Submitted:
07 August 2024
Posted:
07 August 2024
You are already at the latest version
Abstract

Keywords:
1. Introduction
2. Materials and Methods
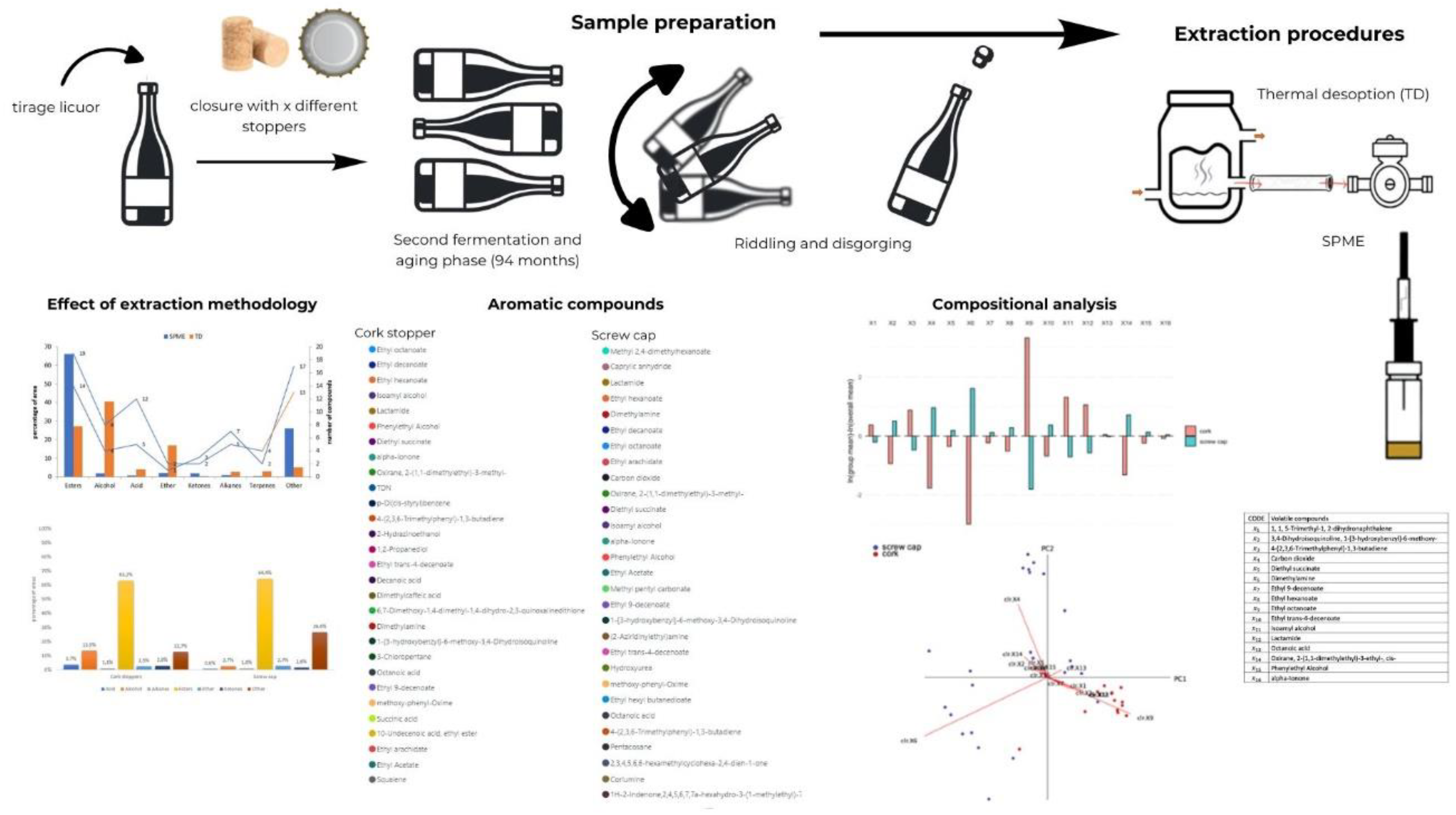
2.1. Closures and Wine Second Fermentation
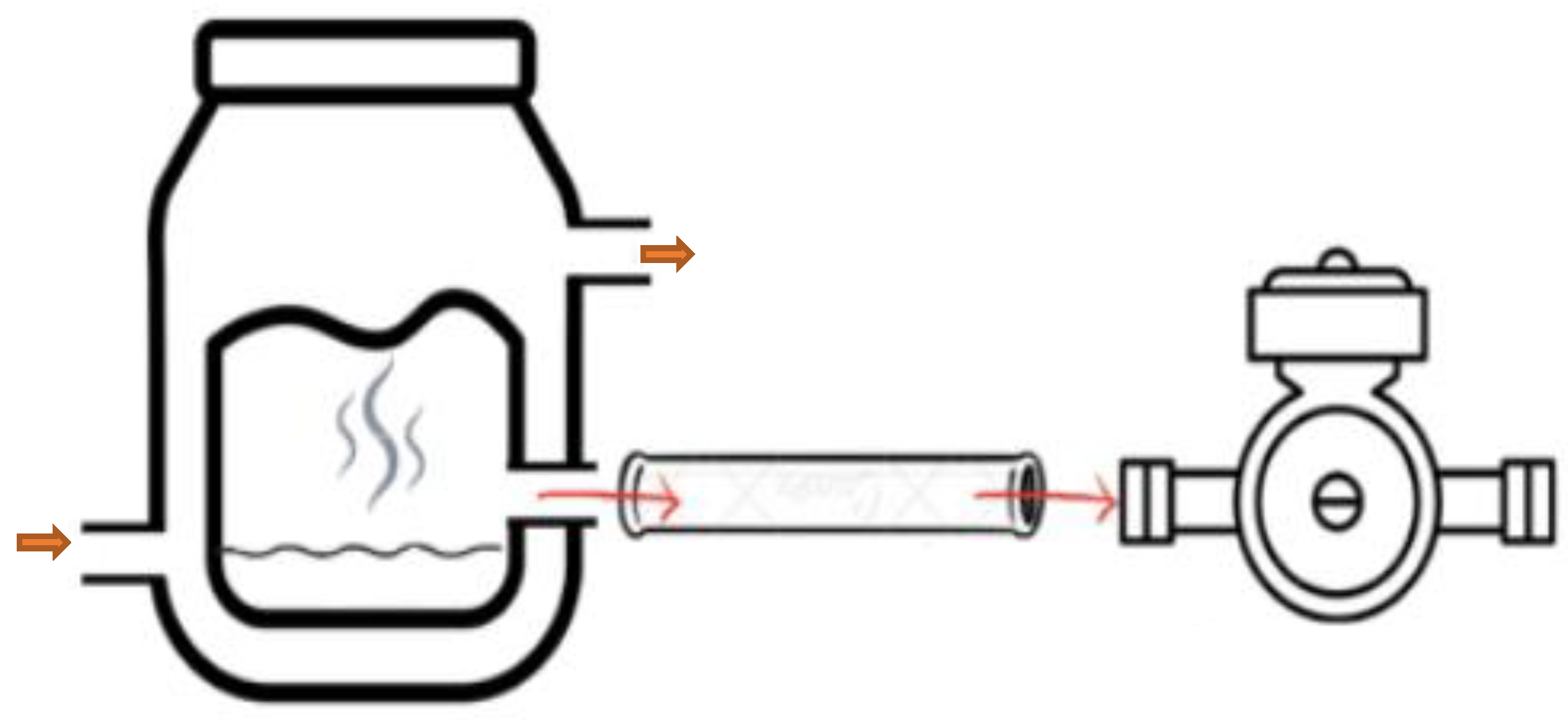
2.2. Extraction of Volatile Compounds
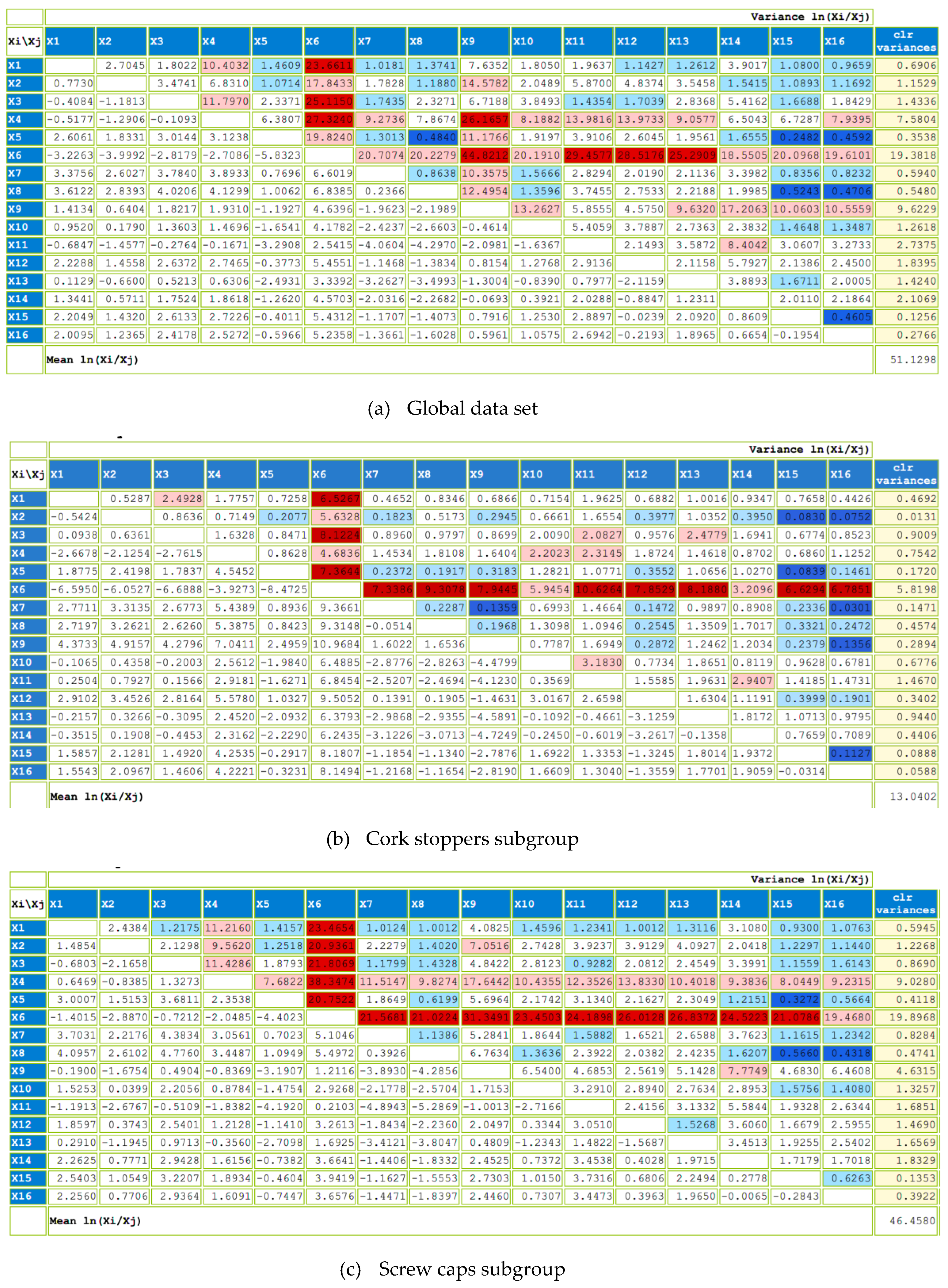
2.3. Compositional Data Analysis
3. Results
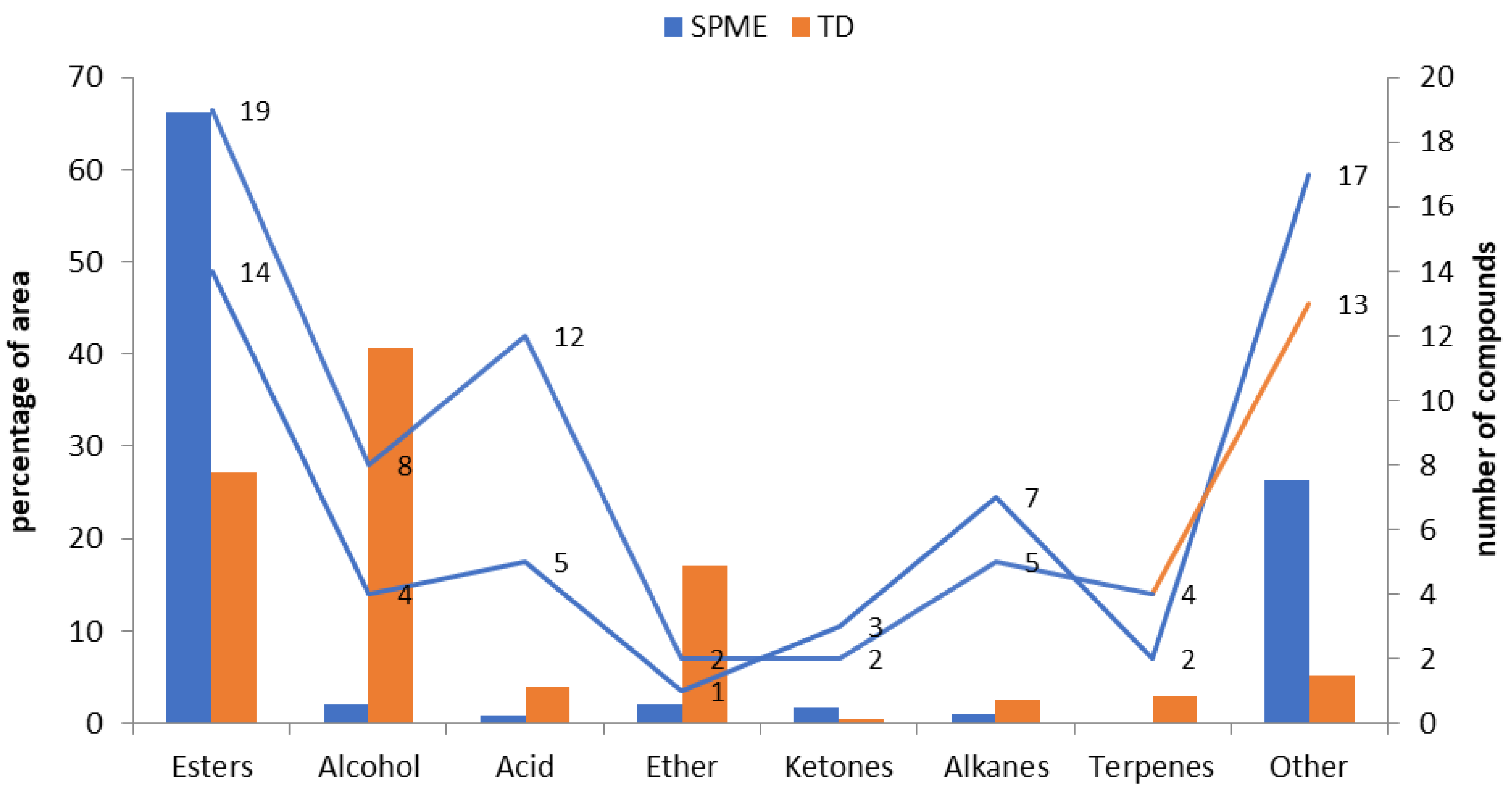
3.1. Effect of Extraction Methodology
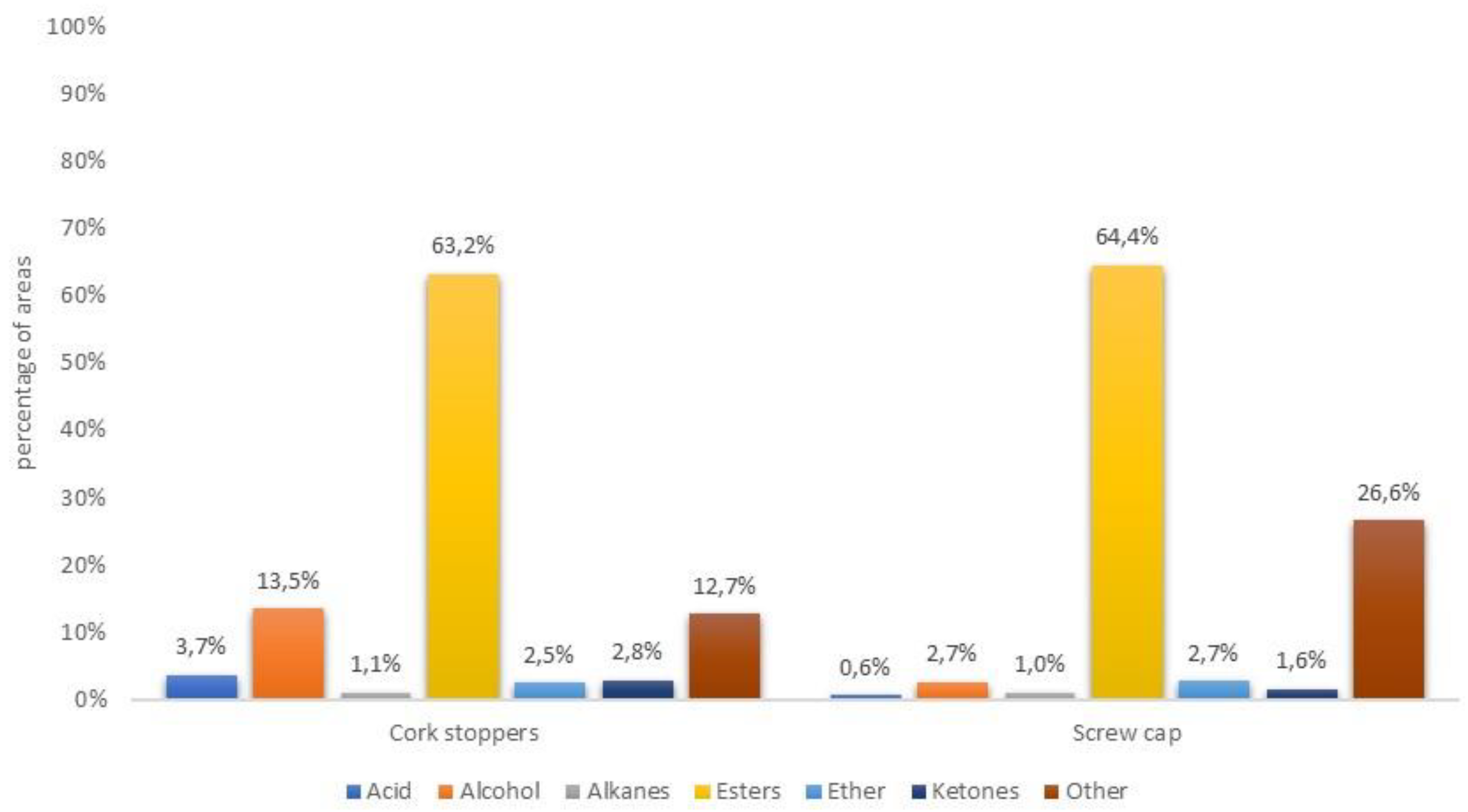
3.2. Aromatic Compounds Versus Type of Closure
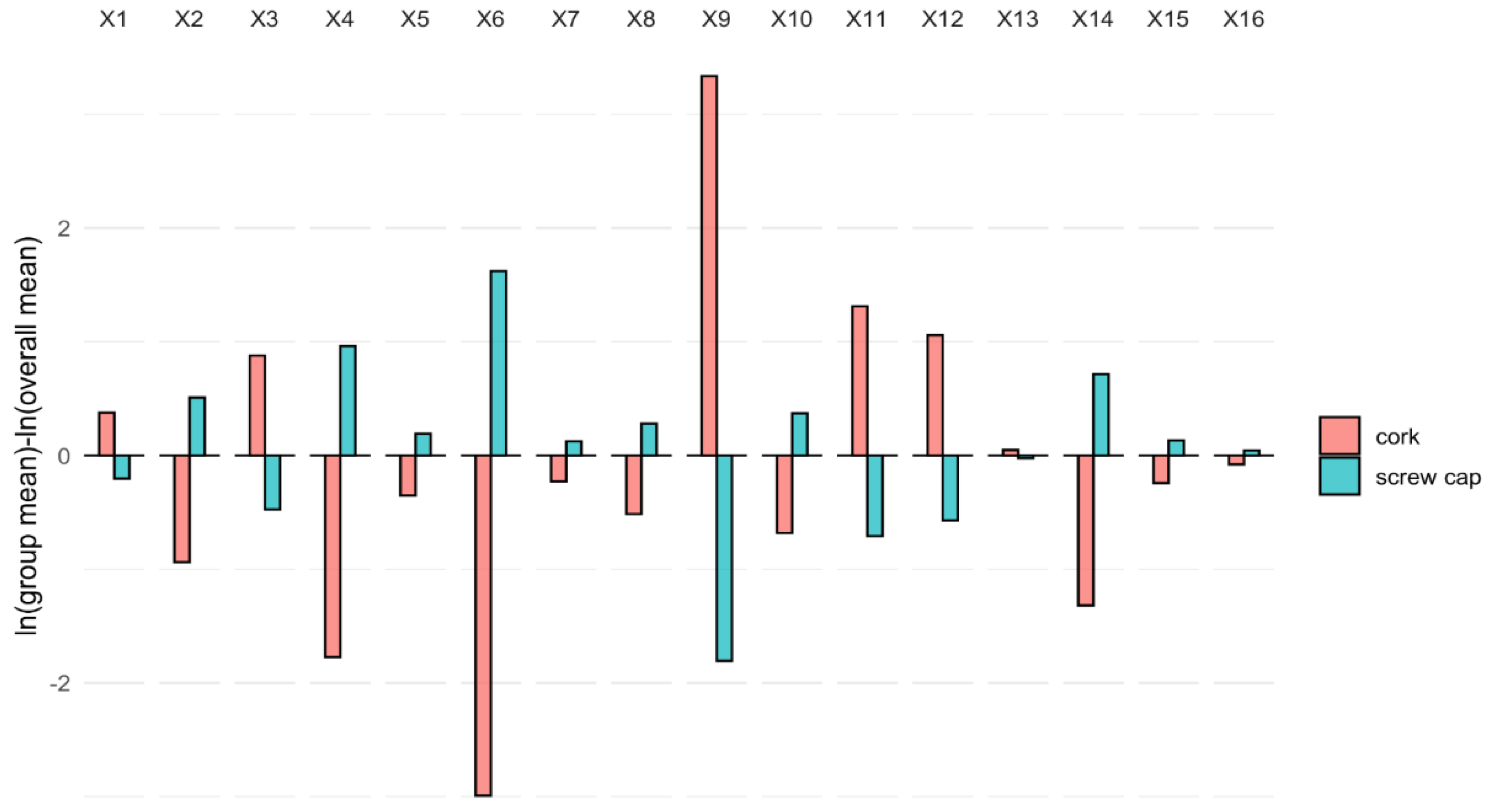
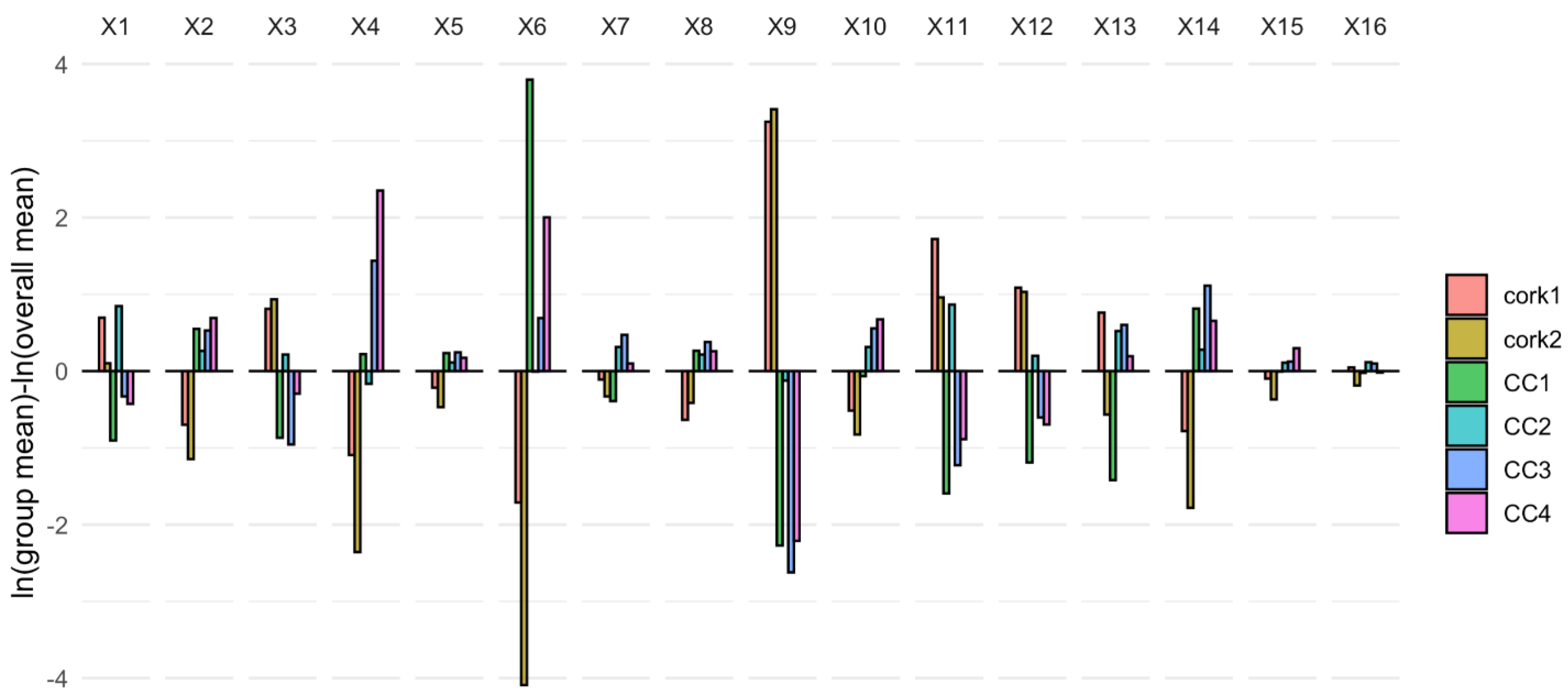
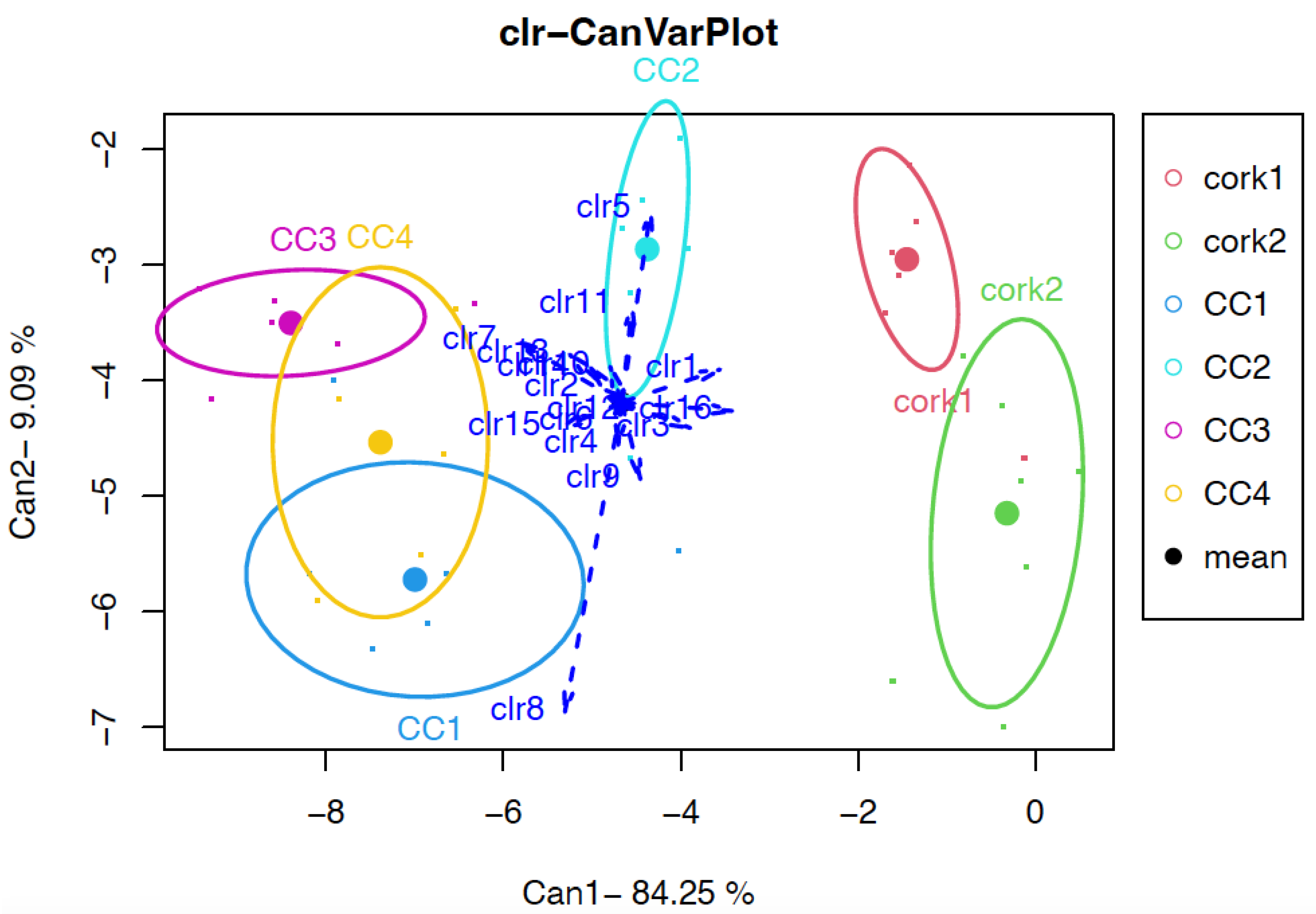
3.3. Effect of the Type of Closure through Compositional Data Analysis
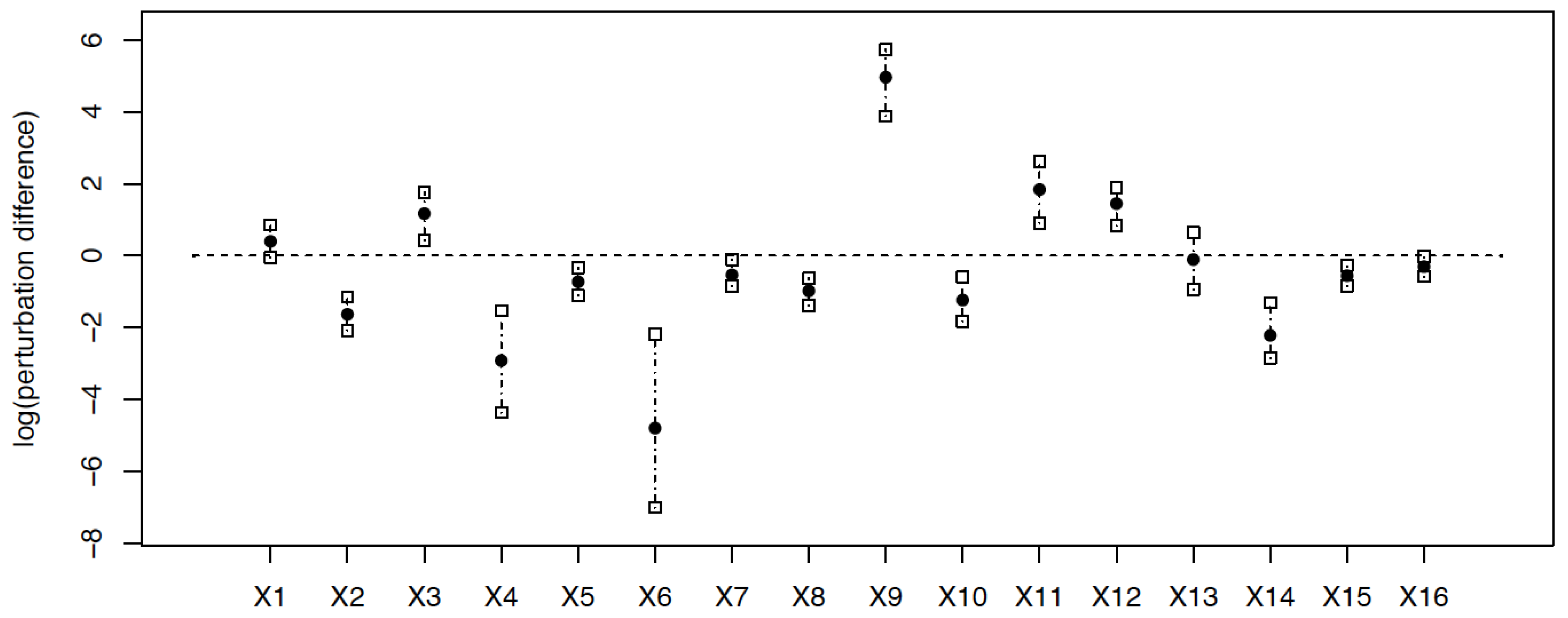
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
| Extracted compounds | cc1 | cc2 | cc3 | cc4 | cork 1 | cork 2 | ||||||
| SPME | TD | SPME | TD | SPME | TD | SPME | TD | SPME | TD | SPME | TD | |
| Acids | ||||||||||||
| 3-Hydroxydodecanoic acid | 0,58 | 0,7 | 1,07 | 0,56 | 0,95 | 0,97 | ||||||
| 3-Methyl-2-propionyl-benzoic acid | 0,4 | |||||||||||
| Acetic acid | 1,25 | 1,81 | ||||||||||
| Alkynyl Stearic Acid | 1,19 | 1,87 | 1,53 | |||||||||
| Aminomethanesulfonic acid | 1,08 | 1,26 | ||||||||||
| Anticopalic acid | 1,19 | |||||||||||
| Decanoic acid | 0,67 | 0,83 | 0,52 | 1,17 | 1,85 | |||||||
| Dimethylcaffeic acid | 1,6 | 0,61 | ||||||||||
| L-Cysteic acid | 0,46 | 0,7 | ||||||||||
| Octanoic acid | 0,39 | 0,58 | 0,46 | 0,43 | 2,55 | 0,92 | 0,7 | |||||
| Oleic Acid | 0,64 | |||||||||||
| Palmitelaidic acid | 0,34 | |||||||||||
| Paullinic acid | 0,61 | |||||||||||
| Succinic acid | 0,23 | 1,67 | ||||||||||
| Undecanoic acid | 0,36 | |||||||||||
| Alcohol | ||||||||||||
| 2,2,4-Trimethyl-3-(3,8,12,16-tetramethyl-heptadeca-3,7,11,15-tetraenyl)-cyclohexanol | 0,95 | |||||||||||
| 2-Propanediol | ||||||||||||
| 10-Methyl-E-11-tridecen-1-ol propionate | 0,59 | 2,56 | 2,23 | |||||||||
| 1-Butanol | 3,75 | |||||||||||
| 1-Hexanol | 0,99 | 0,77 | 1,26 | 1,37 | 1,09 | |||||||
| 15-tetraenyl)-cyclohexanol | ||||||||||||
