1. Introduction
Peach (
Prunus persica L.) ranks as the third most economically significant in temperate regions worldwide, valued for its ornamental blooms and nutritious fruits [
1]. With a long history of cultivation, peaches are grown globally in countries including China, Spain, Italy, Turkey, Iran, USA, Egypt, Chile, and India. In China, the peach cultivation area reached 840 thousand hectares, with a production of approximately 15.8 million tons, as reported by the Food and Agriculture Organization of the United Nations (FAO) 2022 Report [
2]. Beyond fresh consumption, peaches are employed on various forms such as dried fruit snacks, fruit juice, and canned fruit. However, the productivity and quality of peach fruits are greatly threatened by various diseases, resulting in a marked reduction in fruit yield and significantly limiting the development of peach production.
Approximately twenty diseases have been reported in peach, including brown rot caused by
Monilinia fructicola, bacterial spot caused by
Xanthomonas arboricola pv.
pruni (
Xap), bacterial canker caused by
Pseudomonas syringae, and powdery mildew caused by
Podosphaera pannosa, peach leaf curl caused by
Taphrina deformans [
3,
4,
5,
6]. To date, compared with these peach diseases, less attention has been paid to the peach leaf spots caused by fungi. It has been reported that peach leaf spots occur frequently and affect fruit quality and yield [
7,
8,
9]. However, until now, the identification of the peach leaf spot diseases caused by fungi have been rarely reported.
Nigrospora species are widely distributed in nature, with 45 species currently recorded in the MycoBank database [
10]. This genus has a broad-host range and mainly infects plant leaves and stems [
11]. Among them,
N. sphaerica and
N.
oryzae are notable plant pathogens [
12].
N. sphaerica typically infects plant leaves, causing leaf spot disease. In recent years, new hosts of this pathogen have been continuously discovered, such as blueberry plants, watermelon,
Rhododendron simsii, cacao, passion fruit, and olive
[10,12–16]. Furthermore,
N. sphaerica can cause human infections, including onychomycosis and corneal ulcer [
11].
In the summer of 2024, a leaf spot disease occurred in peach orchards in Bazhong City, Sichuan Province, China, with symptoms distinct from the bacterial spots caused by Pseudomonas syringae. This study aims to identify the causal agent of this disease based on morphological and molecular characterization.
2. Materials and Methods
2.1. Sample Collection
Peach leaves with typical lesions were harvested from an orchard in Bazhong City (106°44′35.97″ E, 31°52′12.27″ N), Sichuan Province, China. The collected leaves were wrapped in moistened cotton to retain moisture and placed in a labelled sterile sample bag. The samples were then immediately transported to the laboratory for pathogen isolation. After two weeks, the same-sized healthy leaves were collected from the orchard for pathogenicity testing.
2.2. Isolation and Purification of Pathogenic Fungi
To isolate pathogens, the boundary tissues of the leaf lesions were cut into 1 cm × 1 cm fragments, disinfected in 75% ethanol for 1 min, rinsed with sterile water for three times, then immersed in 3% sodium hypochlorite solution for 1 min, followed by rinsing with sterile water three times. The treated fragments were dried on filter paper and incubated on potato dextrose agar (PDA) plate at 25 °C for two days. The isolated strains were purified by picking the hyphal tip from the colonial margin two or three times [
17].
2.3. Morphological Identification
For morphological identification, the purified strains were cultured on PDA plates at 25 °C for 5–7 days. The colony morphology was observed when the fungal hyphae reached the plate edges. The characteristics of the mycelium and spores were observed under optical microscope (Olympus , Chongqing, China).
