Introduction
A chronic total occlusion (CTO) refers to a completely occluded coronary artery with TIMI 0 flow with duration of ≥3 months [
1]. In series of patients undergoing invasive coronary angiography, the prevalence of at least one CTO was reported to be as 15-20% [
2]. Despite the high frequency of CTO, percutaneous recanalization has traditionally been considered as a technical challenge. As a result of recent technological advances, such as recanalization techniques and equipments coupled with advancing operators’ expertise, the success rate of CTO recanalisation has risen to 80-90% at specialized CTO referral centres [
3].
The benefits of CTO revascularization in percutaneous coronary intervention (PCI) is still controversial. Today, the primary objective of CTO-PCI on top of the medical treatment, is to achieve symptom improvement. Uncertainties regarding the reduction of major adverse cardiovascular events (MACE) persist due to the existing trials being underpowered and yielding conflicting results [
4]. Also, several studies did not show any functional improvement in left ventricle (LV) functions after revascularization [
5,
6]. However, they assessed the systolic function by using the LV ejection fraction (LVEF) and LV diameters by conventional echocardiography. In speckle-tracking echocardiography (STE), image processing algorithms are applied to routine two-dimensional echocardiographic images to identify small, stable myocardial speckles within a defined region of interest. By tracking these speckles frame-by-frame over the cardiac cycle, the distances between them or their spatiotemporal displacement (regional strain velocity vectors) provide valuable information about global and segmental myocardial deformation, so called as myocardial strain analysis [
7].The LV global longitudinal strain (GLS) is the novel parameter to evaluate LV systolic function with superior reproducibility compared to LVEF and has been validated for its efficacy in detecting myocardial ischemia [
8]. In patients with cardiovascular disease, myocardial dysfunction can manifest even when the EF is preserved, potentially correlating with impaired LV longitudinal deformation [
9]. Ischemic cascade begins with impaired in the watershed subendocardial layer, where there is a predominance of longitudinally oriented fibers, so it is not surprising that GLS abnormalities have been reported even in patients with subclinical ischemia [
7]. Furthermore several studies have shown that the LV GLS improves after the recanalization of the CTO [
10,
11].
In the presence of a CTO, the development of collateral vessels can mitigate myocardial necrosis and sustain contractile function within the region distal to the occlusion. The presence of a well-developed (WD) coronary collateral circulation (Rentrop 2-3 classification) has been demonstrated to be able to preserve LV systolic functions in CTO patients [
12,
13]. Moreover, improved coronary collateral circulation can diminish infarct size, thereby lowering the likelihood of ventricular aneurysm formation, reducing future cardiovascular events, and enhancing survival rates [
14]. Although a WD collateral circulation system may adequately perfuse the myocardium at rest, it could potentially be insufficient during periods of increased demand, leading to ischemia [
15].
In the literature, LV functions after recanalization of CTO have been investigated irrespective of the coronary collateral circulation. We hypothesized that among patients with CTO and WD coronary collateralization, there may be no significant improvement in LV functions following revascularization. This could be attributed to the presence of sufficient blood supply at rest, potentially limiting the enhancing effects of recanalization on LV function. Thus, we employed LV GLS analysis in our study to assess whether CTO recanalization provided benefits in restoring LV function. We compared the outcomes between patients with WD and poor coronary collateralization.
Materials and Methods
Patient data: In this multicenter observational study, a cohort of 90 symptomatic patients who underwent planned revascularization of a single major epicardial coronary artery CTO between January 2021 and February 2023, were prospectively screened. 69 patients were enrolled in our study. Symptomatic patients with typical angina pectoris according to Canadian Cardiovascular Society (CCS) under optimum medical therapy (OMT) were included in the study. Patients with atypical symptoms such as exercise dyspnea or intolerance were included if there was stress induced ischemia of ≥10% of global myocardial region in myocardial perfusion scintigraphy (MPS). Patients with recent revascularization for non-CTO lesions, including acute coronary syndromes, could be enrolled if 6 weeks have passed after successful revascularization. Patients with acute coronary syndrome, unstable angina, ≥50% stenosis in a non-CTO artery, unsuccessful CTO-PCI, arrhythmia, moderate to severe valvular disease, cardiomyopathies, akinesia or aneurysm of the target artery area and patients with reduced EF (EF≤40%) were excluded from the study.
