Submitted:
20 December 2024
Posted:
23 December 2024
You are already at the latest version
Abstract
Keywords:
Introduction
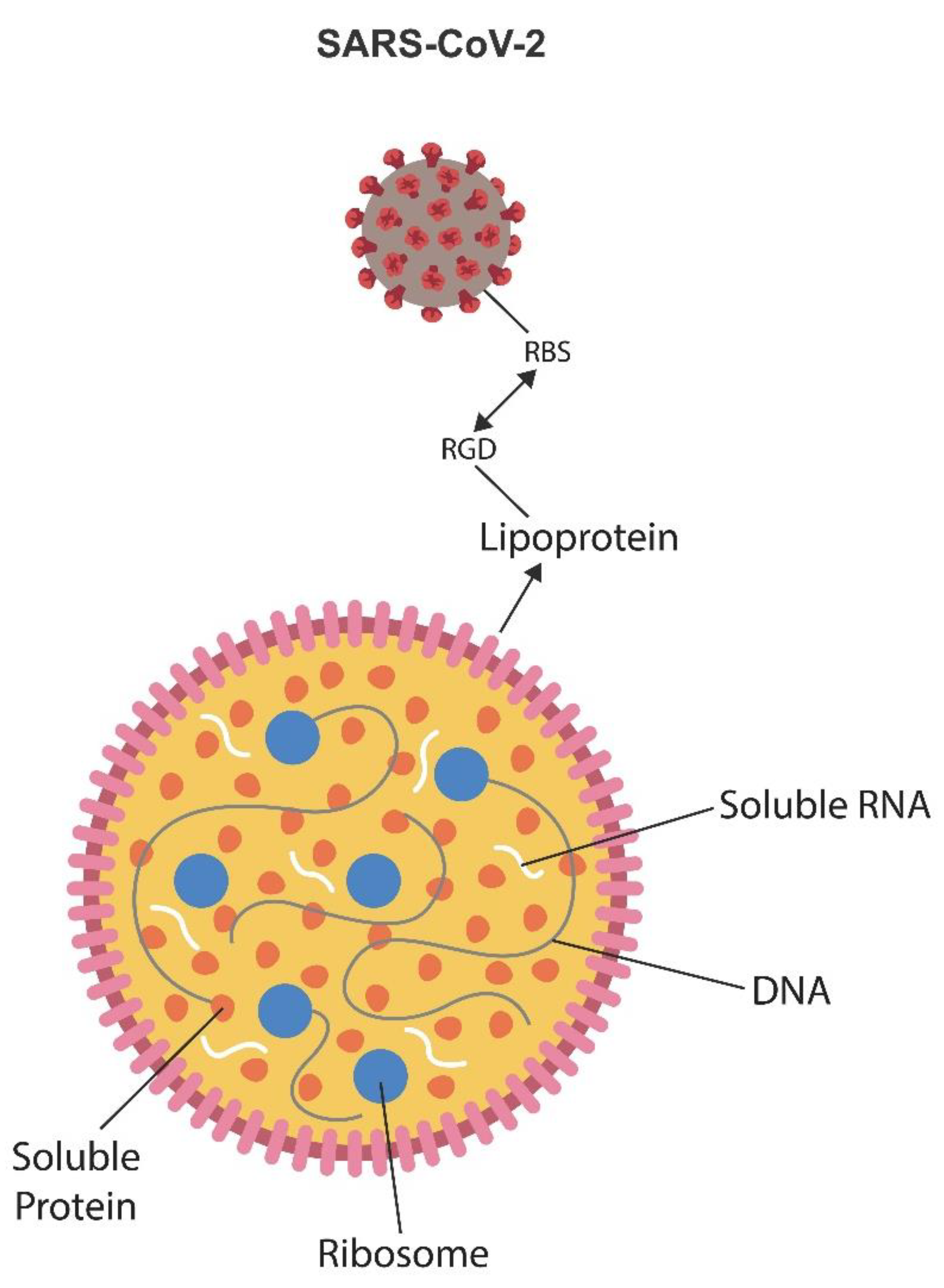
Biological Warfare, Synthetic Microbiology, and Virology
Mycoplasmas, the Ubiquitous and Secretive Slow Killers
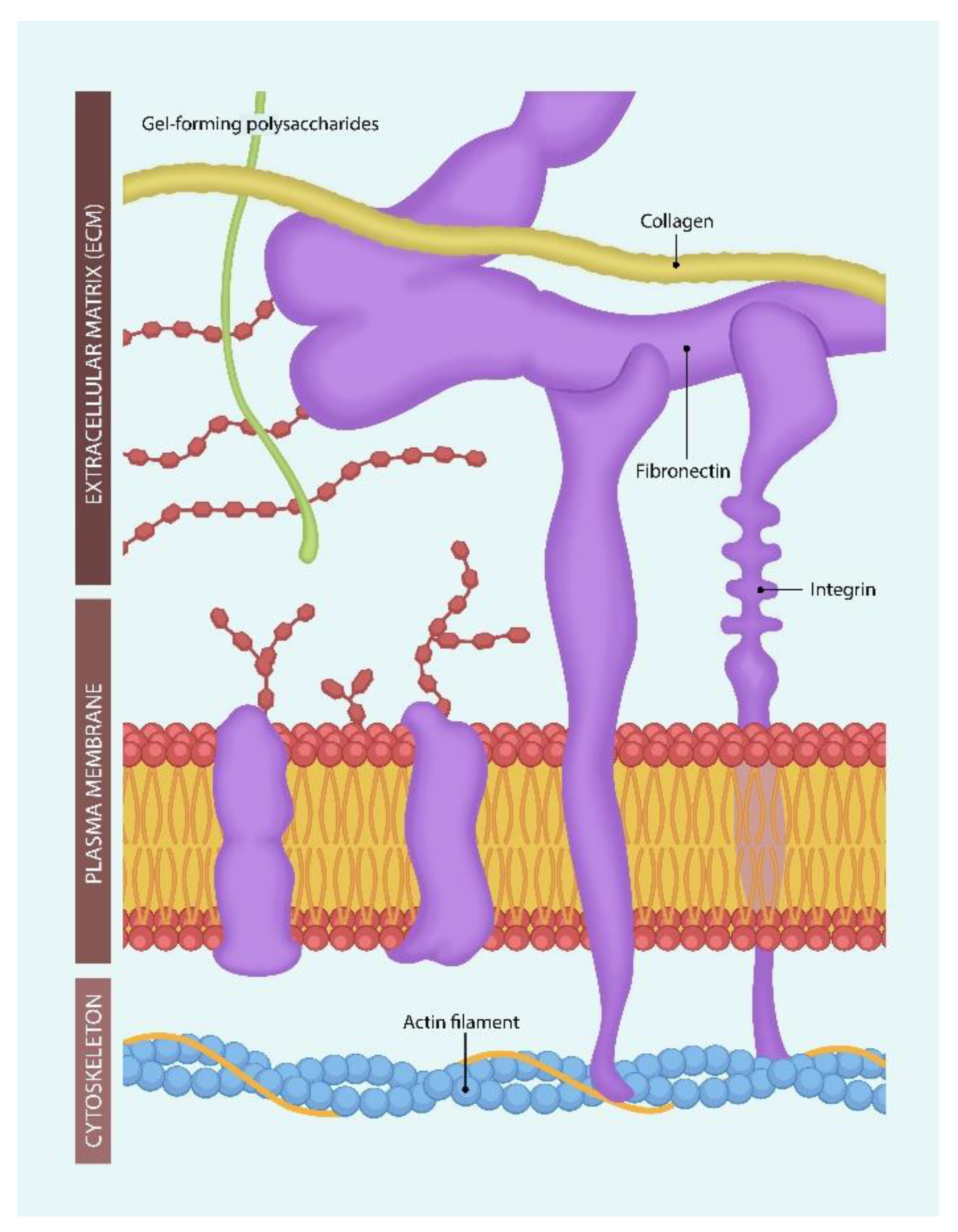
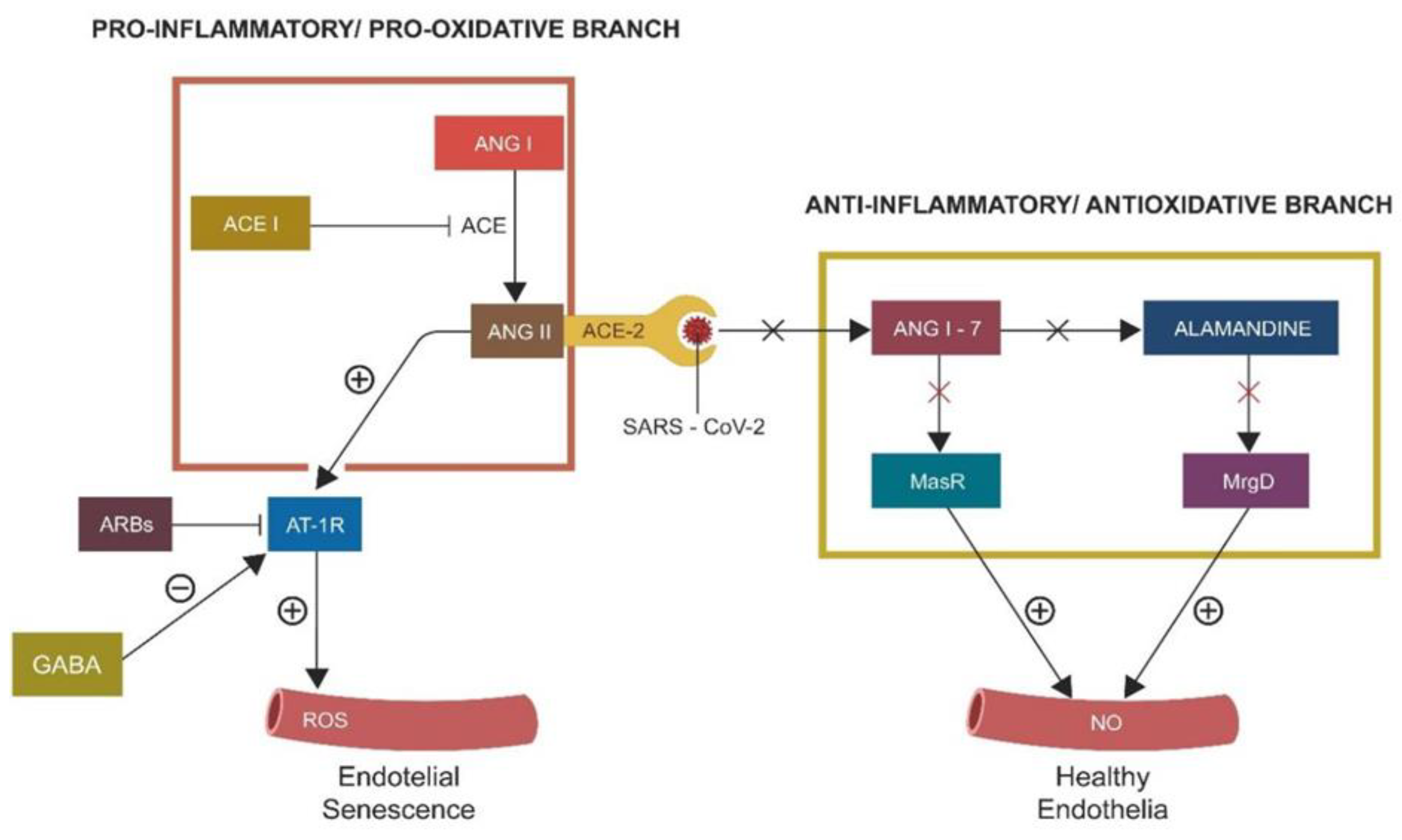
SARS-CoV-2 and Mycoplasma-Induced Premature Endothelial Senescence
Potential Interventions
Phosphoinositide-Dependent Kinase 1 (PDK-1) Inhibitors
AhR Antagonists
Synthetic AhR Antagonists
Senotherapeutics
Mitochondrial Transfer and Transplantation
Conclusions
References
- Michael J. Ainscough. Next Generation Bioweapons: Genetic Engineering and BWUS Air Force. Counterproliferation Center. Future Warfare Series No. 14.
- Nicolson GL, Nasralla MY, Haier J, Pomfret J. High frequency of systemic mycoplasmal infections in Gulf War veterans and civilians with Amyotrophic Lateral Sclerosis (ALS). J Clin Neurosci. 2002 Sep;9(5):525-9. [CrossRef]
- Donta, S.T.; Engel, C.C.; Collins, J.F.; Baseman, J.B.; Dever, L.L.; Taylor, T.; Boardman, K.D.; Kazis, L.E.; Martin, S.E.; Horney, R.A.; et al. Benefits and harms of doxycycline treatment for Gulf War veterans’ illnesses: A randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 2004, 141, 85–94. [Google Scholar] [CrossRef]
- arshis M, Morag B, Mayer M. Mycoplasma cells stimulate in vitro activation of plasminogen by purified tissue-type plasminogen activator. FEMS Microbiol Lett. 1993 Jan 15;106(2):201-4.
