Submitted:
21 October 2024
Posted:
22 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
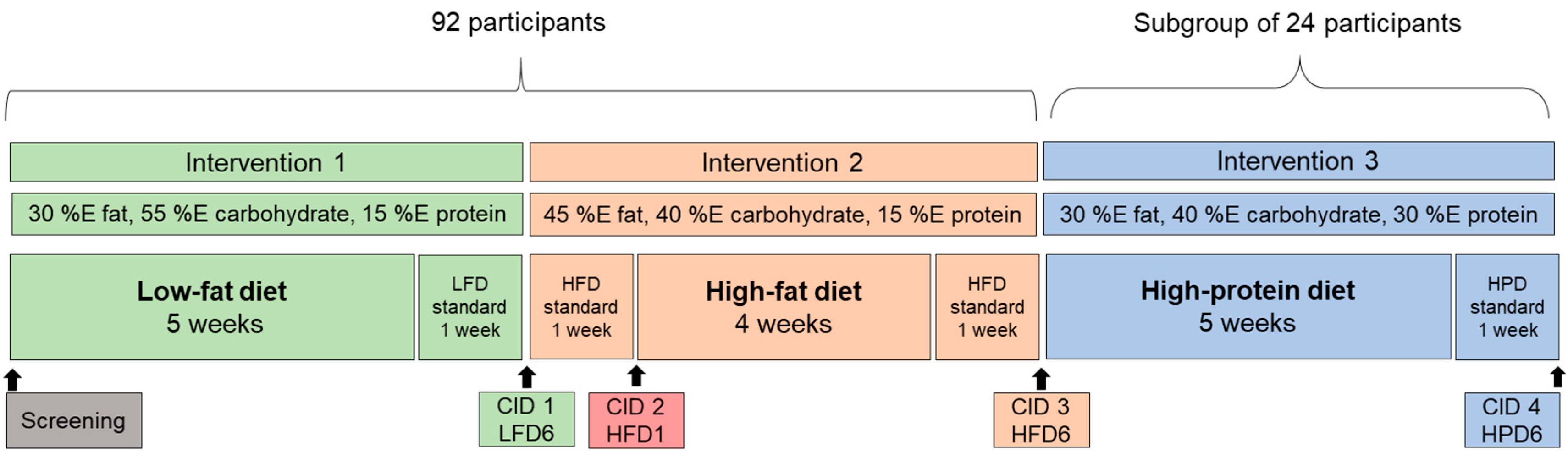
2.1. Study Protocol and Participants
2.2. Blood Parameters
2.3. Heritability
2.4. Statistical Analysis
3. Results
3.1. Participants’ Characteristics and Compliance
3.2. Changes of Serum GCGN and Metabolic Parameters in Response to High Dietary Fat Intake
3.3. Impact of Age and Sex on GCGN Levels
3.4. Changes of Postprandial GCGN in Response to the HFD
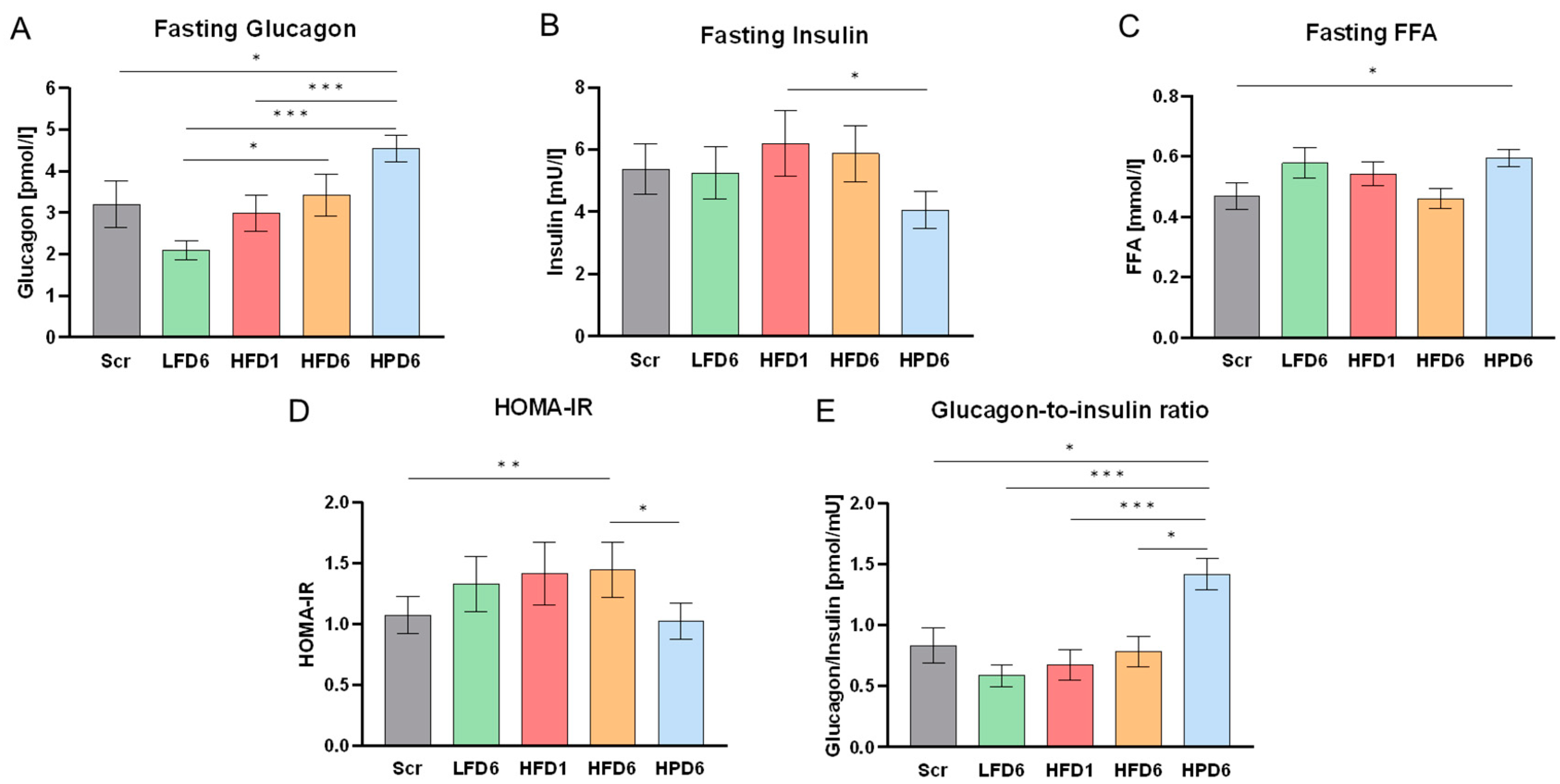
3.5. Serum GCGN and Metabolic Parameter Changes in Response to High Dietary Protein Intake
3.6. Heritability of GCGN Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rohrer, S.; Menge, B.A.; Gruber, L.; Deacon, C.F.; Schmidt, W.E.; Veldhuis, J.D.; Holst, J.J.; Meier, J.J. Impaired crosstalk between pulsatile insulin and glucagon secretion in prediabetic individuals. J Clin Endocrinol Metab 2012, 97, E791–795. [Google Scholar] [CrossRef] [PubMed]
- Menge, B.A.; Gruber, L.; Jorgensen, S.M.; Deacon, C.F.; Schmidt, W.E.; Veldhuis, J.D.; Holst, J.J.; Meier, J.J. Loss of inverse relationship between pulsatile insulin and glucagon secretion in patients with type 2 diabetes. Diabetes 2011, 60, 2160–2168. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.J.; Kjems, L.L.; Veldhuis, J.D.; Lefebvre, P.; Butler, P.C. Postprandial suppression of glucagon secretion depends on intact pulsatile insulin secretion: further evidence for the intraislet insulin hypothesis. Diabetes 2006, 55, 1051–1056. [Google Scholar] [CrossRef]
- Moede, T.; Leibiger, I.B.; Berggren, P.O. Alpha cell regulation of beta cell function. Diabetologia 2020, 63, 2064–2075. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Dattaroy, D.; Pham, J.; Wang, L.; Barella, L.F.; Cui, Y.; Wilkins, K.J.; Roth, B.L.; Hochgeschwender, U.; Matschinsky, F.M.; et al. Intra-islet glucagon signaling is critical for maintaining glucose homeostasis. JCI Insight 2019, 5. [Google Scholar] [CrossRef]
- Meier, J.J.; Deacon, C.F.; Schmidt, W.E.; Holst, J.J.; Nauck, M.A. Suppression of glucagon secretion is lower after oral glucose administration than during intravenous glucose administration in human subjects. Diabetologia 2007, 50, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Dean, E.D. A Primary Role for alpha-Cells as Amino Acid Sensors. Diabetes 2020, 69, 542–549. [Google Scholar] [CrossRef]
- Dean, E.D.; Li, M.; Prasad, N.; Wisniewski, S.N.; Von Deylen, A.; Spaeth, J.; Maddison, L.; Botros, A.; Sedgeman, L.R.; Bozadjieva, N.; et al. Interrupted Glucagon Signaling Reveals Hepatic alpha Cell Axis and Role for L-Glutamine in alpha Cell Proliferation. Cell Metab 2017, 25, 1362–1373.e1365. [Google Scholar] [CrossRef]