CTO of major epicardial artery was defined as complete occlusion with TIMI 0 flow for at least 3 months duration in major coronary artery with a vessel diameter of ≥2.5 mm. The operator judged the suitability and the method of the planned revascularization of the CTO lesion after diagnostic coronary angiography. The procedural approach for CTO included antegrade or retrograde approach adhering to contemporary technical guidelines [
3]. In cases where initial PCI failed, treatment with medical or surgical therapy pursued based on clinical requirements. Five patients who had no suitable image for LV GLS measurements were excluded from the study. Recanalization of CTO failed in 12 patients during coronary angiography, and 4 patients discontinued the echocardiographic follow-up. As a result, 69 patients were enrolled in our study. Medical histories, encompassing all clinical and demographic data including cardiovascular risk factors such as hypertension (HT), diabetes mellitus (DM), dyslipidemia, and smoking, were extracted from electronic medical records and patient interviews. Laboratory results obtained within 24 hours prior to coronary angiography were recorded. Patients were followed up at the first and third month after CTO recanalization, they underwent clinical and echocardiographic evaluations, and were also interviewed about their anginal symptoms.
All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki and the study protocol was reviewed and approved by the local ethical committee.
Canadian Cardiovascular Society grading of angina pectoris: In accordance with the CCS grading system, angina pectoris is graded as follows: Grade I: Ordinary physical activity does not cause angina, such as walking and climbing stairs. Angina with strenuous or rapid or prolonged exertion at work or recreation. Grade II: Slight limitation of ordinary activity. Walking or climbing stairs rapidly, walking uphill, walking or stair climbing after meals, or in cold, or in wind, under emotional stress, or only during the few hours after awakening. Walking more than two blocks on the level and climbing more than one flight of ordinary stairs at a normal pace and in normal conditions. Grade III: Marked limitation of ordinary physical activity. Walking one or two blocks on the level and climbing one flight of stairs in normal conditions and at normal pace. Grade IV: Inability to carry on any physical activity without discomfort [
16].
Grading of the anginal score of the patient was performed at initial visit, also questionned and recorded on the consecutive visits by the attending physician.
Transthoracic echocardiography: Screened patients who met the clinical criteria for study inclusion underwent two-dimensional echocardiographic imaging 24 hours prior to the CTO-PCI. Sequential echocardiographic measurements were conducted in the first and third months following recanalization. Two experienced cardiologists (TST, NO) performed the transthoracic echocardiography (TTE) using the Vivid E9 imaging system (GE Medical Systems, Chicago, USA) and measurements were obtained following the recommendations of the American Society of Echocardiography [
17]. LV dimensions were measured in the parasternal long-axis view at end-systole and end-diastole. LV EF was calculated using the modified Simpson method from four-chamber views.
Left Ventricular Global Longitudinal Strain: An automated function imaging (AFI) method was used in the Echo Pack imaging workstation (GE, Echo Pack imaging systems) to obtain 4-chamber, 2-chamber, and APLAX images with frame rate of 50-70 fps. 2D STE measurements of LV strain were obtained according to the recommendations of the 2015 EACVI/ASE consensus document [
17]. LV strain measurements were omitted from analysis if left ventricular image quality was deemed inadequate or if adjusting the region of interest (ROI) did not enhance tracking quality. LV GLS of >-20% was defined as impaired LV GLS (Negative values in GLS are consigned to longitudinal shortening in systole. Greater degrees of deformation in fact translates to numerically lower strain values and practically higher absolute values of GLS, specifically above 20, suggest normal strain values).
Coronary Angiography: An interventional cardiologist performed coronary angiography according to standard procedures. Following diagnostic coronary angiography, all patients underwent heart team discussion. Patients with typical anginal symptoms under OMT were enrolled directly if they had no exclusion critera. For other symptomatic patients, MPS was ordered to detect ischemia in the target area, since these patients presented with atypical angina or symptoms of effort intolerance, further clarification was required. Patients who were indicated for revascularization by the heart team underwent PCI, performed by experienced interventional cardiologists using femoral access. In accordance with their expertise, the medical team recanalized the CTO, using specialized wires, balloons, and microcatheters. All patients were revascularized with drug eluting stents. Preoperative, perioperative and follow-up medical management, including the antiplatelet regimen, adhered the current guidelines [
18]. Angiographic success was defined as achieving a final angiographic residual stenosis of less than 20% by visual estimate and TIMI III flow following the implantation of stents [
19]. Two blinded interventional cardiologists classified coronary collaterals according to Cohen-Rentrop classification, which was graded as follows: grade 0= no visible collaterals, grade 1= the filling of the side branch via collateral vessels without visualization of the epicardial segment, grade 2= the partial filling of the epicardial coronary artery, and grade 3= the complete filling of the epicardial coronary artery [
20]. Enrolled patients were classified as WD (grades 2 and 3) and poor (grades 0 and 1) coronary collateral groups [
21].
