Submitted:
24 December 2024
Posted:
25 December 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
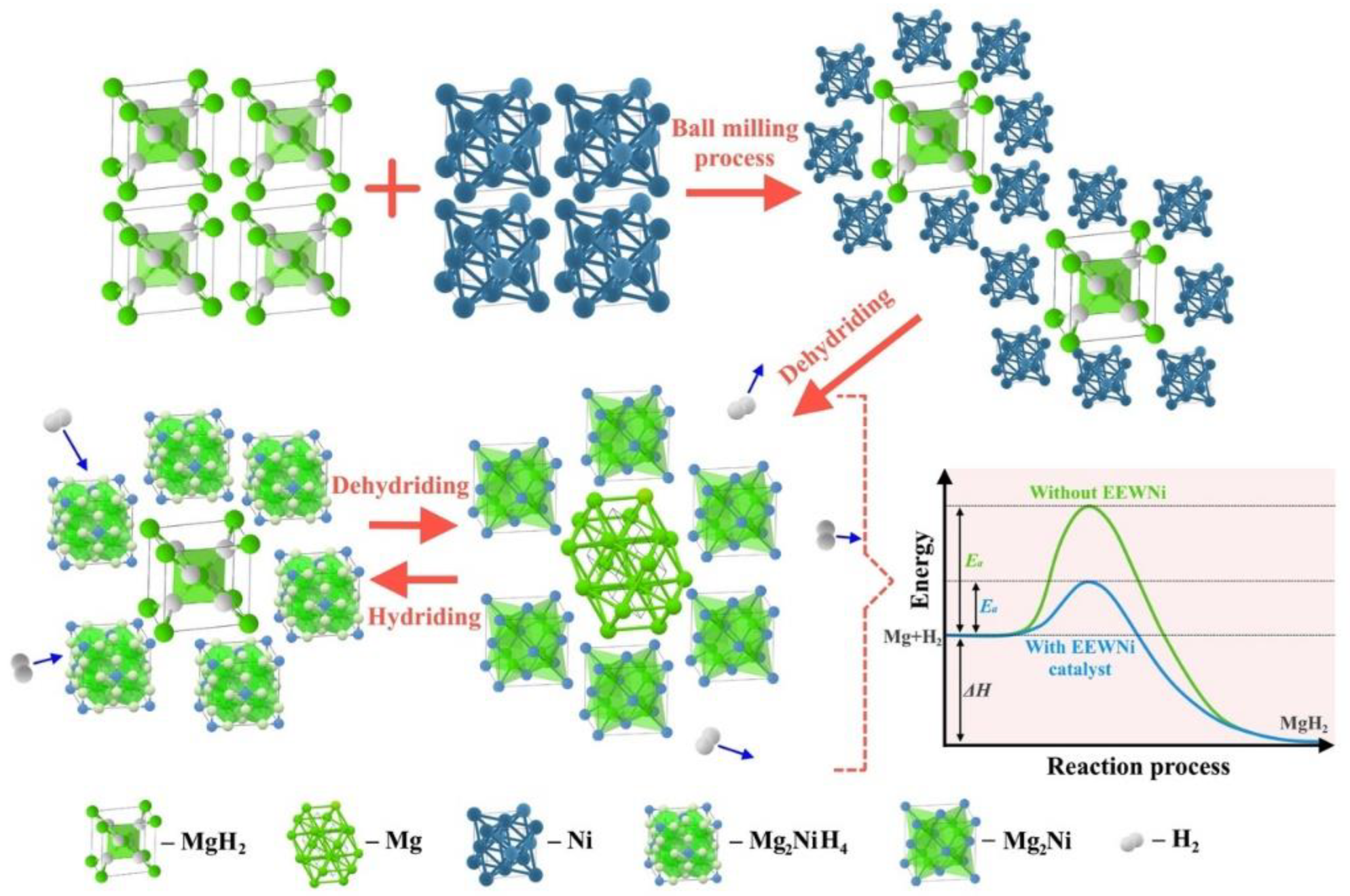
2.1. Materials Preparation
2.2. Analysis and Characterization
3. Results and Discussion
3.1. Composite Characterization
3.2. Hydrogen Storage Properties Of Composite
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bordulev, I.; Kudiiarov, V.; Svyatkin, L.; Syrtanov, M.; Stepanova, E.; Čížek, J.; Vlček, M.; Li, K.; Laptev, R.; Lider, A. Positron Annihilation Spectroscopy Study of Defects in Hydrogen Loaded Zr-1Nb Alloy. Journal of Alloys and Compounds 2019, 798, 685–694. [CrossRef]
- Laptev, R.S.; Lider, A.M.; Bordulev, Y.S.; Kudiiarov, V.N.; Garanin, G.V.; Wang, W.; Kuznetsov, P.V. Investigation of Defects in Hydrogen-Saturated Titanium by Means of Positron Annihilation Techniques. Defect and Diffusion Forum/Diffusion and Defect Data, Solid State Data. Part a, Defect and Diffusion Forum 2015, 365, 232–236. [CrossRef]
- Laptev, R.; Lider, A.; Bordulev, Yu.; Kudiiarov, V.; Garanin, G. HydrogenationInduced Microstructure Changes in Titanium. Journal of Alloys and Compounds 2015, 645, S193–S195. [CrossRef]
- Laptev, R.S.; Kudiiarov, V.N.; Bordulev, Y.S.; Mikhaylov, A.A.; Lider, A.M. GasPhase Hydrogenation Influence on Defect Behavior in Titanium-Based Hydrogen-Storage Material. Progress in Natural Science/Progress in Natural Science 2017, 27, 105–111. [CrossRef]
- Cherdantsev, Y.P.; Chernov, I.P.; Tyurin, Y.I. Methods for studying metalhydrogen systems. Energoatomizdat 2004, 270.
- Faber, M.S.; Lukowski, M.A.; Ding, Q.; Kaiser, N.S.; Jin, S. Earth-Abundant Metal Pyrites (FeS2, CoS2, NiS2, and Their Alloys) for Highly Efficient Hydrogen Evolution and Polysulfide Reduction Electrocatalysis. Journal of Physical Chemistry. C./Journal of Physical Chemistry. C 2014, 118, 21347–21356. [CrossRef]
- Kolachev, B.A. Hydrogen in Metals and Alloys. Metal Science and Heat Treatment 1999, 41, 93–100. [CrossRef]
- Alefeld, G.; Voelkl, J. Hydrogen in Metals I - Basic Properties. 1978, 28.
