang, L.; Depner, M.; Klopp, N.; Ruether, A.; Kumar, A.; Schedel, M.; Vogelberg, C.; von Mutius, E.; von Berg, A.; et al. Unifying Candidate Gene and GWAS Approaches in Asthma. PLoS One 2010, 5, e13894. [Google Scholar] [CrossRef] [PubMed]
- Crisford, H.; Sapey, E.; Rogers, G.B.; Taylor, S.; Nagakumar, P.; Lokwani, R.; Simpson, J.L. Neutrophils in asthma: The good, the bad and the bacteria. Thorax 2021, 76, 835–844. [Google Scholar] [CrossRef]
- Poto, R.; Shamji, M.; Marone, G.; Durham, S.R.; Scadding, G.W.; Varricchi, G. Neutrophil Extracellular Traps in Asthma: Friends or Foes? Cells 2022, 11, 3521. [Google Scholar] [CrossRef] [PubMed]
- Lachowicz-Scroggins, M.E.; Dunican, E.M.; Charbit, A.R.; Raymond, W.; Looney, M.R.; Peters, M.C.; Gordon, E.D.; Woodruff, P.G.; Lefrançais, E.; Phillips, B.R.; et al. Extracellular DNA, Neutrophil Extracellular Traps, and Inflammasome Activation in Severe Asthma. Am. J. Respir. Crit. Care Med. 2019, 199, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Favoreto, S.; Avila, P.C.; Schleimer, R.P. TLR3- and Th2 Cytokine-Dependent Production of Thymic Stromal Lymphopoietin in Human Airway Epithelial Cells. J. Immunol. 2007, 179, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Raychaudhuri, B.; Fisher, C.J.; Farver, C.F.; Malur, A.; Drazba, J.; Kavuru, M.S.; Thomassen, M.J. INTERLEUKIN 10 (IL-10)-MEDIATED INHIBITION OF INFLAMMATORY CYTOKINE PRODUCTION BY HUMAN ALVEOLAR MACROPHAGES. Cytokine 2000, 12, 1348–1355. [Google Scholar] [CrossRef]
- Bang, B.-R.; Chun, E.; Shim, E.-J.; Lee, H.-S.; Lee, S.-Y.; Cho, S.-H.; Min, K.-U.; Kim, Y.-Y.; Park, H.-W. Alveolar macrophages modulate allergic inflammation in a murine model of asthma. Exp. Mol. Med. 2011, 43, 275. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Kim, B.; Hyeon, D.Y.; Jeong, D.; Ryu, J.; Nam, J.S.; Choi, Y.H.; Kim, B.R.; Park, S.C.; Chung, Y.W.; et al. Distinctive CD39+CD9+ lung interstitial macrophages suppress IL-23/Th17-mediated neutrophilic asthma by inhibiting NETosis. Nat. Commun. 2024, 15, 8628. [Google Scholar] [CrossRef] [PubMed]
- Wolterink, R.G.J.K.; KleinJan, A.; van Nimwegen, M.; Bergen, I.; de Bruijn, M.; Levani, Y.; Hendriks, R.W. Pulmonary innate lymphoid cells are major producers of IL -5 and IL -13 in murine models of allergic asthma. Eur. J. Immunol. 2012, 42, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Fang, X.; Zhu, X.; Bai, C.; Zhu, L.; Jin, M.; Wang, X.; Hu, M.; Tang, R.; Chen, Z. IL-131 Type 2 innate lymphoid cells correlate with asthma control status and treatment response. Am. J. Respir. Cell Mol. Biol. 2016, 55, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Ham, J.; Lim, M.; Kim, D.; Kim, H.Y. Memory-like innate lymphoid cells in the pathogenesis of asthma. Front. Immunol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Cayrol, C.; Girard, J.-P. Innate lymphoid cells in asthmatic patients. J. Allergy Clin. Immunol. 2019, 143, 1739–1741. [Google Scholar] [CrossRef]
- Duvall, M.G.; Barnig, C.; Cernadas, M.; Ricklefs, I.; Krishnamoorthy, N.; Grossman, N.L.; Bhakta, N.R.; Fahy, J. V.; Bleecker, E.R.; Castro, M.; et al. Natural killer cell–mediated inflammation resolution is disabled in severe asthma. Sci. Immunol. 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- Altman, M.C.; Whalen, E.; Togias, A.; O’Connor, G.T.; Bacharier, L.B.; Bloomberg, G.R.; Kattan, M.; Wood, R.A.; Presnell, S.; LeBeau, P.; et al. Allergen-induced activation of natural killer cells represents an early-life immune response in the development of allergic asthma. J. Allergy Clin. Immunol. 2018, 142, 1856–1866. [Google Scholar] [CrossRef]
