2. Materials and Methods
In this retrospective, uncontrolled clinical study, medical and surgical records of pseudophakic patients undergoing small-gauge PPV for different retinal pathologies were reviewed. All surgeries were carried out at “S.S. Annunziata Hospital” Eye clinic in Taranto, Italy, between January 2021 and July 2023. The study adhered to the tenets of the Declaration of Helsinki and was approved by the local Institutional Review Board (IRB).
To be included, patients had to fulfill the following inclusion criteria: age of 18 years or older; presence of a regularly signed informed consent for the surgery; pseudophakic status at the time of PPV with a documented minimum period between the previous cataract surgery and the vitreoretinal procedure of 1 year; uncomplicated small-gauge PPV for the following reasons: rhegmatogenous retinal detachment (RRD), epiretinal membrane (ERM), lamellar macular holes (LMH), vitreous hemorrhage (VH) secondary to a posterior vitreous detachment (PVD); a minimum follow-up of 12 months; a comprehensive description of the surgical procedure; clinical data and macular SD-OCT scans (macular cube, radial and raster scans with a line crossing the fovea center) available at 1, 3, 6, 9 and 12 months after surgery and fluoresceine angiograms in those eyes that developed a central macular thickening during the postoperative period.
Patients with a history of exudative AMD, diabetic retinopathy (RD), RVO, uveitis, and uncontrolled glaucoma were excluded. In cases of incomplete clinical data and of poor-quality OCT scans, with a too low resolution to allow a reliable qualitative assessment of the macular condition, patients were excluded as-well.
2.1. Surgical Procedure
All surgeries were performed by a single, experienced vitreoretinal surgeon (F.P.), under peribulbar block (4cc Ropivacaine + 1 cc Lidocaine) or general anesthesia. After a proper disinfection of the surgical field with 5% povidone-iodine (Oftasteril, Alfa Intes Industria Terapeutica Splendore S.r.l., Naples, Italy), a three-ports 23 or 25-gauge (23-G; 25-G) PPV was carried out using the Constellation® Vision System (Alcon Laboratories, Fort Worth, TX). Silicone oil was aspirated from the vitreous chamber, then replaced with balanced salt solution (BSS) or air, after multiple fluid-air exchanges to wash out any emulsion, in eyes scheduled for removal of silicone oil. The core vitrectomy and peripheral shaving were performed with 7500 cuts per min (cpm) cut-rate and linear aspiration of 0–650 mmHg in all the other cases. Triamcinolone acetonide was systematically used for vitreous staining to ensure a better residual vitreous removal as-well-as to confirm the posterior hyaloid detachment. Perfluorcarbon liquid (PFCL) was used to drain the subretinal fluid and stabilize the posterior retina only in RRD cases. In the presence of a PVR complicating a RRD, peeling of epiretinal and subretinal membranes after staining with Trypan Blu (TB) 0.15% + Brilliant Blue G (BBG) 0.05% + Lutein 2%solution (DOUBLE DYNE; Alfa Intes Industria Terapeutica Splendore S.r.l., Naples, Italy), was attempted with vitreoretinal microforceps. Relaxing retinotomies and retinectomies where usually reserved to cases of RRD with severe PVR. Fluid-air exchange was performed to flatten the retina immediately after all the vitreous tractions have been removed. Endolaser retinopexy was applied to all the retinal breaks and along the borders of the retinotomies and retinectomies. Twenty-four % sulfur hexafluoride (SF6), 14% octafluoropropane (C3F8), or silicone oil (1000 centistokes, 5000 centistokes or heavy silicone oil) were alternatively used as endotamponade, according to the complexity of the cases.
After core vitrectomy, peripheral shaving and PVD induction, ERM and internal limiting membrane (ILM) were removed with end-grip vitreoretinal forceps, after staining with Trypan Blu (TB) 0.15% + Brilliant Blue G (BBG) 0.05% + Lutein 2% solution, in patients with idiopathic ERM and LMHs. After ERM + ILM removal, peripheral retinal was carefully evaluated under scleral depression in all cases, to rule-out the presence of peripheral retinal breaks and micro-holes requiring endolaser retinopexy. A partial fluid-air exchange was then caried out. Sclerotomies were systematically closed with 8.0 Vicryl sutures, to reduce the risk of early postoperative hypotony secondary to leaking wounds.
2.2. Definition and Diagnosis of Post-Surgical Macular Edema
Post-vitrectomy CME was defined by an increase in macular thickness of ≥ 10% of the previous OCT value and/or by the presence of more than three circular or ovoid intraretinal hyporeflective spaces in the inner and/or outer layers in the central millimeter of the Early Treatment Diabetic Retinopathy Study (ETDRS) grid and by the identification of subretinal fluid accumulation at the foveal location [
4]. The diagnosis of post-surgical macular edema needed to be further supported by the evidence of late-phase macular and optic disc hyperfluorescence at postoperative fluoresceine angiography (Spectralis HRA + OCT; Heidelberg Engineering, Heidelberg, Germany).
