Submitted:
21 August 2024
Posted:
22 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Enhancing CAR T Cell Therapy Efficacy
2.1. Genetic Engineering of T Cells as a Potential Solution to Lack of Persistent Action In Vivo
2.1.1. CAR T Overexpressing FOXO1
2.1.2. CAR T Overexpressing SUV39H1
2.1.3. CAR T Overexpressing c-Jun
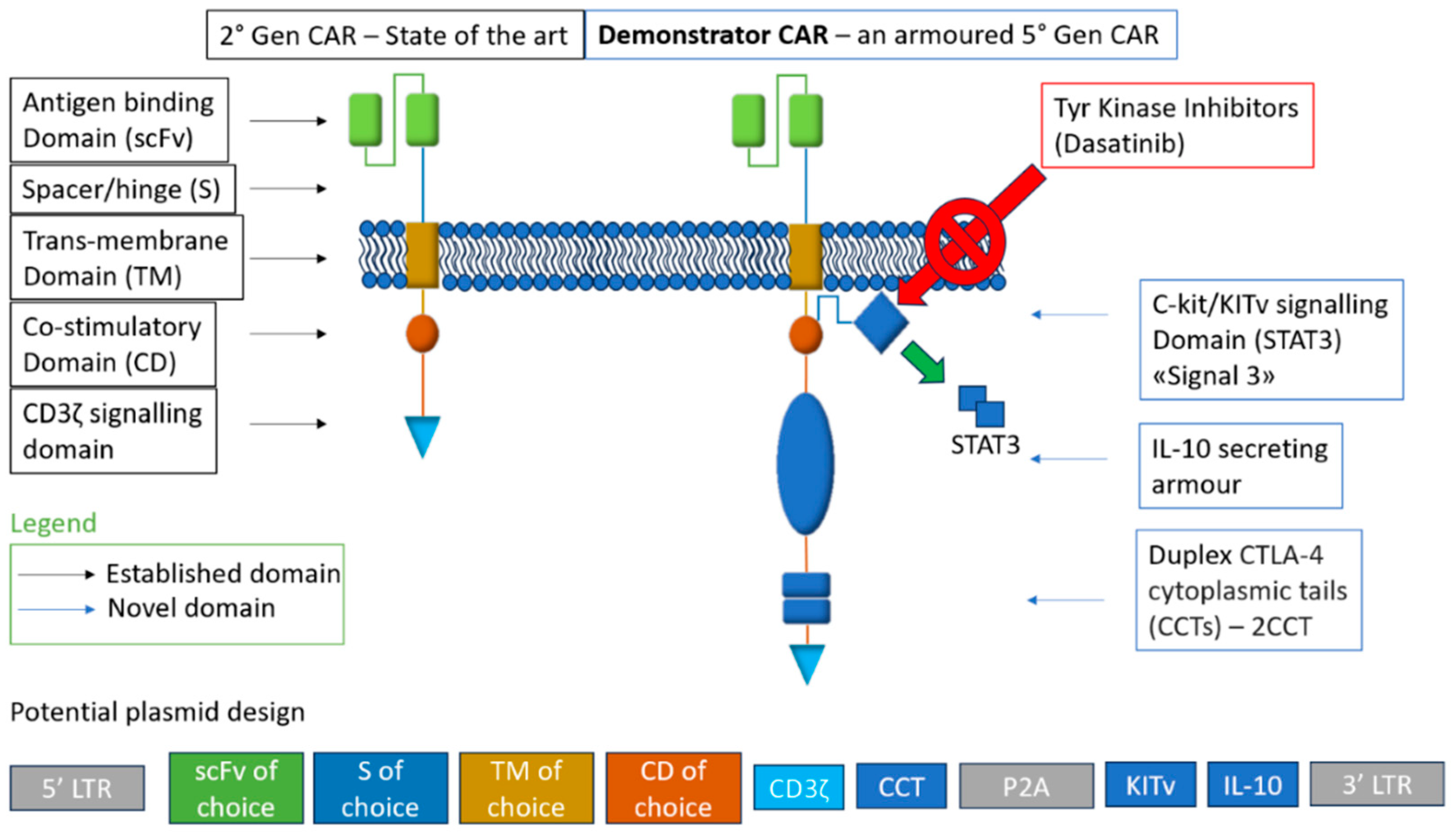
2.2. Construct Engineering of CARs as a Potential Solution to Lack of Persistent Action In Vivo
2.2.1. Metabolic Armouring with IL-10
2.2.2. Dynamic CAR Expression via CTLA-4 Tail
2.2.3. KITv Signaling for Enhanced Functionality
2.3. Construct Engineering of CARs as a Potential Solution to Lack of Efficacy in Solid Tumor Indications: Efficacy through Targeting Regardless of Tumor Type
2.3.1. Nanobody Targeting B7H3 as Antigen Binding Domain
2.3.2. ScFv Targeting nfP2X7 as Antigen Binding Domain
2.3.3. ScFv Amph-Ligand Target as Antigen Binding Domain
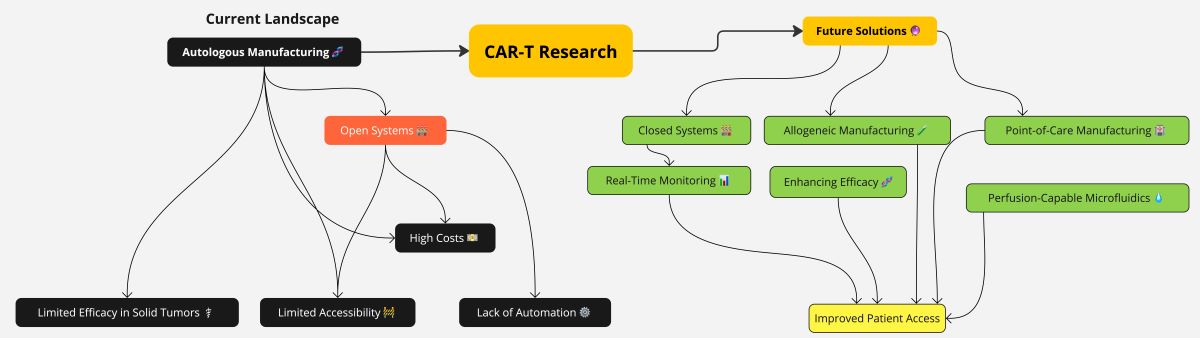
3. CAR T Cell Therapy Manufacturing: Limits and Future Perspectives
3.1. The Limitations of Modern Day ATMP Manufacturing, Including CAR T: Long Times of Production and Costs
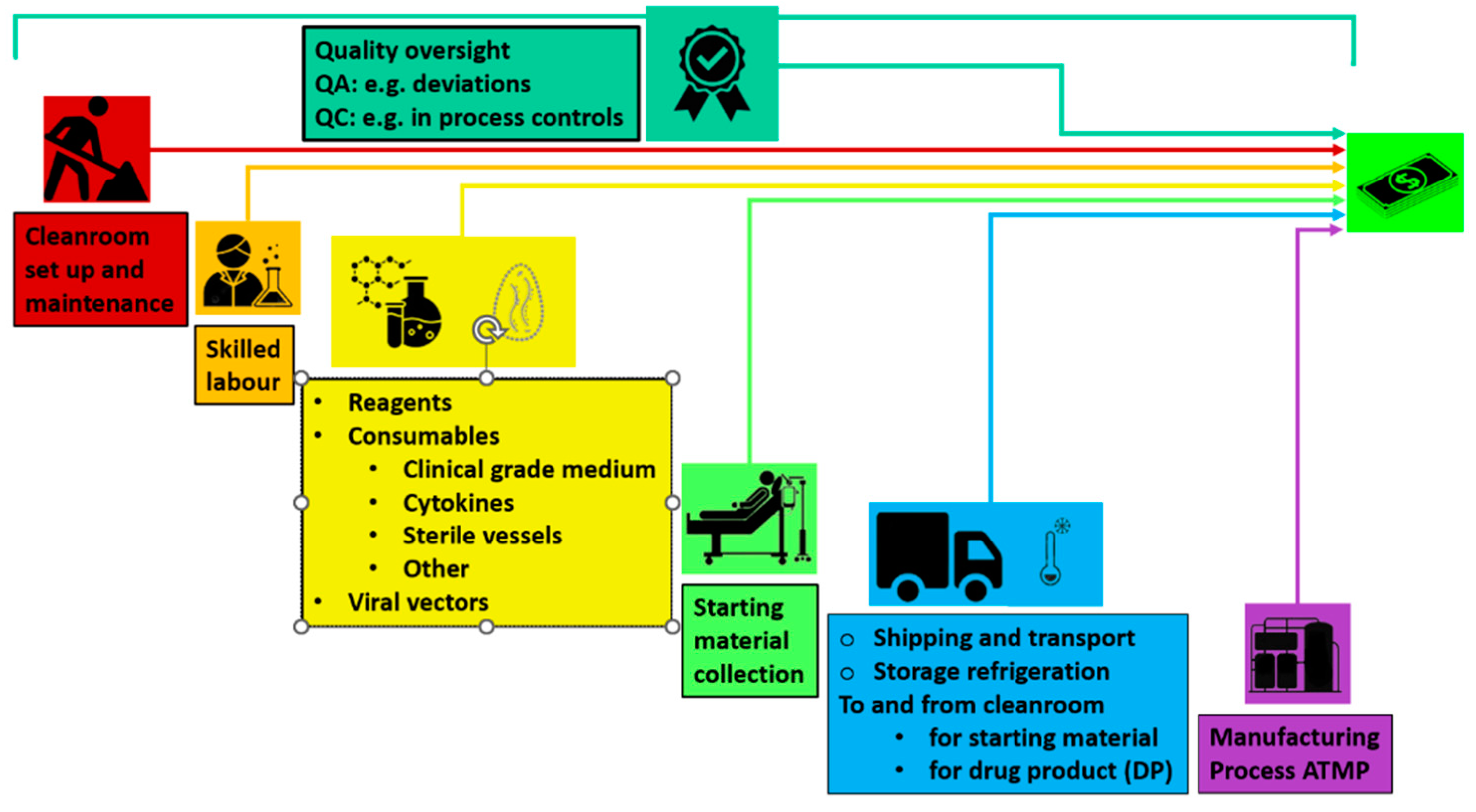
3.1.1. Lowering CAR T Cell Therapy Cost to Improve Access and Expand Clinical Trials
3.2. Newer Manufacturing Methods
3.2.1. Transposons as the Heir to Retro- and Lenti-Viral Transduction
3.2.2. A Mixed AAV-Transposon System to Maximize Yield and Safety
3.2.3. Using Lipid Nanoparticles as a Contender for Transducing CARs, Engineered for Shorter Production Times
3.3. Open versus Closed System for Producing ATMPs
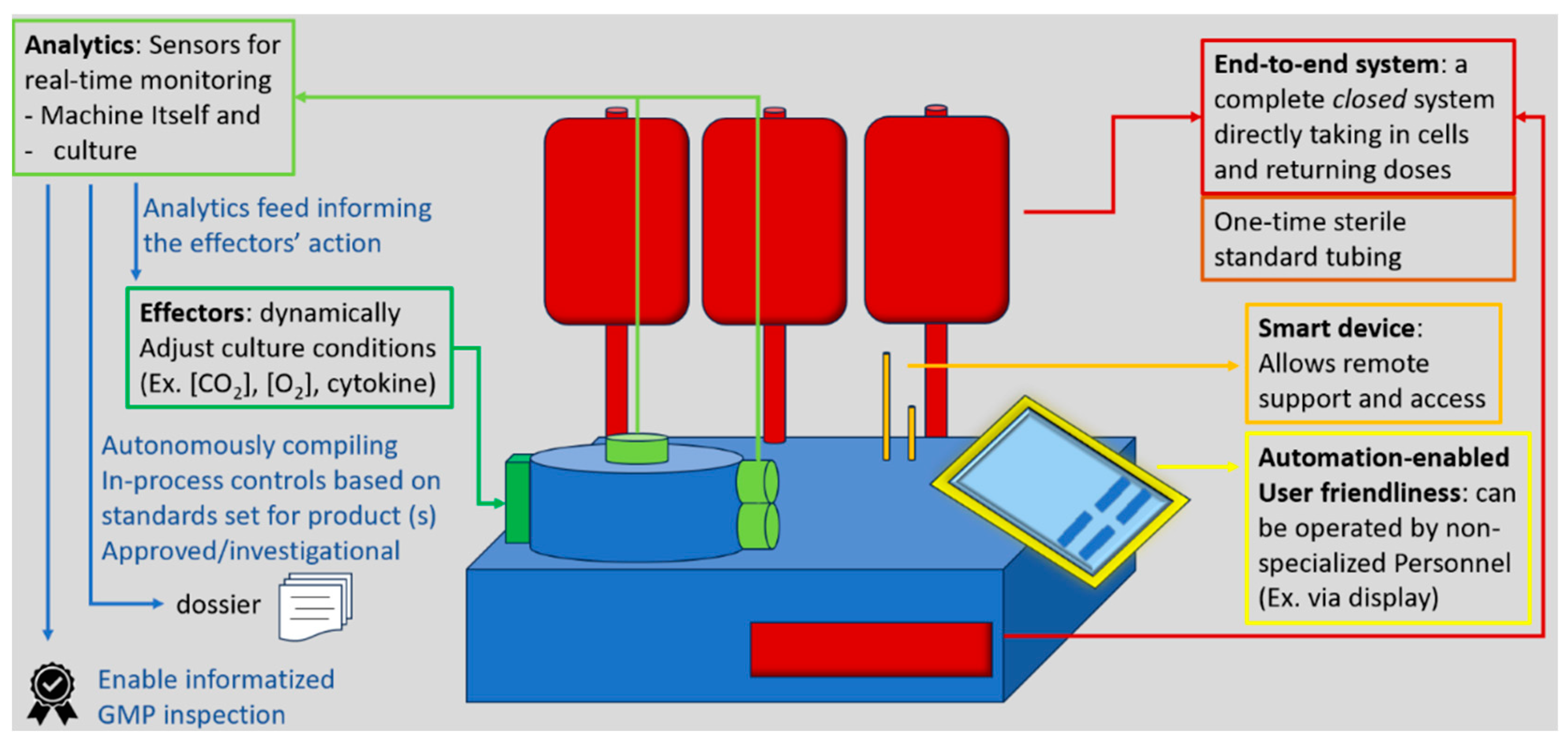
3.3.1. A Closer Look at Closed Systems for CAR T
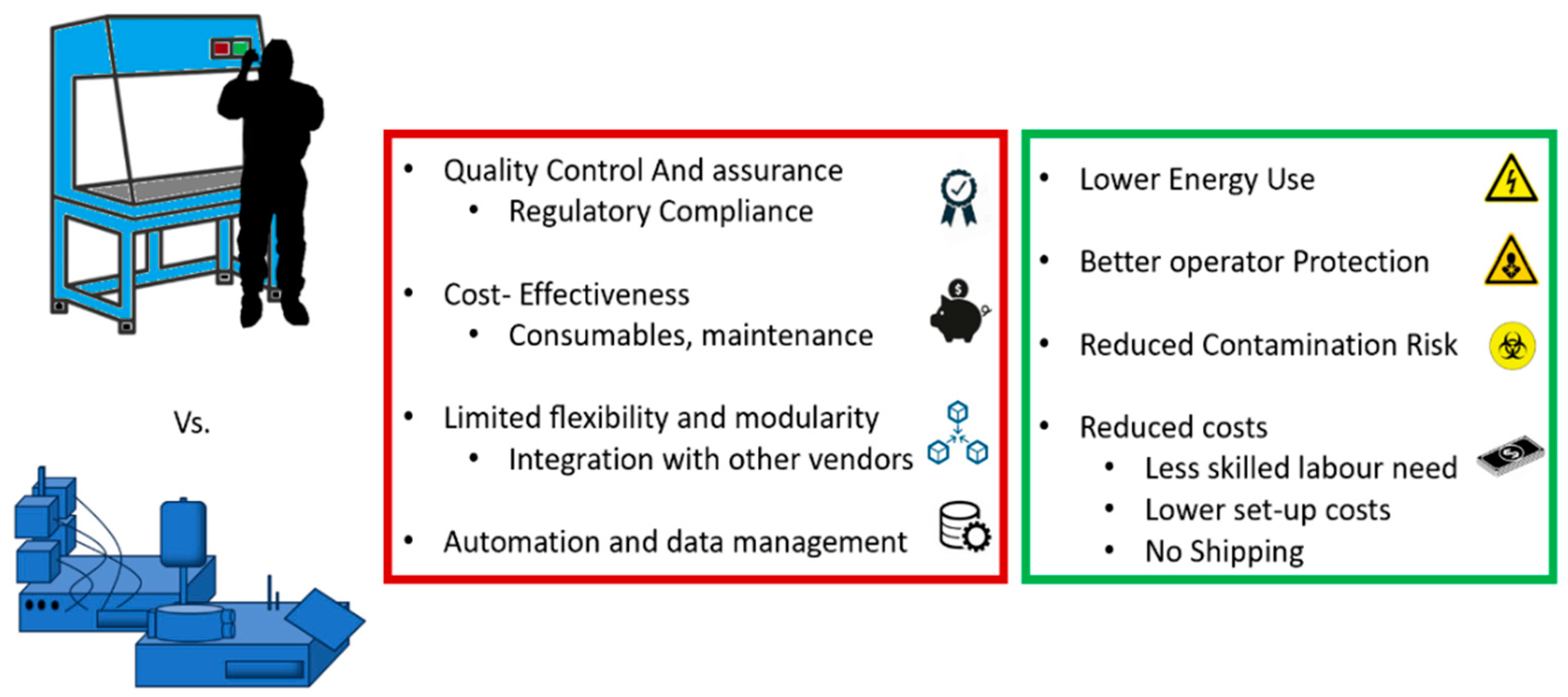
3.3.2. Reports on Real-World Closed Systems Productions
3.3.3. Reports on Real-World Heterologous CAR T Cell Productions: The Italian Data
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chow, A. , Perica, K., Klebanoff, C.A. ; et al. Clinical implications of T cell exhaustion for cancer immunotherapy. Nat Rev Clin Oncol 2022, 19, 775–790. [Google Scholar] [CrossRef] [PubMed]
- Giorgioni, L.; Ambrosone, A.; Cometa, M.F.; Salvati, A.L.; Magrelli, A. CAR-T State of the Art and Future Challenges, A Regulatory Perspective. International Journal of Molecular Sciences. 2023, 24, 11803. [Google Scholar] [CrossRef] [PubMed]
- Cappell, K.M.; Kochenderfer, J.N. Long-term outcomes following CAR T cell therapy: what we know so far. Nat Rev Clin Oncol 2023, 20, 359–371. [Google Scholar] [CrossRef]
