Submitted:
25 November 2024
Posted:
26 November 2024
You are already at the latest version
Abstract
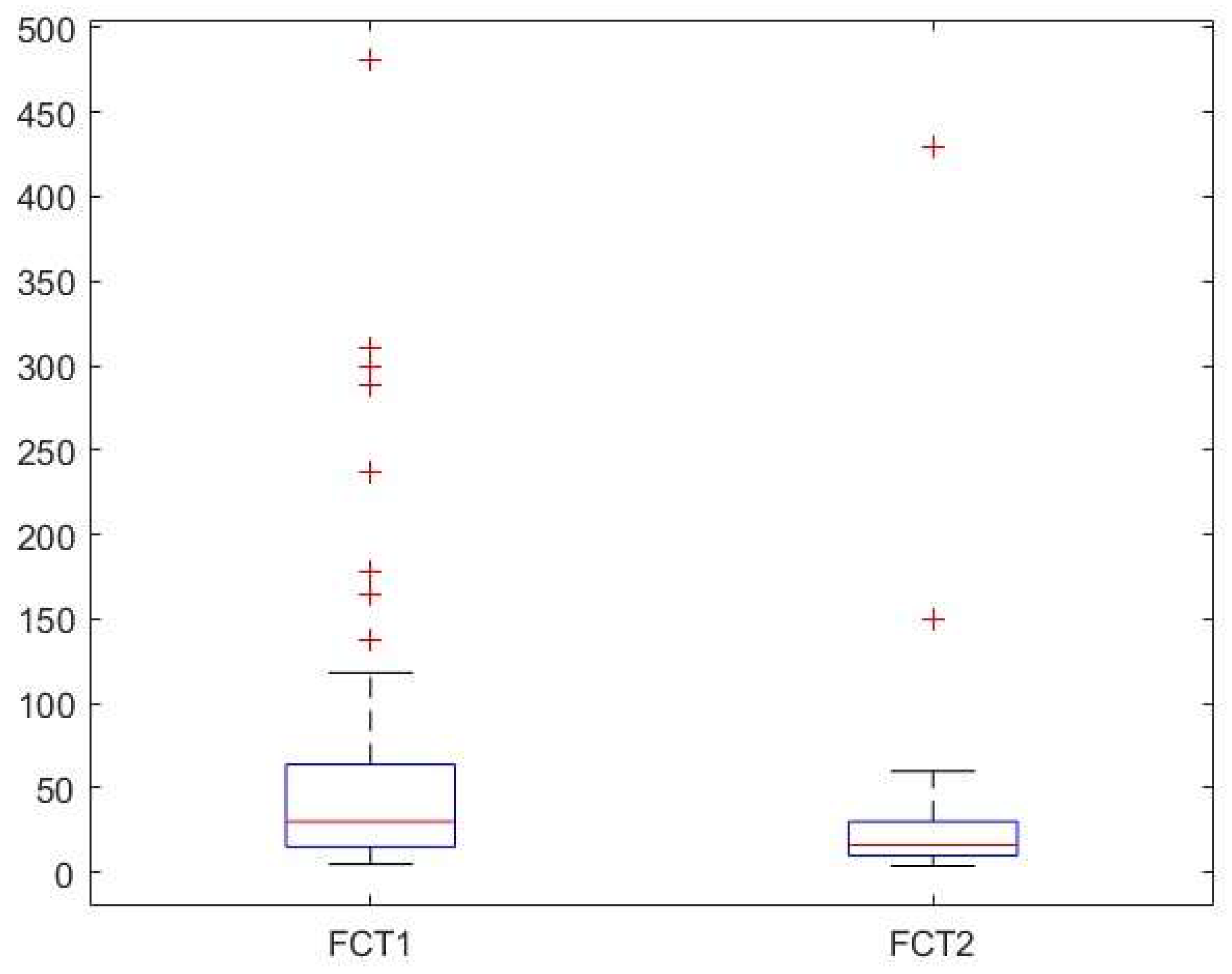
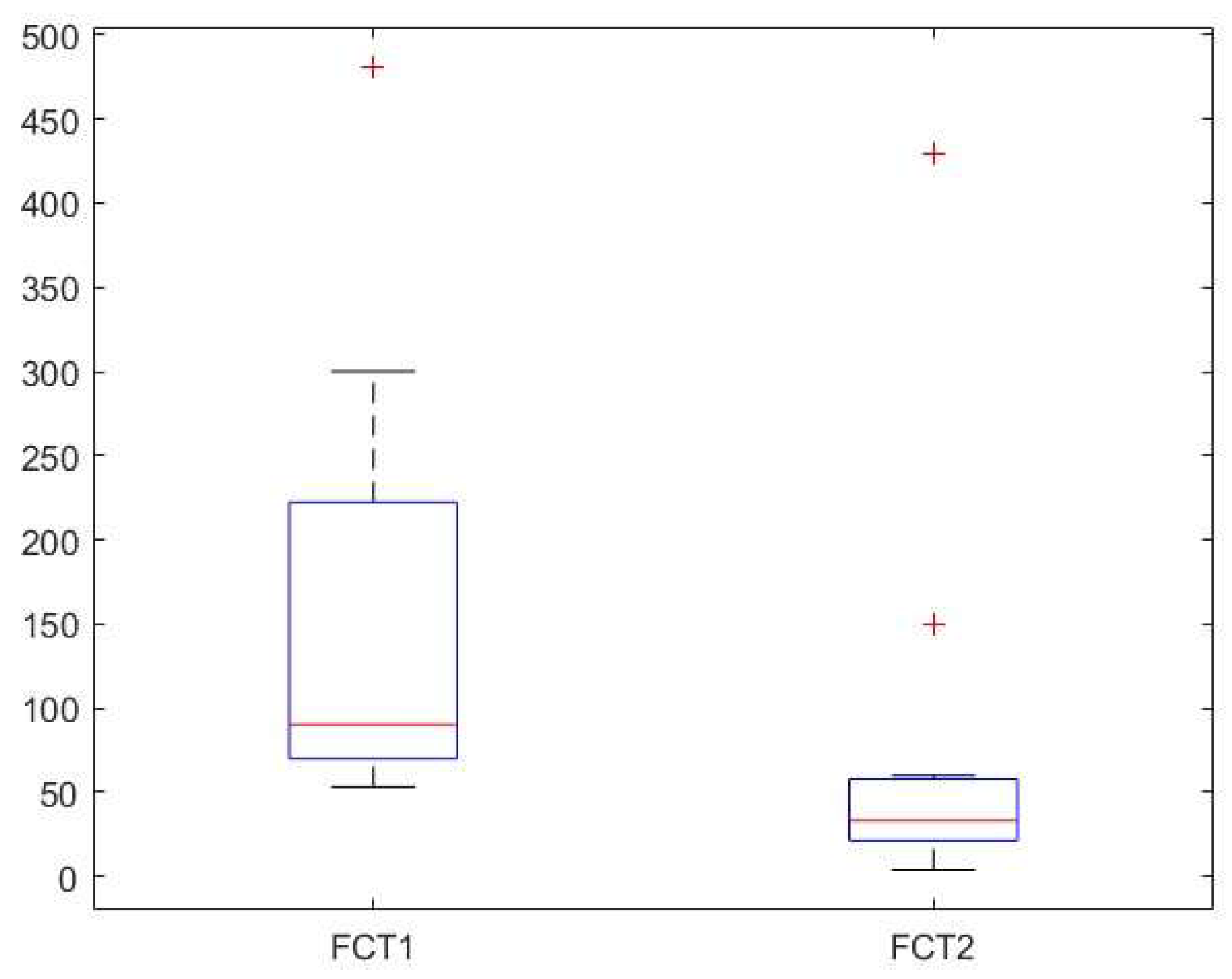
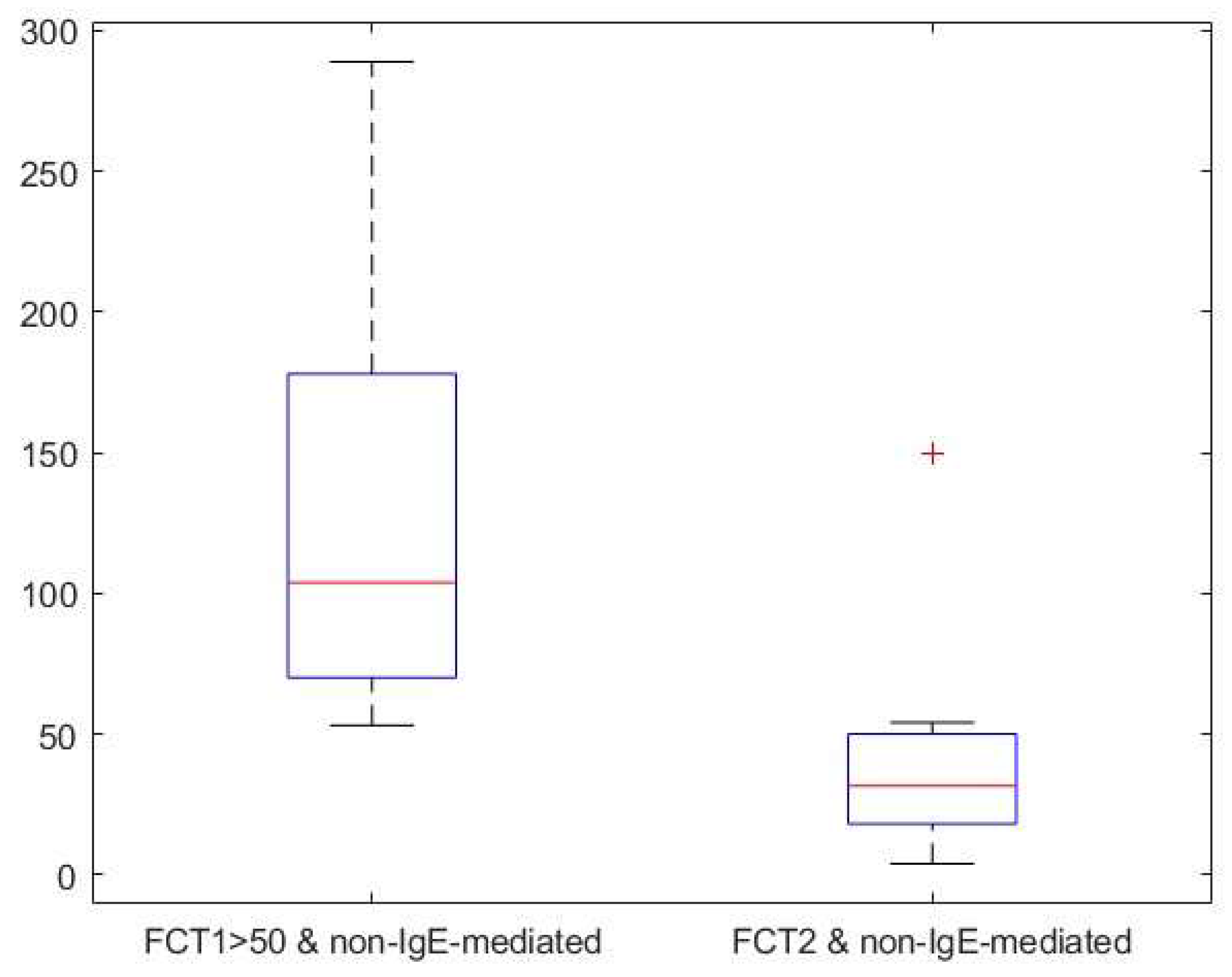
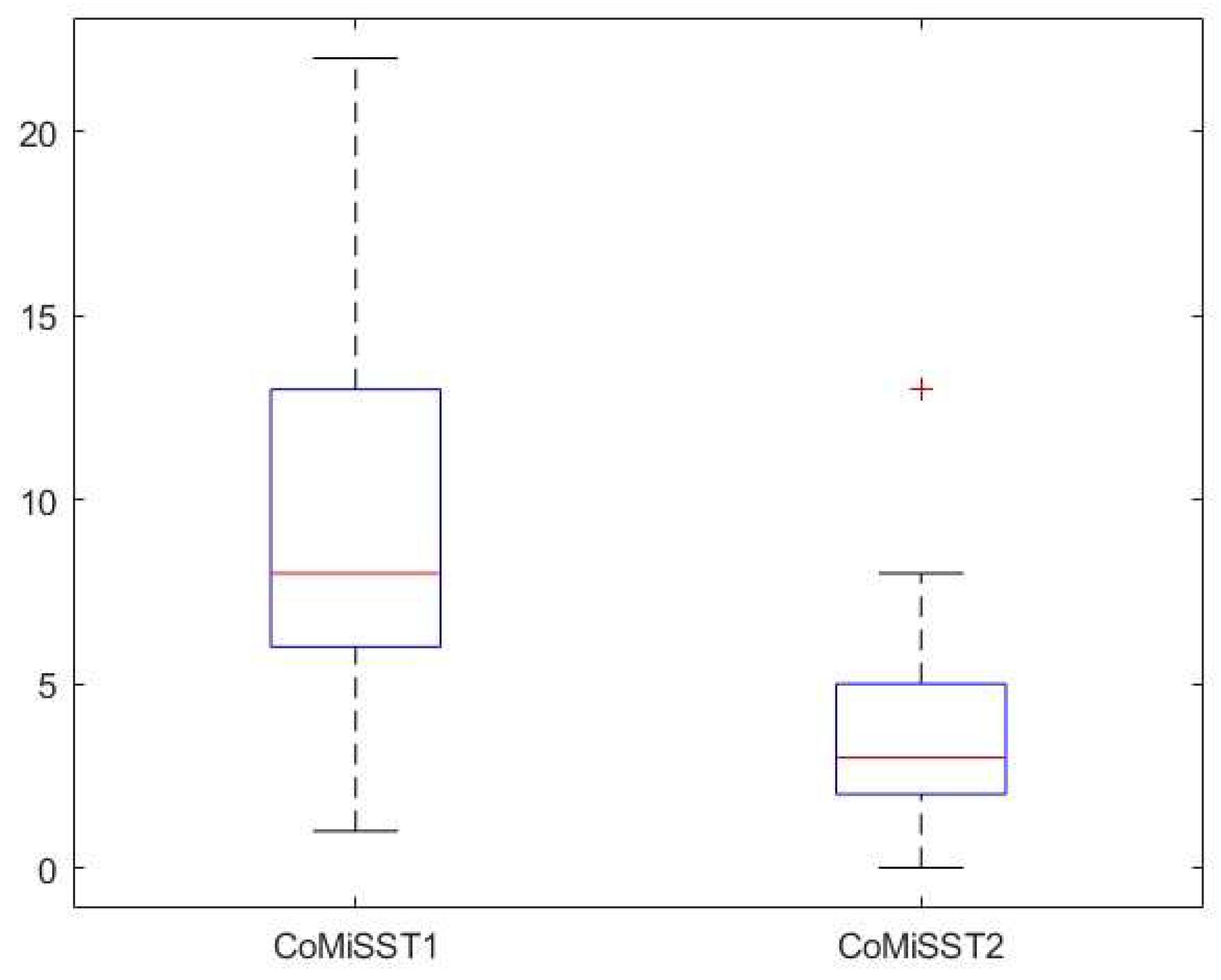
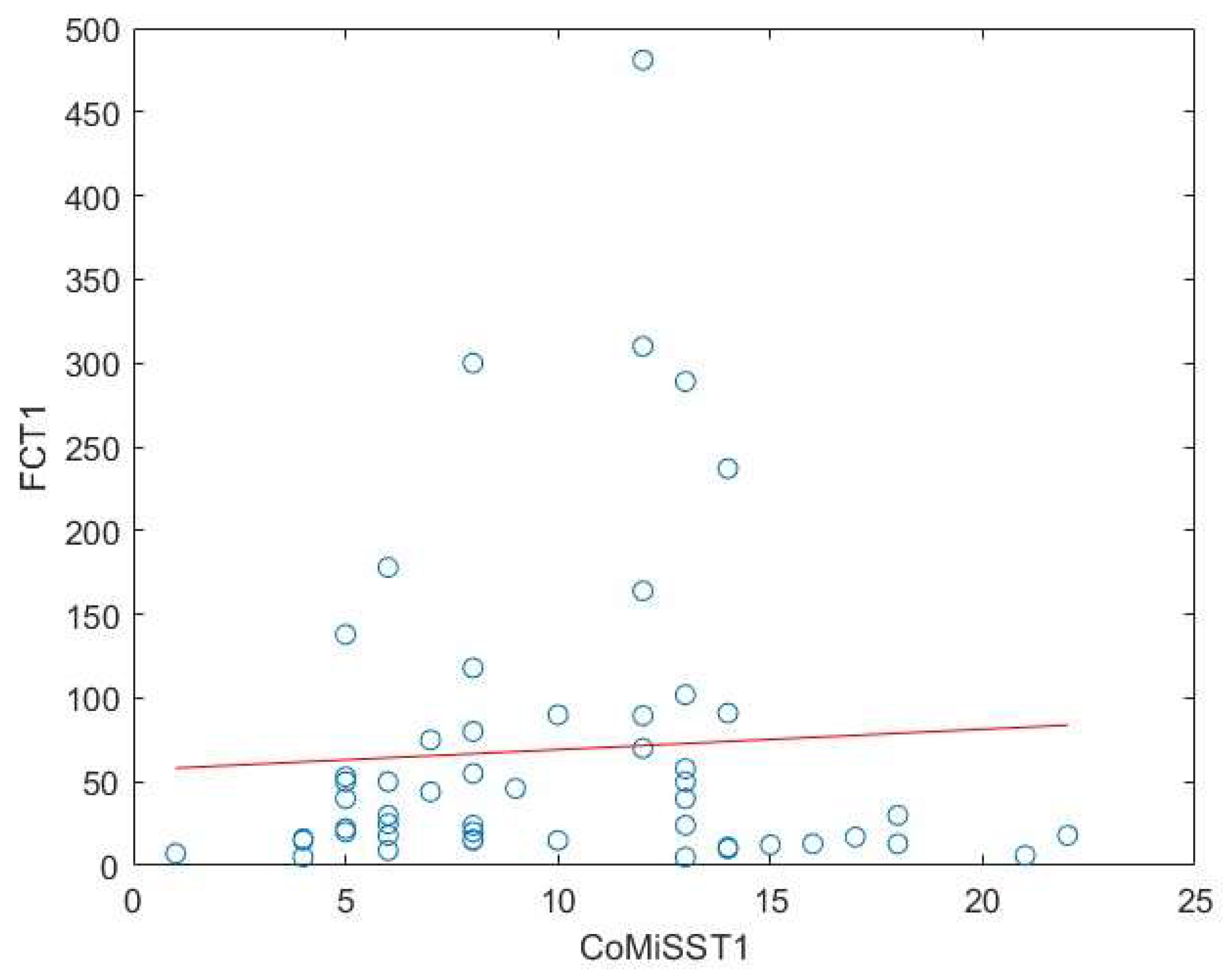
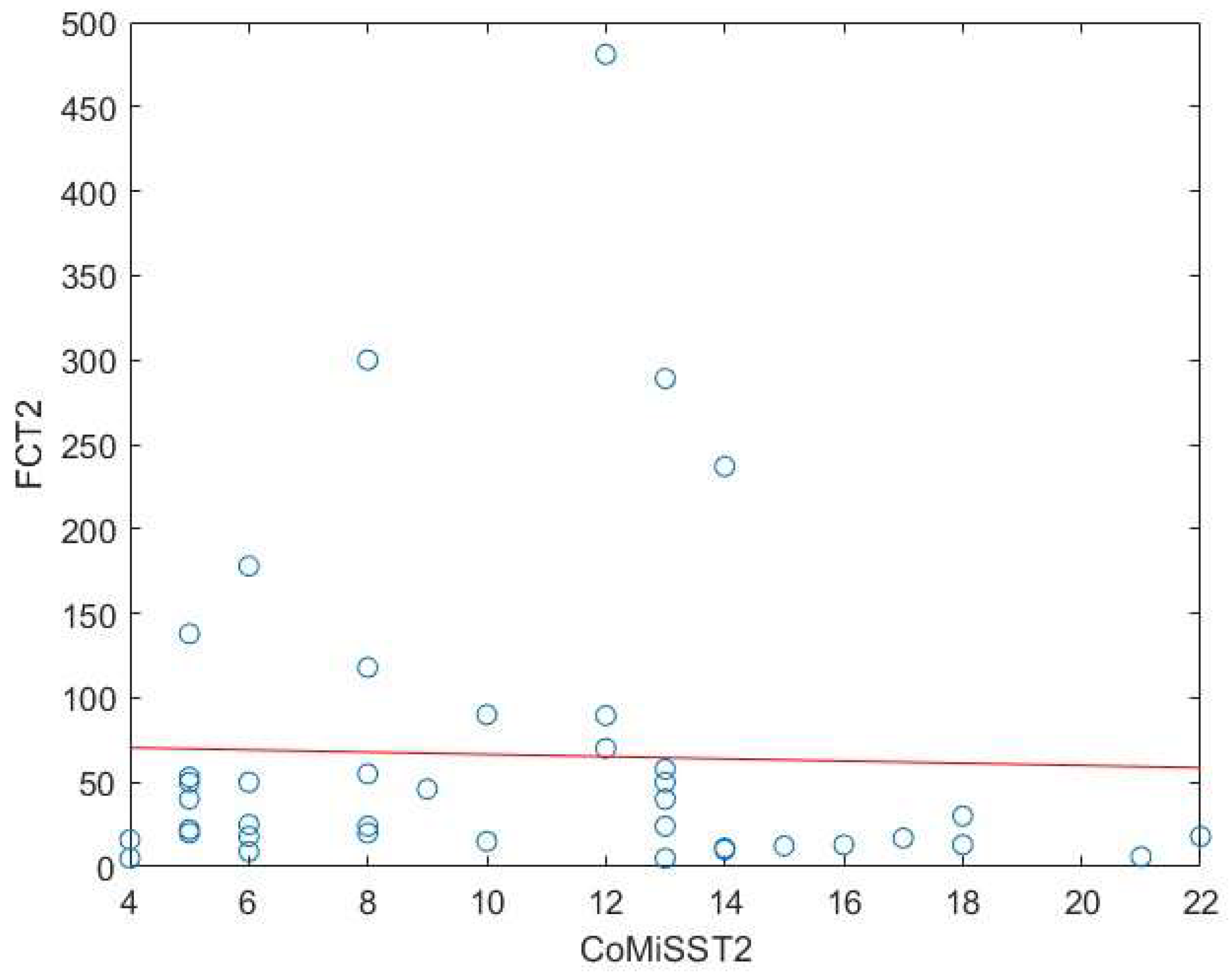
Background: Cow’s milk allergy (CMA) is the most common food allergy in the pediatric age. The oral