Submitted:
30 August 2024
Posted:
31 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
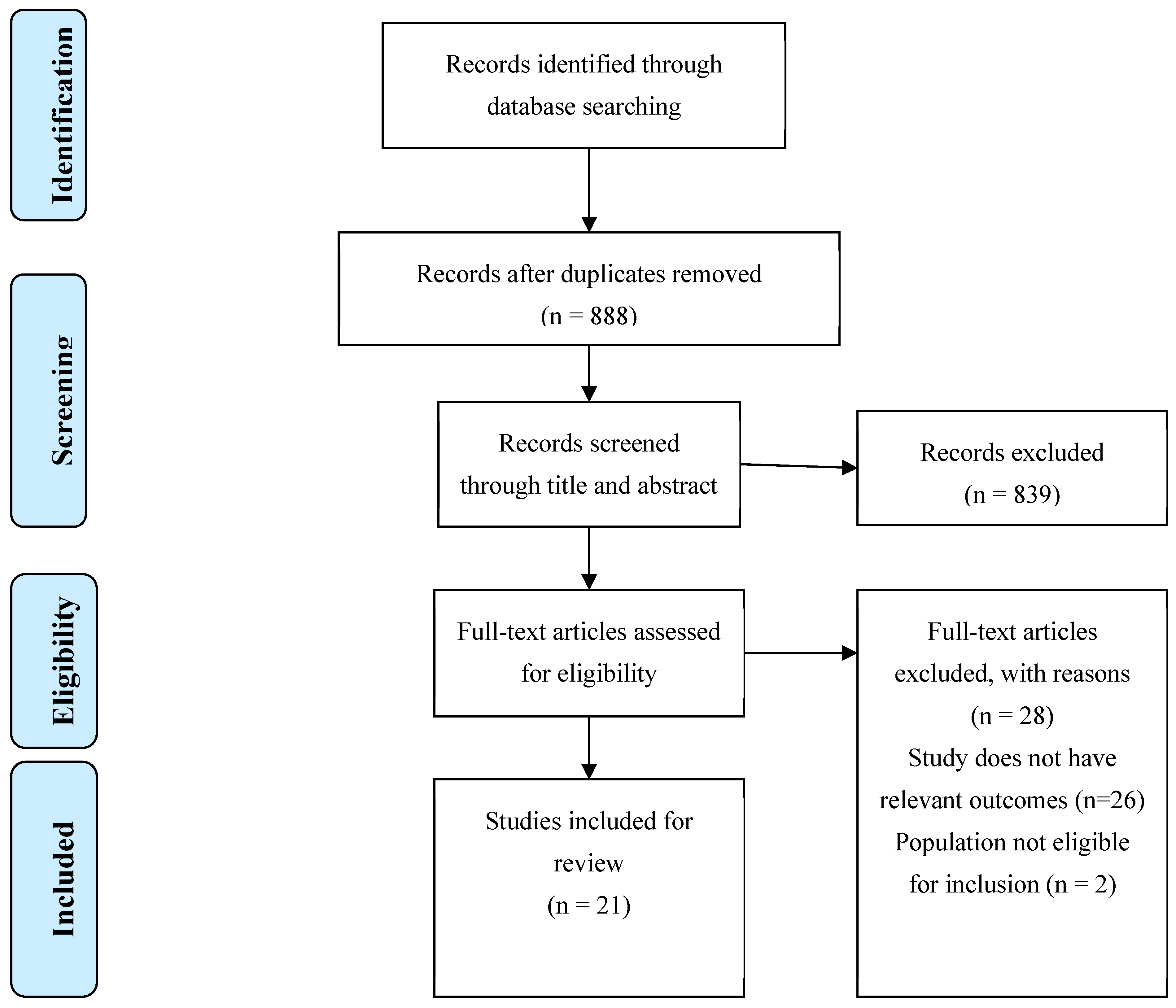
2.1. Search Query and Inclusion Criteria
2.2. Study Selection
3. Results
3.1. Search Results
3.2. Pyridoxamine
3.3. Carnosine
3.4. Aminoguanidine
3.5. Nateglinide and Telmisartan
3.6. Pentoxifylline
3.7. RAGE229
3.8. Pyrogallol-Phloroglucinol-6,6-Bieckol
3.9. Genistein
3.10. Carnosic Acid
3.11. Indian Gooseberry Extract
3.12. Epigallocatechin-3-gallate (EGCG)
4. Discussion
Acknowledgments
Author Contributions
Competing Interests
List of Abbreviations
References
- Hotamisligil KEWaGS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112(12):4. [CrossRef]
- Federation WO. World Obesity Atlas 2023. https://data.worldobesity.org/publications/?cat=19: World Obesity Federation; 2023 march.