- Challenges and innovations in CAR-T cell therapy: a comprehensive analysis Zhao, Y.; Chen, J.; Andreatta, M.; Feng, B.; Xie, Y.Q.; Wenes, M.; Wang, Y.; Gao, M.; Hu, X.; Romero, P.; Carmona, S.; Sun, J.; Guo, Y.; Tang L.
- Doan, A.; Mueller, K.P.; Chen, A.; Rouin, G.T.; Daniel, B.; Lattin, J.; et al. FOXO1 is a master regulator of CAR T memory programming. Res Sq [Preprint]. 2023, rs.3.rs-2802998. https://doi.org/10.21203/rs.3.rs-2802998/v1. Update in: Nature. 2024, 629, 211–218. [CrossRef] [PubMed]
- Chan, J.D. , Scheffler, C.M., Munoz, I. et al. FOXO1 enhances CAR T cell stemness, metabolic fitness and efficacy. Nature 2024, 629, 201–210. [Google Scholar] [CrossRef]
- Jain, N.; Zhao, Z.; Koche, R.P.; Antelope, C.; Gozlan, Y.; et al. Disruption of SUV39H1-Mediated H3K9 Methylation Sustains CAR T-cell Function. Cancer Discov. 2024, 14, 142–157. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agarwal, S.; Wellhausen, N.; Levine, B.L.; June, C.H. Production of Human CRISPR-Engineered CAR-T Cells. J Vis Exp. 2021, 169. [Google Scholar] [CrossRef] [PubMed]
- Lynn, R.C.; Weber, E.W.; Sotillo, E.; Gennert, D.; Xu, P.; et al. c-Jun overexpression in CAR T cells induces exhaustion resistance. Nature. 2019, 576, 293–300. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, D.K.Y. , Adu-Berchie, K., Iyer, S. et al. Enhancing CAR-T cell functionality in a patient-specific manner. Nat Commun 2023, 14, 506. [Google Scholar] [CrossRef]
- Maus, M. V. CD19 CAR T cells for adults with relapsed or refractory acute lymphoblastic leukaemia. Lancet 2021, 398, 466–467. [Google Scholar] [CrossRef]
- Orlando, E. J.; et al. Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Nat. Med 2018, 24, 1504–1506. [Google Scholar] [CrossRef]
- Majzner, R.G.; Mackall, C.L. Tumor antigen escape from CAR T-cell therapy. Cancer Discov. 2018, 8, 1219–1226. [Google Scholar] [CrossRef]
- Vardhana, S. A.; et al. Impaired mitochondrial oxidative phosphorylation limits the self-renewal of T cells exposed to persistent antigen. Nat. Immunol. 2020, 21, 1022–1033. [Google Scholar] [CrossRef]
- Blank, C. U.; et al. Defining ‘T cell exhaustion’. Nat. Rev. Immunol. 2019, 19, 665–674. [Google Scholar] [CrossRef]
- Wherry, E.J.; Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015, 15, 486–499. [Google Scholar] [CrossRef]
- Wherry, E. J.; et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 2007, 27, 670–684. [Google Scholar] [CrossRef]
- Sen, D. R.; et al. The epigenetic landscape of T cell exhaustion. Science 2016, 354, 1165–1169. [Google Scholar] [CrossRef]