- Yavlovich A, Higazi AA, Rottem S. Plasminogen binding and activation by Mycoplasma fermentans. Infect Immun. 2001 Apr;69(4):1977-82. [CrossRef]
- Elizabeth Pennisi,Synthetic Genome Brings New Life to Bacterium.Science328,958-959(2010). [CrossRef]
- Sleator RD. JCVI-syn3.0—A synthetic genome stripped bare! Bioengineered. 2016 Apr 2;7(2):53-6. [CrossRef]
- Hutchison C.A., 3rd, Chuang R.Y., Noskov V.N., Assad-Garcia N., Deerinck T.J., Ellisman M.H., Gill J., Kannan K., Karas B.J., Ma L., et al. Design and synthesis of a minimal bacterial genome. Science. 2016;351:aad6253. [CrossRef]
- Citti C, Dordet-Frisoni E, Nouvel LX, Kuo CH, Baranowski E. Horizontal Gene Transfers in Mycoplasmas (Mollicutes). Curr Issues Mol Biol. 2018;29:3-22. [CrossRef]
- Rosengarten R, Citti C, Glew M, Lischewski A, Droesse M, Much P, Winner F, Brank M, Spergser J. Host-pathogen interactions in mycoplasma pathogenesis: virulence and survival strategies of minimalist prokaryotes. Int J Med Microbiol. 2000 Mar;290(1):15-25. [CrossRef]
- Lai C-C, Wang C-Y, Hsueh P-R. Co-infections among patients with COVID-19: the need for combination therapy with non-anti-SARS-CoV-2 agents? J Microbiol Immunol Infect. 2020;53:505–512. [CrossRef]
- Chaudhry R, Sreenath K, Vinayaraj EV, Sahoo B, Vishnu Narayanan MR, Kiran KVPS, Batra P, Rathor N, Singh S, Mohan A, Bhatnagar S. Mycoplasma pneumoniae co-infection with SARS-CoV-2: A case report. Access Microbiol. 2021 Mar 10;3(3):000212. [CrossRef]
- Arfi Y, Lartigue C, Sirand-Pugnet P, Blanchard A. Beware of Mycoplasma Anti-immunoglobulin Strategies. mBio. 2021 Dec 21;12(6):e0197421. [CrossRef]
- Bransfield RC, Mao C, Greenberg R. Microbes and Mental Illness: Past, Present, and Future. Healthcare (Basel). 2023 Dec 29;12(1):83. [CrossRef]
- Dehhaghi M, Heydari M, Panahi HKS, Lewin SR, Heng B, Brew BJ, Guillemin GJ. The roles of the kynurenine pathway in COVID-19 neuropathogenesis. Infection. 2024 Oct;52(5):2043-2059. [CrossRef]
- Johnson MDL, Younis US, Menghani SV, Addison KJ, Whalen M, Pilon AL, Cress AE, Polverino F, Romanoski CE, Kraft M, Martinez FD, Guerra S, Ledford JG. CC16 Binding to α4β1 Integrin Protects against Mycoplasma pneumoniae Infection. Am J Respir Crit Care Med. 2021 Jun 1;203(11):1410-1418. [CrossRef]
- Zimmermann L, Peterhans E, Frey J. RGD motif of lipoprotein T, involved in adhesion of Mycoplasma conjunctivae to lamb synovial tissue cells. J Bacteriol. 2010 Jul;192(14):3773-9. [CrossRef]
- Vilardi, A., Przyborski, S., Mobbs, C. et al. Current understanding of the interplay between extracellular matrix remodelling and gut permeability in health and disease. Cell Death Discov. 10, 258 (2024). [CrossRef]
- Iyama, K., Zhang, S. & Lo, SC. Effects of Mycoplasmal LAMPs on Receptor Responses to Steroid Hormones in Mammalian Cells. Curr Microbiol 43, 163–169 (2001). [CrossRef]
- Benedetti F, Curreli S, Zella D. Mycoplasmas-Host Interaction: Mechanisms of Inflammation and Association with Cellular Transformation. Microorganisms. 2020 Sep 4;8(9):1351. [CrossRef]
- Seitz-Holland, J., Mulsant, B.H., Reynolds III, C.F. et al. Major depression, physical health and molecular senescence markers abnormalities. Nat. Mental Health 1, 200–209 (2023). [CrossRef]
- Yan B, Liao P, Han Z, Zhao J, Gao H, Liu Y, Chen F, Lei P. Association of aging related genes and immune microenvironment with major depressive disorder. J Affect Disord. 2025 Jan 15;369:706-717. [CrossRef]
