Submitted:
04 September 2024
Posted:
05 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material & Methods
2.1. Sample Collection
2.2. Cell Culture
2.3. Measurement of Circulating Free and Lipid Vesicle-Associated Hsp70 Levels Using the Hsp70-Exo ELISA [35]
2.4. Isolation of Circulating Cells with cmHsp70.1 and EpCAM Antibody-Coupled S-pluriBeads
2.5. Immunohistochemical Staining
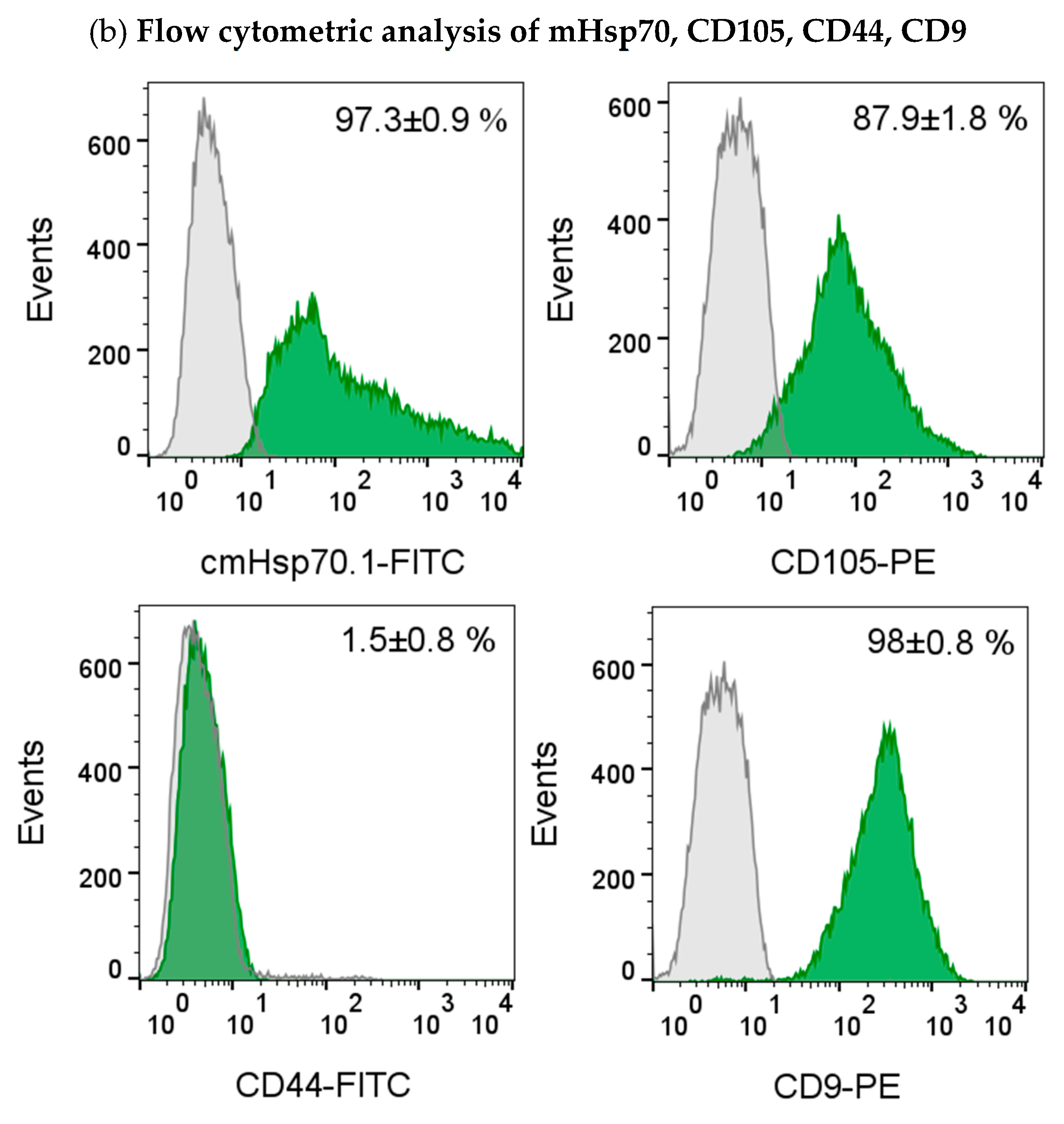
2.6. Flow Cytometry
2.7. RNA Preparation [36,37] and Analysis [38]
2.8. Statistical Evaluations
3. Results
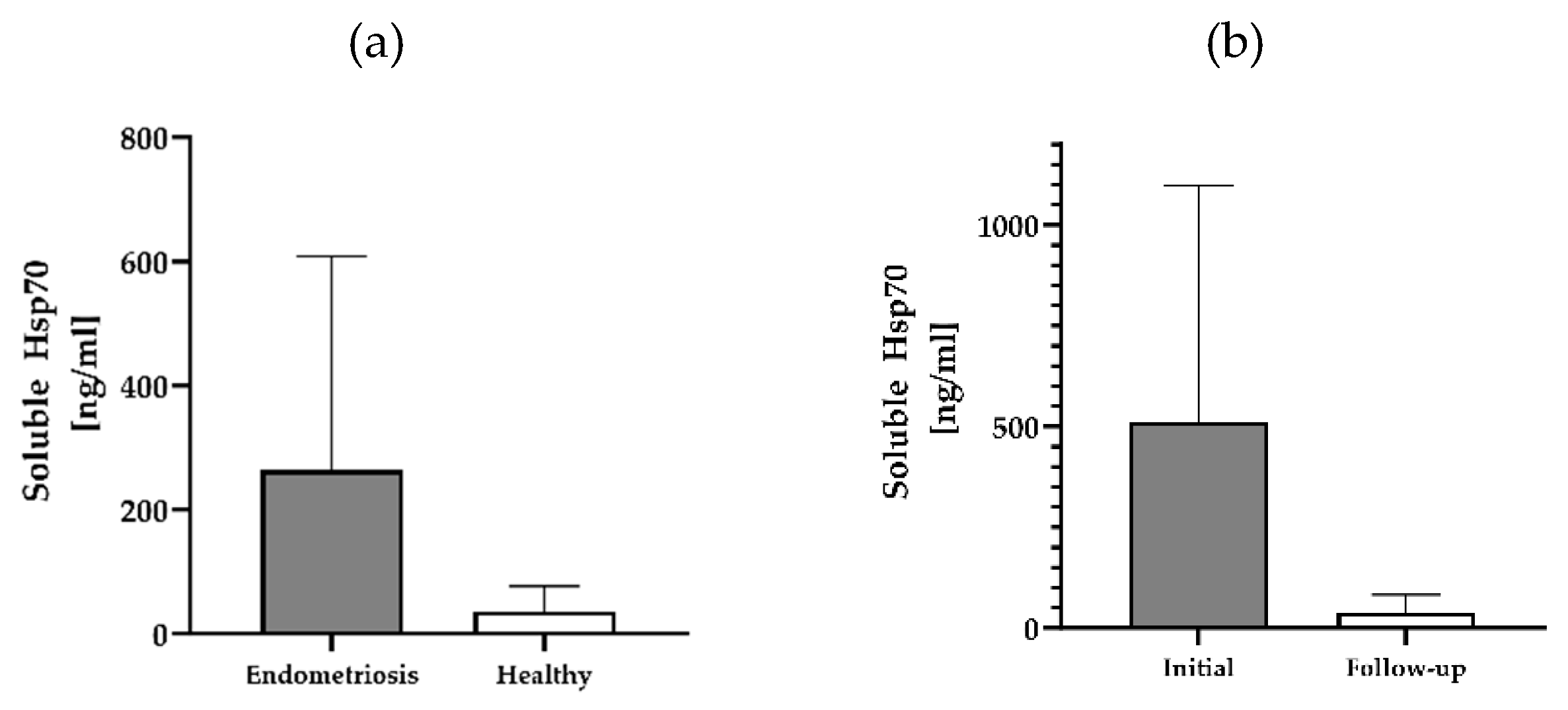
3.1. Circulating Free and Vesicle-Associated Hsp70 Levels in Patients with Endometriosis, Pre- and Post-Surgery and Healthy Controls
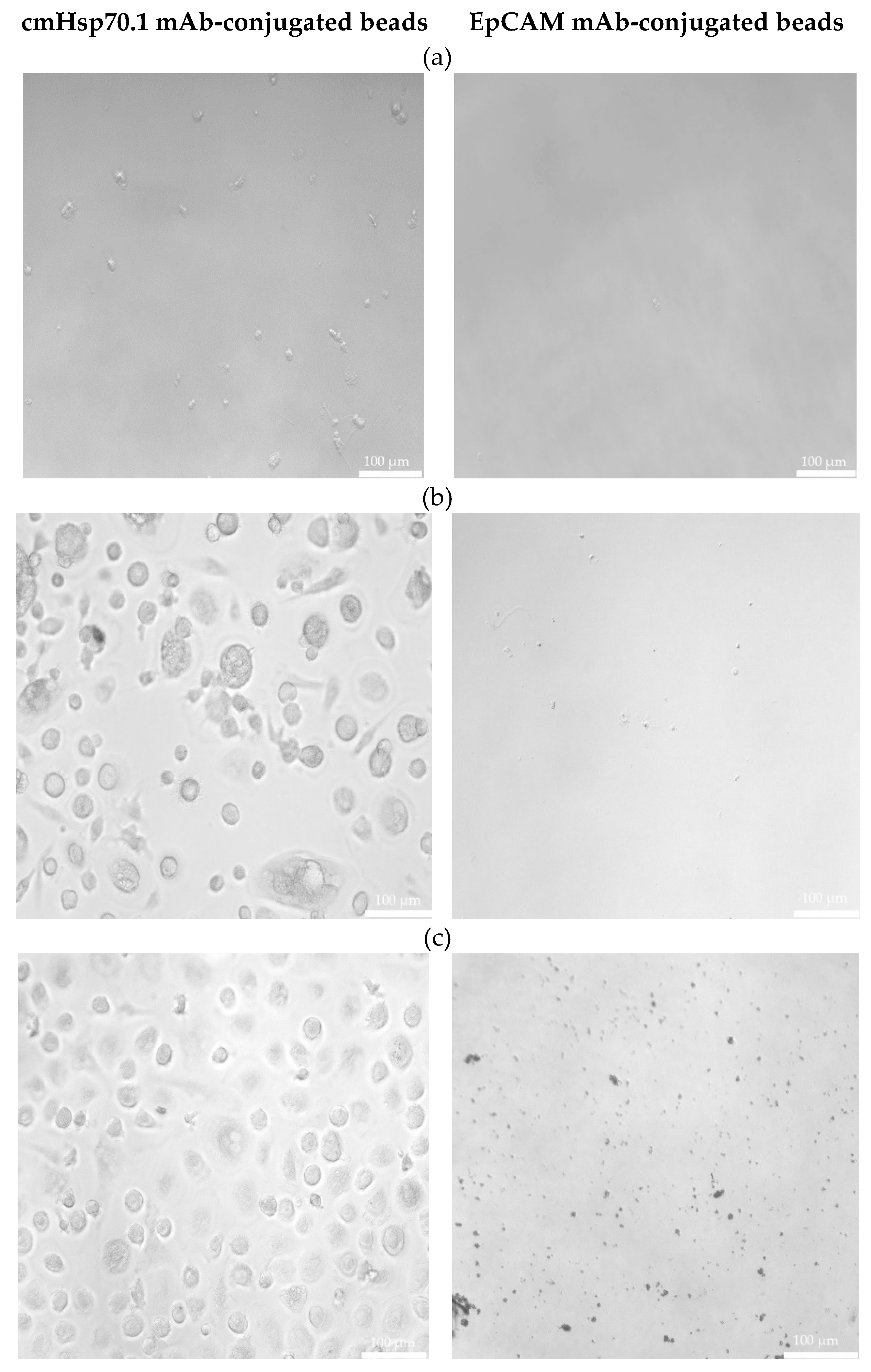
3.2. Circulating Endometriotic Cells (CECs) Can Be Isolated from the Blood of Patients with Confirmed Endometriosis by Bead-Based Approaches Targeting Membrane Hsp70 and EpCAM
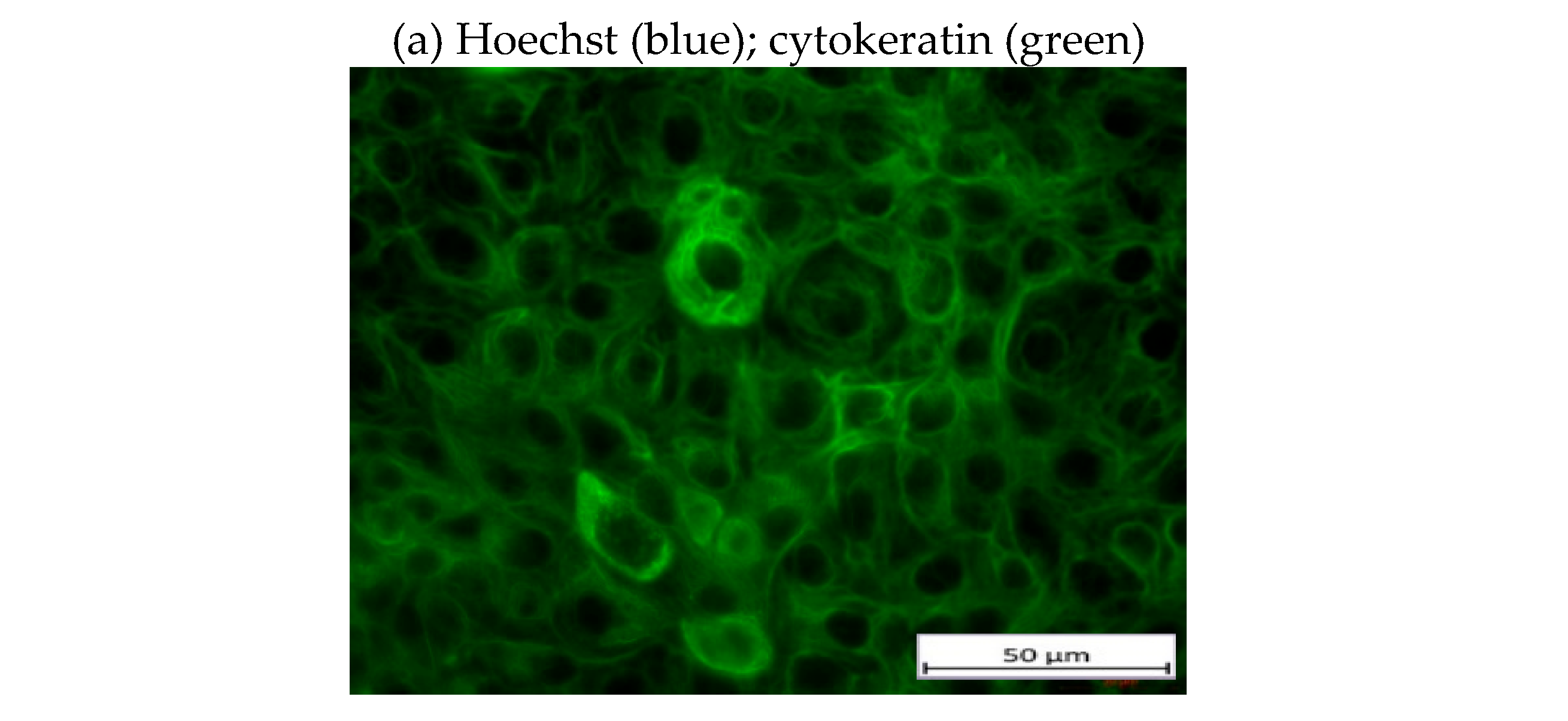
3.3. Morphological Characterization of Cultured CECs from Patients with Endometriosis without and with Extra-Uterine Involvement
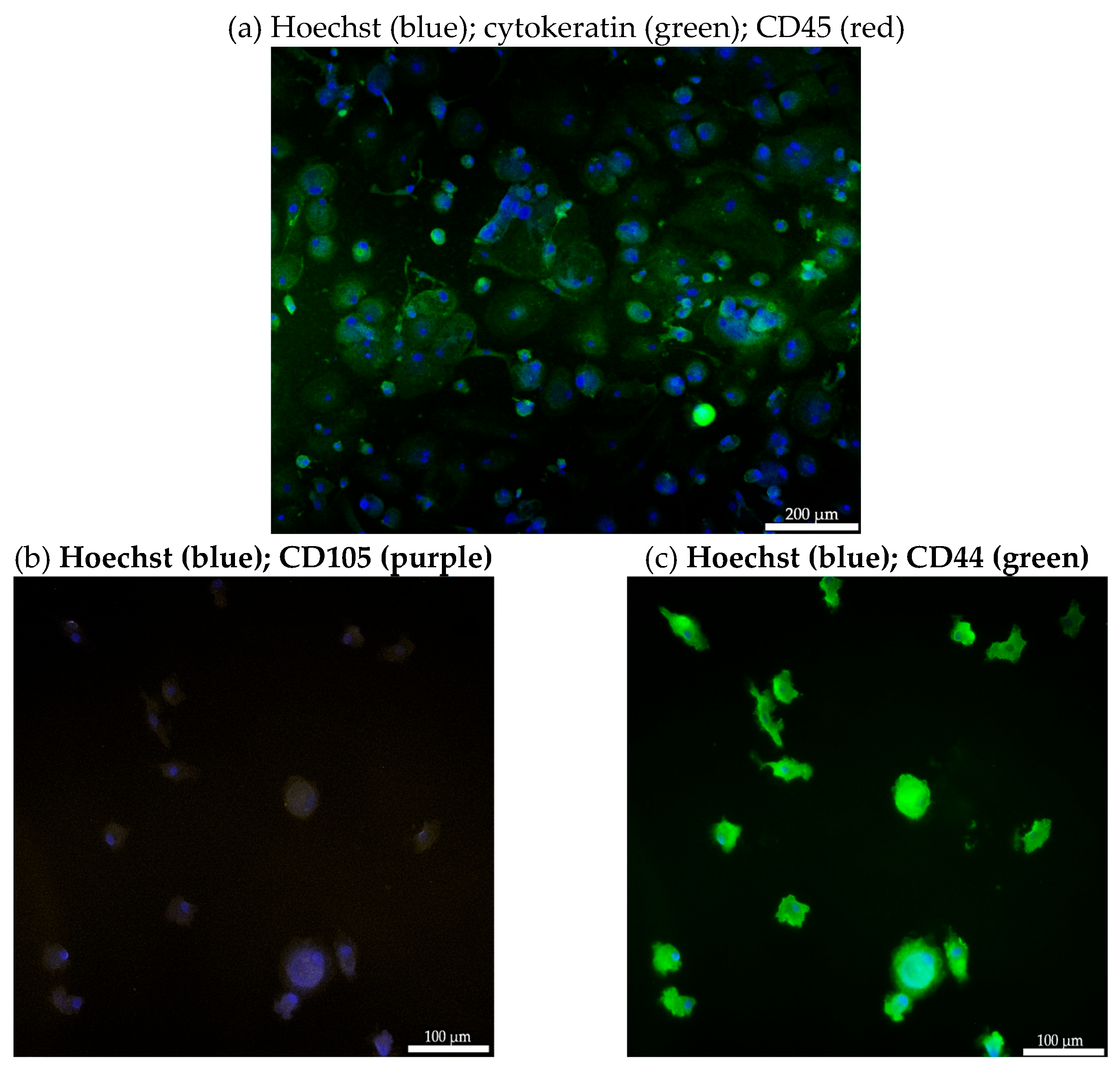
3.4. Immunofluorescent Characterization of Isolated CEC from a Patient with Bladder Endometriosis
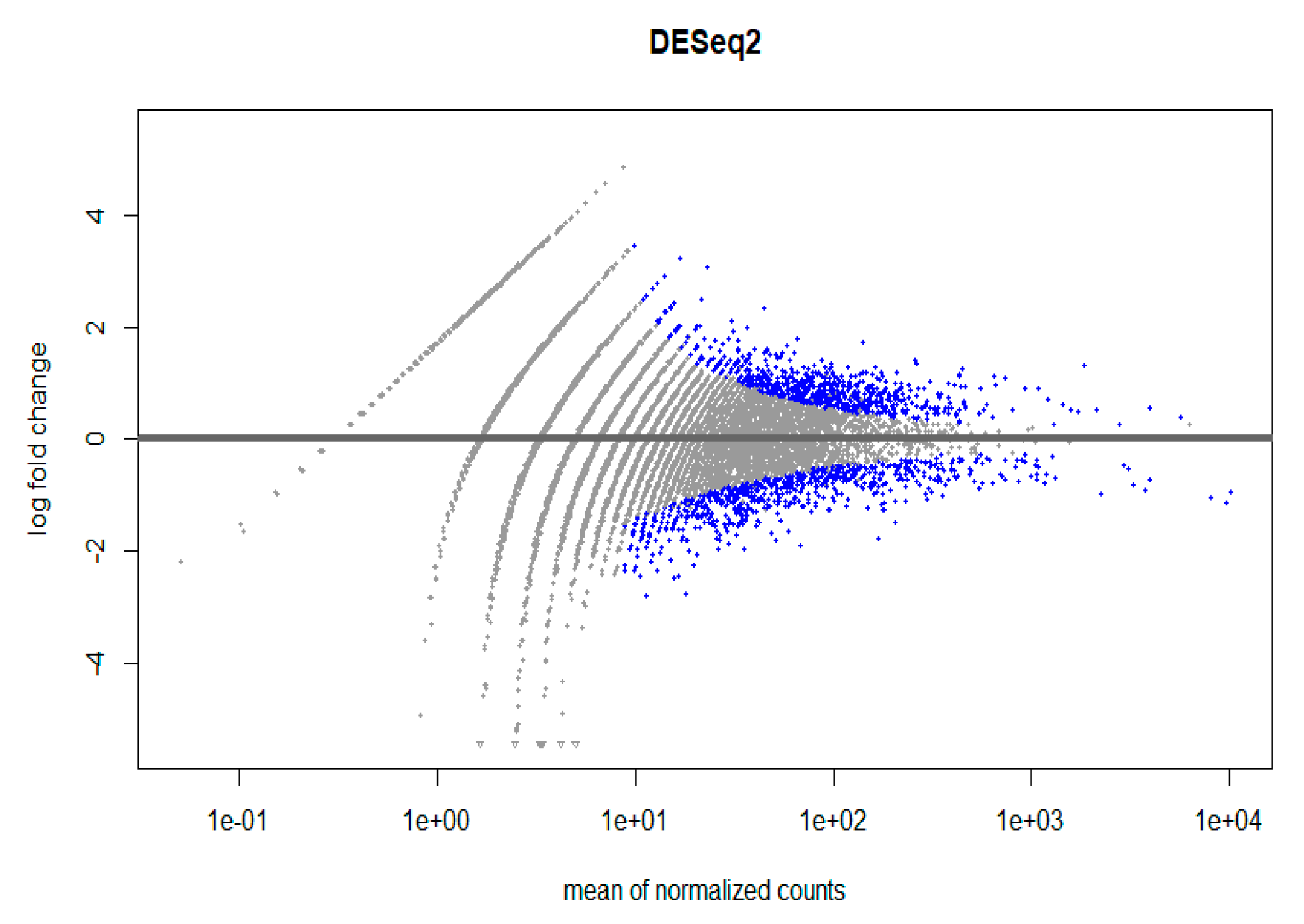
3.5. RNA Analysis of Isolated CECs from a Patient with Lymph Node Endometriosis