- Scharping, N. E.; et al. Mitochondrial stress induced by continuous stimulation under hypoxia rapidly drives T cell exhaustion. Nat. Immunol. 2021, 22, 205–215. [Google Scholar] [CrossRef]
- Yu, Y.-R.; et al. Disturbed mitochondrial dynamics in CD8+ TILs reinforce T cell exhaustion. Nat. Immunol. 2020, 21, 1540–1551. [Google Scholar] [CrossRef]
- Mo, F.; et al. An engineered IL-2 partial agonist promotes CD8+ T cell stemness. Nature 2021, 597, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, D.; et al. IL15 enhances CAR-T cell antitumor activity by reducing mTORC1 activity and preserving their stem cell memory phenotype. Cancer Immunol. Res. 2019, 7, 759–772. [Google Scholar] [CrossRef]
- Zhao, Y. , Chen, J., Andreatta, M. et al. IL-10-expressing CAR T cells resist dysfunction and mediate durable clearance of solid tumors and metastases. Nat Biotechnol 2024. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Cao, H.; Fang, S.Y.; Chow, R.D.; Tang, K.; Majety, M.; Bai, M.; Dong, M.B.; Renauer, P.A.; Shang, X.; Suzuki, K.; Levchenko, A.; Chen, S. CTLA-4 tail fusion enhances CAR-T antitumor immunity. Nat Immunol. 2023, 24, 1499–1510. [Google Scholar] [CrossRef] [PubMed]
- Tivol, E. A.; et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 1995, 3, 541–547. [Google Scholar] [CrossRef]
- Tekguc, M.; Wing, J.B.; Osaki, M.; Long, J.; Sakaguchi, S. Treg-expressed CTLA-4 depletes CD80/CD86 by trogocytosis, releasing free PD-L1 on antigen-presenting cells. Proc. Natl Acad. Sci. 2021. [Google Scholar] [CrossRef]
- Walker, L.S.; Sansom, D.M. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat. Rev. Immunol. 2011, 11, 852–863. [Google Scholar] [CrossRef] [PubMed]
- Schriek, P.; et al. Marginal zone B cells acquire dendritic cell functions by trogocytosis. Science 2022, 375, eabf7470. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; et al. Hijacking TYRO3 from tumor cells via trogocytosis enhances NK-cell effector functions and proliferation. Cancer Immunol. Res. 2021, 9, 1229–1241. [Google Scholar] [CrossRef]
- Huang, J. F.; et al. TCR-Mediated internalization of peptide-MHC complexes acquired by T cells. Science 1999, 286, 952–954. [Google Scholar] [CrossRef]
- Hamieh, M.; et al. CAR T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature 2019, 568, 112–116. [Google Scholar] [CrossRef]
- Olson, M. L.; et al. Low-affinity CAR T cells exhibit reduced trogocytosis, preventing rapid antigen loss, and increasing CAR T cell expansion. Leukemia 2022, 36, 1943–1946. [Google Scholar] [CrossRef]
