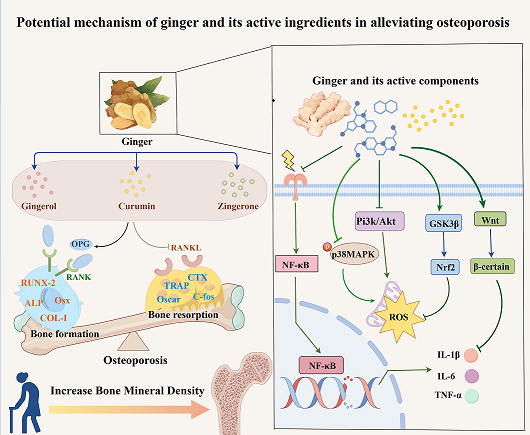
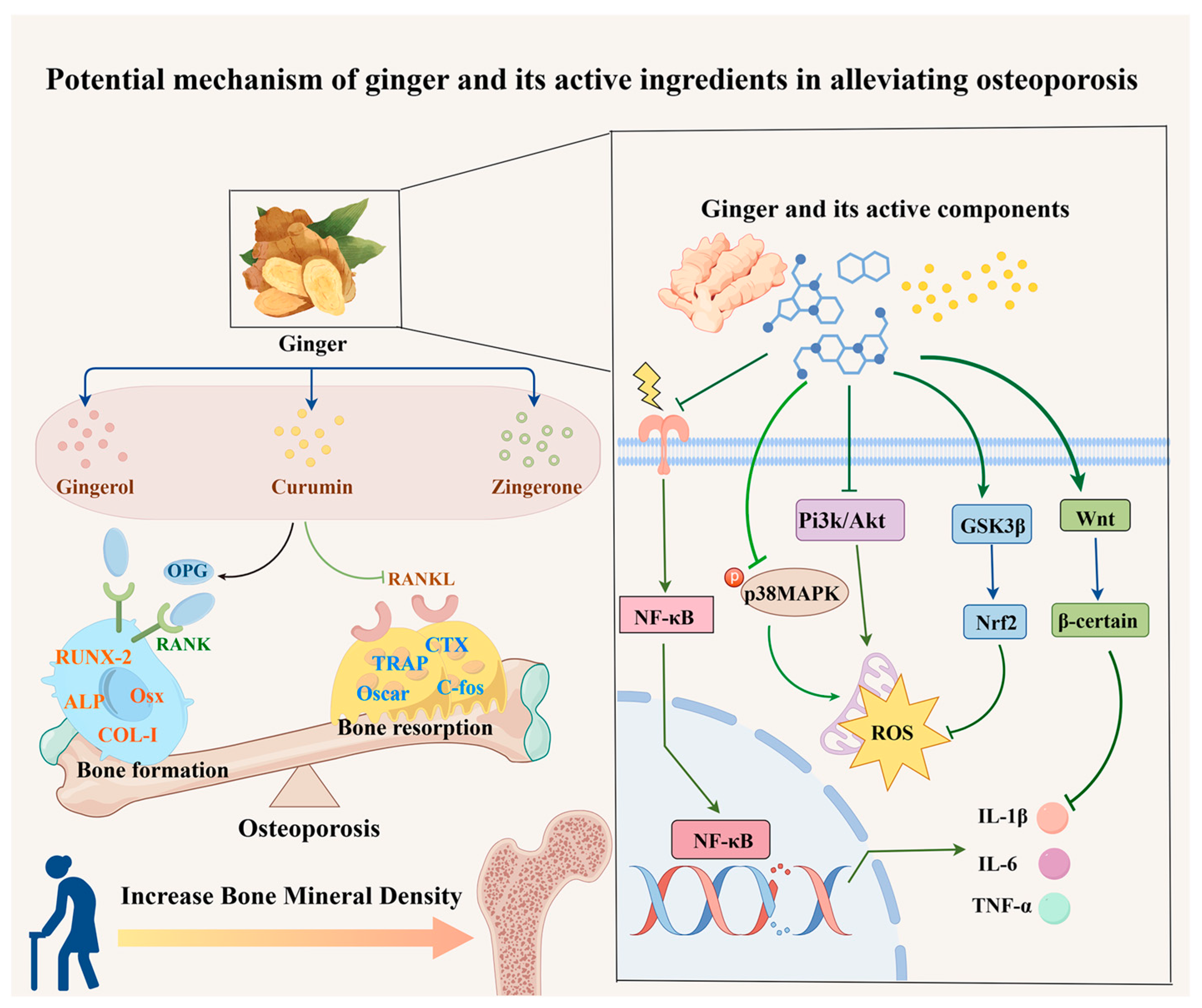
1. Introduction
Osteoporosis (OP) is a metabolic disease caused by an imbalance between bone formation and resorption, resulting in decreased bone mass, increased bone fragility, and a higher risk of fractures [
1]. Globally, over 200 million people are affected by OP [
2]. Epidemiological studies indicate that as populations age, the incidence of OP rises significantly, with projections suggesting that by 2050, more than 50% of brittle fractures in Asia will occur in individuals with OP [
3]. While the exact etiology of OP remains unclear, research identifies genetics, endocrine disorders, aging, nutrition, lifestyle, and intestinal microenvironment disturbances as key contributing factors [
4,
5]. These factors are thought to interact through mechanisms involving oxidative stress, hormonal imbalances, intestinal barrier dysfunction, and other biological pathways [
6,
7].
