1. Introduction
Prostate cancer (PCa) is the most common malignancy among men worldwide and the third leading cause of cancer-related death [
1]. The European Randomized Screening for Prostate Cancer (ERSPC) trial showed a 20% reduction in the PCa-specific mortality among men undergoing screening with serum prostate-specific antigen (PSA) testing and systematic prostate biopsies, compared to those in the control group, after seven years of follow-up [
2]. Recently, the Göteborg Randomized Population-Based Prostate cancer Screening Trial has shown a 29% reduction in PCa-specific mortality after 22 years of follow-up [
3]. These improvements in PCa-specific mortality have been attributed to the increased early detection and treatment of significant PCa, leading to a shift in the focus of population-based screening toward significant cases [
4].
The focus on detecting significant PCa during screening has been facilitated by the widespread use of prostate magnetic resonance imaging (MRI), now commonly employed to select candidates for prostate biopsy and reducing unnecessary procedures due to its high negative predictive value, reaching up to 97% [
5,
6,
7]. MRI also enables targeted biopsies of lesions suspected of having significant PCa through MRI–transrectal ultrasound (TRUS) fusion imaging technology [
8,
9]. Therefore, men suspected of having PCa, identified due to a serum PSA elevation, currently undergo an MRI followed by an MRI-TRUS targeted biopsy of lesions with a PI-RADS score of 3 to 5, followed by a systematic prostate biopsy [
10]. This new approach avoids unnecessary prostate biopsies and decreases the overdetection of insignificant tumors, although uncertain scenarios remain [
11,
12,
13].
Asymptomatic inflammation of the prostate gland has been identified as a frequent non-cancerous cause of serum PSA elevation [
14]. In 1999, the National Institute of Health (NIH) classified this non-symptomatic prostatitis as NIH type IV, which is often present when PCa is not detected [
15]. Furthermore, several studies suggest asymptomatic prostatitis as a PCa mimicker in MRI [
16,
17,
18]. However, the incidence and type of asymptomatic prostatitis in MRI-suspicious lesions remain poorly analyzed [
19].
Our main objective was to analyze the incidence of inflammatory features in targeted biopsies of MRI-suspicious lesions PI-RADS 3 to 5, focusing our attention on chronic, acute, and granulomatous prostatitis. Our secondary objectives were as follows: (i) to analyze the relationship between inflammatory features found in targeted biopsies without PCa and the PI-RADS score; (ii) to analyze the association of inflammatory features in targeted biopsies with PCa according to the aggressiveness of tumors detected; and (iii) to identify if inflammatory features are independent predictors of significant PCa (grade group 2 of higher).
2. Materials and Methods
2.1. Design, Setting, and Participants
This is a prospective study analyzing the inflammatory features reported in 531 MRI-suspicious lesions subjected to targeted biopsies in a consecutive series of prostate biopsies carried out in 364 men suspected of having PCa, between January 1 of 2022 and September 30 of 2023, in one academic center. This study was approved by the ethical committee of the Vall d´Hebron Hospital Campus (PRAG-02/2020).
2.2. Diagnostic Approach for Prostate Cancer Detection
Men suspected of having PCa were identified during an opportunistic PCa screening program from a serum prostate-specific antigen (PSA) level above 3.0 ng/mL and/or a suspicious digital rectal examination (DRE). Men referred to our center underwent a 3 Tesla multiparametric MRI in a Magneton Prisma
® scanner using a pelvic-phased array coil (Siemens Healthcare, Erlangen, Germany), following the European Society of Urologic Radiology guidelines recommendations and reported with the Prostate Imaging-Reporting and Data System (PI-RADS) v 2.1 [