2.3. Treatment of Post-Surgical Macular Edema
Medical therapy, consisting of a combination of topical steroids (preservative-free DEX eye drops 1 mg/ml, administered 4 times daily) plus topical NSAIDs (Bromfenac eye drops 0.9 mg/ml, twice daily), was used for at least one month, in all patients who developed a post-surgical macular edema.
DEX implant 0.7 mg, injected into the vitreous cavity starting from month 3, according to the standard protocol [
17], was reserved to all cases that did not respond to the medical treatment or where CME tended to relapse after topical treatment discontinuation.
2.4. Analysis of the Biomarkers
Qualitative OCT assessment, carried out by two independent expert observers (A.N. and E.G.,) on OCT scans taken one month after surgery, led to the recognition of the following structural biomarkers: intraretinal cysts (IRC), defined by the presence of circular or ovoid intraretinal hyporeflective spaces in the inner and/or outer layers; subretinal fluid (SRF), indicating a subfoveal hyporeflective “cuff” of fluid beneath the neuroepithelium; disorganization of inner retinal layers (DRIL), defined by the complete loss of boundaries between the ganglion cell, inner plexiform, and outer nuclear plexiform layers at the foveal location; disorganization of outer retinal layers (DROL), featured by the complete loss of the EZ + ELM hyperreflective bands at the subfoveal location; hyperreflective foci (HRF), identified as dot-like lesions, less than 30 μm in size, with the same reflectivity of retinal nerve fiber layer.
Main outcomes evaluated were the incidence of PCME in pseudophakic eyes after small-gauge PPV, the change of baseline mean best corrected visual acuity (BCVA) and central macular thickness (CMT) at 12 months in the subset of patients who developed a form of post-vitrectomy CME and were treated consequently, and the safety of intravitreal DEX.
Secondary outcomes were the correlations between the exposure to intravitreal DEX during the FU and specific demographic, clinical and OCT characteristics.
2.5. Statistical Analysis
The qualitative variables are presented as frequencies and percentages, while the quantitative data is presented as means ± standard deviations. No formal sample size calculation was performed. For assessing the change in BCVA and CMT over follow-up, the non-parametric test known as the Wilcoxon rank-sum test was used. For frequencies and percentages, the Fisher test was used. All statistical tests were performed at the p < 0.05 significance level. Univariate and multivariate regression models were performed to assess the relationship between DEX-i at 3,6, and 9 months and each independent variable. The independent variables included gender, age, retinal disease, CMT and OCT biomarkers. The independent variables that were significant at univariate regression analysis were considered in multiple regression model. Statistical analysis was made using STATA 12.1 Statistical Software (StataCorp), 2014, release 12 (College Sta-tion, TX, USA).
3. Results
Medical records of 346 consecutive pseudophakic patients (352 eyes), who underwent small-gauge PPV for RRD (235 eyes), silicone oil removal (43 eyes), ERM (62 eyes) or non-diabetic VH (12 eyes), between January 2021 and July 2023, were identified. Gradable OCT scans, taken between the first and the second month after surgery, were available for all of them. Post-PPV CME, defined as a central foveal thickening with the evidence of intraretinal and/or subretinal fluid, in the absence of any epiretinal component, and further confirmed by the late-phase macular and optic disc hyperfluorescence at fluoresceine angiography, developed in 54 patients (incidence: 15,3 %) within the first two months after surgery.
Of these 54 patients (54 eyes), 6 were excluded for the following reasons: the follow up was shorter than 6 months in 4 eyes and some relevant clinical data were missing in the remaining 2 eyes.
Ultimately, 48 eyes (21 right/27 left) of 48 consecutive patients (15 women and 33 men; mean age 64,1 year ± 4,3, range 57-73) were deemed eligible for the final analysis. Indication for PPV in this population were RRD repair in 36 eyes (75%), 1000 centistokes silicon oil removal in 10 eyes (20.8%) and ERM peeling in 2 eyes (4.1%). Considering only the subgroup of patients who underwent vitreoretinal surgery for RRD repair, the macula was detached in 31 eyes (86,1%), proliferative vitreoretinopathy (PVR) was evident in 14 eyes (38.8%) and a mild VH, that did not impair a proper OCT evaluation of the macula during the preoperative period, was present in the remaining 2 eyes (5.5%).
The mean time (± SD; range) between the previous cataract surgery and the vitreoretinal procedure was 3,8 years (± 2,3; range 1–11) in the study population. A comprehensive clinical assessment, together with reliable OCT scans, were available at 1, 3, 6, 9 and 12 months for all of them.
Additional relevant Demographic and baseline characteristics are listed in
Table 1.