- Geld, P.V.; Ryabov, R.A.; Kodes, E.S. Hydrogen and imperfections of metal structure. Metallurgy 1979, 221.
- Popov, E.; Troev, T.; Petrov, L.; Berovski, K.; Peneva, S.; Kolev, B. Model calculations of positron interaction in materials for ITER. Bulg. Chem. Commun. 2015, 47, 192–199.
- Gainotti, A.; Ghezzi, C.; Manfredi, M.; Zecchina, L. Positron Lifetimes in Metal Hydrides. Nuovo Cimento. B 1968, 56, 47–56. [CrossRef]
- Budziak, A.; Dryzek, J.; Krawczyk, J.; Zieliński, P.M. Calorimetric and Positron Lifetime Measurements Of Hydrogenated Carbon Nanocones. Acta Physica Polonica. A 2010, 117, 574–577. [CrossRef]
- Laptev, R.S.; Bordulev, Y.S.; Kudiiarov, V.N.; Lider, A.M.; Garanin, G.V. Positron Annihilation Spectroscopy of Defects in Commercially Pure Titanium Saturated with Hydrogen. Advanced Materials Research 2014, 880, 134–140. [CrossRef]
- Hautojarvi, P.; Huomo, H.; Puska, M.; Vehanen, A. Vacancy Recovery and Vacancy-Hydrogen Interaction in Niobium and Tantalum Studied by Positrons. Physical Review. B, Condensed Matter 1985, 32, 4326–4331. [CrossRef]
- Kulkova, S. Electron and Positron Characteristics of Group IV Metal Dihydrides. International Journal of Hydrogen Energy 1996, 21, 1041–1047. [CrossRef]
- Aref’ev, K.P.; Boev, O.V.; Imas, O.N.; Lider, A.M.; Surkov, A.S.; Chernov, I.P. Annihilation of Positrons in Hydrogen-Saturated Titanium. Physics of the Solid State 2003, 45, 1–5. [CrossRef]
- Sakaki, K.; Kawase, T.; Hirato, M.; Mizuno, M.; Araki, H.; Shirai, Y.; Nagumo, M. The Effect of Hydrogen on Vacancy Generation in Iron by Plastic Deformation. Scripta Materialia 2006, 55, 1031–1034. [CrossRef]
- Chernov, I.P. Accumulation and elimination of hydrogen defects under radiation and heat treatment of titanium. Fiz. Khimiya Obrab. Mater. 2002, 3, 55–59.
- Takai, K.; Shoda, H.; Suzuki, H.; Nagumo, M. Lattice Defects Dominating Hydrogen-Related Failure of Metals. Acta Materialia 2008, 56, 5158–5167. [CrossRef]
- Middleburgh, S.C.; Voskoboinikov, R.E.; Guenette, M.C.; Riley, D.P. Hydrogen Induced Vacancy Formation in Tungsten. Journal of Nuclear Materials 2014, 448, 270–275. [CrossRef]
- Lin H. J. et al. Symbiotic CeH₂.73/CeO2 catalyst: a novel hydrogen pump // Nano Energy. – 2014. – Vol. 9. – P. 80–87. [CrossRef]
- Bououdina M., Grant D., Walker G. Review on hydrogen absorbing materials—structure, microstructure, and thermodynamic properties // International Journal of Hydrogen Energy. – 2006. – Vol. 31. – N. 2. – P. 177–182. [CrossRef]
- Kojima Y. Hydrogen storage materials for hydrogen and energy carriers // International Journal of Hydrogen Energy. – 2019. – Vol. 44. – N. 33. – P. 18179–18192. [CrossRef]
- Khafidz N. Z. A. K. et al. The kinetics of lightweight solid-state hydrogen storage.
- materials: A review // International Journal of Hydrogen Energy. – 2016. – Vol. 41. –N. 30. – P. 13131–13151. [CrossRef]
- Rusman N. A. A., Dahari M. A review on the current progress of metal hydrides material for solid-state hydrogen storage applications // International Journal of Hydrogen Energy. – 2016. – Vol. 41. – N. 28. – P. 12108–12126. [CrossRef]
- Sakintuna B., Lamari-Darkrim F., Hirscher M. Metal hydride materials for solid hydrogen storage: a review // International journal of hydrogen energy. – 2007. – Vol. 32. – N. 9. – P. 1121–1140. [CrossRef]
- Ismail M. et al. Desorption behaviours of lithium alanate with metal oxide nanopowder additives // International Journal of Electrochemical Science. – 2014. – Vol. 9. – P. 4959–4973. [CrossRef]
- Cortez J. J. et al. Kinetic improvement of H₂ absorption and desorption properties in Mg/MgH₂ by using niobium ethoxide as additive // International Journal of Hydrogen Energy. – 2019. – Vol. 44 – N. 23. – P. 11961–11969. [CrossRef]
- Zhang X. et al. Realizing 6.7 wt% reversible storage of hydrogen at ambient temperature with non-confined ultrafine magnesium hydrides //Energy & Environmental Science. – 2021. – Vol. 14. – N. 4. – P. 2302–2313. [CrossRef]
- Xie X. et al. Recent advances in magnesium-based hydrogen storage materials with multiple catalysts // International Journal of Hydrogen Energy. – 2019. – Vol. 44. – N. 21. – P. 10694–10712. [CrossRef]
- Vigeholm B., Kjøller J., Larsen B. Magnesium for hydrogen storage // Journal of the Less Common Metals. – 1980. – Vol. 74. – N. 2. – P. 341–350. [CrossRef]
- Galey B. et al. Improved hydrogen storage properties of Mg/MgH₂ thanks to the addition of nickel hydride complex precursors // International Journal of Hydrogen Energy. – 2019. – Vol. 44. – N. 54. – P. 28848–28862. [CrossRef]
- Lin H. J. et al. Hydrogen storage properties of Mg–Ce–Ni nanocomposite induced from amorphous precursor with the highest Mg content // International Journal of Hydrogen Energy. – 2012. – Vol. 37. – N. 19. – P. 14329–14335. [CrossRef]
- Yang J., Sudik A., Wolverton C. Activation of hydrogen storage materials in the Li–Mg–N–H system: Effect on storage properties // Journal of Alloys and Compounds. – 2007. – Vol. 430. – N. 1–2. – P. 334–338. [CrossRef]
- Zhang J. et al. State of the art multi-strategy improvement of Mg-based hydrides for hydrogen storage // Journal of Alloys and Compounds. – 2019. – Vol. 782. – P. 796–823. [CrossRef]
- Zaluska A., Zaluski L., Ström–Olsen J. O. Nanocrystalline magnesium for hydrogen storage // Journal of Alloys and Compounds. – 1999. – Vol. 288. – N. 1–2. – P. 217–225. [CrossRef]
- Jinzhe L., Lider A. M., Kudiyarov V. N. Using Ball Milling for Modification of the Hydrogenation/Dehydrogenation Process in Magnesium-Based Hydrogen Storage Materials: An Overview // Metals. – 2019. – Vol. 9. – N. 7. – Article number 768. [CrossRef]