- Tokarew, N.; Ogonek, J.; Endres, S.; von Bergwelt-Baildon, M.; Kobold, S. Teaching an old dog new tricks: next-generation CAR T cells. Br J Cancer. 2019, 120, 26–37. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ghorashian S et al Enhanced CART cell expansion prolonged persistence in pediatric patients with ALL treated with a low-affinity CD19, C.A.R. Nat. Med. 2019, 25, 1408–1414.
- Weber, E. W.; et al. Transient rest restores functionality in exhausted CAR-T cells through epigenetic remodeling. Science 2021. [CrossRef]
- Philip, M.; et al. Chromatin states define tumour-specific T cell dysfunction and reprogramming. Nature 2017, 545, 452–456. [Google Scholar] [CrossRef]
- Rojas-Sutterlin, S.; Lecuyer, E.; Hoang, T. Kit and Scl regulation of hematopoietic stem cells. Curr. Opin. Hematol. 2014, 21, 256–264. [Google Scholar] [CrossRef]
- Linnekin, D. Early signaling pathways activated by c-Kit in hematopoietic cells. Int. J. Biochem. Cell Biol. 1999, 31, 1053–1074. [Google Scholar] [CrossRef]
- Ali, S. Role of c-Kit/SCF in cause and treatment of gastrointestinal stromal tumors (GIST). Gene 2007, 401, 38–45. [Google Scholar] [CrossRef]
- Lennartsson, J.; Rönnstrand, L. Stem cell factor receptor/c-Kit: from basic science to clinical implications. Physiol. Rev. 2012, 92, 1619–1649. [Google Scholar] [CrossRef] [PubMed]
- Raghav, P.K.; Singh, A.K.; Gangenahalli, G. A change in structural integrity of c-Kit mutant D816V causes constitutive signaling. Mutat. Res. 2018, 808, 28–38. [Google Scholar] [CrossRef]
- Kuga, H.; et al. Interferon-γ suppresses transforming growth factor-β-induced invasion of gastric carcinoma cells through cross-talk of Smad pathway in a three-dimensional culture model. Oncogene 2003, 22, 7838–7847. [Google Scholar] [CrossRef]
- Koh, J.; et al. Regulatory (FoxP3+) T cells and TGF-β predict the response to anti-PD-1 immunotherapy in patients with non-small cell lung cancer. Sci. Rep. 2020, 10, 18994. [Google Scholar] [CrossRef]
- Hasegawa, Y.; et al. Transforming growth factor-β1 level correlates with angiogenesis, tumor progression, and prognosis in patients with nonsmall cell lung carcinoma. Cancer 2001, 91, 964–971. [Google Scholar] [CrossRef]
- Stockhammer, P.; et al. Detection of TGF-β in pleural effusions for diagnosis and prognostic stratification of malignant pleural mesothelioma. Lung Cancer 2020, 139, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Omori, I.; et al. D816V mutation in the KIT gene activation loop has greater cell-proliferative and anti-apoptotic ability than N822K mutation in core-binding factor acute myeloid leukemia. Exp. Hematol. 2017, 52, 56–64. [Google Scholar] [CrossRef]
