Submitted:
24 September 2024
Posted:
26 September 2024
You are already at the latest version
Abstract
Keywords:
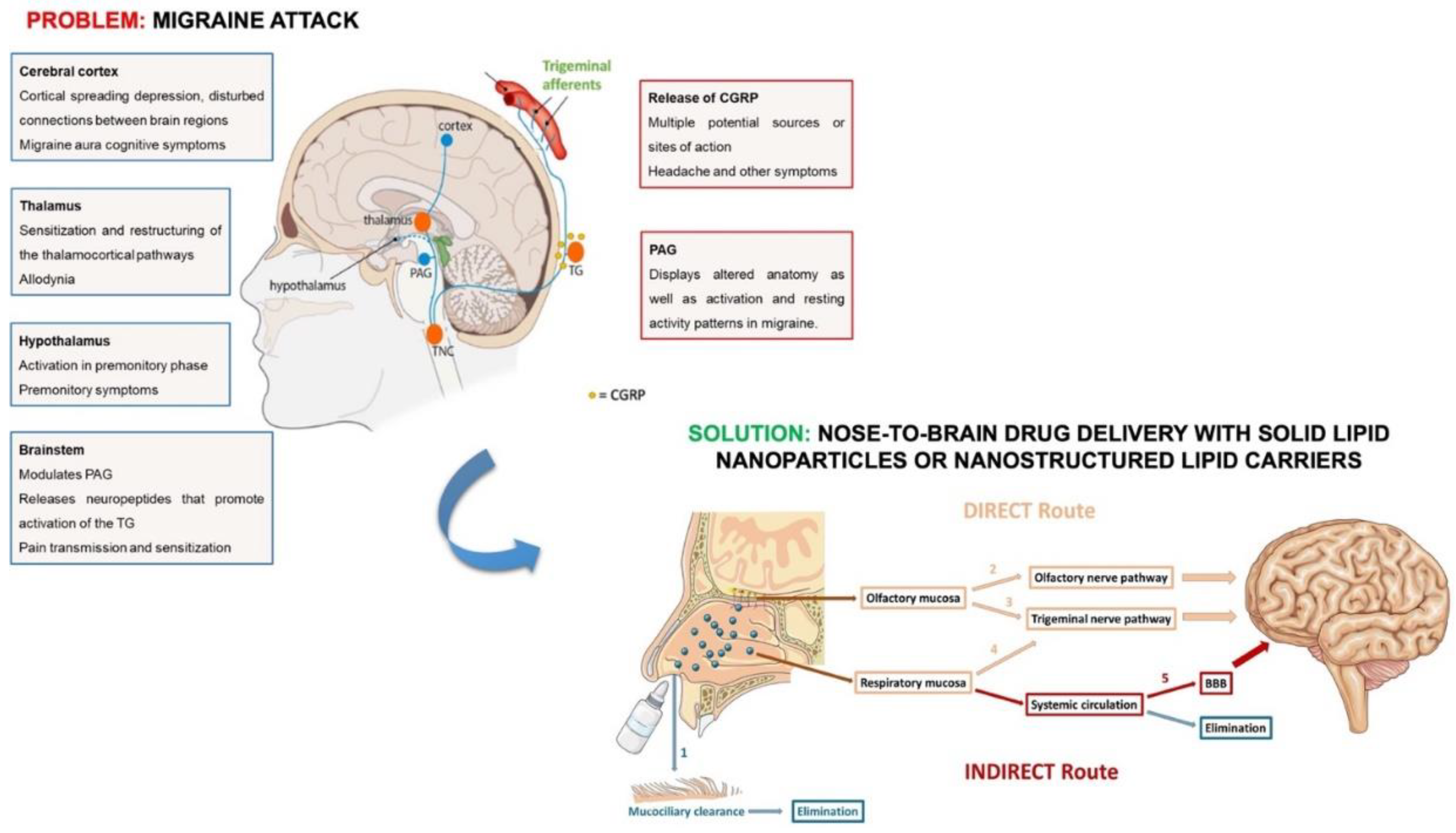
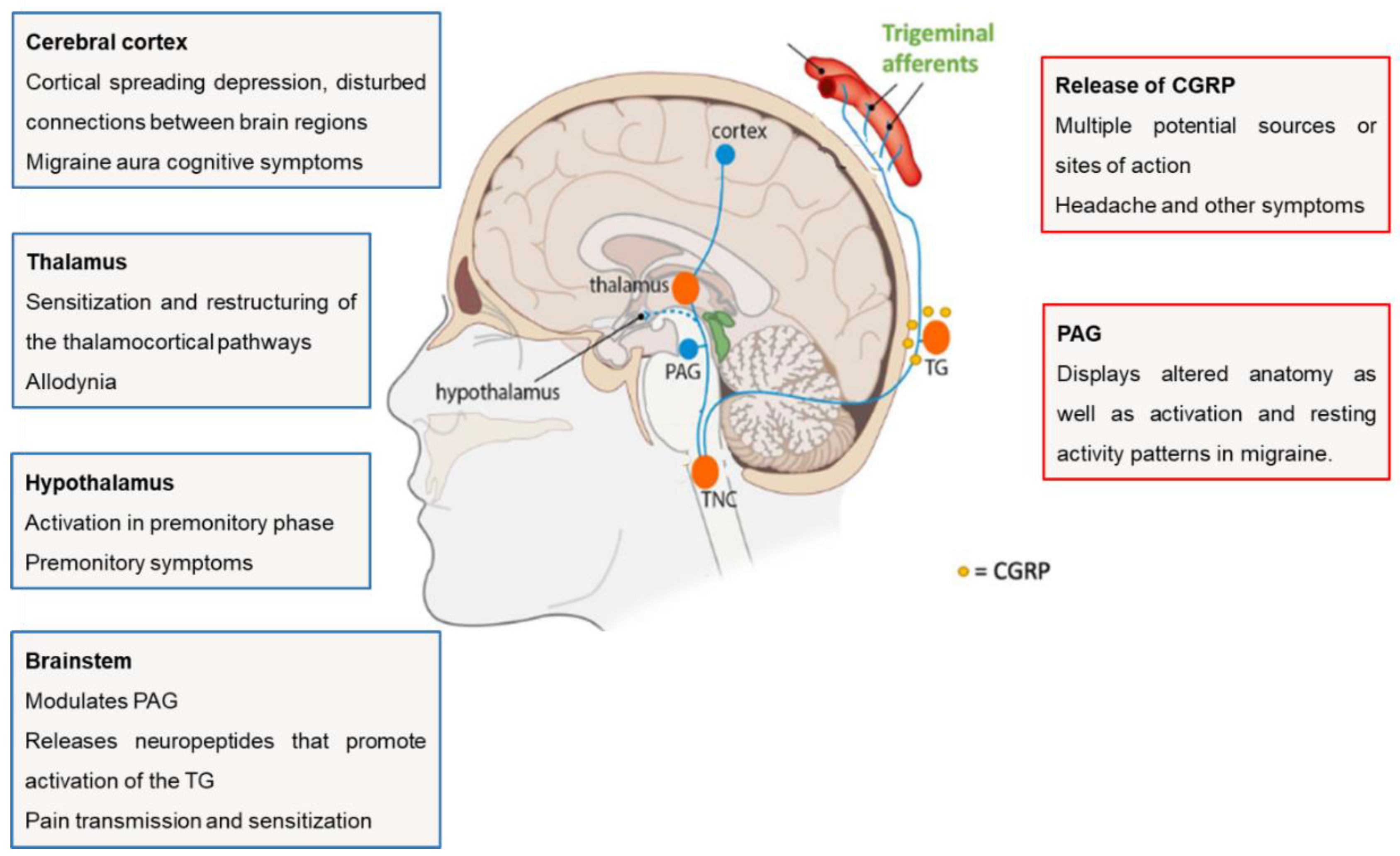
1. Introduction
2. Acute Migraine
2.1. Functional Anatomy and Pathophysiology
2.2. Pharmacological Treatment of Migraine
2.2.1. Acute Treatment
2.2.2. Preventive Treatment
2.2.3. Non-Invasive Strategies to Overcome Treatment Limitations
2.3. Nasal Products Approved for the Treatment of Acute Migraine
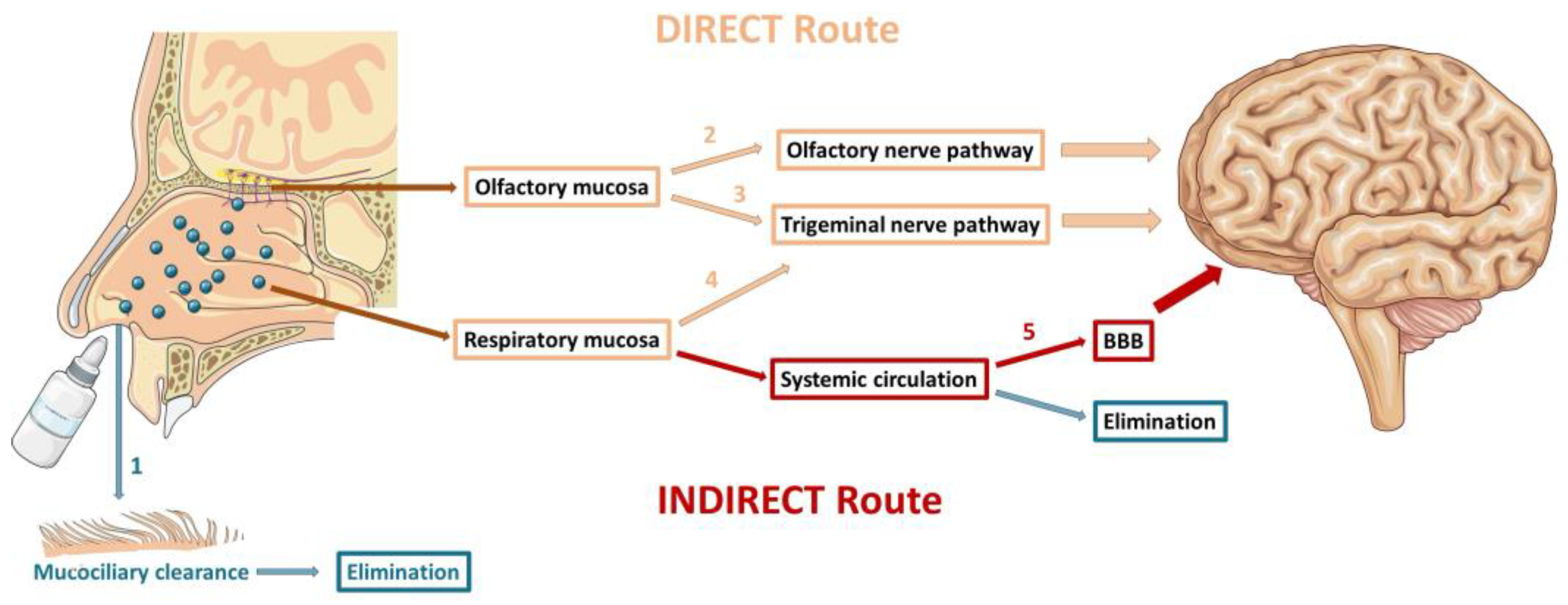
3. Nose-to-Brain Route – An Overview
3.1. Mechanisms of Drug Delivery to the Brain
3.1.1. Olfactory Nerve Pathway
3.1.2. Trigeminal Nerve Pathway
3.1.3. Indirect Transport
3.2. Challenges of Nose-to-Brain Drug Delivery
4. Main Features of SLN and NLC
4.1. Co-Encapsulation of Drugs in SLN and NLC
4.2. Specificities of SLN and NLC for Nose-to-Brain Drug Delivery
4.3. Recent in Vivo Studies with SLN and NLC to Improve the Treatment of Acute Migraine via the Nose-to-Brain Route
5. Conclusions
Funding
References
- Feigin, V.L., et al., The global burden of neurological disorders: translating evidence into policy. Lancet Neurol, 2020. 19(3): p. 255-265. [CrossRef]
- Puledda, F., et al., Migraine: from pathophysiology to treatment. J Neurol, 2023. 270(7): p. 3654-3666. [CrossRef]
- de Boer, I., et al., Place of next generation acute migraine specific treatments among triptans, non-responders and contraindications to triptans and possible combination therapies. Cephalalgia, 2023. 43(2): p. 3331024221143773.
- Mungoven, T.J., L.A. Henderson, and N. Meylakh, Chronic Migraine Pathophysiology and Treatment: A Review of Current Perspectives. Front Pain Res (Lausanne), 2021. 2: p. 705276. [CrossRef]
- Eigenbrodt, A.K., et al., Diagnosis and management of migraine in ten steps. Nat Rev Neurol, 2021. 17(8): p. 501-514. [CrossRef]
- Cooper, W., et al., Delivery of Dihydroergotamine Mesylate to the Upper Nasal Space for the Acute Treatment of Migraine: Technology in Action. J Aerosol Med Pulm Drug Deliv, 2022. 35(6): p. 321-332. [CrossRef]
- Martin, V., et al., Nasal Delivery of Acute Medications for Migraine: The Upper Versus Lower Nasal Space. J Clin Med, 2021. 10(11). [CrossRef]
- John Hoekman, S.R., Sheena K Aurora, and Stephen B Shrewsbury, The Upper Nasal Space—A Novel Delivery Route Ideal for Central Nervous System Drugs. Impel NeuroPharma, Inc., Seattle, WA, USA, 2020. [CrossRef]
- Vidhi Tanna, S.P.S.a.P.R., Exploring Nose to Brain Nano Delivery for Effective Management of Migraine. 2022.
- Kataria, I. and P. Shende, Nose-to-brain lipid nanocarriers: An active transportation across BBB in migraine management. Chem Phys Lipids, 2022. 243: p. 105177. [CrossRef]
- Erdő, F., et al., Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res Bull, 2018. 143: p. 155-170. [CrossRef]