Ginger, derived from the root and stem of the ginger plant (
Zingiber officinale), holds a prominent place in medicine, nutrition, and culinary traditions [
8]. Globally recognized for its medicinal and therapeutic properties, ginger contains a diverse array of bioactive compounds, including gingerol, curcumin, essential oils, polysaccharides, zingerone, protease, and gingerene [
9]. These components are known for their anti-inflammatory, antioxidant, and anti-aging properties, achieved through multiple signaling pathways. Recent research has uncovered ginger’s potential in mitigating OP, making it a promising candidate for natural therapeutic strategies. This article reviews current findings on ginger’s regulatory effects on bone metabolism and explores its active ingredients’ mechanisms in alleviating OP, offering insights into the development of natural treatments for the disease.
2. OP-Alleviating Effect of Ginger
2.1. Basic Research on the OP-Alleviating Effect of Ginger
As a traditional medicinal herb, ginger is increasingly studied for its role in combating OP. Experimental interventions using ginger on various cell types have demonstrated its ability to increase bone density, enhance the expression of osteogenic genes, and suppress osteoclast-related gene activity (
Table 1).
Ginger’s ethanolic extract has been shown to disrupt osteoclast actin ring formation and downregulate Oscar and Trap genes, which are crucial for osteoclast differentiation, effectively inhibiting the transformation of RAW264.7 cells into osteoclasts [
10]. Furthermore, 6-gingerol can repair MG-63 cell damage, suppress IL-6 production, and promote MG-63 cell differentiation into osteoblasts. Additionally, 6-gingerol reduces the inflammatory mediator prostaglandin E2 and hinders osteoclast differentiation associated with inflammation [
12,
13]. Studies also reveal that gingerol and zingerone enhance alkaline phosphatase activity and increase vitamin D levels in human osteosarcoma cell lines, alleviating bone loss [
22]. Ginger ketone influences mesenchymal bone cells by upregulating the expression of genes like alkaline phosphatase, osteocalcin, and Runt-related transcription factor 2 (Runx2), thereby promoting calcium deposition and mineralized nodule formation, which enhance osteogenic differentiation [
18,
19]. Ginger ketone also inhibits NF-κB signaling in osteoclast precursor cells, reducing bone resorption while suppressing F-actin ring formation and osteoclast activity. Another active compound, curcumin, exhibits the ability to suppress reactive oxygen species (ROS) production and enhance osteogenic differentiation in MC3T3-E1 cells [
16].
In OP zebrafish, the relative mRNA expression levels of osteoclast differentiation markers, such as osteoclast-specific protease K, were downregulated following the administration of 10-gingerol, confirming its anti-osteoclast activity [
14]. Additionally, gingerone administration promoted the regeneration of zebrafish tail fins [
23]. In an OP rat model [
11], oral administration of ginger water extract for 28 days improved cervical spine curvature and degeneration, significantly reduced lumbar and sacral vertebral compression, and lowered the lumbar osteoarthritis index. Furthermore, the damaged trabecular bone structure and density were effectively restored, and serum concentrations of tartrate-resistant acid phosphatase decreased significantly. Curcumin has demonstrated therapeutic effects on femoral injuries in OP rats by enhancing alkaline phosphatase activity and stimulating the expression of bone transcription factor Runx2 and other osteoblast differentiation markers, thereby improving bone health [
17]. It also effectively reduces inflammatory cell infiltration and suppresses pro-inflammatory cytokines, including IL-1β, IL-6, and TNF-α, providing a dual benefit of bone regeneration and inflammation alleviation [
24]. These findings suggest that ginger may not only act directly on the skeletal system during OP treatment but also support bone health through its antioxidant properties, reduction of oxidative stress, and inhibition of inflammatory factor production [
25].
Basic research using cell and animal models has established that ginger and its active compounds can upregulate osteogenic gene expression, downregulate osteoclast gene expression, and mitigate OP progression.
2.2. Clinical Studies on the OP-Alleviating Effect of Ginger
Clinical studies have similarly demonstrated the efficacy of ginger in patients with OP. For instance, ginger has been shown to enhance bone density, reduce inflammation, mitigate oxidative stress, and improve overall bone health (
Table 2).