- Zaluska A., Zaluski L., Ström-Olsen J. O. Synergy of hydrogen sorption in ballmilled hydrides of Mg and Mg₂Ni // Journal of Alloys and Compounds. – 1999. – Vol. 289. – N. 1–2. – P. 197–206. [CrossRef]
- Malka I. E., Czujko T., Bystrzycki J. Catalytic effect of halide additives ball milled with magnesium hydride // International Journal of Hydrogen Energy. – 2010. – Vol. 35. – N. 4. – P. 1706–1712. [CrossRef]
- Zadorozhnyy V. Y. et al. Effect of mechanical activation on compactibility of metal hydride materials // Journal of Alloys and Compounds. – 2017. – Vol. 707. – P. 214–219. [CrossRef]
- Huot J., Liang G., Schulz R. Mechanically alloyed metal hydride systems // Applied Physics A. – 2001. – Vol. 72. – N. 2. – P. 187–195. [CrossRef]
- Wu C. Z. et al. Effect of carbon/noncarbon addition on hydrogen storage behaviors of magnesium hydride // Journal of Alloys and Compounds. – 2006. – Vol. 414. – N. 1–2. – P.259–264. [CrossRef]
- Oelerich W., Klassen T., Bormann R. Metal oxides as catalysts for improved hydrogen sorption in nanocrystalline Mg-based materials // Journal of Alloys and Compounds. – 2001. – Vol. 315. – N. 1–2. – P. 237–242. [CrossRef]
- Milošević S. et al. Fast hydrogen sorption from MgH₂–VO2 (B) composite materials // Journal of Power Sources. – 2016. – Vol. 307. – P. 481–488. [CrossRef]
- Polanski M., Bystrzycki J. Comparative studies of the influence of different nanosized metal oxides on the hydrogen sorption properties of magnesium hydride // Journal of Alloys and Compounds. – 2009. – Vol. 486. – N. 1–2. – P. 697–701. [CrossRef]
- Shao H. et al. Preparation and hydrogen storage properties of nanostructured Mg–Ni BCC alloys // Journal of Alloys and Compounds. – 2009. – Vol. 477. – N. 1–2. – P. 301–306. [CrossRef]
- Liu H. et al. Recent advances in hydrogen storage of MgH₂ doped by Ni // IOP Conference Series: Earth and Environmental Science. – IOP Publishing, 2019. – Vol. 267. – N. 2. – Article number 022042. 10.1088/1755-1315/267/2/022042.
- Shao H. et al. Synthesis and hydrogen storage behavior of Mg–Co–H system at nanometer scale // Journal of Solid State Chemistry. – 2004. – Vol. 177. – N. 10. – P. 3626–3632. [CrossRef]
- Pukazhselvan D. et al. Dehydrogenation properties of magnesium hydride loaded with Fe, Fe− C, and Fe− Mg additives // ChemPhysChem. – 2017. – Vol. 18. – N. 3. – P. 287–291. [CrossRef]
- Zhou C. et al. Roles of Ti-based catalysts on magnesium hydride and its hydrogen storage properties // Inorganics. – 2021. – Vol. 9. – N. 5. – Article number 36. [CrossRef]
- Ren C. et al. Hydrogen storage properties of magnesium hydride with V-based additives // The Journal of Physical Chemistry C. – 2014. – Vol. 118. – N. 38. – P. 21778–21784. [CrossRef]
- Liu Y. et al. Magnesium nanoparticles with Pd decoration for hydrogen storage // Frontiers in Chemistry. – 2020. – Vol. 7. – Article number 949. [CrossRef]
- Liu Y. et al. Effect of novel La-based alloy modification on hydrogen storage performance of magnesium hydride: First-principles calculation and experimental investigation // Journal of Power Sources. – 2022. – Vol. 551. – Article number 232187. [CrossRef]
- Spassov T. et al. Mg–Ni–RE nanocrystalline alloys for hydrogen storage // Materials Science and Engineering: A. – 2004. – Vol. 375. – P. 794–799. [CrossRef]
- Jangir M. et al. Catalytic effect of TiF4 in improving hydrogen storage properties of MgH₂ // International Journal of Hydrogen Energy. – 2016. – Vol. 41. – N. 32. – P. 14178– 14183. [CrossRef]
- Park H. R. et al. Hydrogen Sorption of Pure Mg and Niobium (V) Fluoride-Added Mg Alloys Prepared by Planetary Ball Milling in Hydrogen // Korean Journal of Metals and Materials. – 2016. – Vol. 54. – N. 12. – P. 916–924. [CrossRef]
- Lin H. J. et al. Enhanced hydrogen desorption property of MgH₂ with the addition of cerium fluorides // Journal of Alloys and Compounds. – 2015. – Vol. 645. – P. S392-S396. [CrossRef]
- Malka I. E. et al. A study of the ZrF4, NbF5, TaF5, and TiCl3 influences on the MgH₂ sorption properties // International Journal of Hydrogen Energy. – 2011. – Vol. 36. – N. 20. – P. 12909–12917. [CrossRef]
- Rahmalina D. et al. Experimental Evaluation for the Catalytic Effect of Nickel in Micron Size on Magnesium Hydride //WSEAS Transactions on Applied and Theoretical Mechanics. – 2021. – Vol. 16. – P. 293-302. [CrossRef]
- Varin R. A. et al. Catalytic effects of various forms of nickel on the synthesis rate and hydrogen desorption properties of nanocrystalline magnesium hydride (MgH₂) synthesized by controlled reactive mechanical milling (CRMM) //Journal of alloys and compounds. – 2007. – Vol. 432. – N. 1-2. – P. 217-231. [CrossRef]
- Doppiu S., Schultz L., Gutfleisch O. In situ pressure and temperature monitoring during the conversion of Mg into MgH₂ by high-pressure reactive ball milling //Journal of alloys and compounds. – 2007. – Vol. 427. – N. 1-2. – P. 204-208. [CrossRef]
- Liang G. et al. Catalytic effect of transition metals on hydrogen sorption in nanocrystalline ball milled MgH₂–Tm (Tm= Ti, V, Mn, Fe and Ni) systems //Journal of Alloys and Compounds. – 1999. – Vol. 292. – N. 1-2. – P. 247-252. [CrossRef]
- Shang C. X. et al. Mechanical alloying and electronic simulations of (MgH₂+ M) systems (M= Al, Ti, Fe, Ni, Cu and Nb) for hydrogen storage // International Journal of Hydrogen Energy. – 2004. – Vol. 29. – N. 1. – P. 73-80. 10.1016/S0360-3199(03)00045-4.
