1. Introduction
The word “fluorescence” was first coined in 1852 when George Gabriel Stokes published a paper on the change in the refrangibility of light, demonstrating how quinine reacts with ultraviolet light to produce a blue color [
1]. It is typically short-lived (a few nanoseconds) and does not involve intersystem crossing, which is observed when an excited molecule or atom relaxes to a lower energy state by emitting a photon without reversing its spin multiplicity [
2]. Fluorescence emission is intimately linked to the processes of excitation and emission: a photon of a particular wavelength is absorbed by a molecule, which, in turn, excites the molecule to a higher energy state. Subsequently, longer wavelength photons are re-emitted, which is an essential feature of most natural products with inherent optical characteristics [
3]. The photon energy emitted by the molecule is always less than the absorbed photon due to the non-radioactive decay (internal conversion) so, the efficiency or quantum yield of the molecule is always less than 1. Fluorescence is the strongest in the molecule with quantum yield 1. Nevertheless, the molecules having a quantum yield of φ = 0.1 are also quite fluorescent.
Naturally derived fluorescent compounds have attracted increasing attention due to their widespread applications in surgical molecular navigation, live cell imaging [
4], biosensing [
5], chemical sensing, and protein tagging [
6]. Their affinity for biological macromolecules and fluorescence characteristics can justify their use in these different applications [
7]. These affordable and easy-to-use probes can be employed in bioimaging to track and monitor
in-vivo physiological responses in disease situations, identify particular biomolecules, and specifically understand cellular and tissue morphology without invasive methods [
8]. Important groups of fluorescence probes are built on the fundamental structures of secondary metabolites of living organisms, such as coumarins, acridines, quinolones, azulenes, and anthraquinones [
9]. The structure of a fluorescent compound plays a crucial role in its optical properties. For instance, long alkyl chains and halogen atoms produce a high-contrast vibrant mechanical response that may be applied for different applications, including color painting [
10].
Natural products with fluorescent properties can be utilized in fluorescent probes, which have significantly improved the study of cell biology and chemical biology, leading to advancements in biomedical research [
11]. While synthetic modifications and variations in functional groups can enhance the properties of fluorescent compounds, not all natural products are suitable for conversion into biochemically active probes. Epicocconone is a rare but great illustration of how natural fluorescent compounds are not easily converted into biochemical probes although many fluorescent dyes are generated from natural substrates because of their low quantum yield and photostability [
12]. In contrast, some synthetic analogs, such as pyrene, carbostyril 124, and 1,2,4-trioxolane are frequently used as fluorescent agents, because of their higher photophysical qualities and have demonstrated promising results in biomedical applications [
13].
Cancer cells express numerous surface proteins that are generally absent in normal healthy cells. These proteins enable cancer cells to proliferate through alternative metabolic pathways and metastasize from one tissue to another. Many such targets are used for therapeutic intervention but can also serve as diagnostic markers, making them amenable to antibodies, small molecules, and aptamer-based fluorescence targeting and imaging approaches. Fluorescent probes can be valuable in three primary aspects of cancer biology: early cancer diagnostics using cancer biomarkers, monitoring of therapeutic agents to assess their on-target and off-target effects, and evaluating bodily responses to therapeutics, including activation of physiological pathways and immune cell activity [
14]. Yao et al. synthesized and analyzed the pharmacological efficacy and subcellular distribution of some fluorescent 23-hydroxybetulinic acid (HBA) derivatives coupled with coumarin dyes [
15]. Together, these findings provide a fresh perspective on the molecular basis of the potential of the natural substance 23-HBA for skin cancer treatment. Recently, Liu et al. cataloged the inherent fluorescence of substances, such as oils, wines, and honey as an innovative method of combating food fraud. They created a tiny library of fluorescence fingerprints by mapping the distinctive fluorescence of each product over a range of wavelengths, which helped in analyzing the quality of the product [
16]. Moreover, the fluorescent probe-based approach is one of the most effective methods for imaging biological samples in real-time and detecting metal ions and bioactive compounds [
17]. For example, in addition to its anticancer effect, curcumin serves as a fluorescent probe for monitoring noisome migration within cells, which is advantageous for researching its fluorescence-switching abilities [
18]. The high surgical recurrence incidence of pancreatic ductal adenocarcinoma, a lethal aggressive tumor, can be observed and analyzed using a cellular mesenchymal-epithelial transformation factor-targeted near-infrared fluorescence probe [
19].
In addition, natural product-derived fluorescent products have shown analytical utility; for example, Xu et al. prepared widely diffuse and water-soluble fluorescent nanodots from potatoes with quantum yield (0.15) and intense blue fluorescence that can be used as a novel sensing probe for accurate and focused detection of Fe
3+ ions in lake water [
20]. In this review, we discuss recent developments of small fluorescent probes based on natural products and their synthetic analogs that have significant applications in biological, biochemical, and biomedical fields.
2. Natural Fluorescent Compounds
Since ancient times, scientists have been captivated by the usefulness of naturally occurring tiny molecules generated from plants. These organisms have inherent optical properties and fluorescence emission and are useful for tracking medications in biological systems and providing spatial information [
3].
Tryptophan (Trp), tyrosine (Tyr), and phenylalanine (Phe) are the inherent aromatic amino acids that primarily cause the fluorescence signals from folded proteins. The signals from these aromatic amino acids have been consistently investigated to assess protein behavior, such as folding and unfolding, dynamics, pH and temperature dependence, cellular functions, sensing in an aqueous environment, etc. Despite that, the low molar extinction coefficient, low quantum yield, and sensitivity to the environment limit the utilization of these natural fluorophores in
in-vivo applications [
21,
22,
23]. The first naturally occurring fluorescent protein produced by living organisms was green fluorescent protein (GFP), which acts as a light-emitting agent and is widely used in cell imaging and
in-vitro and
in-vivo assays. It was first discovered in the jellyfish
Aequorea victoria and thereafter has been found in other organisms, including the sea pansy
Renilla reniformis and many coelenterates [
24,
25]. When expressed in prokaryotic
(Escherichia coli) or eukaryotic (
Caenorhabditis elegans) cells, the complementary DNA for the protein results in a fluorescent product, which glows without the need for external substrates or cofactors; thus, its expression can be utilized to track the expression and localization of proteins in living organisms [
26]. In addition, it is frequently employed as a marker of gene expression due to its stability and ability to generate chromophores after autocatalytic cyclization [
27]. Modified GFP probes such as GCaMPs have been widely used for monitoring the real-time activity of Ca
2+ ions in both cellular and subcellular settings [
28]. The ability to visualize and investigate the processes and steps of metastasis is complicated and mysterious, and the key to breaking the chain of cancer can be facilitated by using GFP as a tagging agent [
29]. Proteins can be fluorescently tagged with GFP at their N or C-termini for visualization. In addition to GFP, small molecule fluorescent probes are utilized to identify and quantify reactive oxygen and nitrogen species in redox biology and biochemistry. They can function as sensors for the direct assessment of biological events, including pH changes and the concentrations of different cellular ions [
30]. Although the proteins that can be genetically altered have brought a revolution
in vitro and
in vivo studies, they typically have lower luminescence and photo-stability, compared to synthetic fluorescent compounds. To improve these properties, photo stabilizers that covalently connect within the fluorescent proteins have been rediscovered, enhancing the photophysical characteristics of fluorescent proteins [
31].
Moreover, curcumin, coumarin, quinine,
R-phycoerythrin, and perylene are some of the fluorescent compounds isolated as natural products and used for various purposes. Curcumin found in turmeric can inhibit the mutagenesis of DNA and can also be used to visualize functionalized graphene nanosheets, which in turn can be used in various applications, including disease diagnosis, bioimaging, drug delivery and so on.[
32,
33,
34]. Coumarin can be isolated from
Cortex fraxinus and can be used in the detection of metal ions such as Cu
2+, Hg
2+, Mg
2+, and Zn
2+ [
35,
36]. Similarly, quinine, found in rhizomes of
Polygonatum verticullatum, can be used as a fluorescent tracer and is used to estimate a range of soil surface conditions and environments [
35,
37]. In addition, it was also the first fluorescent compound to be used as an antimalarial agent and was discovered by John Frederick William Herschel in 1845 after noticing the emission from an aqueous quinine solution. Since then, it has been used as a fluorescence standard [
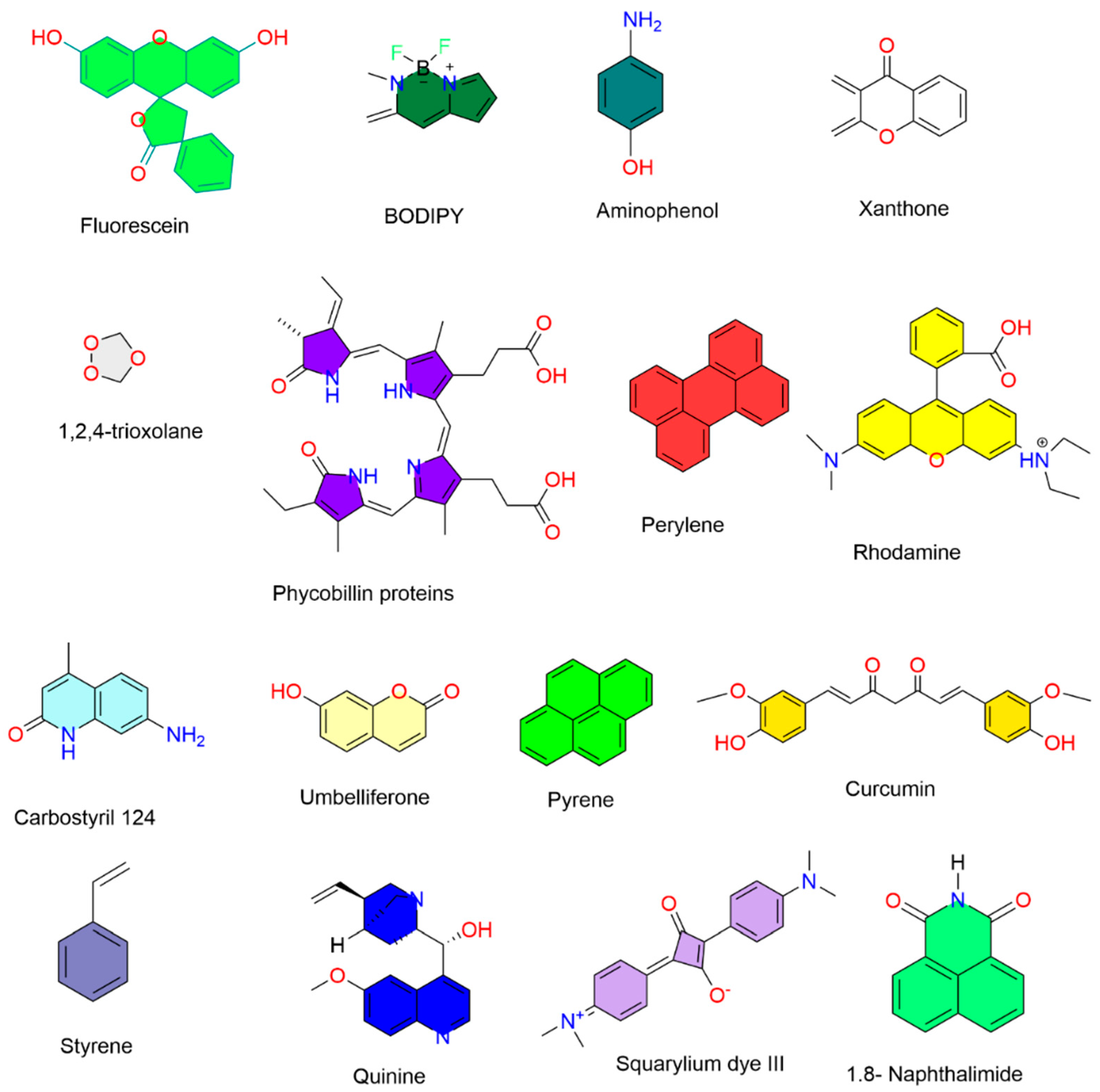
38]. In the same way, the photophysical properties and applications of the sixty-five fluorescent compounds are shown in
Table S1, the chemical structures of the fluorescent compounds reviewed in this article are shown in
Figure S1, the structures of sixteen important naturally fluorescent compounds and their applications are listed in
Table 1, and their chemical structures are shown in
Figure 1.
3. Chemical Structure-Driven Fluorescence Properties
The fluorescence properties can be optimized by altering the chemical structure. For example, the introduction of a long-chain alkyl group enhances optical properties and transforms the donor-acceptor interaction through intramolecular charge transfer (ICT), which regulates the sensitivity of the substance [
10]. Similarly, varying the positions of substituents affects intermolecular interactions, imparting a stronger response to various triggers [
59].
The biological activity of naturally occurring electrophilic substances along with Michael acceptors, such as ring-strained forms such as lactones, lactams, epoxides, or other moieties, is the result of their inherent reactivity with cellular nucleophiles such as cysteine, threonine, serine, or lysine residues, influencing the pathway of finding novel therapeutic targets [
60]. In biomedicine, small fluorescent probes are frequently utilized in processes such as ICT, photoinduced electron transfer (PET) [
61], excited-state intramolecular proton-transfer (ESIPT), stimulated emission depletion (STED) [
62], fluorescence (or Förster) resonance energy transfer (FRET) [
63], fluorescence correlation spectroscopy (FCS), twisted intramolecular charge transfer (TICT) multiphoton fluorescence, and electron donor-acceptor phenomena. These probes exhibit diverse electrical and spectral characteristics and have been proven to have great selectivity and a variety of applications [
64].
The spectral characteristics of fluorescent probes, including wavelength, intensity, and photostability of the fluorescence emission, can be altered by changing the functional groups and molecular structure of the compound [
65]. The absorption of UV or visible light by a conjugated π system requires an energy-matching
-
* energy gap, enabling the excitation of a Highest occupied molecular orbital (HOMO) π electron into the π* (antibonding) orbital or lowest unoccupied molecular orbital (LUMO) orbital. The conjugation of a compound does play a certain role in fluorescence emission. Increased conjugation leads to longer fluorescence emission, which often seems to be correlated with the linkage between alternate double and single bonds, which is related to the energy gap, as illustrated in
Figure 2(A) [
66]. Additionally, the presence of single-bond (sp
3) carbon atoms influences the properties of conjugation through hyperconjugation, albeit to a lesser extent than conjugation and aromatic stabilization [
67].
Figure 2(B) shows how different metabolites demonstrate different fluorescent emissions. However, there are conditions under which this relationship is exactly reversed, such as when a high proportion of the sp
2 domain results in a shorter emission wavelength [
67,
68]. For instance, the extended amount of sp
2 domain in graphene-based materials might subject it to quantum confinement, resulting in the opening of a band gap [
69]. This finding implies that other factors also play a role in controlling the fluorescence emission. The other factors might include the two common forms of the sp
2 domain, zigzag and arm-chair form. Even if the sp
2 domains are the same, the armchair edges are found to have shown short wavelength emission, while the zigzag ones show longer emission [
70].
The change in the photophysical properties due to differences in substitutions is very complex. It differed due to many effects, including the conjugation effect, steric effect, structure effect, and many more. There are instances, where the mobility of electrons gets directly affected by different substituents, the one which enhances the mobility, will also increase the fluorescence emission and vice versa [
71].
In the study conducted by She et al., structure-property correlations were determined, and the fluctuations in optical characteristics due to substituents imparting electronic effects and steric effects were examined in rhodamine derivatives [
72]. The results revealed that, in comparison to electron-releasing groups, electron-withdrawing groups increased fluorescence to a greater extent, as shown in
Figure 2(C); however, this effect was not observed when the substituents were introduced at the meta- or para-positions of the benzene ring. Electron withdrawing groups like the nitro groups affect the photophysical properties of fluorophores by increasing the delocalization of π-electrons in the aromatic ring [
73]. When nitro groups are subjected para to the electron-releasing group, electron displacement occurs, resulting in a change in the difference between the HOMO and LUMO, which in turn changes the dipole moment and affects the photophysical properties, as in 4(5)-nitroimidazole and 5(6)-nitro benzimidazoles. Furthermore, the brightness of chromophores that consist of both electron-releasing and electron-withdrawing groups is enhanced by polarizing the π-system. Additionally, when a larger alkyl group is added to the side chain of the amino acid moiety of the fluorescent brightening agent, the fluorescence intensity of the compound increases [
74]. Furthermore, the fluorophore's brightness and absorption/emission wavelengths can be significantly impacted by the enclosing cavity of the fluorophore because of the creation of a solvation shell [
75]. The creation of aggregates enhances the sensitivity of ESIPT emissions. Attaching the extra fluorescents group to an ESIPT with FRET allows for the development of longer wavelength near-infrared probes, which may detect physiologically relevant analytes in vivo [
76]. Moreover, the photophysical properties and other fluorescence characteristics vary in terms of photobleaching, self-quenching, photostability, and solubility [
77]. Certain carbocyanine dyes have the potential to self-quench, so changes to the carbocyanine structure lead to molecules that glow significantly on proteins, nucleic acids, or other biopolymers, comparable to cyanine dyes with incredible photostability [
78]. Hence, the structure of a compound has a crucial relationship with its photophysical properties, and consequently, in biomedical applications,
Figure 2 shows the relationships between the different substituents and structures and their activity.
