Submitted:
29 November 2024
Posted:
02 December 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
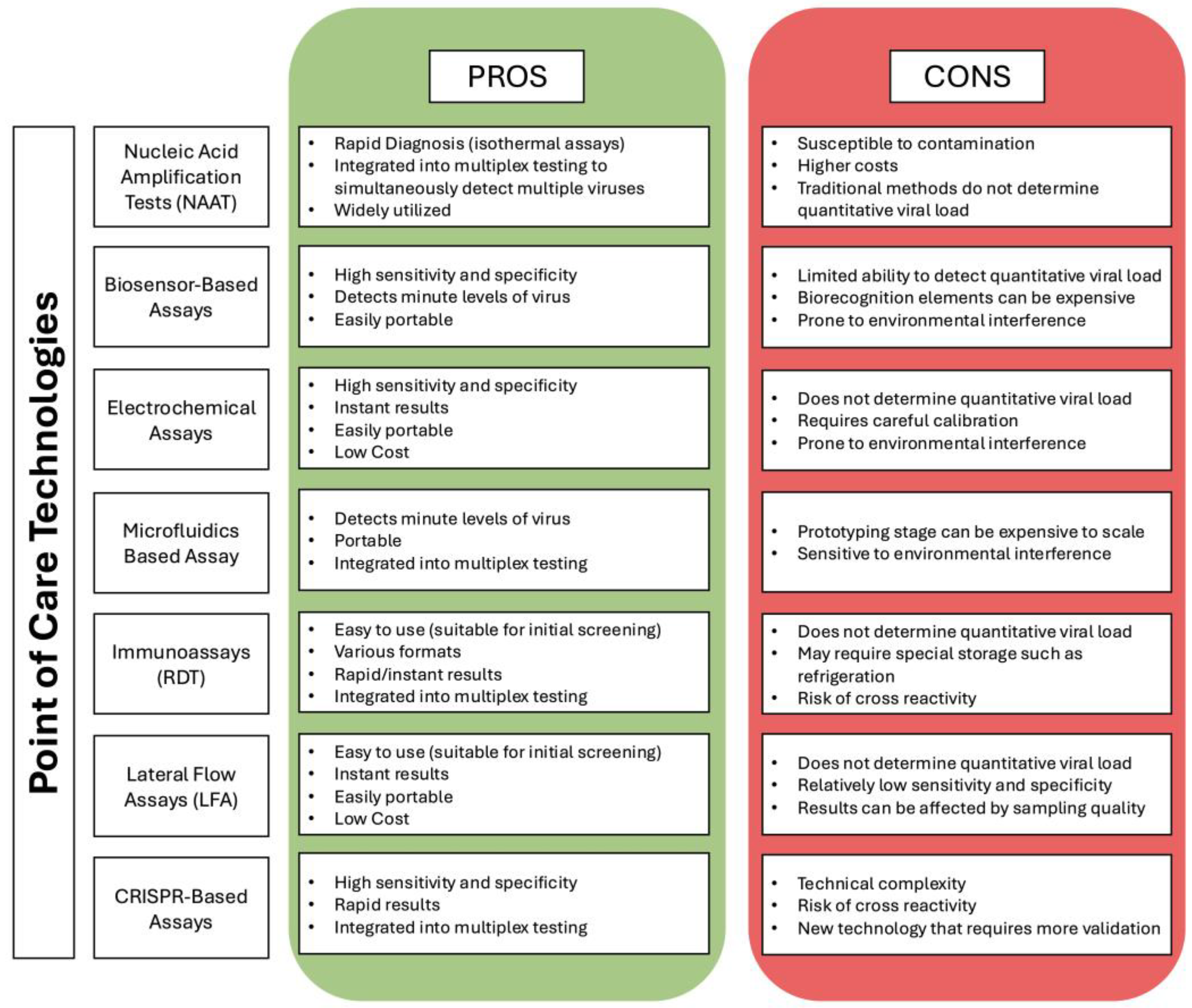
2. Progress in POC Technologies
2.1. Advancements in Nucleic Acid Amplification Tests (NAATs)
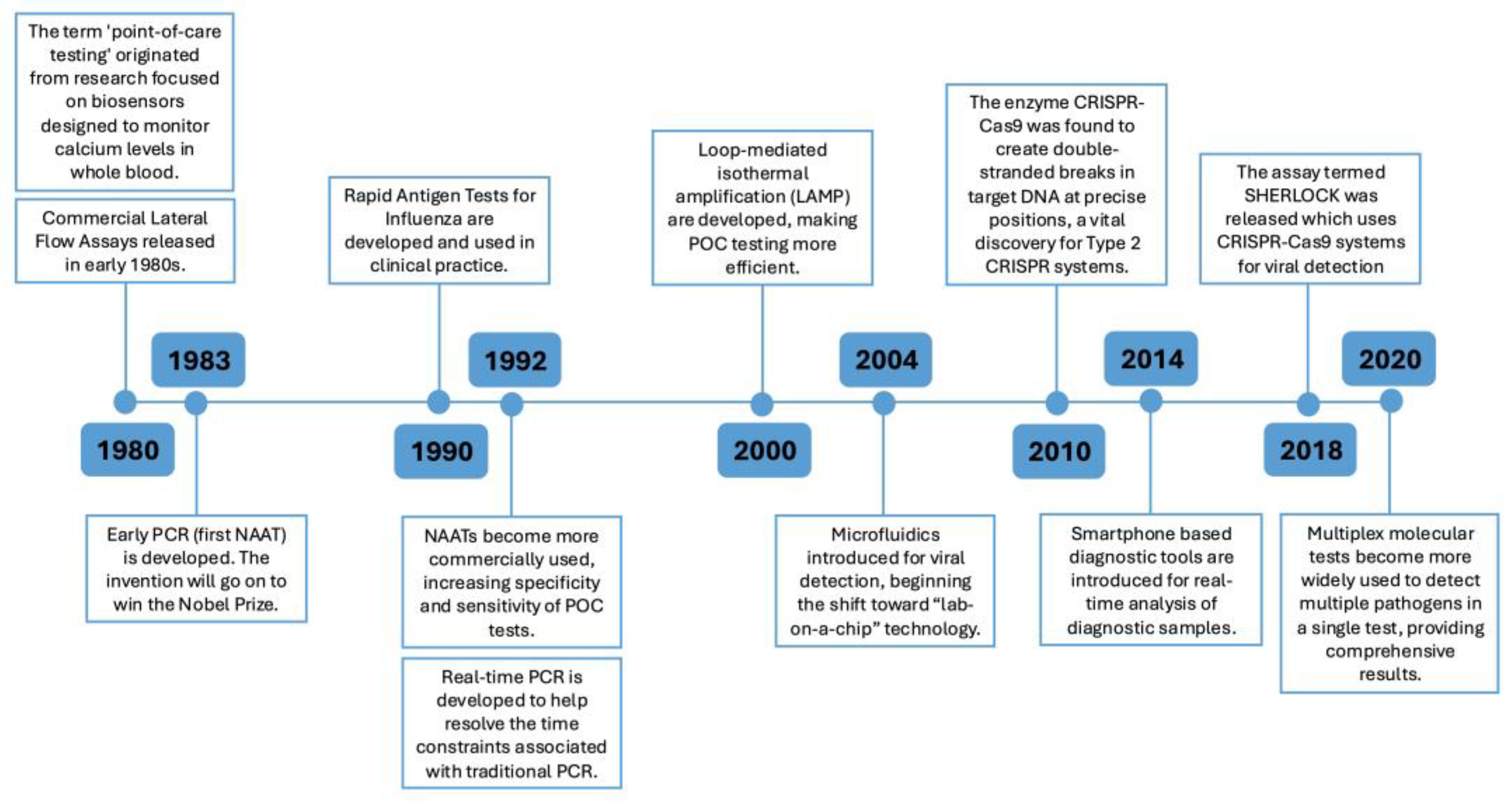
2.2. Biosensors
2.3. Smartphone Based Technologies
2.4. CRISPR-Cas
3. Impact of Viral POC Testing on Diagnosis and Treatment of VPVIs
3.1. Mpox
3.2. HBV
3.3. Respiratory Viruses
3.4. Flaviviruses: Dengue, Zika and Yellow Fever Virus
4. Impact of Viral POC Testing on Surveillance
4.1. Mpox
4.2. HBV
4.3. Respiratory Viruses
4.4. Flaviviruses: Dengue, Zika and Yellow Fever Virus
5. Conclusion
6. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brenzel, L.; Wolfson, L.J.; Fox-Rushby, J.; et al. Vaccine-preventable Diseases. In: Jamison DT, Breman JG, Measham AR.; et al., editors. Disease Control Priorities in Developing Countries. 2nd edition. Washington (DC): The International Bank for Reconstruction and Development / The World Bank; 2006. Chapter 20. Available from: https://www.ncbi.nlm.nih.gov/books/NBK11768/Co-published by Oxford University Press, New York.