- Okun, J.G.; Rusu, P.M.; Chan, A.Y.; Wu, Y.; Yap, Y.W.; Sharkie, T.; Schumacher, J.; Schmidt, K.V.; Roberts-Thomson, K.M.; Russell, R.D.; et al. Liver alanine catabolism promotes skeletal muscle atrophy and hyperglycaemia in type 2 diabetes. Nat Metab 2021, 3, 394–409. [Google Scholar] [CrossRef]
- Gerich, J.E.; Charles, M.A.; Grodsky, G.M. Characterization of the effects of arginine and glucose on glucagon and insulin release from the perfused rat pancreas. J Clin Invest 1974, 54, 833–841. [Google Scholar] [CrossRef]
- Wewer Albrechtsen, N.J.; Pedersen, J.; Galsgaard, K.D.; Winther-Sorensen, M.; Suppli, M.P.; Janah, L.; Gromada, J.; Vilstrup, H.; Knop, F.K.; Holst, J.J. The Liver-alpha-Cell Axis and Type 2 Diabetes. Endocr Rev 2019, 40, 1353–1366. [Google Scholar] [CrossRef] [PubMed]
- Scheen, A.J.; Paquot, N.; Lefebvre, P.J. Investigational glucagon receptor antagonists in Phase I and II clinical trials for diabetes. Expert Opin Investig Drugs 2017, 26, 1373–1389. [Google Scholar] [CrossRef] [PubMed]
- Galsgaard, K.D.; Pedersen, J.; Knop, F.K.; Holst, J.J.; Wewer Albrechtsen, N.J. Glucagon Receptor Signaling and Lipid Metabolism. Front Physiol 2019, 10, 413. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.J.; Zhang, D.; Guerra, M.T.; Brill, A.L.; Goedeke, L.; Nasiri, A.R.; Rabin-Court, A.; Wang, Y.; Peng, L.; Dufour, S.; et al. Glucagon stimulates gluconeogenesis by INSP3R1-mediated hepatic lipolysis. Nature 2020, 579, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.F.; Dufour, S.; Mehal, W.Z.; Shulman, G.I. Glucagon promotes increased hepatic mitochondrial oxidation and pyruvate carboxylase flux in humans with fatty liver disease. Cell Metabolism 2024. [Google Scholar] [CrossRef]
- Briant, L.J.B.; Dodd, M.S.; Chibalina, M.V.; Rorsman, N.J.G.; Johnson, P.R.V.; Carmeliet, P.; Rorsman, P.; Knudsen, J.G. CPT1a-Dependent Long-Chain Fatty Acid Oxidation Contributes to Maintaining Glucagon Secretion from Pancreatic Islets. Cell Reports 2018, 23, 3300–3311. [Google Scholar] [CrossRef]
- Armour, S.L.; Frueh, A.; Chibalina, M.V.; Dou, H.; Argemi-Muntadas, L.; Hamilton, A.; Katzilieris-Petras, G.; Carmeliet, P.; Davies, B.; Moritz, T.; et al. Glucose Controls Glucagon Secretion by Regulating Fatty Acid Oxidation in Pancreatic α-Cells. Diabetes 2023, 72, 1446–1459. [Google Scholar] [CrossRef]
- Gromada, J.; Franklin, I.; Wollheim, C.B. α-Cells of the Endocrine Pancreas: 35 Years of Research but the Enigma Remains. Endocrine Reviews 2007, 28, 84–116. [Google Scholar] [CrossRef]
