Submitted:
17 October 2024
Posted:
18 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Requirements and Notewhorthy Results
2.3. Information Sources, Search Tactics and Further Searches for Primary Studies
2.4. Data Management and Selection Process
2.5. Data Collection Process
2.6. Evaluation of Meta-Bias, Evidence Quality, and Methodological Quality
2.7. Measurement Summary
2.8. Results Summary
3. Results
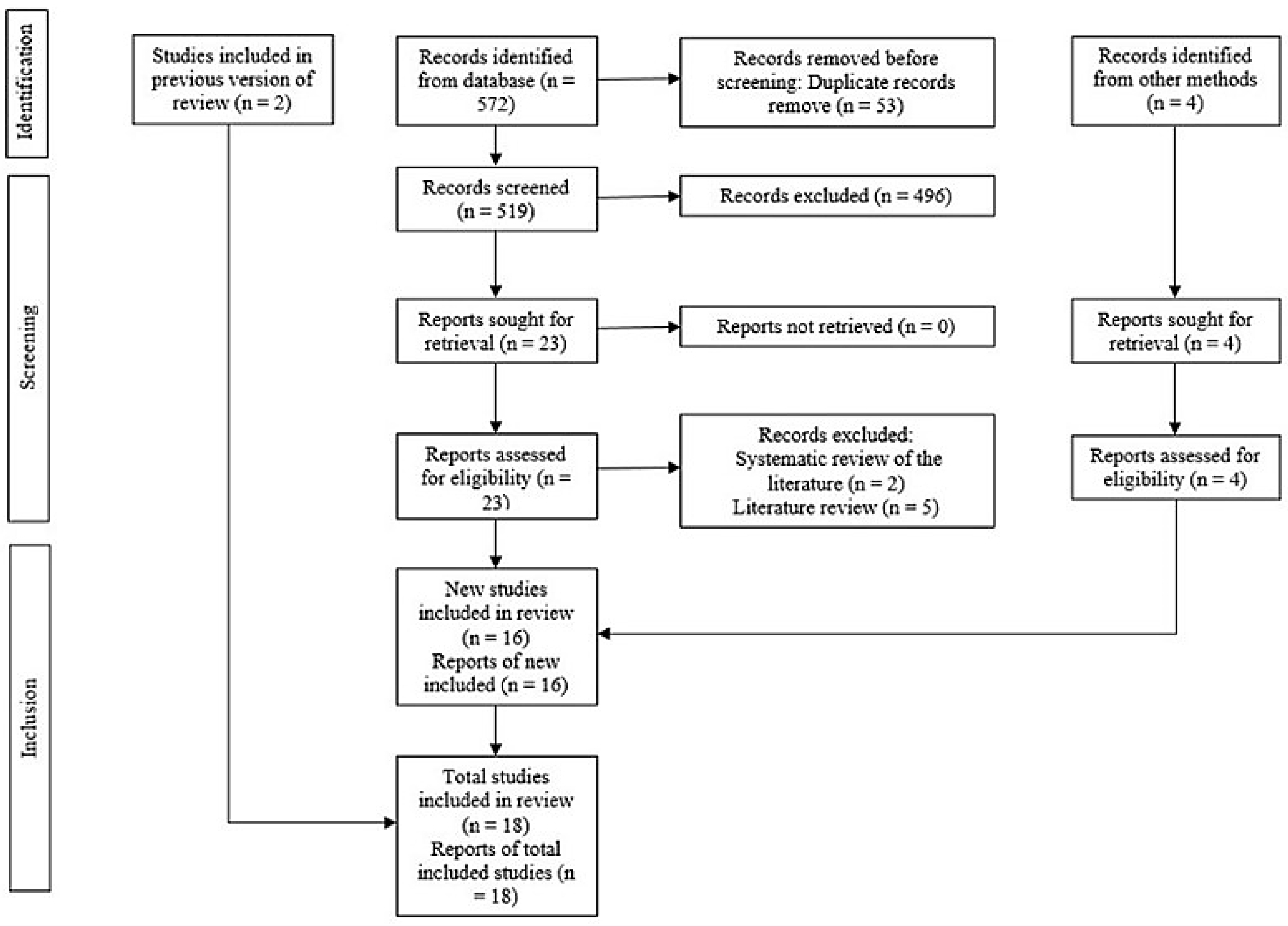
3.1. Examining and Choosing Original Research
3.2. Review and Characteristics of Included Studies
3.3. Assessment of Methodological Quality and Quality of Evidence:
3.4. Overlapping
3.5. Synthesis of Results
3.6. General Association
3.7. Plaque Index
3.8. Gingival Index
3.9. Clinical Attachment Level
3.10. Number of Teeth
3.11. Prevalence
3.12. Probing Depth
3.13. Bleeding on Probing
4. Discussion
4.1. Evidence Summary
4.2. Implications for Clinical Practice
4.3. Implications for Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu C-Z, Yuan Y-H, Liu H-H, Li S-S, Zhang B-W, Chen W, et al. Epidemiologic relationship between periodontitis and type 2 diabetes mellitus. BMC Oral Health. 2020;20(1):204. [CrossRef]
- Al Ansari Y, Shahwan H, Chrcanovic BR. Diabetes mellitus and dental implants: A systematic review and meta-analysis. Materials (Basel). 2022;15(9):3227. [CrossRef]
- Stöhr J, Barbaresko J, Neuenschwander M, Schlesinger S. Bidirectional association between periodontal disease and diabetes mellitus: a systematic review and meta-analysis of cohort studies. Sci Rep. 2021;11(1):13686. [CrossRef]
- Alwithanani N. Periodontal diseases and diabetes mellitus: A systematic review. J Pharm Bioallied Sci. 2023;15(Suppl 1):S54–63. [CrossRef]
- Ahmadinia AR, Rahebi D, Mohammadi M, Ghelichi-Ghojogh M, Jafari A, Esmaielzadeh F, et al. Association between type 2 diabetes (T2D) and tooth loss: a systematic review and meta-analysis. BMC Endocr Disord. 2022;22(1):100. [CrossRef]
- Sanz M, Ceriello A, Buysschaert M, Chapple I, Demmer RT, Graziani F, et al. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International diabetes Federation and the European Federation of Periodontology. Diabetes Res Clin Pract. 2018;137:231–41. [CrossRef]
- Gobin R, Tian D, Liu Q, Wang J. Periodontal diseases and the risk of metabolic syndrome: An updated systematic review and meta-analysis. Front Endocrinol (Lausanne). 2020;11:336. [CrossRef]
- Păunică I, Giurgiu M, Dumitriu AS, Păunică S, Pantea Stoian AM, Martu M-A, et al. The bidirectional relationship between periodontal disease and diabetes mellitus-A review. Diagnostics (Basel). 2023;13(4). [CrossRef]
- Siddiqi A, Zafar S, Sharma A, Quaranta A. Diabetes mellitus and periodontal disease: The call for interprofessional education and interprofessional collaborative care - A systematic review of the literature. J Interprof Care. 2022;36(1):93–101. [CrossRef]