4. Applications for Natural and Synthetic Fluorescent Compounds
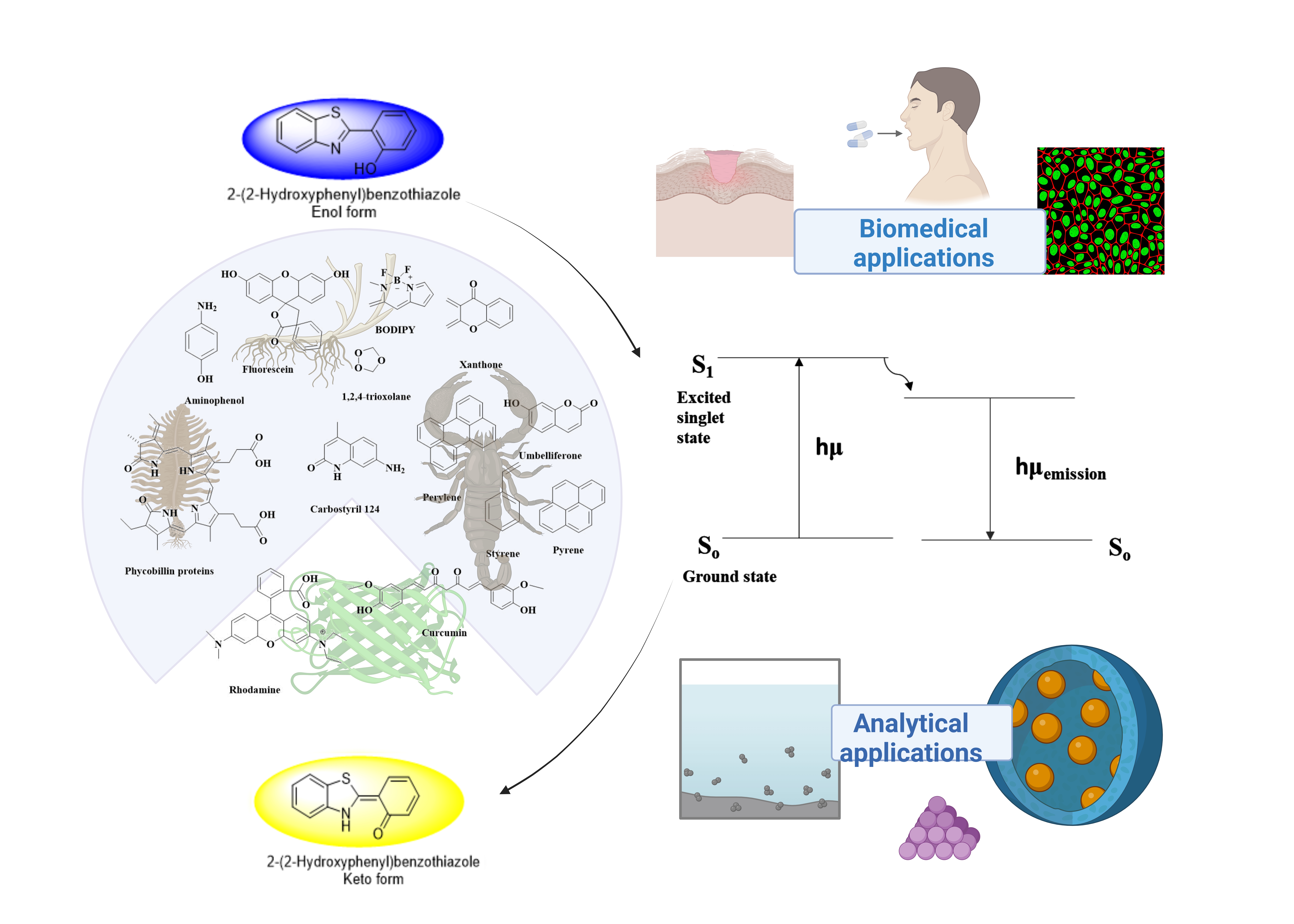
Fluorescent probes and organic dyes derived from natural products and their synthetic analogs have immense potential in biomedicine, environmental monitoring, and food chemistry for detection and imaging, including ion monitoring, physiological and pathological processes in living organisms, tissue penetrations, and in vivo biomarker multimodal imaging [
79]. In this section, the applications of fluorescent natural products and their synthetic analogs are mentioned and categorized into biomedical and analytical.
4.1. Biomedical Applications
Fluorescent compounds have extensive biomedical applications, particularly in cell imaging, medical diagnostics, biosensing, drug discovery, in vivo imaging, disease diagnostics, therapeutics, and theranostics, as shown in
Figure 3. Therefore, natural and synthetic fluorescent compounds span a wide field of research, from basic research to clinical diagnostics and therapy [
79]. These compounds are extensively used in biomedicine due to their biocompatibility, specificity, sensitivity, low toxicity, good water solubility, and eco-friendliness; for example, the use of 1,8-naphthalimide, perylenediimide, morpholine, or N-methylpiperazine improves water solubility, making them appropriate for biomedical applications [
80,
81].
Moreover, fluorescent probes based on ICT, TICT, PET, ESIPT, and FRET are specifically applicable in the biomedical field via distinct mechanisms [
82]. The following are some biomedical applications of fluorescent natural products:
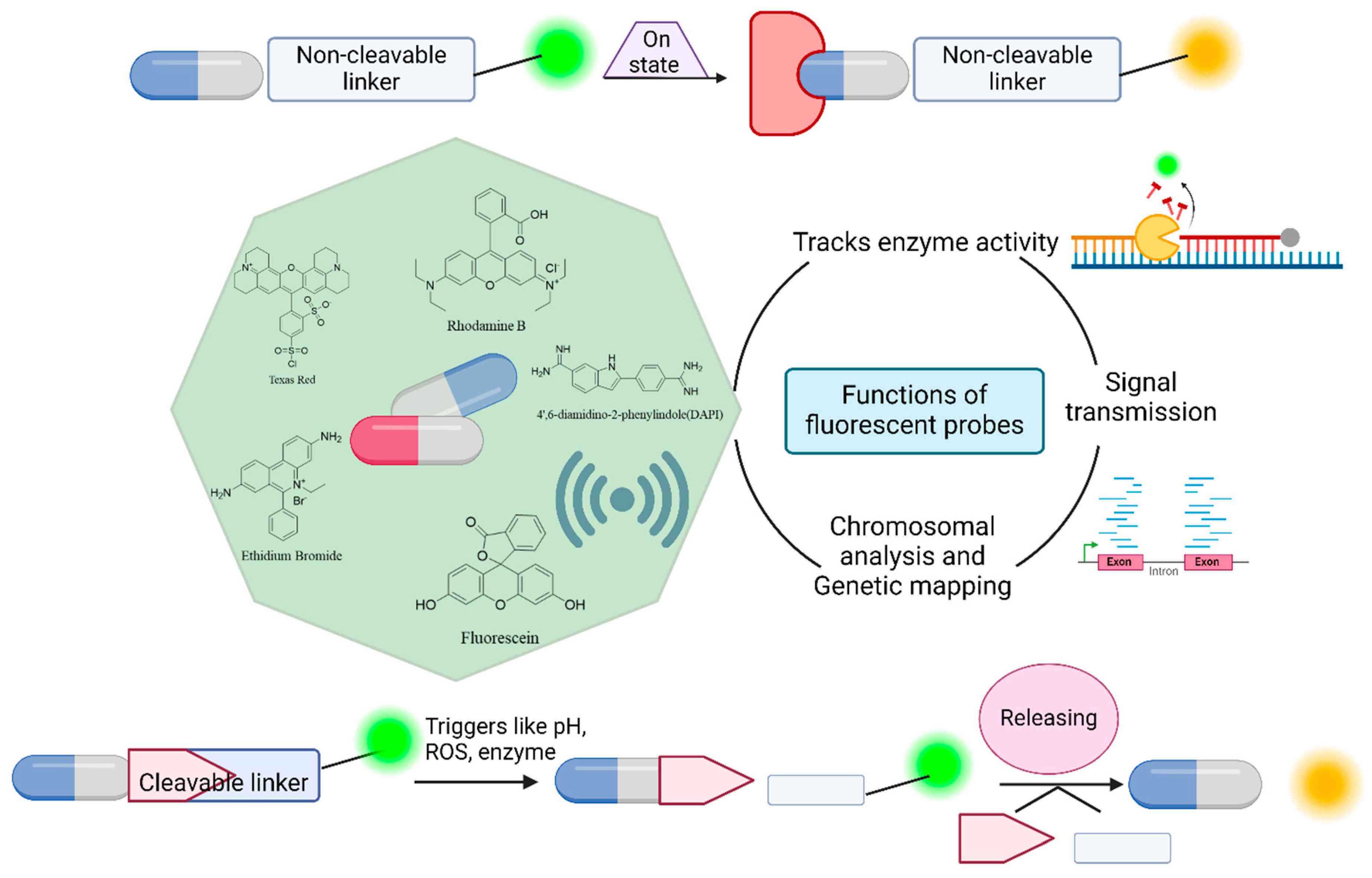
4.1.1. Drug Delivery System and Drug Analysis
The use of fluorescent compounds as probes is crucial for monitoring real-time drug delivery systems. These compounds serve as valuable tools for tracking drug distribution and movement within the body, providing essential information that enhances the standard of medical treatment [
83]. In the drug delivery process, a fluorescent probe is attached to the drug either through a biodegradable linkage or through a nondegradable linkage to form cleavable conjugates or non-cleavable conjugates, respectively, and the cleavable form exhibits dramatic changes in spectral properties when the bond is broken. In contrast, non-cleavable conjugates do not break down even during the drug delivery process [
82]. Under these conditions, the drug is not released because it is in the ‘off’ state, and upon discharge of the medication, the dye produces a fluorescence signal that transforms into an “on” state, as shown in
Figure 4 [
84]. Therefore, real-time study of receptor kinetics during drug delivery is made possible by the application of GFP. Using fluorescence-activated cell sorting (FACS), the released fluorescence may be identified which can be used to sort cells based on biomarkers of interest. This analysis provides valuable information about cell biology without costly mRNA analysis, risky radiolabeled binding experiments, or staining processes [
85]. Fluorescent labeling can help to monitor the drug delivery process by providing information about material transport and distribution throughout the body [
83].
Fluorescent probes have also been found to overcome the problems that arise with the drug delivery process. For example, in the ocular area, fluorescent probes are used to cover the nanocarrier surface, allowing nanosystems to be used as light probes and allowing the formulation of strategies that comprehend the route by which nanocarriers enter the ocular lens [
82]. Recently, Wang et al. developed a probe that couples fluorescent dyes and the protein human serum albumin and exploited the ability of the protein to bind the drug by studying the interaction of tamoxifen (a drug used for breast cancer treatment) with the probe [
86]. This allowed them to study the movement and delivery of the drug, which consequently allowed them to quantify the amount of the drug within the cells. Therefore, they concluded that these fluorescent probes present encouraging opportunities for therapeutic medication dosage recommendations.
Currently, anticancer nanomedicines are efficient for chemotherapy, and fluorescent dyes are added to the surface of nanoparticles (NPs) to enable fluorescence tracing in cells [
87]. For example, green fluorescent fluorescein isothiocyanate-grafted silica NPs were used for in vivo drug administration [
88]. 1,8-Naphthalimide-based fluorescent probes can conjugate chemically with 3-hydroxybenzyl and are successfully used for the detection of tyrosinase enzymes [
80]. This polyphenolic enzyme can be taken as an alarming point of many diseases, such as Alzheimer's disease, Parkinson's disease, and skin cancer [
82].
4.1.2. Optical Sensing and Metabolite Pathway Analysis
Optical sensors are among the most versatile platforms for personalized health parameter monitoring and can completely change the way certain diseases are identified and managed because of the correlation of the variations in light released following excitation with higher energy photons to the presence or absence of a target analyte [
89]. Some commercially available synthetic dyes, such as propidium iodide and propidium azide, are applicable to perform cell viability tests. However, these dyes can be equally toxic and expensive and demonstrate photobleaching effects. Therefore, to overcome these problems, carbon dots from the leaf extract of
Prosopis juliflora can be used for biological applications in cellular imaging studies [
90]. Moreover, fluorescent dyes can be used to monitor enzyme activity, signal transmission, protein transport, cellular integrity, membrane mobility, exocytosis, and endocytosis, along with chromosomal analysis and genetic mapping [
6,
91]. Natural compounds such as quinidine, quinine, cinchonine, and cinchonidine were presented as combinatorial fluorescent molecular logic gates in a study conducted by Agius and Magri [
92], and these fluorescent logic gates can be used as diagnostic criteria for elevated chloride levels in body fluids [
93]. Some natural and unnatural amino acids also exhibit various fluorescent properties based on the attachment with substituents at various positions on the amino acid [
94,
95]. These are widely used in modern cell biology, such as for gene encoding and designing of novel artificial proteins along with the evaluation of cell properties using optical imaging, dynamics, folding, and biomolecular interactions in proteins [
96]. Each enzyme carries out its function, and any changes in its activity invite various serious diseases; therefore, the development of fluorescent probes that permit real-time analysis, allowing straightforward and harmless observation of enzyme activity, might help in analyzing the causes of disease by elucidating metabolic pathway [
97]. It is easier to comprehend the functions of a cell when metabolic activity in the cell is detected. A fluorescent probe, coumarin with an N-(2-hydroxyethyl) carboxamide group at position 3, is capable of fluorescence imaging of fatty acid beta-oxidation activity in the mitochondria of live cells [
98]. Thus, fluorescent compounds can be used for real-time analysis of the concentrations of metabolites and can be used for understanding metabolic processes.
4.1.3. Biomarkers and Cell Imaging
A biomarker is an indicator of a disease or condition typically expressed in the form of biological change, biochemical activity, or cellular processes that can be detected through various physical, chemical, and biochemical methods. Biomarkers can be identified and measured from cell-conditioned media, bodily fluids, tissue specimens, and physical measurements of living systems [
99]. They play an important role in the early detection of diseases. Here, fluorescent probes can identify targets and provide detectable signals indicating aberrant activity [
97]. They are instrumental in monitoring the progression of diseases such as Parkinson’s disease, which is characterized by the unusual build-up of syn-nuclei, oxidative stress due to monoamine oxidase-B (MAO-B)-catalyzed oxidation, impaired mitochondrial function, and neurotransmitter deficits [
100]. To detect this change, different fluorophores, such as coumarin, thioflavin-T, hydroxychrome probes, benzothiazole, benzylidene-imidazolinone, and benzofuranone-based probes, are used. These probes serve as biomarkers by using different mechanisms, including ESIPT, FRET, protein labeling, PET, ICT, structural rearrangement, and conformation modification strategies [
76].
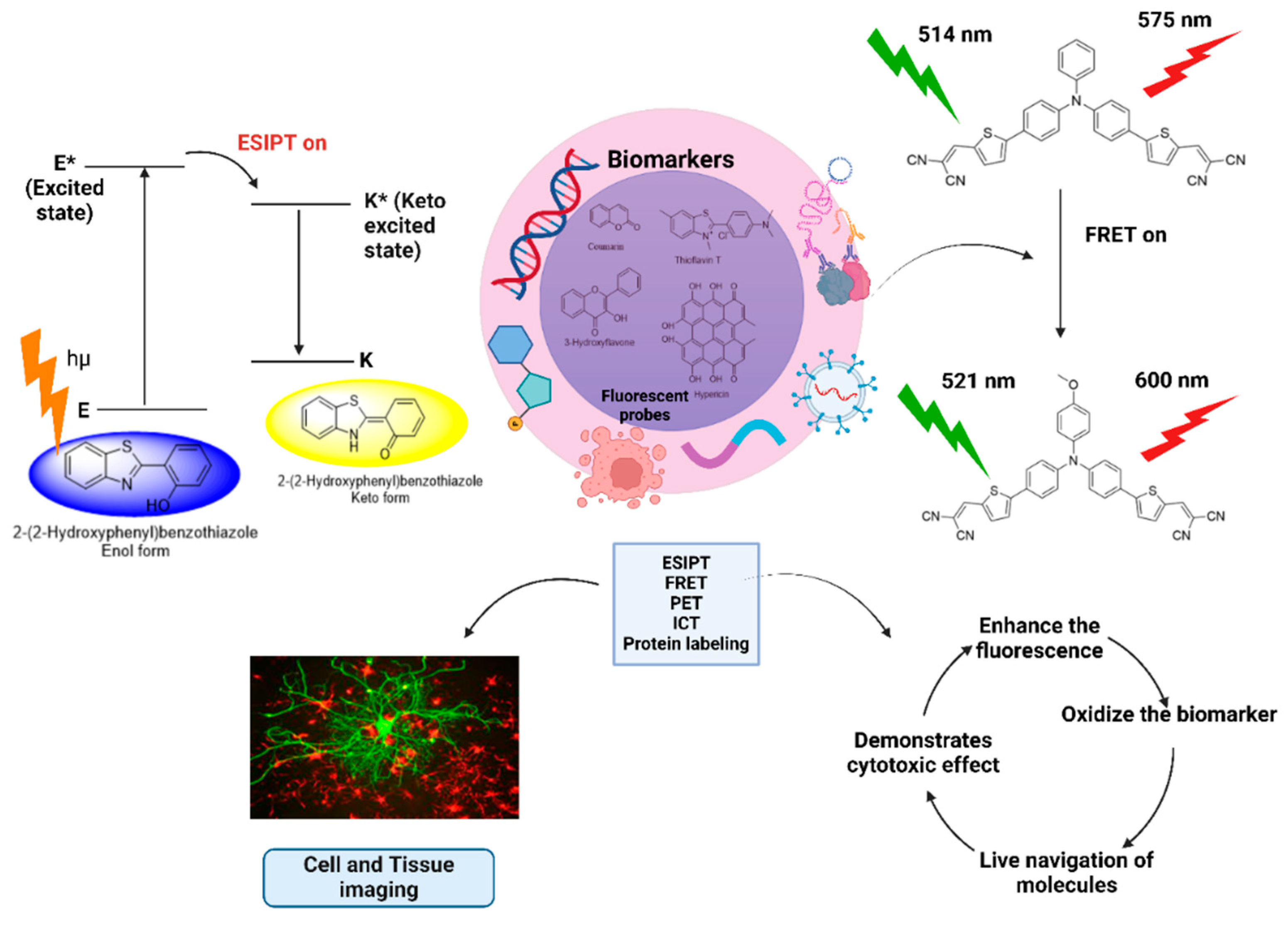
ESIPT involves hydrogen bonding between proton donors, such as -OH, -SH, and -NH
2, and proton acceptors, such as -C=O and C=N [
101]. When light is imparted, the ESIPT sources absorb radiation, jump to a more excited state, and create a photoisomerization phenomenon to create the excited state of its tautomeric form (Keto-enol tautomers). The electron-withdrawing nature of the keto form causes the decay of the excited state, the longer fluorescence is produced, and biomarker detection makes significant use of this phenomenon, as shown in
Figure 5 [
76]. The main strategy for using an ESIPT-based fluorophore is to modify the ESIPT process by first replacing the proton of the proton donor, due to which a short wavelength of the probe from the enol form is released. After interacting, a particular biomarker may initiate the ESIPT process to produce long-wavelength light, which produces different fluorescence [
102], aiding in biomarker detection.
Odyniec et al. created a variety of 3-hydroxy flavone (3-HF) ESIPT boronate fluorescent probes to detect ONOO
- to observe various forms of amyloid-β aggregation [
102]. Similarly, research carried out to determine the efficiency of a new fluorophore, Pl-Cys, a unique ESIPT dye connected to an acrylate group through a hydroxyl moiety, revealed that in the presence of Cys, the acrylate moiety split off from Pl-Cys and Cys and, in turn, interacts with Pl-Cys, triggering the ESIPT phenomenon. This consequently imparted 20 times more fluorescence, which could aid in the detection of various diseases [
103].