- Hotez, P.J. Vaccine Preventable Disease and Vaccine Hesitancy. Med Clin North Am. 2023, 107, 979–987. [Google Scholar] [CrossRef]
- Liu, B.M.; Li, T.; Xu, J.; Li, X.G.; Dong, J.P.; Yan, P.; Yang, J.X.; Yan, L.; Gao, Z.Y.; Li, W.P.; Sun, X.W.; Wang, Y.H.; Jiao, X.J.; Hou, C.S.; Zhuang, H. Characterization of potential antiviral resistance mutations in hepatitis B virus reverse transcriptase sequences in treatment-naïve Chinese patients. Antiviral Res. 2010, 85, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.X.; Liu, B.M.; Li, X.G.; Yan, C.H.; Xu, J.; Sun, X.W.; Wang, Y.H.; Jiao, X.J.; Yan, L.; Dong, J.P.; Hou, C.S.; Abuduheilili, X.; Li, T.; Zhuang, H. Profile of HBV antiviral resistance mutations with distinct evolutionary pathways against nucleoside/nucleotide analogue treatment among Chinese chronic hepatitis B patients. Antivir Ther. 2010, 15, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Li, X.G.; Liu, B.M.; Xu, J.; Liu, X.E.; Ding, H.; Li, T. Discrepancy of potential antiviral resistance mutation profiles within the HBV reverse transcriptase between nucleos(t)ide analogue-untreated and -treated patients with chronic hepatitis B in a hospital in China. J Med Virol. 2012, 84, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yang, J.X.; Yan, L.; Zhuang, H.; Li, T. Novel HBV recombinants between genotypes B and C in 3’-terminal reverse transcriptase (RT) sequences are associated with enhanced viral DNA load, higher RT point mutation rates and place of birth among Chinese patients. Infect Genet Evol. 2018, 57, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Liu, B.; Zhao, C.; Yang, J.; Yan, C.; Yan, L.; Zhuang, H.; Li, T. Amino acid similarities and divergences in the small surface proteins of genotype C hepatitis B viruses between nucleos(t)ide analogue-naïve and lamivudine-treated patients with chronic hepatitis B. Antiviral Res. 2014, 102, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Liu, B.; Hou, J.; Sun, J.; Hao, R.; Xiang, K.; Yan, L.; Zhang, J.; Zhuang, H.; Li, T. Naturally occurring deletions/insertions in HBV core promoter tend to decrease in hepatitis B e antigen-positive chronic hepatitis B patients during antiviral therapy. Antivir Ther. 2015, 20, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Kayser, V.; Ramzan, I. Vaccines and vaccination: history and emerging issues. Hum Vaccin Immunother. 2021, 17, 5255–5268. [Google Scholar] [CrossRef]
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol 2021, 21, 83–100. [Google Scholar] [CrossRef]
- Laboratory Diagnosis of Viral Infections. Fenner’s Veterinary Virology 2017, 105–129. [CrossRef]
- Larkins, M.C.; Thombare, A. Point-of-Care Testing. [Updated 2023 May 29]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK592387/.
- Basharat, S.; Horton, J.; et al. An Overview of Emerging Point-of-Care Tests for Differentiating Bacterial and Viral Infections: CADTH Horizon Scan [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2021 Dec. Available from: https://www.ncbi.nlm.nih.gov/books/NBK594330/.
- Xiao, Y.; Thompson, A.J.; Howell, J. Point-of-Care Tests for Hepatitis B: An Overview. Cells. 2020, 9, 2233. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.L. Quality control issues in point of care testing. Clin Biochem Rev. 2008, 29 Suppl. 1, S79–S82. [Google Scholar]
- Zhang, Z.; Ma, P.; Ahmed, R.; et al. Advanced Point-of-Care Testing Technologies for Human Acute Respiratory Virus Detection. Adv Mater. 2022, 34, e2103646. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; et al. “Virus Detection: From State-of-the-art Laboratories To ...” Wiley Online, 7 Apr. 2022, onlinelibrary.wiley.com/doi/full/10.1002/advs.202105904. Accessed 28 Oct. 2024.