- Costa R, Ríos-Carrasco B, Monteiro L, López-Jarana P, Carneiro F, Relvas M. Association between type 1 diabetes mellitus and periodontal diseases. J Clin Med. 2023;12(3). [CrossRef]
- Natto ZS, Hameedaldain A. Methodological quality assessment of meta-analyses and Systematic Reviews of the relationship between periodontal and systemic diseases. J Evid Based Dent Pract. 2019;19(2):131–9. [CrossRef]
- Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350(jan02 1):g7647. [CrossRef]
- Booth A, Clarke M, Ghersi D, Moher D, Petticrew M, Stewart L. An international registry of systematic-review protocols. Lancet. 2011;377(9760):108–9. [CrossRef]
- Bougioukas KI, Liakos A, Tsapas A, Ntzani E, Haidich A-B. Preferred reporting items for overviews of systematic reviews including harms checklist: a pilot tool to be used for balanced reporting of benefits and harms. J Clin Epidemiol. 2018;93:9–24. [CrossRef]
- Pauletto P, Ruales-Carrera E, Mezzomo LA, Stefani CM, Taba M Jr, Gonçalves RB, et al. Clinical performance of short versus standard dental implants in vertically augmented bone: an overview of systematic reviews. Clin Oral Investig. 2021;25(11):6045–68. [CrossRef]
- Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;j4008. [CrossRef]
- Herrera D, Sanz M, Shapira L, Brotons C, Chapple I, Frese T, et al. Association between periodontal diseases and cardiovascular diseases, diabetes and respiratory diseases: Consensus report of the Joint Workshop by the European Federation of Periodontology (EFP) and the European arm of the World Organization of Family Doctors (WONCA Europe). J Clin Periodontol. 2023;50(6):819–41. [CrossRef]
- Li L-L, Xie X-T, Wu Y, Yan F-H. Advances in research on the mechanism of association between periodontitis and diabetes mellitus. Sichuan Da Xue Xue Bao Yi Xue Ban. 2023;54(1):71–6. [CrossRef]
- George AK, Wills V. Association between periodontal diseases and gestational diabetes mellitus-A review. Kerala Dent J. 2020;43(2):82–4.
- Bansal S, Dhir S, Wangnoo SK. Diabetes mellitus and periodontitis: Relevance of the diabolic duo in India. Apollo Med. 2020;17(4):267–71. [CrossRef]
- Laddha R, Jones AH, Patil A. Relationship between diabetes and periodontitis:A systematic review. Indian J Public Health Res Dev. 2019;10(12):843. [CrossRef]
- Salvi GE, Carollo-Bittel B, Lang NP. Effects of diabetes mellitus on periodontal and peri-implant conditions: update on associations and risks. J Clin Periodontol. 2008;35(8 Suppl):398–409. [CrossRef]
- Mealey BL, Oates TW, American Academy of Periodontology. Diabetes mellitus and periodontal diseases. J Periodontol. 2006;77(8):1289–303. [CrossRef]
- León-Ríos XA, Da Silva Pires S, Gil-Montoya JA. Association between gestational diabetes mellitus and periodontal disease: Systematic review. Clin E Investig En Ginecol Obstet. 2022;49(4):1–11.