- Iljin A. P. , Mostovshchikov A. V. , Nazarenko O. B. , Zmanovsky S. V. Heat release in chemical reaction between micron aluminum powders and water // International Int. J. Hydrog. Energy. – 2019. – Т. 44. – №. 52. – С. 28096-28103.
- Mostovshchikov, A., Gubarev, F., Chumerin, P., Arkhipov, V., Kuznetsov, V., & Dubkova, Y. Solid energetic material based on aluminum micropowder modified by microwave radiation //Crystals. – 2022. – Т. 12. – №. 4. – С. 446.
- Mostovshchikov, A., Gubarev, F., Nazarenko, O., & Pestryakov, A. Influence of short-pulse microwave radiation on thermochemical properties aluminum micropowder //Materials. – 2023. – Т. 16. – №. 3. – С. 951.
- Mostovshchikov A. V., Goldenberg B. G., Nazarenko O. B. Effect of synchrotron radiation on thermochemical properties of aluminum micro-and nanopowders //Materials Science and Engineering: B. – 2022. – Т. 285. – С. 115961.
- Kudiiarov V. N. et al. Microstructure and hydrogen storage properties of MgH₂/MIL-101 (Cr) composite // Journal of Alloys and Compounds. – 2024. – Vol. 976. – Article number 173093. [CrossRef]
- Bordulev I. et al. Positron annihilation spectroscopy complex for structural defect analysis in metal–hydrogen systems // Materials. – 2022. – Vol. 15. – N. 5. – Article number 1823. [CrossRef]