20,
21]. Two- to four-core targeted MRI–transrectal ultrasound fusion imaging biopsies were conducted in men with lesions with PI-RADS scores of 3 to 5, followed by a 12-core systematic biopsy using the Koelis
® system (Koelis Advancing PCa, Grenoble, France) via the transperineal route [
10]. Biopsy material was sent to the pathology department, where expert pathologists classified the detected tumors according to the International Society of Urologic Pathology grade groups. Significant PCa was identified when the grade group was 2 or higher, and insignificant PCa when the grade group was 1 [
22,
23]. Inflammatory features were identified in each analyzed core, and prospectively reported as mild, moderate, or severe chronic prostatitis, acute prostatitis, or granulomatous prostatitis [
24].
2.3. Variables in the Study
The analyzed variables were the age (years), PCa family history (no vs. yes), prostate biopsy type (initial vs. repeated), serum PSA level (ng/mL), DRE (normal vs. suspicious), MRI-prostate volume, and PI-RADS score. Outcome variables were the inflammatory features typified as mild, moderate, and severe chronic prostatitis, acute prostatitis, granulomatous prostatitis, and PCa.
2.4. Diagnostic Approach of Inflammatory Features
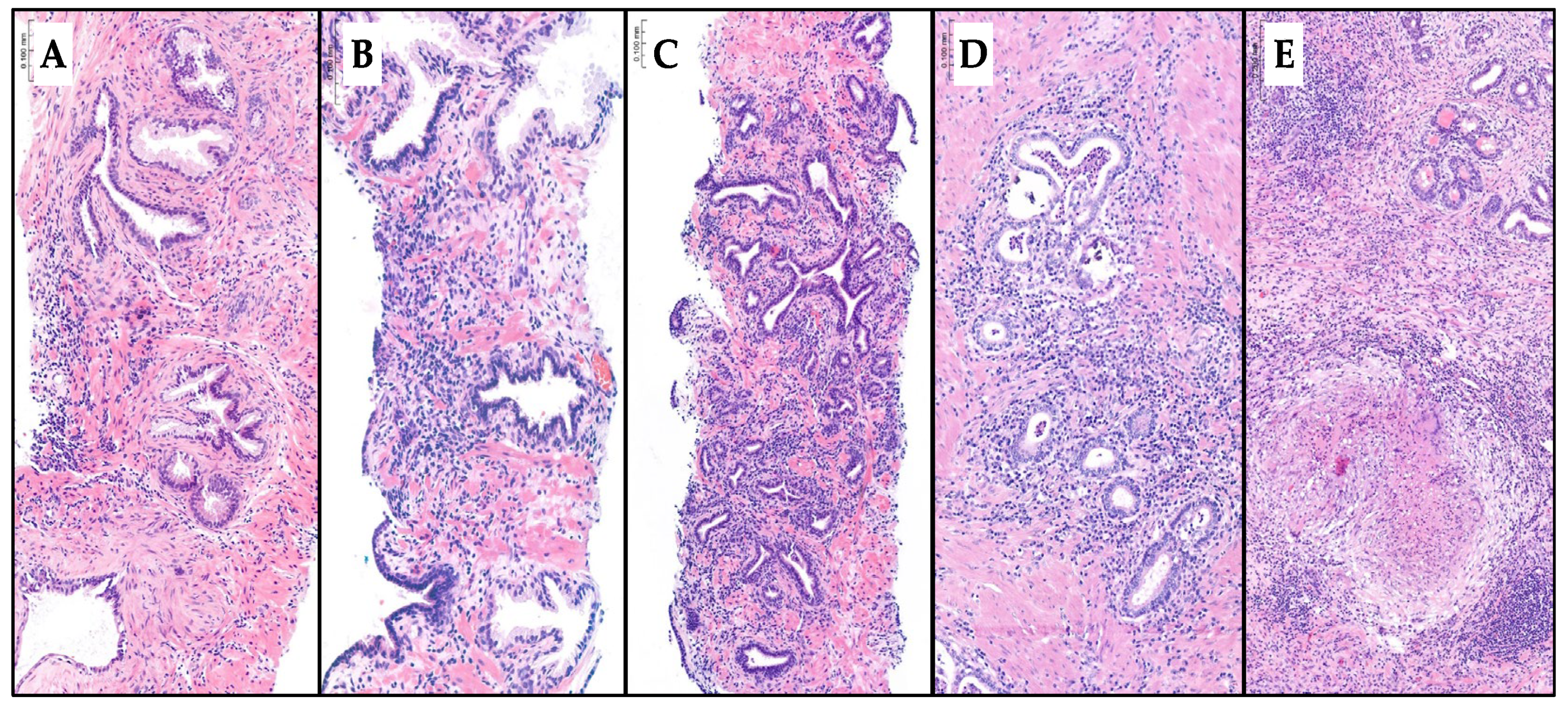
Inflammatory features were recorded as mild, moderate, or severe chronic prostatitis; acute prostatitis; or granulomatous prostatitis. The diagnosis was made in hematoxylin and eosin-stained slides. The type of prostatitis depends on the nature of the observed cell infiltration. If the infiltration is composed of lymphocytes and plasma cells, it is classified as chronic prostatitis. The presence of polymorphonuclear neutrophils and eosinophils indicates acute exacerbation of chronic prostatitis, recorded as acute prostatitis. Granuloma formation is indicative of granulomatous prostatitis. The grading of chronic prostatitis as mild, moderate, or severe is based on inflammatory density. Mild chronic prostatitis typically involves less than 10% of the prostate core volume. Moderate prostatitis affects 10% to more than 50% without confluent inflammatory nests, while severe prostatitis is characterized by more than 50% involvement with confluent inflammatory nests [
25,
26,
27]. When various types of prostatitis coexisted, the most extensive chronic prostatitis was recorded. When acute exacerbation of chronic prostatitis was observed, acute prostatitis was recorded, as well as granulomatous prostatitis when granuloma formation was observed.
Figure 1 represents the type of inflammatory features reported in the hematoxylin and eosin-stained slides.
2.5. Statistical Analysis
The quantitative variables were described as medians with interquartile ranges (IQRs), while the qualitative variables were expressed as percentages. The descriptive quantitative variables were compared with the Mann–Whitney U test and the Kruskal–Wallis test results, and the qualitative variables were compared with chi-square test results. Logistic regression analysis was used to analyze the inflammatory findings as predictors of significant PCa. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated in a univariate and multivariate analysis. Statistical significance was allowed when the p-value was lower than 0.05. Statistical analyses were computed using SPSS® v.29 (IBM, statistical package for social sciences, San Francisco, US).
3. Results
3.1. Characteristics of Study Population and Guided-Biopsy Lesions
The overall characteristics of the study population are summarized in
