Submitted:
25 July 2024
Posted:
25 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
Experimental Design and Cohort
Metabolomics Analysis
Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bello, A. K., Okpechi, I. G., Osman, M. A., Cho, Y., Htay, H., Jha, V., Wainstein, M., & Johnson, D. W. (2022). Epidemiology of haemodialysis outcomes. Nature Reviews Nephrology, 18(6), Article 6. [CrossRef]
- Betjes, M. G. H. (2013). Immune cell dysfunction and inflammation in end-stage renal disease. Nature Reviews. Nephrology, 9(5), 255-265. [CrossRef]
- Thompson, S., James, M., Wiebe, N., Hemmelgarn, B., Manns, B., Klarenbach, S., Tonelli, M., & Alberta Kidney Disease Network. (2015). Cause of Death in Patients with Reduced Kidney Function. Journal of the American Society of Nephrology: JASN, 26(10), 2504-2511. [CrossRef]
- Zuidema, M. Y., & Dellsperger, K. C. (2012). Myocardial Stunning with Hemodialysis: Clinical Challenges of the Cardiorenal Patient. Cardiorenal Medicine, 2(2), 125-133. [CrossRef]
- Wang, Y., & Gao, L. (2022). Inflammation and Cardiovascular Disease Associated With Hemodialysis for End-Stage Renal Disease. Frontiers in Pharmacology, 13, 800950. [CrossRef]
- Cobo, G., Lindholm, B., & Stenvinkel, P. (2018). Chronic inflammation in end-stage renal disease and dialysis. Nephrology Dialysis Transplantation, 33(Suppl 3), iii35-iii40. [CrossRef]
- Kalim, S., Wald, R., Yan, A. T., Goldstein, M. B., Kiaii, M., Xu, D., Berg, A. H., Clish, C., Thadhani, R., Rhee, E. P., & Perl, J. (2018). Extended Duration Nocturnal Hemodialysis and Changes in Plasma Metabolite Profiles. Clinical Journal of the American Society of Nephrology: CJASN, 13(3), 436-444. [CrossRef]
- Velenosi, T. J., Thomson, B. K. A., Tonial, N. C., RaoPeters, A. A. E., Mio, M. A., Lajoie, G. A., Garg, A. X., House, A. A., & Urquhart, B. L. (2019). Untargeted metabolomics reveals N, N, N-trimethyl-L-alanyl-L-proline betaine (TMAP) as a novel biomarker of kidney function. Scientific Reports, 9(1), 6831. [CrossRef]
- Sirich, T. L., Aronov, P. A., Fullman, J., Nguyen, K., Plummer, N. S., & Meyer, T. W. (2017). Untargeted mass spectrometry discloses plasma solute levels poorly controlled by hemodialysis. PLOS ONE, 12(11), e0188315. [CrossRef]
- Watkins, B. A., Friedman, A. N., Kim, J., Borkowski, K., Kaiser, S., Fiehn, O., & Newman, J. W. (2022). Blood Levels of Endocannabinoids, Oxylipins, and Metabolites Are Altered in Hemodialysis Patients. International Journal of Molecular Sciences, 23(17), 9781. [CrossRef]
- Holle, J., Bartolomaeus, H., Löber, U., Behrens, F., Bartolomaeus, T. U. P., Anandakumar, H., Wimmer, M. I., Vu, D. L., Kuhring, M., Brüning, U., Maifeld, A., Geisberger, S., Kempa, S., Schumacher, F., Kleuser, B., Bufler, P., Querfeld, U., Kitschke, S., Engler, D., … Müller, D. (2022). Inflammation in Children with CKD Linked to Gut Dysbiosis and Metabolite Imbalance. Journal of the American Society of Nephrology: JASN, 33(12), 2259-2275. [CrossRef]
- Kim, H. J., Seong, E. Y., Lee, W., Kim, S., Ahn, H.-S., Yeom, J., Kim, K., Kwon, C. H., & Song, S. H. (2021). Comparative analysis of therapeutic effects between medium cut-off and high flux dialyzers using metabolomics and proteomics: Exploratory, prospective study in hemodialysis. Scientific Reports, 11(1), 17335. [CrossRef]
- Tsalik, E. L., Willig, L. K., Rice, B. J., van Velkinburgh, J. C., Mohney, R. P., McDunn, J. E., Dinwiddie, D. L., Miller, N. A., Mayer, E. S., Glickman, S. W., Jaehne, A. K., Glew, R. H., Sopori, M. L., Otero, R. M., Harrod, K. S., Cairns, C. B., Fowler, V. G., Rivers, E. P., Woods, C. W., … Langley, R. J. (2015). Renal systems biology of patients with systemic inflammatory response syndrome. Kidney International, 88(4), 804-814. [CrossRef]