2.4. Molecular Identification and Phylogenetic Analysis
Approximately 0.5 g fresh mycelia were harvested from 7-day-old PDA cultures and ground into a fine powder in liquid nitrogen. Total genomic DNA was extracted using CTAB solution (2 M Tris-HCl, 5 M NaCl, 0.5 M EDTA, 2% (w/v) CTAB, and 0.2% (v/v) mercaptoethanol) [
18]. DNA quantity and quality were assessed using 1.0% (w/v) agarose gel stained with GelRed nucleic acid stain (Vazyme, Nanjing, China). For molecular identification, primer pairs ITS1/ITS4 [
19], EF1-728F/EF1-986R [
20], and Bt2a/Bt2b [
21] were used to amplify the internal transcribed spacer (ITS), partial translation elongation factor 1-alpha (
TEF1-a), and
β-tubulin (TUB) sequences of all isolated fungal strains (
Table 1). PCR was carried out with the following conditions: 35 cycles of denaturation at 95 °C for 30 s, annealing at 52 °C for 30 s, and elongation at 72 °C for 1 min, with an initial denaturation step at 95 °C for 5 min. PCR products were purified using Omega Bio-tek’s E.Z.N.A.® Gel Extraction Kit and cloned into the pMD18-T vector (Takara, Shiga, Japan) for sequencing. The obtained sequences were compared with reference sequences using the BLAST tool on the NCBI website (
https://blast.ncbi.nlm.nih.gov/Blast.cgi). The amplified sequences in this study were aligned with the reference sequences using ClustalW. The phylogenetic tree was constructed using the maximum-likelihood (ML) method via MEGA7.0 software, and the reliability of the tree was assessed using 1000 bootstrap replicates.
2.5. Pathogenicity Test