Salekzamania’s research team [
26]conducted a clinical intervention study on OP patients, comparing the effects of ginger with a placebo. The ginger group consumed a daily dose of ginger tablets, while the control group received an equal amount of placebo. After a 4-month intervention, comprehensive evaluations revealed that the ginger group exhibited increased bone density in the femoral neck and lumbar spine compared to the control group. These findings indicate that ginger enhances bone mass and improves bone health. The ginger group also showed increased antioxidant activity, with higher levels of total antioxidants and superoxide dismutase, alongside decreased malondialdehyde levels. Moreover, serum analysis revealed lower expression levels of IL-6 and TNF-α in the ginger group, suggesting that ginger alleviates bone inflammation and further protects skeletal health. Curcumin’s potential in bone health maintenance was also explored in another clinical study. OP patients received standardized baseline treatment and were randomly assigned to a curcumin group or a placebo group. Over a 6-month intervention, the curcumin group took a specified daily dose of curcumin supplements, while the placebo group continued without therapeutic intervention. Results showed significantly higher bone density in the lumbar spine and femoral neck of the curcumin group compared to the placebo group, alongside notable improvements in quality of life [
27]. Preliminary studies on spinal cord injury patients have similarly demonstrated curcumin’s ability to mitigate bone loss, with increased bone density observed in the femoral neck and hip compared to the control group. The increase in bone mass is evident not only in critical areas such as the femoral neck and lumbar spine but also in the overall improvement of bone density [
28]. This establishes a robust foundation for long-term bone health. Additionally, ginger and its active components exhibit anti-inflammatory properties, significantly reducing the expression levels of pro-inflammatory cytokines such as IL-6 and TNF-α. By enhancing the body’s total antioxidant capacity and superoxide dismutase activity while simultaneously lowering oxidative stress markers such as malondialdehyde, ginger effectively mitigates inflammation and oxidative stress-related damage to bone tissue, fostering a healthier microenvironment for bone maintenance and repair.
Emerging basic and clinical research indicates that ginger and its active ingredients promote osteoblast proliferation and differentiation, inhibit osteoclast activity, and enhance bone formation and repair processes. These effects lead to increased bone density and improved bone microstructure, providing significant therapeutic benefits for OP patients. Furthermore, ginger’s anti-inflammatory and antioxidant properties alleviate bone inflammation and oxidative stress, offering comprehensive protection for bone health.
3. Potential Signaling Pathways Involved in the Ginger Intervention in OP
Ginger and its active ingredients possess diverse biological activities, including anti-inflammatory, antioxidant, antibacterial, and anti-aging effects, demonstrating potential efficacy against OP [
29,
30]. These effects are mediated through the regulation of multiple signaling pathways, such as NF-κB, Wnt/β-catenin, GSK3β/Nrf2, MAPK, and RANK/RANKL/OPG (
Figure 1). Numerous studies have highlighted the pivotal role of these signaling pathways in managing OP, underscoring ginger’s therapeutic potential. This review consolidates recent insights into the mechanisms by which ginger alleviates OP, offering valuable perspectives for its clinical application.
3.1. Ginger and Its Active Ingredients Inhibit the NF-κB Signaling Pathway to Alleviate Osteoporosis
The NF-κB signaling pathway is integral to various immune and inflammatory responses [
31]. It plays a key role in regulating IL-6 and RANKL expression, critical factors in bone remodeling. Ginger and its active compounds regulate this pathway, thereby influencing bone formation processes. Fan’s research demonstrated that curcumin downregulates RelA expression, inhibits the NF-κB pathway, promotes the osteogenic differentiation of mesenchymal stem cells, and alleviates OP [
32]. Another study confirmed that curcumin suppresses NF-κB and IL-6 expression, blocks the NF-κB signaling pathway, increases bone mineral density, and improves trabecular bone microstructure. These findings highlight curcumin’s ability to modulate the NF-κB pathway and mitigate OP. Moreover, in a rat model of testicular injury induced by Shunpa, ginger extract inhibited the NF-κB signaling pathway, exhibiting potent anti-inflammatory effects [
33]. Similarly, 6-gingerol protected the intestinal barrier by modulating the PI3K/Akt and NF-κB pathways, preventing TNF-α-induced alterations in claudin-1 and claudin-2 expression [
34]. Ginger’s suppression of TNF-α further inhibits NF-κB activation, demonstrating anti-inflammatory and anticancer potential [
35]. These findings provide experimental evidence for the mechanisms underlying ginger’s role in alleviating OP and serve as a foundation for further exploration of the NF-κB signaling pathway.