- Organization WH. Obesity https://www.who.int/health-topics/obesity#tab=tab_12024 [.
- Gutowska K, Czajkowski K, Kurylowicz A. Receptor for the Advanced Glycation End Products (RAGE) Pathway in Adipose Tissue Metabolism. Int J Mol Sci. 2023;24(13). [CrossRef]
- Shen CY, Lu CH, Wu CH, Li KJ, Kuo YM, Hsieh SC, et al. The Development of Maillard Reaction, and Advanced Glycation End Product (AGE)-Receptor for AGE (RAGE) Signaling Inhibitors as Novel Therapeutic Strategies for Patients with AGE-Related Diseases. Molecules. 2020;25(23). [CrossRef]
- Jangde N, Ray R, Rai V. RAGE and its ligands: from pathogenesis to therapeutics. Crit Rev Biochem Mol Biol. 2020;55(6):555-75. [CrossRef]
- Ahmad S, Khan H, Siddiqui Z, Khan MY, Rehman S, Shahab U, et al. AGEs, RAGEs and s-RAGE; friend or foe for cancer. Semin Cancer Biol. 2018;49:44-55. [CrossRef]
- C S-B, NA B, CG F, M K, CK N, D P, et al. Advanced glycation end-products regulate extracellular matrix-adipocyte metabolic crosstalk in diabetes. England2019 2019-12-24. 19748 p.
- Z F, L Z, J W. RAGE signalling in obesity and diabetes: focus on the adipose tissue macrophage. Adipocyte. 2020;9(1):563-6. [CrossRef]
- PVM R, JF T, MAC C, JB M, RCG A. Effect of reducing dietary advanced glycation end products on obesity-associated complications: a systematic review. Nutrition reviews. 2019;77(10):725-34. [CrossRef]
- SK M, N V, SK M. Advanced glycation end products induce lipogenesis: regulation by natural xanthone through inhibition of ERK and NF-κB. Journal of cellular physiology. 2014;229(12):1972-80. [CrossRef]
- Villegas-Rodriguez ME, Uribarri J, Solorio-Meza SE, Fajardo-Araujo ME, Cai W, Torres-Graciano S, et al. The AGE-RAGE Axis and Its Relationship to Markers of Cardiovascular Disease in Newly Diagnosed Diabetic Patients. PLoS One. 2016;11(7):e0159175. [CrossRef]
- Vijaykrishnaraj M, Wang K. Dietary natural products as a potential inhibitor towards advanced glycation end products and hyperglycemic complications: A phytotherapy approaches. Biomedicine and Pharmacotherapy. 2021;144. [CrossRef]
- Alderson N, Chachich M, Youssef N, Beattie R, Nachtigal M, Thorpe S, et al. The AGE inhibitor pyridoxamine inhibits lipemia and development of renal and vascular disease in Zucker obese rats. KIDNEY INTERNATIONAL. 2003;63(6):2123-33. [CrossRef]
- EA M, CJ D, MK T, KA L, TL A, NB H, et al. Interactions of the advanced glycation end product inhibitor pyridoxamine and the antioxidant alpha-lipoic acid on insulin resistance in the obese Zucker rat. Metabolism: clinical and experimental. 2008;57(10):1465-72. [CrossRef]
- EM M, CJ D, MK T, KA L, O H, M M, et al. Metabolic interactions of AGE inhibitor pyridoxamine and antioxidant alpha-lipoic acid following 22 weeks of treatment in obese Zucker rats. Life sciences. 2009;84(15):563-8. [CrossRef]
- S H, T G, M T, T I, M M, I O, et al. Effects of pyridoxamine (K-163) on glucose intolerance and obesity in high-fat diet C57BL/6J mice. Metabolism: clinical and experimental. 2009;58(7):934-45. [CrossRef]
- H U-K, S Y, M T, H B, Y S. Pyridoxamine, an inhibitor of advanced glycation end product (AGE) formation ameliorates insulin resistance in obese, type 2 diabetic mice. Protein and peptide letters. 2010;17(9):1177-81. [CrossRef]
- Miura K, Kitahara Y, Kajioka T, Takeuchi M, Yamagishi S. Combination therapy with nateglinide and telmisartan ameliorates insulin resistance in zucker Fatty rats by suppressing advanced glycation end product receptor axis. Horm Metab Res. 2011;43(3):226-8. [CrossRef]