| 2-Hydrazinoethanol | 2,34 | |||||||||||
| 1-Tetradecenol | 0,58 | |||||||||||
| Ethanol | 0,65 | |||||||||||
| Isoamyl alcohol | 43,2 | 1,42 | 32,7 | 20,7 | 34,5 | 2,82 | 47,8 | 1,3 | 49,7 | |||
| Phenylethyl Alcohol | 0,84 | 0,66 | 1,15 | 0,65 | 1,15 | 0,32 | 1,29 | 1,12 | 3,73 | 1,58 | 2,59 | 2,57 |
| Alkanes | ||||||||||||
| 5-methyl-1-Hexane | 2,27 | |||||||||||
| 3-Chloropentane | 1,27 | |||||||||||
| Dotriacontane | 0,62 | |||||||||||
| Hexadecane | 0,78 | 0,68 | 0,93 | |||||||||
| Hexatriacontane | 0,43 | |||||||||||
| n-Hexane | 0,43 | 4,83 | ||||||||||
| Nonacosane | 0,82 | |||||||||||
| Octadecane | 0,38 | 1,56 | ||||||||||
| Octathiocane | 0,78 | 2,23 | ||||||||||
| Pentacosane | 0,8 | 0,46 | ||||||||||
| Tetratriacontane | 0,62 | |||||||||||
| Vinyl decanoate | 0,48 | |||||||||||
| Ester | ||||||||||||
| 10-Undecenoic acid ethyl ester | 0,99 | |||||||||||
| Butyl ethyl succinate | 3,37 | 1,56 | ||||||||||
| Decyl oleate | 0,96 | |||||||||||
| Diethyl Phthalate | 0,44 | 0,71 | 1,29 | 1,19 | ||||||||
| Diethyl succinate | 2,08 | 1,62 | 1,6 | 1,81 | 1,34 | 2,2 | 1 | 2,26 | 2,18 | 2,36 | 3,03 | 2,89 |
| Ethyl 9-decenoate | 1,22 | 1,36 | 0,82 | 0,85 | 1,16 | |||||||
| Ethyl 9-oxononanoate | 0,9 | |||||||||||
| Ethyl Acetate | 4,96 | 2,92 | 3,14 | 6,25 | 3,65 | 2,3 | 4,1 | |||||
| Ethyl arachidate | 9,81 | 1,41 | 0,52 | 1,76 | 1,17 | 6,22 | 2,46 | 4,24 | 2,46 | |||
| Ethyl butyrate | 1,04 | 0,7 | 0,59 | 1,53 | 1,13 | 1,05 | ||||||
| Ethyl cholate | 0,69 | |||||||||||
| Ethyl decanoate | 4,99 | 1,17 | 7,28 | 1,96 | 4,61 | 1,48 | 6,05 | 2,7 | 14,5 | 2,17 | 8,12 | 2,29 |
| Ethyl hexanoate | 7,99 | 1,07 | 6,11 | 2,25 | 5,35 | 2,29 | 4,86 | 3,35 | 10,9 | 4,03 | 9,3 | 0,88 |
| Ethyl hexyl butanedioate | 1,22 | |||||||||||
| Ethyl octanoate | 17,4 | 7,81 | 2,99 | 3,75 | 8,43 | 11 | 30,9 | 7,45 | 49,5 | 5,81 | ||
| Ethyl palmitate | 0,5 | |||||||||||
| Ethyl stearate | 2,4 | |||||||||||
| Ethyl trans-4-decenoate | 0,76 | 0,85 | 0,54 | 0,66 | 0,72 | 2 | 0,8 | |||||
| Hexyl chloroformate | 1,33 | |||||||||||
| Isoamyl lactate | 2,79 | |||||||||||