4. Discussion
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tennfjord, M.K.; Gabrielsen, R.; Tellum, T. Effect of physical activity and exercise on endometriosis-associated symptoms: A systematic review. BMC Womens Health. 2021, 21, 355. [Google Scholar] [CrossRef]
- Bulun, S.E.; Yilmaz, B.D.; Sison, C.; Miyazaki, K.; Bernardi, L.; Liu, S.; Kohlmeier, A.; Yin, P.; Milad, M.; Wei, J. Endometriosis. Endocr Rev. 2019, 40, 1048–1079. [Google Scholar] [CrossRef] [PubMed]
- Mehedintu, C.; Plotogea, M.N.; Ionescu, S.; Antonovici, M. Endometriosis still a challenge. J Med Life. 2014, 7, 349–357. [Google Scholar] [PubMed]
- Mounsey, A.L.; Wilgus, A.; Slawson, D.C. Diagnosis and management of endometriosis. Am Fam Physician. 2006, 74, 594–600. [Google Scholar] [PubMed]
- Chan, R.W.; Schwab, K.E.; Gargett, C.E. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod. 2004, 70, 1738–1750. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Yang, F.; Wang, H.; Liang, S.; Wang, H.; Yang, J.; Lin, J. The role of endometrial stem cells in the pathogenesis of endometriosis and their application to its early diagnosisdagger. Biol Reprod. 2020, 102, 1153–1159. [Google Scholar] [CrossRef]
- Sampson, J.A. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am J Pathol. 1927, 3, 93-110 143.