- Liu, B.M.; Beck, E.M.; Fisher, M.A. The Brief Case: Ventilator-Associated Corynebacterium accolens Pneumonia in a Patient with Respiratory Failure Due to COVID-19. J Clin Microbiol. 2021, 59, e0013721. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, B.M.; Carlisle, C.P.; Fisher, M.A.; Shakir, S.M. The Brief Case: Capnocytophaga sputigena Bacteremia in a 94-Year-Old Male with Type 2 Diabetes Mellitus, Pancytopenia, and Bronchopneumonia. J Clin Microbiol. 2021, 59, e0247220. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, B.; Totten, M.; Nematollahi, S.; Datta, K.; Memon, W.; Marimuthu, S.; Wolf, L.A.; Carroll, K.C.; Zhang, S.X. Development and Evaluation of a Fully Automated Molecular Assay Targeting the Mitochondrial Small Subunit rRNA Gene for the Detection of Pneumocystis jirovecii in Bronchoalveolar Lavage Fluid Specimens. J Mol Diagn. 2020, 22, 1482–1493, Erratum in: J Mol Diagn. 2021, 23, 506. doi: 10.1016/j.jmoldx.2021.01.002. PMID: 33069878. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.M.; Li, N.L.; Wang, R.; Li, X.; Li, Z.A.; Marion, T.N.; Li, K. Key roles for phosphorylation and the Coiled-coil domain in TRIM56-mediated positive regulation of TLR3-TRIF-dependent innate immunity. J Biol Chem. 2024, 300, 107249. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, B.M. History of global food safety, foodborne illness, and risk assessment. In History of Food and Nutrition Toxicology; Aca-demic Press: Washington, DC, USA, 2023; pp. 301–316. [Google Scholar]
- Liu, B.; Forman, M.; Valsamakis, A. Optimization and evaluation of a novel real-time RT-PCR test for detection of parechovirus in cerebrospinal fluid. J Virol Methods. 2019, 272, 113690. [Google Scholar] [CrossRef] [PubMed]
- Gronowski, A.M. Who or What is SHERLOCK? . EJIFCC. 2018, 29, 201–204. [Google Scholar] [PubMed]
- Kaunitz, J.D. The Discovery of PCR: ProCuRement of Divine Power. Dig Dis Sci. 2015, 60, 2230–2231. [Google Scholar] [CrossRef]
- Ongaro, A.E.; Ndlovu, Z.; Sollier, E.; Otieno, C.; Ondoa, P.; Street, A.; Kersaudy-Kerhoas, M. Engineering a sustainable future for point-of-care diagnostics and single-use microfluidic devices. Lab Chip. 2022, 22, 3122–3137. [Google Scholar] [CrossRef]
- Nelson, P.P.; Rath, B.A.; Fragkou, P.C.; Antalis, E.; Tsiodras, S.; Skevaki, C. Current and Future Point-of-Care Tests for Emerging and New Respiratory Viruses and Future Perspectives. Front Cell Infect Microbiol. 2020, 10, 181. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors. 2021, 21, 1109. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, B. V.; Cordeiro, T. A. R.; e Freitas, G. R. O.; Ferreira, L. F.; Franco, D. L. Biosensors for the detection of respiratory viruses: a review. Talanta Open 2020, 2, 100007. [Google Scholar] [CrossRef]
- Ramesh, M.; Janani, R.; Deepa, C.; Rajeshkumar, L. Nanotechnology-Enabled Biosensors: A Review of Fundamentals, Design Principles, Materials, and Applications. Biosensors (Basel). 2022, 13, 40. [Google Scholar] [CrossRef]
- Kobra Salimiyan rizi, The smartphone biosensors for point-of-care detection of human infectious diseases: Overview and perspectives—A systematic review, Current Opinion in Electrochemistry, Volume 32, 2022, 100925, ISSN 2451-9103. [CrossRef]
- Hasan, M.R.; Sharma, P.; Singh, S.; Narang, J. Smartphone-Integrated Wireless Portable Potentiostat to Develop 5th-Generation Dengue Pocket Aptasensor toward Portronicx-Approach.