3.2. Ginger and Its Active Ingredients Activate Wnt/β Catenin Signaling Pathway to Alleviate Osteoporosis
The Wnt/β-catenin signaling pathway plays a pivotal role in regulating bone metabolism. It promotes the proliferation of pre-osteoblasts, enhancing their differentiation into osteoblasts and increasing bone mass. Regarding osteoclasts, the Wnt/β-catenin signaling pathway inhibits bone resorption through Wnt signaling ligands, maintaining bone health [
36]. In a hormone-induced rat model of OP, curcumin treatment significantly increased femoral bone density, serum osteocalcin levels, and the expression of Wnt, β-catenin, and osteoprotegerin (OPG) mRNA, while reducing receptor activator of nuclear factor κ-B ligand (RANKL) mRNA expression. These changes restored bone loss and alleviated OP symptoms [
37]. Additionally, curcumin upregulated transcription factors such as runt-related transcription factor 2 (RUNX2) and OPG, which are critical for osteoblast differentiation, and increased the OPG/RANKL ratio, reactivating the hormone-suppressed Wnt/β-catenin signaling pathway. Similarly, ginger and its bioactive components have been shown to reduce inflammation by modulating the Wnt/β-catenin signaling pathway in various models. For instance, ginger extract inhibits NF-κB and Wnt pathway activation, protecting against inflammatory arthritis [
38]. It also holds potential in cancer therapy, inducing apoptosis in colorectal cancer cells by suppressing the mTOR and Wnt/β-catenin pathways. Collectively, these findings suggest that ginger and its active constituents regulate bone metabolism by modulating the Wnt/β-catenin signaling pathway [
39].
3.3. Ginger and Its Active Ingredients Alleviate Osteoporosis by Affecting the MAPK Signaling Pathway
The MAPK family comprises serine/threonine protein kinases, including p38 MAPK, extracellular signal-regulated kinase (ERK), and c-Jun N-terminal kinase (JNK), all of which are involved in bone remodeling [
40,
41,
42,
43]. Curcumin has been shown to reduce p38 MAPK phosphorylation and enhance the ERK pathway by inhibiting pro-apoptotic protein expression, thereby protecting osteoblasts [
44]. Studies reveal that ginger, through its bioactive components, also modulates the MAPK signaling pathway across various diseases [
45]. For instance, 6-gingerol alleviates neuropathic inflammation in rats by inhibiting signal transduction from p38 MAPK to NF-κB. It also prevents reactive oxygen species (ROS) production and p38 MAPK activation, thereby protecting against intestinal ischemia-reperfusion-induced mucosal damage [
46]. Furthermore, 8-gingerol reduces MAPK protein expression, inhibits myocardial cell apoptosis, and improves cardiac injury [
47]. In a mouse model of traumatic brain injury, curcumin suppresses inflammation by downregulating the p38/MAPK pathway [
48]. These findings collectively suggest that both ginger and curcumin mitigate disease progression by modulating the MAPK signaling pathway. In the context of OP, curcumin has demonstrated efficacy through this mechanism, suggesting that ginger and its bioactive compounds may similarly alleviate OP via MAPK pathway modulation.
3.4. Ginger and Its Active Ingredients Act on the GSK3 β/Nrf2 Signaling Pathway to Alleviate Osteoporosis
The glycogen synthase kinase 3β (GSK3β)/nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway plays a crucial role in cellular signaling, oxidative stress regulation, and inflammation suppression. GSK3β functions as a multifunctional kinase, while Nrf2 is a master regulator of antioxidant defenses, capable of significantly mitigating oxidative stress and slowing OP progression [
49,
50]. Research by Li and colleagues [
51]demonstrated that curcumin effectively reduces oxidative stress by scavenging ROS, thereby activating the GSK3β/Nrf2 pathway and offering robust protection to osteoblasts. Similarly, multiple studies have shown that ginger exerts therapeutic effects via the GSK3β/Nrf2 pathway. For example, 6-gingerol activates Nrf2, enhancing antioxidant capacity and potentially preventing Alzheimer’s disease [
52]. Additionally, 6-shogaol reduces oxidative stress and immune mediator activity in allergic dermatitis through the MAPK/Nrf2 signaling pathway [
53], while ginger oleoresin induces Nrf2 nuclear translocation, decreases ROS generation, and protects mesenchymal stem cells from ionizing damage [
54]. Curcumin has been shown to alleviate OP through GSK3β/Nrf2 pathway activation, and ginger appears to achieve similar effects by targeting this pathway. These findings suggest that the therapeutic potential of ginger and its bioactive constituents in OP may involve the activation of the GSK3β/Nrf2 signaling pathway.
3.5. Ginger and Its Active Ingredients Regulate the RANK/RANKL/OPG Signaling Pathway to Alleviate Osteoporosis
The RANK/RANKL/OPG signaling pathway plays a pivotal role in bone remodeling. RANKL and OPG are transmembrane proteins, while RANK functions as a receptor expressed on osteoclasts. OPG competes with RANKL for binding to RANK, thereby inhibiting osteoclast activation and differentiation as well as suppressing the bone-resorbing activity of mature osteoclasts [
55]. Research has shown that 6-shogaol effectively inhibits ROS production induced by RANKL, thereby modulating the RANKL/OPG balance, exhibiting anti-osteoclast activity, and mitigating bone loss [
56]. Studies demonstrate that drugs stimulating human osteoblasts increase the expression of macrophage colony-stimulating factor and RANKL while reducing OPG expression. Following 6-shogaol intervention, RANKL expression was significantly suppressed, resulting in improved bone resorption [
57]. Additionally, in fractured rat models, six weeks of curcumin treatment reduced RANK and RANKL expression in the femur, decreased the RANKL/OPG ratio, and inhibited osteoclastogenesis, leading to enhanced bone formation over resorption [
58]. These findings suggest that the beneficial effects of ginger on OP may be attributed to its ability to regulate the RANK/RANKL/OPG signaling pathway through its bioactive compounds.
