1. Introduction
Diabetes mellitus is a global health challenge associated with alarming rates of morbidity and mortality. As of 2021, the worldwide count of diabetes reached a staggering 529 million; this figure is projected to escalate exponentially to over 1.3 billion by 2050 [
1]. Besides its pervasive prevalence, diabetes poses a significant threat to affected individuals with respect to adverse cardiovascular events and heightened hospitalization rates when compared to their non-diabetic counterparts. There is now growing recognition of the importance of the macrovascular complications of diabetes besides the traditional focus on microvascular complications (retinopathy, neuropathy, and nephropathy) [
2]. Type 2 diabetes mellitus (T2DM) increases the susceptibility to heart failure (HF) by directly impairing cardiac function as well as through associated comorbidities like hypertension, obesity, metabolic disorder, and renal dysfunction [
3]. The 1974 Framingham Heart Study (n=5209) with an 18-year follow-up underscores the fact that diabetes independently amplifies the risk of HF by 2-fold in men and an astonishing 4-fold in women compared to age-matched controls [
4]. A more recent report highlighted that amongst 3.25 million people in Scotland (>30y old) the incidence of heart failure hospitalization in T2DM was 5.6/1000 person years versus 2.4 in those without [
5]. In patients with T2DM, the prevalence of HF ranges from 9% to 22% and even higher in those aged > 60 years [
6]. In addition, several studies have demonstrated worse outcomes of HF in patients with diabetes. Diabetes accelerates the progression of HF from preclinical stages to more advanced stages [
7]. Given the significant increase in the global prevalence of diabetes over the past decade, and with further increases anticipated in the years ahead, the burden of HF on healthcare systems can only grow [
7]. Accordingly, over the recent years, risk stratification tools have been proposed to estimate HF incidence and to guide treatment in those with diabetes [
8]. In fact, the American Diabetes Association 2022 guidelines highlight the advantages of incorporating HF biomarkers in the risk stratification of patients with diabetes [
9]. In this regard, there is a growing body of evidence for using natriuretic peptides (NP) either as a single variable or as a part of multivariate models for predicting HF in patients with T2DM.
In this review, we aim to summarize the pertinent knowledge about the current use of NPs in the risk stratification and management of patients with T2DM.
2. Pathophysiology of Heart Failure in Diabetes
There is a recognised universal definition of HF since 2021 [
10]. This states that HF is a clinical syndrome with symptoms and/or signs caused by a structural and/or functional cardiac abnormality and corroborated by elevated NP levels and/or objective evidence of pulmonary or systemic congestion. It is characterized by the inability of the heart to pump enough blood and oxygen to support the metabolic needs of the body [
11]. Thus, based on left ventricular ejection fraction (LVEF), the latest guidelines have also classified HF accordingly: HF with reduced ejection fraction (HFrEF; LVEF ≤40%), HF with mildly reduced ejection fraction (HFmrEF; LVEF 41–49%), and HF with preserved ejection fraction (HFpEF; LVEF ≥50%). A subcategory of HF with improved ejection fraction (HFimpEF) is added if baseline LVEF ≤ 40% shows a ≥ 10 point increase on a second measurement i.e. LVEF > 40% [
10]. Though the etiopathology of HF is multifactorial, it is largely influenced by structural and cellular alterations in the presence of chronic comorbid conditions, such as hypertension, diabetes, pulmonary or renal insufficiency, and cancer [
12].
Diabetes remains a major risk factor for the development of HF. Similarly, HF significantly contributes to the progression and worsening of diabetes. The complex interplay between HF and T2DM encompasses a multitude of molecular, cellular, and systemic mechanisms. At the core of their bidirectional relationship lie hyperglycemia and insulin resistance, influenced by common risk factors such as obesity and inflammation [
13]. Diabetes is marked by neurohormonal dysregulation and the activation of the renin-angiotensin-aldosterone system (RAAS), culminating in heightened oxidative stress and inflammation at the endothelial level. This cascade of events results in endothelial dysfunction, a pivotal factor contributing not only to impaired cardiac contractility but also to the initiation and progression of atherosclerosis, thereby exacerbating the development of HF [
14]. In addition, the presence of myocardial lipotoxicity and diabetic cardiomyopathy, characterized by fibrosis and altered energetics, creates a milieu that predisposes individuals to HF [
13]. Furthermore, in the context of diabetes, there is evidence suggesting that hyperactivation of platelets and coagulation dysfunction may contribute to the worsening of HF, adding another layer of complexity to the pathophysiological processes linking these two conditions [
15]. This intricate web of interconnected mechanisms underscores the importance of a comprehensive understanding for the development of targeted therapeutic strategies to mitigate the risk and progression of HF in individuals with diabetes.