Biomolecules in living systems can be modified to exhibit fluorescence, which can facilitate early cancer detection by serving as biomarkers. Biomarkers linked with cancer can be identified based on the abnormal nature of DNA ( DNA tetrahedron as a nanotweezers for the detection of cancer-linked mRNA), and aptamers or antibodies conjugated tetrahedron nanostructures (proteins, RNA, lipids), which specifies cancer states [
104]. Dual-emission ratiometric fluorescence probes are extensively used in the bioimaging of biomarkers for accurate, quantifiable, and real-time studies [
105]. This probe allows us to observe the variations in distinct fluorescence color types that are even visible to the naked eye and correspond to changes in content in detection systems, which helps in easy, quick, and effective target identification [
106]. For example, a coumarin-benzopyrylium-based ratiometric fluorescent probe near infra-red turn-on has been utilized for the detection of Cu(II) at the subcellular level in living cells [
107]. Live surgical molecular navigation, which uses fluorescently labeled markers, allows tumors and nerves to be displayed in real-time in contrasting pseudocolors [
4]. Similarly, sialyl Lewis X (sLeX), a terminal O-glycan structure abundant in cancer cells, can be detected using a diboronic fluorescent (DBF) probe, which acts as a noninvasive detector of prostate cancer cells [
108].
Figure 5 demonstrates the mechanism of fluorescent probes in biomarkers and cell imaging.
4.1.3.1. Fluorescent Probes Aiding in Early Cancer Biomarker Detection
Early detection of cancer biomarkers can be crucial for the prognosis and use of effective therapeutic means for treatment because the late-stage diagnosis adds complexity to the treatment [
109]. The most commonly used methods for the detection/diagnosis of cancer follow biopsy, radiography, genetic testing/profiling, diagnostic imaging, and endoscopic examination which require good sample quality and repeated testing. The efficient choice of detection would be the method that can detect early stages of abnormality /changes in normal healthy cells as a result of the expression of biomarkers in the form of hormones, proteins, enzymes, and sugars [
110,
111,
112,
113]. Major biomarkers for cancers can be detected in circulating tumor cells, nucleic acids, circulating tumor DNA, exosomes, and micro-RNA [
114]. So, the early detection of biomarkers can be critical for effective medical therapies. Among all, the fluorescent-based detection tools and technology can be decisive as they offer numerous advantages like rapid detection, non-invasive, and early detection of biomarkers with high sensitivity and selectivity due to the designing of selective probes for different types of biomarkers [
109,
115].
Epithelial cell adhesion molecule (EpCAM) has been used as a marker for circulating tumor cells (CTCs) due to its strong expression on the surface of many epithelial cancer cells [
116]. Li et al. used a dual-component antibody system with EpCAM and folate receptor alpha (FRα) conjugated to Alexa Fluor® 488 and DAPI to detect CTCs in peripheral blood samples. Van Driel et al. developed a surgical approach utilizing the near-infrared fluorescence dye IRDye800CW conjugated to an EpCAM antibody for targeted visualization of tumor cells [
117] . Mucin 1 (MUC1) is another surface protein involved in tumor cell proliferation; Wang et al. Additionally, Chen et al. developed CdTe quantum dots combined with aptamers to detect CTCs using MUC1 as a cancer biomarker [
118] . Kim et al. combined quantum dots with a siRNA delivery system to target cancer cells, creating a tool that serves as both a bioimaging agent and a gene delivery system [
119].
Wu et. al. has designed and developed the deep tissue imaging BODIPY-based near infrared-I (NIR-I) noninvasive small fluorescent probes for the
in vivo detection of early breast cancer bone metastasis [
120]. In their study, they developed the pH-activatable NIR probes as most of the cancer cells favor acidic environments and achieved the imaging up to 8 mm of tissue depth. Similarly, Tian
et. al. have developed the BODIPY-based radiometric fluorescence sensor for the selective detection of Homocysteine from cysteine and Glutathione which can offer intracellular quantitative analysis of Homocysteine in real-time imaging in neurons, as they crucial for maintaining the redox homeostasis and their imbalance is associated to neuropathology disorder [
121].
Zhang et. al. have developed a π-conjugation system of rhodamine-based near-infrared fluorescence probe for the detection and overexpression of Nitro reductase (NTR) enzyme in hypoxic tumor cells which offers 32-fold fluorescence intensity enhancements and a low detection limit of 1.09 ng/mL [
122].
Li et. al. has developed the FRET (fluorescence resonance energy transfer) nanoprobe (HMSN/DOX/RVRR/PAMAM/TPE) by combining the properties of agglomeration-induced emission fluorophore and FRET pair to quantitative detection of the overexpression of Furin in the triple-negative breast cancer cell line (MDA-MB-468 cell) [
123]. Here, they have used Hollow mesoporous silica nanoparticles (HMSNs) to load antitumor drug doxorubicin (DOX) and the holes or nanopore are blocked or encapsulated by the Furin-specific responsive Arg-Val-Arg-Arg (RVRR) peptides and PAMAM/TPE. The FRET effect in this case in cancer cells is generated by the cleavage of the RVRR peptide by Furin resulting in the release of doxorubicin.
4.1.4. Biosensors
In 1956, Leland C. Clark, Jr. reported the first biosensor for the detection of oxygen, which led to a new development in heart-lung devices used during cardiopulmonary bypass operations [
124]. IUPAC characterizes a biosensor as a chemical sensor that combines a physicochemical sensor with a biologically derived identifying element [
125]. The fluorescent protein sensor has applications in different areas, such as detecting pathogens and monitoring intracellular signaling molecules, and its development has been more than 150 years [
126]. There are many types of fluorescent biosensors, such as nucleic acid biosensors, DNA‒RNA biosensors, quantum dot (QD)-based biosensors, fluorescent protein sensors, organic dyes, and NP-based biosensors [
127]. Fluorescent-based biosensors can be made via natural or synthetic methods, which are particularly applied in drug discovery, food safety, disease diagnosis, environmental analysis, and bioimaging. In addition, fluorescent proteins known as GFP or synthetic fluorophores have been extensively used in protein engineering [
128]. It has also been used in probing the molecular dynamics of macromolecules, metabolites, molecular processes in living cells, and ions [
129]. Traditional sensing techniques lack cellular and subcellular specificity; thus, new techniques are needed to directly quantify the dynamics of target molecules in specific cells and subcellular compartments [
130]. The binding affinity, recognition element for a sensory domain, rate constants, choice of fluorophore, absorption and emission wavelengths, and resolution of sensors are some of the factors that play important roles in developing biosensors [
63]. Chelly et al. developed a fluorescent probe from the medicinal plant
Lavandula multifida and used it as a fluorescent sensor due to its high stability and sensitivity for detecting metal ions, especially Hg
2+ in marine water [
131]. Similarly, Guisán-Ceinos et al. synthesized 3-azo conjugated BODIPY dyes to depict hypoxia-like situations in live cells, and this probe allowed the visualization with strong absorption and large molar absorption coefficients [
132]. While synthetic fluorescent probes impart strong fluorescence, with enhanced other properties, fluorescent natural products have lower toxicity, environmental sustainability, and greater biocompatibility, thereby demonstrating their potential to be used as biosensors with diverse applications.
4.1.5. Cell Counting System and Cellular Proteomics
Accurate quantification and characterization of cells are essential for medical diagnosis and disease treatment. There has been tremendous progress in medicine in terms of analyzing blood cells, performing cell therapy, evaluating the concentration of bacteria and viruses, and calculating the number of dead to live cells to measure the viability of cells with the aid of fluorescence [
133]. Techniques such as fluorescence-based cell counting and viability assessments utilizing genetically encoded fluorescent sensors within cells have contributed to this tremendous progress [
134,
135]. More advanced methods of blood cell counting have produced more reportable parameters, which have been included in the regular complete blood count within the last few years [
134], and these fluorescent sensors, genetically defined in a DNA sequence, are produced within the cells and are predominantly protein-based probes, using at least one fluorescent protein acting as a fluorophore [
135].
One of the developing fields in cell biology is cell proteomics, which aims to create tiny molecule probes to comprehend the function of proteins by analyzing the target that binds to proteins along with the molecular probes derived from nature [
136]. Various fluorescent probes can covalently attach to surfactants, phospholipids, proteins, and polynucleotides, providing various types of information. They facilitate protein tagging by labeling reagents with appropriate functional groups along with fluorescein, rhodamine, and erythrosin derivatives, because of the possibility of covalent bonding between amino groups and sulfydryl groups [
6,
137].
L-phenylalanine,
L-tyrosine, and
L-tryptophan are examples of naturally occurring amino acids with aromatic side chains that have been utilized as intrinsic fluorescence probes to investigate processes such as protein dynamics [
6]. It is possible to directly and easily synthesize optically pure, fluorescent amino acids using inexpensive natural amino acids as the starting material. For instance, using the solid-phase approach, the cyano derivative of
L-phenylalanine, a conformationally defined fluorescence and IR absorption probe, was utilized to research peptide membrane interactions, showing its sensitivity as a microenvironment indicator [
138]. Similarly, the
L-phenylalanine based genetically encodable cyan emitting fluorescent α-amino acid such as 4-phenanthracen-9-yl-l-phenylalanine (Phen-AA), and 4-dibenzothiophen-4-yl-L-phenylalanine (DBT-FAA) have been synthesized and utilized for imaging at cellular and sub-cellular level in live-Hela cells [
139,
140,
141]. Moreover, tyrosine-derived fluorescent unnatural amino acids were synthesized through methods such as the Heck coupling reaction, which have proven useful tools for the synthesis of novel fluorescent amino acids and can be incorporated into peptides for biological studies, including examination of cellular functions, investigations of biological mechanisms, and fabrication of optoelectronic devices [
142]. Tryptophan (Trp), a fluorophore found in proteins, is naturally environmentally sensitive and has been shown in numerous instances in the literature to change its fluorescence spectrum upon substrate or ligand binding [
143]. Similarly, fluorescent amino acid L-(7-hydroxycoumarin-4-yl) ethylglycine closely similar to tryptophan has been incorporated into proteins as a probe for the urea-dependent denaturation of holomyoglobin [
144]. Therefore, these types of natural fluorophore-based fluorescent probes are significant for marking the potential functions of proteins and adding value to the cell counting system.
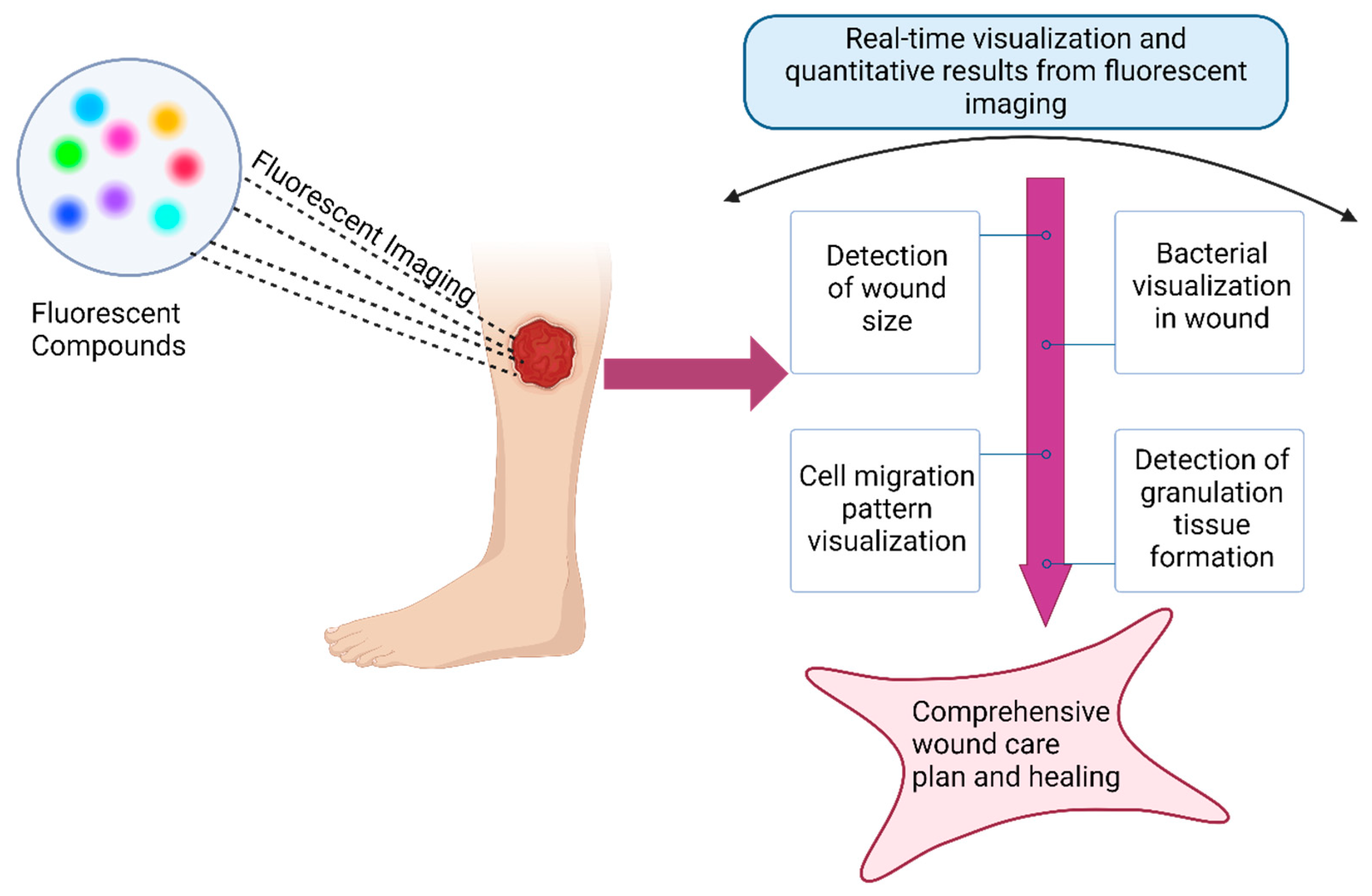
4.1.6. Application for Wound Healing
Wound healing is a complex series of conserved evolutionary processes, including blood clotting, inflammation, cellular proliferation, and remodeling of the extracellular matrix (ECM) [
145]. Hemostasis, the first phase of wound healing, occurs within the first minute to hours after injury and involves forming a platelet plug and releasing growth factors and immune mediators as distress signals to initiate repair. The inflammatory phase overlaps with the initial hemostasis phase during the first 72 hours following injury. During this phase, molecular signals initiate neutrophil and monocyte infiltration to the injury site to eliminate further damage and clear foreign debris. Proliferation occurs between 4 and 21 days and involves reestablishing vascular and granulation tissue and re-epithelialization. New blood vessel formation occurs during this phase, and a number of chemical signals are emitted, including EGF, b-FGF, TGF-α, TGF-β, and VEGF [
146]. The last phase is the remodeling phase, which occurs weeks after the initial injury and continues until scar tissue is formed. The differentiation of myofibroblasts drives remodeling in response to wound contraction. The four stages of wound healing are aided by the proteins and chemical signals produced by each type of cell [
147].
The development of new biotechnologies will help us further understand, diagnose, and treat wounds. During wound healing, cell signaling molecules, including growth factors, chemokines, and cytokines, as well as products of cellular activity, are released into the ECM. Currently, the evaluation of wound healing mainly focuses on the quantitative detection of changes in wound size and granulation tissue formation. However, this method does not reveal what occurs at the molecular level. Luo et al. developed a NIR fluorescent probe, DCM-H
2O
2, that can act as a diagnostic tool for chronic wound healing and for assessing fluctuations in H
2O
2 in chronic wounds in mice and humans [
148]. The probe is synthesized from a penta fluorobenzene sulfonyl ester group as a recognition moiety and dicyanomethylene-benzopyran (DCM) as an NIR fluorophore. This response leads to a large Stokes shift, which emits a fluorescence signal at 695 nm. In another study, Nasir et al. tracked daily wound re-epithelization changes in human wounds over 6 days with the cell tracker dye 5-chloromethylfluorescein diacetate (CMFDA) after developing a serum-free ex vivo human partial thickness wound model. Human skin was obtained from human donors, and the subcutaneous fat was removed [
149]. The fluorescent dye was added to the skin model for 30 min before it was washed with PBS. Imaging was performed with an upright fluorescence microscope where the area of the initial wound and the area of the open wound remaining were calculated. The calculations were used to determine total epithelial wound closure. While their study was only performed utilizing 5-Chloromethylfluorescein diacetate (CMFDA) and cell tracker red CMPTX, there is a possibility of using other dyes or combining multiple fluorescent dyes to visualize cell migration patterns during wound healing. One of the limitations of using fluorescent dyes for the visualization of wounds during the study was the observation of irregular healing and how to interpret factors contributing to that observation [
149].
Irregular wound healing can be caused by the infiltration of bacteria. Microbial infection in wounds is a severe issue that can interfere with healing. Real-time monitoring and early detection of infections can be helpful in the treatment of wounds. Fluorescence imaging is a method used for bacterial visualization in wounds. The implementation of fluorescent imaging was associated with a 49% decrease in antibacterial dressing prescription and a 23% increase in wound healing rates, likely due to early detection of bacterial infection, which leads to better hygienic treatment [
150].
To determine both the type and location of pathogens, the MolecuLight i:X handheld device was developed for quick diagnosis using fluorescent light illuminated by pathogenic bacteria. Moscicka et al. obtained fluorescence images using MolecuLight, where the bacterial load was comparable to that obtained from a microbiology laboratory [
151]. The use of fluorescent biosensor devices allows for early identification of bacterial invasion, which is critical for rapid treatment.
Noninvasive fluorescence imaging is used to visualize wound tissue at the point of care (POC). While MolecuLight i:X can improve the diagnosis of infected wounds, fluorescence imaging does not distinguish whether the bacteria are planktonic or in biofilm form. Le. et al. investigated the diagnostic accuracy of POC fluorescence imaging and compared the results to clinical results obtained during routine wound healing assessments [
152]. They found that 287 out of 350 wounds had bacterial loads, and clinical assessment missed 85% of the lesions. Fluorescence imaging significantly increased the detection rate of bacteria. The use of fluorescence imaging provides an improved assessment of the wound state in real-time compared to clinical assessment. These devices can be used for prompt detection using fluorescence, thereby expediting treatment to reduce bacterial burden and facilitate healing. A schematic representation of the ability of fluorescent compounds to analyze and visualize bacteria in wounds is shown in
Figure 6.