To assess the pathogenicity of the fungal isolates, healthy peach leaves were surface sterilized by immersion in 70% ethanol for 1 min, followed by rinsed in sterilized distilled water three times. The leaves were wounded by pin-pricking the surface with sterilized needle. Subsequently, 5 mm diameter mycelial plugs were obtained from the edges of a fresh colony and inoculated on the wounded leaves. Plugs of PDA were used as controls. The petioles of the inoculated leaves were wrapped with moistened cotton to retain moisture. The inoculated leaves were kept in a plastic box with high humidity at 28°C in the dark for disease development [
22]. To fulfill the Koch’s postulates, re-isolations were made from the diseased leaves. The experiment was repeated three times.
3. Results
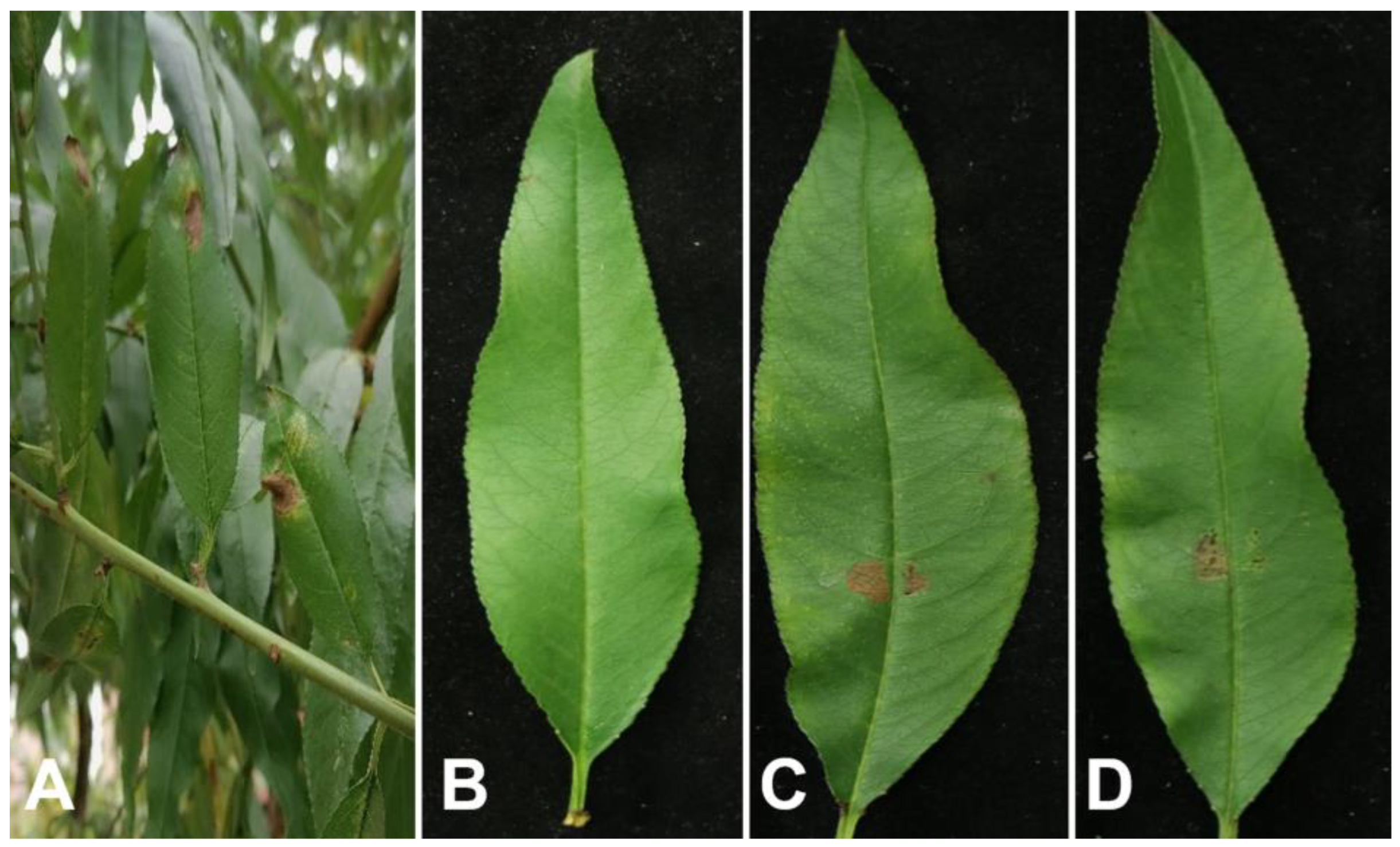
In August 2024, leaf spot symptoms were observed on peach in an orchard in Ba zhong City, Sichuan Province, China. The peach plants with leaf spots accounted for 15% of the total plants. Initially, the lesions appeared small, irregular, and brown with indistinct edges. As the disease progressed, the lesions gradually expanded. Two or more lesions joined together, and the centers of lesions occasionally formed perforations. Additionally, the color of lesions became black or dark brown (
Figure 1A).
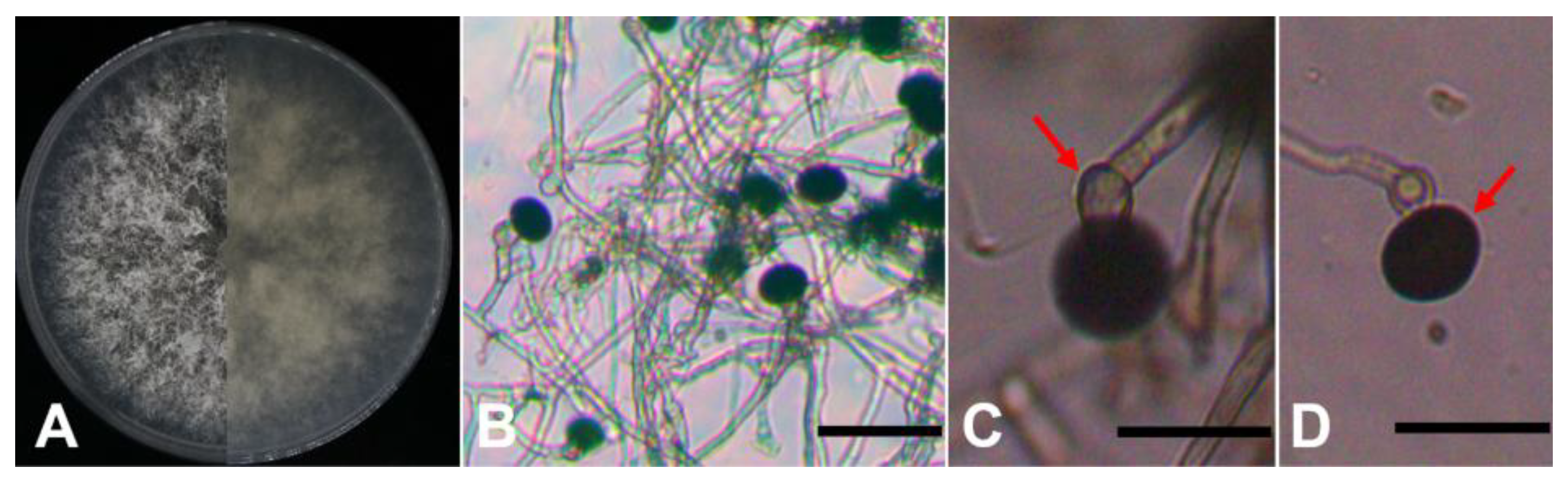
A total of six isolates were obtained from the diseased leaf samples and cultured on PDA plates for 5 days. These isolates exhibited consistent morphological features. Colonies on PDA were white and flocculent, but their reverse side was pale yellow. Under microscopic examination, conidiogenous cells were colorless and subglobose. The conidia were acrogenous, solitary, black and globose with diameters of 15-19 μm (
Figure 2). Based on the morphological characteristics, these isolates were tentatively identified as belonging to the genus
Nigrospora sp.