3. Natriuretic Peptides and Their Clinical Applications in Heart Failure
Natriuretic peptides are a family of hormones that play a crucial role in regulating blood pressure, fluid balance, and cardiovascular health. There are three biologically active peptides in the NP family: atrial natriuretic peptide (ANP), BNP, and C-type natriuretic peptide (CNP) [
16]. First discovered in 1980 by De Bold, initially from the atria of the heart, ANP was found to have potent diuretic and natriuretic effects, leading to increased urinary output and sodium excretion [
17]. Shortly thereafter, BNP and CNP were isolated from the brain, although their major production sites were later found to be the ventricles of the heart and endothelial cells, respectively [
18]. Among these three NPs, BNP stands out for its pivotal role in maintaining cardiovascular homeostasis. It is primarily synthesized and secreted by the ventricles of the heart in response to increased pressure or volume overload [
19]. Once released into the bloodstream, BNP exerts its diverse effects by binding to specific receptors on target cells. These effects include natriuresis, diuresis, vasodilation, anti-inflammatory and anti-fibrotic effects, and antihypertrophic effects [
20]. N-terminal pro-B-type natriuretic peptide (NT-pro BNP) is an inactive precursor molecule of BNP; it is cleaved from pro-BNP to produce the active BNP molecule. Like BNP, NT-pro BNP levels are elevated in response to increased myocardial stress [
21].
The American Heart Association (AHA) recognizes the crucial role of BNP and its precursor NT-pro BNP in both diagnosing and risk-stratifying HF [
22]. The current guidelines emphasize that elevated NT-pro BNP (>125 pg/mL) and BNP (>35 pg/mL) levels provide valuable evidence of the structural and functional alterations of the heart, thus aiding in the diagnosis of HF in both acute and chronic settings. In patients presenting with dyspnea, elevated BNP or NT-pro BNP levels assist in the determination of the need for further evaluation using transthoracic echocardiography. Furthermore, proactive screening based on BNP or NT-pro BNP levels, along with collaborative care involving cardiovascular specialists, proves beneficial for those at risk of developing HF, aiding in the prevention of left ventricular dysfunction or the onset of new HF.
In the context of chronic HF, it is recommended to regularly measure BNP or NT-pro BNP levels for effective risk stratification. The Asia Pacific Society of Cardiology (APSC) developed consensus recommendations on managing chronic HF in the Asia-Pacific region in 2023, acknowledging regional disparities in HF epidemiology and healthcare resources [
23]. Using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system, experts reached a consensus on diagnosis and treatment strategies, emphasizing the significance of NP levels in HF diagnosis. The 19-member expert panel agreed that a plasma concentration of NT-pro BNP <125 pg/mL or BNP <35 pg/mL renders a diagnosis of HF unlikely.
The strong correlation between elevated NP levels and the severity of HF underscores the potential utility of NP-guided therapy. In the EMPEROR-Reduced trial, associations between NT-pro BNP concentrations and the effects of treatment with empagliflozin, a sodium-glucose cotransporter 2 inhibitor (SGLT2i), were evaluated in patients with chronic HFrEF (24). Empagliflozin demonstrated significant reductions in NT-pro BNP concentrations and HF events. Moreover, post-treatment NT-proBNP concentrations following empagliflozin therapy better informed subsequent prognosis compared to pretreatment concentrations [
24].
Recently, the STRONG-HF trial examined the efficacy of intensive treatment involving rapid up-titration of guideline-directed medication and close follow-up post-acute HF admission. Involving 1,078 patients across 87 hospitals in 14 countries, the study found that high-intensity care significantly increased the proportion of patients up-titrated to full drug doses by day 90 and led to greater reductions in key HF indicators like NT-proBNP levels. Notably, high-intensity care substantially reduced the risk of 180-day all-cause death or HF readmission compared to usual care. These findings underscore the importance of NT-proBNP monitoring in optimizing HF management strategies [
25].
The 2024 REVOLUTION-HF (REal WOrLd EdUcaTION in HF) study from Sweden reported on patients with suspected de novo heart failure presenting in outpatient settings [
26]. One of its key findings was that while NT-proBNP levels were quickly tested, there was a significant delay in confirming a HF diagnosis, largely due to long waits for echocardiography. After one year, only 29% of the patients received an HF diagnosis, which contributed to high rates of HF hospitalization and all-cause mortality, particularly in those with elevated NT-proBNP levels. In addition, the uptake of guideline-directed medical therapies (GDMT), such as beta-blockers, renin-angiotensin system inhibitors, and mineralocorticoid receptor antagonists, was limited in the first year of diagnosis. The study highlighted the need for faster initiation of life-saving therapies and a more pragmatic approach, advocating for an NT-proBNP-based rule-in strategy to diagnose HF earlier, without waiting for echocardiography [
26].