- Hasan, M.R.; Sharma, P.; Singh, S.; Narang, J. Smartphone-Integrated Wireless Portable Potentiostat to Develop 5th-Generation Dengue Pocket Aptasensor toward Portronicx-Approach. ACS Appl Bio Mater. 2024, 7, 2299–2308. [Google Scholar] [CrossRef] [PubMed]
- Banik, S.; Melanthota, S.K.; Arbaaz; et al. Recent trends in smartphone-based detection for biomedical applications: a review. Anal Bioanal Chem. 2021, 413, 2389–2406. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhou, X.; Wang, M.; Ren, L. Towards Point of Care CRISPR-Based Diagnostics: From Method to Device. J Funct Biomater. 2023, 14, 97. [Google Scholar] [CrossRef] [PubMed]
- van Dongen, J.E.; Berendsen, J.T.W.; Steenbergen, R.D.M.; Wolthuis, R.M.F.; Eijkel, J.C.T.; Segerink, L.I. Point-of-care CRISPR/Cas nucleic acid detection: Recent advances, challenges and opportunities. Biosens Bioelectron. 2020, 166, 112445. [Google Scholar] [CrossRef]
- Ravichandran, M.; Maddalo, D. Applications of CRISPR-Cas9 for advancing precision medicine in oncology: from target discovery to disease modeling. Front Genet. 2023, 14, 1273994. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, H.; Taguchi, Y.; Nakagawa, R.; et al. Amplification-free RNA detection with CRISPR–Cas13. Commun Biol 2021, 4, 476. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.M. Isothermal nucleic acid amplification technologies and CRISPR-Cas based nucleic acid detection strategies for infectious disease diagnostics. In Manual of Molecular Microbiology; ASM Press: Washington, DC, USA, 2026; In Press. [Google Scholar]
- Luppa, P.B.; Müller, C.; Schlichtiger, A.; Schlebusch, H. Point-of-care testing (POCT): Current techniques and future perspectives. Trends Analyt Chem. 2011, 30, 887–898. [Google Scholar] [CrossRef] [PubMed]
- https://www.surgeryjournal.co.uk/article/S0263-9319(06)00030-5/abstract.
- Mattila, S.; Paalanne, N.; Honkila, M.; Pokka, T.; Tapiainen, T. Effect of Point-of-Care Testing for Respiratory Pathogens on Antibiotic Use in Children: A Randomized Clinical Trial. JAMA Netw Open. 2022, 5, e2216162. [Google Scholar] [CrossRef]
- Brendish, N.J.; Mills, S.; Ewings, S.; Clark, T.W. Impact of point-of-care testing for respiratory viruses on antibiotic use in adults with exacerbation of airways disease. J Infect. 2019, 79, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.M.; Rakhmanina, N.Y.; Yang, Z.; Bukrinsky, M.I. Mpox (Monkeypox) Virus and Its Co-Infection with HIV, Sexually Transmitted Infections, or Bacterial Superinfections: Double Whammy or a New Prime Culprit? Viruses. 2024, 16, 784. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, B.; Panda, D.; Mendez-Rios, J.D.; Ganesan, S.; Wyatt, L.S.; Moss, B. Identification of Poxvirus Genome Uncoating and DNA Replication Factors with Mutually Redundant Roles. J Virol. 2018, 92, e02152–17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rani, I.; Goyal, A.; Shamim, M.; A.; et al. Prevalence of mpox viral DNA in cutaneous specimens of monkeypox-infected patients: a systematic review and meta-analysis. Front Cell Infect Microbiol. 2023, 13, 1179885. [Google Scholar] [CrossRef] [PubMed]
- Deputy, N.P.; Deckert, J.; Chard, A.N.; et al. Vaccine Effectiveness of JYNNEOS against Mpox Disease in the United States. N Engl J Med. 2023, 388, 2434–2443. [Google Scholar] [CrossRef]
- World Health Organzation. Surveillance, case investigation and contact tracing for mpox (monkeypox). 2024 Mar 20; Available from: https://www.who.int/publications/i/item/WHO-MPX-Surveillance-2024.1.
- Cepheid mpox package insert. https://web-support.cepheid.com/Package%20Insert%20Files/302-9630%20Rev.%20A%20Xpert%20Mpox%20IFU%20Xpress%20POC.pdf. Assessed on Nov 25 2024.