Table 1. We note that, among the 364 men suspected of having PCa due to a serum PSA level above 3.0 ng/mL and/or a suspicious DRE, the median age was 68 years, the median serum PSA level was 6.1 ng/mL, and the percentage of suspicious DREs was 20.1%. A total of 25 men (6.9%) had an existing PCa family history, and 41 (11.2%) had previous negative prostate biopsies. A total of 196 men carried one suspicious lesion while 142 (51.7%) presented two and 26 (7.1%) had three suspicious lesions. The percentage of index lesions with a PI-RADS score of 3 was 36.0%, whereas those with lesions with PI-RADS scores of 4 and 5 made up proportions of 42.6% and 21.4%, respectively. The overall detection rate of PCa was 46.1%, with 35.7% being significant PCa and 10.4% being insignificant PCa.
The number of targeted biopsied suspicious lesions was 531, with a median size of 10 mm. A total of 440 (82.9%) suspicious lesions were located in the peripheral zone, 20 (3.8%) were located in the central or transitional zone, and 13.4% were located in the anterior zone. The percentage of lesions with a PI-RADS score of 3 was 48.2%, while 36.2% and 15.6% had PI-RADS scores of 4 and 5, respectively. The overall PCa detection rate per lesion was 29.4%, with 20.3% being significant PCa and 8.5% being insignificant PCa, as shown in
Table 2.
3.2. Distribution of Inflammatory Features in Suspicious Lesions Without and with Prostate Cancer
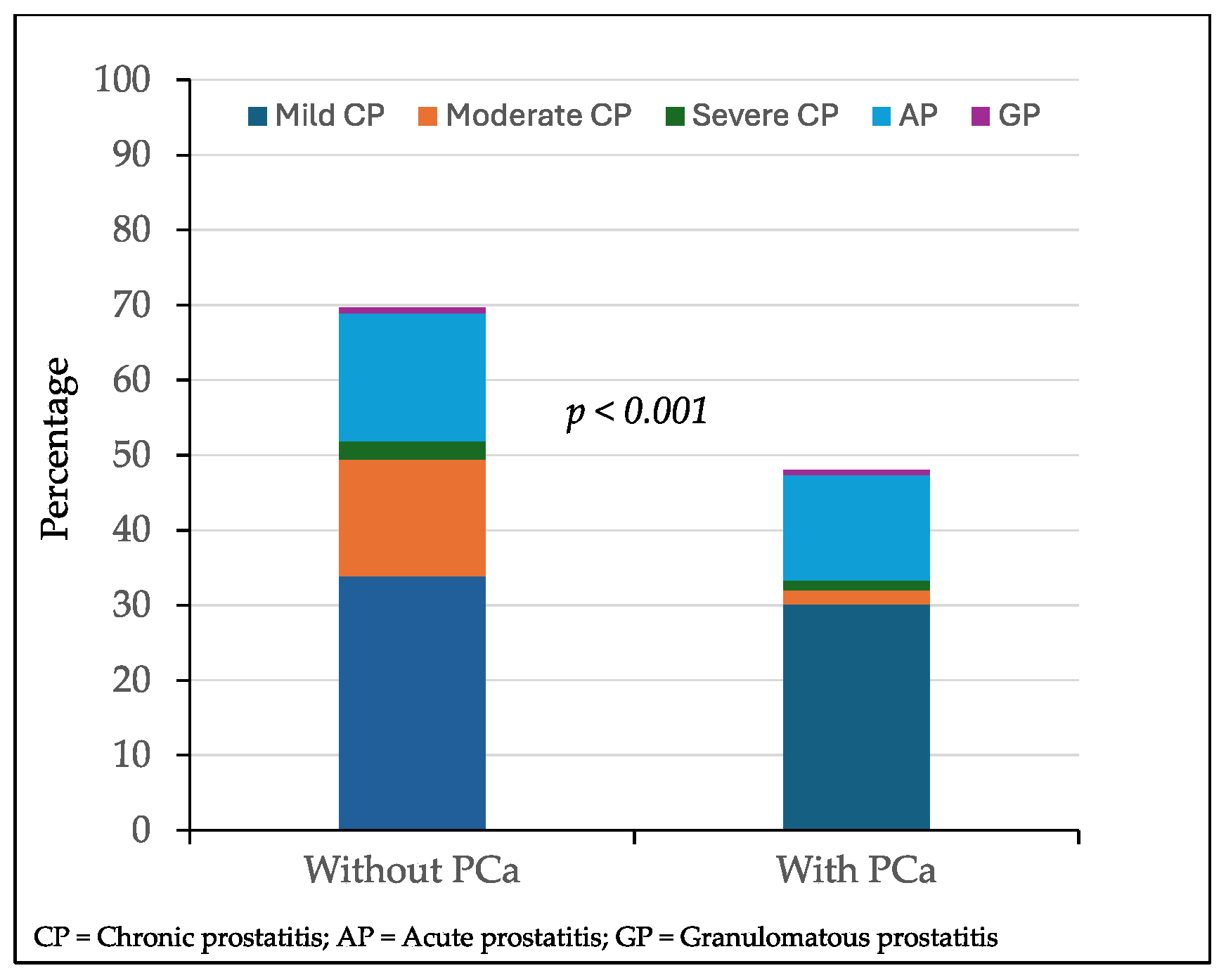
Overall inflammatory features were found in 336 of 531 MRI-suspicious lesions (63.3%). Mild chronic prostatitis was detected in 174 lesions (33.4%), followed by acute prostatitis in 86 lesions (16.5%), moderate chronic prostatitis in 61 (11.7%), severe chronic prostatitis in 11 (2.1%), and granulomatous prostatitis in 4 lesions (0.7%). Overall inflammatory features were found in 261 of 375 lesions with PCa (69.6%) and 75 of 156 with PCa (48.1%), p < 0.001. The cases of mild, moderate, or severe chronic prostatitis, acute prostatitis, and granulomatous prostatitis among the 375 targeted biopsied lesions without PCa and 156 lesions with PCa are represented in
Figure 2. Mild chronic prostatitis was detected in 127 lesions (33.9%) without PCa while it was detected in 47 (30.1%) with PCa (p = 0.128). Moderate chronic prostatitis was observed in 58 lesions (15.5%) without PCa while it was found in 3 (1.9%) with PCa (p < 0.001); severe chronic prostatitis was found in nine (2.4%) and two lesions (1.3%), respectively (p = 0,089). Acute prostatitis was detected in 64 lesions (12.0%) without PCa while it was found in 22 (14.1%) with PCa (p = 0.113). Granulomatous prostatitis was detected in three lesions without PCa (0,1%) and one lesion (0.6%) with PCa (p = 0.568).
3.3. Distribution of Inflammatory Features in Targeted Biopsies Without Prostate Cancer According to the PI-RADS Score
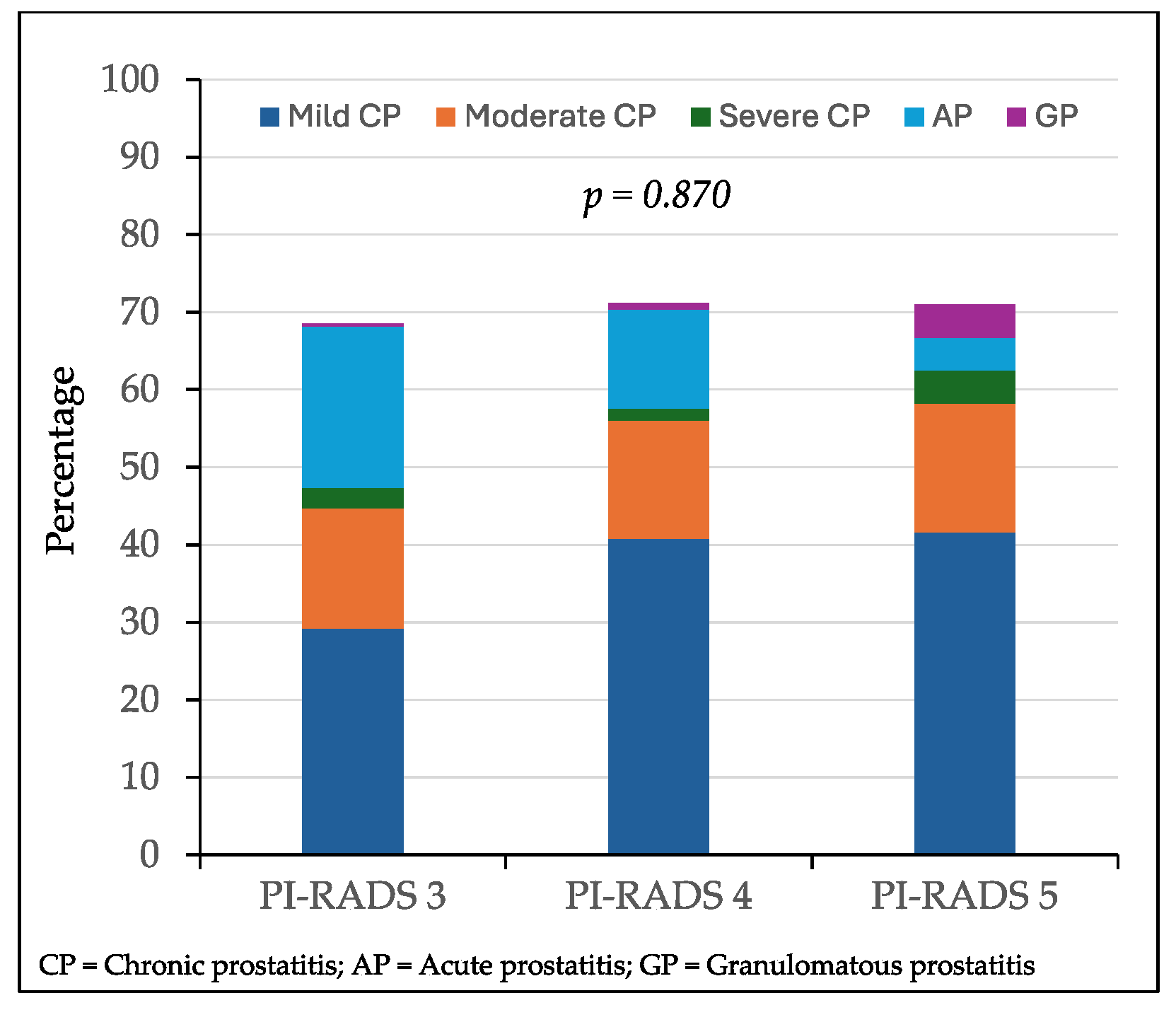
The distribution of mild, moderate, and severe chronic prostatitis, acute prostatitis, and granulomatous prostatitis according to the PI-RADS score of the biopsied lesions without PCa is represented in
Figure 3. There were 66 lesions (29.2%) with PI-RADS 3 mild chronic prostatitis, while 51 (40.8%) lesions had a PI-RADS score of 4 and 10 (41.6%) lesions had a PI-RADS score of 5 (p = 0.014). Moderate chronic prostatitis was detected in 35 (15.5%) lesions with a PI-RADS score of 3, 19 (15.2%) lesions with a PI-RADS score of 4, and 4 (16.6%) lesions with a PI-RADS score of 5 (p = 0.367). Severe chronic prostatitis was found in six lesions (2.6%) with a PI-RADS score of 3, two (1.6%) with a PI-RADS score of 4, and one (4.3%) with a PI-RADS score of 5 (p = 0.536). Acute prostatitis was observed in 47 lesions (20.8%) with a PI-RADS score of 3, 16 (12.8%) with a PI-RADS score of 4, and 1 (2.9%) with a PI-RADS score of 5 (p = 0.006). Granulomatous prostatitis was found in one lesion (0.4%) with a PI-RADS score of 3, one (0.8%) with a PI-RADS score of 4, and one (2.9%) with a PI-RADS score of 5 (p = 0.024). Overall inflammatory symptoms were reported in 155 (68.6%) out of 226 lesions with a PI-RADS score of 3, 89 (71.2%) out of 125 lesions with a PI-RADS score of 4, and 17 (70.8%) out of 24 lesions with a PI-RADS score of 5 (p = 0.870).