Inflammation and oxidative stress are critical factors in alleviating OP. Ginger’s modulation of key pathways such as NF-κB, Wnt/β-catenin, MAPK, GSK3β/Nrf2, and RANK/RANKL/OPG has shown promise in reducing inflammation and oxidative stress, which are central to its therapeutic potential in various diseases. These mechanisms suggest that ginger targets similar pathways in OP. While the precise mechanisms and detailed data on ginger’s role in OP require further exploration, its potential offers significant prospects for advanced research and clinical applications. In summary, ginger and its bioactive components may mitigate oxidative stress and inflammatory responses by targeting multiple signaling pathways. By regulating NF-κB, Wnt/β-catenin, MAPK, GSK3β/Nrf2, and RANK/RANKL/OPG, ginger supports bone metabolism and promotes bone health through an integrated network of mechanisms.
4. Discussion
As a natural plant with both medicinal and dietary value, ginger has garnered considerable attention for its safety, efficacy, affordability, and minimal side effects. Although ginger shows promise in alleviating OP, most studies focus on specific active components’ effects on certain bone cells, with limited clinical evidence available. Current research suggests that ginger alleviates inflammation, inhibits osteoclast differentiation and proliferation, promotes osteoblast growth, and reduces oxidative stress by modulating signaling pathways, including NF-κB, Wnt/β-catenin, MAPK, GSK3β/Nrf2, and RANK/RANKL/OPG. This review highlights ginger’s multi-target, multi-pathway, and multi-component interactions as a potential therapeutic strategy for OP. However, the mechanisms by which ginger and its bioactive compounds regulate bone metabolism remain unclear, and robust clinical evidence is still lacking. Future research should prioritize high-quality clinical studies and foundational experiments to further elucidate ginger’s efficacy in alleviating OP through these pathways. Ginger and its active ingredients exhibit therapeutic effects on OP due to their anti-inflammatory properties. However, the underlying mechanisms and molecular targets remain inadequately elucidated, with limited research exploring related signaling pathways. Further investigations into the mechanisms and targets of ginger’s active components in mitigating OP are essential. Such research could optimize the use of ginger as a natural therapeutic agent and support the development of related studies. In summary, a call to action is needed for researchers to design systematic experimental frameworks and establish effective evaluation systems. These efforts could position ginger and its active ingredients as a viable alternative treatment for OP.
Author Contributions
Writing—original draft preparation,ML,NL.; writing—review and editingML,NL, Conceptualization, CM,FW, and PY; software L D,HP. investigation, YW; funding acquisition and Supervision,LL and DQ, All authors have read and agreed to the published version of the manuscript
Funding
This research was funded by Scientific Research Foundation of Education Department of Yunnan Province of China(202101AZ070001-242、202301AZ070001-004、202101AH070158、2023J0538)Yunnan Provincial Science and Technology Plan Project-Major Science and Technology Special Project Biological Seed Industry and Deep Processing of Agricultural Products(202102AE090031)Yunnan Key Laboratory of Integrated Traditional Chinese and Western Medicine for Chronic Disease in Prevention and Treatment(YPKLG2024-001, YPKLG2024-020). This work was supported by the National Natural Science Foundation of China (81960870, 31960178, 82160923, 82374425); Construction Project of National Traditional Chinese Medicine Clinical Research Base (2018 No. 131); Key Laboratory of Traditional Chinese Medicine for Prevention and Treatment of Neuropsychiatric Diseases, Yunnan Provincial Department of Education; Scientific Research Projects for High-level Talents of Yunnan University of Chinese Medicine (2019YZG01); Young Top-Notch Talent in 10,000 Talent Program of Yunnan Province (YNWR-QNBJ-2019-235); Clinical Cooperative Project of Chinese and Western Medicine for Major and Knotty Diseases; Yunnan Provincial Key Laboratory Construction Project Funding; Yunnan Provincial Key Laboratory of Chinese Medicine Rheumatology and Immunology; Yunnan Provincial Ten Thousands Program Famous Doctor Special; Yunnan Province Qingguo Wang Expert Workstation Construction Project (202005AF150017); Yunnan Applied Basic Research Projects-Union Foundation [2019FF002(-031)]; Applied Basic Research Programs of Science and Technology Commission Foundation of Yunnan Province (2019FA007); Scientific Research Fund Project of Yunnan Provincial Department of Education (2022Y348); and Scientific Research Fund Project of Yunnan Provincial Department of Education (2021Y461).