- Assadpour, S., et al., Harnessing Intranasal Delivery Systems of Sumatriptan for the Treatment of Migraine. Biomed Res Int, 2022. 2022: p. 3692065. [CrossRef]
- Khan, A.R., et al., Progress in brain targeting drug delivery system by nasal route. J Control Release, 2017. 268: p. 364-389. [CrossRef]
- Formica, M.L., et al., On a highway to the brain: A review on nose-to-brain drug delivery using nanoparticles. Applied Materials Today, 2022. 29. [CrossRef]
- Battaglia, L., et al., Lipid nanoparticles for intranasal administration: application to nose-to-brain delivery. Expert Opin Drug Deliv, 2018. 15(4): p. 369-378. [CrossRef]
- Nguyen, T.-T.-L. and H.-J. Maeng, Pharmacokinetics and Pharmacodynamics of Intranasal Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Nose-to-Brain Delivery. Pharmaceutics, 2022. 14(3): p. 572. [CrossRef]
- Pescador Ruschel, M.A. and O. De Jesus, Migraine Headache, in StatPearls. 2023, StatPearls Publishing; Copyright © 2023, StatPearls Publishing LLC.: Treasure Island (FL).
- Belopasova, A.V., et al., Achievements of Recent Decades in the Diagnosis and Study of Migraine Pathogenesis. Human Physiology, 2021. 46(8): p. 870-879. [CrossRef]
- Fan, L., et al., Global, regional, and national time trends in incidence for migraine, from 1990 to 2019: an age-period-cohort analysis for the GBD 2019. J Headache Pain, 2023. 24(1): p. 79. [CrossRef]
- Allais, G., et al., Gender-related differences in migraine. Neurol Sci, 2020. 41(Suppl 2): p. 429-436. [CrossRef]
- Ong, J.J.Y. and M. De Felice, Migraine Treatment: Current Acute Medications and Their Potential Mechanisms of Action. Neurotherapeutics, 2018. 15(2): p. 274-290.
- Dodick, D.W., A Phase-by-Phase Review of Migraine Pathophysiology. Headache, 2018. 58 Suppl 1: p. 4-16.
- Muehlberger, T., What Is Migraine?, in Migraine Surgery. 2018. p. 7-30.
- Gawde, P., et al., Revisiting Migraine: The Evolving Pathophysiology and the Expanding Management Armamentarium. Cureus, 2023. 15(2): p. e34553. [CrossRef]
- M. A. Pescador Ruschel, O.D.J., Migraine Headache. 2023.
- Haanes, K.A. and L. Edvinsson, Pathophysiological Mechanisms in Migraine and the Identification of New Therapeutic Targets. CNS Drugs, 2019. 33(6): p. 525-537. [CrossRef]
- Cohen, C.F., et al., Targeting Nociceptive Neurons and Transient Receptor Potential Channels for the Treatment of Migraine. Int J Mol Sci, 2023. 24(9). [CrossRef]
- van Welie, F.C., et al., Sex-specific metabolic profiling to explain the increased CVD risk in women with migraine: a narrative review. J Headache Pain, 2023. 24(1): p. 64. [CrossRef]
- Yam, M.F., et al., General Pathways of Pain Sensation and the Major Neurotransmitters Involved in Pain Regulation. Int J Mol Sci, 2018. 19(8). [CrossRef]
- Parada, C.A., et al., Primary afferent nociceptor as a target for the relief of pain. Pain Res Treat, 2012. 2012: p. 348043. [CrossRef]
- Guo, S., et al., Role of PACAP in migraine: An alternative to CGRP? Neurobiol Dis, 2023. 176: p. 105946.