- Saibil, H. Chaperone machines for protein folding, unfolding and disaggregation. Nat Rev Mol Cell Biol. 2013, 14, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Radons, J.; Multhoff, G. Immunostimulatory functions of membrane-bound and exported heat shock protein 70. Exerc.Immunol.Rev. 2005, 11, 17–33. [Google Scholar]
- Albakova, Z.; Armeev, G.A.; Kanevskiy, L.M.; Kovalenko, E.I.; Sapozhnikov, A.M. Hsp70 multi-functionality in cancer. Cells. 2020, 9. [Google Scholar] [CrossRef]
- Vostakolaei, M.A.; Hatami-Baroogh, L.; Babaei, G.; Molavi, O.; Kordi, S.; Abdolalizadeh, J. Hsp70 in cancer: A double agent in the battle between survival and death. J Cell Physiol. 2021, 236, 3420–3444. [Google Scholar] [CrossRef]
- Vega, V.L.; Rodriguez-Silva, M.; Frey, T.; Gehrmann, M.; Diaz, J.C.; Steinem, C.; Multhoff, G.; Arispe, N.; De Maio, A. Hsp70 translocates into the plasma membrane after stress and is released into the extracellular environment in a membrane-associated form that activates macrophages. J.Immunol. 2008, 180, 4299–4307. [Google Scholar] [CrossRef]
- Gehrmann, M.; Liebisch, G.; Schmitz, G.; Anderson, R.; Steinem, C.; De, M.A.; Pockley, G.; Multhoff, G. Tumor-specific Hsp70 plasma membrane localization is enabled by the glycosphingolipid Gb3. PLoS.ONE. 2008, 3, e1925. [Google Scholar] [CrossRef] [PubMed]