4. Natriuretic Peptides for HF Risk Stratification and Management in Diabetes
People with diabetes face an increased risk of developing HF, a trend evident in numerous longitudinal observational studies. The heightened risk for HF in diabetes is categorized as stage A, denoting an increased risk without symptoms, structural heart disease, or biomarker evidence of myocardial strain. Those in stage B are asymptomatic but exhibit structural heart disease or functional cardiac abnormalities, making them particularly susceptible to progressing to symptomatic stages C and D [
27]. To mitigate the progression to symptomatic HF, early identification, risk stratification, and treatment of risk factors are paramount. Notably, in T2DM, measuring BNP or NT-pro BNP proves valuable for identifying individuals at risk and predicting HF development, symptom progression, and HF-related mortality. Several randomized controlled trials have underscored the benefits of more intensive risk factor treatment guided by NP levels. Major findings from some of these studies are summarized below:
The St Vincent's Screening to Prevent Heart Failure (STOP-HF) study involved 1374 participants at HF risk (including those with diabetes), randomized into usual care or BNP screening groups. Those with BNP ≥50 pg/mL received echocardiography and collaborative care. Primary outcome showed a lower prevalence of LV dysfunction in the intervention group (5.3% vs 8.7%) after a mean follow-up period of 4.2 years. Moreover, the incidence of cardiovascular hospitalizations was lower in the intervention group. BNP-based screening reduced the composite endpoint of incident asymptomatic LV dysfunction with or without newly diagnosed HF [
28].
The NT-proBNP Selected PreventiOn of cardiac eveNts in a population of diabetic patients without A history of cardiac disease (PONTIAC) study included 300 patients with T2DM and NT-proBNP > 125 pg/mL but without any history of cardiac disease. Participants were randomized into a control group (receiving usual care) and an intensified group, which received additional cardiac outpatient care for the up-titration of RAAS antagonists and beta-blockers and followed up for 2 years. Notably, intensified therapy led to a significant reduction in cardiac hospitalizations/deaths compared to control (hazard ratio: 0.351; p=0.044) [
29].
In the Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care (EXAMINE) trial, two NT-pro BNP measurements (6 months apart) in patients (n = 5,380) with T2DM effectively identified those at the highest risk of developing symptomatic HF. Patients with persistently high or increasing NT-pro BNP levels at 6 months had a significantly higher risk of CV death or HF compared to those with consistently low levels or initial high levels that declined [
30].
The Canagliflozin Cardiovascular Assessment Study (CANVAS) involved 4330 participants with T2DM and either CVD or other risk factors for cardiac events. Plasma NT-proBNP concentrations were measured at baseline, year 1, and year 6, and associations between NT-proBNP levels and various cardiovascular, renal, and mortality outcomes were investigated. NT-pro BNP levels ≥ 125 pg/mL predicted incident hospitalization for HF (hazard ratio 5.40; p < 0.001). Furthermore, it was suggested that elevated NT-pro BNP levels could predict a wide range of deleterious cardiovascular and renal outcomes in T2DM, HF death, and all-cause mortality [
31].
The Thousand and I study assessed the prognostic significance of elevated NT-pro BNP levels in patients (n = 960) with type 1 DM with preserved LVEF and without known heart disease. During a median follow-up of 6.3 years, 121 participants experienced major cardiovascular events (MACE) and 51 died. Higher NT-pro BNP levels were linked to poorer outcomes, with adjusted hazard ratios for MACE at 1.56 and 4.29 per Loge increase for NT-proBNP [
32].