- Ferrao, P.; Gonda, T.J.; Ashman, L.K. Expression of constitutively activated human c-Kit in Myb transformed early myeloid cells leads to factor independence, histiocytic differentiation, and tumorigenicity. Blood 1997, 90, 4539–4552. [Google Scholar] [CrossRef]
- Xiang, Z.; Kreisel, F.; Cain, J.; Colson, A.; Tomasson, M.H. Neoplasia driven by mutant c-KIT is mediated by intracellular, not plasma membrane, receptor signaling. Mol. Cell. Biol. 2007, 27, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Hirota, S.; et al. Gain-of-function mutations of c-Kit in human gastrointestinal stromal tumors. Science 1998, 279, 577–580. [Google Scholar] [CrossRef]
- Falchi, L.; Verstovsek, S. Kit mutations: new insights and diagnostic value. Immunol. Allergy Clin. North Am. 2018, 38, 411–428. [Google Scholar] [CrossRef]
- Abbaspour Babaei, M.; Kamalidehghan, B.; Saleem, M.; Huri, H.Z.; Ahmadipour, F. Receptor tyrosine kinase (c-Kit) inhibitors: a potential therapeutic target in cancer cells. Drug Des. Devel. Ther. 2016, 10, 2443–2459. [Google Scholar] [CrossRef]
- Hou, B.; Tang, Y.; Li, W.; Zeng, Q.; Chang, D. Efficiency of CAR-T therapy for treatment of solid tumor in clinical trials: a meta-analysis. Dis. Markers 2019, 2019, 3425291. [Google Scholar] [CrossRef]
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R. A.; et al. Case report of a serious adverse event following the administration of t cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 2010, 18, 843–851. [Google Scholar] [CrossRef]
- Hirabayashi, K.; et al. Dual-targeting CAR-T cells with optimal co-stimulation and metabolic fitness enhance antitumor activity and prevent escape in solid tumors. Nat. Cancer 2021, 2, 904–918. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, N.; Shi, H. Driving better and safer HER2-specific CARs for cancer therapy. Oncotarget. 2017, 8, 62730–62741. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Rourke, D. M.; et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 2017, 9, eaaa0984. [Google Scholar] [CrossRef]
- Ling, V.; et al. Duplication of primate and rodent B7-H3 immunoglobulin V- and C-like domains: divergent history of functional redundancy and exon loss. Genomics 2003, 82, 365–377. [Google Scholar] [CrossRef]
- Majzner, R. G.; et al. CAR T cells targeting b7-h3, a pan-cancer antigen, demonstrate potent preclinical activity against pediatric solid tumors and brain tumors. Clin. Cancer Res 2019, 25, 2560–2574. [Google Scholar] [CrossRef] [PubMed]