Acknowledgments
The authors would like to thank Figdraw from Researcher’s House for assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, L.; Li, Y.; Gu, J.; etc. Knowledge, Awareness and Perception towards Osteoporosis Risk in China: A Systematic Review. Iran J Public Health 2024, 53, 1009-1020. [CrossRef]
- Sozen, T.; Ozisik, L.; Basaran, N.C. An overview and management of osteoporosis. Eur J Rheumatol 2017, 4, 46-56. [CrossRef]
- Yu, F.; Xia, W. The epidemiology of osteoporosis, associated fragility fractures, and management gap in China. Arch Osteoporos 2019, 14, 32.
- Zhang, Y.W.; Song, P.R.; Wang, S.C.; etc. Diets intervene osteoporosis via gut-bone axis. Gut Microbes 2024, 16, 2295432. [CrossRef]
- Wang, H.; Luo, Y.; Wang, H.; etc. Mechanistic advances in osteoporosis and anti-osteoporosis therapies. Medcomm 2023, 4, e244.
- Goldring, S.R. Inflammatory signaling induced bone loss. Bone 2015, 80, 143-149. [CrossRef]
- De-Ugarte, L.; Balcells, S.; Nogues, X.; etc. Pro-osteoporotic miR-320a impairs osteoblast function and induces oxidative stress. Plos One 2018, 13, e208131. [CrossRef]
- Hu, W.; Yu, A.; Wang, S.; etc. Extraction, Purification, Structural Characteristics, Biological Activities, and Applications of the Polysaccharides from Zingiber officinale Roscoe. (Ginger): A Review. Molecules 2023, 28.
- Liu, Y.; Liu, J.; Zhang, Y. Research Progress on Chemical Constituents of Zingiber officinale Roscoe. Biomed Res Int 2019, 2019, 5370823. [CrossRef]
- Ito, S.; Ohmi, A.; Sakamiya, A.; etc. Ginger hexane extract suppresses RANKL-induced osteoclast differentiation. Biosci Biotech Bioch 2016, 80, 779-785. [CrossRef]
- Zammel, N.; Amri, N.; Chaabane, R.; etc. Proficiencies of Zingiber officinale against spine curve and vertebral damage induced by corticosteroid therapy associated with gonadal hormone deficiency in a rat model of osteoporosis. Biomed Pharmacother 2018, 103, 1429-1435. [CrossRef]
- Fan, J.Z.; Yang, X.; Bi, Z.G. The effects of 6-gingerol on proliferation, differentiation, and maturation of osteoblast-like MG-63 cells. Braz J Med Biol Res 2015, 48, 637-643. [CrossRef]
- Hwang, Y.H.; Kim, T.; Kim, R.; etc. The Natural Product 6-Gingerol Inhibits Inflammation-Associated Osteoclast Differentiation via Reduction of Prostaglandin E(2) Levels. Int J Mol Sci 2018, 19. [CrossRef]
- Zang, L.; Kagotani, K.; Nakayama, H.; etc. 10-Gingerol Suppresses Osteoclastogenesis in RAW264.7 Cells and Zebrafish Osteoporotic Scales. Front Cell Dev Biol 2021, 9, 588093. [CrossRef]
- Yang, C.; Zhu, K.; Yuan, X.; etc. Curcumin has immunomodulatory effects on RANKL-stimulated osteoclastogenesis in vitro and titanium nanoparticle-induced bone loss in vivo. J Cell Mol Med 2020, 24, 1553-1567. [CrossRef]
- Xin, M.; Yang, Y.; Zhang, D.; etc. Attenuation of hind-limb suspension-induced bone loss by curcumin is associated with reduced oxidative stress and increased vitamin D receptor expression. Osteoporosis Int 2015, 26, 2665-2676. [CrossRef]
- Chen, Z.; Xue, J.; Shen, T.; etc. Curcumin alleviates glucocorticoid-induced osteoporosis through the regulation of the Wnt signaling pathway. Int J Mol Med 2016, 37, 329-338. [CrossRef]
- Srinaath, N.; Balagangadharan, K.; Pooja, V.; etc. Osteogenic potential of zingerone, a phenolic compound in mouse mesenchymal stem cells. Biofactors 2019, 45, 575-582. [CrossRef]
- Song, Y.; Mou, R.; Li, Y.; etc. Zingerone Promotes Osteoblast Differentiation Via MiR-200c-3p/smad7 Regulatory Axis in Human Bone Mesenchymal Stem Cells. Med Sci Monitor 2020, 26, e919309. [CrossRef]
- Yang, D.; Tan, Y.; Xie, X.; etc. Zingerone attenuates Ti particle-induced inflammatory osteolysis by suppressing the NF-kappaB signaling pathway in osteoclasts. Int Immunopharmacol 2023, 115, 109720.
