Submitted:
29 August 2024
Posted:
30 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Computational Methods
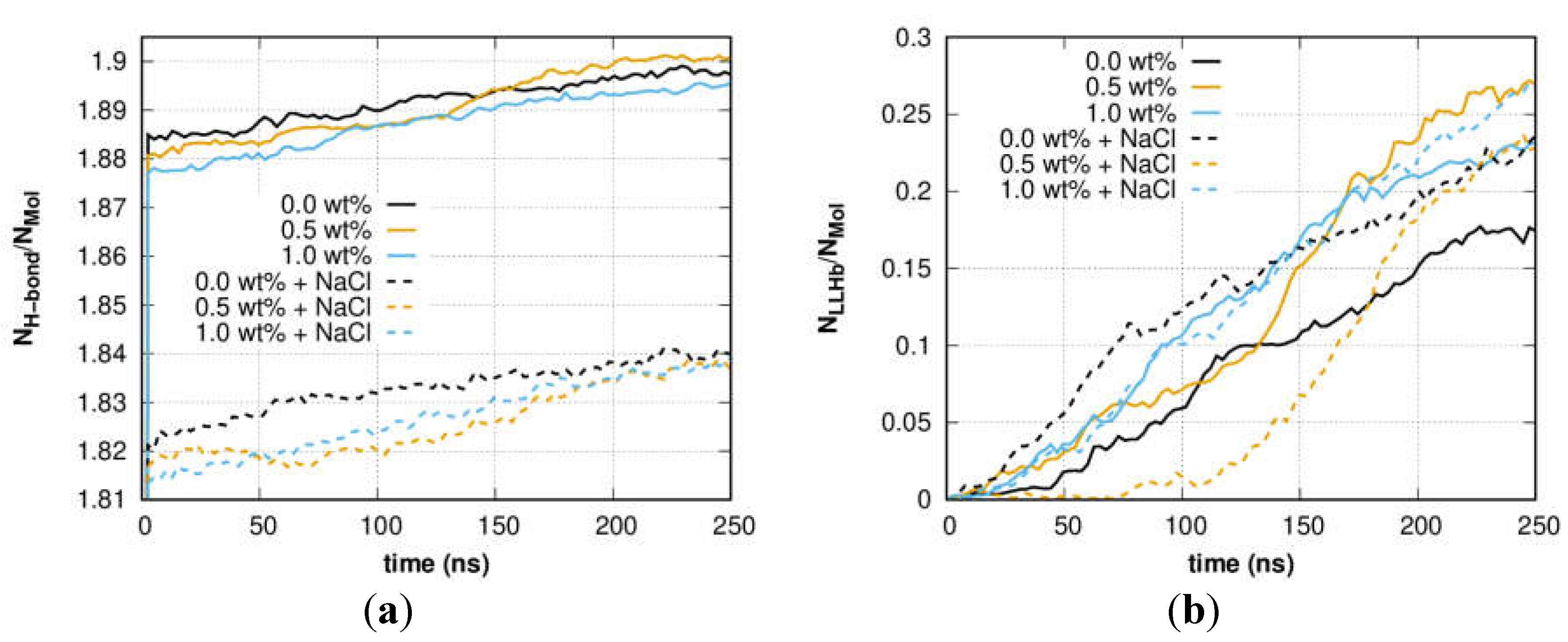
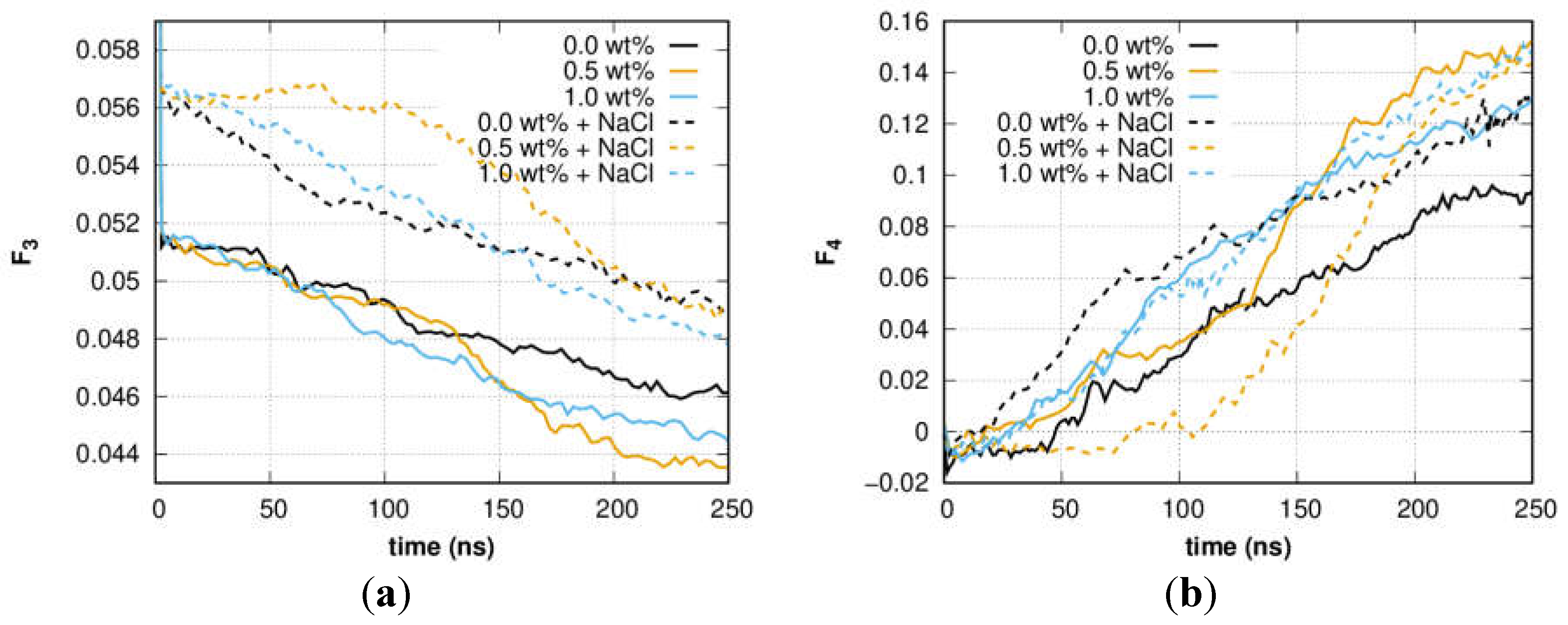
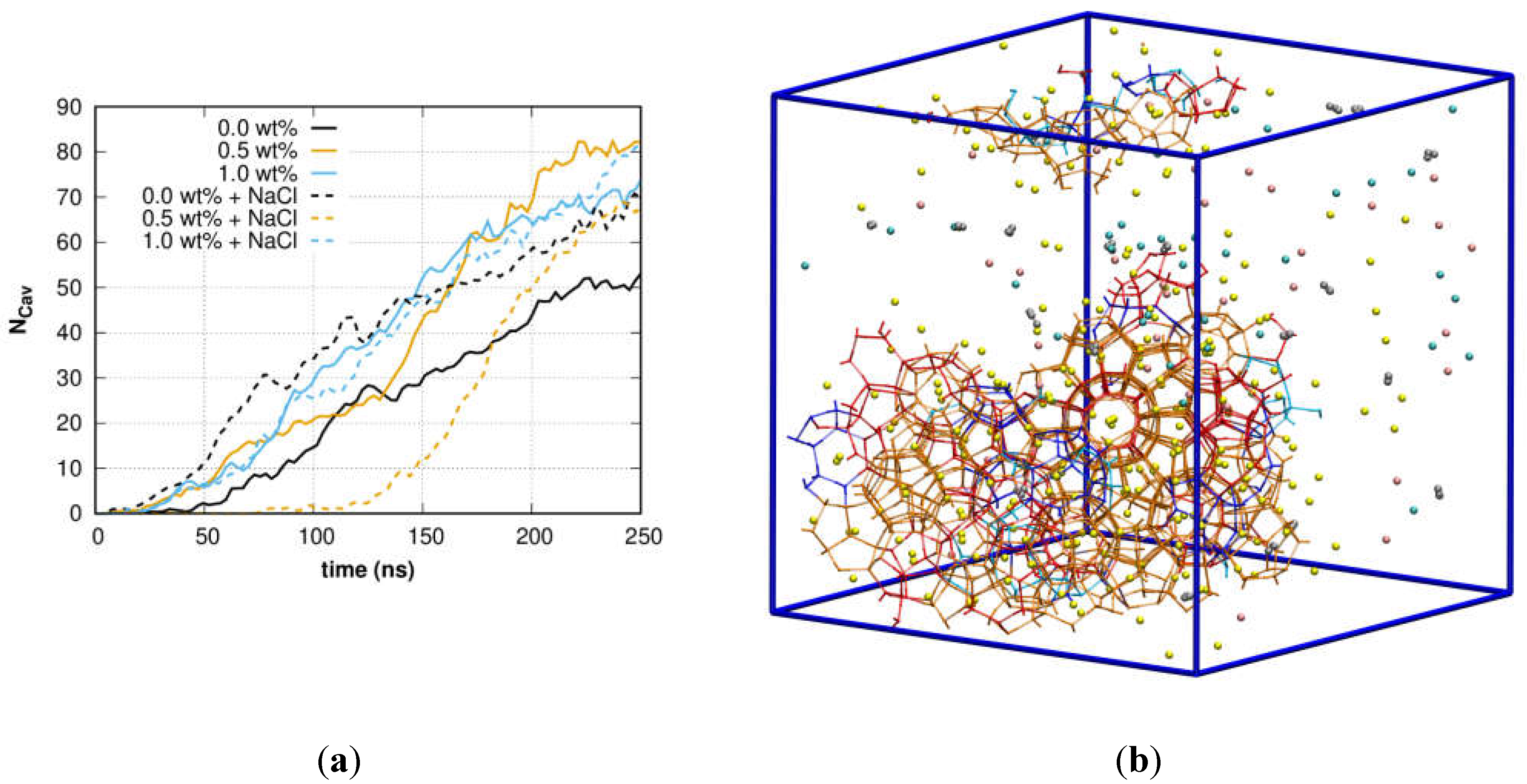
3. Results and Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seyyedattar, M.; Zendehboudi, S.; Butt, S. Technical and Non-Technical Challenges of Development of Offshore Petroleum Reservoirs: Characterization and Production. Nat. Resour. Res. 2020, 29, 2147–2189. [Google Scholar] [CrossRef]
- Fraser, G.S. Impacts of Offshore Oil and Gas Development on Marine Wildlife Resources. In Peak Oil, Economic