- Kontos, F.; et al. B7-H3: an attractive target for antibody-based immunotherapy. Clin. Cancer Res 2021, 27, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Lichtman, E. I.; et al. Preclinical evaluation of b7-h3-specific chimeric antigen receptor t cells for the treatment of acute myeloid leukemia. Clin. Cancer Res 2021, 27, 3141–3153. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, S. M.; et al. ATP in the tumour microenvironment drives expression of nfP2X7, a key mediator of cancer cell survival. Oncogene 2019, 38, 194–208. [Google Scholar] [CrossRef]
- Barden, J.A.; Yuksel, A.; Pedersen, J.; Danieletto, S.; Delprado, W. Non-functional P2X7: a novel and ubiquitous target in human cancer. J. Clin. Cell. Immunol. 2014, 5, 2155–9899. [Google Scholar] [CrossRef]
- Lara, R.; et al. P2X7 in cancer: from molecular mechanisms to therapeutics. Front. Pharm. 2020, 11, 793. [Google Scholar] [CrossRef]
- Park, A. K.; et al. Effective combination immunotherapy using oncolytic viruses to deliver CAR targets to solid tumors. Sci. Transl. Med. 2020. [CrossRef]
- Lam JT et al Inter-patient variation in efficacy of five oncolytic adenovirus candidates for ovarian cancer therapy, J. Gene Med. 2004, 6, 1333–1342. [CrossRef] [PubMed]
- van den Hengel, S. K.; et al. Heterogeneous reovirus susceptibility in human glioblastoma stem-like cell cultures. Cancer Gene Ther. 2013, 20, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Kwong, B.; Irvine, D.J. Membrane anchored immunostimulatory oligonucleotides for in vivo cell modification and localized immunotherapy. Angew. Chem. Int. Ed. 2011, 50, 7052–7055. [Google Scholar] [CrossRef] [PubMed]
- Gulley JL et al Role of antigen spread distinctive characteristics of immunotherapy in cancer treatment, J. Natl Cancer Inst. 2017. [CrossRef]
- Ma, L.; et al. Enhanced CAR-T cell activity against solid tumors by vaccine boosting through the chimeric receptor. Science 2019, 365, 162–168. [Google Scholar] [CrossRef]
- Melero, I.; Castanon, E.; Alvarez, M.; Champiat, S.; Marabelle, A. Intratumoural administration and tumour tissue targeting of cancer immunotherapies. Nat. Rev. Clin. Oncol. 2021. [CrossRef]
- Champiat, S.; et al. Intratumoral immunotherapy: from trial design to clinical practice. Clin. Cancer Res. 2021, 27, 665–679. [Google Scholar] [CrossRef]
- Pugieux-Amarantos, C. General Chapter 5.2.12: Raw Materials of Biological Origin for the Production of Cell-Based and Gene Therapy Medicinal Products. European Pharmacopoeia Training Session on Biologicals, Strasbourg, France, ; https://www.edqm.eu/sites/default/files/cell_and_gene_therapy_by_celine_pugieux-amarantos-bio-training-feb2017.pdf. 7–8 February.