For precise identification, molecular methods were used to amplify the internal transcribed spacer (ITS) region, the partial translation elongation factor 1 alpha (TEF1-a) region, and the β-tubulin region of all isolates via PCR. The fragment sizes of PCR products were about 555 bp, 279 bp, and 434 bp, respectively. These fragments were cloned into the pMD18-T vector and then sequenced. Multiple sequence alignment revealed 100% identity in the ITS, TEF1-a or β-tubulin regions among the six isolates. The ITS, TEF1-a, and β-tubulin sequences of the representative isolate SCBZPP6 were further analyzed via NCBI Blast, which showed that the ITS region sequence of SCBZPP6 matched 100% with N. sphaerica strains (KU553345.1, KC519729.1, and PQ289163.1). The partial TEF1-a sequences of SCBZPP6 showed 99.2-100% identity with N. sphaerica (OM826971.1, MN053315.1, KY019393.1, and KY513872.1), and its β-tubulin region sequence was 99.5-99.7% identical to N. sphaerica (MK408565.1, OM826974.1, and MN719407.1).
Then, the ITS,
TEF1-a, and
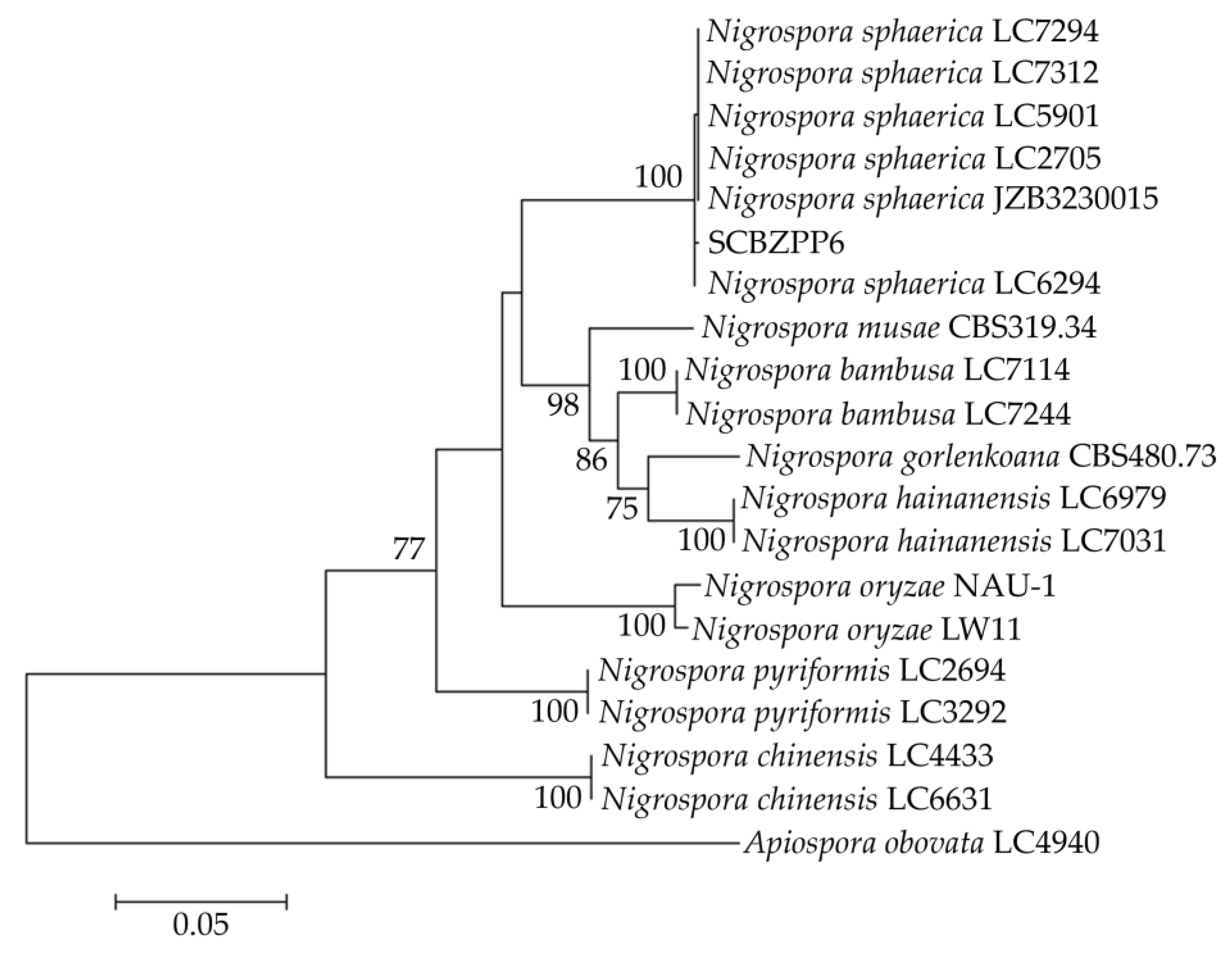
β-tubulin sequences were submitted to the NCBI, received accession numbers (PQ496816, PQ505108, and PQ505109). To analyze homologous relationships, a phylogenetic tree was constructed using the concatenated ITS,
TEF1-α, and
β-tubulin sequences of SCBZPP6 with reference sequences of related strains using the maximum-likelihood (ML) method. The phylogenetic results showed that SCBZPP6 was most closely related to
N. sphaerica (
Figure 3). Based on these findings, we conclude that the isolated pathogens were
N. sphaerica based on morphological and molecular characteristics.
To assess the pathogenicity of SCBZPP6, mycelial plugs of the strain were inoculated onto detached peach leaves. Ten days post inoculation, visible brown spots formed on the leaves inoculated with SCBZPP6, similar to those observed on infected plants in the field, compared with the healthy control leaves (