Growth, and Wildlife Conservation; Gates, J.E., Trauger, D.L., Czech, B., Eds.; Springer New York: New York, NY, 2014; pp. 191–217. ISBN 978-1-4939-1953-6. [Google Scholar]
- Sloan, E.D.; Koh, C.A. Clathrate Hydrates of Natural Gases; Chemical industries; 3rd ed.; CRC Press: Boca Raton, FL, 2008; ISBN 978-1-4200-0849-4. [Google Scholar]
- Millett, J.M.; Wilkins, A.D.; Campbell, E.; Hole, M.J.; Taylor, R.A.; Healy, D.; Jerram, D.A.; Jolley, D.W.; Planke, S.; Archer, S.G.; et al. The Geology of Offshore Drilling through Basalt Sequences: Understanding Operational Complications to Improve Efficiency. Mar. Pet. Geol. 2016, 77, 1177–1192. [Google Scholar] [CrossRef]
- Zhiyuan, W.; Jianbo, Z.; Wenbo, M.; Baojiang, S.; Jinsheng, S.; Jintang, W.; Dahui, L.; Jinbo, W. Formation, Deposition Characteristics and Prevention Methods of Gas Hydrates in Deepwater Gas Wells. Acta Petrolei Sinca 2021, 42, 776–790. [Google Scholar]
- Xia, Z.; Zhao, Q.; Chen, Z.; Li, X.; Zhang, Y.; Xu, C.; Yan, K. Review of Methods and Applications for Promoting Gas Hydrate Formation Process. J. Nat. Gas Sci. Eng. 2022, 101, 104528. [Google Scholar] [CrossRef]
- Carroll, J. Inhibiting Hydrate Formation with Chemicals. In Natural Gas Hydrates; Elsevier, 2020; pp. 163–208 ISBN 978-0-12-821771-9.