- Chen J, Xie X, Lin M, Han H, Wang T, Lei Q, He R. Genes associated with cellular senescence as diagnostic markers of major depressive disorder and their correlations with immune infiltration. Front Psychiatry. 2024 May 31;15:1372386. [CrossRef]
- Diniz, B.S., Reynolds III, C.F., Sibille, E. et al. Major depression and enhanced molecular senescence abnormalities in young and middle-aged adults. Transl Psychiatry 9, 198 (2019). [CrossRef]
- Farooq S, Tunmore J, Wajid Ali M, Ayub M. Suicide, self-harm and suicidal ideation during COVID-19: A systematic review. Psychiatry Res. 2021 Dec;306:114228. [CrossRef]
- Pathirathna, M.L., Nandasena, H.M.R.K.G., Atapattu, A.M.M.P. et al. Impact of the COVID-19 pandemic on suicidal attempts and death rates: a systematic review. BMC Psychiatry 22, 506 (2022). [CrossRef]
- Busl KM, Bleck TP. Treatment of neuroterrorism. Neurotherapeutics. 2012;9(1):139–157. [CrossRef]
- Taubenberger JK, Reid AH, Krafft AE, Bijwaard KE, Fanning TG. Initial genetic characterization of the 1918 “Spanish” influenza virus. Science. 1997 Mar 21;275(5307):1793-6. [CrossRef]
- Becker MM, Graham RL, Donaldson EF, Rockx B, Sims AC, Sheahan T, Pickles RJ, Corti D, Johnston RE, Baric RS, Denison MR. Synthetic recombinant bat SARS-like coronavirus is infectious in cultured cells and in mice. Proc Natl Acad Sci U S A. 2008 Dec 16;105(50):19944-9. [CrossRef]
- Radenkovic D, Chawla S, Pirro M, Sahebkar A, Banach M. Cholesterol in Relation to COVID-19: Should We Care about It? J Clin Med. 2020 Jun 18;9(6):1909. [CrossRef]
- Kočar E, Katz S, Pušnik Ž, Bogovič P, Turel G, Skubic C, Režen T, Strle F, Martins Dos Santos VAP, Mraz M, Moškon M, Rozman D. COVID-19 and cholesterol biosynthesis: Towards innovative decision support systems. iScience. 2023 Aug 31;26(10):107799. [CrossRef]
- Guarnera A, Podda P, Santini E, Paolantonio P, Laghi A. Differential diagnoses of COVID-19 pneumonia: the current challenge for the radiologist-a pictorial essay. Insights Imaging. 2021 Mar 11;12(1):34. PMID: 33704615. [CrossRef]
- Miyashita N, Nakamori Y, Ogata M, Fukuda N, Yamura A, Ishiura Y, Nomura S. Clinical Differences between Community-Acquired Mycoplasma pneumoniae Pneumonia and COVID-19 Pneumonia. J Clin Med. 2022 Feb 12;11(4):964. [CrossRef]
- Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev. 2004 Oct;17(4):697-728, table of contents. [CrossRef]
- Halbedel S, Stülke J. Tools for the genetic analysis of Mycoplasma. Int J Med Microbiol. 2007 Feb;297(1):37-44. [CrossRef]
- Liu Y, Ma Y. Clinical applications of metagenomics next-generation sequencing in infectious diseases. J Zhejiang Univ Sci B. 2024 May 17;25(6):471-484. [CrossRef]
- Fan BE, Lim KGE, Chong VCL, Chan SSW, Ong KH, Kuperan P. COVID-19 and mycoplasma pneumoniae coinfection. Am J Hematol. 2020 Jun;95(6):723-724. Epub 2020 Apr 3. [CrossRef]
- Cheng, Y., Fang, Q. F., & Chen, B. Q. (2024). Clinical Efficacy of Azithromycin in the Treatment of Pediatric Mycoplasma pneumoniae Pneumonia and Its Impact on Platelet Count and D-Dimer Levels. Infection and Drug Resistance, 17, 5195–5202. [CrossRef]
- Liu J, Lu F, Chen Y, Plow E, Qin J. Integrin mediates cell entry of the SARS-CoV-2 virus independent of cellular receptor ACE2. J Biol Chem. 2022 Mar;298(3):101710. [CrossRef]
- Lemańska-Perek A, Krzyżanowska-Gołąb D, Dragan B, Tyszko M, Adamik B. Fibronectin as a Marker of Disease Severity in Critically Ill COVID-19 Patients. Cells. 2022 May 6;11(9):1566. [CrossRef]