- Kim, S.J.; Shin, M.S.; Choi, Y.K. Ameliorative Effects of Zingiber officinale Rosc on Antibiotic-Associated Diarrhea and Improvement in Intestinal Function. Molecules 2024, 29. [CrossRef]
- Abdel-Naim, A.B.; Alghamdi, A.A.; Algandaby, M.M.; etc. Phenolics Isolated from Aframomum meleguta Enhance Proliferation and Ossification Markers in Bone Cells. Molecules 2017, 22. [CrossRef]
- Kim, A.R.; Lim, Y.J.; Jang, W.G. Zingerone stimulates osteoblast differentiation by increasing Smad1/5/9-mediated HO-1 expression in MC3T3-E1 cells and primary mouse calvarial cells. Clin Exp Pharmacol P 2022, 49, 1050-1058.
- Khan, K.; Singh, A.; Mittal, M.; etc. [6]-Gingerol induces bone loss in ovary intact adult mice and augments osteoclast function via the transient receptor potential vanilloid 1 channel. Mol Nutr Food Res 2012, 56, 1860-1873. [CrossRef]
- Zammel, N.; Jedli, O.; Rebai, T.; etc. Kidney injury and oxidative damage alleviation by Zingiber officinale: pharmacokinetics and protective approach in a combined murine model of osteoporosis. 3 Biotech 2022, 12, 112. [CrossRef]
- Salekzamani, Y.; Shakouri, S.K.; Dolatkhah, N.; etc. The effect of ginger and curcumin co-supplementation in postmenopausal women with osteoporosis: a randomised, triple-blind, placebo-controlled clinical trial. J Herb Med 2023, 42, 100746. [CrossRef]
- Usefian, F.; Farshbaf- Khalili, A.; Mirghafourvand, M.; etc. Effect of Curcumin and/or Nigella sativa on bone density and quality of life in postmenopausal women with osteoporosis or osteopenia. Adv Integr Med 2024, 11, 17-23. [CrossRef]
- Hatefi, M.; Ahmadi, M.; Rahmani, A.; etc. Effects of Curcumin on Bone Loss and Biochemical Markers of Bone Turnover in Patients with Spinal Cord Injury. World Neurosurg 2018, 114, e785-e791. [CrossRef]
- Sharifi-Rad, M.; Varoni, E.M.; Salehi, B.; etc. Plants of the Genus Zingiber as a Source of Bioactive Phytochemicals: From Tradition to Pharmacy. Molecules 2017, 22. [CrossRef]
- Mao, Q.Q.; Xu, X.Y.; Cao, S.Y.; etc. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods 2019, 8. [CrossRef]
- Hoesel, B.; Schmid, J.A. The complexity of NF-kappaB signaling in inflammation and cancer. Mol Cancer 2013, 12, 86.
- Fan, D.; Lu, J.; Yu, N.; etc. Curcumin Prevents Diabetic Osteoporosis through Promoting Osteogenesis and Angiogenesis Coupling via NF-kappaB Signaling. Evid-Based Compl Alt 2022, 2022, 4974343.
- Famurewa, A.C.; Ekeleme-Egedigwe, C.A.; Onwe, C.S.; etc. Ginger juice prevents cisplatin-induced oxidative stress, endocrine imbalance and NO/iNOS/NF-kappaB signalling via modulating testicular redox-inflammatory mechanism in rats. Andrologia 2020, 52, e13786.
- Luettig, J.; Rosenthal, R.; Lee, I.M.; etc. The ginger component 6-shogaol prevents TNF-alpha-induced barrier loss via inhibition of PI3K/Akt and NF-kappaB signaling. Mol Nutr Food Res 2016, 60, 2576-2586.
- Habib, S.H.; Makpol, S.; Abdul, H.N.; etc. Ginger extract (Zingiber officinale) has anti-cancer and anti-inflammatory effects on ethionine-induced hepatoma rats. Clinics 2008, 63, 807-813. [CrossRef]
- Yao, Q.; Yu, C.; Zhang, X.; etc. Wnt/beta-catenin signaling in osteoblasts regulates global energy metabolism. Bone 2017, 97, 175-183.
- Chen, Z.; Xue, J.; Shen, T.; etc. Curcumin alleviates glucocorticoid-induced osteoporosis through the regulation of the Wnt signaling pathway. Int J Mol Med 2016, 37, 329-338. [CrossRef]
- Oz, B.; Orhan, C.; Tuzcu, M.; etc. Ginger extract suppresses the activations of NF-kappaB and Wnt pathways and protects inflammatory arthritis. Eur J Rheumatol 2021, 8, 196-201.
- Wee, L.H.; Morad, N.A.; Aan, G.J.; etc. Mechanism of Chemoprevention against Colon Cancer Cells Using Combined Gelam Honey and Ginger Extract via mTOR and Wnt/beta-catenin Pathways. Asian Pac J Cancer Prev 2015, 16, 6549-6556.