- Multhoff, G.; Botzler, C.; Wiesnet, M.; Muller, E.; Meier, T.; Wilmanns, W.; Issels, R.D. A stress-inducible 72-kda heat-shock protein (hsp72) is expressed on the surface of human tumor cells, but not on normal cells. Int.J.Cancer. 1995, 61, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Multhoff, G.; Botzler, C.; Jennen, L.; Schmidt, J.; Ellwart, J.; Issels, R. Heat shock protein 72 on tumor cells: A recognition structure for natural killer cells. J.Immunol. 1997, 158, 4341–4350. [Google Scholar] [CrossRef] [PubMed]
- Gastpar, R.; Gehrmann, M.; Bausero, M.A.; Asea, A.; Gross, C.; Schroeder, J.A.; Multhoff, G. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res. 2005, 65, 5238–5247. [Google Scholar] [CrossRef] [PubMed]
- Seier, S.; Bashiri Dezfouli, A.; Lennartz, P.; Pockley, A.G.; Klein, H.; Multhoff, G. Elevated levels of circulating Hsp70 and an increased prevalence of CD94+/CD69+ NK cells is predictive for advanced stage non-small cell lung cancer. Cancers (Basel). 2022, 14. [Google Scholar] [CrossRef]
- Safi, S.; Messner, L.; Kliebisch, M.; Eggert, L.; Ceylangil, C.; Lennartz, P.; Jefferies, B.; Klein, H.; Schirren, M.; Dommasch, M.; et al. Circulating Hsp70 levels and the immunophenotype of peripheral blood lymphocytes as potential biomarkers for advanced lung cancer and therapy failure after surgery. Biomolecules. 2023, 13. [Google Scholar] [CrossRef]