Smart bandages have emerged to provide physiochemical surveillance of wound healing in real-time as well as treatment. The bandages utilize smart sensors to detect wound infection. Zong et al. designed a series of smart bandages used to accelerate diabetic wound healing and provided real-time fluorescent visualization of hypochlorous acid (HClO) levels. HClO levels are indicative of oxidative stress-related inflammation and delayed wound healing [
153]. In their study, SCy-7, a NIR fluorescent probe, was functionalized with a polymeric ionic liquid cross-linked with hyaluronic acid. This probe was used to develop a fluorescent-based hydrogel wound dressing. Compared with the commercial Tegaderm™ film, the PIL-HA wound dressing was able to generate a fluorescent signal for real-time quantification of HClO levels to monitor wound repair in real time and treat the wound at the same time.
4.1.7. Fluorescent Agents as Targeting Ligands in Tumor
Fluorescent agents can be incorporated with biological markers, like peptides, enzymes, antibodies, or bioactive compounds which can display tumor signals that are specific to tumor cells [
154]. Fluorescent agents especially Near-Infrared fluorescence like heptamethine cyanine, folic acid, fluorochrome, 6-substituted pyrrolo (2,3-d)pyrimidine antifolates, MHI-148, 5-aminolevulinic acid (5-ALA), indocyanine green are used for the diagnosis of the tumor because of their unique and compatible properties, including high extinction coefficients, intense emission, and huge Stoke's shifts [
155,
156,
157] . Amongst them, indocyanine green, 5-ALA, and methylene blue are used for the real-time analysis of tumors and are also considered non-targeted fluorophores, that work by the slow build-up of fluorophores in the tumorous regions, thereby helping in the visualization during the perfusion of tissues [
155]. On the other hand, targeted fluorophores are activated only in specific circumstances, like when they get attached to targeted tissue. This target might be intracellular (enzymes) or extracellular (like matrix metalloproteinases, Cathespins) [
158,
159]. These probes might aid in pinpointing the location of the receptors, evaluate the interaction of the receptor and the ligand, and examine the mode of action of medications [
160]. Furthermore, they should meet certain photophysical rules, such as wavelength, brightness, biostability, photostability, particular tissue accumulation, and pharmacokinetics to be successfully used as targeting probes [
161]. The ligand, the fluorophore and the connector or the linker are the major components of a receptor-targeted probe [
160]. The fluorophores should be highly specific, producing intense fluorescence signals after activation to allow significant target-to-background ratios. For this, mechanisms like PET, FRET, and H-dimer formation play a huge role [
158]. Becker et al. found that after incorporating cyanine dye with octreotate, analog of somatostatin, showed higher fluorescence in tumor tissue and deduced that dye-peptide conjugate formed by the specific interaction of ligand and receptor can be used for the diagnosis of tumor [
162]. Recently, Zhang et al. discovered a specific near-infrared probes, 6-substituted pyrrolo[2,3-d]pyrimidines which showed distinctive affinity towards folate receptors, demonstrating its ability for the diagnosis of tumor [
163]. In addition, Tazwa et al. provide an excellent review of various cell- and tissue-specific fluorescent probes that use adenovirus-based probes in a promoter-dependent manner [
164].
4.1.8. Other Biomedical Applications
Fluorescent compounds are important biochemical tools for a variety of biological research applications, ranging from visualizing cellular structures to monitoring molecular interactions. Some specific tools, such as fluorescein isothiocyanate (FITC), have been widely applied for protein labeling, antibodies, fluorescence microscopy, flow cytometry, and immunohistochemistry [
165]. BODIPY dyes, rhodamine dyes, cyanine 5, and cyanine 3 dyes are versatile fluorophores that have been used for labeling lipids, proteins, nucleic acids, biomolecules, microarray analysis, and multiplexed imaging [
166]. In addition, many fluorophores incorporating NPs have attracted attention for their applicability as biosensors; for instance, a glucose biosensor based on fluorescence was created using the ameliorating effects of silver NPs on cadmium-selenium quantum dots, which resulted in a ninefold increase in fluorescence [
167]. The characteristics of fluorescent probes rely on a range of fluorescence techniques focusing on those that have shown promise in biological sensing and imaging. Furthermore, this shows how fluorescent probes with optimized optical properties are useful for biological analysis [
168].
4.2. Analytical Applications
Due to their high sensitivity, selectivity, and ability to detect and quantify rare analytes, fluorescent compounds have wide-ranging applications in analytical chemistry, as shown in
Figure 7 [
169]. Many common analytical instruments, such as fluorometers, fluorescence microscopes, and plate readers, utilize fluorescence as their detection tool, such as monitoring the concentration of various essential elements like Cu(II), Fe(II), Fe(III), and H
2O
2 (measure of oxidative stress) in live-cells using confocal microscopy [
107,
170,
171,
172] . Fluorescence-based detection has been extensively used for qualitative and quantitative analysis of environmental samples, pharmaceutical agents, nanomaterials, geochemical agents, and atmospheric and interstellar molecules [
167,
173,
174]. Some of the applications related to the analytical field are described below:
4.2.1. Environmental Analysis
Environmental pollutants have become a major concern over the past decade, and these pollutants have numerous harmful effects, including carcinogenesis, growth retardation, endocrine disruption, etc [
175]. Fluorescent natural products can act as molecular probes to monitor changes, such as pH, temperature, and specific pollutants, and can aid in analyzing environmental problems [
173]. They are instrumental in detecting heavy metal contamination and other chemical and biochemical pollutants [
176]. For instance, an ethyl cellulose-modified fluorescent probe was used to detect Al
3+ in the environment and food products, demonstrating high sensitivity and effectiveness in mitigating the hazardous effects of aluminum [
127]. In addition, fluorescent detectors in conjunction with chromatographic techniques have also been pivotal in analyzing contaminants in diverse environmental samples. Much research regarding the determination of polyaromatic hydrocarbons in water, soil, and sediments; pesticides in soil; and water using fluorescent detectors has been performed, and these detectors are effective at quantifying harmful contaminants [
174].
With the use of a smartphone, Shang et al. used an infrared fluorescent probe for the detection of SO
2 derivatives, HSO
3-, in industrial effluent and food samples immediately on the spot, which might help in comprehending the hazardous effects of SO
2 derivatives on nature and living beings [
177]. Similarly, Ali et al. developed an effective donor-acceptor ICT-type probe (tricyanoethylphenyl phenanthroimidazole) for the detection of CO
2, as shown in
Figure 7, and this development of the probe might lead to understanding global climate change and greenhouse effects [
178]. Moreover, a novel ratiometric fluorescent probe, PBQ-AB, with a large Stokes shift and emission wavelength shift, was developed for real-time tracking of hydrazine in environmental soil and water samples, revealing the diverse applications of fluorescent probes in environmental analysis [
179]. Therefore, fluorescent probes have been used as important substrates that play a crucial role in ecological analysis, offering sensitive and effective tools for examining and removing environmental pollutants.
4.2.2. Water Purification
Improving the sustainable decontamination and purification of water is a major concern, and fluorescence spectroscopy has emerged as one of the most promising tools for characterizing organic matter in seawater and freshwater, monitoring diesel pollution, evaluating water treatment processes, detecting insecticides, and assessing the quality of raw sewage. Additionally, fluorescence spectroscopy can be used for discharge detection in wastewater treatment plants [
180]. Nonbiodegradable waste such as antibiotics in water can be converted into nontoxic products such as water and carbon dioxide by photocatalytic reduction [
181]. Similarly, a fluorescence excitation-emission matrix can be used to obtain valuable information on dissolved organic matter in water [
182] and evaluate the impact of wastewater on natural water, as the composition of effluent organic matter is different from that of naturally occurring organic matter (OM) [
183].
Based on the classification provided by Li et al. the fluorophores on wastewater are divided into 2 groups: region Em > 380 nm (fluorophores with limited aromatic rings), and region Em < 380 nm (polycyclic aromatic rings) [
184]. Similarly, the region λ
excitation/λ
emission ~ 225/350 nm is considered as peak T λ
excitation/λ
emission ~ 300-350/400-500 nm is considered as peak C. Phenols, DNA, aromatic amino acids, and lignin are the organic wastewater components falling in the region Em < 380 nm, and fluorophore-like humic acids, flavonoids, and quinones fall under the region Em >380 nm [
185]. The different contaminants present in domestic, industrial, animal waste, and pharmaceutical waste in water can be detected in these regions and can be used as a tool for the identification of category of waste, which is crucial for waste management.
Likewise, the correlation between biochemical oxygen demand (BOD) and peak T provides a direct link to microbial activity in this region, and peak T fluorescence is considered an indicator of the presence/absence of fecal contaminants and sludge systems [
186]. Additionally, with this technique, both online and offline monitoring of wastewater can be performed [
187]. It can convert peak T fluorescence into BOD equivalent values with the use of an internal calibration factor [
188]. On performing real-time fluorescence with some conventional methods, chemical oxygen demand (COD) can be determined for influent and effluent [
189], and the efficiency of treatment methods can also be determined. The ratio of peak T to peak C fluorescence intensity indicated the presence of remnants of farm waste products in the water; hence, fluorescence spectroscopy could be a potential tool for plant- and animal-derived waste control [
190]. Therefore, real-time fluorescence spectroscopy can be a good sign of wastewater management in modern research. Further studies should be performed to determine the negative effects caused by pH, temperature, coagulation, and so on [
191]. In summary, fluorophores have significant applications in modern wastewater management research; however, challenges still need to be addressed.
4.2.3. Application in Other Analytical Sciences
Fluorescence-based methods can be used to characterize structural materials, paints, artwork, and geological structures. Specifically, the chemical composition of the artwork, especially that of an organic material, can be analyzed using fluorescence. X-ray fluorescence (XRF) is routinely used as an analytical method in geochemistry where soils and sediments can be characterized with high accuracy [
192].
5. Current Research Trends
5.1. Fluorescent Nanoparticles, Nanoclusters, Quantum Dots, and Carbon Nanotubes
The incorporation of natural product-based fluorescent probes such as NPs, nanoclusters, QDs, and carbon nanotubes is a promising field of research. These small probes with special features, including size, tunable optical properties, and biocompatibility, have emerged as remarkable approaches for various applications, including environmental sensing, chemosensing, drug administration, etc [
88,
167,
193]. Numerous fluorophores can be tagged and developed into NPs, concentrating the fluorescence signal in a specific region, thereby enhancing the brightness [
194]. They are primarily used in biolabeling as alternatives to traditional organic dyes and have been developed as chemosensors and biosensors whose sensing mechanism relies on the ability of the substrate to either amplify or quench the fluorescence.[
195,
196]
Carbon-based QDs are a novel class of optical nanomaterials that are attractive as fluorescent bioimaging agents due to their low toxicity, aqueous solubility, biocompatibility, high optical performance, and chemical and photochemical stability [
197]. Fe
3O
4 nanoparticles coated by matrix and amine-functionalized quantum dots as key parts of multifunctional nanocarrier system in the nanosphere can be utilized for cancer diagnosis and targeted treatment by dual imaging system; fluorescence and magnetic resonance [
198]. Water-soluble carbon dots derived from bamboo leaves [
199], saffron, and spices [
200] are capable of emitting fluorescence when exposed to light. A growing number of FRET-based applications are using gold NPs (for sensing DNA) and semiconductor QD quenchers because of their remarkable quenching capacity, reduced background signal, enhanced sensitivity, and capacity to mark gold NPs and QDs with various physiologically active groups [
168,
193]. Similarly, photo-switchable fluorescent NPs are extensively used in biological labeling and super-resolution imaging of cellular samples [
168]. Because of their exceptional stability, distinct fluorescent properties, low toxicity, biocompatibility, and potential applications, Au nanoclusters are used for determining analytes in real samples such as serum, lake water, cultured cells, identifying and analyzing materials containing cations, anions, tiny chemical compounds, biomolecules or hydroxyl radicals, and measuring intracellular temperature [
201]. Single-walled carbon nanotube (SWCNT) NIR fluorescence has been used to create optical sensors and monitor nucleic acids, proteins, and drugs by tracking the position and form of SWCNTs incorporated into living cells [
202].
5.1.1. Role in Early Detection of Cancer
Functionalized fluorescent nanoparticles provide the greatest tool for the detection of cancer biomarkers. For example, Federica
et. al., has developed a tool where cancer-related micro-RNA (miR-203) can be quantified in real-time using a fluorescently labeled DNA probe on PEGylated gold nanoparticles (AuNPs) as a reporting of cancer biomarkers in clinical practices [
203]. Similarly, oligonucleotide-modified gold nanoparticle probes hybridized to fluorophore-labeled as “nano flare” have been developed for the detection of mRNA in living cells [
204]. Here, the fluorescence of fluorophore is quenched by the Au NPs. However, in the presence of target mRNA, the oligonucleotide forms a complex which then causes the release of fluorescent reporter sequences from the AuNPs the surface resulting in fluorescent signals like flares. Moreover, these “nano flares” have been developed further by Tang
et. al., for the detection of the expression of multiple mRNAs simultaneously in a single-target assay [
205]. Here, an AuNP core was hybridized with the three short dye-terminated reporter sequences which detect the three different types of tumor-related mRNA targets; c-myc mRNA, TK1 mRNA, and GalNAc-T mRNA were determined by three different fluorescent signals (green, yellow, and red) respectively. Besides these, the Au NPs-based hairpin structures labeled with fluorescein, Texas red, and Cy5 were used as molecular beacons for the detection of three different tumor-suppressor genes [
206]. Kim et al. combined quantum dots with a siRNA delivery system to target cancer cells, creating a tool that serves as both a bioimaging agent and a gene delivery system [
119]. Dong et al.'s 2022 review article provides comprehensive information on various nucleic acid-based nanosensors for tumor RNA monitoring in cancer diagnostics [
207].
Extracellular vesicles are membrane-bound nanoparticles released by cells throughout the body, with typical sizes ranging from 30–1000 nm in diameter. Exosomes, a subclass of extracellular vesicles, are 30–150 nm in diameter. Exosomes and extracellular vesicles hold significant promises as cancer biomarkers and drug-delivery vehicles. Recently, Chen et al. developed a fluorescence-based approach using PKH26 or PbS quantum dots to stain small extracellular vesicles; this allowed the researchers to study vesicle circulation and biodistribution in major organs in a time-dependent manner [
208]. Wu et al. recently developed a ratiometric fluorescence assay to detect plasma exosomes as a cancer diagnostic tool [
209]. This assay employs a Cy5-tagged aptamer targeted against the exosomal surface protein CD63. Previously, Huang et al. developed a fluorescence-based exosome biosensor aimed at gastric cancer-derived exosomes, utilizing rolling circle amplification to enhance the signal, showing great promise for cancer diagnostics [
210].
Hence, the process of incorporating fluorescent probes as NPs has created a wide range of applications.
5.2. Fluorescent Natural Products
Fluorescent natural products are being utilized in several ways in biomedical and bioanalytical research. Recently, the first sustainable, naturally derived affordable luminous carbon dots based on two networked hydrogels (agarose and polyacrylamide) were created, which showed strong mechanical durability, resistance to stretching, self-healing capacity, and Fe
3+ responsiveness. This can be used for creating luminous patterns and data encryption [
211]. Researchers are interested in developing new fluorescent probes followed by their characterization of their applicability in various fields, including biosensing, cellular imaging, and drug discovery [
212,
213]. In a recent study, Dazat et al. used fluorescent natural eutectic solvents (a mixture of fructose and urea, a mixture of citric acid and glucose, and many more) for the improvement of ergosterol detection in mushrooms to analyze fungal contamination in edible mushrooms [
214]. Phytochromes are naturally occurring photoreceptors that are used as sensors because of their ability to respond to near-infrared light and control bacterial metabolism. Similarly, biliverdin, a naturally occurring byproduct of heme catabolism in human cells, serves as the chromophore in all proteins and can be used as a genetically encoded fluorescent probe [
215].
Many natural organophosphorus compounds (pentavalent and trivalent phosphanes, unsaturated phosphanes, and heterophospholes) are being used as optoelectronic materials in the field of electronics, such as field effect transistors, sensors, light emitting diodes, and polymers for organic photovoltaics [
216]. In addition, the electrospinning method is used to prepare many fluorescent nanofibers made from thermosetting polymers, and it has shown significant fluorescence emission, especially when the phenyl ring of a thermoplastic elastomer is confined by its intra- and intermolecular mobility [
217]. Similarly, velocity-, pH-, and polarity (VPP)-targeted probes enable real-time examination of pathogenic microenvironments, which yields vital insights for prompt diagnosis, process tracking, and prediction of pathophysiological processes [
218]. Furthermore, during clinical surgery, an array of fluorophores, namely, methylene blue and indocyanine green, which are synthetic have been used for fluorescence-guided tumor removal after receiving approval from the US Food and Drug Administration for clinical use [
219], but as of now, the use of natural fluorophores in tumor removal is limited and still in the research phase to be successfully used for clinical applications. Natural-based fluorophores often get overshadowed by synthetic ones or their derivatives due to greater fluorescence intensity and stability. However, research on fluorescent natural products has seen significant advancements in recent years, and many researchers are still searching for fluorescent natural products that can aid in many different applications. Overall, research on fluorescent natural products is vibrant and interdisciplinary, with ongoing efforts to explore their diverse applications and unravel their biological and chemical properties.
6. Opportunity and Challenges in Research
Current research on fluorescent probes has provided exciting opportunities for biomedical applications due to their unique properties, biocompatibility, high quantum yield, high specificity, sensitivity, and unique photophysical and photochemical properties. These qualities not only make them novel probes for biological imaging but also open possibilities for other applications like structural and dynamic characteristics of macromolecules. In addition, it offers wide potential for analyzing metabolic pathways, which can accelerate the discovery of new therapeutic agents for curing various diseases. The exploration of complex biological systems at the molecular level can also be performed using fluorescent natural products. In addition, the ability to be used in monitoring pollutants, toxins, and other environmental factors has demonstrated the vast potential of these products; therefore, these compounds can further be utilized for developing new materials with unique properties that can aid in different applications.
There are several challenges in the development of novel probes for fluorescent-based natural products, such as their isolation, characterization, synthetic accessibility, biological compatibility, sensitivity, and quantum yield, as well as their chemical and biochemical properties, such as pH, solvent polarity, temperature, cytotoxicity, pharmacokinetics and pharmacodynamics. Furthermore, the isolation of the fluorescent product in its pure form remains a challenge because the concentration of the fluorescent natural products from complex biological samples is relatively low. In addition, the problem of accurately elucidating the structure of fluorescent natural products represents the next hurdle in the development of fluorophores. Similarly, the limited chemical accessibility and lack of highly active compounds also influence the development of fluorescent natural products.
Nonetheless, there is still much scope for natural fluorescent metabolites in many fields; one of the underappreciated and potential fields could be material science, in which some of the areas of application could be field effect transistors and light-emitting diodes, and many more and these areas could further highlight the importance of natural fluorescent products. In the case of synthetic fluorescent probes, although they have a wide range of applications due to their stability, tailoring ability, and compatibility, the main challenge occurs in terms of toxicity, complexity, cost, and environmental sensitivity. Despite the challenges, there is significant potential for both natural and synthetic fluorescent probes across various fields, and advances in overcoming these challenges will foster their effectiveness.