food challenge (OFC) remains a mainstay of its diagnosis, especially for the non-IgE-mediated type; this test can be risky and time-consuming. Hence, the need to identify biomarkers. Fecal calprotectin (FC) showed variable results, with good reliability and reproducibility in CMA patients. Methods: In this prospective study we enrolled 76 children (age 5-18 months) with CMA-related gastrointestinal and cutaneous symptoms following the European Society of Pediatric Gastroenterology, Hepatology and Nutrition guidelines. Clinical assessments included history, physical examination, skin prick tests, and IgE assays. FC levels and Cow’s Milk Related Symptom Score (CoMiSS) were measured in 51 patients pre- (T1) and post-diet (T2), with a subgroup analysis of 15 patients with elevated baseline FC (>50 mg/kg). Results: FC levels significantly decreased after the elimination diet (median: 30 mg/kg at T1, 16 mg/kg at T2; p <0.01). In the subgroup with higher FC levels, median values dropped from 90 mg/kg to 33 mg/kg (p < 0.01). CoMiSS also improved (median: 8.50 at T1, 3.00 at T2; p <0.01). Linear regression analysis showed no correlation between FC values and the CoMiSS at T1 and T2. Conclusions: The reduction of FC value after an elimination diet suggests that it could be considered a possible biomarker of bowel inflammation