- Kymriah SmPC, 4.2 https://www.ema.europa.eu/en/documents/product-information/kymriah-epar-product-information_en.
- H. Jain, A.Karulkar et al; High Efficacy and Excellent Safety Profile of Actalycabtagene Autoleucel, a Humanized CD19 CAR-T Product in r/r B-Cell Malignancies: A Phase II Pivotal Trial. Blood 2023, 142 (Supplement 1), 4838. [Google Scholar] [CrossRef]
- Cutting-edge CAR-T cancer therapy is now made in India — at one-tenth the cost Smriti Mallapaty Nature 2024, 627, 709–710. [CrossRef]
- India’s First Homegrown CAR T-Cell Therapy Has Roots in NCI Collaboration February 7, 2024, by Linda Wang https://www.cancer.gov/news-events/cancer-currents-blog/2024/nexcar19-car-t-cell-therapy-india-nci-collaboration. February.
- Malhotra, P.; Damodar, S.; Bhat, S.; Thirumalairaj, R.; Raj, R.; et al. . P1409: PHASE-2 STUDY OF VARNIMCABTAGENE AUTOLEUCEL (IMN-003A) FIRST-IN-INDIA INDUSTRY CD19-DIRECTED CAR-T WITH FRACTIONATED INFUSIONS FOR PATIENTS WITH RELAPSED REFRACTORY B CELL MALIGNANCIES: IMAGINE STUDY. HEMASPHERE. 2023, 7, e03326b2. [Google Scholar] [CrossRef] [PubMed Central]
- Magnani, C.F.; Gaipa, G.; Lussana, F.; Belotti, D.; Gritti, G.; et al. Sleeping Beauty-engineered CAR T cells achieve antileukemic activity without severe toxicities. J Clin Invest. 2020, 130, 6021–6033. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ye, L. , Lam, S.Z., Yang, L. et al. AAV-mediated delivery of a Sleeping Beauty transposon and an mRNA-encoded transposase for the engineering of therapeutic immune cells. Nat. Biomed. Eng 2024, 8, 132–148. [Google Scholar] [CrossRef]
- Liu, M.A. A comparison of plasmid DNA and mRNA as vaccine technologies. Vaccines 2019, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Sadelain, M.; Papapetrou, E.P.; Bushman, F.D. Safe harbours for the integration of new DNA in the human genome. Nat. Rev. Cancer 2011, 12, 51–58. [Google Scholar] [CrossRef]
- Querques, I.; et al. A highly soluble Sleeping Beauty transposase improves control of gene insertion. Nat. Biotechnol. 2019, 37, 1502–1512. [Google Scholar] [CrossRef]
- Roth, S.L.; Malani, N.; Bushman, F.D. Gammaretroviral integration into nucleosomal target DNA in vivo. J. Virol. 2011, 85, 7393–7401. [Google Scholar] [CrossRef]
- Kolacsek, O.; et al. Reliable transgene-independent method for determining Sleeping Beauty transposon copy numbers. Mob. DNA 2011, 2, 5. [Google Scholar] [CrossRef]
- Reducing Ex Vivo Culture Improves the Antileukemic Activity of Chimeric Antigen Receptor (CAR) T Cells; S. Ghassemi, M. Milone, Center for Cellular Immunotherapies, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
- Rapid manufacturing of non-activated potent CAR T cells; S. Ghassemi, M. Milone, Center for Cellular Immunotherapies, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
- Antigen Presenting Cell Mimetic Lipid Nanoparticles for Rapid mRNA CAR T Cell Cancer Immunotherapy; A.Metzloff, … C.June, M.Mitchell, Department of Bioengineering, School of Engineering and Applied Science, University of Pennsylvania, Philadelphia.
- Blache, U.; Popp, G.; Dünkel, A.; Koehl, U.; Fricke, S. Potential solutions for manufacture of CAR T cells in cancer immunotherapy. Nat. Commun. 2022, 13, 5225. [Google Scholar] [CrossRef]
- Elsallab, M.; Maus, M.V. Expanding access to CAR T cell therapies through local manufacturing. Nat. Biotechnol. 2023. [Google Scholar] [CrossRef]
- Directive 2009/120/EC https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:242:0003:0012:EN:PDF.
- Guidelines on Good Manufacturing Practice specific to Advanced: Therapy Medicinal Products. EudraLex: The Rules Governing Medicinal Products in the European Union, Volume 4 — Good Manufacturing Practice. European Commission: Brussels, Belgium, 22 November 2017; https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-4/2017_11_22_guidelines_gmp_for_atmps.pdf.