- Liu, Z.G.; Zhao, J.B.; Zhang, C.; etc. The JNK signaling pathway against titanium-particle-induced osteoclastogenesis and bone resorption in vivo. Eur Rev Med Pharmaco 2023, 27, 10301-10312.
- Nagai, T.; Sekimoto, T.; Kurogi, S.; etc. Tmem161a regulates bone formation and bone strength through the P38 MAPK pathway. Sci Rep-Uk 2023, 13, 14639. [CrossRef]
- Guo, J.; Ren, R.; Sun, K.; etc. PERK controls bone homeostasis through the regulation of osteoclast differentiation and function. Cell Death Dis 2020, 11, 847. [CrossRef]
- Liu, Z.G.; Zhao, J.B.; Zhang, C.; etc. The JNK signaling pathway against titanium-particle-induced osteoclastogenesis and bone resorption in vivo. Eur Rev Med Pharmaco 2023, 27, 10301-10312.
- Chen, Z.; Xue, J.; Shen, T.; etc. Curcumin alleviates glucocorticoid-induced osteoporosis by protecting osteoblasts from apoptosis in vivo and in vitro. Clin Exp Pharmacol P 2016, 43, 268-276.
- Torkzadeh-Mahani, S.; Esmaeili-Mahani, S.; Nasri, S.; etc. Ginger Extract Reduces Chronic Morphine-Induced Neuroinflammation and Glial Activation in Nucleus Accumbens of Rats. Addict Health 2019, 11, 66-72. [CrossRef]
- Li, Y.; Xu, B.; Xu, M.; etc. 6-Gingerol protects intestinal barrier from ischemia/reperfusion-induced damage via inhibition of p38 MAPK to NF-kappaB signalling. Pharmacol Res 2017, 119, 137-148.
- Xue, Y.; Zhang, M.; Zheng, B.; etc. [8]-Gingerol exerts anti-myocardial ischemic effects in rats via modulation of the MAPK signaling pathway and L-type Ca(2+) channels. Pharmacol Res Perspe 2021, 9, e852.
- Li, G.; Duan, L.; Yang, F.; etc. Curcumin suppress inflammatory response in traumatic brain injury via p38/MAPK signaling pathway. Phytother Res 2022, 36, 1326-1337.
- Huang, Z.; Jiang, Z.; Zheng, Z.; etc. Methyl 3,4-dihydroxybenzoate inhibits RANKL-induced osteoclastogenesis via Nrf2 signaling in vitro and suppresses LPS-induced osteolysis and ovariectomy-induced osteoporosis in vivo. Acta Bioch Bioph Sin 2022, 54, 1068-1079. [CrossRef]
- Peng, S.; Yao, J.; Liu, Y.; etc. Activation of Nrf2 target enzymes conferring protection against oxidative stress in PC12 cells by ginger principal constituent 6-shogaol. Food Funct 2015, 6, 2813-2823. [CrossRef]
- Li, X.; Chen, Y.; Mao, Y.; etc. Curcumin Protects Osteoblasts From Oxidative Stress-Induced Dysfunction via GSK3beta-Nrf2 Signaling Pathway. Front Bioeng Biotech 2020, 8, 625.
- Lee, C.; Park, G.H.; Kim, C.Y.; etc. [6]-Gingerol attenuates beta-amyloid-induced oxidative cell death via fortifying cellular antioxidant defense system. Food Chem Toxicol 2011, 49, 1261-1269.
- Park, G.; Oh, D.S.; Lee, M.G.; etc. 6-Shogaol, an active compound of ginger, alleviates allergic dermatitis-like skin lesions via cytokine inhibition by activating the Nrf2 pathway. Toxicol Appl Pharm 2016, 310, 51-59. [CrossRef]
- Ji, K.; Fang, L.; Zhao, H.; etc. Ginger Oleoresin Alleviated gamma-Ray Irradiation-Induced Reactive Oxygen Species via the Nrf2 Protective Response in Human Mesenchymal Stem Cells. Oxid Med Cell Longev 2017, 2017, 1480294.
- Yasuda, H. Discovery of the RANKL/RANK/OPG system. J Bone Miner Metab 2021, 39, 2-11. [CrossRef]
- Bezirci, D.; Karsiyaka, H.M.; Ozcan, G.; etc. Prophylactic and therapeutic effects of (6)-shogaol on alveolar bone loss in experimental periodontitis. Eur Oral Res 2024, 58, 37-43. [CrossRef]
- Yeh, I.J.; Chen, S.C.; Yen, M.C.; etc. 6-Shogaol Suppresses 2-Amino-1-Methyl-6-Phenylimidazo [4,5-b] Pyridine (PhIP)-Induced Human 786-O Renal Cell Carcinoma Osteoclastogenic Activity and Metastatic Potential. Nutrients 2019, 11.
- Ilka, S.; Heshmati, A.; Mirabdollahi, S.A.; etc. Effect of turmeric extract on bone healing in an experimental model of femoral bone fracture. Avicenna J Phytomedi 2022, 12, 197-212. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).