Considering all these compelling evidence, the 2024 American Diabetes Association (ADA) practice guidelines recommend screening asymptomatic adults with diabetes using NPs, with a threshold for abnormal values set at BNP ≥50 pg/mL and NT-pro BNP ≥125 pg/mL [
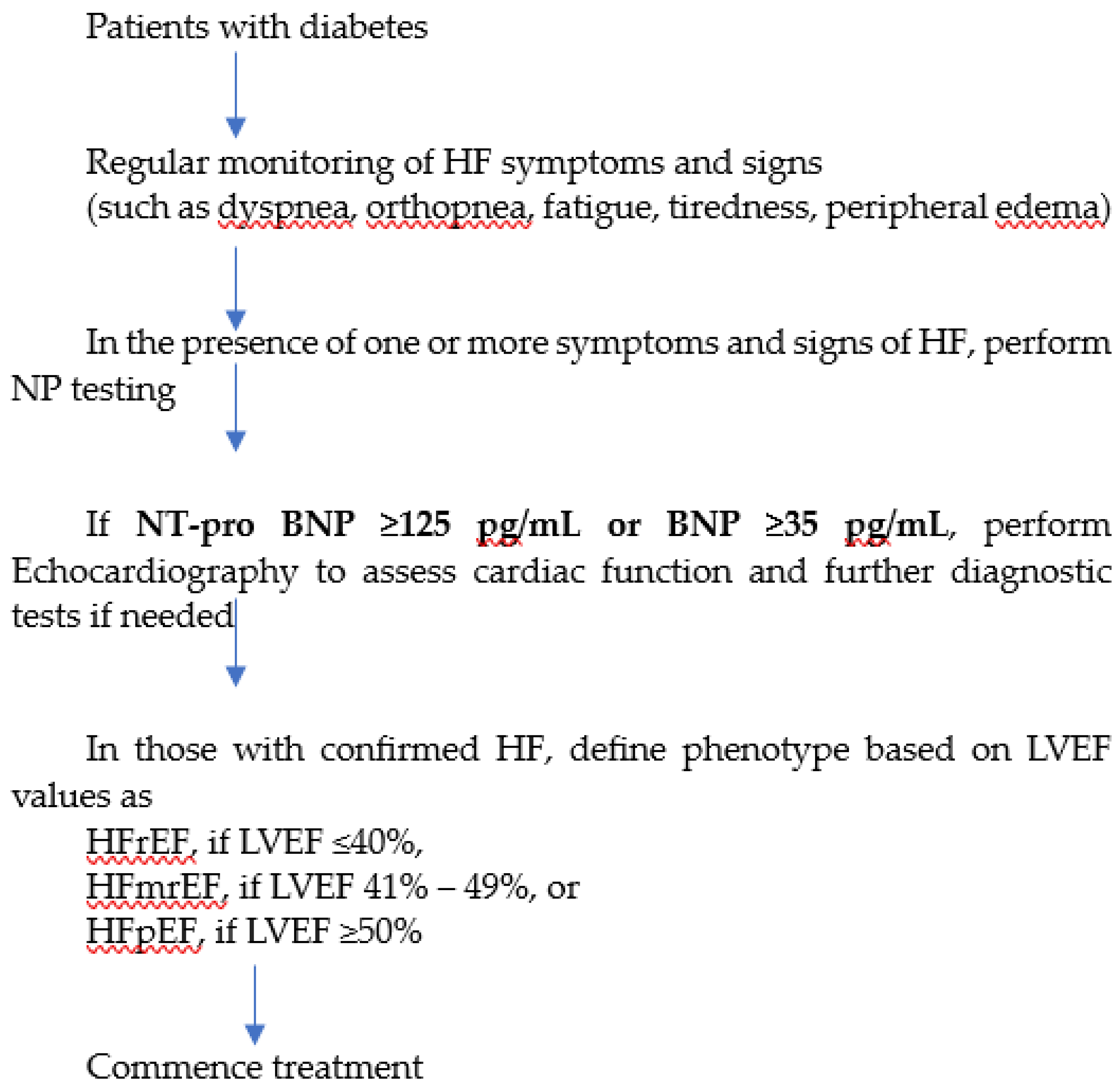
33]. Elevated natriuretic peptide levels, indicative of stage B HF, warrant further evaluation through echocardiography to screen for structural heart disease and diastolic dysfunction. A collaborative, interprofessional approach involving cardiovascular specialists is recommended at this stage to implement guideline-directed medical treatment strategies, potentially averting progression to symptomatic stages of HF. The 2023 European Society of Cardiology (ESC) guidelines recommend that patients with diabetes should be regularly monitored for symptoms and signs of HF (such as dyspnea, fatigue, peripheral edema), and in the presence of one or more symptoms, NPs should be measured to identify those with suspected HF [
34]. The threshold for abnormal values of BNP and NT-pro BNP are ≥35 pg/mL and ≥125 pg/mL, respectively. Accordingly, the diagnostic algorithm for HF in patients with diabetes can be summarized as shown in
Figure 1.
BNP, B-type natriuretic peptide; ECG, electrocardiogram; HF, heart failure; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
With advances in pharmacotherapy over the past decades, the importance of early identification of HF has become even more critical. Early diagnosis allows for the prompt initiation of effective therapies that can slow disease progression, reduce hospitalizations, and improve overall patient outcomes. As treatments evolve, identifying HF in its early stages ensures that patients can benefit fully from these therapeutic innovations [
35]. Numerous studies are currently underway to determine the most effective approach for identifying individuals at the highest risk of developing HF. A recent meta-analysis comprising 30 studies from clinical trials and cohorts (n=44889) aimed to assess the predictive utility of serum NT-proBNP levels in T2DM complications and reported a moderate level of evidence for predicting cardiovascular and all-cause mortalities at NT-proBNP levels of > 100 and >225 pg/mL, respectively [
36]. Two risk scores, namely the WATCH-DM (Weight, Age, Hypertension, Creatinine, High-Density Lipoprotein Cholesterol, Diabetes Control, ECG QRS Duration, Myocardial Infarction, and Coronary Artery Bypass Grafting) and TRS-HF
DM (Thrombolysis in Myocardial Infarction Risk Score for Heart Failure in Diabetes Mellitus), have been developed specifically to predict the risk of HF in individuals with type 2 diabetes [
37]. In a recent study by Patel, selective NT-proBNP testing guided by the WATCH-DM score demonstrated efficiency in identifying a primary prevention population with diabetes (n=6293) at high risk of developing HF [
38]. A two-step screening approach, integrating NT-pro BNP testing, was suggested as an effective method to identify high-risk patients who stand to benefit most from treatment with SGLT2is.
5. Analytical and Clinical Considerations in Natriuretic Peptide Testing
Although the measurement of NP levels is a crucial aspect of HF diagnosis, there are inherent challenges in their laboratory analysis and clinical interpretation that necessitate careful consideration. First, adherence to specific sample requirements and meticulous laboratory analysis procedures is paramount to ensure accurate results. In particular, BNP is prone to proteolytic degradation and is only stable in whole blood for approximately 4 hours at room temperature [
39]. Moreover, for BNP analysis, only ethylenediaminetetraacetic acid (EDTA)-plasma or EDTA-containing whole blood samples can be used. In this regard, NT-pro BNP offers an advantage as it is less vulnerable to degradation, it can be analyzed in serum or heparin plasma and is not significantly affected by moderate hemolysis or freeze-thaw cycles [
40].