Distribution of Inflammatory Findings in Targeted Biopsies of Suspicious Lesions with Prostate Cancer According to Its Aggressiveness
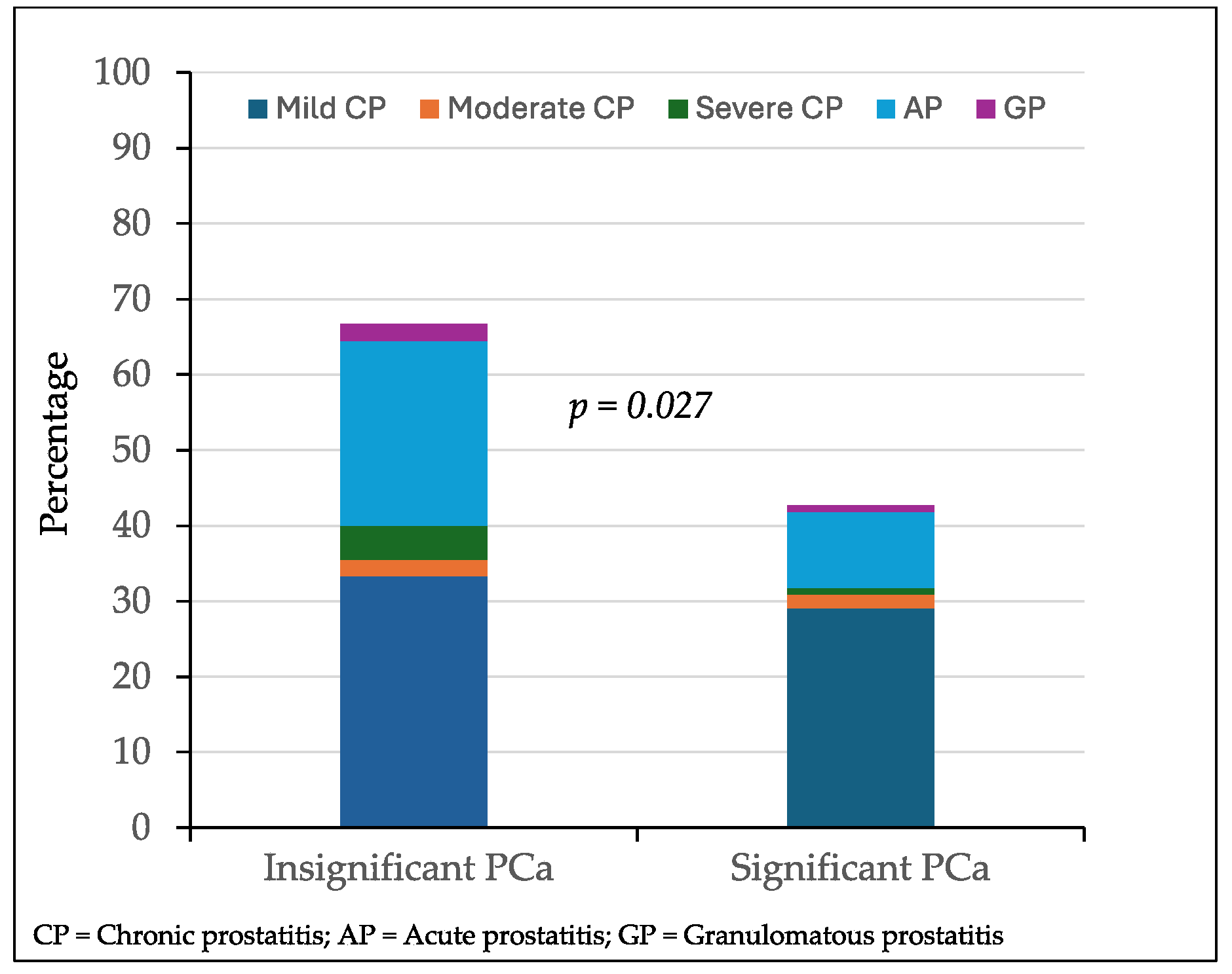
Among 156 targeted biopsied lesions with PCa, inflammatory symptoms were observed in 30 of 45 lesions (66.7%) with insignificant PCa compared to 47 of 110 lesions (42.7%) with significant PCa (p = 0.027). The distribution of the prostatitis type according to the detection of insignificant or significant PCa is represented in
Figure 4. Mild chronic prostatitis was detected in 15 lesions with insignificant PCa (33.3%) and in 32 lesions (29.1%) with significant PCa (p = 0. 453). Moderate chronic prostatitis was detected in one lesion with insignificant PCa (2.2%) and in two (1.8%) with significant PCa (p = 0.673). Severe chronic prostatitis was detected in two lesions with insignificant PCa (2.2%) and in one with significant PCa (p = 0.876). Acute prostatitis was detected in 11 lesions with insignificant PCa (25.5%) and in 11 (10.0%) with significant PCa (p = 0.036). Granulomatous prostatitis was detected in one lesion (p = 1.000).
3.4. Analysis of Associations with Significant Prostate Cancer and Its Prediction of Characte-Ristics of Suspicious Lesions and Reported Inflammatory Findings
The results of a univariate and multivariate analysis of the characteristics of the suspicious targeted biopsied lesions, the reported overall inflammation, and, specifically, the detection of chronic, acute, and granulomatous prostatitis, describing their association with and prediction of significant PCa, are summarized in
Table 3.
We noted in the univariate analysis that the size of the lesion, the PI-RADS score, the detection of inflammatory lesions, and, specifically, chronic prostatitis were associated with the detection of significant PCa in those lesions. However, the location of these lesions (peripheral, central/transition, and anterior) and the detection of acute prostatitis and granulomatous prostatitis were not associated with the detection of significant PCa. The multivariate analysis shows that the lesion size, their location in the prostate gland, and, specifically, the detection of acute prostatitis and granulomatous prostatitis did not predict the detection of significant PCa. On the contrary, the increase in the PI-RADS score of the suspicious lesions increased the risk of significant PCa, while the presence of inflammatory findings, and specifically of chronic prostatitis, decreased the risk of significant PCa.