Preoperative mean BCVA (1.44 ± 0.99 LogMAR) significantly improved at 1 (0,56 ± 0,40 LogMAR), 3 (0.44 ± 0.48 LogMAR), 6 (0.37 ± 0.35 LogMAR), 9 (0.38 ± 0.39 LogMAR) and 12 months (0.32 ± 0.37 LogMAR) in the overall population (P< 0.001 for all the time points).
Table 2.
Mean (± SD) CMT was 347 (± 123.5) μm at one month after PPV and it significantly decreased to 290 μm (± 80.4) at last visit. Mean CMT changes at all time points are reported in
Table 3.
Qualitative analysis of the OCT at month one disclosed the presence of intraretinal fluid in 34 eyes and of subretinal fluid in 14 eyes. Additionally, the disorganization of inner and outer retinal layers (ellipsoid zone + external limiting membrane) at the foveal location and the presence of HRF were evident in 11 (23 %), 10 (20.8%) and 9 (18.7%) eyes respectively. The complete analysis of the OCT biomarkers at month one is reported in
Table 4.
3.1. Treatment Exposure
All patients were treated with topical therapy (DEX eye drops 4 times daily + FANS eye drops 3 times/daily for one month) as first-line treatment. Intravitreal DEX could be administered starting from month 3, only in cases of non-resolving (CMT reduction < 10% of the baseline value with persistence of intraretinal or subretinal fluid), worsened (CMT increase > 10% of the baseline value with the presence of intraretinal or subretinal fluid) or relapsing (CMT increase > 10% when compared to the OCT of the previous evaluation and/or evidence of new subretinal fluid and intraretinal cysts) macular edema.
CME completely regressed after one month of topical medications in 23 eyes (47.9%), without any evidence of recurrence along the entire course of the follow up. The remaining 25 eyes (52%) required one or more intravitreal DEX implant at some point during the follow up, for persistence or recurrence of macular edema.
Intravitreal DEX was administered at 3,6,9 and 12 months in 16 (33.3%), 5 (10.4%), 16 (33.3%) and 4 (8.3%) eyes respectively. Each patient treated with intravitreal DEX received a mean number of 1.64 (± 0.56; range: 1-3) injections between month 3 and 12. More specifically, 10 patients (40%) received a single dexamethasone intravitreal injection, 14 patients (56%) received 2 injections, while one patient (4%) was treated with 3 injections.
Table 5
The mean interval between two consecutive injections was 5.2 (± 1.37; range: 3- 6) months in those patients who required more than one administration.
3.2. Analysis of the Correlations
The correlation analysis, between the mean CMT values taken at different time points, revealed the existence of a statistically significant correlation between mean CMT at month 1 and month 3 (P < 0.001). Additionally, both mean CMT at month 3 and 6 significantly correlated with mean CMT at months 9 and 12 (P < 0.001), while mean CMT at month 9 significantly correlated with mean CMT at last visit (month 12; P < 0.001)
Table 6.
A univariate analysis using a logistic regression model was performed to look at potential correlations between the need for intravitreal DEX at month 3 and specific demographic, clinical characteristics, and predefined structural OCT biomarkers. The indication to start a treatment with intravitreal DEX at month 3 significantly correlated with the mean CMT (p = 0.005), the presence of intraretinal cysts (P = 0.04), DRIL (P = 0.004) and HRF (P = 0.02) at 1 month OCT scans.
When the same univariate logistic regression model was tested with treatment (intravitreal DEX) at month 6, no statistically significant correlations could be found. None of the tested variables appeared to be significantly correlated with the exposure to intravitreal dexamethasone at month 3, in the multivariate logistic regression model.
When the probability of receiving an intravitreal DEX treatment at month 9 was tested for different clinical and OCT variables, a statistically significant correlation with HRF and DROL emerged both in the univariate (HRF: OR = 5.80, P = 0.02; DROL: OR = 7.51, P = 0.01) and multivariate (HRF: OR = 5.53, P = 0.04; DROL: OR = 7.11, P = 0.03) logistic regression models. The results of univariate and multivariate analysis are reported in
Table 7.
3.3. Safety
No severe ocular and non-ocular adverse events were reported for patients who underwent one or more intravitreal DEX administrations. Baseline mean (± SD) intraocular pressure (15.6±3.9 mmHg) at month one did not change significantly during the entire course of the follow-up (
Table 8). Five patients (20%) presented with a mild IOP elevation (> 25 mmHg) at month 6 (3 patients) and 12 (2 patients) that was successfully managed with topical IOP lowering medications. No patients developed glaucoma or required filtering surgery for a chronic, refractory IOP elevation.
4. Discussion
Limited information is available about the incidence of CME after vitrectomy or phacovitrectomy. Leisser et al. analyzed data from 6 studies and showed that the incidence of new intraretinal fluid, detected 3 months after surgery, was 6% and 3% after phacovitrectomy and vitrectomy alone respectively [
18,
19].