- G A, M O, G R, F S, P B, G V, et al. The carbonyl scavenger carnosine ameliorates dyslipidaemia and renal function in Zucker obese rats. Journal of cellular and molecular medicine. 2011;15(6):1339-54. [CrossRef]
- Y Z, R S, P W, H C, Y Z, S S. Carnosic acid as a major bioactive component in rosemary extract ameliorates high-fat-diet-induced obesity and metabolic syndrome in mice. Journal of agricultural and food chemistry. 2015;63(19):4843-52. [CrossRef]
- Baye E, Ukropcova B, Ukropec J, Hipkiss A, Aldini G, de Courten B. Physiological and therapeutic effects of carnosine on cardiometabolic risk and disease. AMINO ACIDS. 2016;48(5):1131-49. [CrossRef]
- Maessen DE, Brouwers O, Gaens KH, Wouters K, Cleutjens JP, Janssen BJ, et al. Delayed Intervention With Pyridoxamine Improves Metabolic Function and Prevents Adipose Tissue Inflammation and Insulin Resistance in High-Fat Diet-Induced Obese Mice. DIABETES. 2016;65(4):956-66. [CrossRef]
- DD X, M Z, N L, JF G, L M, M L. Mediation of inflammation, obesity and fatty liver disease by advanced glycation endoproducts. European review for medical and pharmacological sciences. 2017;21(22):5172-8. [CrossRef]
- Sampath C, Rashid MR, Sang S, Ahmedna M. Green tea epigallocatechin 3-gallate alleviates hyperglycemia and reduces advanced glycation end products via nrf2 pathway in mice with high fat diet-induced obesity. Biomed Pharmacother. 2017;87:73-81. [CrossRef]
- Anderson EJ, Vistoli G, Katunga LA, Funai K, Regazzoni L, Blake Monroe T, et al. A carnosine analog mitigates metabolic disorders of obesity by reducing carbonyl stress. Journal of Clinical Investigation. 2018;128(12):5280-93. [CrossRef]
- J C, S O, M S, K B. Pyrogallol-Phloroglucinol-6,6-Bieckol Alleviates Obesity and Systemic Inflammation in a Mouse Model by Reducing Expression of RAGE and RAGE Ligands. Marine drugs. 2019;17(11). [CrossRef]
- S O, H A, H P, JI L, KY P, D H, et al. The attenuating effects of pyridoxamine on adipocyte hypertrophy and inflammation differ by adipocyte location. The Journal of nutritional biochemistry. 2019;72:108173. [CrossRef]
- Y Z, P W, S S. Dietary Genistein Inhibits Methylglyoxal-Induced Advanced Glycation End Product Formation in Mice Fed a High-Fat Diet. The Journal of nutrition. 2019;149(5):776-87. [CrossRef]
- Inacio MD, Costa MC, Oliveira Lima TF, Figueiredo ID, Motta BP, Spolidorio LC, et al. Pentoxifylline mitigates renal glycoxidative stress in obese mice by inhibiting AGE/RAGE signaling and increasing glyoxalase levels. LIFE SCIENCES. 2020;258. [CrossRef]
- W P, P M, L L, K C, Y Z, W X, et al. Effect of carnosine supplementation on lipid profile, fasting blood glucose, HbA1C and insulin resistance: A systematic review and meta-analysis of long-term randomized controlled trials. Complementary therapies in medicine. 2020;48:102241. [CrossRef]
- MDG VdE, AJHM H, JLJM S, AMA L, PM N, N S, et al. Pyridoxamine reduces methylglyoxal and markers of glycation and endothelial dysfunction, but does not improve insulin sensitivity or vascular function in abdominally obese individuals: A randomized double-blind placebo-controlled trial. Diabetes, obesity & metabolism. 2023;25(5):1280-91. [CrossRef]
- RA W, L A, HH R, B Z, K Q, MB M, et al. Pharmacological antagonism of receptor for advanced glycation end products signaling promotes thermogenesis, healthful body mass and composition, and metabolism in mice. Obesity (Silver Spring, Md). 2023;31(7):1825-43. [CrossRef]