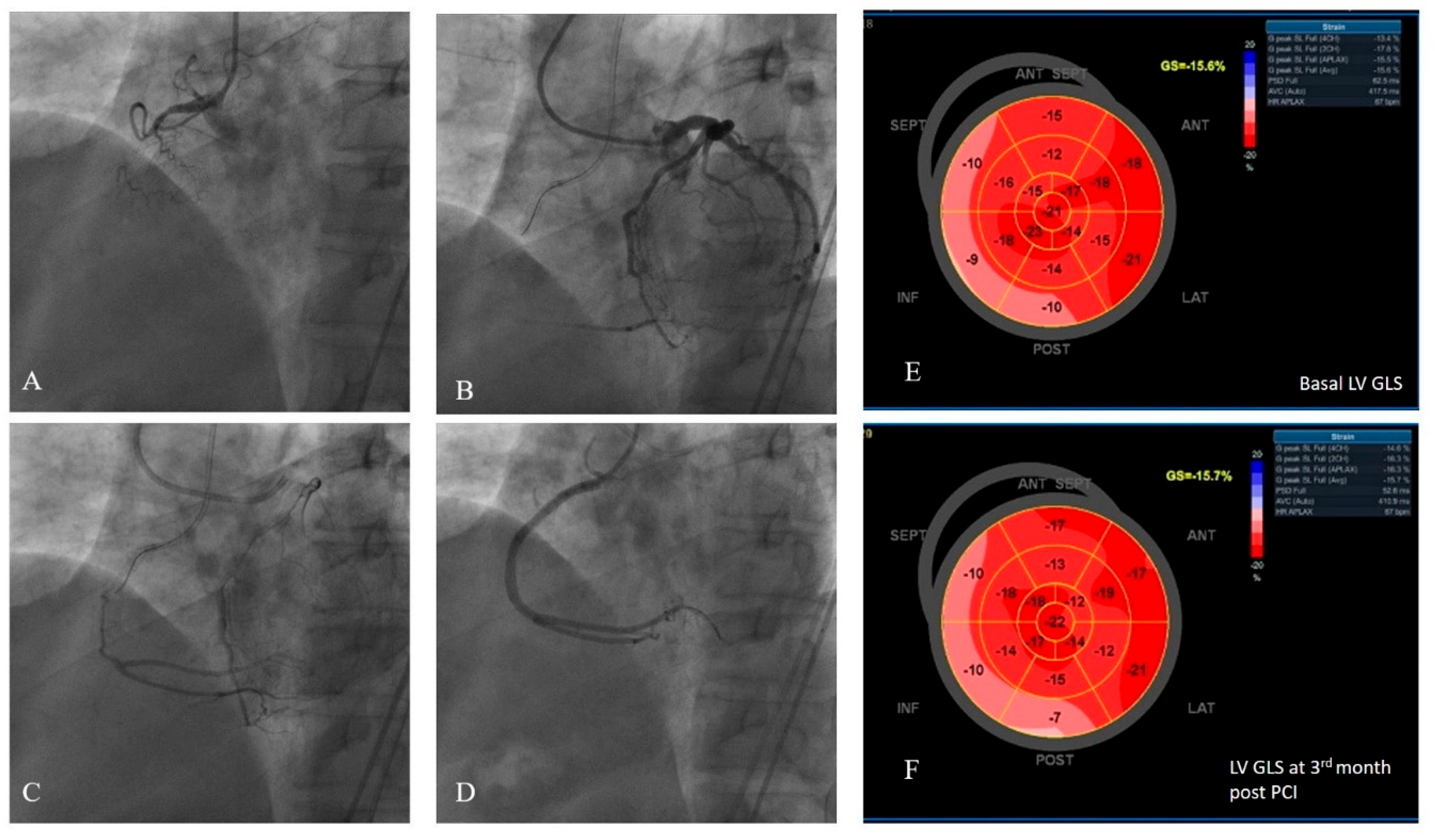
Figure 1 presents an example study patient who had WD collaterals and had successful CTO recanalization, followed-up with consecutive LV GLS measurements recorded at baseline and at the third month.
Figure 1.
An example of a study patients’ LV GLS strain and coronary angiography views. (A) Chronic total occlusion of RCA. (B-C) Dual injection demonstrated the presence of grade 3 collateral circulation from the left coronary arteries to the RCA. (D) The RCA was successfully wired, two drug-eluting stents were implanted, and TIMI 3 flow was demonstrated.(E) Pre-PCI average LV GLS was measured to be -15,6%. (F) Post-PCI third month LV GLS was measured to be -15,7%.LV GLS: left ventricular global longitudinal strain.
Figure 1.
An example of a study patients’ LV GLS strain and coronary angiography views. (A) Chronic total occlusion of RCA. (B-C) Dual injection demonstrated the presence of grade 3 collateral circulation from the left coronary arteries to the RCA. (D) The RCA was successfully wired, two drug-eluting stents were implanted, and TIMI 3 flow was demonstrated.(E) Pre-PCI average LV GLS was measured to be -15,6%. (F) Post-PCI third month LV GLS was measured to be -15,7%.LV GLS: left ventricular global longitudinal strain.