| Isopropyl palmitate | 0,41 | 0,65 | 0,79 | 0,62 | ||||||||
| Methyl 2 4-dimethylhexanoate | 30,9 | 1,88 | 42,1 | 4,05 | 40,7 | 43,6 | 4,07 | |||||
| Methyl pentyl carbonate | 3,59 | |||||||||||
| Nonanoic acid 5-methyl- ethyl ester | 1,05 | 1,21 | 1,55 | 2,34 | 1,51 | |||||||
| Oryctalure | 0,46 | |||||||||||
| Ether | ||||||||||||
| 1-Methyl-1-silacyclopentan-1-ol | 0,28 | |||||||||||
| Oxirane 2-(1 1-dimethylethyl)-3-ethyl | 1,98 | 22,8 | 1,43 | 30,8 | 2 | 33,3 | 2,09 | 4,32 | 1,98 | |||
| ketone | ||||||||||||
| 2,3,4,5,6,6-hexamethylcyclohexa-2,4-dien-1-one | 1,7 | |||||||||||
| 2,2-dimethyl-5-phenylfuran-3-one | 0,61 | 0,61 | ||||||||||
| alpha-Ionone | 1,74 | 0,32 | 1,77 | 0,53 | 0,98 | 0,53 | 1,02 | 0,83 | 4,16 | 2,35 | ||
| Caprolactone | 0,61 | |||||||||||
| Terpenes | ||||||||||||
| D-Limonene | 0,32 | |||||||||||
| Friedelin | 0,62 | 0,95 | 1,96 | |||||||||
| Squalene | 0,99 | 0,73 | 1,3 | 3,36 | 1,19 | 2,96 | 2,02 | |||||
| TDN | 0,59 | 0,18 | 0,48 | 1,94 | 1,27 | 1,07 | 0,69 | |||||
| Other | ||||||||||||
| 2-amino-4,6-dihydro-4,4,6,6-tetramethyl-Thieno[2,3-c]furan-3-carbonitrile | 0,38 | |||||||||||
| (2-Aziridinylethyl)amine | 1,14 | 1,79 | ||||||||||
| 1,1,5-Trimethyl-1,2-dihydronaphthalene | ||||||||||||
| 1-[3-hydroxybenzyl]-6-methoxy-3,4-Dihydroisoquinoline | 1,15 | 1,56 | 0,64 | 0,79 | 2,19 | |||||||
| 12-O-Acetylingol 8-tiglate | 0,74 | |||||||||||
| 1H-2-Indenone,2,4,5,6,7,7a-hexahydro-3-(1-Methylethyl)-7a-methyl | 0,88 | |||||||||||
| 1-Octadecyne | 0,67 | |||||||||||
| 2,3,4-Trimethyllevoglucosan | 4,48 | 3,23 | ||||||||||
| 2,4-dimethyl-1,3-Dioxane | 0,51 | 1,69 | ||||||||||
| 2-Bromooctadecanal | 0,51 | 1,09 | 0,48 | 0,44 | 0,79 | 0,44 | ||||||
| 2-Myristynoyl pantetheine | 0,25 | |||||||||||
| 4-(2,3,6-Trimethylphenyl)-1,3-butadiene | 0,41 | 0,57 | 1,02 | 0,34 | 2,05 | 1,16 | ||||||
| 6,7-Dimethoxy-1,4-dimethyl-1,3-quinoxalinedithione | 1,46 | |||||||||||
| Benzaldehyde | 0,41 | |||||||||||
| Bis(2-ethylhexyl) phthalate | 1,93 | 1,76 | ||||||||||
| Caprylic anhydride | 6,66 | 18,4 | 5,57 | 1,66 | 1,45 | |||||||
| Carbon dioxide | 4,86 | 2,52 | 2,7 | 3,42 | ||||||||
| Corlumine | 0,39 | 0,54 | ||||||||||