- McLaurin, G.; Shin, K.; Alavi, S.; Ripmeester, J.A. Antifreezes Act as Catalysts for Methane Hydrate Formation from Ice. Angew. Chem. Int. ed 2014, 126, 10597–10601. [Google Scholar] [CrossRef]
- Kvamme, B.; Selvåg, J.; Saeidi, N.; Kuznetsova, T. Methanol as a Hydrate Inhibitor and Hydrate Activator. Phys. Chem. Chem. Phys. 2018, 20, 21968–21987. [Google Scholar] [CrossRef]
- Nguyen, N.N.; Nguyen, A.V.; Dang, L.X. The Inhibition of Methane Hydrate Formation by Water Alignment underneath Surface Adsorption of Surfactants. Fuel 2017, 197, 488–496. [Google Scholar] [CrossRef]
- Kvamme, B. Small Alcohols as Surfactants and Hydrate Promotors. Fluids 2021, 6, 345. [Google Scholar] [CrossRef]
- Pandey, J.; Khan, S.; von Solms, N. Screening of Low-Dosage Methanol as a Hydrate Promoter. Energies 2022, 15, 6814. [Google Scholar] [CrossRef]
- Amtawong, J.; Guo, J.; Hale, J.S.; Sengupta, S.; Fleischer, E.B.; Martin, R.W.; Janda, K.C. Propane Clathrate Hydrate Formation Accelerated by Methanol. J. Phys. Chem. Lett. 2016, 7, 2346–2349. [Google Scholar] [CrossRef]
- Devlin, J.P. Catalytic Activity of Methanol in All-Vapor Subsecond Clathrate-Hydrate Formation. J. Chem. Phys. 2014, 140, 164505. [Google Scholar] [CrossRef]
- Choudhary, N.; Kushwaha, O.S.; Bhattacharjee, G.; Chakrabarty, S.; Kumar, R. Molecular Dynamics Simulation and Experimental Study on the Growth of Methane Hydrate in Presence of Methanol and Sodium Chloride. Energy Procedia 2017, 105, 5026–5033. [Google Scholar] [CrossRef]
- Both, A.K.; Gao, Y.; Zeng, X.C.; Cheung, C.L. Gas Hydrates in Confined Space of Nanoporous Materials: New Frontier in Gas Storage Technology. Nanoscale 2021, 13, 7447–7470. [Google Scholar] [CrossRef] [PubMed]
- Casco, M.E.; Silvestre-Albero, J.; Ramírez-Cuesta, A.J.; Rey, F.; Jordá, J.L.; Bansode, A.; Urakawa, A.; Peral, I.; Martínez-Escandell, M.; Kaneko, K.; et al. Methane Hydrate Formation in Confined Nanospace Can Surpass Nature. Nat. Commun. 2015, 6, 6432. [Google Scholar] [CrossRef]
- Yu, K.B.; Yazaydin, A.O. Does Confinement Enable Methane Hydrate Growth at Low Pressures? Insights from Molecular Dynamics Simulations. J. Phys. Chem. C 2020, 124, 11015–11022. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Peng, Y.; Li, Z.; Xie, N.; Zhang, Y.; Wang, W.; Li, Y. Study on Hydrate Formation and Absorption Effect in LNG-High Expansion Foam System. Fuel 2023, 336, 126754. [Google Scholar] [CrossRef]
- Hallbrucker, A.; Mayer, E. Unexpectedly stable nitrogen, oxygen, carbon monoxide and argon clathrate hydrates from vapour-deposited amorphous solid water: an X-ray and two-step differential scanning calorimetry study. J. Chem. Soc. Faraday Trans. 1990, 86, 3785–3792. [Google Scholar] [CrossRef]
- Hallbrucker, A.; Mayer, E. Unexpectedly stable clathrate hydrates formed from microporous vapor-deposited amorphous solid water at low “external” guest pressures and their astrophysical implications. Icarus 1991, 90, 176–180. [Google Scholar] [CrossRef]