Figure 1C-1D). To fulfill Koch’s postulates, the pathogen was re-isolated from the diseased leaf.
4. Discussion
In recent years, climate changes with rising temperatures and increasing precipitation are believed to contribute to an increased occurrence of leaf diseases. The bacterial leaf spot diseases of peach caused by Xanthomonas arboricola pv. pruni (Xap) is well-studied, typically presenting as small, brown and water-soaked lesions. As the lesions enlarge, the centers become surrounded by yellow halos and eventually fall off, forming irregular perforations. In this study, the symptoms of the peach leaf spot were distinguishable from those caused by bacteria. Additionally, the isolates were identified as N. sphaerica. To our knowledge, this is the first report of peach leaf spot disease caused by N. sphaerica.
Nigrospora species have a wide host range, and
N. sphaerica is one of the most reported
Nigrospora species. So far,
N. sphaerica is able to cause diseases in over 40 plants [
10,
15,
16,
23,
24,
25,
26,
27,
28,
29], including vegetables, fruits, flowers, herbs and ornamental trees. The fungus mainly causes leaf spot diseases but also infects twigs, roots, and fruits. It is imperative to identify
Nigrospora species for future study. Historically, identification of
Nigrospora species has relied on morphological characteristics, especially conidial dimensions. However, this approach can lack accuracy, so we used both morphological and molecular characterization to identify the isolates at the species level. Our study provides a foundational understanding for developing effective control measures against this new fungal disease in peach.