| Dimethylamine | 6,31 | 14 | 4,68 | 2,01 | 2,32 | |||||||
| Emulphor | 1,1 | 0,92 | ||||||||||
| Hydroxyurea | 2,52 | |||||||||||
| Isoflavene | 0,76 | |||||||||||
| Lactamide | 2,69 | 4,78 | 14,8 | 3,61 | 6,83 | 4,53 | ||||||
| Laudanosine | 0,56 | |||||||||||
| Longifolenaldehyde | 3 | |||||||||||
| Methoxy-phenyl-Oxime | 0,67 | 1,03 | 0,25 | 0,71 | 1,11 | |||||||
| N-Methylcalycotomine | 0,12 | |||||||||||
| Paromomycin | 0,34 | |||||||||||
| p-Di(cis-styryl)benzene | 2,61 | 0,85 | ||||||||||
| Phenol | 0,68 | |||||||||||
| Code | Global data | Cork stopper | Screw cap |
| 0.008178 | 0.006604 | 0.005742 | |
| 0.017714 | 0.003840 | 0.025362 | |
| 0.005436 | 0.007253 | 0.002908 | |
| 0.004873 | 0.000458 | 0.010966 | |
| 0.110776 | 0.043172 | 0.115416 | |
| 0.000325 | 0.000009 | 0.001414 | |
| 0.239144 | 0.105512 | 0.232965 | |
| 0.302983 | 0.100229 | 0.344981 | |
| 0.033609 | 0.523776 | 0.004749 | |
| 0.021187 | 0.005937 | 0.026394 | |
| 0.004123 | 0.008483 | 0.001745 | |
| 0.075963 | 0.121259 | 0.036875 | |
| 0.009156 | 0.005323 | 0.007681 | |
| 0.031359 | 0.004647 | 0.055165 | |
| 0.074170 | 0.032247 | 0.072831 | |
| 0.061003 | 0.031251 | 0.054808 |
| Code | Global center | Cork 1 | Cork 2 | CC1 | CC2 | CC3 | CC4 |
| 0.008178 | 0.009134 | 0.004938 | 0.003287 | 0.015248 | 0.004281 | 0.004441 | |
| 0.017714 | 0.004912 | 0.003069 | 0.030482 | 0.018456 | 0.021904 | 0.029430 | |
| 0.005436 | 0.006814 | 0.007556 | 0.002265 | 0.005396 | 0.001522 | 0.003370 | |
| 0.004873 | 0.000910 | 0.000251 | 0.006031 | 0.003304 | 0.014947 | 0.042555 | |
| 0.110776 | 0.049711 | 0.037772 | 0.139048 | 0.099106 | 0.103049 | 0.109530 | |
| 0.000325 | 0.000033 | 0.000003 | 0.014368 | 0.000258 | 0.000472 | 0.002000 | |
| 0.239144 | 0.119351 | 0.093734 | 0.160628 | 0.261992 | 0.279475 | 0.219533 | |
| 0.302983 | 0.089439 | 0.109111 | 0.392126 | 0.300740 | 0.322618 | 0.326333 | |
| 0.033609 | 0.482210 | 0.555137 | 0.003440 | 0.023763 | 0.001781 | 0.003061 | |
| 0.021187 | 0.007054 | 0.005056 | 0.019705 | 0.023202 | 0.026922 | 0.034560 | |
| 0.004123 | 0.012845 | 0.005869 | 0.000831 | 0.007845 | 0.000881 | 0.001413 | |