While NPs are regarded as the "gold standard" biomarkers for estimating HF risk, it is essential to recognize that elevated levels of these peptides can occur in conditions other than HF. Conditions such as acute coronary syndrome, chronic kidney disease, pulmonary embolism, sepsis, and atrial fibrillation can lead to elevated levels of NPs. Furthermore, factors such as age, gender, and body mass index can also influence NP levels, complicating their interpretation (10). In addition, the utility of these peptides in HFpEF is subject to debate as their levels tend to be lower in HFpEF compared to HFrEF [
41]. However, current guidelines do not recommend separate NP cut-offs for HFpEF, and the prognosis is similar in patients for a given NP concentration, regardless of LVEF [
20].
The effects of medications on circulating levels of NPs have also been well-recognized. For instance, most drugs used in treating HF, such as SGLT2is, diuretics, RAAS blockers, and dopamine agonists, may lead to elevated NP levels. On the other hand, beta-blockers, digitalis, and aspirin may suppress the circulating levels of BNP and NT-pro BNP [
41,
42]. Neprilysin is an endopeptidase responsible for the lysis of NPs. Neprilysin inhibition (through the use of angiotensin receptor neprilysin inhibitors or ARNIs) may have variable effects on natriuretic peptide assays. Thus, caution is warranted when interpreting BNP results in patients on ARNIs [
43].
6. Future Prospects & Conclusions
B-type natriuretic peptides (BNP and NT-proBNP) have emerged as potential biomarkers in cardiovascular risk assessment and stratification of patients with diabetes. Besides, natriuretic peptide-based therapeutics represent another compelling area of research interest. ARNIs, such as sacubitril/valsartan, have shown efficacy in reducing cardiovascular events and mortality in patients with HF, including those with T2DM [
44]. In addition to their cardiovascular benefits, ARNIs may also impact glucose homeostasis in individuals with diabetes. While some studies have suggested potential improvements in glycemic control with ARNI therapy, others have reported conflicting findings or no significant effect on glucose metabolism [
45]. The mechanisms underlying the effects of ARNIs on glucose homeostasis are not fully understood and may involve complex interactions within the RAAS, NP system, and other pathways influencing insulin sensitivity and glucose metabolism.
While concerns exist that the recognition of HF risk through NP testing might trigger further tests, potentially increasing costs and management complexity, the high negative predictive value of NPs mitigates this by effectively ruling out individuals at minimal risk, thus reducing unnecessary interventions [
2,
46]. Furthermore, early detection of HF risk prompts timely intervention, potentially improving outcomes. Walter demonstrated the cost-effectiveness of NP-based screening in patients with diabetes, underscoring its importance in bridging the diagnostic and treatment gap in HF care, especially when intervention is most impactful [
47]. In addition, a recent study reported the association between plasma microRNAs (miRNAs) and cardiac biomarkers (such as NPs), along with their correlation with cardiac structure, function, and clinical outcomes in patients with symptomatic HF. The study found significant associations between miRNAs and biomarkers with ventricular size and function [
48]. These findings indicate that future research may focus on integrating information from miRNAs with NPs to enhance prognostic assessment in diabetic cardiomyopathy beyond conventional methods.
Overall, NP-guided therapy represents a promising approach for managing cardiovascular risk, especially heart failure, in individuals with diabetes. However, further research is needed to elucidate the precise mechanisms of action and optimize the use of therapies in diabetic populations. Additionally, close monitoring of glycemic control and collaboration between healthcare providers specializing in cardiology and endocrinology is essential to ensure comprehensive management of cardiovascular and metabolic health in patients with diabetes.