7. Conclusions
This review highlights wide-ranging applications of natural products and synthetic-based fluorescent compounds in biomedical and analytical research. We discussed the usefulness of fluorescent probes in cell imaging, biomarker discovery; primarily in cancers and tumors, optical sensing, wound healing, drug delivery systems, as a targeting ligand in tumors and analytical applications including environmental analysis, and water purification. Furthermore, the structural and chemical properties of many natural products are discussed, with an emphasis on their photophysical and photochemical properties, including specific fluorescent properties. This extensive review demonstrates that fluorophores are emerging techniques for understanding biochemical systems that can be used for studying drug effects at cellular and subcellular levels. Fluorescent probes also help to elucidate pharmacokinetics and pharmacodynamics during the drug discovery process. Novel areas of application, such as wound healing, cancer diagnostics, and cellular proteomics, are further discussed. Recently, the rapid growth of fluorescence-based visualization and quantification techniques in both biomedical; imparting its prime role in cancers and environmental analysis has further inspired the development of new natural product-based fluorescent probes and synthetic analogs. Overall, this review will facilitate further research on the photophysical and photochemical properties of different fluorescent compounds that are applicable in biomedical and analytical research.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org, Figure S1: Structures of some of the natural-based fluorescent compounds; Table S1: The list of common fluorescent compounds with general photophysical properties
Author Contributions
Conceptualization, N.P., and N.B.; methodology, S.J.; formal analysis, S.J., A.M., P.B., A.G., R.G., and P.S.; investigation, S.J., A.M., P.B., A.G., R.G., and P.S.; writing—original draft preparation, S.J., A.M., P.B., A.G., R.G., and P.S.; writing—review and editing, A.G., S.P. A., N.B., and N.P.; supervision, N.P., and N.B.; project administration, N.P., and N.B.; funding acquisition, N.P., and N.B.. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Stokes, G.G. On the Change of Refrangibility of Light. Proc. R. Soc. Lond. 1854, 6, 195–200. [Google Scholar] [CrossRef]
- Valeur, B.; Berberan-Santos, M.N. A Brief History of Fluorescence and Phosphorescence before the Emergence of Quantum Theory. J. Chem. Educ. 2011, 88, 731–738. [Google Scholar] [CrossRef]
- Yuan, H.; Jiang, A.; Fang, H.; Chen, Y.; Guo, Z. Optical Properties of Natural Small Molecules and Their Applications in Imaging and Nanomedicine. Advanced Drug Delivery Reviews 2021, 179, 113917. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.T.; Tsien, R.Y. Fluorescence-Guided Surgery with Live Molecular Navigation — a New Cutting Edge. Nat Rev Cancer 2013, 13, 653–662. [Google Scholar] [CrossRef]
- Wang, S.; Han, B.; Chen, M.; Han, Y.; Liu, J.; Wang, G. Construction of Bifunctional Carbon Dots Based Fluorescent/Colorimetric/Smartphone-Assisted Multi-Signal Strategy for Monitoring Alkaline Phosphatase Activity. Materials & Design 2023, 232, 112172. [Google Scholar] [CrossRef]
- Millar, D.P. Time-Resolved Fluorescence Spectroscopy. Current Opinion in Structural Biology 1996, 6, 637–642. [Google Scholar] [CrossRef]
- Wagh, S.B.; Maslivetc, V.A.; La Clair, J.J.; Kornienko, A. Lessons in Organic Fluorescent Probe Discovery. ChemBioChem 2021, 22, 3109–3139. [Google Scholar] [CrossRef]
- Sung, D.-B.; Lee, J.S. Natural-Product-Based Fluorescent Probes: Recent Advances and Applications. RSC Med. Chem. 2023, 14, 412–432. [Google Scholar] [CrossRef]
- Duval, R.; Duplais, C. Fluorescent Natural Products as Probes and Tracers in Biology. Nat. Prod. Rep. 2017, 34, 161–193. [Google Scholar] [CrossRef]
- Fei, G.; Li, S.; Liu, Y.; Carney, J.B.; Chen, T.; Li, Y.; Gao, X.; Chen, J.; Chen, P.; Yue, Y.; et al. Structure–Activity Strategies for Mechanically Responsive Fluorescent Materials: A Molecular Perspective. Chem. Commun. 2024, 60, 10–25. [Google Scholar] [CrossRef]
- Sokolov, P.; Nifontova, G.; Samokhvalov, P.; Karaulov, A.; Sukhanova, A.; Nabiev, I. Nontoxic Fluorescent Nanoprobes for Multiplexed Detection and 3D Imaging of Tumor Markers in Breast Cancer. Pharmaceutics 2023, 15, 946. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, P.A.; Boulangé, A.; Ball, M.; Naudin, B.; Alle, T.; Cosette, P.; Karuso, P.; Franck, X. Design and Synthesis of Epicocconone Analogues with Improved Fluorescence Properties. J. Am. Chem. Soc. 2014, 136, 15248–15256. [Google Scholar] [CrossRef] [PubMed]
- Wangngae, S.; Chansaenpak, K.; Nootem, J.; Ngivprom, U.; Aryamueang, S.; Lai, R.-Y.; Kamkaew, A. Photophysical Study and Biological Applications of Synthetic Chalcone-Based Fluorescent Dyes. Molecules 2021, 26, 2979. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.; Chaurasiya, S.; O’Leary, M.; Han, E.; Fong, Y. Fluorescent Imaging for Cancer Therapy and Cancer Gene Therapy. Molecular Therapy - Oncolytics 2021, 23, 231–238. [Google Scholar] [CrossRef]
- Yao, H.; Wei, G.; Liu, Y.; Yao, H.; Zhu, Z.; Ye, W.; Wu, X.; Xu, J.; Xu, S. Synthesis, Biological Evaluation of Fluorescent 23-Hydroxybetulinic Acid Probes, and Their Cellular Localization Studies. ACS Med. Chem. Lett. 2018, 9, 1030–1034. [Google Scholar] [CrossRef]
- Lim, X. C&EN Talks with Xiaogang Liu, Fluorescence Expert. C&EN Global Enterp 2020, 98, 25–26. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, J.; Tang, Y.; Guo, Z.; Bai, J.; Wu, L.; Su, J.; Cen, S.; Yu, L.; Zhang, D. Geninthiocins E and F, Two New Cyclic Thiopeptides with Antiviral Activities from Soil-Derived Streptomyces Sp. CPCC 200267 Using OSMAC Strategy. J Antibiot 2023, 76, 101–104. [Google Scholar] [CrossRef]
- Puvvada, N.; Rajput, S.; Kumar, B.N.P.; Mandal, M.; Pathak, A. Exploring the Fluorescence Switching Phenomenon of Curcumin Encapsulated Niosomes: In Vitro Real Time Monitoring of Curcumin Release to Cancer Cells. RSC Adv. 2013, 3, 2553. [Google Scholar] [CrossRef]
- Li, D.; Yang, M.; Liang, M.; Mei, C.; Lin, Y.; Yang, F.; Xiao, Y.; Chen, Y.; Wang, F.; Mao, J.; et al. C-Met-Targeted near-Infrared Fluorescent Probe for Real-Time Depiction and Dissection of Perineural Invasion and Lymph Node Metastasis Lesions in Pancreatic Ductal Adenocarcinoma Xenograft Models. Biomater. Sci. 2021, 9, 6737–6752. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, Y.; Liu, S.; Dong, M.; Huang, C. Low-Cost Synthesis of Carbon Nanodots from Natural Products Used as a Fluorescent Probe for the Detection of Ferrum(Iii) Ions in Lake Water. Anal. Methods 2014, 6, 2086. [Google Scholar] [CrossRef]
- Harkiss, A.H.; Sutherland, A. Recent Advances in the Synthesis and Application of Fluorescent α-Amino Acids. Org. Biomol. Chem. 2016, 14, 8911–8921. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Shi, C.; Li, L.; Tang, Y.; Zhang, X.; Liao, S.; Zhang, B.; Sun, C.; Ren, C. A Review on Recent Advances in Amino Acid and Peptide-Based Fluorescence and Its Potential Applications. New J. Chem. 2021, 45, 15180–15194. [Google Scholar] [CrossRef]
- Royer, C.A. Fluorescence Spectroscopy. In Protein Stability and Folding; Humana Press: New Jersey, 1995; ISBN 978-0-89603-301-6. [Google Scholar]
- Chalfie, M. GREEN FLUORESCENT PROTEIN. Photochem & Photobiology 1995, 62, 651–656. [Google Scholar] [CrossRef]
- Shimomura, O.; Johnson, F.H.; Saiga, Y. Extraction, Purification and Properties of Aequorin, a Bioluminescent Protein from the Luminous Hydromedusan, Aequorea. J. Cell. Comp. Physiol. 1962, 59, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, M. Green Fluorescent Protein (GFP): Applications, Structure, and Related Photophysical Behavior. Chem Rev 2002, 102, 759–781. [Google Scholar] [CrossRef]
- Chalfie, M.; Tu, Y.; Euskirchen, G.; Ward, W.W.; Prasher, D.C. Green Fluorescent Protein as a Marker for Gene Expression. Science 1994, 263, 802–805. [Google Scholar] [CrossRef]
- Zhang, Y.; Rózsa, M.; Liang, Y.; Bushey, D.; Wei, Z.; Zheng, J.; Reep, D.; Broussard, G.J.; Tsang, A.; Tsegaye, G.; et al. Fast and Sensitive GCaMP Calcium Indicators for Imaging Neural Populations. Nature 2023, 615, 884–891. [Google Scholar] [CrossRef]
- Condeelis, J.S.; Wyckoff, J.; Segall, J.E. Imaging of Cancer Invasion and Metastasis Using Green Fluorescent Protein. European Journal of Cancer 2000, 36, 1671–1680. [Google Scholar] [CrossRef]
- Martynov, V.I.; Pakhomov, A.A.; Deyev, I.E.; Petrenko, A.G. Genetically Encoded Fluorescent Indicators for Live Cell pH Imaging. Biochimica et Biophysica Acta (BBA) - General Subjects 2018, 1862, 2924–2939. [Google Scholar] [CrossRef]
- Henrikus, S.S.; Tassis, K.; Zhang, L.; Van Der Velde, J.H.M.; Gebhardt, C.; Herrmann, A.; Jung, G.; Cordes, T. Characterization of Fluorescent Proteins with Intramolecular Photostabilization**. ChemBioChem 2021, 22, 3283–3291. [Google Scholar] [CrossRef]
- Anto, R.J.; Kuttan, G.; Kuttan, R.; Sathyanarayana, K.; Rao, M.N.A. Tumor-Reducing and Antioxidant Activities of Sydnone-Substituted Chalcones. J. Clin. Biochem. Nutr. 1994, 17, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Oda, Y. Inhibitory Effect of Curcumin on SOS Functions Induced by UV Irradiation. Mutation Research Letters 1995, 348, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Akbari, E.; Akhavan, O.; Hatamie, S.; Rahighi, R. Curcumin as a Green Fluorescent Label to Revive the Fluorescence Property of Functionalized Graphene Oxide Nanosheets. Journal of Drug Delivery Science and Technology 2018, 45, 422–427. [Google Scholar] [CrossRef]
- Sun, X.; Liu, T.; Sun, J.; Wang, X. Synthesis and Application of Coumarin Fluorescence Probes. RSC Advances 2020, 10, 10826–10847. [Google Scholar] [CrossRef]
- Liu, R.; Sun, Q.; Sun, A.; Cui, J. Isolation and Purification of Coumarin Compounds from Cortex Fraxinus by High-Speed Counter-Current Chromatography. Journal of Chromatography A 2005, 1072, 195–199. [Google Scholar] [CrossRef]
- De Lima, J.L.M.P.; Zehsaz, S.; Tavares, J.L.; De Lima, M.I.P. Brightness of Point Application of Fluorescent Quinine Tracer for Surface Waters. Eng. Sanit. Ambient. 2023, 28, e20220212. [Google Scholar] [CrossRef]
- Jaconiano, Y.R.; Goulart, J.S.; Barros, J.C.; Michel, R.C. From the Periodic Table to Photochemistry with Fluorescent Jellies: A Food Science Celebration of the International Year of the Periodic Table of Chemical Elements. Journal of Food Science Education 2021, 20, 26–30. [Google Scholar] [CrossRef]
- Le Guern, F.; Mussard, V.; Gaucher, A.; Rottman, M.; Prim, D. Fluorescein Derivatives as Fluorescent Probes for pH Monitoring along Recent Biological Applications. IJMS 2020, 21, 9217. [Google Scholar] [CrossRef]
- Poddar, M.; Misra, R. Recent Advances of BODIPY Based Derivatives for Optoelectronic Applications. Coordination Chemistry Reviews 2020, 421, 213462. [Google Scholar] [CrossRef]
- Sletten, E.M.; Swager, T.M. Fluorofluorophores: Fluorescent Fluorous Chemical Tools Spanning the Visible Spectrum. J. Am. Chem. Soc. 2014, 136, 13574–13577. [Google Scholar] [CrossRef]
- Ley, C.; Siedel, A.; Bertaux, T.; Croutxé-Barghorn, C.; Allonas, X. Photochemical Processes of Superbase Generation in Xanthone Carboxylic Salts. Angewandte Chemie 2023, 135, e202214784. [Google Scholar] [CrossRef]
- Olesiejuk, M.; Kudelko, A.; Świątkowski, M. Highly Luminescent 4H-1,2,4-Triazole Derivatives: Synthesis, Molecular Structure and Photophysical Properties. Materials 2020, 13, 5627. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, J.; Xia, S.; Cheng, S.; Shi, Y. 1,2,3-Triazole Derivatives with Anti-Breast Cancer Potential. Current Topics in Medicinal Chemistry 2022, 22, 1406–1425. [Google Scholar] [CrossRef] [PubMed]
- Scheer, H.; Yang, X.; Zhao, K.-H. Biliproteins and Their Applications in Bioimaging. Procedia Chemistry 2015, 14, 176–185. [Google Scholar] [CrossRef]
- Lawrenz, E.; Fedewa, E.J.; Richardson, T.L. Extraction Protocols for the Quantification of Phycobilins in Aqueous Phytoplankton Extracts. J Appl Phycol 2011, 23, 865–871. [Google Scholar] [CrossRef]
- Rostami-Tapeh-Esmail, E.; Golshan, M.; Salami-Kalajahi, M.; Roghani-Mamaqani, H. Perylene-3,4,9,10-Tetracarboxylic Diimide and Its Derivatives: Synthesis, Properties and Bioapplications. Dyes and Pigments 2020, 180, 108488. [Google Scholar] [CrossRef]
- Bucevičius, J.; Gerasimaitė, R.; Kiszka, K.A.; Pradhan, S.; Kostiuk, G.; Koenen, T.; Lukinavičius, G. A General Highly Efficient Synthesis of Biocompatible Rhodamine Dyes and Probes for Live-Cell Multicolor Nanoscopy. Nat Commun 2023, 14, 1306. [Google Scholar] [CrossRef]
- Lee, H.-K.; Cao, H.; Rana, T.M. Design, Microwave-Assisted Synthesis, and Photophysical Properties of Small Molecule Organic Antennas for Luminescence Resonance Energy Transfer. J. Comb. Chem. 2005, 7, 279–284. [Google Scholar] [CrossRef]
- Nizomov, N.; Kholov, A.U.; Ishchenko, A.A.; Ishchenko, V.V.; Khilya, V.P. Electronic Structure and Spectral Fluorescence Properties of Umbelliferone and Herniarin. J Appl Spectrosc 2007, 74, 626–634. [Google Scholar] [CrossRef]
- de Halleux, V.; Mamdouh, W.; De Feyter, S.; De Schryver, F.; Levin, J.; Geerts, Y.H. Emission Properties of a Highly Fluorescent Pyrene Dye in Solution and in the Liquid State. Journal of Photochemistry and Photobiology A: Chemistry 2006, 178, 251–257. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. Photophysics, Photochemistry and Photobiology of Curcumin: Studies from Organic Solutions, Bio-Mimetics and Living Cells. Journal of Photochemistry and Photobiology C: Photochemistry Reviews 2009, 10, 81–95. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Li, Y.; Junge, J.A.; Kim, N.K.; Fraser, S.E.; Zhang, C. Development of Highly Fluorogenic Styrene Probes for Visualizing RNA in Live Cells. ACS Chem. Biol. 2023, 18, 1523–1533. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Han, L.; Leng, X.; Li, Y. Synthesis of Functional Polyethylene via Scandium Catalysted Copolymerization of Ethylene with Triphenylamine- or Carbazole-Substituted Styrene Derivatives. Polymer 2022, 241, 124548. [Google Scholar] [CrossRef]
- Fletcher, A.N. Quinine Sulfate As a Fluorescence Quantum Yield Standard. Photochem & Photobiology 1969, 9, 439–444. [Google Scholar] [CrossRef]
- Law, K.Y. Squaraine Chemistry: Effects of Structural Changes on the Absorption and Multiple Fluorescence Emission of Bis[4-(Dimethylamino)Phenyl]Squaraine and Its Derivatives. J. Phys. Chem. 1987, 91, 5184–5193. [Google Scholar] [CrossRef]
- Cangiotti, M.; Staneva, D.; Ottaviani, M.F.; Vasileva-Tonkova, E.; Grabchev, I. Synthesis and Characterization of Fluorescent PAMAM Dendrimer Modified with 1,8-Naphthalimide Units and Its Cu(II) Complex Designed for Specific Biomedical Application. Journal of Photochemistry and Photobiology A: Chemistry 2021, 415, 113312. [Google Scholar] [CrossRef]
- Abou-Hatab, S.; Spata, V.A.; Matsika, S. Substituent Effects on the Absorption and Fluorescence Properties of Anthracene. J. Phys. Chem. A 2017, 121, 1213–1222. [Google Scholar] [CrossRef]
- Gersch, M.; Kreuzer, J.; Sieber, S.A. Electrophilic Natural Products and Their Biological Targets. Nat. Prod. Rep. 2012, 29, 659–682. [Google Scholar] [CrossRef]
- Escudero, D. Revising Intramolecular Photoinduced Electron Transfer (PET) from First-Principles. Acc. Chem. Res. 2016, 49, 1816–1824. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, C.; Liang, X.; Liu, F.; Yan, X.; Liu, X.; Sun, P.; Zhang, H.; Wang, Y.; Lu, G. Stimulated Emission Depletion (STED) Super-Resolution Imaging with an Advanced Organic Fluorescent Probe: Visualizing the Cellular Lipid Droplets at the Unprecedented Nanoscale Resolution. ACS Materials Lett. 2021, 3, 516–524. [Google Scholar] [CrossRef]
- Verma, A.K.; Noumani, A.; Yadav, A.K.; Solanki, P.R. FRET Based Biosensor: Principle Applications Recent Advances and Challenges. Diagnostics 2023, 13, 1375. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, W.; Yu, X.; Zhang, G.; Su, Z. Synthesis and Biomedical Applications of Fluorescent Nanogels. Polym. Chem. 2016, 7, 5749–5762. [Google Scholar] [CrossRef]
- Rodriguez Burbano, D.C.; Naccache, R.; Capobianco, J.A. Near-IR Triggered Photon Upconversion. In Handbook on the Physics and Chemistry of Rare Earths; Elsevier, 2015; Vol. 47, pp. 273–347 ISBN 978-0-444-63481-8.