| 0.075963 | 0.125506 | 0.116244 | 0.022926 | 0.074176 | 0.030249 | 0.031507 | |
| 0.009156 | 0.010934 | 0.002835 | 0.002196 | 0.012344 | 0.012180 | 0.009241 | |
| 0.031359 | 0.008004 | 0.002879 | 0.070237 | 0.033142 | 0.069526 | 0.050158 | |
| 0.074170 | 0.037497 | 0.027978 | 0.073222 | 0.066240 | 0.061168 | 0.083130 | |
| 0.061003 | 0.035645 | 0.027566 | 0.059207 | 0.054787 | 0.049024 | 0.049738 |
References
- Riu-Aumatell, Montserrat, Jordi Torrens, Susana Buxaderas, y Elvira López-Tamames. 2013. 3. Cava (Spanish Sparkling Wine) Aroma: Composition and Determination Methods. Vol. 3. Diego Muñoz Torrero, Amparo Cortés&Eduardo L. Mariño.
- Alexandre, Hervé, y Michèle Guilloux-Benatier. 2006. «Yeast Autolysis in Sparkling Wine – a Review». Australian Journal of Grape and Wine Research 12(2):119-27. [CrossRef]
- Torrens, Jordi, Pilar Urpí, Montserrat Riu-Aumatell, Stefania Vichi, Elvira López-Tamames, y Susana Buxaderas. 2008. «Different Commercial Yeast Strains Affecting the Volatile and Sensory Profile of Cava Base Wine». International Journal of Food Microbiology 124(1):48-57. [CrossRef]
- Verzeletti, Andrelise, Sergio Echeverrigaray, Alejandro Cardoso, Regina Vanderlinde, y Ana Paula Longaray Delamare. 2016. «Evolution of Aromatic Compounds during the Second Fermentation and Aging of Brazilian Sparkling Wine» editadopor J.-M. Aurand. BIO Web of Conferences 7:02019. [CrossRef]
- Pozo-Bayón, María Ángeles, Adolfo Martínez-Rodríguez, Encarnación Pueyo, y M. Victoria Moreno-Arribas. 2009. «Chemical and biochemical features involved in sparkling wine production: from a traditional to an improved winemaking technology». Trends in Food Science & Technology 20(6):289-99. [CrossRef]
- Gallardo-Chacón, Joan, Stefania Vichi, Elvira López-Tamames, y Susana Buxaderas. 2009. «Analysis of Sparkling Wine Lees Surface Volatiles by Optimized Headspace Solid-Phase Microextraction». Journal of Agricultural and Food Chemistry 57(8):3279-85. [CrossRef]
- Jolly, N., P. Minnaar, M. Booyse, y P. Gerber. 2021. «Bottle Fermented Sparkling Wine: Cork or Crown Closures During the Second Fermentation?» South African Journal of Enology and Viticulture 42(2). [CrossRef]