The incidence of post-PPV CME in pseudophakic patients was 15.3% in our study. This data seems to be in line with those reported in the Literature. The rate of post-PPV CME may vary significantly according to the underling pathology that led to the surgery or to the surgical procedure itself (vitrectomy vs phacovitrectomy). The prevalence of CME was 17.1% in pseudophakic eyes scheduled for PPV for RRD [
4]. A macular thickness increase has been reported in 3.8% to 15.4% of patients undergoing PPV + peeling and phacovitrectomy + peeling for ERM respectively [
19], while no significant differences were observed, in terms of post-PPV CME occurrence rate, between only vitrectomy group and phacovitrectomy group in patients with idiopathic ERM, in similar reports [
20,
21].
Cataract surgery is a well-known risk factor for Irvine-Gass syndrome. Surgical manipulation in the anterior segment may promote an upregulation of inflammatory mediators, which leads to a breakdown of the blood-retinal barrier and increased vascular permeability. Beside the inflammatory response to the surgery, an anterior uveal remodeling, consequent to the crystalline lens removal and its replacement with a thinner and lighter IOL, was hypnotized as a concurrent factor for postsurgical CME in some eyes [
19].
Taken together, these data suggest cataract surgery may act as a relevant stand-alone risk factor for postsurgical CME, therefore a potential source of bias when assessing the true incidence of post-PPV CME [
20].
All patients included in the present study were psudophakic at the time of PPV, with a minimum time between the previous cataract surgery and the vitreoretinal procedure, as short as one year. The exclusion of phakic eyes scheduled for a phacovitrectomy procedure, may have reduced the chance of overrating the true incidence of post-surgical CME.
The exact pathogenetic mechanism responsible for CME occurring after PPV is still poorly understood. Many studies have tried to identify possible risk factors for its occurrence. Age more than 50 years, multiple surgeries, severe PVR, aphakia, endotamponade used and extensive endolaser or cryopexy have been all proposed as risk factors for post-PPV CME in eyes undergoing PPV for RRD repair [
22,
23,
24].
Intraretinal microcystoid changes have been observed after PPV combined with epiretinal membrane and ILM peeling in patients with idiopathic ERMs, especially in those cases whit pre-existing intraretinal cysts[
20], stage 4 ERMs and candidate for a phacovitrectomy procedure [
3].
It has been proposed that the degree of retinal architectural disruption, found in end-stage ERMs, may make the retinal tissue more prone to the mechanical stress induced by ILM peeling maneuvers. This may cause a Muller cell disfunction, secondary to a retrograde trans-synaptic degeneration of inner retinal layers and to an inflammatory process, with a consequent impairment in fluid resorption at the macula. According to this theory, CME observed after ILM peeling for ERMs may derive more from glial degeneration rather than a true blood-retinal barrier disfunction [
3,
25,
26].
The reported incidence of CME after silicone oil endotmaponede ranges between 13.6% to 36%, depending on several factors such as SO viscosity, the grade of PVR complicating the retinal detachment and the interval between PPV and SO removal. Both inflammatory and mechanical components have been postulated as predisposing factors for CME formation in SO filled eyes. SO can promote an inflammatory reaction by impairing the potassium ion buffering function of Muller cells. The adhesive interaction between the SO bubble and the retinal surface may translate into tractional forces to the macula which may promote intraretinal fluid accumulation. CME spontaneous resolution observed after SO removal in most eyes, indicates this process may be reversed with the removal of the endotamponede. There is still a small number of patients though, who do not experience an improvement of their macular condition after SO removal, and who can even progress toward a further macular thickening. This indicates that the pro-inflammatory stimulus may persist for some time, even after the elimination of the SO [
22].
Taken all together, these data suggest, regardless the initial retinal condition treated with PPV, many risk factors can contribute to increase intraocular inflammation [
4].
Patients who underwent PPV for RRD repair (36) represented most of our study population (75%), followed by patients scheduled for silicone oil removal (10 eyes, 20.8%) and idiopathic ERM peeling (2 eyes, 4.1%). Considering the relatively low number of patients included, especially in the ERM peeling group, and the heterogeneity of the retinal pathologies, we did not look at potential risk factors for post-PPV CME. Additionally, the present study was designed to assess the role of different treatment modalities for post-PPV CME, rather than to identify risk factors for this condition.
A low-grade inflammatory response can be considered the leading event that promotes retinal fluid accumulation in eyes that develop a form of post-vitrectomy CME. Inflammatory mediators released during surgery may easily spread in the vitreous chamber, causing a blood-retinal barrier breakdown and extravasation of fluid from retinal vessels, as confirmed by the late leakage and staining of the optic nerve head and the macula, observed at the fluoresceine angiography in these patients [
2,
3]. Fluoresceine angiography is routinely performed in patients who develop any sort of acute macular change, presumably related to inflammation or retinal vascular impairment, after a surgical procedure, at our institution. All eyes included in the study displayed a peculiar pattern at FA, namely a late phase hyperfluorescence at the macula and optic disc, indicating an inflammatory mechanism responsible for CME. Retinal glial disfunction, induced by the surgical trauma and again by the inflammation, may concurrently promote the collection of fluid in the macula of these patients [
3,
25,
27].