LV GLS: left ventricular global longitudinal strain, RCA: right coronary artery, PCI: percutaneous coronary intervention
Results
Baseline characteristics: The study enrolled 69 patients (mean age 64.9±9.4 , 81% male) who underwent CTO-PCI of single epicardial coronary artery. According to the coronary collateral supply to the CTO, patients were classified as having WD (36 patients, 52%) and poor (33 patient, 48%) collaterals. All patients enrolled in the study had typical angina pectoris or significant ischemia was demonstrated in the case of patients with angina equivalent symptoms. According to CCS angina grading system; 28 (41%) patients experienced grade 2 angina, 34 (49%) patients had grade 3 angina, and 7 (10%) patients reported grade 4 angina pectoris. Grade 1 angina pectoris was not observed in the study population. Patients in the poor coronary collateral group demonstrated significantly more severe angina level when compared to the WD collateral group (p=0.02). Demographic and clinical characteristics, laboratory results, and cardiovascular risk factors for the two groups according to the collateral supply is demonstrated in
Table 1. All patients were on appropriate doses of statin, beta blocker, angiotensin receptor blocker (ARBs) or angiotensin converting enzyme inhibitor (ACEi) therapy, and other physician recommended therapies in accordance to the clinical guidelines during the course of the study [
18].
Table 1.
Baseline Characteristics According to Coronary Collateral supply.
Table 1.
Baseline Characteristics According to Coronary Collateral supply.
| Characteristics |
Well-developed Collateral (N=36) |
Poor Collateral (N=33) |
P value |
| Age |
64.7 ± 9.7 |
65.1± 9.3 |
0.9 |
| Male n, (%) |
28 (78) |
28 (85) |
0.5 |
| BSA m² #
|
1.87± 0.1 |
1.86± 0.2 |
0.9 |
| HT n, (%) |
27(75) |
23 (70) |
0.8 |
| DM n,(%) |
22(61) |
19 (58) |
0.8 |
| HL n, (%) |
12(33) |
16 (49) |
0.22 |
| Smoking n, % |
18(50) |
19 (58) |
0.63 |
| Family history n,% |
9(25) |
13 (40) |
0.3 |
| CCS Angina Grading (I/II/III/IV) |
0/24/18/1 |
0/14/16/6 |
0.02 |
| Laboratory Results |
|
|
|
| Fasting glucose mg/dl |
104 ± 8.9 |
98.1± 11.2 |
0.05 |
| Hemoglobin g/dl |
14,05 ±2.1 |
14.1±2.2 |
0.93 |
| Platelet |
259.4± 85.8 |
246.7±57.8 |
0.5 |
| Creatinine (mg/dl) |
0,92±0.18 |
0.86±0.21 |
0.3 |
| TC mg/dl |
191.3±51,4 |
193.5 ±48.8 |
0.9 |
| LDL-C mg/dl |
120.5±40.2 |
129.9±46.4 |
0.4 |
| HDL-C (mg/dl) |
43,6±14.8 |
43.1± 11.01 |
0.9 |
| Echocardiography |
|
|
|
| LVEDD (mm) |
50.8±5.6 |
50.9 ±6.4 |
0.94 |
| LVESD (mm) |
28.9±4.2 |
29.2±4.2 |
0.73 |
| EF% |
54.2±3.4 |
52.2 ±4.1 |
0.04 |
| LV GLS % |
-14.8±1.16 |
-12.8±1.47 |
0.0001 |
| Coronary Angiography |
|
|
|
| CTO artery (LAD/CX/RCA) |
13/6/17 |
14/5/14 |
0.9 |
| Prior CABG n, (%) |
0 |
0 |
- |
| Prior PCI to Target coronary artery n, (%) |
18(50) |
15(46) |
0.81 |
| Prior PCI (all) n, (%) |
18 (49) |
19 (51) |
0.4 |
| Prior MI in CTO area |
9 (25) |
4 (12) |
0.22 |
| Prior MI (all) n, (%) |
9 (25) |
8 (24) |
1 |
Coronary Angiography: All patients were symptomatic and had a CTO at one of the major coronary arteries. None of the patients exhibited secondary arterial stenosis of ≥50%, and all underwent intervention for their single-vessel CTO lesion.
Table 1 summarizes the angiographic characteristics of the patients, encompassing details such as coronary lesions with CTO, history of myocardial infarction, prior PCI, and coronary artery bypass surgery (CABG). Both WD and poor developed collateral groups were statistically similar according to their angiographic characteristics. All revascularization procedures were considered as successful by the attending interventionists, according to criteria defined at the methods section. No complications, such as pericardial tamponade, coronary rupture, MI, or death were observed perioperatively. After revascularization, symptomatic relief of angina or equivalents was reported by 54 patients at the end third month visits (75% of patients in WD group, 81% of patients in poor collateral group, p=0.101). Fifteen patients reported no improvement in their symptoms, with 9 belonging to the WD collateral group and 6 to the poor collateral group. None of the patients reported exacerbation of their complaints.