- Brogna C, Brogna B, Bisaccia DR, Lauritano F, Marino G, Montano L, Cristoni S, Prisco M, Piscopo M. Could SARS-CoV-2 Have Bacteriophage Behavior or Induce the Activity of Other Bacteriophages? Vaccines (Basel). 2022 Apr 29;10(5):708. [CrossRef]
- Lietha D, Izard T. Roles of Membrane Domains in Integrin-Mediated Cell Adhesion. Int J Mol Sci. 2020 Aug 1;21(15):5531. [CrossRef]
- Campbell ID, Humphries MJ. Integrin structure, activation, and interactions. Cold Spring Harb Perspect Biol. 2011 Mar 1;3(3):a004994. [CrossRef]
- Chastney, M.R., Kaivola, J., Leppänen, VM. et al. The role and regulation of integrins in cell migration and invasion. Nat Rev Mol Cell Biol (2024). [CrossRef]
- Harburger DS, Calderwood DA. Integrin signalling at a glance. J Cell Sci. 2009 Jan 15;122(Pt 2):159-63. Erratum in: J Cell Sci. 2009 May 1;122(Pt 9):1472. [CrossRef]
- Arnaout MA, Goodman SL, Xiong JP. Structure and mechanics of integrin-based cell adhesion. Curr Opin Cell Biol. 2007 Oct;19(5):495-507. Epub 2007 Oct 24. PMID: 17928215. [CrossRef]
- Agnati LF, Guidolin D, Carone C, Dam M, Genedani S, Fuxe K. Understanding neuronal molecular networks builds on neuronal cellular network architecture. Brain Res Rev. 2008 Aug;58(2):379-99. [CrossRef]
- Shay JW, Wright WE. Hayflick, his limit, and cellular ageing. Nat Rev Mol Cell Biol. 2000 Oct;1(1):72-6. [CrossRef] [PubMed]
- Rentscher KE, Carroll JE, Polsky LR, Lamkin DM. Chronic stress increases transcriptomic indicators of biological aging in mouse bone marrow leukocytes. Brain Behav Immun Health. 2022 Apr 12;22:100461. [CrossRef]
- Lorenzo EC, Figueroa JE, Demirci DA, El-Tayyeb F, Huggins BJ, Illindala M, Bartley JM, Haynes L, Diniz BS. Unraveling the association between major depressive disorder and senescent biomarkers in immune cells of older adults: a single-cell phenotypic analysis. Front Aging. 2024 Apr 11;5:1376086. [CrossRef]
- Masaldan, S., Clatworthy, S.A., Gamell, C., Meggyesy, P.M., Rigopoulos, A., Haupt, S., Haupt, Y., Denoyer, D., Adlard, P.A., Bush, A.I., & Cater, M.A. (2017). Iron accumulation in senescent cells is coupled with impaired ferritinophagy and inhibition of ferroptosis. Redox Biology, 14, 100–115.
- Crockett MJ, Clark L, Hauser MD, Robbins TW. Serotonin selectively influences moral judgment and behavior through effects on harm aversion. Proc Natl Acad Sci U S A. 2010 Oct 5;107(40):17433-8. [CrossRef]
- Kunieda T, Minamino T, Nishi J, Tateno K, Oyama T, Katsuno T, Miyauchi H, Orimo M, Okada S, Takamura M, Nagai T, Kaneko S, Komuro I. Angiotensin II induces premature senescence of vascular smooth muscle cells and accelerates the development of atherosclerosis via a p21-dependent pathway. Circulation. 2006 Aug 29;114(9):953-60. [CrossRef]
- Logunov DY, Scheblyakov DV, Zubkova OV, Shmarov MM, Rakovskaya IV, Gurova KV, Tararova ND, Burdelya LG, Naroditsky BS, Ginzburg AL, Gudkov AV. Mycoplasma infection suppresses p53, activates NF-kappaB and cooperates with oncogenic Ras in rodent fibroblast transformation. Oncogene. 2008 Jul 31;27(33):4521-31. [CrossRef]
- Schmitt CA, Tchkonia T, Niedernhofer LJ, Robbins PD, Kirkland JL, Lee S. COVID-19 and cellular senescence. Nat Rev Immunol. 2023 Apr;23(4):251-263. Epub 2022 Oct 5. [CrossRef]
- Lefèvre C, Auclair M, Boccara F, Bastard JP, Capeau J, Vigouroux C, Caron-Debarle M. Premature senescence of vascular cells is induced by HIV protease inhibitors: implication of prelamin A and reversion by statin. Arterioscler Thromb Vasc Biol. 2010 Dec;30(12):2611-20. [CrossRef]
