Submitted:
29 November 2024
Posted:
29 November 2024
You are already at the latest version
Abstract
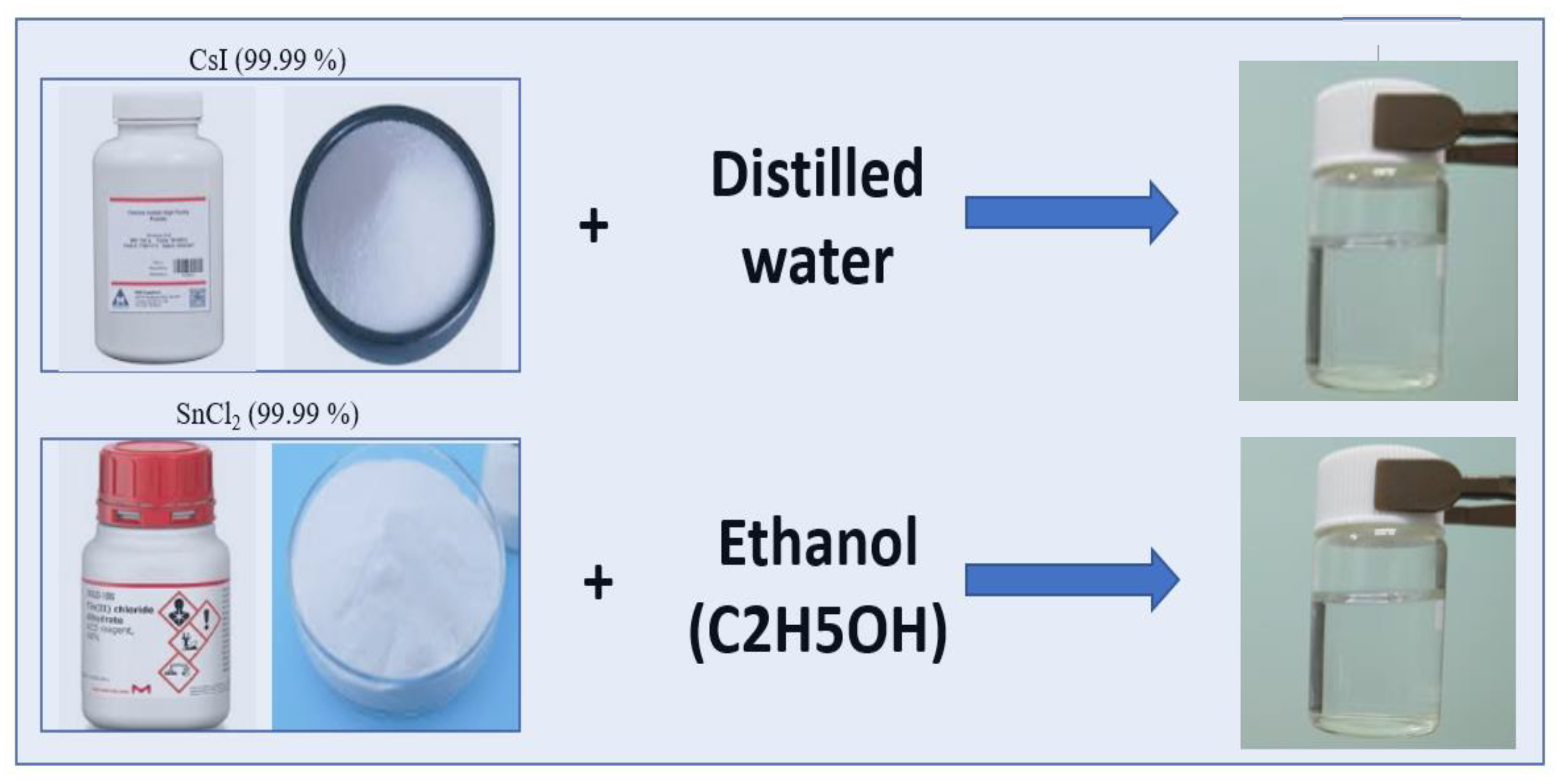
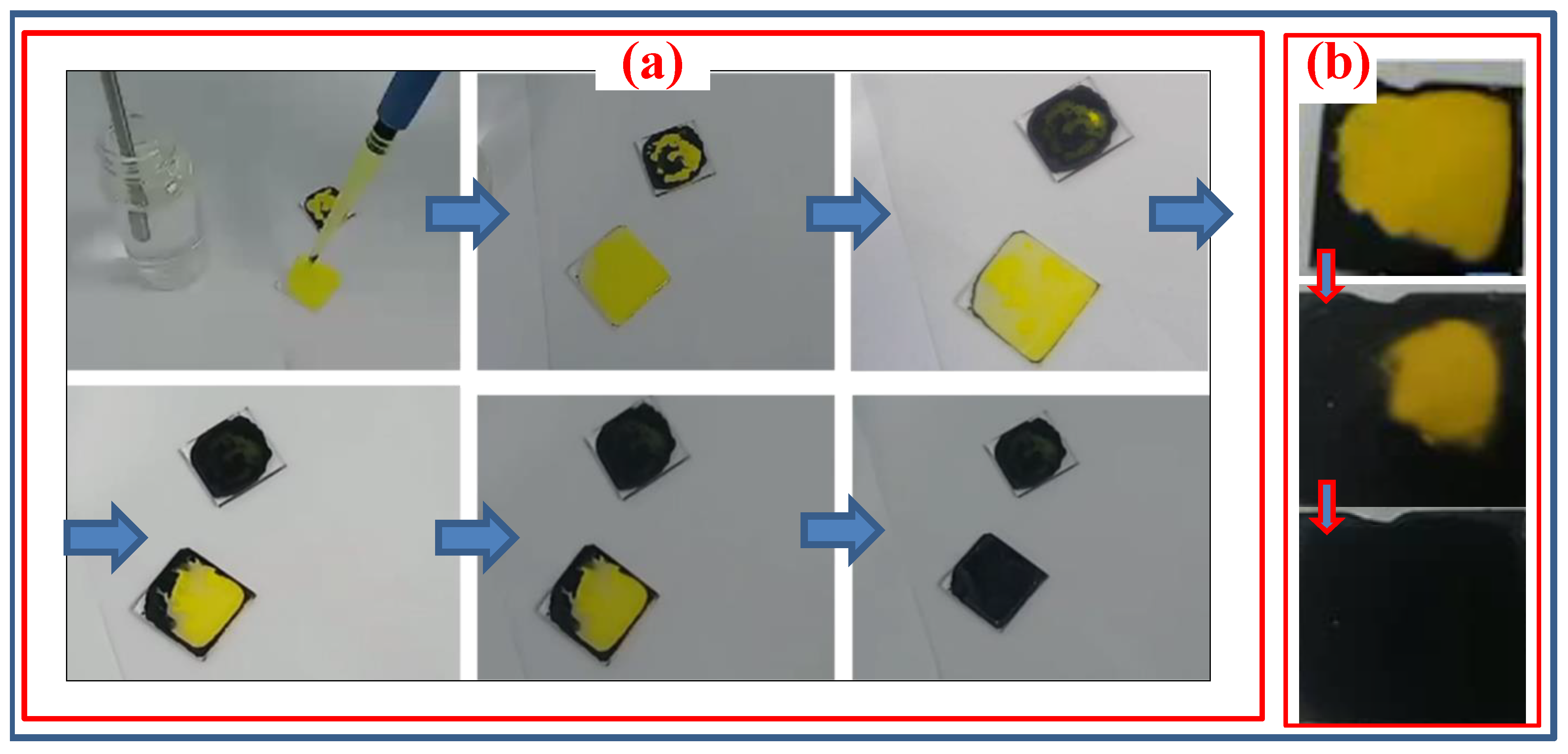
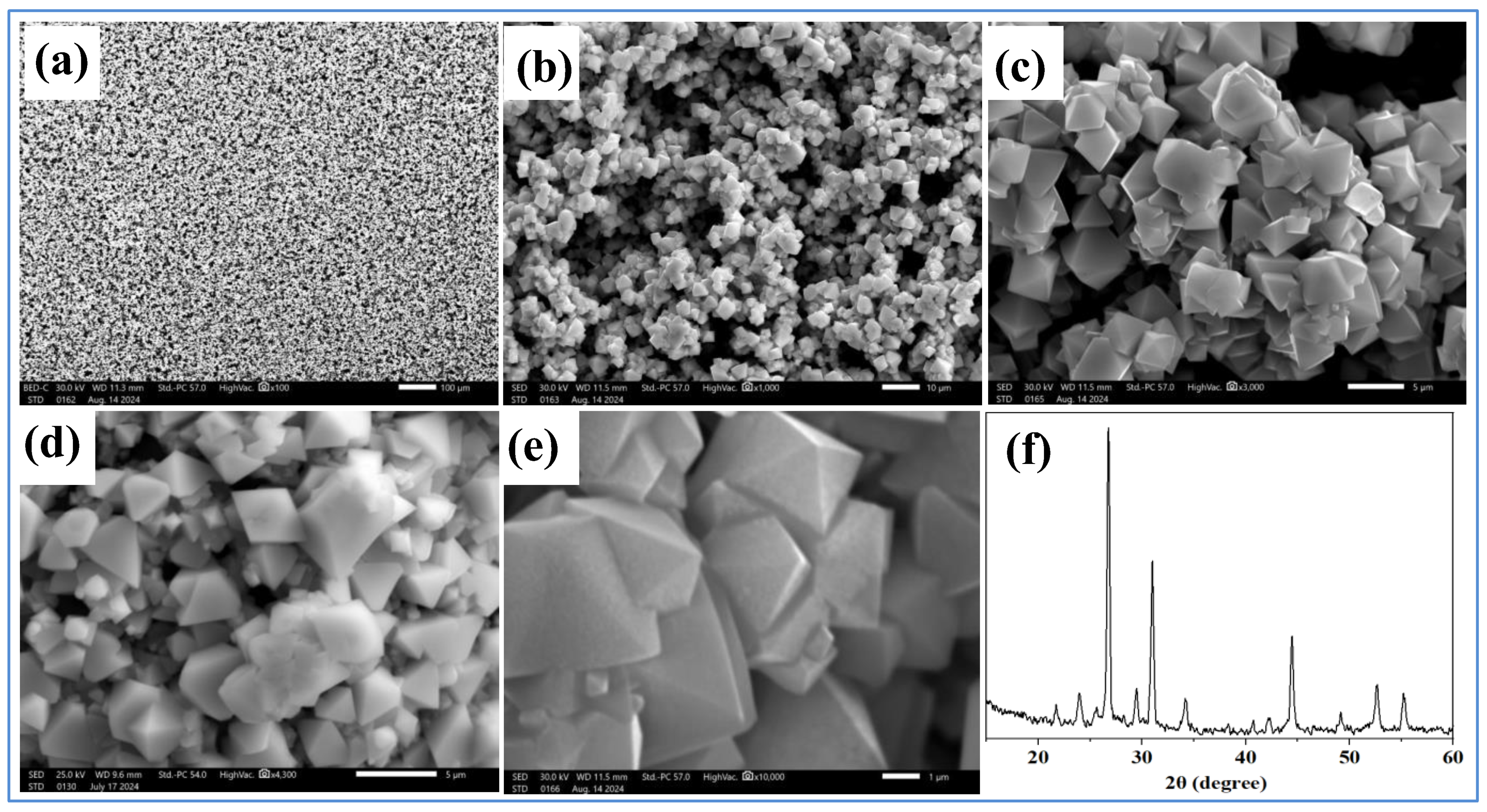
Objective: This study focuses on the synthesis and analysis of the morphology of CsSnI3 crystals and films based on CsI and SnCl2 solutions. The aim of this approach is to synthesize air-stable perovskites and prevent phase transitions of tin-containing perovskite, where previous studies have often reported its rapid oxidation when dissolved in dimethyl sulfoxide. Methods: CsSnI3 crystalline films were obtained from CsI and SnCl2 solutions, in which deionized water and ultrapure ethanol were used for their dissolution. Dissolution and mixing were performed at room temperature. High-purity CsI (99.99%) and SnCl2 (99.99%) powders were used to obtain solutions. To obtain a homogeneous CsSnl3 solution, the SnCl2 solution was added dropwise to the CsI solution and stirred on a magnetic stirrer at 900 rpm. The resulting solution was then applied to FTO