- Zainal Abidin Z, Zainuren ZA, Noor E, Mohd Nor NS, Mohd Saffian S, Abdul Halim R. Periodontal health status of children and adolescents with diabetes mellitus: a systematic review and meta-analysis. Aust Dent J. 2021;66 Suppl 1(S1):S15–26. [CrossRef]
- Zheng M, Wang C, Ali A, Shih YA, Xie Q, Guo C. Prevalence of periodontitis in people clinically diagnosed with diabetes mellitus: a meta-analysis of epidemiologic studies. Acta Diabetol. 2021;58(10):1307–27. [CrossRef]
- Ismail AF, McGrath CP, Yiu CKY. Oral health of children with type 1 diabetes mellitus: A systematic review. Diabetes Res Clin Pract. 2015;108(3):369–81. [CrossRef]
- Dicembrini I, Serni L, Monami M, Caliri M, Barbato L, Cairo F, et al. Type 1 diabetes and periodontitis: prevalence and periodontal destruction-a systematic review. Acta Diabetol. 2020;57(12):1405–12. [CrossRef]
- Rapone B, Corsalini M, Converti I, Loverro MT, Gnoni A, Trerotoli P, et al. Does periodontal inflammation affect type 1 diabetes in childhood and adolescence? A Meta-analysis. Front Endocrinol (Lausanne). 2020;11:278. [CrossRef]
- Graziani F, Gennai S, Solini A, Petrini M. A systematic review and meta-analysis of epidemiologic observational evidence on the effect of periodontitis on diabetes An update of the EFP-AAP review. J Clin Periodontol. 2018;45(2):167–87. [CrossRef]
- Nguyen ATM, Akhter R, Garde S, Scott C, Twigg SM, Colagiuri S, et al. The association of periodontal disease with the complications of diabetes mellitus. A systematic review. Diabetes Res Clin Pract. 2020;165(108244):108244. [CrossRef]
- Tanaka H, Ihana-Sugiyama N, Sugiyama T, Ohsugi M. Contribution of diabetes to the incidence and prevalence of comorbid conditions (cancer, periodontal disease, fracture, impaired cognitive function, and depression): A systematic review of epidemiological studies in Japanese populations. J Epidemiol. 2019;29(1):1–10. [CrossRef]
- Nascimento GG, Leite FRM, Vestergaard P, Scheutz F, López R. Does diabetes increase the risk of periodontitis? A systematic review and meta-regression analysis of longitudinal prospective studies. Acta Diabetol. 2018;55(7):653–67. [CrossRef]
- Ziukaite L, Slot DE, Van der Weijden FA. Prevalence of diabetes mellitus in people clinically diagnosed with periodontitis: A systematic review and meta-analysis of epidemiologic studies. J Clin Periodontol. 2018;45(6):650–62. [CrossRef]
- Mauri-Obradors E, Estrugo-Devesa A, Jane-Salas E, Vinas M, Lopez-Lopez J. Oral manifestations of Diabetes Mellitus. A systematic review. Med Oral Patol Oral Cir Bucal. 2017;0–0. [CrossRef]
- Esteves Lima RP, Cyrino RM, de Carvalho Dutra B, Oliveira da Silveira J, Martins CC, Miranda Cota LO, et al. Association between periodontitis and gestational diabetes mellitus: Systematic review and meta-analysis. J Periodontol. 2016;87(1):48–57. [CrossRef]
- Chávarry NGM, Vettore MV, Sansone C, Sheiham A. The relationship between diabetes mellitus and destructive periodontal disease: a meta-analysis. Oral Health Prev Dent. 2009;7(2):107–27.
- Abariga SA, Whitcomb BW. Periodontitis and gestational diabetes mellitus: a systematic review and meta-analysis of observational studies. BMC Pregnancy Childbirth. 2016;16(1):344. [CrossRef]
- Borgnakke WS, Ylöstalo PV, Taylor GW, Genco RJ. Effect of periodontal disease on diabetes: systematic review of epidemiologic observational evidence. J Periodontol. 2013;84(4 Suppl):S135-52. [CrossRef]
- Bullon P, Jaramillo R, Santos-Garcia R, Rios-Santos V, Ramirez M, Fernandez-Palacin A, et al. Relation of periodontitis and metabolic syndrome with gestational glucose metabolism disorder. J Periodontol. 2014;85(2):e1-8. [CrossRef]
- Al-Khabbaz AK, Al-Shammari KF, Hasan A, Abdul-Rasoul M. Periodontal health of children with type 1 diabetes mellitus in Kuwait: a case-control study. Med Princ Pract. 2013;22(2):144–9. [CrossRef]
- Esteves Lima RP, Miranda Cota LO, Costa FO. Association between periodontitis and gestational diabetes mellitus: a case-control study. J Periodontol. 2013;84(9):1257–65. [CrossRef]
- Chokwiriyachit A, Dasanayake AP, Suwannarong W, Hormdee D, Sumanonta G, Prasertchareonsuk W, et al. Periodontitis and gestational diabetes mellitus in non-smoking females. J Periodontol. 2013;84(7):857–62. [CrossRef]
- Morita I, Inagaki K, Nakamura F, Noguchi T, Matsubara T, Yoshii S, et al. Relationship between periodontal status and levels of glycated hemoglobin. J Dent Res. 2012;91(2):161–6. [CrossRef]
- Xiong X, Elkind-Hirsch KE, Vastardis S, Delarosa RL, Pridjian G, Buekens P. Periodontal disease is associated with gestational diabetes mellitus: a case-control study. J Periodontol. 2009;80(11):1742–9. [CrossRef]
- Dakovic D, Pavlovic MD. Periodontal disease in children and adolescents with type 1 diabetes in Serbia. J Periodontol. 2008;79(6):987–92. [CrossRef]
- Dasanayake AP, Chhun N, Tanner ACR, Craig RG, Lee MJ, Moore AF, et al. Periodontal pathogens and gestational diabetes mellitus. J Dent Res. 2008;87(4):328–33. [CrossRef]
- Saito T, Shimazaki Y, Kiyohara Y, Kato I, Kubo M, Iida M, et al. The severity of periodontal disease is associated with the development of glucose intolerance in non-diabetics: the Hisayama study. J Dent Res. 2004;83(6):485–90. [CrossRef]
- Sun K-T, Chen S-C, Lin C-L, Hsu J-T, Chen I-A, Wu I-T, et al. The association between Type 1 diabetes mellitus and periodontal diseases. J Formos Med Assoc. 2019;118(6):1047–54. [CrossRef]
- Ismail AF, McGrath CP, Yiu CKY. Oral health status of children with type 1 diabetes: a comparative study. J Pediatr Endocrinol Metab. 2017;30(11). [CrossRef]
- Chiu SY-H, Lai H, Yen AM-F, Fann JC-Y, Chen L-S, Chen H-H. Temporal sequence of the bidirectional relationship between hyperglycemia and periodontal disease: a community-based study of 5,885 Taiwanese aged 35-44 years (KCIS No. 32). Acta Diabetol. 2015;52(1):123–31. [CrossRef]
- Habib FA. Evaluation of periodontal status among saudi females with gestational diabetes and its relation to glucose and lipid homeostasis in ohud hospital, Al madina Al-munwarrah. Int J Health Sci (Qassim). 2009;3(2):143–54.