- Yamaguchi, Y.; Matsubara, Y.; Ochi, T.; Wakamiya, T.; Yoshida, Z. How the π Conjugation Length Affects the Fluorescence Emission Efficiency. J. Am. Chem. Soc. 2008, 130, 16442–16442. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, M.; Ren, X.; Shi, W.; Yin, T.; Luo, T.; Lan, Y.; Li, X.; Guan, L. Effect of Conjugation Length on Fluorescence Characteristics of Carbon Dots. RSC Adv. 2023, 13, 27714–27721. [Google Scholar] [CrossRef] [PubMed]
- Liu, M. Optical Properties of Carbon Dots: A Review. NAT 2020, 1, 1–12. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, P.; Zheng, L.; Shi, X.; Zheng, H. Carbon Nanomaterials with Sp or/and Sp Hybridization in Energy Conversion and Storage Applications: A Review. Energy Storage Materials 2020, 26, 349–370. [Google Scholar] [CrossRef]
- Sk, M.A.; Ananthanarayanan, A.; Huang, L.; Lim, K.H.; Chen, P. Revealing the Tunable Photoluminescence Properties of Graphene Quantum Dots. J. Mater. Chem. C 2014, 2, 6954–6960. [Google Scholar] [CrossRef]
- Giri, R.; Rathi, S.S.; Machwe, M.K.; Murti, V.V.S. Effect of Substituents on the Fluorescence and Absorption Spectra of Coumarins. Spectrochimica Acta Part A: Molecular Spectroscopy 1988, 44, 805–807. [Google Scholar] [CrossRef]
- She, M.; Yang, Z.; Hao, L.; Wang, Z.; Luo, T.; Obst, M.; Liu, P.; Shen, Y.; Zhang, S.; Li, J. A Novel Approach to Study the Structure-Property Relationships and Applications in Living Systems of Modular Cu2+ Fluorescent Probes. Sci Rep 2016, 6, 28972. [Google Scholar] [CrossRef]
- Madhura, V.; Kulkarni, M.V.; Badami, S.; Yenagi, J.; Tonannavar, J. Effect of Nitro Groups on the Photo Physical Properties of Benzimidazolone: A Solvatochromic Study. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2011, 84, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.-P.; Luo, J.-C.; Wu, W.; Xiong, J.-F.; Mo, G.-Z.; Wang, Z.-Y. Synthesis and Characterization of Fluorescent Brightening Agents with Chiral 2(5H)-Furanone and Bis-1,2,3-Triazole Structure. Ind. Eng. Chem. Res. 2013, 52, 11850–11857. [Google Scholar] [CrossRef]
- Nienhaus, G.U.; Wiedenmann, J. Structure, Dynamics and Optical Properties of Fluorescent Proteins: Perspectives for Marker Development. ChemPhysChem 2009, 10, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Sedgwick, A.C.; Wu, L.; Han, H.-H.; Bull, S.D.; He, X.-P.; James, T.D.; Sessler, J.L.; Tang, B.Z.; Tian, H.; Yoon, J. Excited-State Intramolecular Proton-Transfer (ESIPT) Based Fluorescence Sensors and Imaging Agents. Chem. Soc. Rev. 2018, 47, 8842–8880. [Google Scholar] [CrossRef] [PubMed]
- Stennett, E.M.S.; Ciuba, M.A.; Levitus, M. Photophysical Processes in Single Molecule Organic Fluorescent Probes. Chem. Soc. Rev. 2014, 43, 1057–1075. [Google Scholar] [CrossRef]
- Steffen, F.D.; Sigel, R.K.O.; Börner, R. An Atomistic View on Carbocyanine Photophysics in the Realm of RNA. Phys. Chem. Chem. Phys. 2016, 18, 29045–29055. [Google Scholar] [CrossRef]
- Hu, X.-L.; Gan, H.-Q.; Meng, F.-D.; Han, H.-H.; Shi, D.-T.; Zhang, S.; Zou, L.; He, X.-P.; James, T.D. Fluorescent Probes and Functional Materials for Biomedical Applications. Front. Chem. Sci. Eng. 2022, 16, 1425–1437. [Google Scholar] [CrossRef]
- Singh, J.-S.; Singh, A.; Garg, N.; Kaur, N.; Singh, N. A Highly Selective Naphthalimide-Based Ratiometric Fluorescent Probe for the Recognition of Tyrosinase and Cellular Imaging. Analyst 2018, 143, 4476–4483. [Google Scholar] [CrossRef]
- Georgiev, N.I.; Said, A.I.; Toshkova, R.A.; Tzoneva, R.D.; Bojinov, V.B. A Novel Water-Soluble Perylenetetracarboxylic Diimide as a Fluorescent pH Probe: Chemosensing, Biocompatibility and Cell Imaging. Dyes and Pigments 2019, 160, 28–36. [Google Scholar] [CrossRef]
- Georgiev, N.I.; Bakov, V.V.; Anichina, K.K.; Bojinov, V.B. Fluorescent Probes as a Tool in Diagnostic and Drug Delivery Systems. Pharmaceuticals 2023, 16, 381. [Google Scholar] [CrossRef]
- Patsenker, L.; Gellerman, G. Fluorescent Reporters for Drug Delivery Monitoring. Israel Journal of Chemistry 2020, 60, 504–518. [Google Scholar] [CrossRef]
- Novo, D.C.; Edgar, K.J. Smart Fluorescent Polysaccharides: Recent Developments and Applications. Carbohydrate Polymers 2024, 324, 121471. [Google Scholar] [CrossRef] [PubMed]
- Arun, K.H.S.; Kaul, C.L.; Ramarao, P. Green Fluorescent Proteins in Receptor Research: An Emerging Tool for Drug Discovery. Journal of Pharmacological and Toxicological Methods 2005, 51, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, X.; Wang, R.; Wang, X.; Li, Z.; Yang, Y.; Zhu, H.-L.; Qian, Y. Engineered Human Serum Albumin for Mapping Drug Fluctuations: Generatation of Pseudo-Fluorescent Proteins by Covalently Coupling Dyes for Probing Tamoxifen in Vitro, in Cellulo, and in Vivo. Chemical Engineering Journal 2024, 484, 149484. [Google Scholar] [CrossRef]
- Fan, J.; Wang, S.; Sun, W.; Guo, S.; Kang, Y.; Du, J.; Peng, X. Anticancer Drug Delivery Systems Based on Inorganic Nanocarriers with Fluorescent Tracers. AIChE Journal 2018, 64, 835–859. [Google Scholar] [CrossRef]
- Lu, J.; Liong, M.; Li, Z.; Zink, J.I.; Tamanoi, F. Biocompatibility, Biodistribution, and Drug-Delivery Efficiency of Mesoporous Silica Nanoparticles for Cancer Therapy in Animals. Small 2010, 6, 1794–1805. [Google Scholar] [CrossRef]
- Tjandra, A.D.; Chang, J.Y.H.; Ladame, S.; Chandrawati, R. Chapter 1.2 - Optical Sensors. In Bioengineering Innovative Solutions for Cancer; Ladame, S., Chang, J.Y.H., Eds.; Academic Press, 2020; pp. 23–45 ISBN 978-0-12-813886-1.
- Prathap, N.; Balla, P.; Shivakumar, M.S.; Periyasami, G.; Karuppiah, P.; Ramasamy, K.; Venkatesan, S. Prosopis Juliflora Hydrothermal Synthesis of High Fluorescent Carbon Dots and Its Antibacterial and Bioimaging Applications. Sci Rep 2023, 13, 9676. [Google Scholar] [CrossRef]
- Cui, C.; Shu, W.; Li, P. Fluorescence In Situ Hybridization: Cell-Based Genetic Diagnostic and Research Applications. Front Cell Dev Biol 2016, 4, 89. [Google Scholar] [CrossRef]
- Agius, N.; Magri, D.C. Cinchona Alkaloids – Acid, Anion-Driven Fluorescent INHIBIT Logic Gates with a Receptor1–Fluorophore–Spacer–Receptor2 Format and PET and ICT Mechanisms. RSC Adv. 2023, 13, 13505–13510. [Google Scholar] [CrossRef]
- Geddes, C.D. Optical Halide Sensing Using Fluorescence Quenching: Theory, Simulations and Applications - a Review. Meas. Sci. Technol. 2001, 12, R53. [Google Scholar] [CrossRef]
- Lang, K.; Chin, J.W. Cellular Incorporation of Unnatural Amino Acids and Bioorthogonal Labeling of Proteins. Chem. Rev. 2014, 114, 4764–4806. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Schultz, P.G. Adding New Chemistries to the Genetic Code. Annu. Rev. Biochem. 2010, 79, 413–444. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Kuru, E.; Sachdeva, A.; Vendrell, M. Fluorescent Amino Acids as Versatile Building Blocks for Chemical Biology. Nat Rev Chem 2020, 4, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Dou, W.-T.; Han, H.-H.; Sedgwick, A.C.; Zhu, G.-B.; Zang, Y.; Yang, X.-R.; Yoon, J.; James, T.D.; Li, J.; He, X.-P. Fluorescent Probes for the Detection of Disease-Associated Biomarkers. Science Bulletin 2022, 67, 853–878. [Google Scholar] [CrossRef]
- Matsumoto, A.; Matsui, I.; Uchinomiya, S.; Katsuma, Y.; Yasuda, S.; Okushima, H.; Imai, A.; Yamamoto, T.; Ojida, A.; Inoue, K.; et al. Spatiotemporally Quantitative in Vivo Imaging of Mitochondrial Fatty Acid β-Oxidation at Cellular-Level Resolution in Mice. American Journal of Physiology-Endocrinology and Metabolism 2023, 325, E552–E561. [Google Scholar] [CrossRef]
- Agnifili, L.; Pieragostino, D.; Mastropasqua, A.; Fasanella, V.; Brescia, L.; Tosi, G.M.; Sacchetta, P.; Mastropasqua, L. Chapter 1 - Molecular Biomarkers in Primary Open-Angle Glaucoma: From Noninvasive to Invasive. In Progress in Brain Research; Bagetta, G., Nucci, C., Eds.; New Trends in Basic and Clinical Research of Glaucoma: A Neurodegenerative Disease of the Visual System, Part B; Elsevier, 2015; Volume 221, pp. 1–32. [Google Scholar]
- Gao, L.; Wang, W.; Wang, X.; Yang, F.; Xie, L.; Shen, J.; Brimble, M.A.; Xiao, Q.; Yao, S.Q. Fluorescent Probes for Bioimaging of Potential Biomarkers in Parkinson’s Disease. Chem. Soc. Rev. 2021, 50, 1219–1250. [Google Scholar] [CrossRef]
- Yang, W.-Y.; Yan, C.-C.; Wang, X.-D.; Liao, L.-S. Recent Progress on the Excited-State Multiple Proton Transfer Process in Organic Molecules. Sci. China Chem. 2022, 65, 1843–1853. [Google Scholar] [CrossRef]
- Odyniec, M.L.; Park, S.-J.; Gardiner, J.E.; Webb, E.C.; Sedgwick, A.C.; Yoon, J.; Bull, S.D.; Kim, H.M.; James, T.D. A Fluorescent ESIPT-Based Benzimidazole Platform for the Ratiometric Two-Photon Imaging of ONOO−in Vitro and Ex Vivo. Chem. Sci. 2020, 11, 7329–7334. [Google Scholar] [CrossRef]
- Lv, H.-M.; Yuan, D.-H.; Liu, W.; Chen, Y.; Au, C.-T.; Yin, S.-F. A Highly Selective ESIPT-Based Fluorescent Probe for Cysteine Sensing and Its Bioimaging Application in Living Cells. Sensors and Actuators B: Chemical 2016, 233, 173–179. [Google Scholar] [CrossRef]
- Huang, R.; He, N.; Li, Z. Recent Progresses in DNA Nanostructure-Based Biosensors for Detection of Tumor Markers. Biosens Bioelectron 2018, 109, 27–34. [Google Scholar] [CrossRef]
- Gui, R.; Jin, H.; Bu, X.; Fu, Y.; Wang, Z.; Liu, Q. Recent Advances in Dual-Emission Ratiometric Fluorescence Probes for Chemo/Biosensing and Bioimaging of Biomarkers. Coordination Chemistry Reviews 2019, 383, 82–103. [Google Scholar] [CrossRef]
- Bu, X.; Fu, Y.; Jin, H.; Gui, R. Specific Enzymatic Synthesis of 2,3-Diaminophenazine and Copper Nanoclusters Used for Dual-Emission Ratiometric and Naked-Eye Visual Fluorescence Sensing of Choline. New J. Chem. 2018, 42, 17323–17330. [Google Scholar] [CrossRef]
- Aydin, Z.; Yan, B.; Wei, Y.; Guo, M. A Novel Near-Infrared Turn-on and Ratiometric Fluorescent Probe Capable of Copper( ii ) Ion Determination in Living Cells. Chem. Commun. 2020, 56, 6043–6046. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, X.; Lu, J.; Wei, D.; Zhou, N.; Li, Y.; Wang, Y.; He, H. Rapid Detection of Prostate Cancer Biomarkers in Living Cells and Urine Samples Using Diboronic Acid Fluorescent Probe. Sensors and Actuators B: Chemical 2023, 392, 134040. [Google Scholar] [CrossRef]
- Tian, M.; Wu, R.; Xiang, C.; Niu, G.; Guan, W. Recent Advances in Fluorescent Probes for Cancer Biomarker Detection. Molecules 2024, 29, 1168. [Google Scholar] [CrossRef]
- Liang, S.-L.; Chan, D.W. Enzymes and Related Proteins as Cancer Biomarkers: A Proteomic Approach. Clinica Chimica Acta 2007, 381, 93–97. [Google Scholar] [CrossRef]
- Wang, Z.; Li, R.; Yang, G.; Wang, Y. Cancer Stem Cell Biomarkers and Related Signalling Pathways. Journal of Drug Targeting 2024, 32, 33–44. [Google Scholar] [CrossRef]
- Yang, Z.; Xie, L.; Han, L.; Qu, X.; Yang, Y.; Zhang, Y.; He, Z.; Wang, Y.; Li, J. Circular RNAs: Regulators of Cancer-Related Signaling Pathways and Potential Diagnostic Biomarkers for Human Cancers. Theranostics 2017, 7, 3106–3117. [Google Scholar] [CrossRef]
- Kontos, C.K.; Scorilas, A. Kallikrein-Related Peptidases (KLKs): A Gene Family of Novel Cancer Biomarkers. Clinical Chemistry and Laboratory Medicine (CCLM) 2012, 50, 1877–1891. [Google Scholar] [CrossRef]
- Jiang, L.; Lin, X.; Chen, F.; Qin, X.; Yan, Y.; Ren, L.; Yu, H.; Chang, L.; Wang, Y. Current Research Status of Tumor Cell Biomarker Detection. Microsyst Nanoeng 2023, 9, 123. [Google Scholar] [CrossRef]
- Ardeshirpour, Y.; Chernomordik, V.; Capala, J.; Hassan, M.; Zielinsky, R.; Griffiths, G.; Achilefu, S.; Smith, P.; Gandjbakhche, A. Using In-Vivo Fluorescence Imaging in Personalized Cancer Diagnostics and Therapy, an Image and Treat Paradigm. Technol Cancer Res Treat 2011, 10, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Xiong, M.; Wang, X.; Lyu, M.; Sun, H.; Cui, Y.; Chen, C.; Jiang, Z.; Sun, F. Regulation of the Function and Expression of EpCAM. Biomedicines 2024, 12, 1129. [Google Scholar] [CrossRef] [PubMed]
- Van Driel, P.B.A.A.; Boonstra, M.C.; Prevoo, H.A.J.M.; Van De Giessen, M.; Snoeks, T.J.A.; Tummers, Q.R.J.G.; Keereweer, S.; Cordfunke, R.A.; Fish, A.; Van Eendenburg, J.D.H.; et al. EpCAM as Multi-Tumour Target for near-Infrared Fluorescence Guided Surgery. BMC Cancer 2016, 16, 884. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Weng, S.; Zhang, F.; Allen, S.; Li, X.; Bao, L.; Lam, R.H.W.; Macoska, J.A.; Merajver, S.D.; Fu, J. Nanoroughened Surfaces for Efficient Capture of Circulating Tumor Cells without Using Capture Antibodies. ACS Nano 2013, 7, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.W.; Jeong, H.Y.; Kang, S.J.; Choi, M.J.; You, Y.M.; Im, C.S.; Lee, T.S.; Song, I.H.; Lee, C.G.; Rhee, K.-J.; et al. Cancer-Targeted Nucleic Acid Delivery and Quantum Dot Imaging Using EGF Receptor Aptamer-Conjugated Lipid Nanoparticles. Sci Rep 2017, 7, 9474. [Google Scholar] [CrossRef]
- Wu, P.; Zhu, Y.; Liu, S.; Xiong, H. Modular Design of High-Brightness pH-Activatable Near-Infrared BODIPY Probes for Noninvasive Fluorescence Detection of Deep-Seated Early Breast Cancer Bone Metastasis: Remarkable Axial Substituent Effect on Performance. ACS Cent. Sci. 2021, 7, 2039–2048. [Google Scholar] [CrossRef]
- Han, Y.; Li, X.; Li, D.; Chen, C.; Zhang, Q.-W.; Tian, Y. Selective, Rapid, and Ratiometric Fluorescence Sensing of Homocysteine in Live Neurons via a Reaction-Kinetics/Sequence-Differentiation Strategy Based on a Small Molecular Probe. ACS Sens. 2022, 7, 1036–1044. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.-F.; Chen, Q.; Cao, X.-Q.; Shen, S.-L. A Novel Near-Infrared Fluorescence off-on Probe for Imaging Hypoxia and Nitroreductase in Cells and in Vivo. Sensors and Actuators B: Chemical 2022, 353, 131145. [Google Scholar] [CrossRef]
- Li, W.; Sun, L.; Zheng, X.; Li, F.; Zhang, W.; Li, T.; Guo, Y.; Tang, D. Multifunctional Nanoprobe Based on Fluorescence Resonance Energy Transfer for Furin Detection and Drug Delivery. Anal. Chem. 2023, 95, 9654–9662. [Google Scholar] [CrossRef]
- Karunakaran, C.; Rajkumar, R.; Bhargava, K. Chapter 1 - Introduction to Biosensors. In Biosensors and Bioelectronics; Karunakaran, C., Bhargava, K., Benjamin, R., Eds.; Elsevier, 2015; pp. 1–68 ISBN 978-0-12-803100-1.