- Kellard, J.A.; Rorsman, N.J.G.; Hill, T.G.; Armour, S.L.; van de Bunt, M.; Rorsman, P.; Knudsen, J.G.; Briant, L.J.B. Reduced somatostatin signalling leads to hypersecretion of glucagon in mice fed a high-fat diet. Mol Metab 2020, 40, 101021. [Google Scholar] [CrossRef]
- Ellingsgaard, H.; Ehses, J.A.; Hammar, E.B.; Van Lommel, L.; Quintens, R.; Martens, G.; Kerr-Conte, J.; Pattou, F.; Berney, T.; Pipeleers, D.; et al. Interleukin-6 regulates pancreatic α-cell mass expansion. Proceedings of the National Academy of Sciences 2008, 105, 13163–13168. [Google Scholar] [CrossRef]
- Eisenstein, A.B.; Strack, I.; Steiner, A. Increased hepatic gluconeogenesis without a rise of glucagon secretion in rats fed a high fat diet. Diabetes 1974, 23, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, O.; Carr, R.D.; Deacon, C.F.; Holst, J.J.; Pacini, G.; Mari, A.; Ahrén, B. Incretin Hormone and Insulin Responses to Oral Versus Intravenous Lipid Administration in Humans. The Journal of Clinical Endocrinology & Metabolism 2011, 96, 2519–2524. [Google Scholar] [CrossRef]
- Carr, R.D.; Larsen, M.O.; Winzell, M.S.; Jelic, K.; Lindgren, O.; Deacon, C.F.; Ahren, B. Incretin and islet hormonal responses to fat and protein ingestion in healthy men. Am J Physiol Endocrinol Metab 2008, 295, E779–784. [Google Scholar] [CrossRef] [PubMed]
- Mandøe, M.J.; Hansen, K.B.; Hartmann, B.; Rehfeld, J.F.; Holst, J.J.; Hansen, H.S. The 2-monoacylglycerol moiety of dietary fat appears to be responsible for the fat-induced release of GLP-1 in humans. Am J Clin Nutr 2015, 102, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Gerich, J.E.; Langlois, M.; Schneider, V.; Karam, J.H.; Noacco, C. Effects of alternations of plasma free fatty acid levels on pancreatic glucagon secretion in man. J Clin Invest 1974, 53, 1284–1289. [Google Scholar] [CrossRef]
- Schuler, R.; Osterhoff, M.A.; Frahnow, T.; Seltmann, A.C.; Busjahn, A.; Kabisch, S.; Xu, L.; Mosig, A.S.; Spranger, J.; Mohlig, M.; et al. High-Saturated-Fat Diet Increases Circulating Angiotensin-Converting Enzyme, Which Is Enhanced by the rs4343 Polymorphism Defining Persons at Risk of Nutrient-Dependent Increases of Blood Pressure. J Am Heart Assoc 2017, 6. [Google Scholar] [CrossRef]
- Machann, J.; Thamer, C.; Schnoedt, B.; Stefan, N.; Haring, H.U.; Claussen, C.D.; Fritsche, A.; Schick, F. Hepatic lipid accumulation in healthy subjects: a comparative study using spectral fat-selective MRI and volume-localized 1H-MR spectroscopy. Magn Reson Med 2006, 55, 913–917. [Google Scholar] [CrossRef]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and Abuse of HOMA Modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef]
- Gannon, M.C.; Nuttall, F.Q. Effect of a high-protein, low-carbohydrate diet on blood glucose control in people with type 2 diabetes. Diabetes 2004, 53, 2375–2382. [Google Scholar] [CrossRef]