- Orbak R, Simsek S, Orbak Z, Kavrut F, Colak M. The influence of type-1 diabetes mellitus on dentition and oral health in children and adolescents. Yonsei Med J. 2008;49(3):357–65. [CrossRef]
- Lalla E, Cheng B, Lal S, Tucker S, Greenberg E, Goland R, et al. Periodontal changes in children and adolescents with diabetes: a case-control study. Diabetes Care. 2006;29(2):295–9. [CrossRef]
- Aren G, Sepet E, Ozdemir D, Dinççağ N, Güvener B, Firatli E. Periodontal health, salivary status, and metabolic control in children with type 1 diabetes mellitus. J Periodontol. 2003;74(12):1789–95. [CrossRef]
- Sbordone L, Ramaglia L, Barone A, Ciaglia RN, Iacono VJ. Periodontal status and subgingival microbiota of insulin-dependent juvenile diabetics: a 3-year longitudinal study. J Periodontol. 1998;69(2):120–8. [CrossRef]
- Firatli E. The relationship between clinical periodontal status and insulin-dependent diabetes mellitus. Results after 5 years. J Periodontol. 1997;68(2):136–40. [CrossRef]
- Pinson M, Hoffman WH, Garnick JJ, Litaker MS. Periodontal disease and type I diabetes mellitus in children and adolescents. J Clin Periodontol. 1995;22(2):118–23. [CrossRef]
- Roy M, Gastaldi G, Courvoisier DS, Mombelli A, Giannopoulou C. Periodontal health in a cohort of subjects with type 1 diabetes mellitus. Clin Exp Dent Res. 2019;5(3):243–9. [CrossRef]
- Babu KLG, Subramaniam P, Kaje K. Assessment of dental caries and gingival status among a group of type 1 diabetes mellitus and healthy children of South India - a comparative study. J Pediatr Endocrinol Metab. 2018;31(12):1305–10. [CrossRef]
- Myllymäki V, Saxlin T, Knuuttila M, Rajala U, Keinänen-Kiukaanniemi S, Anttila S, et al. Association between periodontal condition and the development of type 2 diabetes mellitus-Results from a 15-year follow-up study. J Clin Periodontol. 2018;45(11):1276–86. [CrossRef]
- Chaparro A, Zúñiga E, Varas-Godoy M, Albers D, Ramírez V, Hernández M, et al. Periodontitis and placental growth factor in oral fluids are early pregnancy predictors of gestational diabetes mellitus. J Periodontol. 2018;89(9):1052–60. [CrossRef]
- Winning L, Patterson CC, Neville CE, Kee F, Linden GJ. Periodontitis and incident type 2 diabetes: a prospective cohort study. J Clin Periodontol. 2017;44(3):266–74. [CrossRef]
- Jindal A, Parihar AS, Sood M, Singh P, Singh N. Relationship between severity of periodontal disease and control of diabetes (glycated hemoglobin) in patients with Type 1 diabetes mellitus. J Int Oral Health. 2015;7(Suppl 2):17–20.
- Popławska-Kita A, Siewko K, Szpak P, Król B, Telejko B, Klimiuk PA, et al. Association between type 1 diabetes and periodontal health. Adv Med Sci. 2014;59(1):126–31. [CrossRef]
- Amiri AA, Maboudi A, Bahar A, Farokhfar A, Daneshvar F, Khoshgoeian HR, et al. Relationship between type 2 diabetic retinopathy and periodontal disease in Iranian adults. N Am J Med Sci. 2014;6(4):190.
- Lee K-S, Kim E-K, Kim J-W, Choi Y-H, Mechant AT, Song K-B, et al. The relationship between metabolic conditions and prevalence of periodontal disease in rural Korean elderly. Arch Gerontol Geriatr. 2014;58(1):125–9. [CrossRef]
- Jimenez M, Hu FB, Marino M, Li Y, Joshipura KJ. Type 2 diabetes mellitus and 20-year incidence of periodontitis and tooth loss. Diabetes Res Clin Pract. 2012;98(3):494–500. [CrossRef]
- Southerland JH, Moss K, Taylor GW, Beck JD, Pankow J, Gangula PR, et al. Periodontitis and diabetes associations with measures of atherosclerosis and CHD. Atherosclerosis. 2012;222(1):196–201. [CrossRef]
- Hodge PJ, Robertson D, Paterson K, Smith GLF, Creanor S, Sherriff A. Periodontitis in non-smoking type 1 diabetic adults: a cross-sectional study. J Clin Periodontol. 2012;39(1):20–9. [CrossRef]
- Ruiz DR, Romito GA, Dib SA. Periodontal disease in gestational and type 1 diabetes mellitus pregnant women: Periodontal disease in Brazilian GDM and T1DM pregnant women. Oral Dis. 2011;17(5):515–21. [CrossRef]
- Ide R, Hoshuyama T, Wilson D, Takahashi K, Higashi T. Periodontal disease and incident diabetes: a seven-year study: A seven-year study. J Dent Res. 2011;90(1):41–6. [CrossRef]
- Tagelsir A, Cauwels R, van Aken S, Vanobbergen J, Martens LC. Dental caries and dental care level (restorative index) in children with diabetes mellitus type 1: Dental caries and dental care level in Caries experience in diabetic children. Int J Paediatr Dent. 2011;21(1):13–22. [CrossRef]
- Abrao L, Chagas JK, Schmid H. Periodontal disease and risk for neuropathic foot ulceration in type 2 diabetes. Diabetes Res Clin Pract. 2010;90(1):34–9. [CrossRef]
- Demmer RT, Jacobs DR Jr, Desvarieux M. Periodontal disease and incident type 2 diabetes: results from the First National Health and Nutrition Examination Survey and its epidemiologic follow-up study. Diabetes Care. 2008;31(7):1373–9. [CrossRef]
- Shultis WA, Weil EJ, Looker HC, Curtis JM, Shlossman M, Genco RJ, et al. Effect of periodontitis on overt nephropathy and end-stage renal disease in type 2 diabetes. Diabetes Care. 2007;30(2):306–11. [CrossRef]
- Novak KF, Taylor GW, Dawson DR, Ferguson JE 2nd, Novak MJ. Periodontitis and gestational diabetes mellitus: exploring the link in NHANES III. J Public Health Dent. 2006 Summer;66(3):163–8. [CrossRef]
- Borges-Yáñez SA, Irigoyen-Camacho ME, Maupomé G. Risk factors and prevalence of periodontitis in community-dwelling elders in Mexico. J Clin Periodontol. 2006;33(3):184–94. [CrossRef]
- Mansour AA, Abd-Al-Sada N. Periodontal disease among diabetics in Iraq. MedGenMed. 2005;7(3):2.