- Belosludov, R.V.; Gets, K.V.; Zhdanov, R.K.; Bozhko, Y.Y.; Belosludov, V.R.; Chen, L.-J.; Kawazoe, Y. Molecular Dynamics Study of Clathrate-like Ordering of Water in Supersaturated Methane Solution at Low Pressure. Molecules 2023, 28, 2960. [Google Scholar] [CrossRef]
- Pandey, J.S.; Karantonidis, C.; Karcz, A.P.; von Solms, N. Enhanced CH4-CO2 Hydrate Swapping in the Presence of Low Dosage Methanol. Energies 2020, 13, 5238. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Li, L.; Yan, Y.; Xu, J.; Zhong, J. New Insights into the Kinetic Effects of CH3OH on Methane Hydrate Nucleation. Energy 2023, 263, 125824. [Google Scholar] [CrossRef]
- Kvamme, B. Small Alcohols as Hydrate Promoters. Energy Fuels 2021, 35, 17663–17684. [Google Scholar] [CrossRef]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. P ACKMOL : A Package for Building Initial Configurations for Molecular Dynamics Simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef] [PubMed]
- Abascal, J.L.F.; Sanz, E.; García Fernández, R.; Vega, C. A Potential Model for the Study of Ices and Amorphous Water: TIP4P/Ice. J. Chem. Phys. 2005, 122, 234511. [Google Scholar] [CrossRef] [PubMed]
- Bore, S. L.; Piaggi, P. M.; Car, R.; Paesani, F. Phase diagram of the TIP4P/Ice water model by enhanced sampling simulations. J. Chem. Phys. 2022, 157, 054504. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and Testing of the OPLS All-Atom Force Field on Conformational Energetics and Properties of Organic Liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Nosé, S. A Molecular Dynamics Method for Simulations in the Canonical Ensemble. Mol. Phys. 1984, 52, 255–268. [Google Scholar] [CrossRef]
- Hoover, W.G. Canonical Dynamics: Equilibrium Phase-Space Distributions. Phys. Rev. A 1985, 31, 1695–1697. [Google Scholar] [CrossRef]
- García Fernández, R.; Abascal, J. L.; Vega, C. The melting point of ice Ih for common water models calculated from direct coexistence of the solid-liquid interface. J. Chem. Phys. 2006, 124, 144506. [Google Scholar] [CrossRef]
- Belosludov, V.; Gets, K.; Zhdanov, R.; Malinovsky, V.; Bozhko, Y.; Belosludov, R.; Surovtsev, N.; Subbotin, O.; Kawazoe, Y. The Nano-Structural Inhomogeneity of Dynamic Hydrogen Bond Network of TIP4P/2005 Water. Sci. Rep. 2020, 10, 7323. [Google Scholar] [CrossRef]
- Báez, L.A.; Clancy, P. Computer Simulation of the Crystal Growth and Dissolution of Natural Gas Hydrates a. Annals of the New York Academy of Sciences 1994, 715, 177–186. [Google Scholar] [CrossRef]
- Gao, F.; Gupta, K.M.; Yuan, S.; Jiang, J. Decomposition of CH 4 Hydrate: Effects of Temperature and Salt from Molecular Simulations. Mol. Simul. 2018, 44, 1220–1228. [Google Scholar] [CrossRef]
- Su, Z.; Alavi, S.; Ripmeester, J.A.; Wolosh, G.; Dias, C.L. Methane Clathrate Formation Is Catalyzed and Kinetically Inhibited by the Same Molecule: Two Facets of Methanol. J. Phys. Chem. B 2021, 125, 4162–4168. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Gao, Y.; Zhang, L.; Su, X.; Guo, Y. Study of the Formation of Hydrates with NaCl, Methanol Additive, and Quartz Sand Particles. JMSE 2024, 12, 364. [Google Scholar] [CrossRef]