- Vilas, D., et al., Periaqueductal gray matter echogenicity as a marker of migraine chronification: a case control study. J Headache Pain, 2023. 24(1): p. 41. [CrossRef]
- Chakravarty, A., How triggers trigger acute migraine attacks: a hypothesis. Med Hypotheses, 2010. 74(4): p. 750-3. [CrossRef]
- Todd, A.J., An Historical Perspective: The Second Order Neuron in the Pain Pathway. Front Pain Res (Lausanne), 2022. 3: p. 845211. [CrossRef]
- GOADSBY, Y.E.K.a.P.J., THE PERIAQUEDUCTAL GREY MATTER MODULATES TRIGEMINOVASCULAR INPUT: A ROLE IN MIGRAINE? 2021.
- Hamel, E., Serotonin and Migraine: Biology and Clinical Implications. 2007. [CrossRef]
- Burstein, R., R. Noseda, and D. Borsook, Migraine: multiple processes, complex pathophysiology. J Neurosci, 2015. 35(17): p. 6619-29. [CrossRef]
- Sudershan, A., et al., The complexities of migraine: A debate among migraine researchers: A review. Clin Neurol Neurosurg, 2022. 214: p. 107136. [CrossRef]
- Nicolas, S. and D. Nicolas, Triptans, in StatPearls. 2023, StatPearls Publishing; Copyright © 2023, StatPearls Publishing LLC.: Treasure Island (FL).
- Danesh, A. and P.C.H. Gottschalk, Beta-Blockers for Migraine Prevention: a Review Article. Curr Treat Options Neurol, 2019. 21(4): p. 20. [CrossRef]
- Obermann, S.N.a.M., Topiramate in the prevention and treatment of migraine: efficacy, safety and patient preference. 2009.
- Pavelic, A.R., et al., Monoclonal Antibodies against Calcitonin Gene-Related Peptide for Migraine Prophylaxis: A Systematic Review of Real-World Data. Cells, 2022. 12(1). [CrossRef]
- Ernstsen, C., et al., No additive effect of combining sumatriptan and olcegepant in the GTN mouse model of migraine. Cephalalgia, 2021. 41(3): p. 329-339. [CrossRef]
- van Hoogstraten, W.S. and A. MaassenVanDenBrink, The need for new acutely acting antimigraine drugs: moving safely outside acute medication overuse. J Headache Pain, 2019. 20(1): p. 54.
- Hien Ha, P., and Annika Gonzalez, MD, Migraine Headache Prophylaxis. 2019.
- Diener, H.C., et al., New therapeutic approaches for the prevention and treatment of migraine. Lancet Neurol, 2015. 14(10): p. 1010-22. [CrossRef]
- Ashina, M., et al., Safety and efficacy of eptinezumab for migraine prevention in patients with two-to-four previous preventive treatment failures (DELIVER): a multi-arm, randomised, double-blind, placebo-controlled, phase 3b trial. Lancet Neurol, 2022. 21(7): p. 597-607. [CrossRef]
- Haghdoost, F., et al., Evaluating the efficacy of CGRP mAbs and gepants for the preventive treatment of migraine: A systematic review and network meta-analysis of phase 3 randomised controlled trials. Cephalalgia, 2023. 43(4): p. 3331024231159366. [CrossRef]
- Messina, R., et al., Safety and tolerability of monoclonal antibodies targeting the CGRP pathway and gepants in migraine prevention: A systematic review and network meta-analysis. Cephalalgia, 2023. 43(3): p. 3331024231152169. [CrossRef]
- Vernieri, F., et al., Discontinuing monoclonal antibodies targeting CGRP pathway after one-year treatment: an observational longitudinal cohort study. J Headache Pain, 2021. 22(1): p. 154. [CrossRef]
- Steiner, T.J., et al., Aids to management of headache disorders in primary care (2nd edition) : on behalf of the European Headache Federation and Lifting The Burden: the Global Campaign against Headache. J Headache Pain, 2019. 20(1): p. 57.
- Herman, T.F. and C. Santos, First Pass Effect, in StatPearls. 2023, StatPearls Publishing; Copyright © 2023, StatPearls Publishing LLC.: Treasure Island (FL).
- Costa, C.P., et al., Intranasal delivery of nanostructured lipid carriers, solid lipid nanoparticles and nanoemulsions: A current overview of in vivo studies. Acta Pharm Sin B, 2021. 11(4): p. 925-940. [CrossRef]
- Jeong, S.H., J.H. Jang, and Y.B. Lee, Drug delivery to the brain via the nasal route of administration: exploration of key targets and major consideration factors. J Pharm Investig, 2023. 53(1): p. 119-152. [CrossRef]
- Chattopadhyay, S., S. Das, and K.N. Sarma, Nose-to-Brain Drug Delivery: An Update to the Alternative Path to Successful Targeted Anti-Migraine Drugs. International Journal of Applied Pharmaceutics, 2021: p. 67-75. [CrossRef]
- Correia, A.C., et al., Lipid nanoparticles strategies to modify pharmacokinetics of central nervous system targeting drugs: Crossing or circumventing the blood-brain barrier (BBB) to manage neurological disorders. Adv Drug Deliv Rev, 2022. 189: p. 114485. [CrossRef]
- Pandit, R., L. Chen, and J. Gotz, The blood-brain barrier: Physiology and strategies for drug delivery. Adv Drug Deliv Rev, 2020. 165-166: p. 1-14. [CrossRef]
- Costa, C.P., et al., In Vitro Studies on Nasal Formulations of Nanostructured Lipid Carriers (NLC) and Solid Lipid Nanoparticles (SLN). Pharmaceuticals (Basel), 2021. 14(8). [CrossRef]
- Aurora, S.K., et al., A link between gastrointestinal disorders and migraine: Insights into the gut-brain connection. Headache, 2021. 61(4): p. 576-589. [CrossRef]
- Crowe, T.P. and W.H. Hsu, Evaluation of Recent Intranasal Drug Delivery Systems to the Central Nervous System. Pharmaceutics, 2022. 14(3). [CrossRef]
- C. Duquesnoy , J.P.M., D. Sumner , E. Fuseau, Comparative clinical pharmacokinetics of single doses of sumatriptan following subcutaneous, oral, rectal and intranasal administration. European Journal of Pharmaceutical Sciences, 1998. [CrossRef]
- IMITREX (sumatriptan) nasal spray. 10/09/2023]; Available from: https://gskpro.com/content/dam/global/hcpportal/en_US/Prescribing_Information/Imitrex_Nasal_Spray/pdf/IMITREX-NASAL-SPRAY-PI-PIL.PDF.
- Dihydroergotamine Mesylate Nasal Spray. 10/09/2023]; Available from: https://pi.bauschhealth.com/globalassets/BHC/PI/Migranal-PI.pdf.
- Gallagher, R.M., Acute treatment of migraine with dihydroergotamine nasal spray. Dihydroergotamine Working Group. Arch Neurol, 1996. 53(12): p. 1285-91. [CrossRef]
- ZOMIG (zolmitriptan) nasal spray. 10/09/2023]; Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021450s007lbl.pdf.
- Charlesworth, B.R., et al., Speed of onset and efficacy of zolmitriptan nasal spray in the acute treatment of migraine: a randomised, double-blind, placebo-controlled, dose-ranging study versus zolmitriptan tablet. CNS Drugs, 2003. 17(9): p. 653-67.
- Winner, P., et al., Efficacy and tolerability of zolmitriptan nasal spray for the treatment of acute migraine in adolescents: Results of a randomized, double-blind, multi-center, parallel-group study (TEENZ). Headache, 2016. 56(7): p. 1107-19. [CrossRef]
- ONZETRA ™ XsailTM (sumatriptan nasal powder). 11/09/2023]; Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/206099s000lbl.pdf.
- Obaidi, M., et al., Improved pharmacokinetics of sumatriptan with Breath Powered nasal delivery of sumatriptan powder. Headache, 2013. 53(8): p. 1323-33. [CrossRef]
- Cady, R., A novel intranasal breath-powered delivery system for sumatriptan: a review of technology and clinical application of the investigational product AVP-825 in the treatment of migraine. Expert Opin Drug Deliv, 2015. 12(9): p. 1565-77. [CrossRef]
- TOSYMRA™ (sumatriptan) nasal spray. 12/09/2023]; Available from: https://www.upsher-smith.com/wp-content/uploads/TOS-MI.pdf.
- Munjal, S., et al., A multicenter, open-label, long-term safety and tolerability study of DFN-02, an intranasal spray of sumatriptan 10 mg plus permeation enhancer DDM, for the acute treatment of episodic migraine. J Headache Pain, 2017. 18(1): p. 31. [CrossRef]
- Munjal, S., et al., A Randomized Trial Comparing the Pharmacokinetics, Safety, and Tolerability of DFN-02, an Intranasal Sumatriptan Spray Containing a Permeation Enhancer, With Intranasal and Subcutaneous Sumatriptan in Healthy Adults. Headache, 2016. 56(9): p. 1455-1465. [CrossRef]
- TRUDHESATM (dihydroergotamine mesylate) nasal spray. 11/09/2023]; Available from: https://www.trudhesa.com/trudhesa-prescribing-information.pdf.