- Campus G, Salem A, Uzzau S, Baldoni E, Tonolo G. Diabetes and periodontal disease: a case-control study. J Periodontol. 2005;76(3):418–25. [CrossRef]
- Saremi A, Nelson RG, Tulloch-Reid M, Hanson RL, Sievers ML, Taylor GW, et al. Periodontal disease and mortality in type 2 diabetes. Diabetes Care. 2005;28(1):27–32. [CrossRef]
- Siudikiene J, Maciulskiene V, Dobrovolskiene R, Nedzelskiene I. Oral hygiene in children with type I diabetes mellitus. Stomatologija. 2005;7(1):24–7.
- Noma H, Sakamoto I, Mochizuki H, Tsukamoto H, Minamoto A, Funatsu H, et al. Relationship between periodontal disease and diabetic retinopathy. Diabetes Care. 2004;27(2):615. [CrossRef]
- Marugame T, Hayasaki H, Lee K, Eguchi H, Matsumoto S. Alveolar bone loss associated with glucose tolerance in Japanese men: Original article. Diabet Med. 2003;20(9):746–51. [CrossRef]
- Zielinski MB, Fedele D, Forman LJ, Pomerantz SC. Oral health in the elderly with non-insulin-dependent diabetes mellitus. Spec Care Dentist. 2002;22(3):94–8. [CrossRef]
- Kawamura M, Fukuda S, Kawabata K, Iwamoto Y. Comparison of health behaviour and oral/medical conditions in non-insulin-dependent (type II) diabetics and non-diabetics. Aust Dent J. 1998;43(5):315–20. [CrossRef]
- Collin HL, Uusitupa M, Niskanen L, Kontturi-Närhi V, Markkanen H, Koivisto AM, et al. Periodontal findings in elderly patients with non-insulin dependent diabetes mellitus. J Periodontol. 1998;69(9):962–6. [CrossRef]
- Firatli E, Yilmaz O, Onan U. The relationship between clinical attachment loss and the duration of insulin-dependent diabetes mellitus (IDDM) in children and adolescents. J Clin Periodontol. 1996;23(4):362–6. [CrossRef]
- Thorstensson H, Kuylenstiema J, Hugoson A. Medical Status and complications in relation to periodontal disease experience in insulin-dependent diabetics. J Clin Periodontol. 1996;23(3):194–202. [CrossRef]
- Sbordone L, Ramaglia L, Barone A, Ciaglia RN, Tenore A, Iacono VJ. Periodontal status and selected cultivable anaerobic microflora of insulin-dependent juvenile diabetics. J Periodontol. 1995;66(6):452–61. [CrossRef]
- de Pommereau V, Dargent-Paré C, Robert JJ, Brion M. Periodontal status in insulin-dependent diabetic adolescents. J Clin Periodontol. 1992;19(9):628–32. [CrossRef]
- Sandholm L, Swanljung O, Rytömaa I, Kaprio EA, Mäenpää J. Periodontal status of Finnish adolescents with insulin-dependent diabetes mellitus. J Clin Periodontol. 1989;16(10):617–20. [CrossRef]
- Lavigne SE, Forrest JL. An umbrella review of systematic reviews examining the relationship between type 2 diabetes and periodontitis: Position paper from the Canadian Dental Hygienists Association. Can J Dent Hyg. 2021;55(1):57–67.
- Seitz MW, Listl S, Bartols A, Schubert I, Blaschke K, Haux C, et al. Current knowledge on correlations between highly prevalent dental conditions and chronic diseases: An umbrella review. Prev Chronic Dis. 2019;16(180641):E132. [CrossRef]
- Moher D. The problem of duplicate systematic reviews. BMJ. 2013;347(aug14 4):f5040. [CrossRef]
| Database | Search strategy | Number of studies |
|---|---|---|
| Pubmed | (("Periodontal disease") OR ("gingivitis") OR ("periodontitis")) AND (("Diabetes mellitus") OR ("DM1") OR ("DM2") OR ("gestacional diabetes") OR ("type 1 diabetes") OR ("type 2 diabetes")) AND ("association") AND (("systematic review") OR ("meta-analysis") OR ("systematic review and meta-analysis")) | 66 |
| Cochrane database | #1 MeSH descriptor: [Periodontal Diseases] explode all trees #2 MeSH descriptor: [Periodontitis] in all MeSH products #3 MeSH descriptor: [Gingivitis] explode all trees #4 ("Periodontal disease"):ti,ab,kw OR ("gingivitis"):ti,ab,kw OR ("periodontitis"):ti,ab,kw #5 #1 OR #2 OR #3 OR #4 #6 MeSH descriptor: [Diabetes Mellitus] explode all trees #7 MeSH descriptor: [Diabetes Mellitus, Type 2] explode all trees #8 MeSH descriptor: [Diabetes Mellitus, Type 1] explode all trees #9 MeSH descriptor: [Diabetes, Gestational] explode all trees #10 ("Diabetes mellitus"):ti,ab,kw OR ("DM1"):ti,ab,kw OR ("DM2"):ti,ab,kw OR ("gestacional diabetes"):ti,ab,kw OR ("type 1 diabetes mellitus"):ti,ab,kw #11 ("type 2 diabetes mellitus"):ti,ab,kw #12 #6 OR #7 OR #8 OR #9 OR #10 OR #11 #13 MeSH descriptor: [Association] explode all trees #14 ("association"):ti,ab,kw #15 #13 OR #14 #16 #5 AND #12 AND #15 |
1 |
| Scielo | (("Periodontal disease") OR ("gingivitis") OR ("periodontitis")) AND (("Diabetes mellitus") OR ("DM1") OR ("DM2") OR ("gestacional diabetes") OR ("type 1 diabetes") OR ("type 2 diabetes")) AND ("association") AND (("systematic review") OR ("meta-analysis") OR ("systematic review and meta-analysis")) | 3 |