4. Discussion
The present study demonstrates that inflammatory features are frequently detected in targeted biopsies of MRI-suspicious lesions without PCa, consistent with findings previously reported in systematic biopsies [
28,
29]. In the pre-MRI era, Schatterman et al. observed that inflammation was present in nearly all systematic biopsies after excluding pre-malignant lesions and symptomatic prostatitis. They also noted that increases in the serum PSA level and PSA density were usually typically associated with epithelial disruption caused by the aggressiveness of inflammatory infiltrates rather than their extent [
29]. Our findings reveal that prostatitis features in the targeted biopsies of lesions without PCa were more common than those observed in targeted biopsies where PCa was present. The overall percentage of inflammatory features across the targeted lesions of various PI-RADS scores without PCa remained stable, in contrast with the decrease reported in other studies [
30]. However, we observed an increased prevalence of mild chronic prostatitis in lesions with PI-RADS scores of 4 and 5 compared to those with a PI-RADS score of 3. The incidence of intermediate and severe chronic prostatitis was consistent across the PI-RADS categories, while the incidence of acute prostatitis decreased with higher PI-RADS scores. Granulomatous prostatitis, an uncommon inflammatory condition, was exclusively observed in lesions with PI-RADS scores of 4 and 5. Previous studies have suggested that granulomatous prostatitis tends to appear in the lesions with the highest PI-RADS scores, with some exploring radiomic features to differentiate it from significant PCa [
31,
32,
33,
34,
35,
36]. In our study, we observed a significantly increased incidence of mild chronic prostatitis and acute prostatitis in lesions with insignificant PCa compared to those with significant PCa. Notably, granulomatous prostatitis was observed in the lesions with significant and insignificant PCa [
31,
32,
33].
The incidence of inflammatory features was lower in the targeted biopsies of lesions with significant PCa compared to those with insignificant PCa. In this study, we observed that detection of inflammation in the targeted biopsies of MRI-suspicious lesions was an independent predictor of a lower likelihood of detecting significant PCa. Consistent with our findings, previous studies have reported that both acute prostatitis [
37,
38] and chronic prostatitis [
37,
39,
40,
41,
42,
43] are associated with a reduced risk of future PCa.
The high percentage of inflammatory features in negative targeted biopsies of suspicious lesions suggest that these features can mimic PCa in MRI findings. Although our study was not specifically designed to confirm this hypothesis, the high incidence of inflammatory features in suspicious lesions without PCa supports this possibility.
In 2016, Jyoti et al. conducted a retrospective analysis of 228 MRI-suspicious lesions in 137 men suspected of having PCa. PCa was detected in 55 lesions (24.1%), while inflammatory features were identified in 62 lesions (27.2%) without PCa, with incidences of 58% with a PI-RADS score of 3, 39% with a PI-RADS score of 4, and 3% with a PI-RADS score of 5. Their study suggested that the prevalence of inflammatory features decreases with higher PI-RADS scores [
44]. In contrast, we showed a higher incidence of inflammatory features, at approximately 70% across all the PI-RADS categories. Notably, we observed an increased prevalence of mild chronic prostatitis with PI-RADS scores of 4 and 5 compared to PI-RADS 3 lesions. Conversely, the incidence of acute prostatitis decreased progressively with higher PI-RADS scores, and granulomatous prostatitis emerged as a rare finding in PI-RADS 4 and 5 lesions. These discrepancies may reflect differences in the radiomic characteristics of various types of prostatitis. Jyoti et al.’s study did not specify the types of inflammatory lesions identified, reporting a high median prostate volume and analyzing the lesions irrespective of the presence or absence of PCa. Some authors have specifically highlighted granulomatous prostatitis as a major mimicker of PCa, particularly in lesions with the highest PI-RADS scores [
31,
32,
33,
34,
35,
36].
In 2018, Gordestsky et al. analyzed the incidence of benign findings in 41 retrospectively selected cases with prior negative biopsies, examining 62 MRI-suspicious lesions sampled through targeted biopsies and additional systematic biopsies. The authors reported inflammatory features in 29% of the targeted biopsies and 14.6% of the systematic biopsies. Chronic prostatitis was identified in 10% of the targeted biopsies and 7.1% of the systematic biopsies, while granulomatous prostatitis was detected in 3.2% and 2.5%, respectively [
45]. Although our study did not compare the incidence of inflammatory features between targeted and systematic biopsies, we did assess the incidence of inflammatory features in targeted biopsies according to the presence of PCa. The incidence of inflammatory features was higher in lesions without PCa compared to those with PCa, and that in significant PCa lesions was lower than in those with insignificant PCa.
In 2019, Rourke at al. retrospectively identified 43 men who consecutively underwent biopsies for 61 suspicious lesions observed on pre-biopsy prostate MRIs. These men received both targeted and systematic biopsies. Pathologists subsequently reviewed the pathology, identifying inflammation in 43 lesions (70.5%). Inflammatory features were found in 54.5% of the targeted biopsies without PCa, compared to 40% of the cases where PCa was detected only through systematic biopsies. While they reported a similar incidence of inflammatory features, we observed a lower incidence in the suspicious lesions with PCa. However, no association was found between inflammatory lesions and the PI-RADS scores or the aggressiveness of the PCa, consistent with the findings of our study [
19].
In 2020, Pepe et al. analyzed the detection of granulomatous prostatitis in 105 men with PI-RADS 5 lesions who underwent targeted prostate biopsies. PCa was detected in 91 cases (86.7%), with significant PCa identified in 89 cases (84.5%). Among 16 men without significant PCa, granulomatous prostatitis was detected in 6 (37.7%). The authors reported that these men underwent specific antibiotic treatment, after which none required repeated biopsies. All the patients demonstrated their serum PSA level and PSA density, along with a reduction in their PI-RADS score to 3 or lower [
33]. Bertelli et al. recently analyzed 11 cases of granulomatous prostatitis, including 4 cases with PI-RADS 4 lesions and 6 with PI-RADS 5 lesions, as reported using PI-RADS version 2.1. Among them, seven cases involved nonspecific lesions, while five men had previously received Bacillus Calmette–Guérin vesical instillations to prevent recurrences of non-muscle-invasive bladder cancer. All the lesions exhibited a low signal intensity on T2-weighted images, restricted diffusion with hyperintensity in Diffusion-Weighted Imaging, and low Appa-rent Diffusion Coefficient values. On Dynamic Contrast-Enhanced imaging, most cases of specific granulomatous prostatitis demonstrated high peaks and persistent enhancement. Additionally, most of these latter lesions showed an avascular core and peripheral rim enhancement. This study reinforces the association between granulomatous prostatitis and high-PI-RADS-score lesions, consistent with our findings, and suggests potential role of radiomic features for differentiating these lesions from those of significant PCa [
34]. Recent studies have also highlighted promising functional imaging techniques for diffe-rentiating chronic prostatitis from significant PCa, such as quantitative contrast-enhanced perfusion kinetics on multiparametric MRI [
17,
46,
47,
48].
One of the strengths of the current study is that a large, prospective, and correlative cohort of cases was analyzed, focusing on the type and extent of prostatitis observed in targeted biopsies. However, several limitations should be noted. First, the analysis was restricted to asymptomatic prostatitis, excluding other benign or pre-malignant conditions that may also mimic PCa. Second, the study was not designed to identify radiomic characteristics capable of differentiating PCa from inflammatory features. Furthermore, the diversity and extent of inflammatory features complicate the interpretation of the fin-dings. Lastly, while targeted core biopsies provide valuable insights, they do not fully represent the entire tissue of suspicious lesions, nor do prostate biopsies capture the patho-logy of the whole prostate gland.