- Zhang, L., Xie, F., Tang, H., Zhang, X., Hu, J., Zhong, X., Gong, N., Lai, Y., Zhou, M., Tian, J., Zhou, Z., Xie, L., Hu, Z., Zhu, F., Jiang, J., & Nie, J. (2022). Gut microbial metabolite TMAO increases peritoneal inflammation and peritonitis risk in peritoneal dialysis patients. Translational Research, 240, 50-63. [CrossRef]
- Daniels, J. R., Ma, J. Z., Cao, Z., Beger, R. D., Sun, J., Schnackenberg, L., Pence, L., Choudhury, D., Palevsky, P. M., Portilla, D., & Yu, L.-R. (2021). Discovery of Novel Proteomic Biomarkers for the Prediction of Kidney Recovery from Dialysis-Dependent AKI Patients. Kidney360, 2(11), 1716-1727. [CrossRef]
- Sun, J., Cao, Z., Schnackenberg, L., Pence, L., Yu, L.-R., Choudhury, D., Palevsky, P. M., Portilla, D., & Beger, R. D. (2021). Serum metabolite profiles predict outcomes in critically ill patients receiving renal replacement therapy. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences, 1187, 123024. [CrossRef]
- Missailidis, C., Hällqvist, J., Qureshi, A. R., Barany, P., Heimbürger, O., Lindholm, B., Stenvinkel, P., & Bergman, P. (2016). Serum Trimethylamine-N-Oxide Is Strongly Related to Renal Function and Predicts Outcome in Chronic Kidney Disease. PloS One, 11(1), e0141738. [CrossRef]
- Ferrantelli, E., Farhat, K., Ederveen, A. L. H., Reiding, K. R., Beelen, R. H. J., van Ittersum, F. J., Wuhrer, M., & Dotz, V. (2018). Effluent and serum protein N-glycosylation is associated with inflammation and peritoneal membrane transport characteristics in peritoneal dialysis patients. Scientific Reports, 8(1), 979. [CrossRef]
- Su, X., Gao, Y., & Yang, R. (2022). Gut Microbiota-Derived Tryptophan Metabolites Maintain Gut and Systemic Homeostasis. Cells, 11(15), 2296. [CrossRef]
- Wojciech, L., Png, C. W., Koh, E. Y., Kioh, D. Y. Q., Deng, L., Wang, Z., Wu, L.-Z., Hamidinia, M., Tung, D. W., Zhang, W., Pettersson, S., Chan, E. C. Y., Zhang, Y., Tan, K. S., & Gascoigne, N. R. (2023). A tryptophan metabolite made by a gut microbiome eukaryote induces pro-inflammatory T cells. The EMBO Journal, 42(21), e112963. [CrossRef]
- Shang, H., Huang, C., Xiao, Z., Yang, P., Zhang, S., Hou, X., & Zhang, L. (2023). Gut microbiota-derived tryptophan metabolites alleviate liver injury via AhR/Nrf2 activation in pyrrolizidine alkaloids-induced sinusoidal obstruction syndrome. Cell & Bioscience, 13(1), 127. [CrossRef]
- Wishart, D. S., Guo, A., Oler, E., Wang, F., Anjum, A., Peters, H., Dizon, R., Sayeeda, Z., Tian, S., Lee, B. L., Berjanskii, M., Mah, R., Yamamoto, M., Jovel, J., Torres-Calzada, C., Hiebert-Giesbrecht, M., Lui, V. W., Varshavi, D., Varshavi, D., … Gautam, V. (2022). HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Research, 50(D1), D622-D631. [CrossRef]
- Wu, N., Ma, Y.-C., Gong, X.-Q., Zhao, P.-J., Jia, Y.-J., Zhao, Q., Duan, J.-H., & Zou, C.-G. (2023). The metabolite alpha-ketobutyrate extends lifespan by promoting peroxisomal function in C. elegans. Nature Communications, 14(1), 240. [CrossRef]
- Boo, Y. C. (2020). Emerging Strategies to Protect the Skin from Ultraviolet Rays Using Plant-Derived Materials. Antioxidants (Basel, Switzerland), 9(7), 637. [CrossRef]
- Korhonen, E., Piippo, N., Hytti, M., Kaarniranta, K., & Kauppinen, A. (2023). Cis-urocanic acid improves cell viability and suppresses inflammasome activation in human retinal pigment epithelial cells. Biochemical Pharmacology, 216, 115790. [CrossRef]
- Meotti, F. C., Jameson, G. N. L., Turner, R., Harwood, D. T., Stockwell, S., Rees, M. D., Thomas, S. R., & Kettle, A. J. (2011). Urate as a physiological substrate for myeloperoxidase: Implications for hyperuricemia and inflammation. The Journal of Biological Chemistry, 286(15), 12901-12911. [CrossRef]
- Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD - PubMed. (s. f.). Recuperado 9 de abril de 2024, de https://pubmed.ncbi.nlm.nih.