substrates heated on a hotplate without spin coating. The samples were heated to temperatures from 60 0C to 130 0C, where, depending on the rate of evaporation of the liquid, the process of formation of crystalline and thin-film structures was controlled. Results: Stable CsSnI3 films were obtained. SEM and X-ray diffraction analyses of the obtained cesium tin triiodide films deposited on conventional FTO glass substrates were performed. X-ray diffraction patterns of synthesized perovskite crystals and films were obtained. Conclusion: The synthesized CsSnI3 perovskite thin films retained their black perovskite phase for more than 4 months, indicating their long-term stability. Based on the results and long-term stability performance, it can be concluded that the black phases of CsSnI3 are suitable for various applications such as photovoltaic devices.
Keywords:
1. Introduction
2. Experimental Methodology
3. Results and Discussion
Conclusions
Author Contribution
Funding Acknowledgements
Conflicts of Interest
Abbreviation List
References
- Dada M, Popoola P. Recent advances in solar photovoltaic materials and systems for energy storage applications: a review. Beni-Suef University Journal of Basic and Applied Sciences 2023, 12, 1–15. [Google Scholar] [CrossRef]
- Polman A, Knight M, Garnett EC at al. Photovoltaic materials: Present efficiencies and future challenges. Science, 2016; 352. [CrossRef]
- Shah, A. Amorphous silicon solar cells. Solar Cells and Modules 2020, 4, 139–161. [Google Scholar] [CrossRef]
- Jin Y, Seok J, & Yu K. Highly efficient silicon-based thin-film Schottky barrier photodetectors. ACS Photonics 2023, 10, 1302–1309. [Google Scholar] [CrossRef]
- Yan J, Saunders BR. Third-generation solar cells: a review and comparison of polymer: fullerene, hybrid polymer and perovskite solar cells. Rsc Advances 2014, 4, 43286–43314. [Google Scholar] [CrossRef]
- https://www.nrel.gov/pv/cell-efficiency.html.
- Luo J, Im J, Mayer MT, Nazeeruddin MK, at al. Water photolysis at 12.3% efficiencyvia perovskite photovoltaics and earth-abundant catalysts. Science 2014, 345, 1593. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