- Chen S-Y, Huang Y-N, Lin J-A, Yen G-C. Effect of Indian gooseberry extract on improving methylglyoxal-associated leptin resistance in peripheral tissues of high-fat diet-fed rats. JOURNAL OF FOOD AND DRUG ANALYSIS. 2024;32(1). [CrossRef]
- Metz TO, Alderson NL, Chachich ME, Thorpe SR, Baynes JW. Pyridoxamine Traps Intermediates in Lipid Peroxidation Reactions in Vivo: EVIDENCE ON THE ROLE OF LIPIDS IN CHEMICAL MODIFICATION OF PROTEIN AND DEVELOPMENT OF DIABETIC COMPLICATIONS*. Journal of Biological Chemistry. 2003;278(43):42012-9. [CrossRef]
- Sabliov CM, Astete CE. 12 - Encapsulation and controlled release of antioxidants and vitamins. In: Garti N, editor. Delivery and Controlled Release of Bioactives in Foods and Nutraceuticals: Woodhead Publishing; 2008. p. 297-330. [CrossRef]
- Thornalley PJ. Use of aminoguanidine (Pimagedine) to prevent the formation of advanced glycation endproducts. Archives of Biochemistry and Biophysics. 2003;419(1):31-40. [CrossRef]
- Freedman BI, Wuerth J-P, Cartwright K, Bain RP, Dippe S, Hershon K, et al. Design and Baseline Characteristics for the Aminoguanidine Clinical Trial in Overt Type 2 Diabetic Nephropathy (ACTION II). Controlled Clinical Trials. 1999;20(5):493-510. [CrossRef]
- Bolton WK, Cattran DC, Williams ME, Adler SG, Appel GB, Cartwright K, et al. Randomized trial of an inhibitor of formation of advanced glycation end products in diabetic nephropathy. Am J Nephrol. 2004;24(1):32-40. [CrossRef]
- Kajioka T, Miura K, Kitahara Y, Yamagishi S. Potential utility of combination therapy with nateglinide and telmisartan for metabolic derangements in Zucker Fatty rats. Horm Metab Res. 2007;39(12):889-93. [CrossRef]
- Langlais P, Yi Z, Finlayson J, Luo M, Mapes R, De Filippis E, et al. Global IRS-1 phosphorylation analysis in insulin resistance. Diabetologia. 2011;54(11):2878-89. [CrossRef]
- Lee JH, Ko JY, Oh JY, Kim CY, Lee HJ, Kim J, et al. Preparative isolation and purification of phlorotannins from Ecklonia cava using centrifugal partition chromatography by one-step. Food Chem. 2014;158:433-7. [CrossRef]
- Lo CY, Li S, Tan D, Pan MH, Sang S, Ho CT. Trapping reactions of reactive carbonyl species with tea polyphenols in simulated physiological conditions. Mol Nutr Food Res. 2006;50(12):1118-28. [CrossRef]
- Saini R, Sharma N, Oladeji OS, Sourirajan A, Dev K, Zengin G, et al. Traditional uses, bioactive composition, pharmacology, and toxicology of Phyllanthus emblica fruits: A comprehensive review. J Ethnopharmacol. 2022;282:114570. [CrossRef]
- Huang Y-N, Chen S-Y, Lin J-A, Chiang IC, Yen G-C. Phyllanthus emblica L. extract alleviates leptin resistance and lipid accumulation by inhibiting methylglyoxal production. Food Bioscience. 2023;53:102619. [CrossRef]
- Aruwa CE, Sabiu S. Adipose tissue inflammation linked to obesity: A review of current understanding, therapies and relevance of phyto-therapeutics. Heliyon. 2024;10(1):e23114. [CrossRef]
- Wu Y, Zong M, Wu H, He D, Li L, Zhang X, et al. Dietary Advanced Glycation End-Products Affects the Progression of Early Diabetes by Intervening in Carbohydrate and Lipid Metabolism. Mol Nutr Food Res. 2022;66(11):e2200046. [CrossRef]
- Goran MI, Alderete TL. Targeting Adipose Tissue Inflammation to Treat the Underlying Basis of the Metabolic Complications of Obesity. In: Drewnowski A, Rolls BJ, editors. Obesity Treatment and Prevention: New Directions: 73rd Nestlé Nutrition Institute Workshop, Carlsbad, Calif, September 2011. 73: S.Karger AG; 2012. p. 0.