| Scopus | (TITLE-ABS-KEY ((("Periodontal disease") OR ("gingivitis") OR ("periodontitis"))) AND TITLE-ABS-KEY ((("Diabetes mellitus") OR ("DM1") OR ("DM2") OR ("gestacional diabetes") OR ("type 1 diabetes") OR ("type 2 diabetes"))) AND TITLE-ABS-KEY (("association")) AND TITLE-ABS-KEY ((("systematic review") OR ("meta-analysis") OR ("systematic review and meta-analysis")))) AND ( LIMIT-TO (SRCTYPE , "j")) AND ( LIMIT-TO (DOCTYPE , "re")) | 92 |
| Google Scholar | "Periodontal disease" + "Diabetes mellitus" OR "gestacional diabetes" + "association" + "systematic review" -"in vitro" -"literature" | 410 |
| OpenGrey | ("Periodontal disease") AND (("Diabetes mellitus") OR ("gestacional diabetes")) AND ("association") AND ("systematic review") | 0 |
| Author | Reason for exclusion |
|---|---|
| Herrera et al. [17] | Literature review |
| Li et al. [18] | |
| George et al. [19] | |
| Bansal et al. [20] | |
| Laddha et al. [21] | |
| Salvi et al. [22] | Systematic review of the literature |
| Mealey et al. [23] |
| Authors | Year | Study design | Included study design | Number of studies in the qualitative analysis | Number of studies in the quantitative analysis | Type of diabetes | Outcomes | Conclusions | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Costa et al. [10] | 2023 | SR | CCs, Cs, CSs and RCTs | 15 | 0 | T1DM | Most studies confirm the association between T1DM and PDs. | The prevalence and severity of PD was higher in patients with T1DM compared to healthy subjects. | ||
| León-Ríos et al. [24] | 2022 | SR | CCs, Cs and CSs | 8 | 0 | GDM | In most studies an association between PD and GDM was verified. | PD increases the risk of developing GDM. | ||
| Zainal et al. [25] | 2021 | SR with MA | CCs and CSs | 11 | 11 | DM | PI | SMD = 0.54 (0.20 - 0.87) | The duration of DM in children and adolescents affects periodontal status even with a difference of 1 year. Thus, children and adolescents with DM and early PD will progress to periodontitis without intervention. | |
| GI | SMD = 0.63 (0.39 - 0.87) | |||||||||
| BP | SMD = 0.32 (0.07 - 0.58) | |||||||||
| PDT | SMD = 0.67 (0.23 - 1.11) | |||||||||
| CAL | SMD = 0.79 (0.52 - 1.05) | |||||||||
| Zheng et al. [26] | 2021 | SR with MA | CCs, Cs and CSs | 40 | 27 | DM | P | OR = 1.85 (1.61 - 2.11) | The prevalence and severity of periodontitis are higher in patients with diabetes than in non-diabetic populations. | |
| PDT | MD = 0.23 (0.17 - 0.29) | |||||||||
| PI | MD = 0.20 (0.18 - 0.23) | |||||||||
| CAL | MD = 0.39 (0.28 - 0.50) | |||||||||
| NT | MD = -2.14 (-2.87 - -1.40) | |||||||||
| BP | MD = 7.90 (4.24 - 11.56) | |||||||||
| Stöhr et al. [3] | 2021 | SR with MA | Cs | 15 | 15 | DM | GA | RR = 1.26 (1.12 - 1.41) | The findings show a positive bidirectional association between PD and DM. | |
| Dicembrini et al. [28] | 2020 | SR with MA | CCs, Cs and CSs | 19 | 19 | T1DM | P | OR = 0.19 (0.08 - 0.37) | The present data confirm that T1DM is a relevant risk factor for the development of PD. | |
| GA | OR = 2.52 (1.33 - 4.76) | |||||||||
| CAL | SMD = 0.47 (0.37 - 0.57) | |||||||||
| Nguyen et al. [31] | 2020 | SR | Ls, CCs and CSs | 14 | 0 | DM | Higher risks of diabetic complications have been reported in people with diabetes and periodontitis compared to those with diabetes who do not have periodontitis. | This review systematically addressed current epidemiological data that provide evidence that periodontitis is associated with an increased risk of developing diabetic complications compared to patients without periodontitis. | ||
| Rapone et al. [29] | 2020 | SR with MA | CCs and CSs | 10 | 10 | T1DM | BP | SMD = 0.65 (0.08 - 1.23) | Although evidence suggests that there may be an association between PD and T1DM in children and adolescents, study designs and methodological limitations make interpretation of the current research difficult. | |
| CAL | SMD = 0.82 (0.59 - 1.04) | |||||||||
| GI | SMD = 0.46 (0.08 - 0.84) | |||||||||
| PI | SMD = 0.71 (0.19 - 1.22) | |||||||||
| PDT | SMD = 0.36 (0.16 - 0.55) | |||||||||
| Tanaka et al. [32] | 2019 | SR | SRs, Cs, CCs and CSs | 33 | 0 | DM | Although several cohort studies and MAs evaluated cancer development in diabetes, there was little epidemiological evidence for the other conditions. | In Japan, there is little evidence on the effect of diabetes on the incidence and prevalence of PD. | ||
| Nascimento et al. [33] | 2018 | SR with MA | Ls | 13 | 6 | DM | GA | RR = 1.86 (1.25 - 2.77) | This study provides evidence that diabetes is associated with an increased risk of onset and progression of periodontitis in adults. | |
| Ziukaite et al. [34] | 2018 | SR with MA | CCs, Cs and CSs | 29 | 21 | DM | GA | OR = 2.59 (2.12 - 3.15) | The overall prevalence and odds of having diabetes are higher in populations with periodontitis compared to people without periodontitis. | |