- Hu, J.-R., Coresh, J., Inker, L. A., Levey, A. S., Zheng, Z., Rebholz, C. M., Tin, A., Appel, L. J., Chen, J., Sarnak, M. J., & Grams, M. E. (2018). Serum metabolites are associated with all-cause mortality in chronic kidney disease. Kidney international, 94(2), 381-389. [CrossRef]
- Guizoni, D. M., Vettorazzi, J. F., Carneiro, E. M., & Davel, A. P. (2020). Modulation of endothelium-derived nitric oxide production and activity by taurine and taurine-conjugated bile acids. Nitric Oxide, 94, 48-53. [CrossRef]
- Choucair, I., Nemet, I., Li, L., Cole, M. A., Skye, S. M., Kirsop, J. D., Fischbach, M. A., Gogonea, V., Brown, J. M., Tang, W. H. W., & Hazen, S. L. (2020). Quantification of bile acids: A mass spectrometry platform for studying gut microbe connection to metabolic diseases. Journal of Lipid Research, 61(2), 159-177. [CrossRef]
- Duranton, F., Cohen, G., De Smet, R., Rodriguez, M., Jankowski, J., Vanholder, R., Argiles, A., & European Uremic Toxin Work Group. (2012). Normal and pathologic concentrations of uremic toxins. Journal of the American Society of Nephrology: JASN, 23(7), 1258-1270. [CrossRef]
- Pongratz, G., & Straub, R. H. (2023). Chronic Effects of the Sympathetic Nervous System in Inflammatory Models. Neuroimmunomodulation, 30(1), 113-134. [CrossRef]
- Hunter, R. W., Lawson, C., Galitsiou, E., Gifford, F., & Neary, J. J. (2016). Pyroglutamic acidosis in association with therapeutic paracetamol use. Clinical Medicine, 16(6), 524-529. [CrossRef]
- Gamarra, Y., Santiago, F. C., Molina-López, J., Castaño, J., Herrera-Quintana, L., Domínguez, Á., & Planells, E. (2019). Pyroglutamic acidosis by glutathione regeneration blockage in critical patients with septic shock. Critical Care (London, England), 23(1), 162. [CrossRef]
- Liu, Y., Zhu, Q., Guo, G., Xie, Z., Li, S., Lai, C., Wu, Y., Wang, L., & Zhong, S. (2024). Causal associations of genetically predicted gut microbiota and blood metabolites with inflammatory states and risk of infections: A Mendelian randomization analysis. Frontiers in Microbiology, 15, 1342653. [CrossRef]
- Lu, G., Zhou, J., Yang, T., Li, J., Jiang, X., Zhang, W., Gu, S., & Wang, J. (2022). Landscape of Metabolic Fingerprinting for Diagnosis and Risk Stratification of Sepsis. Frontiers in Immunology, 13, 883628. [CrossRef]
- Li, D., Lu, X., Xu, G., Liu, S., Gong, Z., Lu, F., Xia, X., Jiang, J., Wang, H., Zou, F., & Ma, X. (2023). Dihydroorotate dehydrogenase regulates ferroptosis in neurons after spinal cord injury via the P53-ALOX15 signaling pathway. CNS Neuroscience & Therapeutics, 29(7), 1923-1939. [CrossRef]
- Liu, Y., Tan, S., Wu, Y., & Tan, S. (2022). The Emerging Role of Ferroptosis in Sepsis. DNA and Cell Biology, 41(4), 368-380. [CrossRef]
- Zhang, S., Zhao, D., Yang, Z., Wang, F., Yang, S., & Wang, C. (2024). Circulating mitochondria promoted endothelial cGAS-derived neuroinflammation in subfornical organ to aggravate sympathetic overdrive in heart failure mice. Journal of Neuroinflammation, 21(1), 27. [CrossRef]