| Electronic Database | Search Date | Search Strategy | Nº of results |
| MEDLINE(PubMed) | 23/05/2024 | ((obese OR obesity[MeSH Terms])) AND ((advanced glycation end products[MeSH Terms]) OR (Receptor for Advanced Glycation End Products[MeSH Terms]) OR (RAGE) OR (sRAGE)) AND ((inhibitor) OR (inhibition) OR (antagonist) OR (small molecule) OR (liraglutide) OR (pyridoxamine) OR (azeliragon) OR (S100-derived peptide) OR (HMGB1-derived Peptide) OR (alagebrium) OR (carnosine) OR (FPS-ZM1) OR (GM-1111) OR (semaglutide) OR (GLP1 analog)) AND ((adipose tissue metabolism) OR (adipose tissue dysfunction) OR (metabolic inflammation) OR (insulin resistance)) | 245 |
| SCOPUS | 23/05/2024 | ALL ( obese OR obesity ) AND ALL ( "advanced glycation end products" OR "Receptor for Advanced Glycation End Products" OR "RAGE" OR "sRAGE" ) AND ALL ( inhibitor OR inhibition OR antagonist OR "small molecule" OR liraglutide OR pyridoxamine OR azeliragon OR "S100-derived peptide" OR "HMGB1-derived Peptide" OR "alagebrium" OR "carnosine" OR "FPS-ZM1" OR "GM-1111" OR "semaglutide" OR glp1 AND analog ) AND TITLE-ABS-KEY ( "adipose tissue metabolism" OR "adipose tissue dysfunction" OR "metabolic inflammation" OR "insulin resistance") | 588 |
| Web of Science | 23/05/2024 | ALL=(Obese OR Obesity) AND ALL=((advanced glycation end products) OR (Receptor for Advanced Glycation End Products) OR (RAGE) OR (sRAGE)) AND ALL=((inhibitor) OR (inhibition) OR (antagonist) OR (small molecule) OR (liraglutide) OR (pyridoxamine) OR (azeliragon) OR (S100-derived peptide) OR (HMGB1-derived Peptide) OR (alagebrium) OR (carnosine) OR (FPS-ZM1) OR (GM-1111) OR (semaglutide) OR (GLP1 analog)) AND ALL=((adipose tissue metabolism) OR (adipose tissue dysfunction) OR (metabolic inflammation) OR (insulin resistance)) | 104 |
| Authors | Year | Study type | Methodology | Key findings |
| Alderson N. et al.[14] | 2003 | Animal study | Three groups of Zucker rats were studied: lean, untreated fatty and fa/fa treated with PM (2 g/Ldrinking water) for 32 weeks. | PM inhibited the increases in AGE/ALEs in collagen, and significantly decreased the rise in plasma triglycerides, cholesterol, and creatinine, corrected hypertension and thickening of the vascular wall in Zucker fa/fa rats. |
| Muellenbach E. et al. [15] |
2008 | Animal study | Obese Zucker rats were assigned to either a control group or to a treatment group receiving daily injections of the R-ALA (92 mg/kg) or PM (60 mg/kg), individually or in combination, for 6 weeks. | Individual and combined treatments with R-ALA and PM significantly (P < .05) reduced markers of oxidative stress. Combination treatment resulted in the largest reductions of fasting plasma glucose (23%), insulin (16%), and free fatty acids (24%) and of muscle triglycerides (45%). It also elicited the greatest enhancement of whole-body insulin sensitivity. |
| Muellenbach E. et al.[16] |
2009 | Animal study | Female Obese Zucker rats received vehicle (OV), PM (OP, 60 mg/kg body wt), racemic ALA (rac-ALA; OM, 92 mg/kg), the R- (+)-enantiomer of ALA (R-ALA; OR, 92 mg/kg), or combined treatments with PM and rac-ALA (OPM) or PM and R-ALA (OPR), daily for 22 weeks. | Individual and combined treatments with PM, rac-LA, and R-LA significantly inhibited markers of oxidative damage, and triglyceride levels, and plasma free fatty acids, with the greatest decrease (26%) elicited in OPR. Insulin resistance was lowered (20%) only in combined treatment. |