- Gorska, M.M. Natural killer cells in asthma. Curr. Opin. Allergy Clin. Immunol. 2017, 17, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Bratke, K.; Fritz, L.; Nokodian, F.; Geißler, K.; Garbe, K.; Lommatzsch, M.; Virchow, J.C. Differential regulation of PD-1 and its ligands in allergic asthma. Clin. Exp. Allergy 2017, 47, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, S.; Furuhashi, K.; Horiguchi, R.; Nihashi, F.; Yasui, H.; Karayama, M.; Suzuki, Y.; Hozumi, H.; Enomoto, N.; Fujisawa, T.; et al. Conventional type 2 lung dendritic cells are potent inducers of follicular helper T cells in the asthmatic lung. Allergol. Int. 2021, 70, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Banafea, G.H.; Bakhashab, S.; Alshaibi, H.F.; Natesan Pushparaj, P.; Rasool, M. The role of human mast cells in allergy and asthma. Bioengineered 2022, 13, 7049–7064. [Google Scholar] [CrossRef]
- Kaur, D.; Gomez, E.; Doe, C.; Berair, R.; Woodman, L.; Saunders, R.; Hollins, F.; Rose, F.R.; Amrani, Y.; May, R.; et al. IL -33 drives airway hyper-responsiveness through IL -13-mediated mast cell: airway smooth muscle crosstalk. Allergy 2015, 70, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Brusselle, G.G.; Maes, T.; Bracke, K.R. Eosinophils in the Spotlight: Eosinophilic airway inflammation in nonallergic asthma. Nat. Med. 2013, 19, 977–979. [Google Scholar] [CrossRef]
- Varricchi, G.; Senna, G.; Loffredo, S.; Bagnasco, D.; Ferrando, M.; Canonica, G.W. Reslizumab and Eosinophilic Asthma: One Step Closer to Precision Medicine? Front. Immunol. 2017, 8. [Google Scholar] [CrossRef]
- Kouro, T.; Takatsu, K. IL-5- and eosinophil-mediated inflammation: from discovery to therapy. Int. Immunol. 2009, 21, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Pelaia, C.; Paoletti, G.; Puggioni, F.; Racca, F.; Pelaia, G.; Canonica, G.W.; Heffler, E. Interleukin-5 in the Pathophysiology of Severe Asthma. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Menzella, F.; Lusuardi, M.; Galeone, C.; Taddei, S.; Zucchi, L. Profile of anti-IL-5 mAb mepolizumab in the treatment of severe refractory asthma and hypereosinophilic diseases. J. Asthma Allergy 2015, 105. [Google Scholar] [CrossRef] [PubMed]
- McBrien, C.N.; Menzies-Gow, A. The Biology of Eosinophils and Their Role in Asthma. Front. Med. 2017, 4. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, M.; Carter, H.; Espindola, M.S.; Doyle, T.J.; Lee, J.S.; Merriam, L.T.; Zhang, F.; Kawano-Dourado, L.; Sparks, J.A.; Hogaboam, C.M.; et al. Immune mechanisms in fibrotic interstitial lung disease. Cell 2024, 187, 3506–3530. [Google Scholar] [CrossRef]
- Ishikawa, G.; Liu, A.; Herzog, E.L. Evolving Perspectives on Innate Immune Mechanisms of IPF. Front. Mol. Biosci. 2021, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Gao, W.; Zhai, Z.; Zhu, W. Immune mechanisms and novel therapies for idiopathic pulmonary fibrosis. Pharm. Sci. Adv. 2024, 2, 100030. [Google Scholar] [CrossRef]
- Warheit-Niemi, H.I.; Hult, E.M.; Moore, B.B. A pathologic two-way street: how innate immunity impacts lung fibrosis and fibrosis impacts lung immunity. Clin. Transl. Immunol. 2019, 8, 1–13. [Google Scholar] [CrossRef]
- Achaiah, A.; Fraser, E.; Saunders, P.; Hoyles, R.K.; Benamore, R.; Ho, L.-P. Neutrophil levels correlate with quantitative extent and progression of fibrosis in IPF: results of a single-centre cohort study. BMJ Open Respir. Res. 2023, 10, e001801. [Google Scholar] [CrossRef]