Echocardiographic Measurements: All patients underwent baseline TTE within 24 hours prior to the PCI. Mean basal LVEDD and LVESD were 51.03±6.04 mm and 29,02±4,19 mm, respectively and there were no statistically significant difference between the collateral groups. None of the patients had EF<40% and mean basal EF of the study group was 53.2±3.86. Although the difference was slight, the basal EF of the WD collateral group was statistically higher than that of the poor collateral group (54.2±3,4 vs 52.2±4.1 respectively, p=0.04). The basal LV GLS values of the study population were impaired with a mean value of -13,8±1,66. The basal LV GLS of the WD collateral group was significantly lower (meaning less impaired as the absolute value is higher) than that of the poor collateral group (-14.8±1,16 vs -12.8±1.47, respectively, p<0.0001) (
Table 1).
All echocardiographic parameters were remonitored at the first and third months following the PCI. One-way ANOVA was used to analyse repeated measures of LVEDD, EF, and LV GLS. Basal, first and third month measurements of GLS were all found to be improved significantly (-13.8±1.66 vs.-15.3±1.29 vs. -15.5±1.26, respectively, p= 0.0001). However, there was no improvement in EF (53.2±3,86 vs. 53.3±3.8 vs. 53.4±3.7, respectively, p= 0.7) and in LVEDD values ( 51.03±6.04 mm vs. 50.9±5.9 mm vs. 50.8±5.9 mm, respectively, p=0.06) (
Table 2).
Table 2.
Repeated Measures of Echocardiographic Parameters (n=69).
Table 2.
Repeated Measures of Echocardiographic Parameters (n=69).
| Echocardiographic parameters |
Pre-PCI |
Post-PCI M1 |
Post-PCI M3 |
P value (all) |
P value-
Pre-PCI vs. Post-PCI M1 |
P value-
Pre-PCI vs. Post-PCI M3 |
P value-
Post-PCI M1 vs. Post-PCI M3 |
| LVEDD mm |
51.03±6.04 |
50.9±5.9 |
50.8±5.9 |
0.06 |
0.2 |
0.2 |
0.3 |
| EF% |
53.2±3.86 |
53.3±3.8 |
53.4±3.7 |
0.7 |
0.10 |
0.30 |
0.08 |
| LV GLS% |
-13.8±1.66 |
-15.3±1.29 |
-15.5±1.26 |
0.0001 |
0.0001 |
0.0001 |
0.001 |
A mixed-ANOVA method was employed to assess differences between the WD and poor collateral groups across repeated measures, as well as within the repeated measures of each group. There was no significant difference in EF between the WD and poor collateral groups (p=0.56). Furthermore, no significant difference was observed within the repeated measures of EF in WD and poor collateral groups individually (
Table 3). LV GLS values differed significantly between the WD and poor collateral groups at baseline, first and third month follow-ups, with poor collateral group revealing significant improvement when compared to WD group (WD collateral GLS: -14.8±1.16 ; -15.2±1.13; -15.2±1.1, respectively vs. poor collateral GLS: -12.8±1.47; -15.5±1.45; -15.9±1.33, respectively, p<0.0001). LV GLS was found to improve significantly from baseline to the first month, significance preserved also at the third month, in the poor collateral group. However, there were no significant differences between the baseline, first month, and third month measures in the WD collateral group.
Table 3 and
Figure 2 represents the mixed ANOVA measurements in details.
The intraobserver (ICC: 0.97, 95% CI: 0.93–0.98) and interobserver (ICC: 0.94, 95% CI: 0.88–0.96) agreement of the LV strain measurements was excellent.
Table 3.
Comparison of Echocardiographic Parameters According to Coroner Collateral Classification (Well-developed collateral, n=36, poor collateral, n=33).
Table 3.
Comparison of Echocardiographic Parameters According to Coroner Collateral Classification (Well-developed collateral, n=36, poor collateral, n=33).