- Li, D.; Wilkins, K.; McCollum, A.M.; et al. Evaluation of the GeneXpert for Human Monkeypox Diagnosis. Am J Trop Med Hyg. 2017, 96, 405–410. [Google Scholar] [CrossRef]
- Damhorst, G.; McLendon, K.; Morales, E.; et al. Detection of Mpox virus with the Cepheid Xpert Mpox assay in oropharyngeal, anorectal and cutaneous lesion swab specimens. Open Forum Infect Dis. 2023, 10 (Suppl. 2), ofad500. [Google Scholar] [CrossRef]
- Suñer, C.; Ubals, M.; Tarín-Vicente, E.J.; et al. Viral dynamics in patients with monkeypox infection: a prospective cohort study in Spain. Lancet Infect Dis. 2023, 23, 445–453. [Google Scholar] [CrossRef]
- Thompson, P.; Morgan, C.E.; Ngimbi, P.; et al. Arresting vertical transmission of hepatitis B virus (AVERT-HBV) in pregnant women and their neonates in the Democratic Republic of the Congo: a feasibility study [published correction appears in Lancet Glob Health. 2021, 9, e1507. doi: 10.1016/S2214-109X(21)00468-X]. Lancet Glob Health. Lancet Glob Health 2021, 9, e1600–e1609. [Google Scholar] [CrossRef]
- Chahal, H.S.; Peters, M.G.; Harris, A.M.; McCabe, D.; Volberding, P.; Kahn, J.G. Cost-effectiveness of Hepatitis B Virus Infection Screening and Treatment or Vaccination in 6 High-risk Populations in the United States. Open Forum Infect Dis. 2018, 6, ofy353 Published 2018 Dec 26. [Google Scholar] [CrossRef]
- Ioannou, G.N. Hepatitis B virus in the United States: infection, exposure, and immunity rates in a nationally representative survey. Ann Intern Med. 2011, 154, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Marcuccilli, F.; Chevaliez, S.; Muller, T.; et al. Multicenter Evaluation of the Cepheid Xpert® HBV Viral Load Test. Diagnostics (Basel). 2021, 11, 297. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.; Tekoaua, R.; Li, X.; Locarnini, S. Real-world application of the Xpert® HBV viral load assay on serum and dried blood spots. J Med Virol. 2021, 93, 3707–3713. [Google Scholar] [CrossRef]
- Khounvisith, V.; Saysouligno, S.; Souvanlasy, B.; et al. Hepatitis B virus and other transfusion-transmissible infections in child blood recipients in Lao People’s Democratic Republic: a hospital-based study. Arch Dis Child. 2023, 108, 15–19. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States. Recommendations of the Advisory Committee on Immunization Practices (ACIP) Part 1 Immunization of Infants, Children, and Adolescents. MMWR Morb Mortal Wkly Rep. 2005, 54, 1–23. [Google Scholar]
- Liu, B.M.; Martins, T.B.; Peterson, L.K.; Hill, H.R. Clinical significance of measuring serum cytokine levels as inflammatory biomarkers in adult and pediatric COVID-19 cases: A review. Cytokine. 2021, 142, 155478. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- LeMessurier, K.S.; Rooney, R.; Ghoneim, H.E.; Liu, B.; Li, K.; Smallwood, H.S.; Samarasinghe, A.E. Influenza A virus directly modulates mouse eosinophil responses. J Leukoc Biol. 2020, 108, 151–168. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, B.M.; Hill, H.R. Role of Host Immune and Inflammatory Responses in COVID-19 Cases with Underlying Primary Immunodeficiency: A Review. J Interferon Cytokine Res. 2020, 40, 549–554. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, B.M.; Mulkey, S.B.; Campos, J.M.; DeBiasi, R.L. Laboratory diagnosis of CNS infections in children due to emerging and re-emerging neurotropic viruses. Pediatr Res. 2024, 95, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Liu, B. Universal PCR Primers Are Critical for Direct Sequencing-Based Enterovirus Genotyping. J Clin Microbiol. 2016, 55, 339–340. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, B.; Li, N.L.; Shen, Y.; Bao, X.; Fabrizio, T.; Elbahesh, H.; Webby, R.J.; Li, K. The C-Terminal Tail of TRIM56 Dictates Antiviral Restriction of Influenza A and B Viruses by Impeding Viral RNA Synthesis. J Virol. 2016, 90, 4369–4382. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stamm, B.D.; Tamerius, J.; Reddy, S.; et al. The Influence of Rapid Influenza Diagnostic Testing on Clinician Decision-Making for Patients With Acute Respiratory Infection in Urgent Care. Clin Infect Dis. 2023, 76, 1942–1948. [Google Scholar] [CrossRef]
- Jian, M.J.; Chung, H.Y.; Chang, C.K.; et al. Clinical Comparison of Three Sample-to-Answer Systems for Detecting SARS-CoV-2 in B. 1.1.7 Lineage Emergence. Infect Drug Resist. 2021, 14, 3255–3261. [Google Scholar] [CrossRef]