- Collins KL, Younis US, Tanyaratsrisakul S, Polt R, Hay M, Mansour HM, Ledford JG. Angiotensin-(1-7) Peptide Hormone Reduces Inflammation and Pathogen Burden during Mycoplasma pneumoniae Infection in Mice. Pharmaceutics. 2021 Oct 4;13(10):1614. [CrossRef]
- Servant G, Escher E, Guillemette G. The angiotensin II binding site on Mycoplasma hyorhynis is structurally distinct from mammalian AT1 and AT2 receptors. Regul Pept. 1998 Jan 2;73(1):35-41. [CrossRef] [PubMed]
- Gong S, Deng F. Renin-angiotensin system: The underlying mechanisms and promising therapeutical target for depression and anxiety. Front Immunol. 2023 Jan 24;13:1053136. [CrossRef]
- Herbert KE, Mistry Y, Hastings R, Poolman T, Niklason L, Williams B. Angiotensin II-mediated oxidative DNA damage accelerates cellular senescence in cultured human vascular smooth muscle cells via telomere-dependent and independent pathways. Circ Res. 2008 Feb 1;102(2):201-8. [CrossRef]
- Sinha JK, Ghosh S, Raghunath M. Progeria: a rare genetic premature ageing disorder. Indian J Med Res. 2014 May;139(5):667-74. PMID: 25027075; PMCID: PMC4140030. [PubMed]
- Harhouri K, Frankel D, Bartoli C, Roll P, De Sandre-Giovannoli A, Lévy N. An overview of treatment strategies for Hutchinson-Gilford Progeria syndrome. Nucleus. 2018 Jan 1;9(1):246-257. [CrossRef]
- S. An, S. Cho, J. Kang, S. Lee, H. Kim, D. Min, E. Son, K. Cho, Inhibition of 3-phosphoinositide–dependent protein kinase 1 (PDK1) can revert cellular senescence in human dermal fibroblasts, Proc. Natl. Acad. Sci. U.S.A.117 (49) 31535-31546. (2020). [CrossRef]
- Sonnenburg, Erica Dutil et al. The Phosphoinositide-dependent Kinase, PDK-1, Phosphorylates Conventional Protein Kinase C Isozymes by a Mechanism That Is Independent of Phosphoinositide 3-Kinase. Journal of Biological Chemistry, Volume 276, Issue 48, 45289–45297.
- Moghbelinejad S, Alizadeh S, Mohammadi G, Khodabandehloo F, Rashvand Z, Najafipour R, Nassiri-Asl M. The effects of quercetin on the gene expression of the GABAA receptor α5 subunit gene in a mouse model of kainic acid-induced seizure. J Physiol Sci. 2017 Mar;67(2):339-343. [CrossRef]
- Saleem A, Qurat-Ul-Ain, Akhtar MF. Alternative Therapy of Psychosis: Potential Phytochemicals and Drug Targets in the Management of Schizophrenia. Front Pharmacol. 2022 May 17;13:895668. [CrossRef]
- Elisabetsky E, Costa-Campos L. The alkaloid alstonine: a review of its pharmacological properties. Evid Based Complement Alternat Med. 2006 Mar;3(1):39-48. [CrossRef]
- Olayinka JN, Akawa OB, Ogbu EK, Eduviere AT, Ozolua RI, Soliman M. Apigenin attenuates depressive-like behavior via modulating monoamine oxidase A enzyme activity in chronically stressed mice. Curr Res Pharmacol Drug Discov. 2023 Jul 11;5:100161. [CrossRef]
- Żmudzka E, Lustyk K, Głuch-Lutwin M, Wolak M, Jaśkowska J, Kołaczkowski M, Sapa J, Pytka K. Novel Multimodal Salicylamide Derivative with Antidepressant-like, Anxiolytic-like, Antipsychotic-like, and Anti-Amnesic Activity in Mice. Pharmaceuticals (Basel). 2023 Jan 24;16(2):175. [CrossRef]
- McGovern K, Castro AC, Cavanaugh J, Coma S, Walsh M, Tchaicha J, Syed S, Natarajan P, Manfredi M, Zhang XM, Ecsedy J. Discovery and Characterization of a Novel Aryl Hydrocarbon Receptor Inhibitor, IK-175, and Its Inhibitory Activity on Tumor Immune -7163.MCT-21-0984.