| Parameters divided by Collateral Classification |
Pre-PCI |
Post-PCI 1st month |
Post-PCI 3rd month |
P value (all) |
P value
Pre-PCI vs. Post-PCI 1st month |
P value
Pre-PCI vs. Post-PCI 3rd month |
P value
Post-PCI 1st month vs. Post-PCI 3rd month |
| WD Collateral |
EF% |
54.1±3.37 |
54.2±3.3 |
54.3±3.2 |
0.56 |
1.0 |
0.5 |
0.3 |
| Poor Collateral |
52.2±4.14 |
52.4±4.1 |
52.4±4.09 |
0.09 |
0.24 |
1.0 |
| WD Collateral |
LV GLS%
|
-14.8±1.16 |
-15.2±1.13 |
-15.2±1.1 |
0.0001 |
0.07 |
0.054 |
1.0 |
| Poor Collateral |
-12.8±1.47 |
-15.5±1.45 |
-15.9±1.33 |
0.0001 |
0.0001 |
0.0001 |
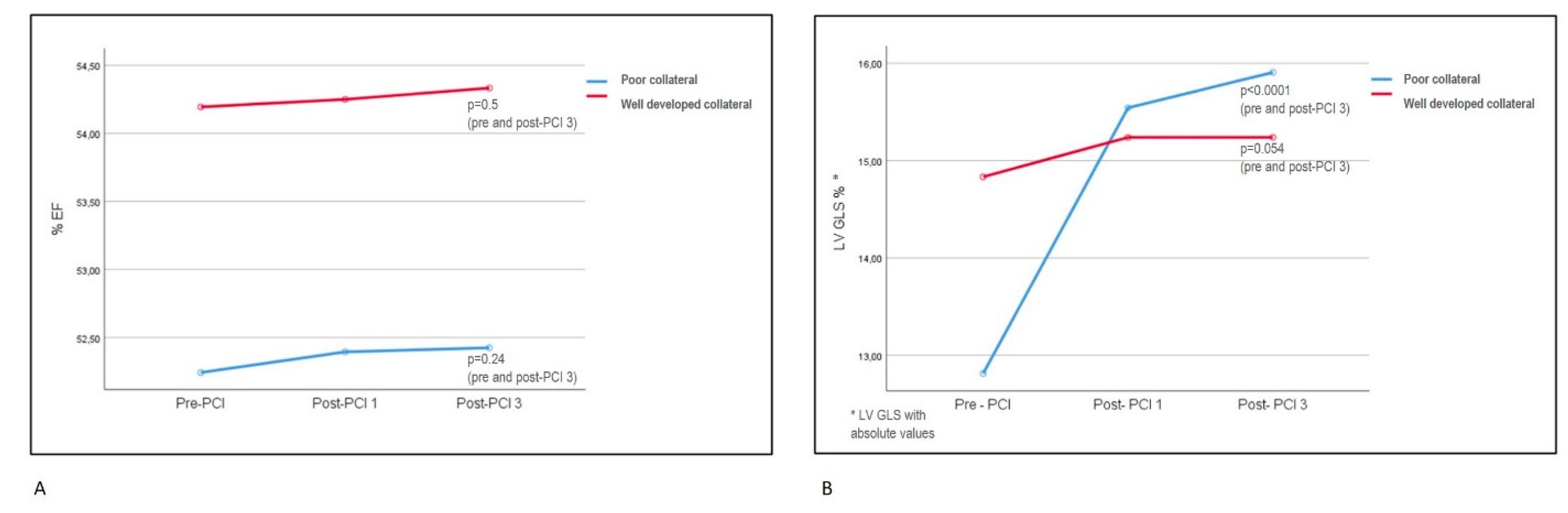
Figure 2.
Mixed- design ANOVA analysis of repeated measures of EF and LV GLS in WD and poor collateral groups. (Please note that absolute values of LV GLS measurement were used in the chart and p-values in the figure were provided for the pre-PCI and third month post-PCI periods; detailed p-values were presented in
Table 3). A: Repeated measures of EF did not show any differences between collateral groups and also within the groups. B: LV GLS improvement in poor collateral group was significantly higher than the WD group. All LV GLS values of consecutive follow-ups showed improvement in the poor collateral group, but not in the WD group, when examined separately.
Figure 2.
Mixed- design ANOVA analysis of repeated measures of EF and LV GLS in WD and poor collateral groups. (Please note that absolute values of LV GLS measurement were used in the chart and p-values in the figure were provided for the pre-PCI and third month post-PCI periods; detailed p-values were presented in
Table 3). A: Repeated measures of EF did not show any differences between collateral groups and also within the groups. B: LV GLS improvement in poor collateral group was significantly higher than the WD group. All LV GLS values of consecutive follow-ups showed improvement in the poor collateral group, but not in the WD group, when examined separately.
EF: ejection fraction; LV GLS: left ventricular global longitudinal strain; PCI: percutaneous coronary intervention, Post-PCI1: first month after PCI. Post-PCI3: third month after PCI.
Discussion
In this trial of patients with symptomatic stable coronary artery disease and single-vessel CTO, successful PCI procedure resulted in progressively improved LV function measured by LV GLS in the poor collateral supply group. However, no such improvement was observed in the WD collateral supply group. There was no significant improvement in EF for both collateral groups. The study cohort consisted of a highly selective group of patients with a single vessel CTO and no significant lesions in non-CTO vessels. Additionally, these patients exhibited either typical anginal symptoms or significant ischemia in the CTO territory, without akinesia or dyskinesia indicative of non-viable myocardium. Consequently, our study demonstrated the impact of CTO revascularization on LV strain values based on collateral supply levels. To the best of our knowledge, no prior articles have explored this specific topic.