- Lobinger, D.; Gempt, J.; Sievert, W.; Barz, M.; Schmitt, S.; Nguyen, H.T.; Stangl, S.; Werner, C.; Wang, F.; Wu, Z.; et al. Potential role of Hsp70 and activated NK cells for prediction of prognosis in glioblastoma patients. Front Mol Biosci. 2021, 8, 669366. [Google Scholar] [CrossRef]
- Rothammer, A.; Sage, E.K.; Werner, C.; Combs, S.E.; Multhoff, G. Increased heat shock protein 70 (Hsp70) serum levels and low NK cell counts after radiotherapy - potential markers for predicting breast cancer recurrence? Radiat Oncol. 2019, 14, 78. [Google Scholar] [CrossRef]
- Xanthopoulos, A.; Samt, A.K.; Guder, C.; Taylor, N.; Roberts, E.; Herf, H.; Messner, V.; Trill, A.; Holzmann, K.L.K.; Kiechle, M.; et al. Hsp70-a universal biomarker for predicting therapeutic failure in human female cancers and a target for CTC isolation in advanced cancers. Biomedicines. 2023, 11. [Google Scholar] [CrossRef]
- Scully, O.J.; Bay, B.H.; Yip, G.; Yu, Y. Breast cancer metastasis. Cancer Genomics Proteomics. 2012, 9, 311–320. [Google Scholar] [PubMed]
- Allard, W.J.; Matera, J.; Miller, M.C.; Repollet, M.; Connelly, M.C.; Rao, C.; Tibbe, A.G.; Uhr, J.W.; Terstappen, L.W. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004, 10, 6897–6904. [Google Scholar] [CrossRef] [PubMed]