in CMA patients. Further studies are necessary to confirm these data and to evaluate and standardize the use of FC for diagnosis and follow-up of CMA.
Keywords:
1. Introduction
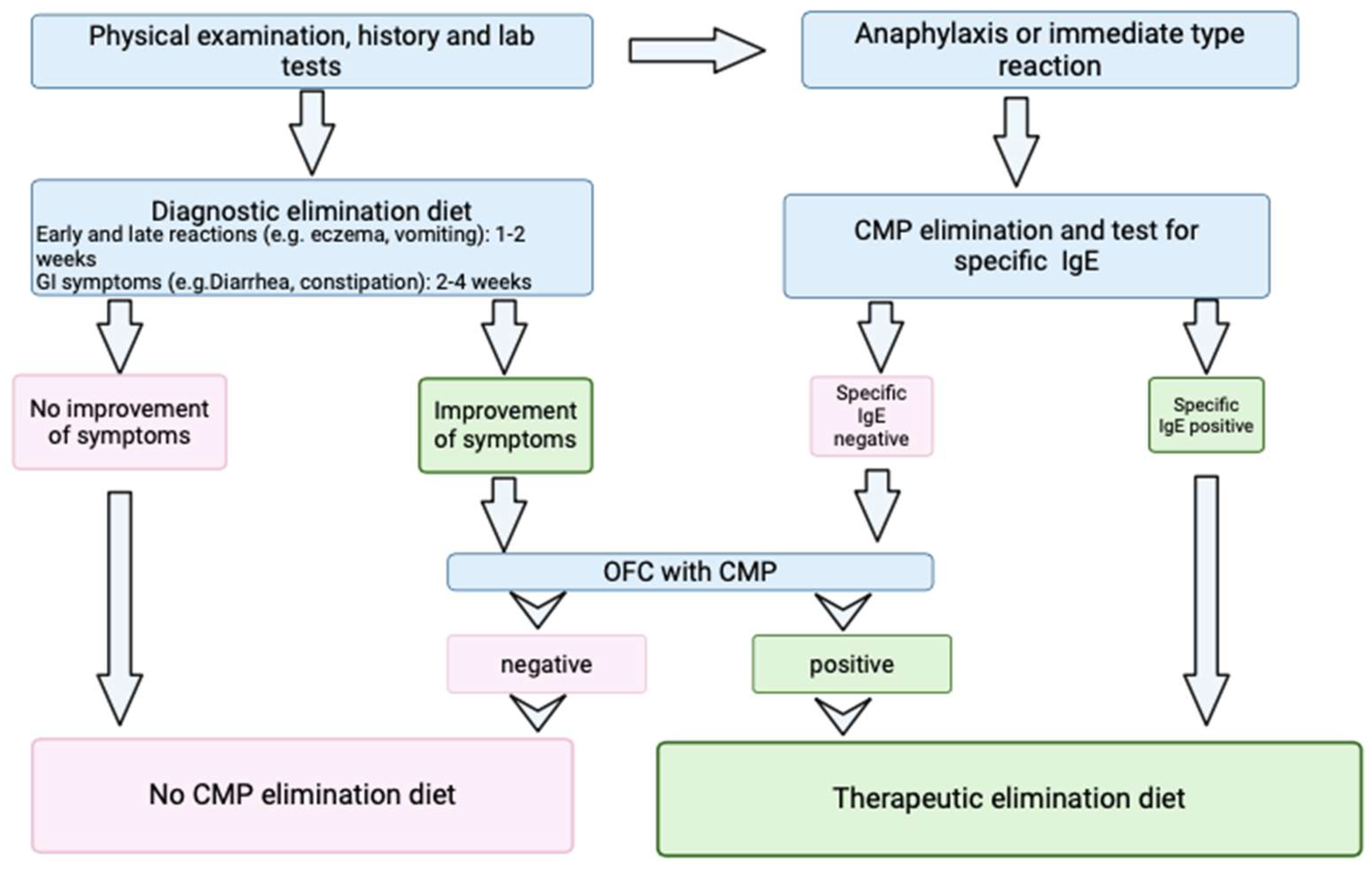
2. Materials and Methods
2.1. Study Design
2.1.1. Fecal Calprotectin Measurement
2.1.2. Ethics
2.1.3. Informed Consent Statement
2.1.3. Statistical Analysis
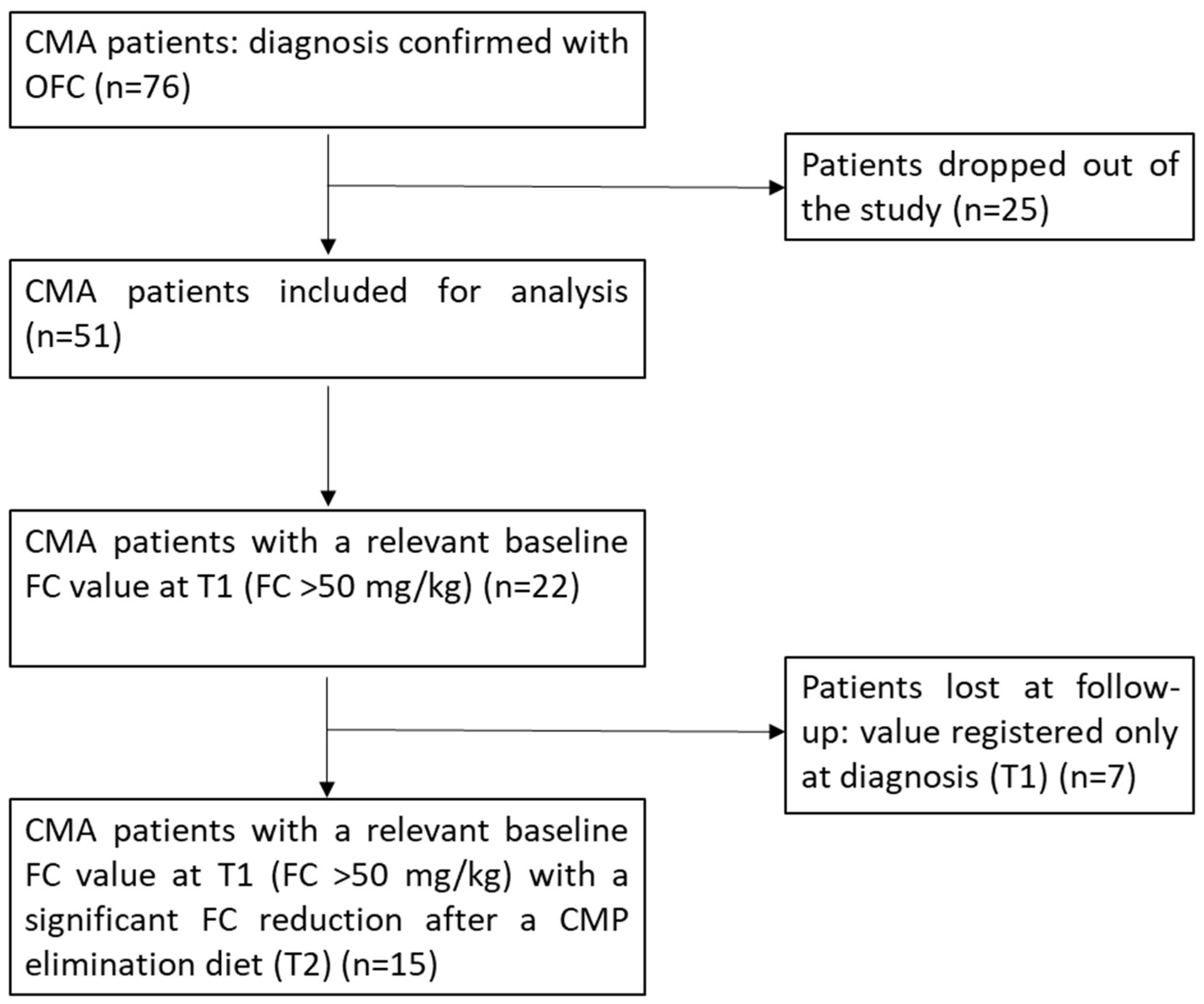
3. Results
3.1. Characteristics of Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Licari, A., Manti, S., Marseglia, A., et al. Food allergies: Current and future treatments. Med. 2019, 55, 1–13 https:// doi:10.3390/medicina55050120. [CrossRef]
- Arvola, T. Ruuska, T., Keränen, J.,et al. Rectal bleeding in infancy: Clinical, allergological, and microbiological examination. Ped. 2006, 117(4):e760-e768. https://doi.org/10.1542/peds.2005-1069. 10.1542/peds.2005-1069.