The decision whether or not to recanalize a CTO presents a significant dilemma for the cardiologists. A recent systematic review and meta-analysis including 17 cohort studies with a total of 11493 patients, compared the use of OMT and PCI in patients with known CTO [
22]. The randomized studies in the meta-analysis indicated a potential improvement with PCI in overall mortality, cardiac death, repeat revascularization, and MACE; however, these improvements did not reach statistical significance [
22]. More randomized controlled trials (RCTs) are needed for a definitive answer. Despite this, there is currently no evidence to advise against PCI in CTO, and there may potentially be some benefits. Today, angina or angina equivalent symptoms resistant to OMT remain to be the main indication for the CTO recanalization, as MACE reduction at follow-up remains uncertain based on the currently available data [
4]. Evidence supporting CTO-PCI as an effective tool for symptom relief and improvement in quality of life was primarily based on three randomized controlled trials [
23,
24,
25]. In the design of our trial, we enrolled the symptomatic patients on OMT and verified the significant ischemia for the ones with atypical or possible angina equivalent symptoms, consistent with the recent guidelines [
18]. Symptomatic improvement after revascularization was achieved in 78% of the study population, with statistically similar outcomes observed between the subgroups of collateral supply. In the literature, anginal improvement after CTO PCI was reported to be around 70% with Seattle angina questionnare (SAQ) in EURO-CTO trial at the end of first year of revascularization. Miyashita et al. reported the symptomatic recovery rate after CTO PCI as 75% at the end of 1 year and improvement in symptoms were questionned by patient interviews at visits [
26]. The recovery rate in our trial was slightly higher (78%). We employed patient interviews to determine symptoms, which may have resulted in a higher reported rate of well-being. Additionally, the final visit in our trial was conducted at the third month post-revascularization, which may be too early to
capture potential restenotic events that could provoke anginal symptoms in the future. However, demonstrating the high anginal recovery rate following single vessel CTO revascularization in patients with clear PCI indications may underscore the importance of appropriate patient selection.
The possibility of functional recovery of the LV is another consideration for CTO revascularization; however, its role remains subject to debate [
4,
27]. In a meta-analysis of 34 studies including symptomatic patients with large ischemic burden or LV dysfunction, it was found that CTO-PCI induces an improvement of 4.44% in EF (p < 0.01) [
28]. CTO-PCI may show benefit especially for patients with more severe LV dysfunction, significant myocardial inducible perfusion defects and viability, as shown by previous trials [
29,
30,
31]. Although, these findings were not confirmed in the randomized REVASC trial. In this study, the mean baseline LVEF was only mildly to moderately reduced in both the OMT and CTO-PCI groups. LV functions, such as EF and segmental wall thickening (SWT), were assessed using magnetic resonance imaging (MRI). The functional recovery in the CTO territory revealed no differences between the invasive and non-invasive treatment groups (change in EF: 0.9 vs 0.7, p=0.79, change in SWT: 4.1 vs 6.0, p=0.57) [
6]. Schumacher et al, consistent with the findings of ISCHEMIA trial, had suggested that patients with stable coronary disease and impaired contractility constituted a subgroup where extensive ischemia relief via coronary revascularization may provide a protective effect, which is less pronounced in patients with preserved LV function [
32,
33,
34]. In the TOSCA study, basal LV dysfunction was shown to be an independent predictor of improvement in EF after CTO-PCI [
35]. In our trial, the study population had preserved EF and were symptomatic under OMT or had significant ischemia. No significant changes in EF were observed at the 3 month follow-up after revascularization, either in the entire group or between the groups based on their CTO collateral supply. However, the poor collateral supply group had a slightly, but significantly lower EF when compared to the WD group at the pre-PCI echocardiography (52.2% vs 54.2%, p=0.04). Poor collateral supply possibly leading to myocardial hibernation may be the cause of this slight difference in basal LV functions.