Author Contributions
Conceptualization, D.T. and T.C.A.; formal analysis, D.T.; investigation, D.T.; resources, D.T. and T.C.A.; data curation, D.T.; writing—original draft preparation, D.T.; writing—review and editing, T.C.A.; visualization, D.T. and T.C.A.; All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ARNI |
angiotensin receptor neprilysin inhibitor |
| BNP |
B-type natriuretic peptide |
| NT-pro BNP |
N-terminal pro-B-typ-e natriuretic peptide |
| RAAS |
Renin-angiotensin-aldosterone system |
| SGLT2i |
sodium-glucose cotransporter 2 inhibitor |
| T2DM |
Type 2 diabetes mellitus |
References
- GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2023, 402, 203–234. [Google Scholar] [CrossRef] [PubMed]
- Pop-Busui, R.; Januzzi, J.L.; Bruemmer, D.; Butalia, S.; Green, J.B.; Hortonx, W.B.; et al. Heart Failure: An Underappreciated Complication of Diabetes. A Consensus Report of the American Diabetes Association. Diabetes Care. 2022, 45, 1670–1690. [Google Scholar] [CrossRef] [PubMed]
- Palazzuoli, A.; Iacoviello, M. Diabetes leading to heart failure and heart failure leading to diabetes: epidemiological and clinical evidence. Heart Fail Rev. 2023, 28, 585–596. [Google Scholar] [CrossRef]
- Kannel, W.B.; McGee, D.L. Diabetes and cardiovascular disease. The Framingham study. JAMA, 1979, 241, 2035–2038. [Google Scholar] [CrossRef]
- McAllister, D.A.; Read, S.H.; Kerssens, J.; Livingstone, S.; McGurnaghan, S.; Jhund, P.; Petrie, J.; Sattar, N.; Fischbacher, C.; Kristensen, S.L.; et al. Incidence of hospitalization for heart failure and case-fatality among 3.25 million people with and without diabetes mellitus. Circulation. 2018, 138, 2774–2786. [Google Scholar] [CrossRef]
- Dunlay, S.M.; Givertz, M.M.; Aguilar, D.; Allen, L.A.; Chan, M.; Desai, A.S.; Deswal, A.; Dickson, V.V.; Kosiborod, M.N.; Lekavich, C.L.; et al. American Heart Association Heart Failure and Transplantation Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; and the Heart Failure Society of America. Type 2 Diabetes Mellitus and Heart Failure: A Scientific Statement From the American Heart Association and the Heart Failure Society of America: This statement does not represent an update of the 2017 ACC/AHA/HFSA heart failure guideline update. Circulation. 2019, 140, e294–e324. [Google Scholar]
- Verma, S.; Pandey, A.; Bhatt, D. Forecasting Heart Failure Risk in Diabetes. J Am Coll Cardiol, 2022, 79, 2294–2297. [Google Scholar] [CrossRef]
- Cannistraci, R.; Mazzetti, S.; Mortara, A.; Perseghin, G.; Ciardullo, S. Risk stratification tools for heart failure in the diabetes clinic. Nutr Metab Cardiovasc Dis, 2020, 30, 1070–1079. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022, 45, S144–S174. [Google Scholar] [CrossRef]
- Bozkurt, B.; Coats, A.J.S.; Tsutsui, H.; Abdelhamid, C.M.; Adamopoulos, S.; Albert, N.; Anker, S.D.; Atherton, J.; Böhm, M.; Butler, J.; et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur J Heart Fail. 2021, 23, 352–380. [Google Scholar]
- Emdin, M.; Vittorini, S.; Passino, C.; Clerico, A. Old and new biomarkers of heart failure. Eur J Heart Fail. 2009, 11, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Schwinger, R.H.G. Pathophysiology of heart failure. Cardiovasc Diagn Ther. 2021, 11, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Elendu, C.; Amaechi, D.C.; Elendu, T.C.; Ashna, M.; Ross-Comptis, J.; Ansong, S.O.; Egbunu, E.O.; Okafor, G.C.; Jingwa, K.A.; Akintunde, A.A.; et al. Heart failure and diabetes: Understanding the bidirectional relationship. Medicine (Baltimore). 2023, 102, e34906. [Google Scholar] [CrossRef] [PubMed]
- Panchal, K.; Lawson, C.; Chandramouli, C.; Lam, C.; Khunti, K.; Zaccardi, F. Diabetes and risk of heart failure in people with and without cardiovascular disease: systematic review and meta-analysis. Diabetes Res Clin Pract. 2023, 207, 111054. [Google Scholar] [CrossRef] [PubMed]
- Sobczak, A.I.S.; Stewart, A.J. Coagulatory Defects in Type-1 and Type-2 Diabetes. Int J Mol Sci, 2019, 20, 6345. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Nishikimi, T.; Kuwahara, K. Atrial and brain natriuretic peptides: Hormones secreted from the heart. Peptides. 2019, 111, 18–25. [Google Scholar] [CrossRef]
- de Bold, A.J.; Borenstein, H.B.; Veress, A.T.; Sonnenberg, H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 1981, 28, 89–94. [Google Scholar] [CrossRef]
- Pandey, K.N. Emerging Roles of Natriuretic Peptides and their Receptors in Pathophysiology of Hypertension and Cardiovascular Regulation. J Am Soc Hypertens. 2008, 2, 210–226. [Google Scholar] [CrossRef]
- Bhatia, V.; Nayyar, P.; Dhindsa, S. Brain natriuretic peptide in diagnosis and treatment of heart failure. J Postgrad Med. 2003, 49, 182–185. [Google Scholar]