- Dhillon, S., Zavegepant: First Approval. Drugs, 2023. 83(9): p. 825-831. [CrossRef]
- Kahwaji, S.C.F.D.A.K.C.I., Physiology, Nasal. 2023: StatPearls [Internet].
- Lofts, A., et al., Using the Intranasal Route to Administer Drugs to Treat Neurological and Psychiatric Illnesses: Rationale, Successes, and Future Needs. CNS Drugs, 2022. 36(7): p. 739-770. [CrossRef]
- Torres, J., et al., Intranasal Lipid Nanoparticles Containing Bioactive Compounds Obtained from Marine Sources to Manage Neurodegenerative Diseases. Pharmaceuticals (Basel), 2023. 16(2). [CrossRef]
- Keller, L.A., O. Merkel, and A. Popp, Intranasal drug delivery: opportunities and toxicologic challenges during drug development. Drug Deliv Transl Res, 2022. 12(4): p. 735-757. [CrossRef]
- Lee, D. and T. Minko, Nanotherapeutics for Nose-to-Brain Drug Delivery: An Approach to Bypass the Blood Brain Barrier. Pharmaceutics, 2021. 13(12). [CrossRef]
- Bahadur, S., et al., Intranasal Nanoemulsions for Direct Nose-to-Brain Delivery of Actives for CNS Disorders. Pharmaceutics, 2020. 12(12). [CrossRef]
- Moradi, F. and N. Dashti, Targeting neuroinflammation by intranasal delivery of nanoparticles in neurological diseases: a comprehensive review. Naunyn Schmiedebergs Arch Pharmacol, 2022. 395(2): p. 133-148. [CrossRef]
- Bharadwaj, V.N., et al., Intranasal Administration for Pain: Oxytocin and Other Polypeptides. Pharmaceutics, 2021. 13(7). [CrossRef]
- Costa, C., et al., Nose-to-brain delivery of lipid-based nanosystems for epileptic seizures and anxiety crisis. J Control Release, 2019. 295: p. 187-200. [CrossRef]
- Yang, R., et al., Getting Drugs Across Biological Barriers. Adv Mater, 2017. 29(37). [CrossRef]
- Sigurdsson, H.H., J. Kirch, and C.M. Lehr, Mucus as a barrier to lipophilic drugs. Int J Pharm, 2013. 453(1): p. 56-64. [CrossRef]
- Martins, P.P., H.D.C. Smyth, and Z. Cui, Strategies to facilitate or block nose-to-brain drug delivery. Int J Pharm, 2019. 570: p. 118635. [CrossRef]
- Hong, S.S., et al., Liposomal Formulations for Nose-to-Brain Delivery: Recent Advances and Future Perspectives. Pharmaceutics, 2019. 11(10). [CrossRef]
- Landoni, M.I.V.a.F., Penetration Enhancers for the Development of Intranasal Formulations for Use in Equines. International Journal of Equine Science, 2022. Vol 1: p. 16–32.
- Li Du, L.C., Fangfang Liu, Wenya Wang, Hongyun Huang, Chapter Eight - Nose-to-brain drug delivery for the treatment of CNS disease: New development and strategies. 2023.
- Patharapankal, E.J., et al., Nose-to-Brain (N2B) Delivery: An Alternative Route for the Delivery of Biologics in the Management and Treatment of Central Nervous System Disorders. Pharmaceutics, 2023. 16(1). [CrossRef]
- Alexander, A. and S. Saraf, Nose-to-brain drug delivery approach: A key to easily accessing the brain for the treatment of Alzheimer's disease. Neural Regen Res, 2018. 13(12): p. 2102-2104.
- Pires, P.C., et al., Strategies to Improve Drug Strength in Nasal Preparations for Brain Delivery of Low Aqueous Solubility Drugs. Pharmaceutics, 2022. 14(3). [CrossRef]
- Sorokowski, P., et al., Sex Differences in Human Olfaction: A Meta-Analysis. Front Psychol, 2019. 10: p. 242. [CrossRef]
- Rassu, G., et al., The Role of Combined Penetration Enhancers in Nasal Microspheres on In Vivo Drug Bioavailability. Pharmaceutics, 2018. 10(4). [CrossRef]
- Giunchedi, P., E. Gavini, and M.C. Bonferoni, Nose-to-Brain Delivery. Pharmaceutics, 2020. 12(2).
- Sasaki, K., et al., Effective nose-to-brain drug delivery using a combination system targeting the olfactory region in monkeys. J Control Release, 2023. 359: p. 384-399. [CrossRef]
- Chung, S., et al., The nose has it: Opportunities and challenges for intranasal drug administration for neurologic conditions including seizure clusters. Epilepsy Behav Rep, 2023. 21: p. 100581. [CrossRef]
- Alabsi, W., et al., Nose-to-Brain Delivery of Therapeutic Peptides as Nasal Aerosols. Pharmaceutics, 2022. 14(9). [CrossRef]
- Wang, Z., et al., Nose-to-Brain Delivery. J Pharmacol Exp Ther, 2019. 370(3): p. 593-601.
- Gänger, S. and K. Schindowski, Tailoring Formulations for Intranasal Nose-to-Brain Delivery: A Review on Architecture, Physico-Chemical Characteristics and Mucociliary Clearance of the Nasal Olfactory Mucosa. Pharmaceutics, 2018. 10(3).
- Tekade, A.R., P.S. Mittha, and C.S. Pisal, Nanostructured Lipid Carriers for Nose to Brain Delivery Targeting CNS: Diversified Role of Liquid Lipids for Synergistic Action. Adv Pharm Bull, 2022. 12(4): p. 763-771. [CrossRef]
- Musielak, E., A. Feliczak-Guzik, and I. Nowak, Synthesis and Potential Applications of Lipid Nanoparticles in Medicine. Materials (Basel), 2022. 15(2). [CrossRef]
- Xu, L., et al., Lipid Nanoparticles for Drug Delivery. Advanced NanoBiomed Research, 2021. 2(2).
- Viegas, C., et al., Solid Lipid Nanoparticles vs. Nanostructured Lipid Carriers: A Comparative Review. Pharmaceutics, 2023. 15(6). [CrossRef]
- Shan, X., et al., Current approaches of nanomedicines in the market and various stage of clinical translation. Acta Pharm Sin B, 2022. 12(7): p. 3028-3048. [CrossRef]
- Pasarin, D., et al., Coating Materials to Increase the Stability of Liposomes. Polymers (Basel), 2023. 15(3). [CrossRef]
- Alshaer, W., et al., Quality by Design Approach in Liposomal Formulations: Robust Product Development. Molecules, 2022. 28(1). [CrossRef]
- Scioli Montoto, S., G. Muraca, and M.E. Ruiz, Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects. Front Mol Biosci, 2020. 7: p. 587997. [CrossRef]
- Müller, R.H., K. Mäder, and S. Gohla, Solid lipid nanoparticles (SLN) for controlled drug delivery - a review of the state of the art. Eur J Pharm Biopharm, 2000. 50(1): p. 161-77.
- Mehnert, W. and K. Mäder, Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev, 2001. 47(2-3): p. 165-96.
- Shidhaye, S.S., et al., Solid lipid nanoparticles and nanostructured lipid carriers--innovative generations of solid lipid carriers. Curr Drug Deliv, 2008. 5(4): p. 324-31. [CrossRef]
- Müller, R.H., M. Radtke, and S.A. Wissing, Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev, 2002. 54 Suppl 1: p. S131-55.
- Hald Albertsen, C., et al., The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv Drug Deliv Rev, 2022. 188: p. 114416. [CrossRef]
- Oehlke, K., et al., Edible solid lipid nanoparticles (SLN) as carrier system for antioxidants of different lipophilicity. PLoS One, 2017. 12(2): p. e0171662. [CrossRef]
- Fonseca-Santos, B., et al., Formulating SLN and NLC as Innovative Drug Delivery Systems for Non-Invasive Routes of Drug Administration. Curr Med Chem, 2020. 27(22): p. 3623-3656. [CrossRef]
- Satapathy, M.K., et al., Solid Lipid Nanoparticles (SLNs): An Advanced Drug Delivery System Targeting Brain through BBB. Pharmaceutics, 2021. 13(8). [CrossRef]
- Dhiman, N., et al., Lipid Nanoparticles as Carriers for Bioactive Delivery. Front Chem, 2021. 9: p. 580118. [CrossRef]
- Kaul, S., et al., Role of Nanotechnology in Cosmeceuticals: A Review of Recent Advances. J Pharm (Cairo), 2018. 2018: p. 3420204. [CrossRef]
- Pucek, A., A. Lewińska, and K.A. Wilk, Co-encapsulating solid lipid nanoparticles for multifunctional therapeutics: Preparation and characterization. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2016. 510: p. 11-21. [CrossRef]
- Janaína Artem Ataide, J.C.C., Érica Mendes dos Santos, Viviane Beraldo-Araujo ,Jéssica Ribeiro Alves Silva ,Karine Cappuccio de Castro, André Moreni Lopes, Nina Filipczak, Satya Siva Kishan Yalamarty, Vladimir P. Torchilin and Priscila Gava Mazzola, Co-Encapsulation of Drugs for Topical Application—A Review. 2023. [CrossRef]
- Renault-Mahieux, M., et al., Co-encapsulation of flavonoids with anti-cancer drugs: A challenge ahead. Int J Pharm, 2022. 623: p. 121942. [CrossRef]
- Bhattacharjee, S., Craft of Co-encapsulation in Nanomedicine: A Struggle To Achieve Synergy through Reciprocity. ACS Pharmacol Transl Sci, 2022. 5(5): p. 278-298. [CrossRef]
- Palliyage, G.H., et al., Novel Curcumin-Resveratrol Solid Nanoparticles Synergistically Inhibit Proliferation of Melanoma Cells. Pharm Res, 2021. 38(5): p. 851-871. [CrossRef]
- Amerigos Daddy, J.C.K., et al., Co-Encapsulation of Mitoxantrone and beta-Elemene in Solid Lipid Nanoparticles to Overcome Multidrug Resistance in Leukemia. Pharmaceutics, 2020. 12(2).