| Graziani et al. [30] | 2018 | SR | CCs, Cs and CSs | 20 | 0 | DM | Healthy individuals with periodontitis have poor glycemic control and an increased risk of developing diabetes. People affected by diabetes show impaired glycemic control if they also suffer from periodontitis and a significantly higher prevalence of diabetes-related complications. There is limited evidence available on GDM and T1DM. | Periodontitis has a significant impact on the control, incidence, and complications of diabetes. | ||
| Mauri-Obradors et al. [35] | 2017 | SR | Ls and CSs | 19 | 0 | DM | PD was more prevalent among diabetic patients. | There are multiple oral manifestations associated with DM, one of them is PD. | ||
| Lima et al. [36] | 2016 | SR with MA | CCs and CSs | 8 | 7 | GDM | GA | CSs | OR = 1.67 (1.20 - 2.32) | Scientific evidence cannot affirm a positive association between periodontitis and GDM. |
| CCs | OR = 1.69 (0.68 - 4.21) | |||||||||
| Abariga et al. [38] | 2016 | SR with MA | CCs, Cs and CSs | 10 | 10 | GDM | GA | OR = 2.08 (1.21 - 3.58) | The MAs suggests that periodontitis is associated with a statistically significant increased risk of GDM compared to women without periodontitis. | |
| Ismail et al. [27] | 2015 | SR | CCs and Ls | 28 | 0 | T1DM | Most studies reported significantly greater plaque accumulation and higher GI in children with T1DM. Cohort studies reported no significant differences in periodontal parameters over time. | There is evidence that children with T1DM show poorer periodontal health with greater plaque accumulation compared to healthy children. | ||
| Borgnakke et al. [39] | 2013 | SR | CCs, Cs, CSs, Ls and Rs | 16 | 0 | DM | A small amount of evidence supports the significant adverse effects of PD on glycemic control, diabetes complications, and the development of T2DM and possibly GDM. | Current evidence suggests that PD negatively affects diabetes outcomes. | ||
| Chávarry et al. [37] | 2009 | SR with MA | Ls and CSs | 57 | 16 | DM | CAL | T1DM | MD = 0.26 (-0.00 - 0.53) | T2DM can be considered a risk factor for periodontitis. More studies are needed to confirm the harmful effects of T1DM on PD. |
| T2DM | MD = 1.00 (0.15 - 1.84) | |||||||||
| PDT | T1DM | MD = 0.11 (-0.03 - 0.25) | ||||||||
| T2DM | MD = 0.46 (0.01 - 0.91) | |||||||||
| Authors | Year | AMSTAR – 2 | Overall confidence | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2* | 3 | 4* | 5 | 6 | 7* | 8 | 9* | 10 | 11* | 12 | 13* | 14 | 15* | 16 | |||
| Costa et al. [10] | 2023 | Yes | Yes | Yes | Yes partial | Yes | No | Yes partial | Yes | Yes | Yes | No meta-analysis | Yes | Yes | No meta-analysis | Yes | High | |
| León-Ríos et al. [24] | 2022 | Yes | Yes partial | Yes | Yes partial | Yes | No | Yes partial | Yes | Yes | Yes | No meta-analysis | Yes | Yes | No meta-analysis | Yes | High | |
| Zainal et al. [25] | 2021 | Yes | Yes | Yes | Yes partial | No | Yes | Yes partial | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Zheng et al. [26] | 2021 | Yes | Yes | Yes | Yes partial | Yes | Yes | Yes partial | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Stöhr et al. [3] | 2021 | Yes | Yes | Yes | Yes partial | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Dicembrini et al. [28] | 2020 | Yes | Yes partial | Yes | Yes partial | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Critically low |
| Nguyen et al. [31] | 2020 | Yes | No | Yes | No | Yes | Yes | No | Yes | Yes | Yes | No meta-analysis | Yes | Yes | No meta-analysis | Yes | Critically low | |
| Rapone et al. [29] | 2020 | Yes | Yes partial | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Critically low |
| Tanaka et al. [32] | 2019 | Yes | Yes | Yes | Yes partial | Yes | Yes | No | Yes | Yes | Yes | No meta-analysis | Yes | Yes | No meta-analysis | No | Low | |
| Nascimento et al. [33] | 2018 | Yes | Yes | Yes | Yes partial | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Ziukaite et al. [33] | 2018 | Yes | Yes partial | Yes | Yes partial | Yes | Yes | Yes partial | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Graziani et al. [30] | 2018 | Yes | Yes partial | Yes | Yes partial | Yes | Yes | Yes partial | Yes | Yes | Yes | No meta-analysis | Yes | Yes | No meta-analysis | Yes | High | |
| Mauri-Obradors et al. [36] | 2017 | Yes | Yes partial | Yes | Yes | Yes | Yes | Yes partial | Yes | Yes | Yes | No meta-analysis | Yes | Yes | No meta-analysis | Yes | High | |
| Lima et al. [37] | 2016 | Yes | Yes | Yes | Yes partial | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | High |
| Abariga et al. [38] | 2016 | Yes | Yes partial | Yes | Yes partial | No | Yes | Yes partial | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Ismail et al. [27] | 2015 | Yes | No | Yes | Yes partial | Yes | Yes | Yes partial | Yes | No | Yes | No meta-analysis | No | Yes | No meta-analysis | Yes | Critically low | |