- Fjelltveit, E.B.; Cox, R.J.; Østensjø, J.; et al. Point-of-Care Influenza Testing Impacts Clinical Decision, Patient Flow, and Length of Stay in Hospitalized Adults. J Infect Dis. 2022, 226, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, H.; van Genne, M.; Lizarazo-Forero, E.; Niesters, H.G.M.; Gard, L. Evaluation of the QIAstat-Dx RP2.0 and the BioFire FilmArray RP2.1 for the Rapid Detection of Respiratory Pathogens Including SARS-CoV-2. Front. Microbiol. 2022, 13, 854209. [Google Scholar] [CrossRef] [PubMed]
- Fistera, D.; Kikull, K.; Risse, J.; Herrmann, A.; Brachmann, M.; Kill, C. Point-of-care PCR testing of SARS-CoV-2 in the emergency department: Influence on workflow and efficiency. PLoS One. 2023, 18, e0288906. [Google Scholar] [CrossRef] [PubMed]
- Larkin, HD. NIH Pilots Telehealth Program for COVID-19. JAMA. 2023, 329, 363–364. [Google Scholar] [CrossRef] [PubMed]
- González-Parra, G.; Mahmud, M.S.; Kadelka, C. Learning from the COVID-19 pandemic: A systematic review of mathematical vaccine prioritization models. Infect Dis Model. 2024, 9, 1057–1080. [Google Scholar] [CrossRef]
- Loeffelholz, M.J.; Alland, D.; Butler-Wu, S.M.; et al. Multicenter Evaluation of the Cepheid Xpert Xpress SARS-CoV-2 Test. J Clin Microbiol. 2020, 58, e00926–20. [Google Scholar] [CrossRef] [PubMed]
- Barker, K.R.; Small, L.N.; Thai, D.V.; Sohn, K.Y.; Rosella, L.C. Evaluating the Ability to ID (COVID-19) NOW: a Large Real-World Prospective Evaluation of the Abbott ID NOW COVID-19 Assay. Microbiol Spectr. 2022, 10, e0051322. [Google Scholar] [CrossRef]
- Dinnes, J.; Sharma, P.; Berhane, S.; et al. Rapid, point-of-care antigen tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. 2022, 7, CD013705 Published 2022 Jul 22. [Google Scholar] [CrossRef]
- Chong, Z.-S.; Wright, G. J.; Sharma, S. Investigating Cellular Recognition Using CRISPR/Cas9 Genetic Screening. Trends Cell Biol. 2020, 30, 619–627. [Google Scholar] [CrossRef]
- Sinclair, W.; Omar, M. Enterovirus. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK562330/.
- Cong, L.; Ran, F. A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; et al. Multiplex Genome Engineering Using CRISPR/Cas Systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Schechter, M. Prioritization of antiretroviral therapy in patients with high CD4 counts, and retention in care: lessons from the START and Temprano trials. J Int AIDS Soc. 2018, 21, e25077. [Google Scholar] [CrossRef]
- Jallow, M.M.; Mendy, M.P.; Barry, M.; A.; et al. Real-Time Enterovirus D68 Outbreak Detection through Hospital Surveillance of Severe Acute Respiratory Infection, Senegal, 2023. Emerg Infect Dis. 2024, 30, 1687–1691. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.A.; Zilouchian, H.; Younas, M.A.; Asghar, W. Dengue Detection: Advances in Diagnostic Tools from Conventional Technology to Point of Care. Biosensors (Basel). 2021, 11, 206. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, N.L.; Wang, J.; Shi, P.Y.; Wang, T.; Miller, M.A.; Li, K. Overlapping and distinct molecular determinants dictating the antiviral activities of TRIM56 against flaviviruses and coronavirus. J Virol. 2014, 88, 13821–13835. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, D.; Li, N.L.; Wei, D.; Liu, B.; Guo, F.; Elbahesh, H.; Zhang, Y.; Zhou, Z.; Chen, G.Y.; Li, K. The E3 ligase TRIM56 is a host restriction factor of Zika virus and depends on its RNA-binding activity but not miRNA regulation, for antiviral function. PLoS Negl Trop Dis. 2019, 13, e0007537. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pang, J.; Chia, P.Y.; Lye, D.C.; Leo, Y.S. Progress and Challenges towards Point-of-Care Diagnostic Development for Dengue. J Clin Microbiol. 2017, 55, 3339–3349. [Google Scholar] [CrossRef] [PubMed]
- Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition. Geneva: World Health Organization; 2009. 4, LABORATORY DIAGNOSIS AND DIAGNOSTIC TESTS. Available from: https://www.ncbi.nlm.nih.gov/books/NBK143156/.
- Ooi, E.E. Challenges in prevaccination screening for previous dengue infection. Lancet Glob Health. 2021, 9, e4–e5. [Google Scholar] [CrossRef]
- World Health Organization (22 August 2024). Disease Outbreak News; Mpox in African Region. Available at: https://www.who.int/emergencies/disease-outbreak-news/item/2024-DON528.