- Gupta, S., S. Wairkar, and L.K. Bhatt, Isotretinoin and α-tocopherol acetate-loaded solid lipid nanoparticle topical gel for the treatment of acne. J Microencapsul, 2020. 37(8): p. 557-565. [CrossRef]
- Andrade, L.M., et al., Improved tacrolimus skin permeation by co-encapsulation with clobetasol in lipid nanoparticles: Study of drug effects in lipid matrix by electron paramagnetic resonance. Eur J Pharm Biopharm, 2017. 119: p. 142-149. [CrossRef]
- Lacatusu , N.B., G. Badea , M. Mihaila , C. Ott , R. Stan , A. Meghea, Advanced bioactive lipid nanocarriers loaded with natural and synthetic anti-inflammatory actives. Chemical Engineering Science, 2019. [CrossRef]
- Nasr, M., Development of an optimized hyaluronic acid-based lipidic nanoemulsion co-encapsulating two polyphenols for nose to brain delivery. Drug Deliv, 2016. 23(4): p. 1444-52. [CrossRef]
- Gugleva, V. and V. Andonova, Drug delivery to the brain – lipid nanoparticles-based approach. Pharmacia, 2023. 70(1): p. 113-120.
- Shankar, R., M. Joshi, and K. Pathak, Lipid Nanoparticles: A Novel Approach for Brain Targeting. Pharm Nanotechnol, 2018. 6(2): p. 81-93. [CrossRef]
- Cunha, S., et al., Thermosensitive in situ hydrogels of rivastigmine-loaded lipid-based nanosystems for nose-to-brain delivery: characterisation, biocompatibility, and drug deposition studies. Int J Pharm, 2022. 620: p. 121720. [CrossRef]
- Sara Cunha, C.P.C., João Nuno Moreira, José Manuel Sousa Lobo, Ana Catarina Silva, Using the quality by design (QbD) approach to optimize formulations of lipid nanoparticles and nanoemulsions: A review. [CrossRef]
- Correia, A.C., et al., Design of experiment (DoE) as a quality by design (QbD) tool to optimize formulations of lipid nanoparticles for nose-to-brain drug delivery. Expert Opin Drug Deliv, 2023.
- Cunha, S., et al., Double Optimization of Rivastigmine-Loaded Nanostructured Lipid Carriers (NLC) for Nose-to-Brain Delivery Using the Quality by Design (QbD) Approach: Formulation Variables and Instrumental Parameters. Pharmaceutics, 2020. 12(7). [CrossRef]
- Cunha, S., et al., Improving Drug Delivery for Alzheimer's Disease Through Nose-to-Brain Delivery Using Nanoemulsions, Nanostructured Lipid Carriers (NLC) and in situ Hydrogels. Int J Nanomedicine, 2021. 16: p. 4373-4390.
- (CHMP), C.F.M.P.F.H.U., GUIDELINE ON THE PHARMACEUTICAL QUALITY OF INHALATION AND NASAL PRODUCTS. London, 21 June 2006.
- Patel, D., B. Patel, and S. Wairkar, Intranasal delivery of biotechnology-based therapeutics. Drug Discov Today, 2022. 27(12): p. 103371. [CrossRef]
- Gandh, R. STEPS TO SUCCESS IN NOSE-TO-BRAIN DRUG DELIVERY. 2022.
- Jagruti B. Prajapati, G.C.P., Nose to brain delivery of Rotigotine loaded solid lipid nanoparticles: Quality by design based optimization and characterization. 2021. [CrossRef]
- Yasir, M., et al., Glyceryl behenate-based solid lipid nanoparticles as a carrier of haloperidol for nose to brain delivery: formulation development, in-vitro, and in-vivo evaluation. Brazilian Journal of Pharmaceutical Sciences, 2022. 58. [CrossRef]
- Prakash Ramalingam, P.G., D. S. Prabakaran, Pardeep K. Gupta, Sriramakamal Jonnalagadda, Karthivashan Govindarajan, Revuri Vishnu, Kalaiselvi Sivalingam, Srushti Sodha, Dong-Kug Choi & Young Tag Ko Lipid Nanoparticles Improve the Uptake of α-Asarone Into the Brain Parenchyma: Formulation, Characterization, In Vivo Pharmacokinetics, and Brain Delivery. 2020.
- Islam, S.U., et al., Intranasal Delivery of Nanoformulations: A Potential Way of Treatment for Neurological Disorders. Molecules, 2020. 25(8). [CrossRef]
- Akel, H. and I. Csóka, Formulation and In Vitro Comparison Study between Lipid-Based and Polymeric-Based Nanoparticles for Nose-to-Brain Delivery of a Model Drug for Alzheimer’s Disease, in The 1st International Electronic Conference on Pharmaceutics. 2020.
- Mohanty, D., et al., Development of Atomoxetine-Loaded NLC In Situ Gel for Nose-to-Brain Delivery: Optimization, In Vitro, and Preclinical Evaluation. Pharmaceutics, 2023. 15(7). [CrossRef]
- Teaima, M.H., et al., Lyophilized Nasal Inserts of Atomoxetine HCl Solid Lipid Nanoparticles for Brain Targeting as a Treatment of Attention-Deficit/Hyperactivity Disorder (ADHD): A Pharmacokinetics Study on Rats. Pharmaceuticals (Basel), 2023. 16(2). [CrossRef]
- Arora, D., et al., QbD-based rivastigmine tartrate-loaded solid lipid nanoparticles for enhanced intranasal delivery to the brain for Alzheimer's therapeutics. Front Aging Neurosci, 2022. 14: p. 960246.
- Zafar, A., et al., Formulation of intranasal surface engineered nanostructured lipid carriers of rotigotine: Full factorial design optimization, in vitro characterization, and pharmacokinetic evaluation. Int J Pharm, 2022. 627: p. 122232. [CrossRef]
- Taha, E., et al., Cod liver oil nano-structured lipid carriers (Cod-NLCs) as a promising platform for nose to brain delivery: Preparation, in vitro optimization, ex vivo cytotoxicity & in vivo biodistribution utilizing radioiodinated zopiclone. Int J Pharm X, 2023. 5: p. 100160. [CrossRef]
- Ter Horst, J.P., et al., Implementation of Quality by Design (QbD) Principles in Regulatory Dossiers of Medicinal Products in the European Union (EU) Between 2014 and 2019. Ther Innov Regul Sci, 2021. 55(3): p. 583-590. [CrossRef]
- Boyuklieva, R. and B. Pilicheva, Micro- and Nanosized Carriers for Nose-to-Brain Drug Delivery in Neurodegenerative Disorders. Biomedicines, 2022. 10(7). [CrossRef]
- Rinaldi, F., et al., Chitosan Glutamate-Coated Niosomes: A Proposal for Nose-to-Brain Delivery. Pharmaceutics, 2018. 10(2). [CrossRef]
- Yermak, I.M., V.N. Davydova, and A.V. Volod'ko, Mucoadhesive Marine Polysaccharides. Mar Drugs, 2022. 20(8). [CrossRef]
- Pandey, V., et al., Formulation strategies for nose-to-brain delivery of therapeutic molecules, in Drug Delivery Systems. 2020. p. 291-332. [CrossRef]
- Doub, W.H., et al., Laboratory Performance Testing of Aqueous Nasal Inhalation Products for Droplet/Particle Size Distribution: an Assessment from the International Pharmaceutical Aerosol Consortium on Regulation and Science (IPAC-RS). AAPS PharmSciTech, 2023. 24(7): p. 208. [CrossRef]
- Bioavailability and Bioequivalence Studies for Nasal Aerosols and Nasal Sprays for Local Action. Guidance for Industry 2003; Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070111.pdf.