| Borgnakke et al. [39] | 2013 | Yes | Yes partial | Yes | Yes partial | Yes | Yes | Yes partial | Yes | Yes | Yes | No meta-analysis | Yes | Yes | No meta-analysis | Yes | High | |
| Chávarry et al. [37] | 2009 | Yes | Yes partial | Yes | Yes partial | Yes | Yes | Yes partial | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | High |
| Primary studies | Systematic reviews that included the primary studies | Times that primary studies were included |
|---|---|---|
| Bullon et al. [40] | Zheng et al. [26], Graziani et al. [30], Lima et al. [36], Abariga et al. [38] | 4 |
| Al-Khabbaz et al. [41] | Zainal et al. [25], Dicembrini et al. [28], Rapone et al. [29], Ismail et al. [27] | 4 |
| Esteves Lima et al. [42] | León-Ríos et al. [24], Graziani et al. [30], Lima et al. [36], Abariga et al. [38] | 4 |
| Chokwiriyachit et al. [43] | Zheng et al. [26], Graziani et al. [30], Lima et al. [36], Abariga et al. [38] | 4 |
| Morita et al. [44] | Stöhr et al. [3], Tanaka et al. [32], Nascimento et al. [33], Borgnakke et al. [39] | 4 |
| Xiong et al. [45] | Zheng et al. [26], Lima et al. [36], Abariga et al. [38], Borgnakke et al. [39] | 4 |
| Dakovic et al. [46] | Zainal et al. [25], Dicembrini et al. [28], Rapone et al. [29], Ismail et al. [27] | 4 |
| Dasanayake et al. [47] | Zheng et al. [26], Lima et al. [36], Abariga et al. [38], Borgnakke et al. [39] | 4 |
| Saito et al. [48] | Zheng et al. [26], Tanaka et al. [32], Borgnakke et al. [39], Chávarry et al. [37] | 4 |
| Sun et al. [49] | Costa et al. [10], Stöhr et al. [3], Dicembrini et al. [28] | 3 |
| Ismail et al. [50] | Costa et al. [10], Zainal et al. [25], Rapone et al. [29] | 3 |
| Chiu et al. [51] | Stöhr et al. [3], Nascimento et al. [33], Graziani et al. [30] | 3 |
| Habib [52] | Zheng et al. [26], Lima et al. [36], Abariga et al. [38] | 3 |
| Orbak et al. [53] | Zainal et al. [25], Rapone et al. [29], Ismail et al. [27] | 3 |
| Lalla et al. [54] | Zainal et al. [25], Rapone et al. [29], Ismail et al. [27] | 3 |
| Aren et al. [55] | Zainal et al. [25], Ismail et al. [27], Chávarry et al. [36] | 3 |
| Sbordone et al. [56] | Nascimento et al. [33], Ismail et al. [27], Chávarry et al. [37] | 3 |
| Firatli [57] | Nascimento et al. [33], Ismail et al. [27], Chávarry et al. [37] | 3 |
| Pinson et al. [58] | Dicembrini et al. [28], Ismail et al. [27], Chávarry et al. [37] | 3 |
| Roy et al. [59] | Costa et al. [10], Dicembrini et al. [28] | 2 |
| Babu et al. [60] | Zainal et al. [25], Rapone et al. [29] | 2 |
| Myllymäki et al. [61] | Zheng et al. [26], Stöhr et al. [3] | 2 |
| Chaparro et al. [62] | León-Ríos et al. [24], Zheng et al. [26] | 2 |
| Winning et al. [63] | Zheng et al. [26], Stöhr et al. [3] | 2 |
| Jindal et al. [64] | Costa et al. [10], Dicembrini et al. [28] | 2 |
| Popławska-Kita et al. [65] | Costa et al. (10), Dicembrini et al. (28) | 2 |
| Amiri et al. [66] | Nguyen et al. [31], Graziani et al. [30] | 2 |
| Lee et al. [67] | Nascimento et al. [33], Ziukaite et al. [34] | 2 |
| Jimenez et al. [68] | Stöhr et al. [3], Nascimento et al. [33] | 2 |
| Southerland et al. [69] | Nguyen et al. [31], Borgnakke et al. [39] | 2 |
| Hodge et al. [70] | Zheng et al. [26], Dicembrini et al. [28] | 2 |
| Ruiz et al. [71] | Lima et al. [36], Abariga et al. [38] | 2 |
| Ide et al. [72] | Stöhr et al. [3], Borgnakke et al. [39] | 2 |
| Tagelsir et al. [73] | Zainal et al. [25], Ismail et al. [27] | 2 |
| Abrao et al. [74] | Nguyen et al. [31], Borgnakke et al. [39] | 2 |
| Demmer et al. [75] | Stöhr et al. [3], Borgnakke et al. [39] | 2 |
| Shultis et al. [76] | Nguyen et al. [31], Borgnakke et al. [39] | 2 |
| Novak et al. [77] | Lima et al. [36], Abariga et al. [38] | 2 |
| Borges-Yáñez [78] | Ziukaite et al. [34], Chávarry et al. [37] | 2 |
| Mansour et al. [79] | Zheng et al. [26], Chávarry et al. [37] | 2 |
| Campus et al. [80] | Zheng et al. [26], Chávarry et al. [37] | 2 |
| Saremi et al. [81] | Nguyen et al. [31], Borgnakke et al. [39] | 2 |
| Siudikiene et al. [82] | Zainal et al. [25], Ismail et al. [27] | 2 |
| Noma et al. [83] | Nguyen et al. [31], Borgnakke et al. [39] | 2 |
| Marugame et al. [84] | Tanaka et al. [32], Chávarry et al. [37] | 2 |
| Zielinski et al. [85] | Zheng et al. [26], Chávarry et al. [37] | 2 |
| Kawamura et al. [86] | Zheng et al. [26], Chávarry et al. [37] | 2 |
| Collin et al. [87] | Zheng et al. [26], Chávarry et al. [37] | 2 |
| Firatli et al. [88] | Ismail et al. [27], Chávarry et al. [37] | 2 |
| Thorstensson et al. [89] | Nguyen et al. [31], Borgnakke et al. [39] | 2 |
| Sbordone et al. [90] | Ismail et al. [27], Chávarry et al. [37] | 2 |
| de Pommereau et al. [91] | Ismail et al. [27], Chávarry et al. [37] | 2 |
| Sandholm et al. [92] | Ismail et al. [27], Chávarry et al. [37] | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