| Hagiwara S. et al. [17] | 2009 | Animal study | C57BL/6J mice were divided into 3 groups: low-fat diet, high-fat diet, and high-fat diet with pyridoxamine treatment, for 12 weeks | Body and adipose tissue weights of PM treatment group were diminished. PM also diminished serum AGE; increased antioxidant enzyme expression; and improved dysregulation of adipocytokines in adipose tissues. PM treatment improved blood glucose levels and fasting hyperinsulinemia. |
| Unoki-Kubota H. et al. [18] |
2010 | Animal study | Of 37 KK-Ay/Ta Jcl mice, 21 aged at 5 weeks were followed up to 15 weeks of age, and body weight and food consumption were monitored during the study periods. The rest of 5-week-old KK- Ay mice were divided randomly into four groups; untreated control group, 24, 120, 240 mg/l PM treatment, and followed for 5 more weeks. | PM treatment dose-dependently decreased fasting insulin levels and improved insulin sensitivity in KK-Ay mice of 10 weeks old, but it did not affect fasting blood glucose levels. |
| Miura K. et al. [19] | 2011 | Animal study | Male Zucker fatty rats were divided into 4 groups (n = 6 each): vehicle (VEH), 50 mg / kg of NAT, 5 mg / kg of TEL, or both (NAT / TEL). Treatment was administered for 6 weeks. |
Combination therapy with NAT and TEL, but not each monotherapy, inhibited IRS-1 serine phosphorylations at 307 and 636 / 639 residues and restored the decrease in IRS-1 tyrosine phosphorylation in the liver. It also reduced levels of AGEs, hepatic RAGE expression and hepatic AGE-RAGE index. |
| Aldini G. et al[20]. | 2011 | Animal study | Zucker fa/fa rats with obesity were randomly divided into three groups of 12 each: untreated control with obesity, L-CAR treated and D-CAR- treated. D-CAR and L-CAR were administered to fa/fa rats in the drinking water (24 hr drug dose of 30 mg/kg) for 24 weeks. | L-CAR and D-CAR restrained the development of dyslipidaemia, hypertension and renal injury. Body weight was reduced. L-CAR and D- CAR-fed rats, after 24 weeks, had plasma cholesterol and triglycerides levels reduced, but plasma glucose levels weren’t affeced. L- CAR and D-CAR restrained the development of hyperinsulinemia and improved insulin resistance. |
| Zhao Y. et al.[21] | 2015 | Animal study | Male C57BL/6J mice were given a low-fat diet, a high-fat diet or a high-fat diet supplemented with either 0.14% CA- enriched rosemary extract or 0.28% CA-enriched RE or 0.5% of commercial RE, for a period of 16 weeks. | RE supplementation significantly reduced body weight gain, percent of fat, plasma ALT, AST, glucose, insulin levels, liver weight, liver triglyceride, and free fatty acid levels It also decreased the levels of plasma and liver AGEs, and the liver expression of RAGE. |
| Baye E. et al. [22] | 2016 | Narrative review | Not disclosed | Carnosine supplementation reduced fasting insulin, decreased insulin resistance and reduced insulin secretion in healthy humans with obesity or overweight. |
| Maessen D. et al. [23] | 2016 | Animal study | Male C57BL/6J 12-week-old mice were divided into three groups. The low- fat diet group, and 2 high fat diet groups. After 6 weeks of HFD, one group started to receive PM (2 g/L) in the drinking water (HFD + PM) for 18 weeks. Male db/db mice were included in the study at an age of 6 weeks. They were also treated with PM in their drinking water for 18 weeks. | Delayed intervention with PM protected against HFD-induced body weight gain, hyperglycemia and hypercholesterolemia. PM also inhibited impaired glucose and insulin resistance in HFD-induced and db/db obese mice. PM prevented expansion of adipose tissue, adipocyte hypertrophy and attenuated expression of proinflammatory genes in visceral adipose tissue. |