- Sijs, R., et al., Drop size measurement techniques for sprays: Comparison of image analysis, phase Doppler particle analysis, and laser diffraction. AIP Advances, 2021. 11(1). [CrossRef]
- Thomas, B.J.e.a., Analytical method development for characterizing ingredient-specific particle size distributions of nasal spray suspension products. Journal of Pharmaceutical Sciences, Volume 110, Issue 7, 2778 - 2788, 2021.
- Le Guellec, S., S. Ehrmann, and L. Vecellio, In vitro - in vivo correlation of intranasal drug deposition. Adv Drug Deliv Rev, 2021. 170: p. 340-352. [CrossRef]
- Liu, Q., et al., Scientific Considerations for the Review and Approval of First Generic Mometasone Furoate Nasal Suspension Spray in the United States from the Bioequivalence Perspective. Aaps j, 2019. 21(2): p. 14. [CrossRef]
- Hallworth, G.W. and J.M. Padfield, A comparison of the regional deposition in a model nose of a drug discharged from metered aerosol and metered-pump nasal delivery systems. J Allergy Clin Immunol, 1986. 77(2): p. 348-53.
- Chari, S., K. Sridhar, and C. Kleinstreuer, Effects of subject-variability on nasally inhaled drug deposition, uptake, and clearance. Journal of Aerosol Science, 2022. 165. [CrossRef]
- Williams, G. and J.D. Suman, In Vitro Anatomical Models for Nasal Drug Delivery. Pharmaceutics, 2022. 14(7). [CrossRef]
- Jinxiang Xi, Z.W., Danielle Nevorski, Thomas White, and Yue Zhou, Nasal and Olfactory Deposition with Normal and Bidirectional Intranasal Delivery Techniques: In Vitro Tests and Numerical Simulations. 2017. [CrossRef]
- Walenga RL, D.S., Newman B, Babiskin A, Zhao L., In Silico and Experimental Methods to Support Generic Nasal Drug Product (NDP) Development, in Respiratory Drug Delivery 2021. Volume , 2021: 141-150. 2021.
- Chen, J.Z., W.H. Finlay, and A. Martin, In Vitro Regional Deposition of Nasal Sprays in an Idealized Nasal Inlet: Comparison with In Vivo Gamma Scintigraphy. Pharm Res, 2022. 39(11): p. 3021-3028. [CrossRef]
- Chen, J.Z., et al., In vitro assessment of an idealized nose for nasal spray testing: Comparison with regional deposition in realistic nasal replicas. Int J Pharm, 2020. 582: p. 119341. [CrossRef]
- Pina Costa, C., et al., In situ hydrogel containing diazepam-loaded nanostructured lipid carriers (DZP-NLC) for nose-to-brain delivery: development, characterization and deposition studies in a 3D-printed human nasal cavity model. Int J Pharm, 2023. 644: p. 123345. [CrossRef]
- Pardeshi, C.V. and V.S. Belgamwar, Improved brain pharmacokinetics following intranasal administration of N,N,N-trimethyl chitosan tailored mucoadhesive NLCs. Materials Technology, 2019. 35(5): p. 249-266.
- Som Chaudhury, S., K. Sinha, and C. Das Mukhopadhyay, Intranasal route: The green corridor for Alzheimer's disease therapeutics. Journal of Drug Delivery Science and Technology, 2021. 66.
- Pires, P.C. and A.O. Santos, Nanosystems in nose-to-brain drug delivery: A review of non-clinical brain targeting studies. J Control Release, 2018. 270: p. 89-100. [CrossRef]
- Youssef, N., et al., A novel nasal almotriptan loaded solid lipid nanoparticles in mucoadhesive in situ gel formulation for brain targeting: Preparation, characterization and in vivo evaluation. Int J Pharm, 2018. 548(1): p. 609-624. [CrossRef]
- Salem, L.H., et al., Coated Lipidic Nanoparticles as a New Strategy for Enhancing Nose-to-Brain Delivery of a Hydrophilic Drug Molecule. J Pharm Sci, 2020. 109(7): p. 2237-2251. [CrossRef]
- Tripathi, D., et al., Augmented Brain Delivery of Cinnarizine Through Nanostructured Lipid Carriers Loaded in situ Gel: in vitro and Pharmacokinetic Evaluation. BioNanoScience, 2021. 11(1): p. 159-171. [CrossRef]
- Bakshi, V., P.R. Amarachinta, and A.K. Chettupalli, Design, Development and Optimization of Solid Lipid Nanoparticles of Rizatriptan for Intranasal delivery: Invitro & Invivo assessment. Materials Today: Proceedings, 2022. 66: p. 2342-2357. [CrossRef]
- Masjedi, M., et al., Nose-to-brain delivery of sumatriptan-loaded nanostructured lipid carriers: preparation, optimization, characterization and pharmacokinetic evaluation. J Pharm Pharmacol, 2020. 72(10): p. 1341-1351. [CrossRef]
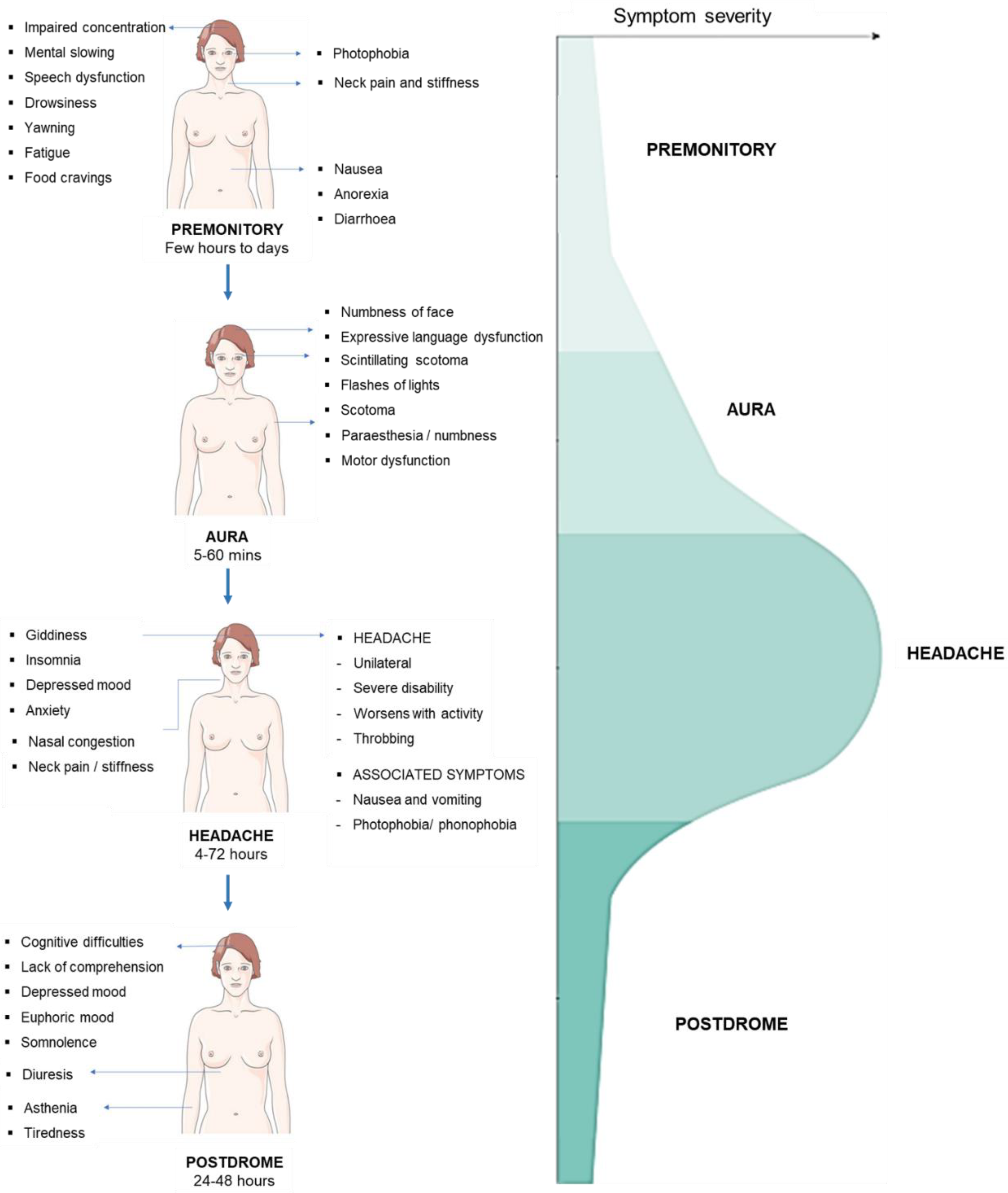
| Treatment | Drug class | Drug | AEs | Contraindications | References | ||
|---|---|---|---|---|---|---|---|
| Acute | First-line medication | ||||||
| Non-steroidal anti-inflammatory drugs (NSAIDs) | Acetylsalicylic acid | Gastric effects | Patients with inflammatory bowel disease, renal dysfunction and who have had gastric bypass surgery. | [5,21] | |||
| Ibuprofen | |||||||
| Naproxen | |||||||
| Diclofenac potassium | |||||||
| Other simple analgesics | Paracetamol | Gastrointestinal effects | Patients with hepatic disease and renal failure. | ||||
| Antiemetic drugs | Metoclopramide | Drowsiness, weight gain, blurred vision, cardiac arrhythmias, urinary retention, extrapyramidal symptoms, and infertility | Patients with gastrointestinal bleeding, epilepsy, renal failure, cardiac arrhythmia, and Parkinson’s disease. | ||||
| Chlorpromazine | |||||||
| Prochlorperazine | |||||||
| Second-line medication | |||||||
| Triptans | Sumatriptan | Nausea, dizziness, coronary vasoconstriction, chest pressure and tingling in the limbs | Patients with cardio- or cerebrovascular disease, uncontrolled hypertension, ischemic bowel, pregnant patients, or those who have used another triptan in the last 24 h. | [3,39] | |||
| Zolmitriptan | |||||||
| Rizatriptan | |||||||
| Naratriptan | |||||||
| Almotriptan | |||||||
| Frovatriptan | |||||||
| Third-line medication | |||||||
| Ditans | Lasmiditan | Dizziness, nausea and somnolence | Pregnant women and patients using drugs that are P-glycoprotein substrates. | [2,3,5] | |||
| Gepants | Ubrogepant | Fatigue and nausea | Patients with hypersensitivity and hepatic impairment. | ||||
| Rimegepant | |||||||
| Preventive | First-line medication | ||||||
| Beta blockers | Metoprolol | Dizziness, cold hands or feet and difficulties in sleeping | Patients with asthma, cardiac failure, Raynaud disease, atrioventricular block and diabetes mellitus. | [5,40,41] | |||
| Propranolol | |||||||
| Anticonvulsant | Topiramate | Fatigue, cognitive disturbance, weight loss and paresthesia | Pregnant and lactating patients; and patients with nephrolithiasis and glaucoma. | ||||
| Second-line medication | |||||||
| Antidepressant | Amitriptyline | Dry mouth, fatigue, dizziness and sweating | Patients with age ˂ 6 years, glaucoma, prostatic adenoma hyperplasia and heart insufficiency. | [5,41] | |||