- Vandenplas, Y., Koletzko, S., Isolauri, E., et al. Guidelines for the diagnosis and management of cow’s milk protein allergy in infants. Arch. Dis. Child. 2007, 92, 902–908 https://doi.org/10.1136/adc.2006.110999. [CrossRef]
- Koletzko,S., Niggemann, B.,Arato, A. et al. Diagnostic approach and management of cow’s-milk protein allergy in infants and children: ESPGHAN GI committee practical guidelines. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 221–229 https://doi.org/10.1097/MPG.0b013e31825c9482. [CrossRef]
- Sicherer,SH. Epidemiology of food allergy. J.Allergy Clin. Immunol. 2011;127:594–602. https://doi.org/10.1016/j.jaci.2010.11.044. [CrossRef]
- Rona, R. J., Keil, T., Summers, C. et al. The prevalence of food allergy: A meta-analysis. J. Allergy Clin. Immunol. 2007, 120, 638–646. https://doi.org/10.1016/j.jaci.2007.05.026. [CrossRef]
- Høst, A. et al. Clinical course of cow’s milk protein allergy/intolerance and atopic diseases in childhood. Pediatr. Allergy Immunol. Suppl. 2002, 13, 23–28. https://doi.org/10.1034/j.1399-3038.13.s.15.7.x. [CrossRef]
- Ambroszkiewicz, J., Gajewska, J., Chełchowska, M. et al. Assessment of inflammatory markers in children with cow’s milk allergy treated with a milk-free diet. Nutrients 2021, 13, 1–13 . https://doi.org/10.3390/nu13041057. [CrossRef]
- Anvari, S., Miller, J., Yeh, C. et al. IgE-Mediated Food Allergy. Clin. Rev. Allergy Immunol.2019, 57, 244–260 https://doi.org/10.1007/s12016-018-8710-3. [CrossRef]
- Ferrara, M., Coppola, L., Coppola, A., et al. Iron deficiency in childhood and adolescence: retrospective review. Hemat. 2006, 11:183–6, https://doi.org/10.1080/10245330600775105. [CrossRef]
- Trillo Belizón, C., Ortega Páez, E., Medina Claros, AF., et al. Faecal calprotectin as an aid to the diagnosis of non-IgE mediated cow’s milk protein allergy. An. Pediatr. 2016 84:318, https://doi.org/10.1016/j.anpedi.2015.07.007. [CrossRef]
- Mori, F., Barni, S., Cianferoni, A., et al. Cytokine expression in CD3+ cells in an infant with food protein-induced enterocolitis syndrome (FPIES): case report. Clin. Dev. Immunol. 2009;67:81-93. https://doi:10.1155/2009/ 679381. [CrossRef]
- Zhang, S., Sicherer, S., Berin, M.C., Pathophysiology of Non-IgE-Mediated Food Allergy. Immunotargets Ther. 2021, 10, 431–446. https://doi.org/10.2147/ITT.S284821. [CrossRef]
- Berni Canani, R. De Filippis, F., Nocerino, R. et al. Gut microbiota composition and butyrate production in children affected by non-IgE-mediated cow’s milk allergy. Sci. Rep. 2018, 8, 1–10 https://doi.org/10.1038/s41598-018-30428-3. [CrossRef]
- Labrosse, R., Graham, F. & Caubet, J. C. Non-ige-mediated gastrointestinal food allergies in children: An update. Nutrients 2020, 12, 1–28 https://doi.org/10.3390/nu12072086. [CrossRef]
- Burris, A.D., Burris, J., Cow’s Milk Protein Allergy in Term and Preterm Infants: Clinical Manifestations, Immunologic Pathophysiology, and Management Strategies. Neoreviews. 2020, 21(12):e79, https://doi.org/10.1542/neo.21-12-e795. [CrossRef]
- Burks, W.; Tang, M., Sicherer, S. et al. ICON: Food allergy. J. Allergy Clin. Immunol. 2012, 129, 906–9, https://doi.org/10.1016/j.jaci.2012.02.001. [CrossRef]
- Boyce, J.A., Assa’ad, A., Burks, A.W., et al. NIAID-Sponsored Expert Panel. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010, 126(6 Suppl), S1–S58. https://doi.org/10.1016/j.jaci.2010.10.007. [CrossRef]
- Vandenplas, Y.; Bajerova, K.; Dupont, C. et al. The Cow’s Milk Related Symptom Score: The 2022 Update. Nutrients,2022, 14(13), 2682. https://doi.org/10.3390/nu14132682. [CrossRef]
- Saarinen, K.M., Pelkonen, A.S., Mäkelä, M.J., Clinical course and prognosis of cow’s milk allergy are dependent on milk-specific IgE status. J Allergy Clin Immunol. 2005, 116(4), 869–875. https://doi.org/10.1016/j.jaci.2005.06.018. [CrossRef]
- Isaksen, B., Calprotectin inhibits matrix metalloproteinases by sequestration of zinc. Mol Pathol. 2001, 54(5), 289–292. https://doi.org/10.1136/mp.54.5.289. [CrossRef]
- Stríz, I.; Trebichavský, I. Calprotectin—A Pleiotropic Molecule in Acute and Chronic Inflammation. Physiol. Res. 2004, 53(3), 245–253.