- Kang, S.; Lee, A.G.; Im, S.; Oh, S.J.; Yoon, H.J.; Park, J.H.; Pak, Y.K. A Novel Aryl Hydrocarbon Receptor Antagonist HBU651 Ameliorates Peripheral and Hypothalamic Inflammation in High-Fat Diet-Induced Obese Mice. Int. J. Mol. Sci. 2022, 23, 14871. [Google Scholar] [CrossRef]
- Yan L., Li H., Qian Y., Zhang J., Cong S., Zhang X., Wu L., Wang Y., Wang M., Yu T. Transcutaneous vagus nerve stimulation: A new strategy for Alzheimer’s disease intervention through the brain-gut-microbiota axis? Front. Aging Neurosci. 2024;16:1334887. [CrossRef]
- Karpiński P., Żebrowska-Różańska P., Kujawa D., Łaczmański Ł., Samochowiec J., Jabłoński M., Plichta P., Piotrowski P., Bielawski T., Misiak B. Gut microbiota alterations in schizophrenia might be related to stress exposure: Findings from the machine learning analysis. Psychoneuroendocrinology. 2023;155:106335. [CrossRef]
- Seeman, M.V. Subjective Overview of Accelerated Aging in Schizophrenia. Int. J. Environ. Res. Public Health 2023, 20, 737. [Google Scholar] [CrossRef] [PubMed]
- Kozato N., Mishra M., Firdosi M. New-onset psychosis due to COVID-19. BMJ Case Rep. 2021;14:e242538. [CrossRef]
- Solis G.M., Kardakaris R., Valentine E.R., Bar-Peled L., Chen A.L., Blewett M.M., McCormick M.A., Williamson J.R., Kennedy B., Cravatt B.F., et al. Translation attenuation by minocycline enhances longevity and proteostasis in old post-stress-responsive organisms. eLife. 2018;7:e40314. [CrossRef]
- Suda, M., Shimizu, I., Katsuumi, G. et al. Senolytic vaccination improves normal and pathological age-related phenotypes and increases lifespan in progeroid mice. Nat Aging 1, 1117–1126 (2021). [CrossRef]
- Liesa M, Shirihai OS. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 2013 Apr 2;17(4):491-506. [CrossRef]
- Trumpff C, Michelson J, Lagranha CJ, Taleon V, Karan KR, Sturm G, Lindqvist D, Fernström J, Moser D, Kaufman BA, Picard M. Stress and circulating cell-free mitochondrial DNA: A systematic review of human studies, physiological considerations, and technical recommendations. Mitochondrion. 2021 Jul;59:225-245. [CrossRef]
- Boyapati RK, Dorward DA, Tamborska A, Kalla R, Ventham NT, Doherty MK, Whitfield PD, Gray M, Loane J, Rossi AG, Satsangi J, Ho GT. Mitochondrial DNA Is a Pro-Inflammatory Damage-Associated Molecular Pattern Released During Active IBD. Inflamm Bowel Dis. 2018 Sep 15;24(10):2113-2122. [CrossRef]
- Konaka H, Kato Y, Hirano T, Tsujimoto K, Park J, Koba T, Aoki W, Matsuzaki Y, Taki M, Koyama S, Itotagawa E, Jo T, Hirayama T, Kawai T, Ishii KJ, Ueda M, Yamaguchi S, Akira S, Morita T, Maeda Y, Nishide M, Nishida S, Shima Y, Narazaki M, Takamatsu H, Kumanogoh A. Secretion of mitochondrial DNA via exosomes promotes inflammation in Behçet’s syndrome. EMBO J. 2023 Oct 16;42(20):e112573. [CrossRef]
- Van Bogart K, Engeland CG, Sliwinski MJ, Harrington KD, Knight EL, Zhaoyang R, Scott SB, Graham-Engeland JE. The Association Between Loneliness and Inflammation: Findings From an Older Adult Sample. Front Behav Neurosci. 2022 Jan 11;15:801746. [CrossRef]
- Ali Pour P, Hosseinian S, Kheradvar A. Mitochondrial transplantation in cardiomyocytes: foundation, methods, and outcomes. Am J Physiol Cell Physiol. 2021 Sep 1;321(3):C489-C503. Epub 2021 Jun 30. PMID: 34191626. [CrossRef]
- Fairley LH, Grimm A, Eckert A. Mitochondria Transfer in Brain Injury and Disease. Cells. 2022 Nov 14;11(22):3603. [CrossRef]
- Stefano GB, Büttiker P, Weissenberger S, Esch T, Anders M, Raboch J, Kream RM, Ptacek R. Independent and sensory human mitochondrial functions reflecting symbiotic evolution. Front Cell Infect Microbiol. 2023 Jun 14;13:1130197. [CrossRef]
- Kmita H, Pinna G, Lushchak VI. Potential oxidative stress related targets of mitochondria-focused therapy of PTSD. Front Physiol. 2023 Nov 13;14:1266575. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