The paramount role of inflammation can be inferred by the satisfactory response to a given anti-inflammatory therapy in these patients [
22].
Medical treatment, based on the administration of topical steroids and NSAIDs eyedrops, is generally considered a safe and effective option in patients with PCME. The good ocular penetration, combined with a low rate of adverse events, make topical therapy a fairly good first line option in these cases [
5]. Patients who fail to respond to medical treatment, and cases where CME tends to relapse as soon as the topical medication is discontinued, may benefit from more invasive treatments like intravitreal steroids [
28,
29] or anti-VEGF [
30].
Intravitreal DEX implant is a biodegradable corticosteroid implant, which provides sustained release of 700 μg DEX into the vitreous for up to 6 months [
9]. Results from different clinical studies showed intravitreal DEX implant can promote a significative long-term CMT reduction and a visual acuity improvement in vitrectomized eyes that develop PCME after RRD surgery and ERM peeling, both in naïve and refractory to medical therapy eyes [
8,
9,
11,
31].
Twenty-five eyes (52%) did not show a satisfactory response to the medical therapy in our study, so they were candidate for intravitreal DEX implant starting from month 3. BCVA improvement, together with a significative CMT reduction, indicated intravitreal therapy is effective in promoting macular fluid resolution and visual recovery in this population.
While CME resolution was achieved after a single injection in 10 patients (40%), the remaining 15 patients required two or more injections by the end of the 12-months FU, with a mean number of 1.64 treatment per patient and a mean interval between two consecutive injections of 5.2 months. The maximum mean CMT reduction was reported at month 6, 3 months after the injection, with a recurrence of macular edema at month 9 in 10 patients (62.5 % of the population that received the first injection at month 3).
This observation seems in line with similar reports in the Literature [
10,
32]. The maximum effect of intravitreal DEX is usually observed one month from the administration, with a progressive loss of the effect over the next period. This can explain why approximatively 44% of patients need further dexamethasone injections after a mean period of 6 months [
9,
32].
The role of OCT structural biomarkers in several macular pathologies has grown in popularity over the past few years. High resolution OCT scans enable the identification of even subtle anatomical changes in different retinal layers at the macula. These structural elements may correlate with the visual function and predict the response to a treatment. The presence of subretinal fluid and of HRF were shown to be predictive of a better anatomical and functional response to intravitreal DEX in DME [
33,
34,
35]. The detection of MAs in the nasal macula may indicate resistance to anti-VEGF therapy in patients with DME [
36].
Very little evidence on the role of clinical and structural biomarkers as indicators of response to intravitreal DEX, in patients with post-PPV CME, can be found in the Literature. Fressinger et al. investigated the efficacy of intravitreal DEX in eyes that developed CME after PPV for ERM or macula-on RD and tried to identify functional and morphological OCT predictors of response. Their results indicated the presence of SRF, the integrity of EZ and a worse baseline BCVA were all predictive of a better functional outcome after intravitreal DEX [
10].
The existence of potential correlations between mean CMT values, measured at different time points, and between the intravitreal DEX treatment exposure and specific demographic, clinical characteristics, and predefined structural OCT biomarkers, were investigated in our study.
Mean CMT at month 1 significantly correlated with mean CMT at month 3, while CMT values at month 3 and 6, both correlated with mean CMT at month 9 and 12. These correlations suggest most of the patients who displayed a more pronounced central macular thickening at first month, still have a clinically significant macular edema at month 3, despite the medical therapy. Additionally, the correlations between the mean CMT at month 3 and 6 with that measured at month 9 and 12, may suggest CME tend to relapse approximatively 6 months after the dexamethasone injection.
The univariate logistic regression model revealed that the indication for treatment with intravitreal DEX at month 3, significantly correlated with the mean CMT at month 1 and with the existence of intraretinal fluid, DRIL and HRF at 1 month OCT. The need for a second DEX intravitreal injection at month 9 was significantly correlated with the presence of HRF and DROL at the OCT both in the univariate and multivariate linear regression models. Presence of HRF and DROL at the OCT, indicates an 8 and 6-folds increase respectively of the chance to be treated with an additional intravitreal DEX injection, 9 months after surgery, for CME recurrence.
A greater CMT at month 1 is suggestive of a more severe macular edema, that may not resolve completely after medical therapy. Patients who presented with a thicker macula 1 month after vitrectomy, will eventually continue to display persistent fluid at month 3, thus requiring intravitreal treatment.
HRF, considered aggregated of activated microglia cells, located in both inner and outer retina in patients with macular edema secondary to different retinal etiologies [
33], are known to be associated with increased inflammation in the retina [
35,
37,
38].
Conflicting evidence, on the role of HRF as biomarkers of response to intravitreal steroids for diabetic macular edema, still exists to date.