- Chaffer, C.L.; Weinberg, R.A. A perspective on cancer cell metastasis. Science. 2011, 331, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002, 2, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Alix-Panabieres, C.; Pantel, K. Circulating tumor cells: Liquid biopsy of cancer. Clin Chem. 2013, 59, 110–118. [Google Scholar] [CrossRef]
- Cristofanilli, M. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. Semin Oncol. 2006, 33, S9–14. [Google Scholar] [CrossRef]
- Nieto, M.A.; Huang, R.Y.; Jackson, R.A.; Thiery, J.P. Emt: 2016. Cell. 2016, 166, 21–45. [Google Scholar] [CrossRef]
- Cano, A.; Perez-Moreno, M.A.; Rodrigo, I.; Locascio, A.; Blanco, M.J.; Del Barrio, M.G.; Portillo, F.; Nieto, M.A. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000, 2, 76–83. [Google Scholar] [CrossRef]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef]
- He, P.; Dai, Q.; Wu, X. New insight in urological cancer therapy: From epithelial-mesenchymal transition (EMT) to application of nano-biomaterials. Environ Res. 2023, 229, 115672. [Google Scholar] [CrossRef]
- Breuninger, S.; Stangl, S.; Werner, C.; Sievert, W.; Lobinger, D.; Foulds, G.A.; Wagner, S.; Pickhard, A.; Piontek, G.; Kokowski, K.; et al. Membrane Hsp70-a novel target for the isolation of circulating tumor cells after epithelial-to-mesenchymal transition. Front Oncol. 2018, 8, 497. [Google Scholar] [CrossRef]
- Annemiek, W.N. Pathogenesis of endometriosis. Best Practice & Research Clinical Obstetrics & Gynaecology. 2004, 18, 233-244.