Future well-designed radiomic studies focusing on the differentiation of specific inflammatory features and their extent in MRI-suspicious lesions could provide valuable insights into identifying true mimickers of significant PCa. Such advancements could help reduce unnecessary prostate biopsies, particularly in uncertain scenarios characterized by low detection rates of significant PCa and the persistent overdetection of insignificant PCa [
49].
5. Conclusions
Asymptomatic inflammatory features were observed in approximately 65% of the MRI-suspicious lesions, occurring more frequently in lesions with PCa than in those without. The most common inflammatory finding was mild chronic prostatitis, followed by acute prostatitis, moderate chronic prostatitis, severe chronic prostatitis, and granulomatous prostatitis. Granulomatous prostatitis was rare and primarily found in lesions with PI-RADS scores of 4 and 5. The inflammatory findings remained consistent across the PI-RADS categories in lesions without PCa. However, their incidence was significantly higher in lesions without PCa compared to those with PCa, as well as in lesions with insignificant PCa compared to those with significant PCa. Overall inflammatory features were identified as an independent predictor of a lower likelihood of detection of significant PCa. These findings suggest that overall inflammatory features could serve as potential mimickers of significant PCa.
Author Contributions
Conceptualization, J.M.; methodology, J.M., B.M.; data curation, A.C., M.E.S., A.A., O.M.; formal analysis: J.M., B.M.; writing—original draft preparation, J.M.; writing—review and editing, M.E.S., A.A., B.M., O.M., E.T. X.X.; supervision, J.M..; project administration, J.M., O.M.; funding acquisition, O.M., J.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministerio de Asuntos Económicos y Transformación Digital (SP) (MIA.2021.M02.0005) and the Instituto de Salut Carlos III (SP) (PI20/01666).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the Vall d´Hebron Hospital and Research Institute ((PRAG-02/2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available upon request to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, and Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, Denis LJ, Recker F, Berenguer A, Maattanen L, Bangma CH, Aus G, Villers A, Rebillard X, van der Kwast T, Blijenberg BG, Moss SM, de Koning HJ, and Auvinen A. Screening and prostate-cancer mortality in a randomized European study. N. Engl. J. Med. 2009, 360, 1320–1328. [Google Scholar] [CrossRef] [PubMed]
- Frånlund M, Månsson M, Godtman RA, Aus G, Holmberg E, Kollberg KS, Lodding P, Pihl CG, Stranne J, Lilja H, and Hugosson J Results from 22 years of Followup in the Göteborg Randomized Population-Based Prostate Cancer Screening Trial. J. Urol. 2022, 208, 292–300. [CrossRef] [PubMed]
- Van Poppel, H.; Albreht, T.; Basu, P.; Hogenhout, R.; Collen, S.; Roobol, M. Serum PSA-based early detection of prostate cancer in Europe and globally: past, present and future. Nat. Rev. Urol. 2022, 19, 562–572. [Google Scholar] [CrossRef]
- Itatani, R.; Namimoto, T.; Atsuji, S.; Katahira, K.; Morishita, S.; Kitani, K.; Hamada, Y.; Kitaoka, M.; Nakaura, T.; Yamashita, Y. Negative predictive value of multiparametric MRI for prostate cancer detection: outcome of 5-year follow-up in men with negative findings on initial MRI studies. Eur. J. Radiol. 2014, 83, 1740–1745. [Google Scholar] [CrossRef]
- Moldovan, P.C.; Van den Broeck, T.; Sylvester, R.; Marconi, L.; Bellmunt, J.; van den Bergh, R.C.; Bolla, M.; Briers, E.; Cumberbatch, M.G.; Fossati, N.; Gross, T.; Henry, A.M.; Joniau, S.; van der Kwast, T.H.; Matveev, V.B.; van der Poel, H.G.; De Santis, M.; Schoots, I.G.; Wiegel, T.; Yuan, C.Y.; Cornford, P.; Mottet, N.; Lam, T.B.; Rouvière, O. What Is the Negative Predictive Value of Multiparametric Magnetic Resonance Imaging in Excluding Prostate Cancer at Biopsy? A Systematic Review and Meta-analysis from the European Association of Urology Prostate Cancer Guidelines Panel. Eur. Urol. 2017, 71, 618–629. [Google Scholar] [CrossRef]
- Sathianathen, N.J.; Omer, A.; Harriss, E.; Davies, L.; Kasivisvanathan, V.; Punwani, S.; Moore, C.M.; Kastner, C.; Barrett, T.; Van Den Bergh, R.C.; Eddy, B.A.; Gleeson, F.; Macpherson, R.; Bryant, R.J.; Catto, J.W.F.; Murphy, D.G.; Hamdy, F.C.; Ahmed, H.U.; Lamb, A.D. Negative Predictive Value of Multiparametric Magnetic Resonance Imaging in the Detection of Clinically Significant Prostate Cancer in the Prostate Imaging Reporting and Data System Era: A Systematic Review and Meta-analysis. Eur. Urol. 2020, 78, 402–414. [Google Scholar] [CrossRef]
- Kasivisvanathan, V.; Dufour, R.; Moore, C.M.; Ahmed, H.U.; Abd-Alazeez, M.; Charman, S.C.; Freeman, A.; Allen, C.; Kirkham, A.; van der Meulen, J.; Emberton, M. Transperineal magnetic resonance image targeted prostate biopsy versus transperineal template prostate biopsy in the detection of clinically significant prostate cancer. J. Urol. 2013, 189, 860–866. [Google Scholar] [CrossRef]
- Kasivisvanathan, V.; Rannikko, A.S.; Borghi, M.; Panebianco, V.; Mynderse, L.A.; Vaarala, M.H.; Briganti, A.; Budäus, L.; Hellawell, G.; Hindley, R.G.; Roobol, M.J.; Eggener, S.; Ghei, M.; Villers, A.; Bladou, F.; Villeirs, G.M.; Virdi, J.; Boxler, S.; Robert, G.; Singh, P.B.; Venderink, W.; Hadaschik, B.A.; Ruffion, A.; Hu, J.C.; Margolis, D.; Crouzet, S.; Klotz, L.; Taneja, S.S.; Pinto, P.; Gill, I.; Allen, C.; Giganti, F.; Freeman, A.; Morris, S.; Punwani, S.; Williams, N.R.; Brew-Graves, C.; Deeks, J.; Takwoingi, Y.; Emberton, M.; Moore, C.M. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N. Engl. J. Med. 2018, 378, 1767–1777. [Google Scholar] [CrossRef]