| Calcium channel blocker | Flunarizine | Fatigue, weight gain, depression, hyperkinesia, tremor, parkinsonism and gastrointestinal side effects | Patients with familial parkinsonism, focal dystonia and depression. | ||||
| Anticonvulsant | Valproic acid | Fatigue dizziness, tremorand elevation of liver enzymes/disturbance in liver function | Patients with liver failure, pregnancy, alcoholism and polycystic ovaries. | ||||
| Third-line medication | |||||||
| Calcitonin gene-related peptide monoclonal antibodies | Erenumab | Constipation, gastric pain, and chest pain | Patients with inflammatory bowel disease, coronary heart disease, chronic obstructive pulmonary disease and subarachnoid hemorrhage. | [5,42] | |||
| Fremanezumab | |||||||
| Galcanezumab | |||||||
| Drug | Product details | Brand name | Key results | References |
|---|---|---|---|---|
| Sumatriptan | Dose: 5, 10, or 20 mg Liquid formulation delivered via traditional nasal spray |
IMITREX® | Pharmacokinetic studies demonstrated a Cmax blood of 69.5 ng/mL and 12.9 ng/mL following subcutaneous and nasal administration of sumatriptan, respectively. Pharmacokinetic studies demonstrated that the mean bioavailability following nasal administration is 15.8 %, compared with the subcutaneous route. Greater percentage of patients had headache relief 2 hours after treatment with 10 or 20 mg of IMITREX® vs. placebo. Frequent AEs include nasal cavity/sinuses discomfort, burning, dizziness, nausea, vomiting, unusual taste and throat discomfort. |
[61,62] |
| Dihydroergotamine mesylate | Dose: 2 mg Liquid formulation delivered to the respiratory region |
MIGRANAL® | Pharmacokinetic studies demonstrated that the mean bioavailability following nasal administration is 32%, compared with the intravenous administration. Greater percentage of patients had headache relief 4 hours after treatment with 2 mg of MIGRANAL® vs. placebo. Frequent AEs include rhinitis, nausea, unusual taste, application site reactions and dizziness. |
[63,64] |
| Zolmitriptan | Dose: 2.5 or 5 mg Liquid formulation delivered to the nasopharynx and lower nasal space |
ZOMIG® | Pharmacokinetic studies demonstrated that the mean bioavailability following nasal administration is 102%, compared with the oral tablet. Greater percentage of patients had headache relief 2 hours after treatment with 2.5 or 5 mg of ZOMIG® vs. placebo. One multi-attack trial for adults showed that the headache response with ZOMIG® was consistently maintained during the 2 hours. Frequent AE include unusual taste (adolescents), paresthesia, hyperesthesia and somnolence. |
[65,66,67] |
| Sumatriptan | Dose: 22 mg Nasal powder delivered via breath to the upper nasal space |
ONZETRATM XsailTM | Pharmacokinetic studies demonstrated that administration of sumatriptan nasal powder (ONZETRATM XsailTM) resulted in 27% higher Cmax (20.8 vs 16.4 ng/mL) and 75% higher early exposure (AUC0-15 min, 2.1 vs. 1.2 ng*hour/mL) comparative to the sumatriptan nasal spray (IMITREX®). Pharmacokinetic studies demonstrated that the mean bioavailability following nasal administration is 19 %, compared with the subcutaneous route. Greater percentage of patients had headache relief 2 hours after treatment with 22 mg ONZETRATM XsailTM vs. placebo. Frequent AEs include unusual taste, nasal discomfort and rhinorrhea. |
[68,69,70] |
| Sumatriptan | Dose: 10 mg Liquid formulation containing a permeation-enhancing excipient (0.2% 1-O-n-Dodecyl-β-D-maltopyranoside) |
TOSYMRA™ | Pharmacokinetic studies comparing a single dose of 10 mg TOSYMRATM to 20 mg IMITREX® demonstrated that TOSYMRATM was more rapidly absorbed, with Cmax values of 63.9 and 21.4 ng/mL and AUC0–2hr values of 48.4 and 24.7 ng*hour/mL for TOSYMRATM and IMITREX®, respectively. Pharmacokinetic studies demonstrated that the mean bioavailability following nasal administration is 58 %, compared with the subcutaneous route. Greater percentage of patients had headache relief 2 hours after treatment with 10 mg TOSYMRATM vs. placebo. Frequent AEs include application site pain and reaction, unusual taste, upper respiratory infection, sinusitis and nasopharyngitis. |
[71,72,73] |
| Dihydroergotamine mesylate | Dose: 1.45 mg Liquid formulation delivered to the upper nasal space |
TrudhesaTM | Greater percentage of patients had headache relief 4 hours after treatment with 2 mg TrudhesaTM vs. placebo. In patients with migraine-associated nausea, photophobia, and phonophobia at baseline there was a lower incidence of these symptoms at 2- and 4-hours following administration of TrudhesaTM nasal spray vs. placebo. Frequent AEs include application site reaction, rhinitis, nausea, vomiting, somnolence, pharyngitis and diarrhea. |
[54,74] |
| Zavegepant | Dose: 10 mg Liquid formulation delivered via nasal spray |
Zavzpret™ | Greater percentage of patients had headache relief 2 hours after treatment with 10 mg ZavzpretTM vs. placebo. Frequent AEs include unusual taste, nausea, nasal discomfort, and vomiting. |
[75] |
| Limitations | Strategies | Description | References | |
|---|---|---|---|---|
| Mucociliary clearance mechanism | Increased contact time of the formulation with the nasal mucosa for improved absorption of the drug |
Absorption enhancers: cyclodextrins, sodium hyaluronate, Cremophor RH40, chitosan and cyclopentyladenosin | [54,55,60] | |
| Mucoadhesive agents: chitosan, and carboxymethylcellulose | [13,54] | |||
| Viscosity enhancers: pectin, Pluronic®, Carbopol®, cellulose derivatives and chitosan | [13] | |||
| Mucoadhesive systems: nanoparticulate drug delivery systems | [13,14,55,56,60] | |||
| Enzymatic and P-glycoprotein activity | Disturb the normal function of enzymes in the nasal epithelium | Enzyme modulators: P-glycoprotein inhibitors and CYP450 inhibitors | [55,56,60] | |
| Protection of drugs against enzymatic degradation and efflux transport mechanisms | Nanoparticulate drug delivery systems | [56] | ||
| Systemic absorption | Prevent deposition of the formulation in the respiratory region | Delivery devices designed to deposit the formulation in the olfactory region: ViaNase™, SipNose, OptiMist™, Precision Olfactory Device (POD®), VersiDoser®, VRx2TM, DARTTM and MAD NasalTM | [55,56,60] | |
| Tight junctions | Transiently decrease nasal epithelial tight junctions’ tightness | Compounds that modulate the permeability of tight junctions: chitosan, 12-O-tetradecanotlophorbol-13-acetate (TPA), papaverine, poly-L-arginine and bisindolylmaleimide | [60] | |