- Sotnikova, N.Y.; Antsiferova, Y.S.; Posiseeva, L.V.; Shishkov, D.N.; Posiseev, D.V.; Filippova, E.S. Mechanisms regulating invasiveness and growth of endometriosis lesions in rat experimental model and in humans. Fertil Steril. 2010, 93, 2701–2705. [Google Scholar] [CrossRef]
- Werner, C.; Stangl, S.; Salvermoser, L.; Schwab, M.; Shevtsov, M.; Xanthopoulos, A.; Wang, F.; Dezfouli, A.B.; Tholke, D.; Ostheimer, C.; et al. Hsp70 in liquid biopsies - a tumor-specific biomarker for detection and response monitoring in cancer. Cancers (Basel). 2021, 13, 3706. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Lersch, R.; De Andrade Kratzig, N.; Strong, A.; Friedrich, M.J.; Weber, J.; Engleitner, T.; Ollinger, R.; Yen, H.Y.; Kohlhofer, U.; et al. In vivo interrogation of regulatory genomes reveals extensive quasi-insufficiency in cancer evolution. Cell Genom. 2023, 3, 100276. [Google Scholar] [CrossRef]
- Macosko, E.Z.; Basu, A.; Satija, R.; Nemesh, J.; Shekhar, K.; Goldman, M.; Tirosh, I.; Bialas, A.R.; Kamitaki, N.; Martersteck, E.M.; et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell. 2015, 161, 1202–1214. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. Clusterprofiler 4.0: A universal enrichment tool for interpreting omics data. Innovation (Camb). 2021, 2, 100141. https://doi.org/10.1016/j.xinn.2021.100141. [CrossRef]
- Gunther, S.; Ostheimer, C.; Stangl, S.; Specht, H.M.; Mozes, P.; Jesinghaus, M.; Vordermark, D.; Combs, S.E.; Peltz, F.; Jung, M.P.; et al. Correlation of Hsp70 serum levels with gross tumor volume and composition of lymphocyte subpopulations in patients with squamous cell and adeno non-small cell lung cancer. Front Immunol. 2015, 6, 556. [Google Scholar] [CrossRef] [PubMed]
- Manek, R.; Pakzamir, E.; Mhawech-Fauceglia, P.; Pejovic, T.; Sowter, H.; Gayther, S.A.; Lawrenson, K. Targeting src in endometriosis-associated ovarian cancer. Oncogenesis. 2016, 5, e251. [Google Scholar] [CrossRef]
- Kleimenova, T.; Polyakova, V.; Linkova, N.; Drobintseva, A.; Medvedev, D.; Krasichkov, A. The expression of kisspeptins and matrix metalloproteinases in extragenital endometriosis. Biomedicines. 2024, 12. [Google Scholar] [CrossRef]
- Lenci, R.E.; Rachakonda, P.S.; Kubarenko, A.V.; Weber, A.N.; Brandt, A.; Gast, A.; Sucker, A.; Hemminki, K.; Schadendorf, D.; Kumar, R. Integrin genes and susceptibility to human melanoma. Mutagenesis. 2012, 27, 367–373. [Google Scholar] [CrossRef]
- Huang, Z.X.; Mao, X.M.; Wu, R.F.; Huang, S.M.; Ding, X.Y.; Chen, Q.H.; Chen, Q.X. Rhoa/rock pathway mediates the effect of oestrogen on regulating epithelial-mesenchymal transition and proliferation in endometriosis. J Cell Mol Med. 2020, 24, 10693–10704. [Google Scholar] [CrossRef]
- Zhang, G.; Hou, S.; Li, S.; Wang, Y.; Cui, W. Role of STAT3 in cancer cell epithelial-mesenchymal transition (review). Int J Oncol. 2024, 64. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wu, F.; Qin, C.; Lin, Y. Paradoxical role of phosphorylated STAT3 in normal fertility and the pathogenesis of adenomyosis and endometriosisdagger. Biol Reprod. 2024, 110, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Keyoumu, K.; Yu, R.; Wen, D.; Jiang, H.; Liu, X.; Di, X.; Zhang, S. Extracellular matrix marker LAMC2 targets ZEB1 to promote tnbc malignancy via up-regulating CD44/STAT3 signaling pathway. Mol Med. 2024, 30, 61. [Google Scholar] [CrossRef] [PubMed]
- Inui, H.; Kawakita, T.; Murayama, M.; Nakagawa, T.; Sasada, H.; Shinohara, A.; Aragaki, R.; Kagawa, T.; Kadota, Y.; Kato, T.; et al. Effects of STAT inhibitors in mouse models of endometriosis. Reprod Sci. 2023, 30, 2449–2456. [Google Scholar] [CrossRef]
- Choi, J.; Jo, M.; Lee, E.; Kim, S.E.; Lee, D.Y.; Choi, D. Dienogest attenuates stat3 activation in ovarian endometriotic cysts. Eur J Obstet Gynecol Reprod Biol. 2024, 294, 217–221. [Google Scholar] [CrossRef]
- Chopyak, V.V.; Koval, H.D.; Havrylyuk, A.M.; Lishchuk-Yakymovych, K.A.; Potomkina, H.A.; Kurpisz, M.K. Immunopathogenesis of endometriosis - a novel look at an old problem. Cent Eur J Immunol. 2022, 47, 109–116. [Google Scholar] [CrossRef]
- Salvermoser, L.; Flisikowski, K.; Dressel-Bohm, S.; Nytko, K.J.; Rohrer Bley, C.; Schnieke, A.; Samt, A.K.; Tholke, D.; Lennartz, P.; Schwab, M.; et al. Elevated circulating Hsp70 levels are correlative for malignancies in different mammalian species. Cell Stress Chaperones. 2022. [CrossRef]
- Lee, H.W.; Lee, E.H.; Kim, S.H.; Roh, M.S.; Jung, S.B.; Choi, Y.C. Heat shock protein 70 (Hsp70) expression is associated with poor prognosis in intestinal type gastric cancer. Virchows Arch. 2013, 463, 489–495. [Google Scholar] [CrossRef]
- Botzler, C.; Schmidt, J.; Luz, A.; Jennen, L.; Issels, R.; Multhoff, G. Differential Hsp70 plasma-membrane expression on primary human tumors and metastases in mice with severe combined immunodeficiency. Int.J.Cancer. 1998, 77, 942–948. [Google Scholar] [CrossRef]