Some authors demonstrated that the presence of HRF is associated with an excellent response to intravitreal steroids [
35], while other reports showed baseline numbers of HRF on SD-OCT may be a predictive indicator of early recurrence of macular edema after intravitreal DEX implantation for DME [
39].
Accepting the letter evidence, a higher baseline number of HRF may indicate a more severe retinal inflammation with a more pronounced macular edema, maybe resistant to topical therapy. This hypothesis could explain why patients, who presented with a higher number of HRF at baseline, did require intravitreal therapy more frequently, in our study.
It is worth to note that our assumption is purely speculative, since no precise evidence of the impact of HRF on the response intravitreal steroid treatment, in patients with post vitrectomy macular edema, can be found into the Literature to date.
The role that IRF, DRILS and DROL may play in defining the response to intravitreal steroids in these patients is less clear, with no conclusive evidence that can be derived from the Literature.
We hypnotized that the presence of intraretinal cysts, DRIL and DROL at baseline may be suggestive of a more severe macular edema, with a higher degree of intraretinal degeneration. In a recent report the odds of having DRIL was greater in DME eyes with an increased retinal thickness at the fovea [
40]. The presence of large cystoid spaces in the inner retinal layers may also contribute to generate distortion into the retina, thus opening the way to DRIL. Conversely, the absence of DRIL was significantly correlated with the presence of SRF, which is known to be more commonly seen in acute DME [
41]. This might indicate DRIL as a sign of chronicity of macular edema.
Taken together, these observations may support the hypothesis that eyes with post-vitrectomy CME, associated with the presence of IRF, DRIL and DROL at the OCT, may be affected by a more severe condition that requires a more aggressive treatment, rather than a topical therapy alone.
When a multivariate logistic regression model was carried out, none of the tested variables correlated with the exposure to the intravitreal treatment at month 3. The disagreement between the two models could be explained, at least in part, by the relative reduced number of cases included in our study.
Repeated treatments with intravitreal DEX over a prolonged period, may generate some safety concerns, especially when we come to the risk of uncontrolled IOP elevation. IOP rise >25 mm Hg has been reported in approximatively 26% of patients treated with DEX for different retinal conditions. Overall, more than 90% of eyes with IOP elevation can be successfully managed with medical therapy, with only 0.5% eyes required filtering surgery [
42].
An IOP increase > 25 mmHg was evident in 20% of patients in our study and it was managed medically in all cases. No cases of severe ocular adverse events like endophthalmitis, retinal detachment or vitreous hemorrhage were reported.
The relatively small sample size, the retrospective nature, the lack of a control group and the heterogeneity of the retinal pathologies that required surgery, represent the major limitations of the present study. Additionally, correlations between clinical, OCT biomarkers and visual function were not investigated. This is mainly due to the fact we feel that, at least some degree of inner and outer retinal disruption (DRIL and DROL) observed in some patients, may be related to the preexisting retinal pathology, rather than to the post-vitrectomy CME.
To the best of our knowledge, this is the first study where the role of specific OCT biomarkers, such as DRIL, IRF, DROL and HRF, as predictors of exposure to DEX intravitreal treatment in pseudophakic eyes that developed CME after a vitreoretinal procedure, was investigated.
The strict inclusion criteria (only pseudophakic patients could be enrolled), the long follow-up, the availability of comprehensive clinical and OCT data for all the enrolled patients, and the use of fluoresceine angiography to confirm the diagnosis of post-surgical CME, further increase the reliability of our results.
In conclusion, approximatively 50% of pseudophakic patients, who develop a CME after vitreoretinal surgery, may benefit from topical therapy alone. Intravitreal DEX implant seems to warrant a sustained CMT reduction and a VA recovery in patients who show an incomplete response to topical medications after 2 months from the diagnosis. The identification of specific biomarkers at the OCT performed one month after surgery, may indicate a more severe CME, and could help the clinician to stratify those patients who will probably require an intravitreal approach in the next future.