Author Contributions
Conceptualization, W.G.; methodology, H.L. and H.W.; validation, H.L. and H.W.; formal analysis, H.L. and H.W; investigation, H.L. and H.W.; writing original draft preparation, H.L. and I.S.; writing—review and editing, W.G.; supervision, W.G.; project administration, W.G.; funding acquisition, W.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (No. 32072377).
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Rawandoozi, Z.J.; Hartmann, T.P.; Carpenedo, S.; Gasic, K.; da Silva Linge, C.; Cai, L.; Van de Weg, E.; Byrne, D.H. Identification and characterization of QTLs for fruit quality traits in peach through a multi-family approach. BMC Genomics 2020, 21, 522. [Google Scholar] [CrossRef]
- Karim, M.M.; Usman, H.M.; Tan, Q.; Hu, J.-J.; Fan, F.; Hussain, R.; Luo, C.-X. Fungicide resistance in Colletotrichum fructicola and Colletotrichum siamense causing peach anthracnose in China. Pesticide Biochemistry and Physiology 2024, 203, 106006. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.-X.; Schnabel, G.; Hu, M.; De Cal, A. Global distribution and management of peach diseases. Phytopathology Research 2022, 4, 30. [Google Scholar] [CrossRef]
- Dong, J.; Shi, H.; Wu, Y.; Yang, L.; Zhu, F.; Ji, Z. Identification and pathogenicity analysis of Fusarium spp. on peach in China. BMC Microbiology 2023, 23, 211. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Manawasinghe, I.S.; He, Z.; Zhang, W.; Liu, M.; Song, J.; Li, S.; Fan, Z.; Yan, J. Microfungi Associated with Peach Branch Diseases in China. Journal of Fungi 2024, 10, 217. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, L.; Cao, J.; Zhu, Y.; Zhang, L.; Jin, W.; Zhu, F.; Ji, Z. Molecular and Biological Characterization of Two New Species Causing Peach Shoot Blight in China. Plant Disease 2022, 106, 182–189. [Google Scholar] [CrossRef]
- Jin, S.; Wang, N.; Zhou, Z.; Ji, Z.; Zhang, J. Identification of Bacillus species causing bacterial leaf spot of peach in China. Journal of Phytopathology 2022, 170, 811–819. [Google Scholar] [CrossRef]
- Inoue, K.; Nasu, H. Black Spot of Peach Caused by Alternaria alternata (Fr.) Keissler. Journal of General Plant Pathology 2000, 66, 18–22. [Google Scholar] [CrossRef]
- Ji, Z.L.; Zhang, S.W.; Zhu, F.; Wan, B.X.; Liang, R.Z. First Report of Arthrinium arundinis Causing Leaf Edge Spot of Peach in China. Plant Disease 2020, 104, 3077. [Google Scholar] [CrossRef]
- Petrović, E.; Vrandečić, K.; Ćosić, J.; Đermić, E.; Godena, S. First Report of Nigrospora Species Causing Leaf Spot on Olive (Olea europaea L.). Horticulturae 2023, 9, 1067. [Google Scholar] [CrossRef]
- Wang, M.; Liu, F.-l.; Crous, P.W.; Cai, L.-Z. Phylogenetic reassessment of Nigrospora: Ubiquitous endophytes, plant and human pathogens. Persoonia : Molecular Phylogeny and Evolution of Fungi 2017, 39, 118–142. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.W.; Rehman, A.; Ahmad, S.; Sarwar, M.; Nawaz, A.; Khan, S.M.; Ali, S.; Aslam, S.; Mannan, A. First report of Nigrospora sphaerica causing leaf spot of date palm in Pakistan. J. Plant Pathol. 2020, 102, 223. [Google Scholar] [CrossRef]
- Ismail, S.I.; Abd Razak, N.F. First Report of Nigrospora sphaerica Causing Leaf Spot on Watermelon (Citrullus lanatus) in Malaysia. Plant Disease 2021, 105, 488–488. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cernava, T.; Zhou, X.; Yang, L.; Baccelli, I.; Wang, J.; Gou, Y.; Sang, W.; Chen, X. First Report of Passion Fruit Leaf Blight Caused by Nigrospora sphaerica in China. Plant Disease 2022, 106, 323. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Liu, L.; Javed, K.; Lin, J.; Li, Z.; Ding, H. First Report of Nigrospora sphaerica Causing Leaf Spot on Rhododendron simsii in China. Plant Disease 2024, 108, 1395. [Google Scholar] [CrossRef]