- Furtado, Isabel, Paulo Lopes, Ana Sofia Oliveira, Filipa Amaro, Maria de Lourdes Bastos, Miguel Cabral, Paula Guedes de Pinho, y Joana Pinto. 2021. «The Impact of Different Closures on the Flavor Composition of Wines during Bottle Aging». Foods 10(9):2070. [CrossRef]
- Vidal, Jean-Claude, Soline Caille, Alain Samson, y Jean-Michel Salmon. 2017. «Impact of eight closures in controlled industrial conditions on the shelf life of two (red and rosé) wines». OENO One 51(4):387-99. [CrossRef]
- Azevedo, J., I. Fernandes, P. Lopes, I. Roseira, M. Cabral, N. Mateus, y V. Freitas. 2014. «Migration of Phenolic Compounds from Different Cork Stoppers to Wine Model Solutions: Antioxidant and Biological Relevance». European Food Research and Technology 239(6):951-60. [CrossRef]
- Hopfer, Helene, Jenny Nelson, Alyson E. Mitchell, Hildegarde Heymann, y Susan E. Ebeler. 2013. «Profiling the trace metal composition of wine as a function of storage temperature and packaging type». Journal of Analytical Atomic Spectrometry 28(8):1288-91.
- Silva, S. P., M. A. Sabino, E. M. Fernandes, V. M. Correlo, L. F. Boesel, y R. L. Reis. 2005. «Cork: Properties, Capabilities and Applications». International Materials Reviews 50(6):345-65. [CrossRef]
- Campos, P., H. Daly-Hassen, y P. Ovando. 2007a. «Cork Oak Forest Management in Spain and Tunisia: Two Case Studies of Conflicts between Sustainability and Private Income». International Forestry Review 9(2):610-26. [CrossRef]
- Liger-Belair, Gérard, y Clara Cilindre. 2021. «Recent Progress in the Analytical Chemistry of Champagne and Sparkling Wines». Annual Review of Analytical Chemistry 14(1):21-46. [CrossRef]
- Pereira, H. 1988. «Chemical Composition and Variability of Cork from Quercus Suber L.» Wood Science and Technology 22(3):211-18. [CrossRef]
- Duarte, Ana Paula, y João Carlos Bordado. 2015. «Cork–a renewable raw material: forecast of industrial potential and development priorities». Frontiers in Materials 2:2.
- Gil, Luís. 2014. «Cork: a strategic material». Frontiers in chemistry 2:16.