| Xiong D-D. et al. [24] | 2017 | Animal study | Sprague Dawley (SD) rats were randomly divided into control, model and AGEs inhibitor groups. Obesity fatty liver model was prepared by the application of high-fat diet and subcutaneous injection of CCl4 at 0.2 mg/100 g for six weeks. Aminoguanidine (100 mg/kg·d) was given by subcutaneous injection for 6 weeks. | Administration of aminoguanidine significantly improved liver functions, improved the metabolism of fatty acids and lowered TNF-α or IL-6 levels. |
| Sampath C. et al. [25] | 2017 | Animal study | Dietary EGCG was tested in C57BL/6 mice placed on a high-fat diet with or without ECGC for 17 weeks, compared to a control group placed on low-fat diet for the same period. | Dietary EGCG significantly reduced weight gain, plasma glucose, insulin level, liver and kidney weight. EGCG also decreased levels of AGEs in plasma and liver, and inhibited RAGE expression. |
| Anderson E. et al. [26] | 2018 | Animal study | C57BL6/J female mice were crossed with male GPx4+/– mice. At 8 to 12 weeks of age, WT and GPx4+/– male age-matched littermates were randomly assigned to groups. Mice were fed either a control or a HFHS diet for 25 weeks. After 8 weeks of the HFHS diet, half of the mice in the HFHS diet were administered carnosinol (45 mg/kg/day) until study termination at 20 weeks. | In models of diet-induced obesity and metabolic syndrome, carnosinol dose-dependently reduced HNE adduct formation in liver and skeletal muscle, and mitigated inflammation, dyslipidemia, insulin resistance, and steatohepatitis. |
| Choi J. et al. [27] | 2019 | Animal study | C57BL/6N male mice were fed a 45% high-fat diet for 8 weeks. Mice were separated into three groups: control, DIO/saline, DIO/PPB. Isolated PPB was dissolved in 0.9% saline and each group was orally administrated saline and PPB (2.5 mg/kg) daily for 4 weeks. | In visceral fat, PPB significantly inhibited RAGE ligands, reduced the RAGE expression, and reduced binding ratio between RAGE and RAGE ligands. PPB reduced differentiation of macrophages in visceral fat into M1-type and related pro-inflammatory cytokines. |
| Oh S. et al. [28] | 2019 | Animal study | Sprague-Dawley rats were divided into three groups and fed a 45% high-fat diet or a normal diet for 8 weeks. Rats in the pyridoxamine treated group were fed a HFD for 4 weeks and then pyridoxamine (2 mg/day dissolved in 1 ml of saline) for another 4 weeks with feeding a 45% high-fat diet. | Pyridoxamine reduced HFD-induced weight gain, adipocyte size, RAGE ligand accumulations, AGE-RAGE ligands binding, decreased macrophage M1 polarization and increased M2 polarization in visceral fat tissues, but not in subcutaneous tissues. PM induced Glo-1 expression in visceral fat in the HFD group. |
| Zhao Y. et al[29]. | 2019 | Animal study | Male C57BL/6J mice (n = 15) were fed a LF diet or a VHF diet alone or including 0.25% genistein for 16 weeks in study 1. In study 2, 75 similar mice were fed the LF diet or the HF diet or in combination with up to 0.2% MGO in water (HFM) and 0.067% or 0.2% dietary genistein for 18 weeks. | Body weight gain, fat deposits, dyslipidemia, hyperglycemia, and fatty liver were ameliorated by dietary genistein. Plasma MGO and plasma, liver and kidney AGEs concentration were significantly lower with genistein. Genistein upregulated the expressions of GLO- 1 and 2 |