- Comford, P.; Tilki, D.; Bergh, R.C.N.V.D.; Briers, E. ; D.Ebetli; Meerleer, G.D.; Santis, M.D.; Gillessen, A.; Leeders, A.M.H.V.; J, O.; Oort, I.M.C.; Schoots, I.G.; Stranne, J.; Wiegel, T. EAU - EANM - ESTRO - ESUR - ISUP - SIOG Guidelines on Prostate Cancer. 2024. http://uroweb.org/guidelines/compilations-of-all-guidelines/. 2024. [Google Scholar]
- Schoots, I.G.; Padhani, A.R.; Rouvière, O.; Barentsz, J.O.; Richenberg, J. Analysis of Magnetic Resonance Imaging-directed Biopsy Strategies for Changing the Paradigm of Prostate Cancer Diagnosis. Eur. Urol. Oncol. 2020, 3, 32–41. [Google Scholar] [CrossRef]
- Maggi, M.; Panebianco, V.; Mosca, A.; Salciccia, S.; Gentilucci, A.; Di Pierro, G.; Busetto, G.M.; Barchetti, G.; Campa, R.; Sperduti, I.; Del Giudice, F.; Sciarra, A. Prostate Imaging Reporting and Data System 3 Category Cases at Multiparametric Magnetic Resonance for Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. Focus. 2020, 6, 463–478. [Google Scholar] [CrossRef] [PubMed]
- Morote, J.; Campistol, M.; Triquell, M.; Celma, A.; Regis, L.; de Torres, I.; Semidey, M.E.; Mast, R.; Santamaria, A.; Planas, J.; Trilla, E. Improving the Early Detection of Clinically Significant Prostate Cancer in Men in the Challenging Prostate Imaging-Reporting and Data System 3 Category. Eur. Urol. Open. Sci. 2022, 37, 38–44. [Google Scholar] [CrossRef]
- Nadler, R.B.; Humphrey, P.A.; Smith, D.S.; Catalona, W.J.; Ratliff, T.L. Effect of inflammation and benign prostatic hyperplasia on elevated serum prostate specific antigen levels. J. Urol. 1995, 154, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Krieger, J.N.; Nyberg, L.; Nickel, J.C. NIH consensus definition and classification of prostatitis. JAMA. 1999, 282, 236–237. [Google Scholar] [CrossRef] [PubMed]
- Nagel, K.N.; Schouten, M.G.; Hambrock, T.; Litjens, G.J.; Hoeks, C.M.; ten Haken, B.; Barentsz, J.O.; Fütterer, J.J. Differentiation of prostatitis and prostate cancer by using diffusion-weighted MR imaging and MR-guided biopsy at 3 T. Radiology 2013, 267, 164–172. [Google Scholar] [CrossRef]
- Uysal, A.; Karaosmanoğlu, A.D.; Karcaaltıncaba, M.; Akata, D.; Akdogan, B.; Baydar, D.E.; Ozmen, M.N. Prostatitis, the Great Mimicker of Prostate Cancer: Can We Differentiate Them Quantitatively With Multiparametric MRI. Am. J. Roentgenol. 2020, 215, 1104–1112. [Google Scholar] [CrossRef]
- Bertelli, E.; Zantonelli, G.; Cinelli, A.; Pastacaldi, S.; Agostini, S.; Neri, E.; Miele, V. Granulomatous Prostatitis, the Great Mimicker of Prostate Cancer: Can Multiparametric MRI Features Help in This Challenging Differential Diagnosis. Diagnostics (Basel). 2022, 12, 2302. [Google Scholar] [CrossRef]
- Rourke, E.; Sunnapwar, A.; Mais, D.; Kukkar, V.; DiGiovanni, J.; Kaushik, D.; Liss, M.A. Inflammation appears as high Prostate Imaging-Reporting and Data System scores on prostate magnetic resonance imaging (MRI) leading to false positive MRI fusion biopsy. Investig. Clin. Urol. 2019, 60, 388–395. [Google Scholar] [CrossRef]
- Barentsz, J.O.; Richenberg, J.; Clements, R.; Choyke, P.; Verma, S.; Villeirs, G.; Rouviere, O.; Logager, V.; Futterer, J.J. ESUR prostate MR guidelines 2012. Eur. Radiol. 2012, 22, 746–757. [Google Scholar] [CrossRef]
- Turkbey, B.; Rosenkrantz, A.B.; Haider, M.A.; Padhani, A.R.; Villeirs, G.; Macura, K.J.; Tempany, C.M.; Choyke, P.L.; Cornud, F.; Margolis, D.J.; Thoeny, H.C.; Verma, S.; Barentsz, J.; Weinreb, J.C. Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2. Eur Urol. 2019, 76, 340–351. [Google Scholar] [CrossRef]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A.; Grading, C. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am J Surg Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef]
- Epstein, J.I.; Zelefsky, M.J.; Sjoberg, D.D.; Nelson, J.B.; Egevad, L.; Magi-Galluzzi, C.; Vickers, A.J.; Parwani, A.V.; Reuter, V.E.; Fine, S.W.; Eastham, J.A.; Wiklund, P.; Han, M.; Reddy, C.A.; Ciezki, J.P.; Nyberg, T.; Klein, E.A. A Contemporary Prostate Cancer Grading System: A Validated Alternative to the Gleason Score. Eur. Urol. 2016, 69, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Yebes, A.; Toribio-Vazquez, C.; Martinez-Perez, S.; Quesada-Olarte, J.M.; Rodriguez-Serrano, A.; Álvarez-Maestro, M.; Martinez-Piñeiro, L. Prostatitis: A Review. Curr. Urol. Rep. 2023, 24, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Irani, J.; Levillain, P.; Goujon, J.M.; Bon, D.; Doré, B.; Aubert, J. Inflammation in benign prostatic hyperplasia: correlation with prostate specific antigen value. J. Urol. 1997, 157, 1301–1303. [Google Scholar] [CrossRef] [PubMed]
- Sciarra, A.; Mariotti, G.; Salciccia, S.; Autran Gomez, A.; Monti, S.; Toscano, V.; Di Silverio, F. Prostate growth and inflammation. J. Steroid. Biochem. Mol Biol. 2008, 108, 254–260. [Google Scholar] [CrossRef]
- Sessa, F.; Nicoletti, R.; De Nunzio, C.; Porreca, A.; Magrini, S.M.; Mirone, V.; Tubaro, A.; Serni, S.; Gontero, P.; Noale, M.; Maggi, S.; Gacci, M.; On, B.O.T.P.-I.S.G. Inflammation and Prostate Cancer: Pathological Analysis from Pros-IT CNR 2. Cancers (Basel). 2023, 15, 630. [Google Scholar] [CrossRef]
- Vasavada, S.R.; Dobbs, R.W.; Kajdacsy-Balla, A.A.; Abern, M.R.; Moreira, D.M. Inflammation on Prostate Needle Biopsy is Associated with Lower Prostate Cancer Risk: A Meta-Analysis. J. Urol. 2018, 199, 1174–1181. [Google Scholar] [CrossRef]
- Schatteman, P.H.; Hoekx, L.; Wyndaele, J.J.; Jeuris, W.; Van Marck, E. Inflammation in prostate biopsies of men without prostatic malignancy or clinical prostatitis: correlation with total serum PSA and PSA density. Eur. Urol. 2000, 37, 404–412. [Google Scholar] [CrossRef]
- Yli-Hemminki, T.H.; Laurila, M.; Auvinen, A.; Määttänen, L.; Huhtala, H.; Tammela, T.L.; Kujala, P.M. Histological inflammation and risk of subsequent prostate cancer among men with initially elevated serum prostate-specific antigen (PSA) concentration in the Finnish prostate cancer screening trial. BJU. Int. 2013, 112, 735–741. [Google Scholar] [CrossRef]