- Jia, H.; Fan, F.; Wang, Q.; Shen, Z.; Wang, Y.; Sun, H.; Pei, P.; Li, C.; Lv, K.; Huang, P. Molecular Insights into the Dual Promotion–Inhibition Effects of NaCl at Various Concentrations on the CO2 Hydrate Growth: A Molecular Simulation Study. Langmuir 2024, 40, 9012–9019. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.N.; Nguyen, A.V. Recent Insights into the Anomalous Dual Nature (Both Promotion and Inhibition) of Chemical Additives on Gas Hydrate Formation. Chem. Eng. J. 2023, 475, 146362. [Google Scholar] [CrossRef]
- Xu, J.; Du, S.; Hao, Y.; Yang, X.; Zhang, J. Molecular Simulation Study of Methane Hydrate Formation Mechanism in NaCl Solutions with Different Concentrations. Chem. Phys. 2021, 551, 111323. [Google Scholar] [CrossRef]
- Lauricella, M.; Meloni, S.; English, N.J.; Peters, B.; Ciccotti, G. Methane Clathrate Hydrate Nucleation Mechanism by Advanced Molecular Simulations. J. Phys. Chem. C 2014, 118, 22847–22857. [Google Scholar] [CrossRef]
- Jacobson, L.C.; Hujo, W.; Molinero, V. Amorphous Precursors in the Nucleation of Clathrate Hydrates. J. Am. Chem. Soc. 2010, 132, 11806–11811. [Google Scholar] [CrossRef]
- Belosludov, V.R.; Gets, K.V.; Zhdanov, R.K.; Bozhko, Yu.Yu.; Belosludov, R.V.; Chen, L.-J. Collective Effect of Transformation of a Hydrogen Bond Network at the Initial State of Growth of Methane Hydrate. Jetp Lett. 2022, 115, 124–129. [Google Scholar] [CrossRef]
- Choudhary, N.; Kushwaha, O. S.; Bhattacharjee, G.; Chakrabarty, S.; Kumar, R. Macro and molecular level insights on gas hydrate growth in the presence of Hofmeister salts. Ind. Eng. Chem. Res. 2020, 59, 20591–20600. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, D.; Hei, Y.; Sun, X.; Chen, J.; Lv, Q.; Li, X.; Hou, X.; Xiao, Y. Effects of different concentrations of methanol on the decomposition of methane hydrate: insights from molecular dynamics simulations. J. Mater. Res. Technol. 2023, 24, 7283–7290. [Google Scholar] [CrossRef]
- Semenov, A.P.; Tulegenov, T.B.; Mendgaziev, R.I.; Stoporev, A.S.; Istomin, V.A.; Vinokurov, V.A. Effect of Methanol on the Kinetics of Nucleation and Growth of Methane Hydrate. Chem Technol Fuels Oils 2023, 59, 667–672. [Google Scholar] [CrossRef]
- Semenov, A.P.; Tulegenov, T.B.; Mendgaziev, R.I.; Stoporev, A.S.; Istomin, V.A.; Sergeeva, D.V.; Lednev, D.A.; Vinokurov, V.A. Dual Nature of Methanol as a Thermodynamic Inhibitor and Kinetic Promoter of Methane Hydrate Formation in a Wide Concentration Range. J. Mol. Liq. 2024, 403, 124780. [Google Scholar] [CrossRef]
- Sowa, B.; Zhang, X. H.; Hartley, P. G.; Dunstan, D. E.; Kozielski, K. A.; Maeda, N. Formation of ice, tetrahydrofuran hydrate, and methane/propane mixed gas hydrates in strong monovalent salt solutions. Energy Fuels 2014, 28, 6877–6888. [Google Scholar] [CrossRef]
- Sowa, B.; Zhang, X. H.; Kozielski, K. A.; Hartley, P. G.; Dunstan, D. E.; Maeda, N. Nucleation Probability Distributions of Methane−Propane Mixed Gas Hydrates in Salt Solutions and Urea. Energy Fuels 2015, 29, 6259–6270. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