- Fujita, Y.; Gotto, A.M.; Unger, R.M. Basal and Postprotein Insulin and Glucagon Levels During a High and Low Carbohydrate Intake and Their Relationships to Plasma Triglycerides. Diabetes 1975, 24, 552–558. [Google Scholar] [CrossRef]
- Shimy, K.J.; Feldman, H.A.; Klein, G.L.; Bielak, L.; Ebbeling, C.B.; Ludwig, D.S. Effects of Dietary Carbohydrate Content on Circulating Metabolic Fuel Availability in the Postprandial State. J Endocr Soc 2020, 4, bvaa062. [Google Scholar] [CrossRef] [PubMed]
- Alsalim, W.; Tura, A.; Pacini, G.; Omar, B.; Bizzotto, R.; Mari, A.; Ahrén, B. Mixed meal ingestion diminishes glucose excursion in comparison with glucose ingestion via several adaptive mechanisms in people with and without type 2 diabetes. Diabetes Obes Metab 2016, 18, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Ohneda, A.; Parada, E.; Eisentraut, A.M.; Unger, R.H. Characterization of response of circulating glucagon to intraduodenal and intravenous administration of amino acids. The Journal of Clinical Investigation 1968, 47, 2305–2322. [Google Scholar] [CrossRef] [PubMed]
- Forslund, A.H.; Hambræus, L.; van Beurden, H.; Holmbäck, U.; El-Khoury, A.E.; Hjorth, G.; Olsson, R.; Stridsberg, M.; Wide, L.; Åkerfeldt, T.; et al. Inverse relationship between protein intake and plasma free amino acids in healthy men at physical exercise. American Journal of Physiology-Endocrinology and Metabolism 2000, 278, E857–E867. [Google Scholar] [CrossRef] [PubMed]
- Fernstrom, J.D.; Wurtman, R.J.; Hammarstrom-Wiklund, B.; Rand, W.M.; Munro, H.N.; Davidson, C.S. Diurnal variations in plasma concentrations of tryptophan, tryosine, and other neutral amino acids: effect of dietary protein intake. Am J Clin Nutr 1979, 32, 1912–1922. [Google Scholar] [CrossRef]
- Nie, C.; He, T.; Zhang, W.; Zhang, G.; Ma, X. Branched Chain Amino Acids: Beyond Nutrition Metabolism. Int J Mol Sci 2018, 19. [Google Scholar] [CrossRef]
- Vanweert, F.; Schrauwen, P.; Phielix, E. Role of branched-chain amino acid metabolism in the pathogenesis of obesity and type 2 diabetes-related metabolic disturbances BCAA metabolism in type 2 diabetes. Nutr Diabetes 2022, 12, 35. [Google Scholar] [CrossRef]
- Prodhan, U.K.; Milan, A.M.; Thorstensen, E.B.; Barnett, M.P.G.; Stewart, R.A.H.; Benatar, J.R.; Cameron-Smith, D. Altered Dairy Protein Intake Does Not Alter Circulatory Branched Chain Amino Acids in Healthy Adults: A Randomized Controlled Trial. Nutrients 2018, 10. [Google Scholar] [CrossRef]