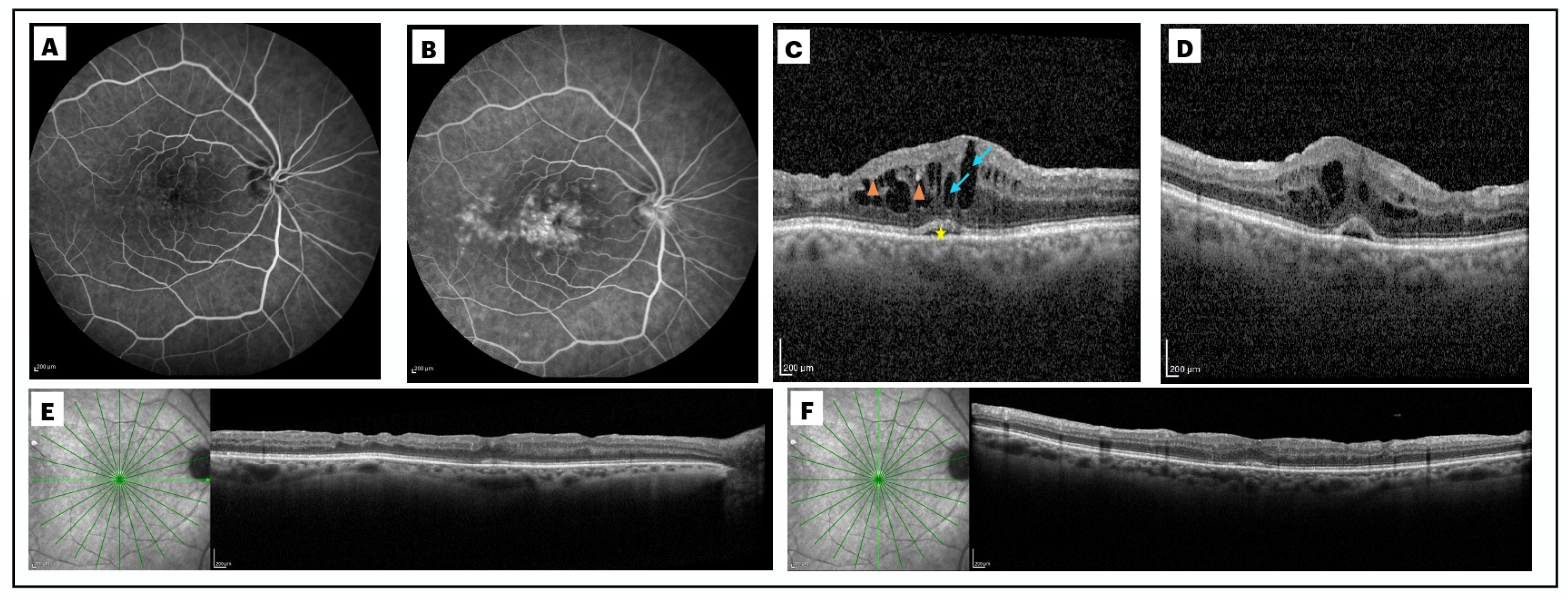
Figure 1.
Post-vitrectomy macular edema after surgery for rhegmatogenous retinal detachment (RRD) in the right eye of a 65-year-old lady. Early (A) and late (B) phase fluoresceine angiography, performed one month after surgery, show the classical perifoveal petaloid staining pattern and late leakage of the optic disc. A significant central macular thickening (CMT; 633 μm), associated to the presence of intraretinal cysts (arrows) and subretinal fluid (star), can be observed at 1-month spectral domain optical coherence tomography (SD OCT). Best corrected visual acuity (BCVA) was 20/200 in this eye (C-D). SD-OCT scans taken at month 3 (E-F), reveal a complete intraretinal and subretinal fluid resolution, with a CMT of 205 μm, and a BCVA of 20/30, after topical therapy.
Figure 1.
Post-vitrectomy macular edema after surgery for rhegmatogenous retinal detachment (RRD) in the right eye of a 65-year-old lady. Early (A) and late (B) phase fluoresceine angiography, performed one month after surgery, show the classical perifoveal petaloid staining pattern and late leakage of the optic disc. A significant central macular thickening (CMT; 633 μm), associated to the presence of intraretinal cysts (arrows) and subretinal fluid (star), can be observed at 1-month spectral domain optical coherence tomography (SD OCT). Best corrected visual acuity (BCVA) was 20/200 in this eye (C-D). SD-OCT scans taken at month 3 (E-F), reveal a complete intraretinal and subretinal fluid resolution, with a CMT of 205 μm, and a BCVA of 20/30, after topical therapy.