- Tanase, D.M.; Radu, S.; Al Shurbaji, S.; Baroi, G.L.; Florida Costea, C.; Turliuc, M.D.; Ouatu, A.; Floria, M. Natriuretic Peptides in Heart Failure with Preserved Left Ventricular Ejection Fraction: From Molecular Evidences to Clinical Implications. Int J Mol Sci. 2019, 20, 2629. [Google Scholar] [CrossRef]
- Cao, Z.; Jia, Y.; Zhu, B. BNP and NT-proBNP as Diagnostic Biomarkers for Cardiac Dysfunction in Both Clinical and Forensic Medicine. Int J Mol Sci. 2019, 20, 1820. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022, 145, e895–e1032. [Google Scholar] [CrossRef] [PubMed]
- Sim, D.; Lin, W. , Sindone, A., Yingchoncharoen, T., Prameswari, H.S., Mohd Ghazi, A., Pin, L.C.; Louis, T.; Aw, T.C.; Michael-Joseph, A.; et al. Asian Pacific Society of Cardiology Consensus Statements on the Diagnosis and Management of Chronic Heart Failure. Journal of Asian Pacific Society of Cardiology, 2023, 2, e10. [Google Scholar] [CrossRef]
- Januzzi, J.L. Jr.; Zannad, F.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Ferreira, J.P.; Sattar, N.; Verma, S.; Vedin, O.; et al. Prognostic Importance of NT-proBNP and Effect of Empagliflozin in the EMPEROR-Reduced Trial. J Am Coll Cardiol. 2021, 78, 1321–1332. [Google Scholar] [CrossRef]
- Mebazaa, A.; Davison, B.; Chioncel, O.; Cohen-Solal, A.; Diaz, R.; Filippatos, G.; Metra, M.; Ponikowski, P.; Sliwa, K.; Voors, A.A.; et al. Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): a multinational, open-label, randomised, trial. Lancet. 2022, 400, 1938–1952. [Google Scholar] [CrossRef]
- Reza, N.; Pellicori, P.; Starling, R.C. REVOLUTION in Heart Failure Care and SELECT Highlights From the European Society of Cardiology-Heart Failure Association Heart Failure & World Congress on Acute Heart Failure 2024. J Card Fail. 2024, Jun 3:S1071-9164(24)00192-1. Epub ahead of print. [CrossRef]
- Oktay, A.A., Paul, T.K., Koch, C.A.; et al. Diabetes, Cardiomyopathy, and Heart Failure. [Updated 2023 Sep 26]. In: Feingold, K.R.; Anawalt, B.; Blackman, M.R.; et al., editors. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560257/ (accessed on 5 February 2024).
- Ledwidge, M.; Gallagher, J.; Conlon, C.; Tallon, E.; O'Connell, E.; Dawkins, I.; Watson, C.; O'Hanlon, R.; Bermingham, M.; Patle, A.; et al. Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA. 2013, 310, 66–74. [Google Scholar] [CrossRef]
- Huelsmann, M.; Neuhold, S.; Resl, M.; Strunk, G.; Brath, H.; Francesconi, C.; Adlbrecht, C.; Prager, R.; Luger, A.; Pacher, R.; et al. PONTIAC (NT-proBNP selected prevention of cardiac events in a population of diabetic patients without a history of cardiac disease): a prospective randomized controlled trial. J Am Coll Cardiol. 2013, 62, 1365–1372. [Google Scholar] [CrossRef]
- Jarolim, P.; White, W.B.; Cannon, C.P.; Gao, Q.; Morrow, D.A. Serial Measurement of Natriuretic Peptides and Cardiovascular Outcomes in Patients With Type 2 Diabetes in the EXAMINE Trial. Diabetes Care. 2018, 41, 1510–1515. [Google Scholar] [CrossRef]
- Januzzi, J.L. Jr.; Xu, J.; Li. J.; Shaw, W.; Oh, R.; Pfeifer, M.; Butler, J.; Sattar, N.; Mahaffey, K.W.; Neal, B.; et al. Effects of Canagliflozin on Amino-Terminal Pro-B-Type Natriuretic Peptide: Implications for Cardiovascular Risk Reduction. J Am Coll Cardiol. 2020, 76, 2076–2085.
- Rørth, R.; Jørgensen, P.G.; Andersen, H.U.; Christoffersen, C.; Gøtze, J.P.; Køber, L.; Rossing, P.; Jensen, M.T. Cardiovascular prognostic value of echocardiography and N terminal pro B-type natriuretic peptide in type 1 diabetes: the Thousand & 1 Study. Eur J Endocrinol. 2020, 182, 481–488. [Google Scholar]
- American Diabetes Association Professional Practice Committee. 10. Cardiovascular Disease and Risk Management: Standards of Care in Diabetes-2024. Diabetes Care. 2024, 47, S179–S218. [Google Scholar] [CrossRef]
- Marx, N.; Federici, M.; Schütt, K.; Müller-Wieland, D.; Ajjan, R.A.; Antunes, M.J.; Christodorescu, R.M.; Crawford, C.; Di Angelantonio, E.; Eliasson, B. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023, 44, 4043–4140. [Google Scholar] [PubMed]
- Beavers, C.J. Heart Failure Pharmacotherapy Over the Past 30 Years: Boats Against the Current. J Card Fail. 2024, 30, 1044–1046. [Google Scholar] [CrossRef] [PubMed]
- Ramzi, Z.S. (2023). N-Terminal Prohormone Brain Natriuretic Peptide as a Prognostic Biomarker for the Risk of Complications in Type 2 Diabetes: A Systematic Review and Meta-Analysis. Lab Med. 2023, 54, 339–351. [Google Scholar] [CrossRef]
- Segar, M.W.; Patel, K.V.; Hellkamp, A.S.; Vaduganathan, M.; Lokhnygina Y, Green JB, Wan SH, Kolkailah AA, Holman RR, Peterson ED.; et al. Validation of the WATCH-DM and TRS-HFDM Risk Scores to Predict the Risk of Incident Hospitalization for Heart Failure Among Adults With Type 2 Diabetes: A Multicohort Analysis. J Am Heart Assoc. 2022, 11, e024094.