- Villanueva, J.D.; Solpot, T.C.; Tangonan, N.G. Nigrospora sphaerica causing Leaf Blight Disease of Cacao in the Philippines. Indian Phytopathology 2023, 76, 915–922. [Google Scholar] [CrossRef]
- Hao, Y.; Aluthmuhandiram, J.V.S.; Chethana, K.W.T.; Manawasinghe, I.S.; Li, X.; Liu, M.; Hyde, K.D.; Phillips, A.J.L.; Zhang, W. Nigrospora Species Associated with Various Hosts from Shandong Peninsula, China. Mycobiology 2020, 48, 169–183. [Google Scholar] [CrossRef]
- Lee, S.B.; Milgroom, M.G.; Taylor, J.W. A rapid, high yield mini-prep method for isolation of total genomic DNA from fungi. Fungal Genetics Reports 1988, 35, 23–24. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W. 38—Amplification and direct sequencing of fungal ribosomal RNA Genes for phylogenetics. In PCR—Protocols and Applications—A Laboratory Manual; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press, Inc.: Cambridge, MA, USA, 1990; pp. 315–322.Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Woudenberg, J.H.C.; Aveskamp, M.M.; Gruyter, J.; Spiers, A.G.; Crous, P. Multiple Didymella teleomorphs are linked to the Phoma clematidina morphotype. Persoonia 2009, 22, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Shi, J.; Li, B.; Wang, S.; Zhang, W.; Shang, M.; Wang, Y.; Liu, B. Occurrence of Neopestalotiopsis clavispora Causing Apple Leaf Spot in China. Agronomy 2024, 14, 1658. [Google Scholar] [CrossRef]
- Bansal, N.; Juwantha, R.; Pandey, S. Morphology and ITS-based phylogeny revealed Nigrospora sphaerica causing leaf spot and blight disease of Bambusa polymorpha in India. Physiological and Molecular Plant Pathology 2024, 132, 102315. [Google Scholar] [CrossRef]
- Khoo, Y.W.; Khaw, Y.S.; Tan, H.T.; Li, S.-F.; Chong, K.P. First Report of Stem Brown Spot on ‘Thai Gold’ Selenicereus megalanthus in Malaysia Caused by Nigrospora sphaerica. Plant Disease 2023, 107, 223. [Google Scholar] [CrossRef] [PubMed]
- Sadid, K.N.E.; Hossain, M.A.; Munshi, A.R.; Karim, M.R. First detection, isolation and molecular characterization of banana leaf spot diseases caused by Nigrospora sphaerica and Neocordana musae in Bangladesh. Archives of Phytopathology and Plant Protection 2023, 56, 1112–1125. [Google Scholar] [CrossRef]
- Liu, J.; Deng, Y.; Liu, Q.; Zhang, K.; Zhao, Y.; Ma, T.; Chang, W.; Wang, H. First Report of Leaf Blight on Pumpkin Caused by Nigrospora sphaerica in China. Plant Disease 2024, 108, 2574. [Google Scholar] [CrossRef]
- Li, J.; Xu, J.; Wang, H.; Wu, C.; Zheng, J.; Zhang, C.; Han, Y. First Report of Fungal Pathogens Causing Leaf Spot on Sorghum–Sudangrass Hybrids and Their Interactions with Plants. Plants 2023, 12, 3091. [Google Scholar] [CrossRef]
- Zeng, Y.; Luo, M.-Y.; Yang, M.-f.; Jiang, Y.-L. First Report of Leaf Spot on Zanthoxylum bungeanum Caused by Nigrospora sphaerica in China. Plant Disease 2024, 108, 812. [Google Scholar] [CrossRef]
- Tejaswini, G.S.; Mahadevakumar, S.; Joy, J.; Chandranayaka, S.; Niranjan Raj, S.; Devi, L.; Sowjanya, R.; Sowmya, R. First Report of Nigrospora sphaerica Associated with Leaf Spot Disease of Crossandra infundibuliformis in India. Plant Disease 2023, 107, 2218. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).