- Strianese, M.; Staiano, M.; Ruggiero, G.; Labella, T.; Pellecchia, C.; D’Auria, S. Fluorescence-Based Biosensors. In Spectroscopic Methods of Analysis: Methods and Protocols; Bujalowski, W.M., Ed.; Humana Press: Totowa, NJ, 2012; ISBN 978-1-61779-806-1. [Google Scholar]
- Wu, D.; Sedgwick, A.C.; Gunnlaugsson, T.; Akkaya, E.U.; Yoon, J.; James, T.D. Fluorescent Chemosensors: The Past, Present and Future. Chem. Soc. Rev. 2017, 46, 7105–7123. [Google Scholar] [CrossRef]
- Zhou, Y. Biomedical Applications of Fluorescent Biosensors. TNS 2023, 23, 265–271. [Google Scholar] [CrossRef]
- Wang, H.; Nakata, E.; Hamachi, I. Recent Progress in Strategies for the Creation of Protein-Based Fluorescent Biosensors. ChemBioChem 2009, 10, 2560–2577. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, K.A.; Taylor, D.L. Fluorescent-Protein Biosensors: New Tools for Drug Discovery. Trends in Biotechnology 1998, 16, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Okumoto, S.; Jones, A.; Frommer, W.B. Quantitative Imaging with Fluorescent Biosensors. Annual Review of Plant Biology 2012, 63, 663–706. [Google Scholar] [CrossRef] [PubMed]
- Chelly, M.; Chelly, S.; Ferlazzo, A.; Neri, G.; Bouaziz-Ketata, H. Lavandula Multifida as a Novel Eco-Friendly Fluorescent-Blue Material for Mercury Ions Sensing in Seawater at Femto-Molar Concentration. Chemosphere 2024, 352, 141409. [Google Scholar] [CrossRef]
- Guisán-Ceinos, S.; Rivero, A.; Romeo-Gella, F.; Simón-Fuente, S.; Gómez-Pastor, S.; Calvo, N.; Orrego, A.H.; Guisán, J.M.; Corral, I.; Sanz-Rodriguez, F.; et al. Turn-on Fluorescent Biosensors for Imaging Hypoxia-like Conditions in Living Cells. J. Am. Chem. Soc. 2022, 144, 8185–8193. [Google Scholar] [CrossRef]
- Trinh, K.T.L.; Lee, N.Y. Recent Methods for the Viability Assessment of Bacterial Pathogens: Advances, Challenges, and Future Perspectives. Pathogens 2022, 11, 1057. [Google Scholar] [CrossRef]
- Briggs, C. Quality Counts: New Parameters in Blood Cell Counting. International Journal of Laboratory Hematology 2009, 31, 277–297. [Google Scholar] [CrossRef]
- Carter, K.P.; Young, A.M.; Palmer, A.E. Fluorescent Sensors for Measuring Metal Ions in Living Systems. Chem. Rev. 2014, 114, 4564–4601. [Google Scholar] [CrossRef]
- Wright, M.H.; Sieber, S.A. Chemical Proteomics Approaches for Identifying the Cellular Targets of Natural Products. Nat. Prod. Rep. 2016, 33, 681–708. [Google Scholar] [CrossRef]
- Chen, X.; Huang, Z.; Huang, L.; Shen, Q.; Yang, N.-D.; Pu, C.; Shao, J.; Li, L.; Yu, C.; Huang, W. Small-Molecule Fluorescent Probes Based on Covalent Assembly Strategy for Chemoselective Bioimaging. RSC Adv 2022, 12, 1393–1415. [Google Scholar] [CrossRef] [PubMed]
- De Zotti, M.; Bobone, S.; Bortolotti, A.; Longo, E.; Biondi, B.; Peggion, C.; Formaggio, F.; Toniolo, C.; Dalla Bona, A.; Kaptein, B.; et al. 4-Cyano-α-Methyl-l-Phenylalanine as a Spectroscopic Marker for the Investigation of PeptaibioticMembrane Interactions. Chemistry & Biodiversity 2015, 12, 513–527. [Google Scholar] [CrossRef]
- Gupta, A.; Garreffi, B.P.; Guo, M. Facile Synthesis of a Novel Genetically Encodable Fluorescent α-Amino Acid Emitting Greenish Blue Light. Chem. Commun. 2020, 56, 12578–12581. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Yan, B.; Guo, M. Synthesis, Characterization, and Cellular Imaging of a Novel Cyan Emitting Fluorescent α-Amino Acid. Journal of Biological Chemistry 2023, 299, 103251. [Google Scholar] [CrossRef]
- Gupta, A.; Yan, B.; Guo, M. Synthesis, Characterization, and Cellular Imaging of a Cyan Emitting Fluorescent Alpha-Amino Acid for Live-Cell Protein Imaging. In Proceedings of the PROTEIN SCIENCE.; WILEY 111 RIVER ST, HOBOKEN 07030-5774, NJ USA, 2023; Vol. 32.
- Cheruku, P.; Huang, J.-H.; Yen, H.-J.; Iyer, R.S.; Rector, K.D.; Martinez, J.S.; Wang, H.-L. Tyrosine-Derived Stimuli Responsive, Fluorescent Amino Acids. Chem. Sci. 2015, 6, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Demchenko, A.P. Optimization of Fluorescence Response in the Design of Molecular Biosensors. Anal Biochem 2005, 343, 1–22. [Google Scholar] [CrossRef]
- Wang, J.; Xie, J.; Schultz, P.G. A Genetically Encoded Fluorescent Amino Acid. J. Am. Chem. Soc. 2006, 128, 8738–8739. [Google Scholar] [CrossRef]
- Takeo, M.; Lee, W.; Ito, M. Wound Healing and Skin Regeneration. Cold Spring Harb Perspect Med 2015, 5, a023267. [Google Scholar] [CrossRef]
- Brown, B.N.; Badylak, S.F. The Role of the Host Immune Response in Tissue Engineering and Regenerative Medicine. In Principles of Tissue Engineering; Elsevier, 2014; pp. 497–509 ISBN 978-0-12-398358-9.
- Ellis, S.; Lin, E.J.; Tartar, D. Immunology of Wound Healing. Curr Derm Rep 2018, 7, 350–358. [Google Scholar] [CrossRef]
- Luo, X.; Cheng, S.; Zhang, W.; Dou, K.; Wang, R.; Yu, F. Near-Infrared Fluorescence Probe for Indication of the Pathological Stages of Wound Healing Process and Its Clinical Application. ACS Sens. 2024, 9, 810–819. [Google Scholar] [CrossRef]
- Nasir, N.A.M.; Paus, R.; Ansell, D.M. Fluorescent Cell Tracer Dye Permits Real-time Assessment of Re-epithelialization in a Serum-free Ex Vivo Human Skin Wound Assay. Wound Repair Regeneration 2019, 27, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Price, N. Routine Fluorescence Imaging to Detect Wound Bacteria Reduces Antibiotic Use and Antimicrobial Dressing Expenditure While Improving Healing Rates: Retrospective Analysis of 229 Foot Ulcers. Diagnostics 2020, 10, 927. [Google Scholar] [CrossRef] [PubMed]
- Mościcka, P.; Cwajda-Białasik, J.; Jawień, A.; Szewczyk, M.T. Fluorescence - Modern Method of the Diagnosis of Chronic Wounds on the Example of Venous Leg Ulcer. Postepy Dermatol Alergol 2023, 40, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Le, L.; Baer, M.; Briggs, P.; Bullock, N.; Cole, W.; DiMarco, D.; Hamil, R.; Harrell, K.; Kasper, M.; Li, W.; et al. Diagnostic Accuracy of Point-of-Care Fluorescence Imaging for the Detection of Bacterial Burden in Wounds: Results from the 350-Patient Fluorescence Imaging Assessment and Guidance Trial. Adv Wound Care (New Rochelle) 2021, 10, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Liu, P.; Zha, R.; Zong, B.; Wang, Y.; Fang, H.; Wong, W.-L.; Li, C. An Ionic Liquid-Functionalized near-Infrared Fluorescent Hydrogel Dressing for Promoting Wound Healing and Real-Time Monitoring Hypochlorous Acid at the Diabetic Wound Site. Sensors and Actuators B: Chemical 2023, 394, 134405. [Google Scholar] [CrossRef]
- Paganin-Gioanni, A.; Bellard, E.; Paquereau, L.; Ecochard, V.; Golzio, M.; Teissié, J. Fluorescence Imaging Agents in Cancerology. Radiology and Oncology 2010, 44. [Google Scholar] [CrossRef]
- Neijenhuis, L.K.A.; De Myunck, L.D.A.N.; Bijlstra, O.D.; Kuppen, P.J.K.; Hilling, D.E.; Borm, F.J.; Cohen, D.; Mieog, J.S.D.; Steup, W.H.; Braun, J.; et al. Near-Infrared Fluorescence Tumor-Targeted Imaging in Lung Cancer: A Systematic Review. Life 2022, 12, 446. [Google Scholar] [CrossRef]
- Shi, C.; Wu, J.B.; Pan, D. Review on Near-Infrared Heptamethine Cyanine Dyes as Theranostic Agents for Tumor Imaging, Targeting, and Photodynamic Therapy. J. Biomed. Opt 2016, 21, 050901. [Google Scholar] [CrossRef]
- Yeh, C.-S.; Su, C.-H.; Ho, W.-Y.; Huang, C.-C.; Chang, J.-C.; Chien, Y.-H.; Hung, S.-T.; Liau, M.-C.; Ho, H.-Y. Tumor Targeting and MR Imaging with Lipophilic Cyanine-Mediated near-Infrared Responsive Porous Gd Silicate Nanoparticles. Biomaterials 2013, 34, 5677–5688. [Google Scholar] [CrossRef]
- Kobayashi, H.; Choyke, P.L. Target-Cancer-Cell-Specific Activatable Fluorescence Imaging Probes: Rational Design and in Vivo Applications. Acc. Chem. Res. 2011, 44, 83–90. [Google Scholar] [CrossRef]
- Zhang, R.R.; Schroeder, A.B.; Grudzinski, J.J.; Rosenthal, E.L.; Warram, J.M.; Pinchuk, A.N.; Eliceiri, K.W.; Kuo, J.S.; Weichert, J.P. Beyond the Margins: Real-Time Detection of Cancer Using Targeted Fluorophores. Nat Rev Clin Oncol 2017, 14, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, S.; Zhang, H.; Xu, H. Design and Application of Receptor-Targeted Fluorescent Probes Based on Small Molecular Fluorescent Dyes. Bioconjugate Chem. 2021, 32, 4–24. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Yu, F.; Lv, C.; Choo, J.; Chen, L. Fluorescent Chemical Probes for Accurate Tumor Diagnosis and Targeting Therapy. Chem. Soc. Rev. 2017, 46, 2237–2271. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.; Hessenius, C.; Licha, K.; Ebert, B.; Sukowski, U.; Semmler, W.; Wiedenmann, B.; Grötzinger, C. Receptor-Targeted Optical Imaging of Tumors with near-Infrared Fluorescent Ligands. Nat Biotechnol 2001, 19, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, Z.; Guo, L.; Zhang, H.; Su, T.; Tan, Z.; Ren, Q.; Zhang, C.; Fu, Y.; Xing, R.; et al. Discovery of Novel Tumor-Targeted near-Infrared Probes with 6-Substituted Pyrrolo[2,3-d]Pyrimidines as Targeting Ligands. European Journal of Medicinal Chemistry 2023, 262, 115914. [Google Scholar] [CrossRef]
- Tazawa, H.; Shigeyasu, K.; Noma, K.; Kagawa, S.; Sakurai, F.; Mizuguchi, H.; Kobayashi, H.; Imamura, T.; Fujiwara, T. Tumor-targeted Fluorescence Labeling Systems for Cancer Diagnosis and Treatment. Cancer Science 2022, 113, 1919. [Google Scholar] [CrossRef]
- Biochemistry (Moscow). Available online: https://link.springer.com/journal/10541 (accessed on 7 May 2024).
- Lavis, L.D.; Raines, R.T. Bright Building Blocks for Chemical Biology. ACS Chem Biol 2014, 9, 855–866. [Google Scholar] [CrossRef]
- Malekzad, H.; Zangabad, P.S.; Mirshekari, H.; Karimi, M.; Hamblin, M.R. Noble Metal Nanoparticles in Biosensors: Recent Studies and Applications. Nanotechnol Rev 2017, 6, 301–329. [Google Scholar] [CrossRef]
- Tian, X.; Murfin, L.C.; Wu, L.; Lewis, S.E.; James, T.D. Fluorescent Small Organic Probes for Biosensing. Chem. Sci. 2021, 12, 3406–3426. [Google Scholar] [CrossRef]
- Nath, P.; Mahtaba, K.R.; Ray, A. Fluorescence-Based Portable Assays for Detection of Biological and Chemical Analytes. Sensors 2023, 23, 5053. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, Y.; Liu, Z.; Guo, M. A Novel Profluorescent Probe for Detecting Oxidative Stress Induced by Metal and H2O2 in Living Cells. Chem. Commun. 2010, 46, 4472. [Google Scholar] [CrossRef] [PubMed]
- Maiti, S.; Aydin, Z.; Zhang, Y.; Guo, M. Reaction-Based Turn-on Fluorescent Probes with Magnetic Responses for Fe 2+ Detection in Live Cells. Dalton Trans. 2015, 44, 8942–8949. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Aydin, Z.; Zhang, Y.; Liu, Z.; Guo, M. A Turn-on Fluorescent Sensor for Imaging Labile Fe 3+ in Live Neuronal Cells at Subcellular Resolution. ChemBioChem 2012, 13, 1569–1573. [Google Scholar] [CrossRef] [PubMed]
- Hudson, N.; Baker, A.; Reynolds, D. Fluorescence Analysis of Dissolved Organic Matter in Natural, Waste and Polluted Waters—A Review. River Research & Apps 2007, 23, 631–649. [Google Scholar] [CrossRef]
- Brouwer, E.R.; Hermans, A.N.J.; Lingeman, H.; Brinkman, U.A.T. Determination of Polycyclic Aromatic Hydrocarbons in Surface Water by Column Liquid Chromatography with Fluorescence Detection, Using on-Line Micelle-Mediated Sample Preparation. Journal of Chromatography A 1994, 669, 45–57. [Google Scholar] [CrossRef]
- Shetty, S.S.; Deepthi, D.; Harshitha, S.; Sonkusare, S.; Naik, P.B.; Kumari, N.S.; Madhyastha, H. Environmental Pollutants and Their Effects on Human Health. Heliyon 2023, 9, e19496. [Google Scholar] [CrossRef]
- Shin, Y.-H.; Gutierrez-Wing, M.T.; Choi, J.-W. Review—Recent Progress in Portable Fluorescence Sensors. J. Electrochem. Soc. 2021, 168, 017502. [Google Scholar] [CrossRef]
- Shang, Z.; Wu, M.; Meng, Q.; Jiao, Y.; Zhang, Z.; Zhang, R. A Near-Infrared Fluorescent Probe for Rapid and on-Site Detection of Sulfur Dioxide Derivative in Biological, Food and Environmental Systems. Journal of Hazardous Materials 2024, 465, 133165. [Google Scholar] [CrossRef]
- Ali, R.; Razi, S.S.; Shahid, M.; Srivastava, P.; Misra, A. Off – On – Off Fluorescence Behavior of an Intramolecular Charge Transfer Probe toward Anions and CO 2. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2016, 168, 21–28. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Meng, Z.; Li, M.; Zhang, C.; Yang, L.; Yang, Y.; Xu, X.; Wang, S. Development of a Ratiometric Fluorescent Probe with Large Stokes Shift and Emission Wavelength Shift for Real-Time Tracking of Hydrazine and Its Multiple Applications in Environmental Analysis and Biological Imaging. Journal of Hazardous Materials 2022, 422, 126891. [Google Scholar] [CrossRef]
- Ahmad, S.R.; Reynolds, D.M. Synchronous Fluorescence Spectroscopy of Wastewater and Some Potential Constituents. Water Research 1995, 29, 1599–1602. [Google Scholar] [CrossRef]
- Jaffari, Z.H.; Lam, S.-M.; Sin, J.-C.; Zeng, H.; Mohamed, A.R. Magnetically Recoverable Pd-Loaded BiFeO3 Microcomposite with Enhanced Visible Light Photocatalytic Performance for Pollutant, Bacterial and Fungal Elimination. Separation and Purification Technology 2020, 236, 116195. [Google Scholar] [CrossRef]
- Coble, P.G. Characterization of Marine and Terrestrial DOM in Seawater Using Excitation-Emission Matrix Spectroscopy. Marine Chemistry 1996, 51, 325–346. [Google Scholar] [CrossRef]
- Wang, X.-H.; Wang, X.; Huppes, G.; Heijungs, R.; Ren, N.-Q. Environmental Implications of Increasingly Stringent Sewage Discharge Standards in Municipal Wastewater Treatment Plants: Case Study of a Cool Area of China. Journal of Cleaner Production 2015, 94, 278–283. [Google Scholar] [CrossRef]
- Li, W.; Xu, Z.; Wu, Q.; Li, Y.; Shuang, C.; Li, A. Characterization of Fluorescent-Dissolved Organic Matter and Identification of Specific Fluorophores in Textile Effluents. Environ Sci Pollut Res 2015, 22, 4183–4189. [Google Scholar] [CrossRef]
- Weishaar, J.L.; Aiken, G.R.; Bergamaschi, B.A.; Fram, M.S.; Fujii, R.; Mopper, K. Evaluation of Specific Ultraviolet Absorbance as an Indicator of the Chemical Composition and Reactivity of Dissolved Organic Carbon. Environ. Sci. Technol. 2003, 37, 4702–4708. [Google Scholar] [CrossRef]
- Sorensen, J.P.R.; Lapworth, D.J.; Marchant, B.P.; Nkhuwa, D.C.W.; Pedley, S.; Stuart, M.E.; Bell, R.A.; Chirwa, M.; Kabika, J.; Liemisa, M.; et al. In-Situ Tryptophan-like Fluorescence: A Real-Time Indicator of Faecal Contamination in Drinking Water Supplies. Water Research 2015, 81, 38–46. [Google Scholar] [CrossRef]
- Tartakovsky, B.; Lishman, L.A.; Legge, R.L. Application of Multi-Wavelength Fluorometry for Monitoring Wastewater Treatment Process Dynamics. Water Research 1996, 30, 2941–2948. [Google Scholar] [CrossRef]
- Carstea, E.M.; Bridgeman, J.; Baker, A.; Reynolds, D.M. Fluorescence Spectroscopy for Wastewater Monitoring: A Review. Water Research 2016, 95, 205–219. [Google Scholar] [CrossRef]
- Galinha, C.F.; Carvalho, G.; Portugal, C.A.M.; Guglielmi, G.; Oliveira, R.; Crespo, J.G.; Reis, M.A.M. Real-Time Monitoring of Membrane Bioreactors with 2D-Fluorescence Data and Statistically Based Models. Water Science and Technology 2011, 63, 1381–1388. [Google Scholar] [CrossRef]
- Baker, A. Fluorescence Properties of Some Farm Wastes: Implications for Water Quality Monitoring. Water Research 2002, 36, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.; Bro, R.; Stedmon, C. Chemometric Analysis of Organic Matter Fluorescence. In; 2014; pp. 339–375 ISBN 978-1-139-04545-2.