- Vormittag, P.; Gunn, R.; Ghorashian, S.; Veraitch, F.S. A guide to manufacturing CAR T cell therapies. Curr. Opin. Biotechnol. 2018, 53, 164–181. [Google Scholar] [CrossRef]
- Mitra, A.; et al. From bench to bedside: the history and progress of CAR T cell therapy. Front. Immunol. 2023, 14, 1188049. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi, S.; et al. Rapid manufacturing of non-activated potent CAR T cells. Nat. Biomed. Eng. 2022, 6, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi, S.; et al. Reducing ex vivo culture improves the antileukemic activity of chimeric antigen receptor (CAR) T cells. Cancer Immunol. Res. 2018, 6, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Abou-El-Enein, M.; et al. Scalable manufacturing of CAR T cells for cancer immunotherapy. Blood Cancer Discov. 2021, 2, 408–422. [Google Scholar] [CrossRef] [PubMed]
- Ganeeva, I.; et al. Recent advances in the development of bioreactors for manufacturing of adoptive cell immunotherapies. Bioengineering 2022, 9, 808.
- Baudequin, T.; Nyland, R.; Ye, H. Objectives, benefits and challenges of bioreactor systems for the clinical-scale expansion of T lymphocyte cells. Biotechnol. Adv. 2021, 49, 107735. [Google Scholar] [CrossRef]
- Ravindran, S.; et al. Microbioreactors and perfusion bioreactors for microbial and mammalian cell culture. Biotechnology and Bioengineering (IntechOpen, 2019). [CrossRef]
- Dai, X.; et al. Scaling up the manufacturing process of adoptive T cell immunotherapy. Biotechnol. J. 2019, 14, 1800239. [Google Scholar] [CrossRef]
- Tian, Y.; Hu, R.; Du, G.; Xu, N. Microfluidic chips: emerging technologies for adoptive cell immunotherapy. Micromachines 2023, 14, 877. [Google Scholar] [CrossRef]
- Lee, K.S.; Boccazzi, P.; Sinskey, A.J.; Ram, R.J. Microfluidic chemostat and turbidostat with flow rate, oxygen, and temperature control for dynamic continuous culture. Lab Chip 2011, 11, 1730–1739. [Google Scholar] [CrossRef]
- McLaurin, C.; Pham, Q.L.; Mahadevan, J. Do more with less: fit-for-purpose tools to speed up upstream process development for continuous biomanufacturing. Integrated Continuous Biomanufacturing V ( 2022.
- Quinn, W. J.; et al. Lactate limits T cell proliferation via the NAD(H) redox state. Cell Rep. 2020, 33, 108500. [Google Scholar] [CrossRef]
- Bai Z et al Single-cell antigen-specific landscape of CART infusion product identifies determinants of CD19-positive relapse in patients with, A.L.L. Sci. Adv. 2022, 8, eabj2820.
- Baradez, M.-O.; Biziato, D.; Hassan, E.; Marshall, D. Application of Raman spectroscopy and univariate modelling as a process analytical technology for cell therapy bioprocessing. Front. Med. 2018, 5, 47. [Google Scholar] [CrossRef]
- Amini, A.; Veraitch, F. Glucose deprivation enriches for central memory T cells during chimeric antigen receptor-T cell expansion. Cytotherapy 2019, 21, S30–S31. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, J.; Wang, K.; Wang, B.; Zhang, C. Current status and opportunities in adaptive data analysis for therapeutic cell manufacturing. Curr. Opin. Biomed. Eng. 2021, 20, 100351. [Google Scholar] [CrossRef]
- Van Beylen, K.; et al. Real-time cell growth control using a lactate-based model predictive controller. Processes 2023, 11, 22. [Google Scholar] [CrossRef]
- Klysz, D. D.; et al. Inosine induces stemness features in CAR-T cells and enhances potency. Cancer Cell 2024, 42, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Shalabi, H.; Qin, H.; Su, A.; Yates, B.; et al. CD19/22 CART cells in children young adults with B-ALL: phase 1 results development of a novel bicistronic, C. A.R. Blood. 2022, 140, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives Set, al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. 2018, 378, 439–448. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shah, B.D.; Ghobadi, A.; Oluwole, O.O.; Logan, A.C.; et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet. 2021, 398, 491–502. [Google Scholar] [CrossRef]
- Asnani, M.; Hayer, K.E.; Naqvi, A.S.; Zheng, S.; Yang, S.Y.; et al. Retention of CD19 intron 2 contributes to CART-19 resistance in leukemias with subclonal frameshift mutations in CD19. Leukemia. 2020, 34, 1202–1207. [Google Scholar] [CrossRef]
- Mock, U.; Nickolay, L.; Philip, B.; Cheung, G.W.; Zhan, H.; Johnston ICD, et al. Automated manufacturing of chimeric antigen receptor T cells for adoptive immunotherapy using CliniMACS Prodigy. Cytotherapy. 2016, 18, 1002–1011. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.N.; Johnson, B.D.; Schneider, D.; Zhu, F.; Szabo, A.; Keever-Taylor, C.A.; et al. Bispecific anti-CD20, anti-CD19 CAR T cells for relapsed B cell malignancies: a Phase 1 dose escalation and expansion trial. Nat Med. 2020, 26, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Fry, T.J.; Shah, N.N.; Orentas, R.J.; Stetler-Stevenson, M.; Yuan, C.M.; Ramakrishna, S.; et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018, 24, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Del Bufalo, F.; Becilli, M.; Rosignoli, C.; De Angelis, B.; Algeri, M.; et al. Allogeneic donor-derived second-generation CD19-directed CAR-T cells for the treatment of pediatric relapsed/refractory, B. C.P.-A.L.L. Blood. 2023, 142, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Zanini, C. , Severina, F., Lando, G., Fanizza, C., Cesana, E., Desidera, D., & Bonifacio, M. (2020). Good Design Practices for an integrated containment and production system for Advanced Therapies. Biotechnology and bioengineering. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