- Markova, M.; Hornemann, S.; Sucher, S.; Wegner, K.; Pivovarova, O.; Rudovich, N.; Thomann, R.; Schneeweiss, R.; Rohn, S.; Pfeiffer, A.F.H. Rate of appearance of amino acids after a meal regulates insulin and glucagon secretion in patients with type 2 diabetes: a randomized clinical trial. Am J Clin Nutr 2018, 108, 279–291. [Google Scholar] [CrossRef]
- Moundras, C.; Remesy, C.; Demigne, C. Dietary protein paradox: decrease of amino acid availability induced by high-protein diets. American Journal of Physiology-Gastrointestinal and Liver Physiology 1993, 264, G1057–G1065. [Google Scholar] [CrossRef]
- Matthews, D.E.; Campbell, R.G. The effect of dietary protein intake on glutamine and glutamate nitrogen metabolism in humans. Am J Clin Nutr 1992, 55, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.D.; Matthews, D.E.; Bier, D.M.; Wen, Z.M.; Young, V.R. Response of alanine metabolism in humans to manipulation of dietary protein and energy intakes. Am J Physiol 1986, 250, E39–46. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.A.; Faloona, G.R.; Unger, R.H. The effect of alanine on glucagon secretion. J Clin Invest 1971, 50, 2215–2218. [Google Scholar] [CrossRef] [PubMed]
- Galsgaard, K.D.; Jepsen, S.L.; Kjeldsen, S.A.S.; Pedersen, J.; Wewer Albrechtsen, N.J.; Holst, J.J. Alanine, arginine, cysteine, and proline, but not glutamine, are substrates for, and acute mediators of, the liver-α-cell axis in female mice. American Journal of Physiology-Endocrinology and Metabolism 2020, 318, E920–E929. [Google Scholar] [CrossRef]
- Dandona, P.; Ghanim, H.; Abuaysheh, S.; Green, K.; Batra, M.; Dhindsa, S.; Makdissi, A.; Patel, R.; Chaudhuri, A. Decreased insulin secretion and incretin concentrations and increased glucagon concentrations after a high-fat meal when compared with a high-fruit and -fiber meal. Am J Physiol Endocrinol Metab 2015, 308, E185–191. [Google Scholar] [CrossRef]
- Radulescu, A.; Gannon, M.C.; Nuttall, F.Q. The Effect on Glucagon, Glucagon-Like Peptide-1, Total and Acyl-Ghrelin of Dietary Fats Ingested with and without Potato. The Journal of Clinical Endocrinology & Metabolism 2010, 95, 3385–3391. [Google Scholar] [CrossRef]
- Sloth, B.; Due, A.; Larsen, T.M.; Holst, J.J.; Heding, A.; Astrup, A. The effect of a high-MUFA, low-glycaemic index diet and a low-fat diet on appetite and glucose metabolism during a 6-month weight maintenance period. Br J Nutr 2009, 101, 1846–1858. [Google Scholar] [CrossRef]
- Raben, A.; Holst, J.J.; Madsen, J.; Astrup, A. Diurnal metabolic profiles after 14 d of an ad libitum high-starch, high-sucrose, or high-fat diet in normal-weight never-obese and postobese women. Am J Clin Nutr 2001, 73, 177–189. [Google Scholar] [CrossRef]