| Chelating agents: disodium ethylenediaminetetraacetate (EDTA) | [77] | |||
| Absorption enhancers: polysorbate 80, propylene glycol, and polyethylene glycol 400 | [77] | |||
| Physicochemical characteristics of drug molecules | Increase the nasal permeability of hydrophilic drugs | Nanoparticulate systems | [54,55,95] | |
| Absorption enhancers: cyclodextrins and chitosan | ||||
| Increase the nasal permeability of lipophilic drugs | Nanoparticulate drug delivery systems | [14] | ||
| Prodrugs | [13] | |||
| Damage to the nasal mucosa | Appropriately select the excipients of the formulation | Excipients generally recognized as safe (GRAS) | [96] | |
| Keep nasal mucosa moist | Humectants: glycerin, sorbitol, and mannitol | [13] | ||
| Formulations with pH similar to the nasal cavity (5.5-6.5) | pH adjustment and buffers: citric acid, sodium chloride, sodium hydroxide, hydrochloric acid, and potassium phosphate | [77] | ||
| Isotonic formulations | Isotonizing excipients: glycerin, sodium chloride, glucose or dextrose | [84] | ||
| Insufficient in vivo studies in humans | Use non-human primates with anatomical and physiological resemblance to humans | Preclinical investigations with cynomolgus monkey (Macaca fascicularis) | [97] | |
| Parameters | SLN | NLC | References | |
|---|---|---|---|---|
| Bioavailability | Ability to penetrate through several anatomical barriers increasing the bioavailability of drugs | [103,117,118,119] | ||
| Protection | Protection of the encapsulated molecules from enzymatic degradation | |||
| Drug loading | Effective for encapsulating lipophilic drugs | |||
| Drug delivery | Improved ability to transport drugs to the site of therapeutic action | |||
| Functionality | Possibility of attaching specific ligands to the surface of nanoparticles to improve drug targeting | |||
| Excipients | Use of generally recognized as safe (GRAS) excipients, including physiological biocompatible lipids | |||
| Cytotoxicity | Low or absence of toxicity related to the use of GRAS excipients | |||
| Solvents | No need of organic solvents in the production methods | |||
| Scale-up | Ease of transferring production methods to an industrial scale | |||
| Application | Versatility as drug/active ingredient delivery systems for various routes and used in different areas (food, cosmetic, pharmaceutical) | |||
| Storage stability | Possible particle aggregation and drug leakage during storage | Improved physical stability and reduced drug leakage during storage | ||
| Drug | Formulations tested | Constituents of SLN and NLC | AUC0-t brain ± SD AUC 0-t blood ± SD (µg*hour/mL) |
Tmax brain (h) |
Cmax brain± SD Cmax blood ± SD (µg/mL) |
DTE (%) | DTP (%) | Relevant results | References |
|---|---|---|---|---|---|---|---|---|---|
| Almotriptan malate (ALM) | IN ALM-loaded SLN in-situ gel | Solid lipid: Precirol® ATO 5 Emulsifier(s): Polyvinyl alcohol (PVA) and Poloxamer 188 |
7.87 ± 0.09 8.77 ± 0.08 |
0.17 | 2.41 ± 0.04 2.69 ± 0.02 |
335.7 | 70.21 | Higher Cmax brain of IN ALM-loaded SLN in-situ gel (1.7-fold vs. IN free ALM in-situ gel and 2-fold vs. IV ALM solution); Faster onset of IN ALM-loaded SLN in-situ gel (Tmax brain = 0.17 h); The toxicological results indicated the higher safety profile of IN ALM-loaded SLN in-situ gel for nasal administration. |
[172] |
| IN free ALM in-situ gel | 6.25 ± 0.03 9.15 ± 0.07 |
2 | 1.43 ± 0.02 3.09 ± 0.05 |
255.1 | 60.80 | ||||
| IV ALM solution | 3.32 ± 0.04 12.43 ± 0.09 |
0.5 | 1.23 ± 0.02 3.20 ± 0.06 |
- | - | ||||
| Almotriptan malate (ALM) | IN ALM-loaded NLC | Solid lipid: Compritol® ATO 888 Liquid lipid: Labrafil® M 2125 CS Emulsifier(s): Tween® 80 and Lauroglycol |
27291.00 ± 0.02 15348.60 ± 0.03 |
0.17 | 3.44 ± 0.03 1.54 ± 0.02 |
- | - | Higher Cmax brain of IN ALM-loaded NLC (7.2-fold vs. IN ALM solution and 6.6-fold vs. oral marketed formulation); Faster onset of IN ALM-loaded NLC (Tmax brain = 0.17 h); The toxicological results indicated the IN ALM-loaded NLC as safe for nasal administration. |
[173] |
| IN ALM solution | 3387.00 ± 0.05 2541.60 ± 0.05 |
0.33 | 0.48 ± 0.04 0.25 ± 0.03 |
- | - | ||||
| Oral marketed ALM formulation (tablet) | 5982.60 ± 0.03 7579.20 ± 0.04 |
1 | 0.52 ± 0.05 0.58 ± 0.03 |
- | - | ||||
| Cinnarizine (CIN) | IN CIN-loaded NLC in situ gel | Solid lipid: Cetyl palmitate Liquid lipid: Oleic acid Emulsifier(s): Poloxamer 188 and Soya lecithin |
108000 ± 111.5 41076 ± 57.46 |
1 | 786.65 ± 7.4 345.29 ± 11.2 |
- | - | Higher Cmax brain of IN CIN-loaded NLC in situ gel (2.1-fold vs. IN CIN solution). | [174] |
| IN CIN solution | 48432 ± 55.81 54210 ± 81.9 |
1 | 380.73 ± 2.41 471.31 ± 7.5 |
- | - | ||||
| Rizatriptan (RZT) | IN RZT-loaded SLN | Solid lipid: Compritol® ATO 888 Emulsifier(s): Tween® 80 |
1824.82 1894.80 |
1 | 583.20 955.18 |
50.52* | -97.88* | Higher Cmax brain of IN RZT-loaded SLN (1.7-fold vs. IV RZT-loaded SLN and 7.3-fold vs. oral marketed formulation); Faster onset of IN RZT-loaded SLN (Tmax brain = 1 h); DTE value of IN RZT-loaded SLN not indicated a more effective drug brain targeting after IN administration vs. IV administration. |
[175] |
| IV RZT-loaded SLN | 2375.10 1246.06 |
2 | 351.29 175.12 |
- | - | ||||
| Oral marketed Rizatriptan formulation (tablet) | 841.39 -1432.59 |
4 | 79.84 103.12 |
- | - | ||||
| Sumatriptan | IN Sumatriptan-loaded NLC | Solid lipid: Stearic acid and Cholesterol Liquid lipid: Triolein Emulsifier(s): Brij® 35 and Brij® 72 |
0.57 0.19 |
2 | 0.18 0.08 |
258.02 | 61.23 | Higher Cmax brain of IN Sumatriptan-loaded NLC (9.4-fold vs. IV Sumatriptan-loaded NLC, 5.6-fold vs. IN Sumatriptan solution and 7.4-fold vs. IV Sumatriptan solution). | [176] |
| IV Sumatriptan-loaded NLC | 0.07 0.06 |
2 | 0.02 0.05 |
- | - | ||||
| IN Sumatriptan solution | 0.07 0.10 |
1 | 0.03 0.07 |
- | - | ||||
| IV Sumatriptan solution | 0.04 0.36 |
1 | 0.02 0.23 |
- | - | ||||
| Zolmitriptan | IN Zolmitriptan-loaded SLN | Solid lipid: Glyceryl monostearate Emulsifier(s): Soya lecithin and Poloxamer 188 |
0.04 ± 2.45 - |
0.5 | 0.04 ± 1.32 - |
- | - | Higher Cmax brain of IN Zolmitriptan-loaded SLN (2-fold vs. IN Marketed formulation and 2.3-fold vs. IN Zolmitriptan solution); IN Zolmitriptan-loaded SLN showed a higher Tmax value (0.5 h) due to a slower drug release pattern. |
[10] |
| IN marketed Zolmitriptan formulation (Zolmist® nasal spray) | 0.02 ± 1.65 - |
0.17 | 0.03 ± 2.50 - |
- | - | ||||
| IN Zolmitriptan solution | 0.02 ± 1.25 - |
0.17 | 0.03 ± 1.56 - |
- | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