- Rais-Bahrami, S.; Nix, J.W.; Turkbey, B.; Pietryga, J.A.; Sanyal, R.; Thomas, J.V.; Gordetsky, J.B. Clinical and multiparametric MRI signatures of granulomatous prostatitis. Abdom. Radiol. (NY). 2017, 42, 1956–1962. [Google Scholar] [CrossRef]
- Moon, S.K.; You, M.W.; Lim, J.W. Granulomatous Prostatitis Mimicking Prostate Cancer. Urology 2020, 137, e3–e5. [Google Scholar] [CrossRef] [PubMed]
- Pepe, P.; Pennisi, M. Negative Biopsy Histology in Men With PI-RADS Score 5 in Daily Clinical Practice: Incidence of Granulomatous Prostatitis. Clin. Genitourin. Cancer 2020, 18, e684–e687. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, E.; Zantonelli, G.; Cinelli, A.; Pastacaldi, S.; Agostini, S.; Neri, E.; Miele, V. Granulomatous Prostatitis, the Great Mimicker of Prostate Cancer: Can Multiparametric MRI Features Help in This Challenging Differential Diagnosis. Diagnostics (Basel). 2022, 23, 12. [Google Scholar] [CrossRef] [PubMed]
- Gaudiano, C.; Renzetti, B.; De Fino, C.; Corcioni, B.; Ciccarese, F.; Bianchi, L.; Schiavina, R.; Droghetti, M.; Giunchi, F.; Brunocilla, E.; Fiorentino, M. Multiparametric magnetic resonance imaging for the differential diagnosis between granulomatous prostatitis and prostate cancer: a literature review to an intriguing diagnostic challenge. Front. Oncol. 2023, 13, 1178430. [Google Scholar] [CrossRef] [PubMed]
- Ogreden, E.; Oguz, U.; Demirelli, E.; Tok, D.S.; Aslan, S.; Tok, B.; Çiftçi, O.Ç.; Akyol, S.; Altun, E.; Ahmadlı, C.; Semiz, U.; Batur, H. The role of multiparametric magnetic resonance ımaging in the diagnosis of granulomatous prostatitis mimicking prostate cancer. Abdom. Radio.l (NY). 2024, 49, 2305–2310. [Google Scholar] [CrossRef]
- Moreira, D.M.; Nickel, J.C.; Gerber, L.; Muller, R.L.; Andriole, G.L.; Castro-Santamaria, R.; Freedland, S.J. Baseline prostate inflammation is associated with a reduced risk of prostate cancer in men undergoing repeat prostate biopsy: Results from the REDUCE study: Inflammation and Prostate Cancer. Cancer 2014, 120, 190–196. [Google Scholar] [CrossRef]
- Hu, J.C.; Palapattu, G.S.; Kattan, M.W.; Scardino, P.T.; Wheeler, T.M. The association of selected pathological features with prostate cancer in a single-needle biopsy accession. Hum Pathol. 1998, 29, 1536–1538. [Google Scholar] [CrossRef]
- Karakiewicz, P.I.; Benayoun, S.; Bégin, L.R.; Duclos, A.; Valiquette, L.; McCormack, M.; Bénard, F.; Saad, F.; Perrotte, P. Chronic inflammation is negatively associated with prostate cancer and high-grade prostatic intraepithelial neoplasia on needle biopsy. Int J Clin. Pract. 2007, 61, 425–430. [Google Scholar] [CrossRef]
- Abouassaly, R.; Tan, N.; Moussa, A.; Jones, J.S. Risk of prostate cancer after diagnosis of atypical glands suspicious for carcinoma on saturation and traditional biopsies. J. Urol. 2008, 180, 911–914. [Google Scholar] [CrossRef]
- Bassett, W.W.; Bettendorf, D.M.; Lewis, J.M.; Loughlin, K.R. Chronic periglandular inflammation on prostate needle biopsy does not increase the likelihood of cancer on subsequent biopsy. Urology 2009, 73, 845–849. [Google Scholar] [CrossRef]
- Amini, E.; Pishgar, F.; Ayati, M.; Jamshidian, H.; Arbab, A.; Gooshe, M.; Nowroozi, M.R. Transition Zone Prostate-specific Antigen Density Could Better Guide the Rebiopsy Strategy in Men With Prostate Inflammation at Initial Biopsy. Urology 2015, 86, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Porcaro, A.B.; Novella, G.; Balzarro, M.; Martignoni, G.; Brunelli, M.; Cacciamani, G.; Cerruto, M.A.; Artibani, W. Prostate chronic inflammation type IV and prostate cancer risk in patients undergoing first biopsy set: Results of a large cohort study. Asian J. Urol. 2015, 2, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Jyoti, R.; Jina, N.H.; Haxhimolla, H.Z. In-gantry MRI guided prostate biopsy diagnosis of prostatitis and its relationship with PIRADS V.2 based score. J. Med. Imaging Radiat. Oncol. 2016, 61, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Gordetsky, J.B.; Ullman, D.; Schultz, L.; Porter, K.K.; Del Carmen Rodriguez Pena, M.; Calderone, C.E.; Nix, J.W.; Ullman, M.; Bae, S.; Rais-Bahrami, S. Histologic Findings Associated with False Positive Multiparametric Magnetic Resonance Imaging Performed for Prostate Cancer Detection. Hum. Pathol. 2018, 76, 68–75. [Google Scholar] [CrossRef]
- Schatteman, P.H.; Hoekx, L.; Wyndaele, J.J.; Jeuris, W.; Van Marck, E. Inflammation in prostate biopsies of men without prostatic malignancy or clinical prostatitis: correlation with total serum PSA and PSA density. Eur. Urol. 2000, 37, 404–412. [Google Scholar] [CrossRef]
- Sureka, B.; Elhence, P.; Khera, P.S.; Choudhary, G.R.; Pandey, H.; Garg, P.K.; Yadav, K.; Goel, A. Quantitative contrast-enhanced perfusion kinetics in multiparametric MRI in differentiating prostate cancer from chronic prostatitis: results from a pilot study. Br. J. Radiol. 2019, 92, 20190181. [Google Scholar] [CrossRef]
- Al-Monajjed, R.; Radtke, J.P.; Thomas, M.; Boschheidgen, M.; Drewes, L.R.; Ullrich, T.; Rau, T.; Esposito, I.; Antoch, G.; Albers, P.; Lopez-Cotarelo, C.; Schimmöller, L. Multiparametric MRI characteristics of prostatitis and atrophy in the peripheral zone in men without prostate cancer. Eur. J. Radiol. 2023, 169, 111151. [Google Scholar] [CrossRef]
- Antolin, A.; Roson, N.; Mast, R.; Arce, J.; Almodovar, R.; Cortada, R.; Maceda, A.; Escobar, M.; Trilla, E.; Morote, J. The Role of Radiomics in the Prediction of Clinically Significant Prostate Cancer in the PI-RADS v2 and v2.1 Era: A Systematic Review. Cancers (Basel). 2024, 16, 2951. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).