- Amaro, Filipa, Joana Almeida, Ana Sofia Oliveira, Isabel Furtado, Maria De Lourdes Bastos, Paula Guedes De Pinho, y Joana Pinto. 2022. «Impact of Cork Closures on the Volatile Profile of Sparkling Wines during Bottle Aging». Foods 11(3):293. [CrossRef]
- Pinto, Joana, Ana Sofia Oliveira, Paulo Lopes, Isabel Roseira, Miguel Cabral, Maria De Lourdes Bastos, y Paula Guedes De Pinho. 2019. «Characterization of Chemical Compounds Susceptible to Be Extracted from Cork by the Wine Using GC-MS and 1H NMR Metabolomic Approaches». Food Chemistry 271:639-49. [CrossRef]
- Oliveira, Graça, y Augusta Costa. 2012. «How resilient is Quercus suber L. to cork harvesting? A review and identification of knowledge gaps». Forest Ecology and Management 270:257-72. [CrossRef]
- Kurz-Besson, Cathy, Dennis Otieno, Raquel Lobo Do Vale, Rolf Siegwolf, Markus Schmidt, Alastair Herd, Carla Nogueira, Teresa Soares David, Jorge Soares David, John Tenhunen, João Santos Pereira, y Manuela Chaves. 2006. «Hydraulic Lift in Cork Oak Trees in a Savannah-Type Mediterranean Ecosystem and Its Contribution to the Local Water Balance». Plant and Soil 282(1-2):361-78. [CrossRef]
- Pinheiro, Antonio Cipriano, Nuno Almeida Ribeiro, Peter Surovy, y Alfredo Goncalves Ferreira. 2008. «Economic Implications of Different Cork Oak Forest Management Systems». International Journal of Sustainable Society 1(2):149. [CrossRef]
- Bosch-Fusté, Joan, M. Riu-Aumatell, J. Guadayol, J. Caixach, E. Lopeztamames, y S. Buxaderas. 2007. «Volatile Profiles of Sparkling Wines Obtained by Three Extraction Methods and Gas Chromatography–Mass Spectrometry (GC–MS) Analysis». Food Chemistry 105(1):428-35. [CrossRef]
- Francioli, Sonia, Maria Guerra, Elvira López-Tamames, Josep M. Guadayoi, y JosepCaixach. 1999. «Aroma of sparkling wines by headspace/solid phase microextraction and gas chromatography/mass spectrometry». American journal of enology and viticulture 50(4):404-8.
- Riu-Aumatell, M., J. Bosch-Fusté, E. López-Tamames, y S. Buxaderas. 2006. «Development of volatile compounds of cava (Spanish sparkling wine) during long ageing time in contact with lees». Food Chemistry 95(2):237-42. [CrossRef]
- Torrens, Jordi, Montserrat Riu-Aumatell, Stefania Vichi, Elvira López-Tamames, y Susana Buxaderas. 2010. «Assessment of Volatile and Sensory Profiles between Base and Sparkling Wines». Journal of Agricultural and Food Chemistry 58(4):2455-61. [CrossRef]
- Coelho, Elisabete, Manuel A. Coimbra, J. M. F. Nogueira, y Sílvia M. Rocha. 2009. «Quantification approach for assessment of sparkling wine volatiles from different soils, ripening stages, and varieties by stir bar sorptive extraction with liquid desorption». Analytica Chimica Acta 635(2):214-21. [CrossRef]
- Herrero, Paula, Pilar Sáenz-Navajas, Laura Culleré, Vicente Ferreira, Amelie Chatin, Vincent Chaperon, François Litoux-Desrues, y Ana Escudero. 2016. «Chemosensory characterization of Chardonnay and Pinot Noir base wines of Champagne. Two very different varieties for a common product». Food Chemistry 207:239-50. [CrossRef]
- Culbert, J. A., R. Ristic, L. A. Ovington, A. J. Saliba, y K. L. Wilkinson. 2017. «Influence of Production Method on the Sensory Profile and Consumer Acceptance of Australian Sparkling White Wine Styles: Sensory Profiles of Australian Sparkling Wines». Australian Journal of Grape and Wine Research 23(2):170-78. [CrossRef]
- Bingman, Matthew T., Claire E. Stellick, Jordanne P. Pelkey, Jared M. Scott, y Callie A. Cole. 2020. «Monitoring cider aroma development throughout the fermentation process by headspace solid phase microextraction (HS-SPME) gas chromatography–mass spectrometry (GC-MS) analysis». Beverages 6(2):40.
- Madrera, Roberto Rodríguez, Ana García Hevia, Noemí Palacios García, y Belén Suárez Valles. 2008. «Evolution of aroma compounds in sparkling ciders». LWT-Food Science and Technology 41(10):2064-69.
- Ocvirk, Miha, Nataša Kočar Mlinarič, y Iztok J. Košir. 2018. «Comparison of Sensory and Chemical Evaluation of Lager Beer Aroma by Gas Chromatography and Gas Chromatography/Mass Spectrometry». Journal of the Science of Food and Agriculture 98(10):3627-35. [CrossRef]
- Riu-Aumatell, M., P. Miró, A. Serra-Cayuela, S. Buxaderas, y E. López-Tamames. 2014. «Assessment of the aroma profiles of low-alcohol beers using HS-SPME–GC-MS». Food Research International 57:196-202. [CrossRef]
- Minnaar, P. P., P. Gerber, y M. Booyse. 2021. «Phenolic Compounds in Cork-Closed Bottle-Fermented Sparkling Wines». South African Journal of Enology and Viticulture 42(1). [CrossRef]
- Zhang, Zhouyao, y Janusz Pawliszyn. 1993. «Headspace solid-phase microextraction». Analytical chemistry 65(14):1843-52.
- Pati, Sandra, Maria Tufariello, Pasquale Crupi, Antonio Coletta, Francesco Grieco, y Ilario Losito. 2021. «Quantification of volatile compounds in wines by HS-SPME-GC/MS: Critical issues and use of multivariate statistics in method optimization». Processes 9(4):662.
- Tufariello, Maria, Sandra Pati, Lorenzo Palombi, Francesco Grieco, y Ilario Losito. 2022. «Use of multivariate statistics in the processing of data on wine volatile compounds obtained by HS-SPME-GC-MS». Foods 11(7):910.
- Zhang, Lin, Qianqian Liu, Yuanyuan Li, Shuzhen Liu, Qian Tu, y Chunlong Yuan. 2023. «Characterization of wine volatile compounds from different regions and varieties by HS-SPME/GC-MS coupled with chemometrics». Current Research in Food Science 6:100418.
- Cheng, Zeng, David T. Mannion, Maurice G. O’Sullivan, Song Miao, Joseph P. Kerry, y Kieran N. Kilcawley. 2021. «Comparison of automated extraction techniques for volatile analysis of whole milk powder». Foods 10(9):2061.
- Salinas, M. Rosario, Gonzalo L. Alonso, y F. Javier Esteban-Infantes. 1994. «Adsorption-Thermal Desorption Gas Chromatography Applied to the Determination of Wine Aromas». Journal of Agricultural and Food Chemistry 42(6):1328-31. [CrossRef]
- Baltussen, E., C. Cramers, y P. Sandra. 2002. «Sorptive Sample Preparation – a Review». Analytical and Bioanalytical Chemistry 373(1-2):3-22. [CrossRef]
- Woolfenden, Elizabeth. 2021. «Thermal desorption gas chromatography». Pp. 267-323 en Gas chromatography. Elsevier.
- Jové, Patricia, Anna Pareras, Raquel De Nadal, y Maria Verdum. 2021. «Development and Optimization of a Quantitative Analysis of Main Odorants Causing off Flavours in Cork Stoppers Using Headspace Solid-phase Microextraction Gas Chromatography Tandem Mass Spectrometry». Journal of Mass Spectrometry 56(5):e4728. [CrossRef]
- Aitchison, J. (1982). The statistical analysis of compositional data. Journal of the Royal Statistical Society: Series B (Methodological), 44(2), 139-160.
- Korhoňová, M., K. Hron, D. Klimčíková, L. Müller, P. Bednář, y P. Barták. 2009. «Coffee aroma—statistical analysis of compositional data». Talanta 80(2):710-15.
- Pawlowsky-Glahn, Vera, Juan José Egozcue, y Raimon Tolosana-Delgado. 2015. Modeling and analysis of compositional data. John Wiley & Sons.
- Martín Fernández, Josep Antoni, Pepus Daunis-i-Estadella, y Glòria Mateu i Figueras. 2015. «On the interpretation of differences between groups for compositional data». SORT: statistics and operations research transactions, 2015, vol. 39, núm. 2, p. 231-252.
- Mateu-Figueras, G., V. Pawlowsky-Glahn, y J. J. Egozcue. 2008. «The normal distribution in some constrained sample spaces.
- Louw, Leanie, Karolien Roux, Andreas Tredoux, Oliver Tomic, Tormod Naes, Hélène H. Nieuwoudt, y Pierre Van Rensburg. 2009. «Characterization of Selected South African Young Cultivar Wines Using FTMIR Spectroscopy, Gas Chromatography, and Multivariate Data Analysis». Journal of Agricultural and Food Chemistry 57(7):2623-32. [CrossRef]
- Makris, Dimitris P., Stamatina Kallithraka, y Andreas Mamalos. 2006. «Differentiation of young red wines based on cultivar and geographical origin with application of chemometrics of principal polyphenolic constituents». Talanta 70(5):1143-52.