Conventional echocardiography method allows us to identify significant LV dysfunction, but not subclinical dysfunction and recovery. The two-dimensional (2D) speckle tracking of LV strain is an established technique used in the evaluation of detailed LV systolic functions. GLS represents the overall change in myocardial length during a cardiac cycle. It has been shown to sensitively reflect damage to the subendocardial myocardium, the region most prone to ischemic changes [
36]. Regarding the LV contractile functions, LV GLS can provide more accurate and early information than the classical EF measurements, with demonstrated feasibility and reproducibility [
17]. Subtle changes in contractility, whether decreases due to damage or increases due to recovery, can be effectively detected through strain analysis [
37,
38]. As a result, GLS assessed by 2D speckle tracking analysis appears to be a highly suitable method for evaluating changes in myocardial ischemic burden. In the literature, successful CTO-PCI was shown to improve LV function measured by strain echocardiography, especially if viability and ischemia was evident [
39,
40]. In a cohort of 70 CTO patients attempted for revascularization, LV GLS values showed significant improvement at the ninth month following successful PCI, whereas the failed PCI group exhibited no change [
5]. Wang et al. demonstrated that LV EF values tended to improve after the third month of CTO PCI; however, GLS values began to recover as early as the first day after successful revascularization [
11]. Sotomi et al. demonstrated the significant improvement in GLS, but not in EF, among patients undergoing single vessel CTO PCI, significance beginning from the first day post-procedure and persisting at three months (GLS pre-PCI, 1 day post-PCI and 3 months post-PCI were given respectively: -12.8±4.2, -14.3±4.1, -14.3±4.4, p=0.023). They also searched for the regional longitudinal strain change in the CTO area and CTO collateral donor vessel area, both showing significant improvement after CTO-PCI (p=0.044 and p=0.034, respectively) [
10]. They hypothesized that not only collateral recipient CTO area, but also collateral donor vessel area could be under ischemic risk. This may be the case especially in diseased donor vessels, where collateral supply to the CTO area may lead to reduction of blood flow of the donor vessel itself, known as the “steal phenomenon” [
41]. Our study investigated the potential impact of collateral supply grade on GLS, a sensitive tool for subtle ischemic changes, in patients undergoing single vessel CTO-PCI. We found that GLS values improved in the poor collateral group, but not in the WD collateral group. Our study patients had only one CTO lesion, and the remaining coronaries were free from significant stenosis (≥50% stenosis in non-CTO arteries were excluded). Therefore, it was anticipated that collateral donor vessel flow would not fall below ischemic thresholds, and regional strain in the donor vessel territory would remain unchanged after revascularization. This could explain the lack of improvement in GLS in the WD collateral group, as both the collateral donor and recipient coronary territory were expected to receive adequate blood flow to maintain contractile function. However, inefficient coronary supply under increased demand appeared evident, given that all patients were either symptomatic or had significant provoked ischemia.
Patients with CTO typically develop collateralization of the distal vessel at varying degrees. It can contribute to the relief of ischemia and anginal symptoms, as well as to the preservation of ventricular function [
12,
13]. In a meta-analysis of 12 studies and 6529 patients, the WD collateralization in coronary artery disease was associated with a higher survival rate and yielded a 36% reduction in mortality risk compared to poor collateralization [
42]. Numerous studies had demonstrated that a WD collateral circulation improved survival and LV function by maintaining adequate blood supply, thereby preserving metabolic function and preventing necrosis [
43,
44]. A strong correlation was observed between the degree of coronary collateral circulation and significantly smaller myocardial infarctions, along with preserved LV EF [
14,
45]. Many physicians may view robust coronary collateral circulation as indicative of a favorable prognosis in patients with CTO, and consequently, they may often recommend medical therapy over revascularization. Nevertheless, during exercise, the functional reserve of these collaterals is known to be limited, leading to ischemia in more than 90% of patients with well-collateralized occlusions [
43]. Li et al. assessed 1124 CTO patients based on their treatment strategies within subgroups according to collateral degree. WD collateral circulation was not associated with a lower risk of cardiac death or MACE among patients with CTO, regardless of whether they received revascularization or not (for WD and poor collateral groups; cardiac death: 4.2% vs 4.1%; adjusted hazard ratio [HR], 1.01; p= 0.97 and MACEs: 23.6% vs 23.2%; adjusted HR, 1.07; p= 0.59) [
46]. In a large cohort of 954 CTO patients undergoing revascularization or OMT, the degree of collateral supply did not correlate with long-term mortality. However, patients who underwent revascularization through CABG or PCI exhibited significantly lower mortality rates compared to those managed with medical therapy alone (p < 0.0001) [
47]. In the context of CTO revascularization, there does not seem to be a corresponding improvement in prognosis according to the degree of collateral supply [
48]. A plausible explanation for these results may stem from the grading technique typically employed for collateral assessment. The classical angiographic grading system, described by Rentrop et al., evaluates the effectiveness of collaterals in filling the occluded arterial segment rather than assessing the function and quality directly [
20]. Although these anatomic methods are practical, they do not correlate with functional perfusion necessarily [
41]. A study by Werner et al had revealed that there was no relationship between Rentrop collateral grade classification and collateral function assessment in CTO patients [
49]. However, these functional assessments determining flow and pressure indices, are technically challenging and operator dependent. Today, physiological assessment of collateral supply is not used in clinical practice and remain to be a research tool. In our study, Rentrop collateral grading was used and patients were grouped according to the supply grade. Our clinical and LV functional outcomes across groups at pre-PCI and post-PCI follow-ups appeared to align with the collateral grading scores. Patients with WD collateral supply had significantly higher EF and GLS values when compared to poor supply group at the initial visit (EF: 54.2±3.4 vs 52.2±4.1, p=0.04, GLS: -14.8±1.6 vs -12.8±1.47, p=0.0001). This difference observed during the baseline examination may be attributed to the potentially larger area of hibernating myocardium in the poor collateral group. This mechanism can also explain the significant improvement of GLS observed at post-PCI follow-ups, which was evident again only in the poor collateral group.