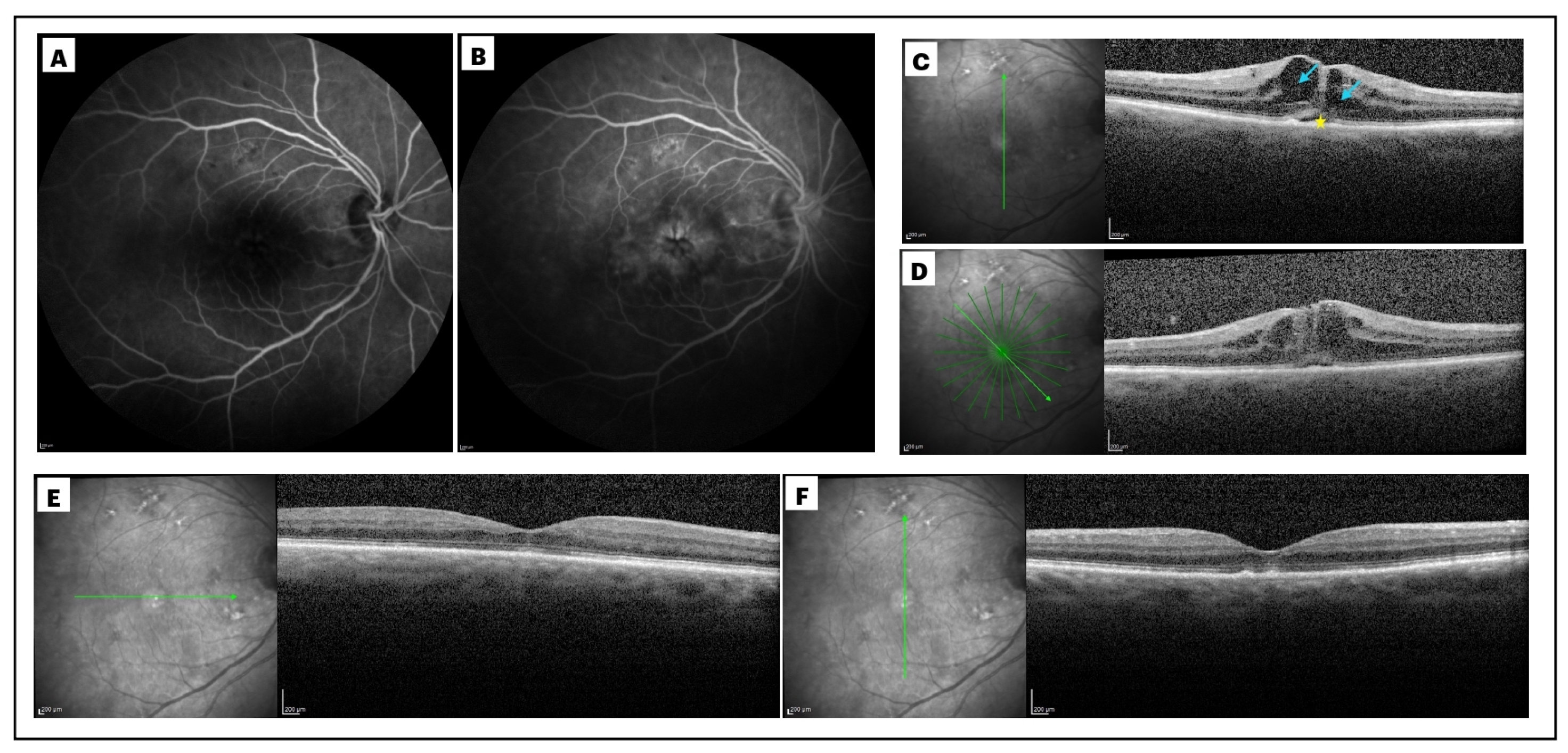
Figure 2.
Right eye of a 73-year-old male patient who developed macular edema one month after a pars plana vitrectomy with epiretinal membrane (ERM) peeling. The extensive late-phase macular leakage, with a petaloid patter, and the hyperfluorescence of the optic disc, were evident at the late frames of the fluoresceine angiography performed 1 month after surgery (B), when compared to the early phase of the exam (A). Spectral domain optical coherence tomography (SD OCT) at month 1 (C), revealed a post-vitrectomy macular edema with a central macular thickness (CMT) of 576 μm and presence of large intraretinal cysts (arrow), sub retinal fluid at the fovea (star) and hypereflective foci (HRF, arrowheads). The condition showed a very negligible improvement at month 3 (CMT: 556 μm), after 2 months of topical therapy, with the persistence of sub retinal fluid and only a slight reduction of intraretinal cysts (D). Best corrected visual acuity (BCVA) was 20/400 at this point. This patient was treated with a dexamethasone (DEX) intravitreal implant at month 3. SD-OCT performed at month 6, 3 months after intravitreal DEX administration, shows a significant CMT reduction (289 μm), with a complete resolution of intraretinal and sub retinal fluid (final BCVA of 20/50).
Figure 2.
Right eye of a 73-year-old male patient who developed macular edema one month after a pars plana vitrectomy with epiretinal membrane (ERM) peeling. The extensive late-phase macular leakage, with a petaloid patter, and the hyperfluorescence of the optic disc, were evident at the late frames of the fluoresceine angiography performed 1 month after surgery (B), when compared to the early phase of the exam (A). Spectral domain optical coherence tomography (SD OCT) at month 1 (C), revealed a post-vitrectomy macular edema with a central macular thickness (CMT) of 576 μm and presence of large intraretinal cysts (arrow), sub retinal fluid at the fovea (star) and hypereflective foci (HRF, arrowheads). The condition showed a very negligible improvement at month 3 (CMT: 556 μm), after 2 months of topical therapy, with the persistence of sub retinal fluid and only a slight reduction of intraretinal cysts (D). Best corrected visual acuity (BCVA) was 20/400 at this point. This patient was treated with a dexamethasone (DEX) intravitreal implant at month 3. SD-OCT performed at month 6, 3 months after intravitreal DEX administration, shows a significant CMT reduction (289 μm), with a complete resolution of intraretinal and sub retinal fluid (final BCVA of 20/50).