- ZIEGENHAGEN, M.W.; ZABEL, P.; ZISSEL, G.; SCHLAAK, M.; MÜLLER-QUERNHEIM, J. Serum Level of Interleukin 8 Is Elevated in Idiopathic Pulmonary Fibrosis and Indicates Disease Activity. Am. J. Respir. Crit. Care Med. 1998, 157, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Takemasa, A.; Ishii, Y.; Fukuda, T. A neutrophil elastase inhibitor prevents bleomycin-induced pulmonary fibrosis in mice. Eur. Respir. J. 2012, 40, 1475–1482. [Google Scholar] [CrossRef]
- Yan, S.; Li, M.; Liu, B.; Ma, Z.; Yang, Q. Neutrophil extracellular traps and pulmonary fibrosis: an update. J. Inflamm. 2023, 20, 2. [Google Scholar] [CrossRef] [PubMed]
- Mora, A.L.; Torres-González, E.; Rojas, M.; Corredor, C.; Ritzenthaler, J.; Xu, J.; Roman, J.; Brigham, K.; Stecenko, A. Activation of Alveolar Macrophages via the Alternative Pathway in Herpesvirus-Induced Lung Fibrosis. Am. J. Respir. Cell Mol. Biol. 2006, 35, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Novak, C.M.; Sethuraman, S.; Luikart, K.L.; Reader, B.F.; Wheat, J.S.; Whitson, B.; Ghadiali, S.N.; Ballinger, M.N. Alveolar macrophages drive lung fibroblast function in cocultures of IPF and normal patient samples. Am. J. Physiol. - Lung Cell. Mol. Physiol. 2023, 324, L507–L520. [Google Scholar] [CrossRef]
- Adams, T.S.; Schupp, J.C.; Poli, S.; Ayaub, E.A.; Neumark, N.; Ahangari, F.; Chu, S.G.; Raby, B.A.; DeIuliis, G.; Januszyk, M.; et al. Single-cell RNA-seq reveals ectopic and aberrant lung-resident cell populations in idiopathic pulmonary fibrosis. Sci. Adv. 2020, 6. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Q.; Xiang, J.; Zhao, M.; Meng, Y.; Hu, X.; Li, T.; Nie, Y.; Sun, H.; Yan, T.; et al. Novel strategy of combined interstitial macrophage depletion with intravenous targeted therapy to ameliorate pulmonary fibrosis. Mater. Today Bio 2023, 20, 100653. [Google Scholar] [CrossRef] [PubMed]
- Otaki, N.; Motomura, Y.; Terooatea, T.; Thomas Kelly, S.; Mochizuki, M.; Takeno, N.; Koyasu, S.; Tamamitsu, M.; Sugihara, F.; Kikuta, J.; et al. Activation of ILC2s through constitutive IFNγ signaling reduction leads to spontaneous pulmonary fibrosis. Nat. Commun. 2023, 14, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Cruz, T.; Jia, M.; Sembrat, J.; Tabib, T.; Agostino, N.; Bruno, T.C.; Vignali, D.; Sanchez, P.; Lafyatis, R.; Mora, A.L.; et al. Reduced Proportion and Activity of Natural Killer Cells in the Lung of Patients with Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2021, 204, 608–610. [Google Scholar] [CrossRef] [PubMed]
- Bocchino, M.; Zanotta, S.; Capitelli, L.; Galati, D. Dendritic Cells Are the Intriguing Players in the Puzzle of Idiopathic Pulmonary Fibrosis Pathogenesis. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Silva-Cardoso, S.C.; Tao, W.; Angiolilli, C.; Lopes, A.P.; Bekker, C.P.J.; Devaprasad, A.; Giovannone, B.; van Laar, J.; Cossu, M.; Marut, W.; et al. CXCL4 Links Inflammation and Fibrosis by Reprogramming Monocyte-Derived Dendritic Cells in vitro. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Tao, X.; Li, B.; Cao, H.; Chen, H.; Zou, Y.; Tao, H.; Mu, M.; Wang, W.; Xu, K. C-X-C-Chemokine-Receptor-Type-4 Inhibitor AMD3100 Attenuates Pulmonary Inflammation and Fibrosis in Silicotic Mice. J. Inflamm. Res. 2022, Volume 15, 5827–5843. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