- Chou, R.; Dana, T.; Bougatsos, C.; Blazina, I.; Khangura, J.; Zakher, B. Screening for Hepatitis B Virus Infection in Adolescents and Adults: A Systematic Review to Update the U.S. Preventive Services Task Force Recommendation. Ann Intern Med. 2014, 161, 31–45. [Google Scholar] [CrossRef]
- Servant-Delmas, A.; Ly, T.D.; Hamon, C.; Houdah, A.K.; Laperche, S. Comparative Performance of Three Rapid HBsAg Assays for Detection of HBs Diagnostic Escape Mutants in Clinical Samples. J Clin Microbiol. 2015, 53, 3954–3955. [Google Scholar] [CrossRef]
- Liu, B.M.; Hayes, A.W. Mechanisms and Assessment of Genotoxicity of Metallic Engineered Nanomaterials in the Human Environment. Biomedicines. 2024, 12, 2401. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, B.M. Epidemiological and clinical overview of the 2024 Oropouche virus disease outbreaks, an emerging/re-emerging neurotropic arboviral disease and global public health threat. J Med Virol. 2024, 96, e29897. [Google Scholar] [CrossRef] [PubMed]
- Mueller-Breckenridge, A.J.; Garcia-Alcalde, F.; Wildum, S.; et al. Machine-learning based patient classification using Hepatitis B virus full-length genome quasispecies from Asian and European cohorts. Sci Rep. 2019, 9, 18892. [Google Scholar] [CrossRef] [PubMed]
- Piermatteo, L.; D’Anna, S.; Bertoli, A.; Bellocchi, M.; Carioti, L.; Fabeni, L.; Alkhatib, M.; Frazia, S.L.; Lichtner, M.; Mastroianni, C.; Sanctis, G.D. Unexpected rise in the circulation of complex HBV variants enriched of HBsAg vaccine-escape mutations in HBV genotype-D: potential impact on HBsAg detection/quantification and vaccination strategies. Emerging Microbes & Infections 2023, 12. [Google Scholar] [CrossRef]
- Geyer, R.E.; Kotnik, J.H.; Lyon, V.; et al. Diagnostic Accuracy of an At-Home, Rapid Self-test for Influenza: Prospective Comparative Accuracy Study. JMIR Public Health Surveill. 2022, 8, e28268. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Evaluation of rapid influenza diagnostic tests for influenza A (H3N2)v virus and updated case count--United States, 2012. MMWR Morb Mortal Wkly Rep. 2012, 61, 619–621. [Google Scholar]
- Scott A. Harper, John S. Bradley, Janet A. Englund, Thomas M. File, Stefan Gravenstein, Frederick G. Hayden, Allison J. McGeer, Kathleen M. Neuzil, Andrew T. Pavia, Michael L. Tapper, Timothy M. Uyeki, Richard K. Zimmerman, Seasonal Influenza in Adults and Children—Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management: Clinical Practice Guidelines of the Infectious Diseases Society of America, Clinical Infectious Diseases, Volume 48, Issue 8, 15 April 2009, Pages 1003–1032. [CrossRef]
- Iliescu, F.S.; Ionescu, A.M.; Gogianu, L.; et al. Point-of-Care Testing-The Key in the Battle against SARS-CoV-2 Pandemic. Micromachines (Basel). 2021, 12, 1464. [Google Scholar] [CrossRef]
- Sakthivel, D.; Delgado-Diaz, D.; McArthur, L.; Hopper, W.; Richards, J.S.; Narh, C.A. Point-of-Care Diagnostic Tools for Surveillance of SARS-CoV-2 Infections. Front. Public Health 2021, 9, 766871. [Google Scholar] [CrossRef]
- Hirano, J.; Murakami, K.; Hayashi, T. CRISPR-Cas9-Based Technology for Studying Enteric Virus Infection. Front. Genome Ed. 2022, 4, 888878. [Google Scholar] [CrossRef] [PubMed]
- Abedi, G.R.; Watson, J.T.; Nix, W.A.; Oberste, M.S.; Gerber, S.I. Enterovirus and Parechovirus Surveillance - United States, 2014-2016. MMWR Morb Mortal Wkly Rep. 2018, 67, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Raafat, N.; Blacksell, S.D.; Maude, R.J. A review of dengue diagnostics and implications for surveillance and control. Trans R Soc Trop Med Hyg. 2019, 113, 653–660. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).