- Kudiiarov V. et al. The phase transitions behavior and defect structure evolution in magnesium hydride/single-walled carbon nanotubes composite at hydrogen sorption-desorption processes // Journal of Alloys and Compounds. – 2023. – Vol. 953. – Article number 170138. [CrossRef]
- Kudiiarov V. N. et al. The defect structure evolution in magnesium hydride/metalorganic framework structures MIL-101 (Cr) composite at high temperature hydrogen sorptiondesorption processes // Journal of Alloys and Compounds. – 2023. – Vol. 966. – Article number 171534. [CrossRef]
- Kudiiarov V. et al. The phase transitions behavior and defect structure evolution in magnesium hydride/single-walled carbon nanotubes composite at hydrogen sorption-desorption processes // Journal of Alloys and Compounds. – 2023. – Vol. 953. – Article number 170138. [CrossRef]
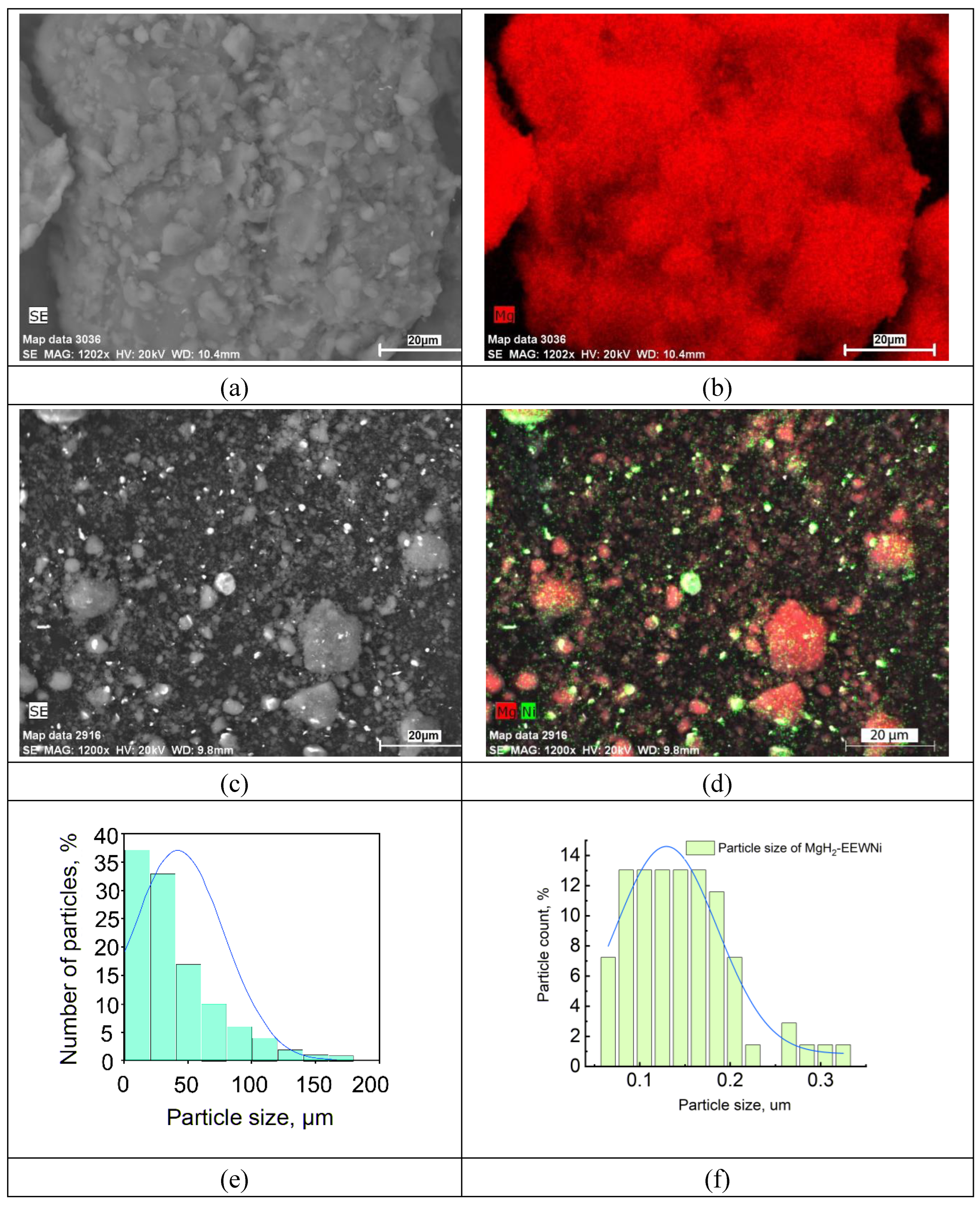
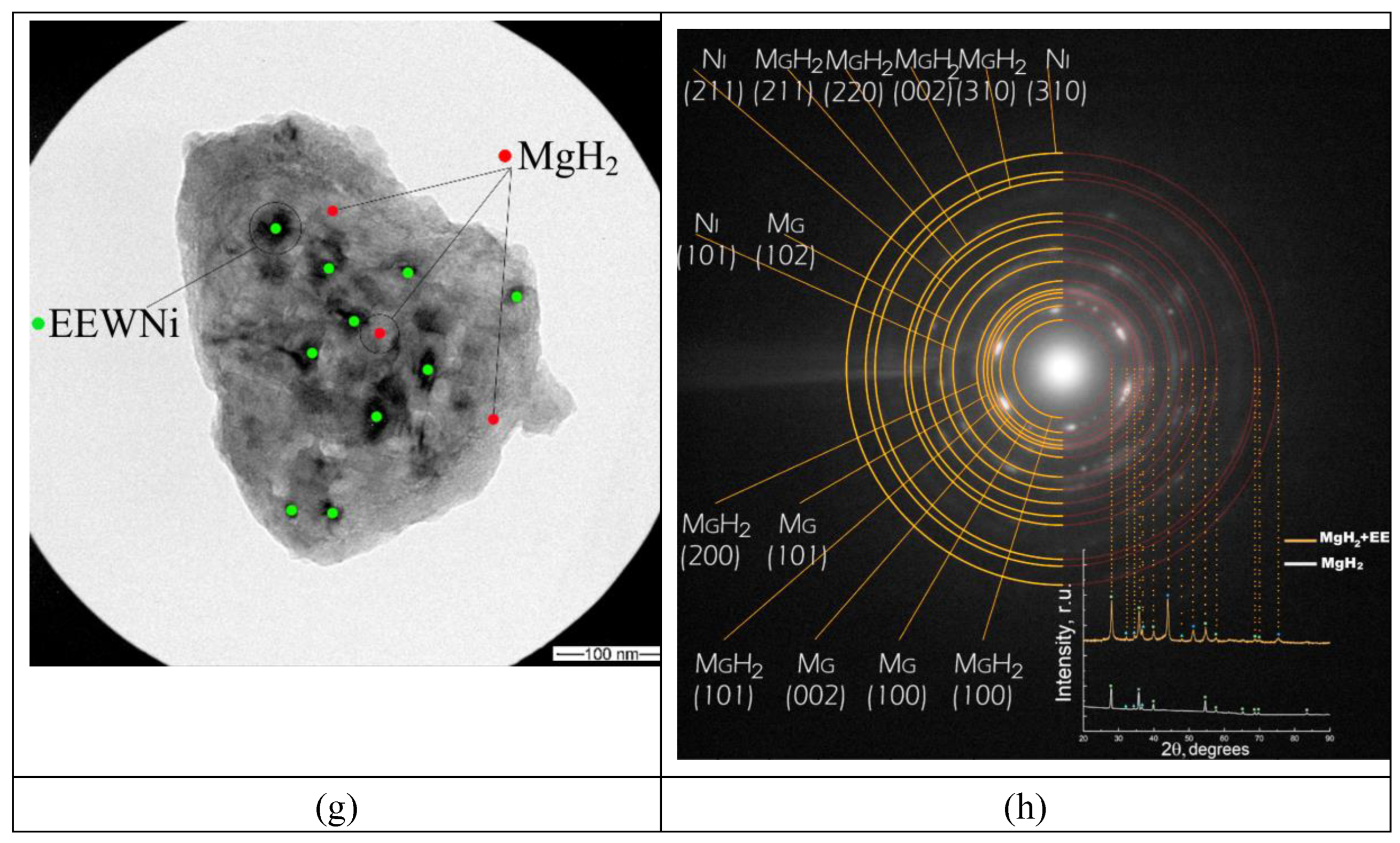
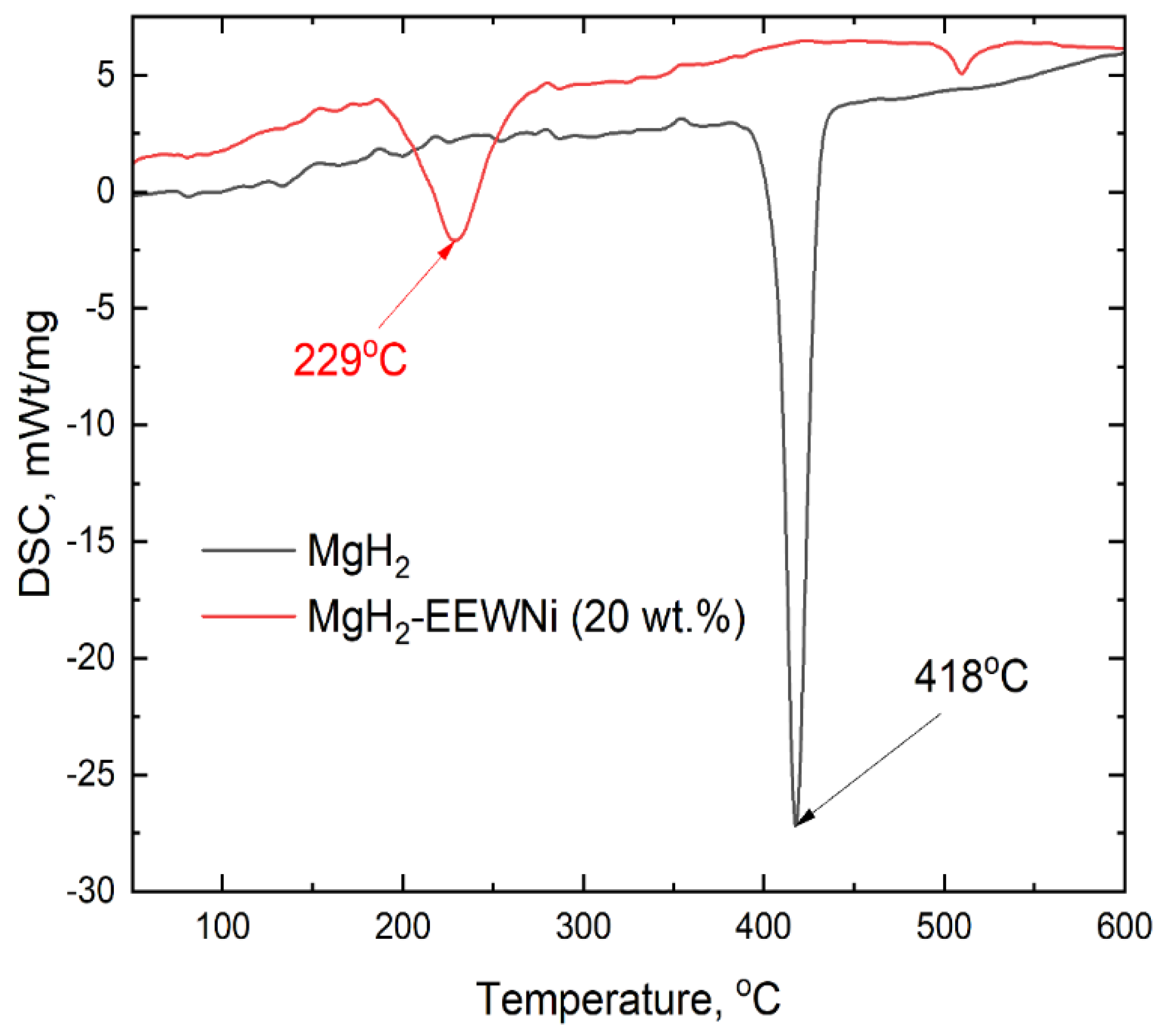
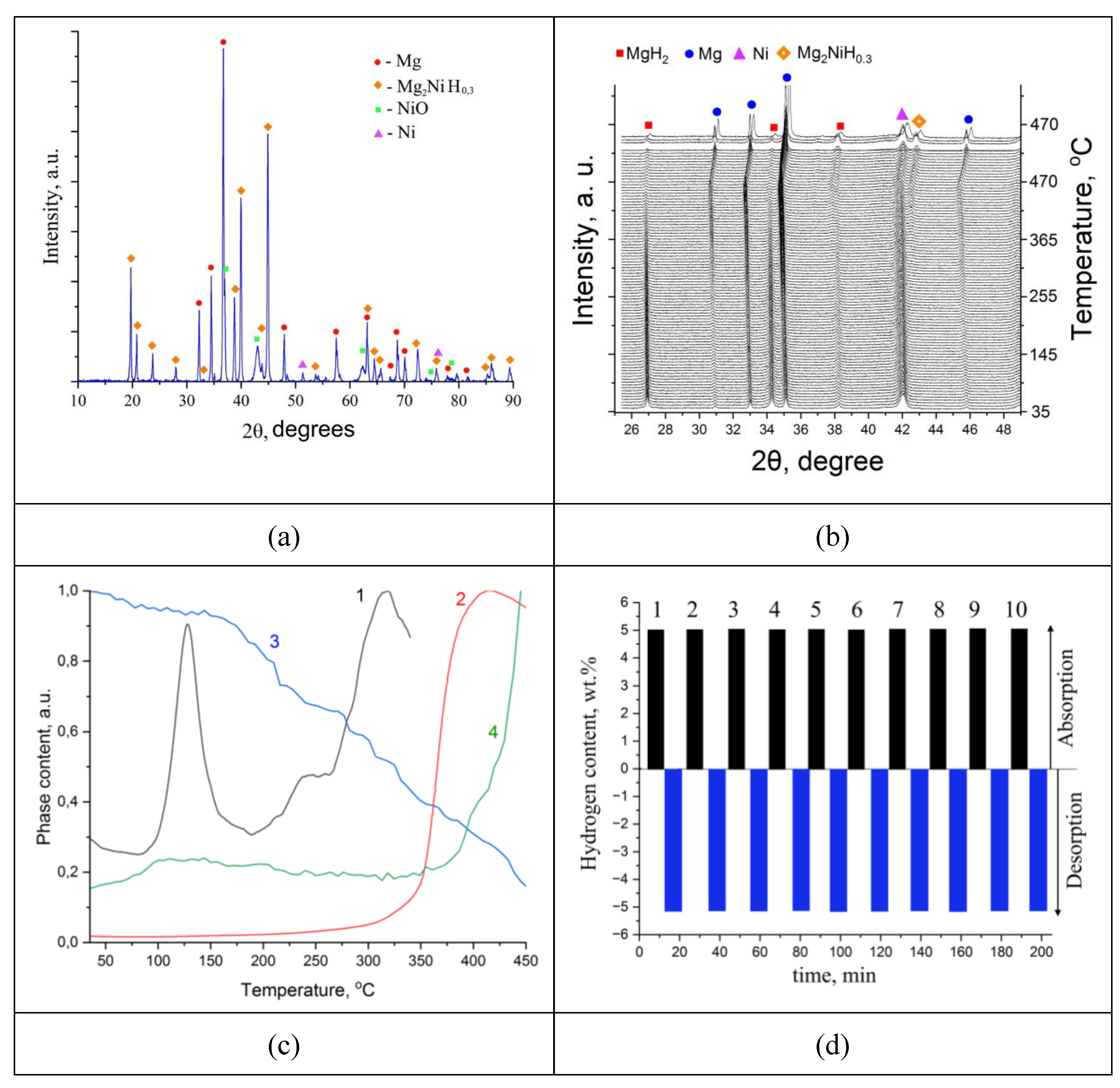
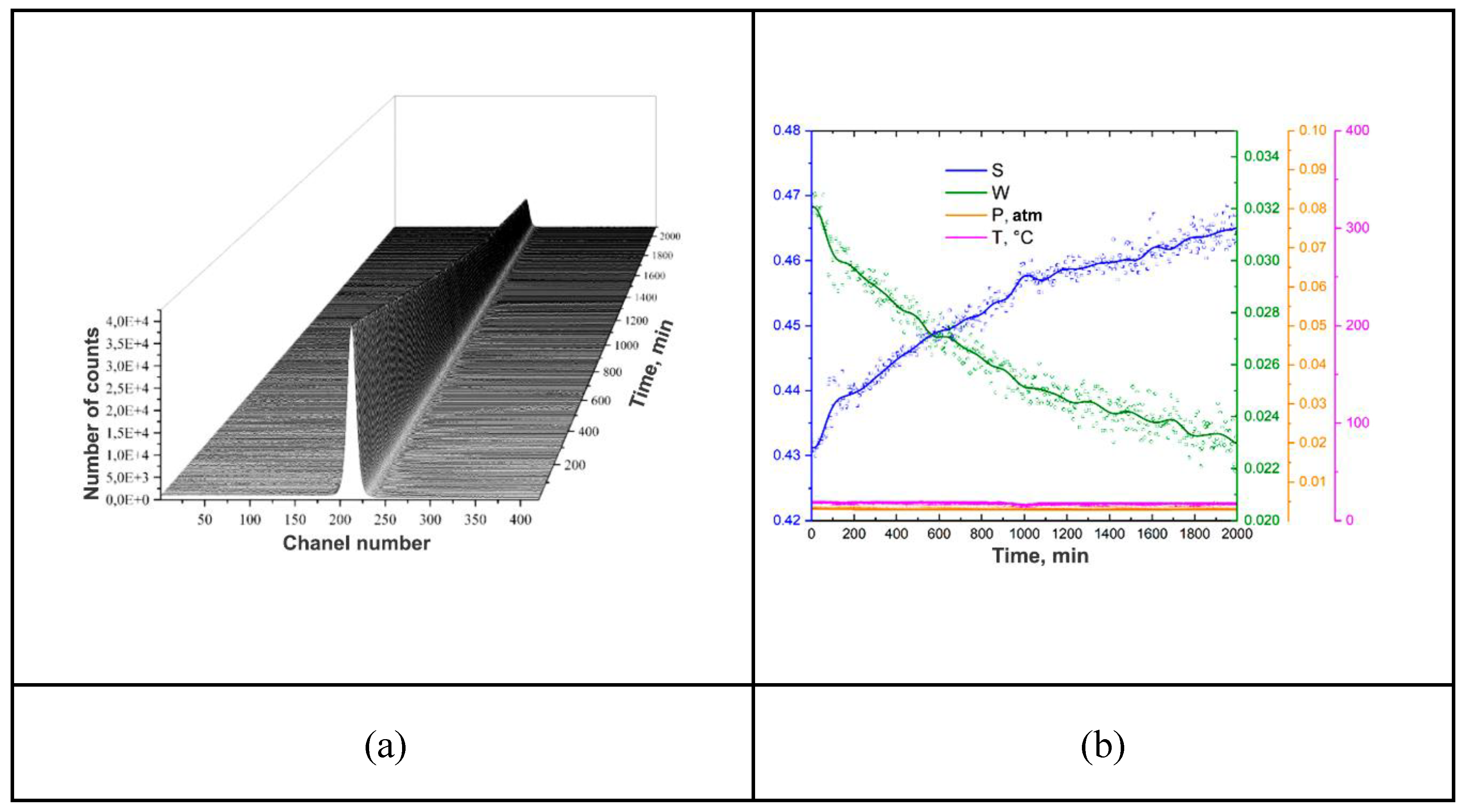
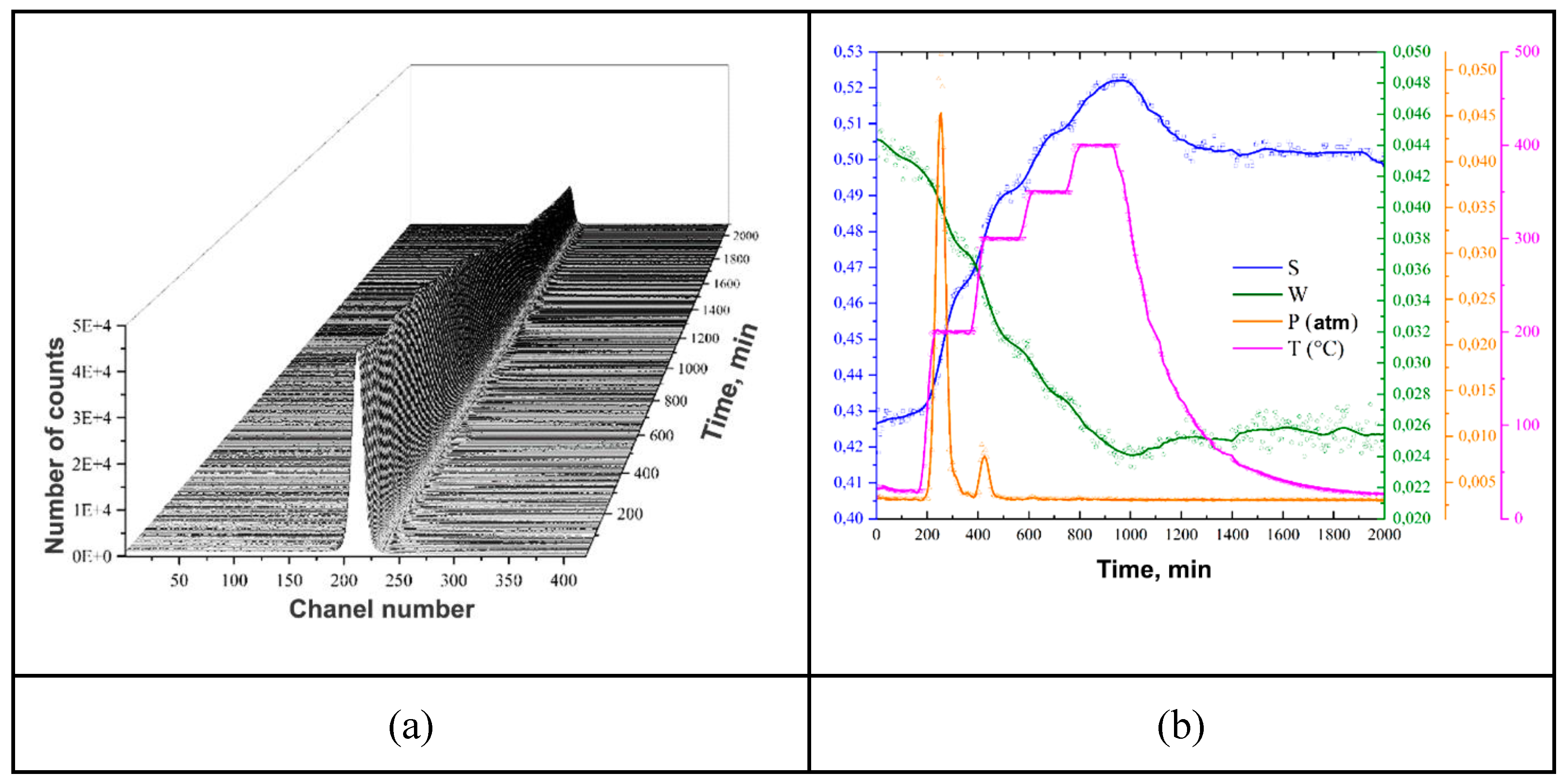
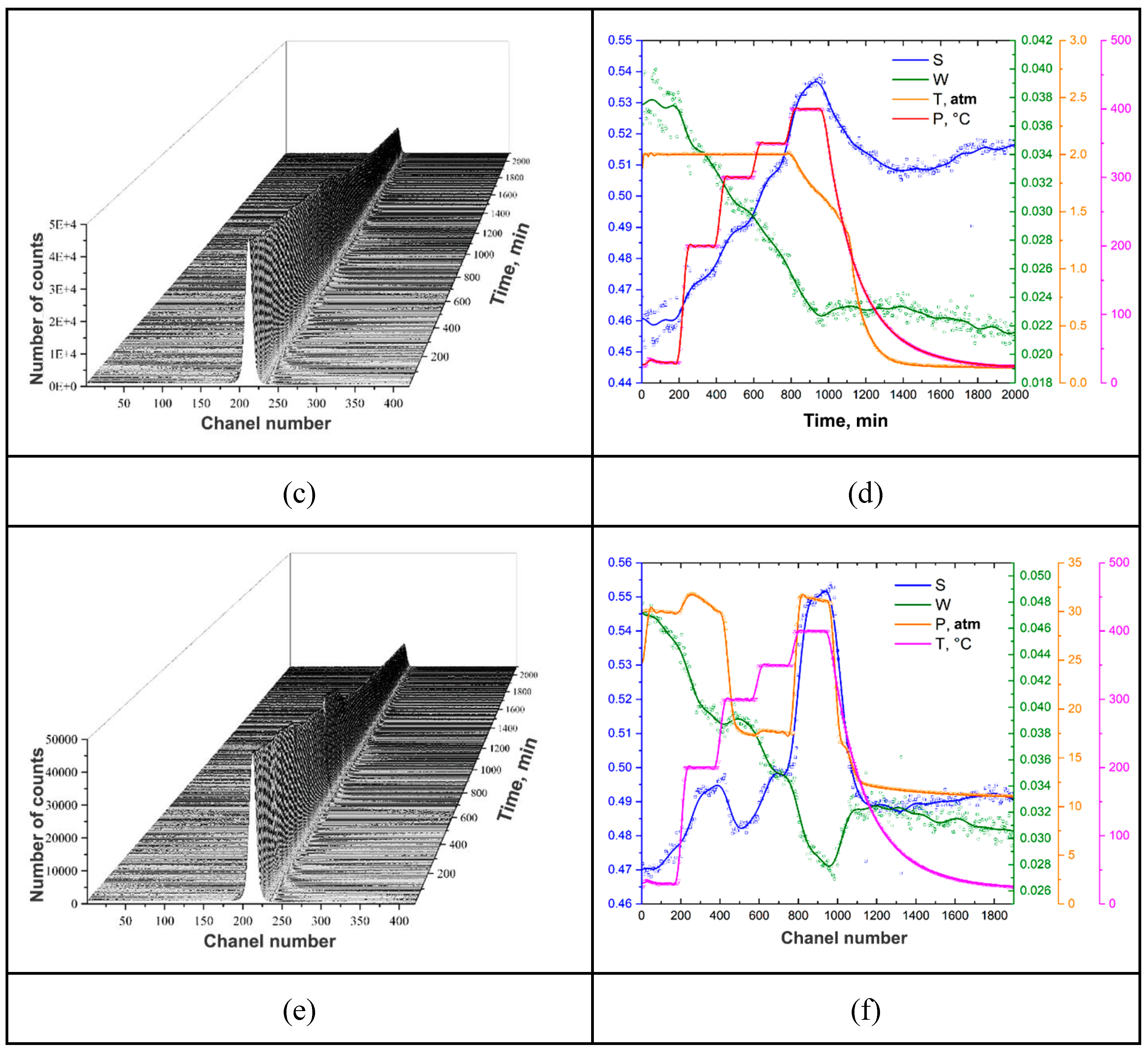
| Sample | Phase | Phase content, vol.% | Crystallite size, nm | Strains, ×10-3 |
| MgH₂ | Mg | 24 | 90 | 0.31 |
| MgH₂ | 76 | 70 | 2.32 | |
| MgH₂-EEWNi | Mg | 18 | 31 | 1.76 |
| MgH₂ | 61 | 24 | 3.53 | |
| Ni | 21 | 31 | 3.84 |
| Sample | Thickness, μm | The backscattering coefficient of positrons | The absorption coefficient of positrons, cm-1 | Intensity without taking into account the contribution of the source |
| MgH₂–20 wt.%-EEWNi | 5000 | 0.25 | 73 | 50 |
| Copper source (⁶⁴Cu) | 10 | 0.35 | 345 | - |
| MgH₂–20 wt.%-EEWNi | 5000 | 0.25 | 73 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