- Patel, K.V.; Segar, M.W.; Klonoff, D.C.; Khan, M.S.; Usman, M.S.; Lam, C.S.P.; Verma, S.; DeFilippis, A.P.; Nasir, K.; Bakker, S.J.L.; et al. Optimal Screening for Predicting and Preventing the Risk of Heart Failure Among Adults With Diabetes Without Atherosclerotic Cardiovascular Disease: A Pooled Cohort Analysis. Circulation. 2024, 149, 293–304. [Google Scholar] [CrossRef]
- García de Guadiana-Romualdo, L.; Ramos-Arenas, V.; Campos-Rodríguez, V.; Consuegra-Sánchez, L.; Albaladejo-Otón, M.D. In vitro stability of B-type natriuretic peptide (BNP) in plasma stored under different conditions when measured with the Lumipulse® assay. Scand J Clin Lab Invest. 2019, 79, 455–458. [Google Scholar] [CrossRef]
- Revuelta-López, E.; Barallat, J.; Cserkóová, A.; Gálvez-Montón, C.; Jaffe, A.S.; Januzzi, J.L.; Bayes-Genis, A. Pre-analytical considerations in biomarker research: focus on cardiovascular disease. Clin Chem Lab Med. 2021, 59, 1747–1760. [Google Scholar] [CrossRef]
- Lee, D.J.W.; Aw, T.C. Natriuretic Peptides in Clinical Practice: A Current Review. J Immunol Sci. 2023, 7, 28–34. [Google Scholar] [CrossRef]
- Troughton, R.W.; Richards, A.M.; Yandle, T.G.; Frampton, C.M.; Nicholls, M.G. The effects of medications on circulating levels of cardiac natriuretic peptides. Ann Med. 2007, 39, 242–260. [Google Scholar] [CrossRef]
- Ibrahim, N.; McCarthy, C.P.; Shrestha, S.; Gaggin, H.K.; Mukai, R.; Szymonifka, J.; Apple, F.S.; Burnett, J.C. Jr.; Iyer, S.; Januzzi, J.L. Jr. Effect of Neprilysin Inhibition on Various Natriuretic Peptide Assays. J Am Coll Cardiol. 2019, 73, 1273–1284. [Google Scholar] [CrossRef]
- Pascual-Figal, D.; Bayés-Genis, A.; Beltrán-Troncoso, P.; Caravaca-Pérez, P.; Conde-Martel, A.; Crespo-Leiro, M.G.; Delgado, J.F.; Díez, J.; Formiga, F.; Manito, N. Sacubitril-Valsartan, Clinical Benefits and Related Mechanisms of Action in Heart Failure With Reduced Ejection Fraction. A Review. Front Cardiovasc Med. 2021, 11, 754499. [Google Scholar] [CrossRef]
- Tsai, M.L.; Lin, Y.; Lin, M.S.; Tsai, T.H.; Yang, N.I.; Wang, C.Y.; Hsieh, I.C.; Hung, M.J.; Chen, T.H. Comparing angiotensin receptor-neprilysin inhibitors with sodium-glucose cotransporter 2 inhibitors for heart failure with diabetes mellitus. Diabetol Metab Syndr. 2023, 15, 110. [Google Scholar] [CrossRef] [PubMed]
- Huelsmann, M.; Neuhold, S.; Strunk, G.; Moertl, D.; Berger, R.; Prager, R.; Abrahamian, H.; Riedl, M.; Pacher, R.; Luger, A.; et al. NT-proBNP has a high negative predictive value to rule-out short-term cardiovascular events in patients with diabetes mellitus. Eur Heart J. 2008, 29, 2259–2264. [Google Scholar] [CrossRef] [PubMed]
- Walter, E.; Arrigo, M.; Allerstorfer, S.; Marty, P.; Hülsmann, M. Cost-effectiveness of NT-proBNP-supported screening of chronic heart failure in patients with or without type 2 diabetes in Austria and Switzerland. J Med Econ. 2023, 26, 1287–1300. [Google Scholar] [CrossRef] [PubMed]
- Spahillari, A.; Jackson, L.; Varrias, D.; Michelhaugh, S.A.; Januzzi, J.L.; Shahideh, B.; Daghfal, D.; Valkov, N.; Murtagh, G.; Das, S. MicroRNAs are associated with cardiac biomarkers, cardiac structure and function and incident outcomes in heart failure. ESC Heart Fail. 2024, 11, 1400–1410. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).