- Chanteloup, G.; Cordonnier, M.; Isambert, N.; Bertaut, A.; Hervieu, A.; Hennequin, A.; Luu, M.; Zanetta, S.; Coudert, B.; Bengrine, L.; et al. Monitoring Hsp70 exosomes in cancer patients' follow up: A clinical prospective pilot study. J Extracell Vesicles. 2020, 9, 1766192. [Google Scholar] [CrossRef]
- Yu, M.; Stott, S.; Toner, M.; Maheswaran, S.; Haber, D.A. Circulating tumor cells: Approaches to isolation and characterization. J Cell Biol. 2011, 192, 373–382. [Google Scholar] [CrossRef]
- Riethdorf, S.; Fritsche, H.; Muller, V.; Rau, T.; Schindlbeck, C.; Rack, B.; Janni, W.; Coith, C.; Beck, K.; Janicke, F.; et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: A validation study of the cellsearch system. Clin Cancer Res. 2007, 13, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Kralickova, M.; Lagana, A.S.; Ghezzi, F.; Vetvicka, V. Endometriosis and risk of ovarian cancer: What do we know? Arch Gynecol Obstet. 2020, 301, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Brilhante, A.V.; Augusto, K.L.; Portela, M.C.; Sucupira, L.C.; Oliveira, L.A.; Pouchaim, A.J.; Nobrega, L.R.; Magalhaes, T.F.; Sobreira, L.R. Endometriosis and ovarian cancer: An integrative review (endometriosis and ovarian cancer). Asian Pac J Cancer Prev. 2017, 18, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Barnard, M.E.; Farland, L.V.; Yan, B.; Wang, J.; Trabert, B.; Doherty, J.A.; Meeks, H.D.; Madsen, M.; Guinto, E.; Collin, L.J.; et al. Endometriosis typology and ovarian cancer risk. JAMA. 2024. [CrossRef]
- Kim, W.T.; Ryu, C.J. Cancer stem cell surface markers on normal stem cells. BMB Rep. 2017, 50, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Barak, V.; Goike, H.; Panaretakis, K.W.; Einarsson, R. Clinical utility of cytokeratins as tumor markers. Clin Biochem. 2004, 37, 529–540. [Google Scholar] [CrossRef]
- Lane, E.B.; Alexander, C.M. Use of keratin antibodies in tumor diagnosis. Semin Cancer Biol. 1990, 1, 165–179. [Google Scholar]
- Ullah, I.; Subbarao, R.B.; Rho, G.J. Human mesenchymal stem cells - current trends and future prospective. Biosci Rep. 2015, 35. [Google Scholar] [CrossRef]
- Miller-Rhodes, P. A guide to mesenchymal stem cell (msc) markers. 2023,.
- Wu, L.; Amjad, S.; Yun, H.; Mani, S.; De Perrot, M. A panel of emerging EMT genes identified in malignant mesothelioma. Sci Rep. 2022, 12, 1007. [Google Scholar] [CrossRef]
- Nielsen, J.S.; Mcnagny, K.M. Novel functions of the CD34 family. J Cell Sci. 2008, 121, 3683–3692. [Google Scholar] [CrossRef]
- Li, J.; Yang, R.; Yang, H.; Chen, S.; Wang, L.; Li, M.; Yang, S.; Feng, Z.; Bi, J. NCAM regulates the proliferation, apoptosis, autophagy, EMT, and migration of human melanoma cells via the Src/Akt/mTOR/Cofilin signaling pathway. J Cell Biochem. 2020, 121, 1192–1204. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Wei, F.; Zhang, J.; Li, B. Mir-134 suppresses the migration and invasion of non-small cell lung cancer by targeting ITGB1. Oncol Rep. 2017, 37, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Zeng, J.J.; Yang, Y.; Ruge, F.; Lane, J.; Hargest, R.; Jiang, W.G. Expression of ALCAM in clinical colon cancer and relationship with patients' treatment responses. In Vivo. 2023, 37, 1117–1128. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wan, Y.; Su, Z.; Li, J.; Han, M.; Zhou, C. Mesenchymal stem cell-derived exosomal microrna-3940-5p inhibits colorectal cancer metastasis by targeting integrin alpha6. Dig Dis Sci. 2021, 66, 1916–1927. [Google Scholar] [CrossRef]
| Endometriosis | Distant organ involvement | Bead functionalization | |
|---|---|---|---|
| Patient ID | cmHsp70.1 mAb | EpCAM mAb | |
| 01 | No | 150 | 589 |
| 02 | Bladder | 1,013 | 1,845 |
| 03 | No | 120 | 98 |
| 04 | No | 552 | 30 |
| 05 | No | 34 | 57 |
| 06 | Bladder, Rectum | 4,963 | 770 |
| 07 | Bladder | 346,896 | 50 |
| 08 | Bladder | 214 | 199 |
| 09 | No | 98 | 139 |
| 10 | Lymph node | 12,935 | 379 |
| 10: follow-up | 186,648 | 6 | |
| 11 | No | 890 | 435 |
| 11: follow up | 219 | 384 | |
| 12 | No | 467 | 384 |
| 12: follow up | 123 | 156 | |
| Healthy donors | |||
| 01 | No | 0 | 0 |
| 02 | No | 0 | 0 |
| Genes | Function | Base mean | Log2 fold change | Padj |
|---|---|---|---|---|
| EpCAM mAb | ||||
| SRC | Progression Cytoskeletal reorganization |
44 | 1.1 | 4E−3 |
| FGR | Src kinase | 230.5 | 1.1 | 2E−13 |
| MMP2-AS1 | Extracellular matrix remodeling | 11.8 | 1.7 | 4E−3 |
| CDK5RAP3 | Cell cycle regulation | 10.2 | 2.1 | 7E−4 |
| CDKN1A | Cell cycle regulation | 51.9 | 1.3 | 1E−4 |
| RGCC | Cell cycle regulation | 94.8 | 1.3 | 3E−8 |
| ITGAM | Cell adhesion and migration | 20 | 1 | 1E−4 |
| ITGAE | Cell adhesion and migration | 29.5 | 1.4 | 1E−3 |
| cmHsp70.1mAb | ||||
| ROCK2 | EMT Cytoskeletal organization |
66.2 | -1.8 | 4E−5 |
| STAT3 | EMT Stemness-related marker |
107.8 | -1 | 1E−3 |
| CD82 | Cell adhesion | 134.2 | -1 | 2E−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