- Canani, R.B., de Horatio, L.T., Terrin, G., et al. Combined use of noninvasive tests is useful in the initial diagnostic approach to a child with suspected inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2006, 42(1), 9–15. https://doi.org/10.1097/01.mpg.0000187818.76954.9a. [CrossRef]
- von Roon, A.C., Karamountzos, L., Purkayastha, S., et al. Diagnostic precision of fecal calprotectin for inflammatory bowel disease and colorectal malignancy. Am J Gastroenterol. 2007, 102(4), 803–813. https://doi.org/10.1111/j.1572-0241.2007.01126.x. [CrossRef]
- Chang, M.H., Chou, J.W., Chen, S.M., et al.. Faecal calprotectin as a novel biomarker for differentiating between inflammatory bowel disease and irritable bowel syndrome. Mol Med Rep. 2014 10(1), 522–526. https://doi.org/10.3892/mmr.2014.2180. [CrossRef]
- Berni Canani, R., Rapacciuolo, L., Romano, M.T., et al. Diagnostic value of faecal calprotectin in pediatric gastroenterology clinical practice. Dig Liver Dis. 2004, 36(7), 467–470. https://doi.org/10.1016/j.dld.2004.02.009. [CrossRef]
- Xiong, L. J., Xie, X. L., Li, Y. et al. . Current status of fecal calprotectin as a diagnostic or monitoring biomarker for cow’s milk protein allergy in children: a scoping review. World J. Pediatr. 2021, 17, 63–70 https://doi.org/10.1007/s12519-020-00364-2. [CrossRef]
- Vandenplas, Y., Broekaert, I., Domellöf, M., et al. An ESPGHAN Position Paper on the Diagnosis, Management, and Prevention of Cow’s Milk Allergy. J ped gastr nut, 2024, 78(2), 386–413. https://doi.org/10.1097/MPG.0000000000003897F. [CrossRef]
- Lieberman, J.A. S. & Sicherer, S. H. Diagnosis of food allergy: epicutaneous skin tests, in vitro tests, and oral food challenge. Curr Allergy Asthma Rep 2011,Feb;11(1): 58–64. https://doi.org/10.1007/s11882-010-0149-4. [CrossRef]
- Ricciuto, A. & Griffiths, A. M. Clinical value of fecal calprotectin. Critical Reviews in Clinical Laboratory Sciences 2019, 56(5), 307–320. https://doi.org/10.1080/10408363.2019.1619159. [CrossRef]
- Ayling, R. M. & Kok, K. Fecal Calprotectin. Adv. Clin. Chem. 2018, 87, 161–190. https://doi.org/10.1016/bs.acc.2018.07.005. [CrossRef]
- Zhu, Q., Li, F., Wang, J. et al. Upregulation of calprotectin in mild IgE-mediated ovalbumin hypersensitivity. Oncotarget 2017,8, 37342–37354 https://doi.org/10.18632/oncotarget.16954. [CrossRef]
- Baldassarre, M. E. et al. Lactobacillus GG Improves Recovery in Infants with Blood in the Stools and Presumptive Allergic Colitis Compared with Extensively Hydrolyzed Formula Alone. J. Pediatr. 2010, 156, 397–401 https://doi.org/10.1016/j.jpeds.2009.09.012. [CrossRef]
- Belizón, C. T. et al. Calprotectina fecal como apoyo al diagnóstico en la alergia a las proteínas de leche de vaca no IgE mediada. An. Pediatr.2016, 84, 318–323.
- Beşer, Ö. F. et al. Can fecal calprotectin level be used as a markers of inflammation in the diagnosis and follow-up of cow’s milk protein allergy? All. asth. & imm. res, 2014, 6(1), 33–38. https://doi.org/10.4168/aair.2014.6.1.33. [CrossRef]
- Zhang, Z. H., Wang, W., Zhang, X. H. et al. Fecal Calprotectin in Children with Cow’s Milk Protein Allergy: A Systematic Review and Meta-Analysis. Int. Arch. All. Immunol. 2022, 183, 1189–1197 https://doi.org/10.1159/000525961. [CrossRef]
- Qiu, L., Wang, J., Ren, F. et al. Can fecal calprotectin levels be used to monitor infant milk protein allergies? Allergy, Asthma Clin. Immunol. 2021, 17(1), 132. https://doi.org/10.1186/s13223-021-00636-0. [CrossRef]
- Roca, M. Donat, E., Rodriguez Varela, A.,et al. Fecal calprotectin and eosinophil-derived neurotoxin in children with non-ige-mediated cow’s milk protein allergy. J. Clin. Med. 2021, 10(8), 1595. https://doi.org/10.3390/jcm10081595. [CrossRef]
- Koninckx, C. R. Donat, E., Benninga, M. A., et al. The Use of Fecal Calprotectin Testing in Paediatric Disorders: A Position Paper of the European Society for Paediatric Gastroenterology and Nutrition Gastroenterology Committee. J. Pediatr. Gastroenterol. Nutr. 2021, 72(4), 617–640. https://doi.org/10.1097/MPG.0000000000003046. [CrossRef]
- Merras-Salmio, L., Kolho, K. L., Pelkonen, A. S., et al. Markers of gut mucosal inflammation and cow’s milk specific immunoglobulins in non-IgE cow’s milk allergy. Clin. Transl. All. 2014, 4(1), 8. https://doi.org/10.1186/2045-7022-4-8. [CrossRef]
- Lendvai-Emmert, D., Emmert, V., Makai, A., et al. Fecal calprotectin levels in pediatric cow’s milk protein allergy. Front. Pediatr. 2022, 10, 945212. https://doi.org/10.3389/fped.2022.945212. [CrossRef]
- Zain-Alabedeen, S., Kamel, N., Amin, M., et al. Fecal Calprotectin and Cow’s Milk-Related-Symptoms Score in Children with Cow’s Milk Protein Allergy. Pediatr. Gastroenterol. Hepatol. Nutr. 2023, 26(1), 43–49. https://doi.org/10.5223/pghn.2023.26.1.43. [CrossRef]
| Patients’ characteristics | CMA (N=51) | |||
| Boys/girls | 34/17 (67/33%) | |||
| Age (years, median [IQR] | 1,3 [0.7-2.2] | |||
| Weight (kg)- [median] | 10.5 [8,8,12.8] | |||
| Height (cm) [median] | 78.0 [73.5, 90.5] | |||
| Diagnosis of CMA (IgE/non IgE) | 25/26 (49/51%) | |||
| IgE tot (elevated/not elevated) | 22/28 (56/ 44%) | |||
| IgE milk (elevated/not elevated) | 29/21 (58/42%) | |||
| IgE alpha-album (elevated/not elevated) | 13/37 (74/26%) | |||
| IgE beta-lactoglobulin (elevated/not elevated) | 18/55 (25/75%) | |||
| IgE casein (elevated/not elevated) | 14/36 (28/72%) | |||
| Symptoms | ||||
| Vomiting | 20 (44%) | |||
| Dhiarrea/Constipation | 37 (73%) | |||
| Abdominal pain | 21 (41%) | |||
| Skin involvement (urticaria, dermatitis) | 38 (75%) | |||
| Calprotectin (mg/kg) (median, [IQR]) | 30 [15.5, 63.9] | 16.1 [10.0, 30.0] | ||
| ComiSS | 8.5 [6.0, 13.0] | 3.0 [2.0, 5.0] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