- Romani, A.; Clementi, C.; Miliani, C.; Favaro, G. Fluorescence Spectroscopy: A Powerful Technique for the Noninvasive Characterization of Artwork. Acc. Chem. Res. 2010, 43, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Chen, W. Nanoparticle Fluorescence Based Technology for Biological Applications. j nanosci nanotechnol 2008, 8, 1019–1051. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, M. Advantages of Fluorescent Nanoparticles. Available online: https://www.news-medical.net/life-sciences/Advantages-of-Fluorescent-Nanoparticles.aspx (accessed on 7 May 2024).
- Dubertret, B.; Skourides, P.; Norris, D.J.; Noireaux, V.; Brivanlou, A.H.; Libchaber, A. In Vivo Imaging of Quantum Dots Encapsulated in Phospholipid Micelles. Science 2002, 298, 1759–1762. [Google Scholar] [CrossRef] [PubMed]
- Ruedas-Rama, M.J.; Hall, E.A.H. Analytical Nanosphere Sensors Using Quantum Dot−Enzyme Conjugates for Urea and Creatinine. Anal. Chem. 2010, 82, 9043–9049. [Google Scholar] [CrossRef]
- Das, S.; Mondal, S.; Ghosh, D. Carbon Quantum Dots in Bioimaging and Biomedicines. Front Bioeng Biotechnol 2023, 11, 1333752. [Google Scholar] [CrossRef]
- Cho, H.-S.; Dong, Z.; Pauletti, G.M.; Zhang, J.; Xu, H.; Gu, H.; Wang, L.; Ewing, R.C.; Huth, C.; Wang, F.; et al. Fluorescent, Superparamagnetic Nanospheres for Drug Storage, Targeting, and Imaging: A Multifunctional Nanocarrier System for Cancer Diagnosis and Treatment. ACS Nano 2010, 4, 5398–5404. [Google Scholar] [CrossRef]
- Yang, X.; Wang, D.; Luo, N.; Feng, M.; Peng, X.; Liao, X. Green Synthesis of Fluorescent N,S-Carbon Dots from Bamboo Leaf and the Interaction with Nitrophenol Compounds. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2020, 239, 118462. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Hghighat Sefat, S.; Kazemifard, N.; Rezaei, B.; Moradi, F. A Novel One-Step and Green Synthesis of Highly Fluorescent Carbon Dots from Saffron for Cell Imaging and Sensing of Prilocaine. Sensors and Actuators B: Chemical 2017, 253, 451–460. [Google Scholar] [CrossRef]
- Sun, J.; Jin, Y. Fluorescent Au Nanoclusters: Recent Progress and Sensing Applications. J. Mater. Chem. C 2014, 2, 8000–8011. [Google Scholar] [CrossRef]
- Huang, H.; Zou, M.; Xu, X.; Liu, F.; Li, N.; Wang, X. Near-Infrared Fluorescence Spectroscopy of Single-Walled Carbon Nanotubes and Its Applications. TrAC Trends in Analytical Chemistry 2011, 30, 1109–1119. [Google Scholar] [CrossRef]
- Degliangeli, F.; Kshirsagar, P.; Brunetti, V.; Pompa, P.P.; Fiammengo, R. Absolute and Direct MicroRNA Quantification Using DNA–Gold Nanoparticle Probes. J. Am. Chem. Soc. 2014, 136, 2264–2267. [Google Scholar] [CrossRef] [PubMed]
- Seferos, D.S.; Giljohann, D.A.; Hill, H.D.; Prigodich, A.E.; Mirkin, C.A. Nano-Flares: Probes for Transfection and mRNA Detection in Living Cells. J. Am. Chem. Soc. 2007, 129, 15477–15479. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Chang, C.; Pan, W.; Tang, B. A Multicolor Nanoprobe for Detection and Imaging of Tumor-Related mRNAs in Living Cells. Angew Chem Int Ed 2012, 51, 7426–7430. [Google Scholar] [CrossRef]
- Song, S.; Liang, Z.; Zhang, J.; Wang, L.; Li, G.; Fan, C. Gold-Nanoparticle-Based Multicolor Nanobeacons for Sequence-Specific DNA Analysis. Angew Chem Int Ed 2009, 48, 8670–8674. [Google Scholar] [CrossRef]
- Dong, F.; Yan, W.; Dong, W.; Shang, X.; Xu, Y.; Liu, W.; Wu, Y.; Wei, W.; Zhao, T. DNA-Enabled Fluorescent-Based Nanosensors Monitoring Tumor-Related RNA toward Advanced Cancer Diagnosis: A Review. Front. Bioeng. Biotechnol. 2022, 10, 1059845. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, Y.; Tao, Z. Fluorescence Tracking of Small Extracellular Vesicles In Vivo. Pharmaceutics 2023, 15, 2297. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, Z.; Chai, Y.; Zhang, A.; He, S.; Liu, X.; Yuan, H.; Tan, L.; Ding, L.; Wu, Y. One-Step and Label-Free Ratiometric Fluorescence Assay for the Detection of Plasma Exosome towards Cancer Diagnosis. Talanta 2024, 271, 125700. [Google Scholar] [CrossRef]
- Huang, R.; He, L.; Li, S.; Liu, H.; Jin, L.; Chen, Z.; Zhao, Y.; Li, Z.; Deng, Y.; He, N. A Simple Fluorescence Aptasensor for Gastric Cancer Exosome Detection Based on Branched Rolling Circle Amplification. Nanoscale 2020, 12, 2445–2451. [Google Scholar] [CrossRef]
- Xie, S.; Chen, Y.; Guo, Z.; Luo, Y.; Tan, H.; Xu, L.; Xu, J.; Zheng, J. Agar/Carbon Dot Crosslinked Polyacrylamide Double-Network Hydrogels with Robustness, Self-Healing, and Stimulus-Response Fluorescence for Smart Anti-Counterfeiting. Mater. Chem. Front. 2021, 5, 5418–5428. [Google Scholar] [CrossRef]
- Liu, B.; Zhuang, J.; Wei, G. Recent Advances in the Design of Colorimetric Sensors for Environmental Monitoring. Environ. Sci.: Nano 2020, 7, 2195–2213. [Google Scholar] [CrossRef]
- Lussier, F.; Staufer, O.; Platzman, I.; Spatz, J.P. Can Bottom-Up Synthetic Biology Generate Advanced Drug-Delivery Systems? Trends in Biotechnology 2021, 39, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Dazat, R.E.; Mammana, S.B.; Canizo, B.V.; Silva, M.F.; Gomez, F.J.V. Enhanced Fluorescence Detection of Ergosterol by Hydrophobic Fluorescent Natural Deep Eutectic Solvent. Green Analytical Chemistry 2022, 3, 100026. [Google Scholar] [CrossRef]
- Chernov, K.G.; Redchuk, T.A.; Omelina, E.S.; Verkhusha, V.V. Near-Infrared Fluorescent Proteins, Biosensors, and Optogenetic Tools Engineered from Phytochromes. Chem. Rev. 2017, 117, 6423–6446. [Google Scholar] [CrossRef]
- Shameem, M.A.; Orthaber, A. Organophosphorus Compounds in Organic Electronics. Chemistry – A European Journal 2016, 22, 10718–10735. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ding, Y.; Tebyetekerwa, M.; Xie, Y.; Wang, L.; Li, H.; Hu, R.; Wang, Z.; Qin, A.; Tang, B.Z. Fluorescent Aggregation-Induced Emission (AIE)-Based Thermosetting Electrospun Nanofibers: Fabrication, Properties and Applications. Mater. Chem. Front. 2019, 3, 2491–2498. [Google Scholar] [CrossRef]
- Wang, S.; Ren, W.X.; Hou, J.-T.; Won, M.; An, J.; Chen, X.; Shu, J.; Kim, J.S. Fluorescence Imaging of Pathophysiological Microenvironments. Chem. Soc. Rev. 2021, 50, 8887–8902. [Google Scholar] [CrossRef]
- Troyan, S.L.; Kianzad, V.; Gibbs-Strauss, S.L.; Gioux, S.; Matsui, A.; Oketokoun, R.; Ngo, L.; Khamene, A.; Azar, F.; Frangioni, J.V. The FLARETM Intraoperative Near-Infrared Fluorescence Imaging System: A First-in-Human Clinical Trial in Breast Cancer Sentinel Lymph Node Mapping. Ann Surg Oncol 2009, 16, 2943–2952. [Google Scholar] [CrossRef]
Figure 1.
Chemical structures of selected natural products and synthetic-based fluorescent compounds. Color represents the fluorescence of the respective structures.
Figure 1.
Chemical structures of selected natural products and synthetic-based fluorescent compounds. Color represents the fluorescence of the respective structures.
Figure 2.
Structure-activity relationship of the fluorescent probes. (A) The effect of conjugation on the electronic excited state and its effect on fluorescence intensity—an increase in conjugation results in increasing fluorescence intensity. (B) Structures of different fluorescent compounds with their emission colors. (C) Effect of the electron-donating group and electron-withdrawing groups on the intensity and quantum yield of rhodamine.
Figure 2.
Structure-activity relationship of the fluorescent probes. (A) The effect of conjugation on the electronic excited state and its effect on fluorescence intensity—an increase in conjugation results in increasing fluorescence intensity. (B) Structures of different fluorescent compounds with their emission colors. (C) Effect of the electron-donating group and electron-withdrawing groups on the intensity and quantum yield of rhodamine.
Figure 3.
Schematic representation of biomedical applications of fluorescent probes.
Figure 3.
Schematic representation of biomedical applications of fluorescent probes.
Figure 4.
The working mechanism of fluorescent probes in drug delivery systems, drug analysis, and their function as sensors. Drugs attached with probes through cleavable linkage or non-cleavable linkage allow to evaluate its delivery system properly and its other function as a sensor helps to analyze the metabolite pathway, enzyme activity, chromosomal analysis, and genetic mapping.
Figure 4.
The working mechanism of fluorescent probes in drug delivery systems, drug analysis, and their function as sensors. Drugs attached with probes through cleavable linkage or non-cleavable linkage allow to evaluate its delivery system properly and its other function as a sensor helps to analyze the metabolite pathway, enzyme activity, chromosomal analysis, and genetic mapping.
Figure 5.
Mode of action of fluorescent probes in biomarkers and cell imaging. Fluorescent probes undergo different mechanisms, such as ESIPT, FRET, PET, ICT, and protein labeling, to intensify the fluorescence properties, followed by real-time analysis of molecules, thereby aiding in cell and tissue imaging.
Figure 5.
Mode of action of fluorescent probes in biomarkers and cell imaging. Fluorescent probes undergo different mechanisms, such as ESIPT, FRET, PET, ICT, and protein labeling, to intensify the fluorescence properties, followed by real-time analysis of molecules, thereby aiding in cell and tissue imaging.
Figure 6.
Application of fluorescent compounds in wound healing. Fluorescent compounds allow real-time visualization and analysis of wounds, leading to an understanding of the healing process.
Figure 6.
Application of fluorescent compounds in wound healing. Fluorescent compounds allow real-time visualization and analysis of wounds, leading to an understanding of the healing process.
Figure 7.
Schematic representation of the analytical applications of fluorescent probes. With the incorporation of fluorescent natural products into NPs, nanoclusters exhibit enhanced properties, increasing their utility in different fields, including water purification and environmental monitoring.
Figure 7.
Schematic representation of the analytical applications of fluorescent probes. With the incorporation of fluorescent natural products into NPs, nanoclusters exhibit enhanced properties, increasing their utility in different fields, including water purification and environmental monitoring.
Table 1.
The list of common fluorescents with general photophysical properties.
Table 1.
The list of common fluorescents with general photophysical properties.
| S.N. |
Fluorescent compound |
Derivatives |
Excitation phenomenon |
Photophysical properties |
Solvent |
Biomedical applications |
References |
| |
|
|
|
Absorptions maximum(nm) |
Emission max(nm) |
Molar excitation coefficient (M-1 cm-1) |
quantum yield (%) |
|
|
|
| 1 |
Fluorescein |
carboxyfluorescein, fluorescein di-acetate (FDA), carboxyfluorescein di-acetate (CFDA) |
Electronic excitations |
490 |
515 |
88000 |
0.93 |
0.01 M NaOH |
cellular pH imaging, cancer therapy, bacterial growth, and duel sensor |
[39] |
| 2 |
BODIPY |
4,4-Difluoro-4-bora-3a, 4a-diaza-s-indacene |
Electron excitations |
505 |
515 |
> 50,000 |
0.94 |
Toluene |
Tunable electronic and photonic properties, dye-sensitized solar cells (DSSCs) |
[40] |
| 3 |
p-Aminophenol |
Fluorofluorophores (Chromene, Squaraine, Oxazine) |
Electronic excitation |
363-641 |
435 – 664 |
1.9 × 104-1.68 × 105
|
0.03 – 0.89 |
Ethanol |
Allows the creation of extremely fluorescent nanoemulsions; holds promise as brilliant bioimaging agents |
[41] |
| 4 |
Xanthone |
xanthone-2-carboxylic, dimethylxanthenone-4-arcetic acid |
donor-acceptor phenomenon |
338 |
355 – 400 nm |
6828 |
0.97 |
Acetonitrile |
photobase generation mechanism,
monitor the photocatalysis reactions, photostability |
[42] |
| 5 |
1,2,4-trioxolane |
4H-1,2,4-triazole,4-alkyl-3,5-bis(4-bromophenyl)-4H-1,2,4-triazoles |
π-conjugated systems |
472 |
545 |
5 × 10 ^5 |
>0.98 |
Dichloromethane |
Used for the detection of cell morphological changes, anticancer agents |
[43,44] |
| 6 |
phycobiliprotein |
B-phycoerythrin, allophycocyanin, phycocyanin,
phycoerythrin |
Electronic excitation |
495 – 655 |
580 – 660 |
1.9 –2.41 × 10 6
|
0.98 |
Aqueous |
bioimaging,
diagnosis of human metabolic disorder, chelating agents |
[45,46] |
| 7 |
Perylene |
Perylene-3,4,9,10-tetracarboxylic diimide (PTCDI), |
donor-π-acceptor, strong π-π intermolecular interactions |
440 |
444 nm |
34000 |
0.95 |
Toluene |
successfully enter living cells without destroying their morphology, intracellular biomedical application |
[47] |
| 8 |
Rhodamine |
Si-rhodamine,4-carboxyrhodamines |
donor-acceptor of electron |
512 |
530 |
8.57 |
0.86 |
PBS |
labeling of intracellular structures, isomeric tuning Halo-tagged and SNAP-tagged proteins |
[48] |
| 9 |
Carbostyril 124 |
carbostyril (cs124,7-amino-4-methyl-2(1H)-quinolinone), |
Excitation and emission |
348 |
381 |
16000 |
0.97 |
Water |
used as a small molecule organic antenna for Lanthanide-based FRET |
[49] |
| 10 |
Umbelliferone (7-hydroxy coumarin) |
4-methyl umbelliferone |
Proton donor-acceptor phenomena |
323 – 370 |
396 – 480 |
12500 – 21,500 |
0.30 –0.87 |
Dimethylformamide, chloroform, water, ethanol, water with acid or base |
Aggregates around bacterial, fungal regions and deals it with excited-state hydrogen transfer reactions, |
[50] |
| 11 |
Pyrene |
1,3,6,8-tetrakis(4-n-monoethyleneglycol-(2-propynyl) ether) pyrene , 1,3,6,8-tetrakis(4-n-diethyleneglycol-(2-propynyl)ether)pyrene and |
dipolar donor-π-acceptor (D-π-A) |
378 nm |
422 nm |
100000 |
0.99 |
Methanol |
on account of their strong emission of fluorescence in live cells, detection of reactive oxygen species (ROS) |
[51] |
| 12 |
Curcumin |
mono-biotinylated curcumin |
Proton donor-acceptor phenomena |
408 – 430 nm |
450 – 560 nm |
55000 |
0.0104 – 0.174 |
cyclohexane |
fluorescence intensity is higher in the cancerous cells than normal cells, helps to understand the nature and membrane polarity of cells, binds to lipids of membrane and proteins of cells, useful for knowing the changes in the structure in the host |
[52,53] |
| 13 |
Styrene |
triphenylamine or carbazole-substituted styrene derivatives |
conjugations phenomenon |
426 – 518 |
538 – 606 |
29400 – 40300 |
0.02 – 0.037 |
DMSO |
high thermal stability, optoelectronic properties, and charge-transport material. |
[54,55] |
| 14 |
Quinine |
quinolines |
electronic excitation |
350 |
450 |
1.09 × 10^4 |
0.55 |
0.5 N Sulphuric acid |
anti-malarial and precursor for modern synthetic anti-plasmodial quinolines |
[56] |
| 15 |
Squarylium dye III |
indodicarbocyanine dye D3,
squarylium dye D1 |
Donar -acceptor -Donar of electron |
627.6 |
676 |
30900 |
0.65 |
Methylene chloride |
photosensitizers, biolabeling and chemosensory materials for analytical uses in the biomedical field |
[57] |
| 16 |
1,8-naphthalimide |
4-N-methylpiperazine-1,8-napthalimide |
conjugations phenomenon |
398-416 |
494-525 |
(3.87-4.12) × 104
|
0.011-0.594 |
Methanol |
antimicrobial, antibacterial, and anticancer agents |
[58] |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).