- Luukkonen, P.K.; Sädevirta, S.; Zhou, Y.; Kayser, B.; Ali, A.; Ahonen, L.; Lallukka, S.; Pelloux, V.; Gaggini, M.; Jian, C.; et al. Saturated Fat Is More Metabolically Harmful for the Human Liver Than Unsaturated Fat or Simple Sugars. Diabetes Care 2018, 41, 1732–1739. [Google Scholar] [CrossRef]
- Zhang, J.; Pivovarova-Ramich, O.; Kabisch, S.; Markova, M.; Hornemann, S.; Sucher, S.; Rohn, S.; Machann, J.; Pfeiffer, A.F.H. High Protein Diets Improve Liver Fat and Insulin Sensitivity by Prandial but Not Fasting Glucagon Secretion in Type 2 Diabetes. Front Nutr 2022, 9, 808346. [Google Scholar] [CrossRef]
- Knudsen, J.G.; Hamilton, A.; Ramracheya, R.; Tarasov, A.I.; Brereton, M.; Haythorne, E.; Chibalina, M.V.; Spegel, P.; Mulder, H.; Zhang, Q.; et al. Dysregulation of Glucagon Secretion by Hyperglycemia-Induced Sodium-Dependent Reduction of ATP Production. Cell Metab 2019, 29, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Gar, C.; Haschka, S.J.; Kern-Matschilles, S.; Rauch, B.; Sacco, V.; Prehn, C.; Adamski, J.; Seissler, J.; Wewer Albrechtsen, N.J.; Holst, J.J.; et al. The liver-alpha cell axis associates with liver fat and insulin resistance: a validation study in women with non-steatotic liver fat levels. Diabetologia 2021, 64, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Szczepaniak, L.S.; Nurenberg, P.; Leonard, D.; Browning, J.D.; Reingold, J.S.; Grundy, S.; Hobbs, H.H.; Dobbins, R.L. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab 2005, 288, E462–468. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, M.E.; Wait, J.B.; Koech, J.; Gordon, A.N.; Coch, R.W.; Svendsen, B.; Finan, B.; D’Alessio, D.A.; Campbell, J.E. Glucagon lowers glycemia when β cells are active. JCI Insight 2019, 4. [Google Scholar] [CrossRef]

| Baseline characteristics (mean ± SD) |
All participants n=92 |
HPD subgroup n=24 |
|---|---|---|
| Sex [female/male] | 58/34 | 10/14 |
| Zygosity [mono/di] | 68/24 | 20/4 |
| Age [y] | 31 ± 14 | 39 ± 15 |
| BMI [kg/m²] | 23 ± 3 | 24 ± 2 |
| WHR | 0.81 ± 0.07 | 0.84 ± 0.06 |
| Systolic BP [mm Hg] | 118 ± 13 | 117 ± 11 |
| Diastolic BP [mm Hg] | 74 ± 9 | 76 ± 6 |
| Fasting insulin [mU/L] | 5.21 ± 3.68 | 5.38 ± 4.00 |
| Fasting glucose [mmol/L] | 4.78 ± 0.48 | 5.05 ± 0.46 |
| HbA1c [%] | 5.0 ± 0.4 | 5.0 ± 0.4 |
| HbA1c [mmol/mol] | 31 ± 5 | 31 ± 5 |
| Fasting glucagon [pmol/L] | 3.97 ± 2.71 | 3.21 ± 2.75 |
| Fasting total cholesterol [mmol/L] | 4.58 ± 0.93 | 4.69 ± 0.98 |
| Fasting HDL cholesterol [mmol/L] | 1.38 ± 0.35 | 1.32 ± 0.34 |
| Fasting LDL cholesterol [mmol/L] | 2.73 ± 0.77 | 2.89 ± 0.83 |
| Fasting triglycerides [mmol/L] | 0.99 ± 0.44 | 0.94 ± 0.38 |
| Fasting FFA [mmol/L] | 0.52 ± 0.26 | 0.47 ± 0.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