- Shimizu, Hideaki, Fumikazu Akamatsu, Aya Kamada, Kazuya Koyama, Kazuhiro Iwashita, y Nami Goto-Yamamoto. 2020. «Variation in the mineral composition of wine produced using different winemaking techniques». Journal of bioscience and bioengineering 130(2):166-72.
- Palarea-Albaladejo, Javier, y Josep Antoni Martín-Fernández. 2015. «zCompositions—R package for multivariate imputation of left-censored data under a compositional approach». Chemometrics and Intelligent Laboratory Systems 143:85-96.
- Dı́az-Regañon, Dolores Huerta, Rosario Salinas, Taisir Masoud, y Gonzalo Alonso. 1998. «Adsorption-thermal desorption-gas chromatography applied to volatile compounds of Madrid region wines». Journal of Food Composition and Analysis 11(1):54-69.
- Panighel, Annarita, y Riccardo Flamini. 2014. «Applications of solid-phase microextraction and gas chromatography/mass spectrometry (SPME-GC/MS) in the study of grape and wine volatile compounds». Molecules 19(12):21291-309.
- Whiton, R. S. , y Bruce W. Zoecklein. 2000. «Optimization of headspace solid-phase microextraction for analysis of wine aroma compounds». American journal of enology and viticulture 51(4):379-82.
- Ziółkowska, Angelika, Erwin Wąsowicz, y Henryk H. Jeleń. 2016. «Differentiation of wines according to grape variety and geographical origin based on volatiles profiling using SPME-MS and SPME-GC/MS methods». Food Chemistry 213:714-20.
- Ubeda, Cristina, Ingeborg Kania-Zelada, Rubén del Barrio-Galán, Marcela Medel-Marabolí, Mariona Gil, y Álvaro Peña-Neira. 2019. «Study of the changes in volatile compounds, aroma and sensory attributes during the production process of sparkling wine by traditional method». Food Research International 119:554-63.
- Benucci, Ilaria, Martina Cerreti, Diamante Maresca, Gianluigi Mauriello, y Marco Esti. 2019. «Yeast cells in double layer calcium alginate–chitosan microcapsules for sparkling wine production». Food chemistry 300:125174.
- JagatićKorenika, Ana-Marija, DarkoPreiner, Ivana Tomaz, y Ana Jeromel. 2020. «Volatile profile characterization of Croatian commercial sparkling wines». Molecules 25(18):4349.
- Voce, Sabrina, DomenŠkrab, UrskaVrhovsek, Franco Battistutta, Piergiorgio Comuzzo, y Paolo Sivilotti. 2019. «Compositional Characterization of Commercial Sparkling Wines from Cv. RibollaGialla Produced in Friuli Venezia Giulia». European Food Research and Technology 245(10):2279-92. [CrossRef]
- Polášková, Pavla, JulianHerszage, y Susan E. Ebeler. 2008. «Wine flavor: chemistry in a glass». Chemical Society Reviews 37(11):2478-89.
- He, Yang, Xinyuan Wang, Penghui Li, YingchiLv, Hailong Nan, Liankui Wen, y Zhitong Wang. 2023. «Research progress of wine aroma components: A critical review». Food Chemistry 402:134491.
- Cichewicz, Robert H., y Samir A. Kouzi. 2004. «Chemistry, Biological Activity, and Chemotherapeutic Potential of Betulinic Acid for the Prevention and Treatment of Cancer and HIV Infection». Medicinal Research Reviews 24(1):90-114. [CrossRef]
- Francioli, Sonia, Jordi Torrens, Montserrat Riu-Aumatell, Elvira López-Tamames, y Susana Buxaderas. 2003. «Volatile compounds by SPME-GC as age markers of sparkling wines». American Journal of Enology and Viticulture 54(3):158-62.
- Dziadas, Mariusz, y Henryk H. Jeleń. 2010. «Analysis of terpenes in white wines using SPE–SPME–GC/MS approach». Analytica chimica acta 677(1):43-49.
- Genovese, Alessandro, Simona A. Lamorte, Angelita Gambuti, y Luigi Moio. 2013. «Aroma of Aglianico and Uva di Troia grapes by aromatic series». Food research international 53(1):15-23.
- Caliari, Vinícius, VívianMariaBurin, Jean Pierre Rosier, y Marilde T. BordignonLuiz. 2014. «Aromatic Profile of Brazilian Sparkling Wines Produced with Classical and Innovative Grape Varieties». Food Research International 62:965-73. [CrossRef]
- Torchio, Fabrizio, Susana RíoSegade, Vincenzo Gerbi, Enzo Cagnasso, Manuela Giordano, Simone Giacosa, y Luca Rolle. 2012. «Changes in varietal volatile composition during shelf-life of two types of aromatic red sweet Brachetto sparkling wines». Food research international 48(2):491-98.
- Ferreira, Vicente, y Ricardo Lopez. 2019. «The actual and potential aroma of winemaking grapes». Biomolecules 9(12):818.
- Xu, Minwei, Zhao Jin, Yang Lan, Jiajia Rao, y Bingcan Chen. 2019. «HS-SPME-GC-MS/olfactometry combined with chemometrics to assess the impact of germination on flavor attributes of chickpea, lentil, and yellow pea flours». Food Chemistry 280:83-95.
- Ancín-Azpilicueta, Carmen, Nerea Jiménez-Moreno, José Antonio Moler, Rodrigo Nieto-Rojo, y Henar Urmeneta. 2016. «Effects of Reduced Levels of Sulfite in Wine Production Using Mixtures with Lysozyme and Dimethyl Dicarbonate on Levels of Volatile and Biogenic Amines». Food Additives & Contaminants: Part A 33(10):1518-26. [CrossRef]
- Liu, N., Y. Y. Song, G. F. Dang, D. Q. Ye, X. Gong, y Y. L. Liu. 2015.«Effect of wine closures on the aroma properties of Chardonnay wines after four years of storage». South African Journal of Enology and Viticulture 36(3):296-303.
- Lee, Dong-Hyun, Bo-Sik Kang, y Hyun-Jin Park. 2011. «Effect of Oxygen on Volatile and Sensory Characteristics of Cabernet Sauvignon during Secondary Shelf Life». Journal of Agricultural and Food Chemistry 59(21):11657-66. [CrossRef]
- Anderson, Marti J. 2001. «A New Method for Non-parametric Multivariate Analysis of Variance». Austral Ecology 26(1):32-46. [CrossRef]
- Anderson, Marti J., y Daniel C. I. Walsh. 2013. «PERMANOVA, ANOSIM, and the Mantel Test in the Face of Heterogeneous Dispersions: What Null Hypothesis Are You Testing?» Ecological Monographs 83(4):557-74. [CrossRef]


| Type | Code | Closure description |
|---|---|---|
| Cork stopper | Cork 1 | Agglomerated cork stopper with 31 mm of diameter |
| Cork stopper | Cork 2 | Agglomerated cork stopper with 32 mm of diameter |
| Screw cap | CC1 | Polyethylene screw cap consists of a cell polyethylene foam disc with a very fine structure. |
| Screw cap | CC2 | Saranexis composed of PE covered on both sides with PVDC |
| Screw cap | CC3 | Daraform is a non- polyvinylidene (PVC) compound based on polyolefinic raw materials |
| Screw cap | CC4 | Saranex + araldite is composed of PE covered on both sides with PVDC and with the addition of an additional glue such as Varnishe (Araldite) |
| Base wine | Base wine after the addition of a “tirage solution” | ||
| Total acidity by sulfuric (g L-1) | 4.05 | Density (g cm3) | 998.9 |
| Sugar (GAP) (g L-1) | 23.1 | Turbidity (NTU) | 35.9 |
| NFA (mg L-1) | 63.0 | Free SO2 (mg L-1) | 7 |
| Free SO2 (mg L-1) | 8.0 | Temperature (ºC) | 17 |
| Sucrose | 21.3 | ||
| Code | Volatile compounds | % zeros replaced |
| 1, 1, 5-Trimethyl-1, 2-dihydronaphthalene | 75.7% | |
| 3,4-Dihydroisoquinoline, 1-[3-hydroxybenzyl]-6-methoxy- | 62.2% | |
| 4-(2,3,6-Trimethylphenyl)-1,3-butadiene | 70.3% | |
| Carbon dioxide | 70.3% | |
| Diethyl succinate | 0.0% | |
| Dimethylamine | 70.3% | |
| Ethyl 9-decenoate | 13.5% | |
| Ethyl hexanoate | 0.0% | |
| Ethyl octanoate | 56.8% | |
| Ethyl trans-4-decenoate | 32.4% | |
| Isoamyl alcohol | 73.0% | |
| Lactamide | 51.4% | |
| Octanoic acid | 56.8% | |
| Oxirane, 2-(1,1-dimethylethyl)-3-ethyl-, cis- | 46.0% | |
| Phenylethyl Alcohol | 2.7% | |
| alpha-Ionone | 21.6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