| Inacio M. et al. [30] | 2020 | Animal study | C57BL-6J mice were fed a high-fat diet for 14 weeks and treated with 50 mg/kg pentoxifylline during the last 7 weeks. | Pentoxifylline reduced body weight gain, improved insulin sensitivity and glucose tolerance and downregulated biomarkers of glycoxidative stress. |
| Peng W. et al. [31] | 2020 | Systematic review and meta-analysis | PubMed, Scopus and Web of sciences were investigated to identify relevant articles up to June 2019. Inclusion criteria: (1) RCTs, (2) Carnosine use versus any control, (3) intervention for at least 2 weeks. Exclusion criteria: (1) animal studies; (2) studies that surveyed the effect of carnosine along with other components. | Carnosine use versus control for at least 2 weeks showed reduced HbA1C levels in intervention vs control groups. triglyceride levels were not significant reduced. No significant change in HOMA-IR, Cholesterol, fasting blood sugar, or HDL-C. |
| Van den Eynde M. et al.[32] | 2023 | Randomized controlled trial | Individuals with abdominal obesity were randomized to an 8- week intervention with either placebo (n = 36), 25 mg PM (n = 36) or 200 mg PM (n = 36). | PM reduces MGO, AGEs, sVCAM-1 and sICAM-1. No treatment effects on insulin sensitivity, vascular function or other functional outcome measurements. |
| Wilson R. et al. [33] | 2023 | Animal study | RAGE229 (150 parts per million [ppm], approximately 30 mg/kg/d or 50 ppm, approximately 10 mg/kg/d;) was administered to lean mice and mice with obesity undergoing diet-induced weight loss. | RAGE229 reduced body mass and adiposity and improved glucose, insulin, and lipid metabolism in male mice with obesity undergoing weight loss. |
| Chen S.-Y. et al. [34] | 2024 | Animal study | Sprague-Dawley rats were randomly allocated to six groups. The experimental groups included Control, HFD, LWEIG (WEIG, 250 mg kg/b.w./daily), HWEIG (WEIG, 500 mg kg/b.w./daily), ALA (1 mg kg/b.w./daily), and GA (GA, 100 mg kg/b.w./daily), which were administered for 112 days. |
WEIG and GA prevented leptin resistance and MGO, and AGEs accumulation in the liver, kidney, and perinephric fat. WEIG and GA supplementation increased adiponectin, glutathione peroxidase, superoxide dismutase and catalase, and decreased IL-6, IL-1b, TNF-a in the peripheral tissues. |
|
Abbreviations: AGE – advanced glycation end products; RAGE – receptor for advanced glycation end products; ROS- reactive oxygen species; PM- pyridoxamine; ALE- advanced lipoxidation end products; R- ALA – α-lipoic acid; L-CAR – L-carnosine; D-CAR – D- carnosine ; CA – carnosic acid; RE – rosemary extract; HFD – high-fat diet; EGCG – epigallocatechin-3-gallate; HFHS – high-fat/high-sucrose diet; HNE – 4-Hydroxynonenal; DIO – diet-induced obesity; PPB – Pyrogallol-Phloroglucinol-6,6-Bieckol; Glo 1/2 – glyoxalase 1 /2; HOMA – IR – Homeostatic Model Assessment for Insulin Resistance; MGO – methylglyoxal; sVCAM-1 – soluble vascular cell adhesion molecule 1; sICAM-1 – soluble intercellular adhesion molecule-1; LWEIG – low- water extract of Indian gooseberry fruit; WEIG – water extract of Indian gooseberry fruit; HWEIG – High - water extract of Indian gooseberry fruit; GA - gallic acid | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