- Claiborne, M. D., Sengupta, S., Zhao, L., Arwood, M. L., Sun, I.-M., Wen, J., Thompson, E. A., Mitchell-Flack, M., Laiho, M., & Powell, J. D. (2022). Persistent CAD activity in memory CD8+ T cells supports rRNA synthesis and ribosomal biogenesis required at rechallenge. Science Immunology, 7(71), eabh4271. [CrossRef]
- Qin, C., Rao, Y., Yuan, H., Wang, T.-Y., Zhao, J., Espinosa, B., Liu, Y., Zhang, S., Savas, A. C., Liu, Q., Zarinfar, M., Rice, S., Henley, J., Comai, L., Graham, N. A., Chen, C., Zhang, C., & Feng, P. (2022). SARS-CoV-2 couples evasion of inflammatory response to activated nucleotide synthesis. Proceedings of the National Academy of Sciences of the United States of America, 119(26), e2122897119. [CrossRef]
- Tu, H.-F., Ko, C.-J., Lee, C.-T., Lee, C.-F., Lan, S.-W., Lin, H.-H., Lin, H.-Y., Ku, C.-C., Lee, D.-Y., Chen, I.-C., Chuang, Y.-H., Del Caño-Ochoa, F., Ramón-Maiques, S., Ho, C.-C., Lee, M.-S., & Chang, G.-D. (2021). Afatinib Exerts Immunomodulatory Effects by Targeting the Pyrimidine Biosynthesis Enzyme CAD. Cancer Research, 81(12), 3270-3282. [CrossRef]
- Mao, X., Yang, Q., Chen, D., Yu, B., & He, J. (2019). Benzoic Acid Used as Food and Feed Additives Can Regulate Gut Functions. BioMed Research International, 2019, 5721585. [CrossRef]
- Sun, B., Wang, X., Liu, X., Wang, L., Ren, F., Wang, X., & Leng, X. (2020). Hippuric Acid Promotes Renal Fibrosis by Disrupting Redox Homeostasis via Facilitation of NRF2-KEAP1-CUL3 Interactions in Chronic Kidney Disease. Antioxidants (Basel, Switzerland), 9(9), 783. [CrossRef]
- Huang, M., Wei, R., Wang, Y., Su, T., Li, P., & Chen, X. (2018). The uremic toxin hippurate promotes endothelial dysfunction via the activation of Drp1-mediated mitochondrial fission. Redox Biology, 16, 303-313. [CrossRef]
- Shang, F., Wang, S.-C., Hsu, C.-Y., Miao, Y., Martin, M., Yin, Y., Wu, C.-C., Wang, Y.-T., Wu, G., Chien, S., Huang, H.-D., Tarng, D.-C., Shiu, Y.-T., Cheung, A. K., Huang, P.-H., Chen, Z., & Shyy, J. Y.-J. (2017). MicroRNA-92a Mediates Endothelial Dysfunction in CKD. Journal of the American Society of Nephrology: JASN, 28(11), 3251-3261. [CrossRef]
- Navik, U., Sheth, V. G., Khurana, A., Jawalekar, S. S., Allawadhi, P., Gaddam, R. R., Bhatti, J. S., & Tikoo, K. (2021). Methionine as a double-edged sword in health and disease: Current perspective and future challenges. Ageing Research Reviews, 72, 101500. [CrossRef]
- Błaszczyk, I., Grucka-Mamczar, E., Kasperczyk, S., & Birkner, E. (2009). Influence of methionine upon the concentration of malondialdehyde in the tissues and blood of rats exposed to sodium fluoride. Biological Trace Element Research, 129(1-3), 229-238. [CrossRef]
- Li, H., Cai, L., Liang, M., Wang, Z., Zhang, Y., Wu, Q., & Yang, L. (2020). Methionine augments endogenous antioxidant capacity of rice protein through stimulating MSR antioxidant system and activating Nrf2-ARE pathway in growing and adult rats. European Food Research and Technology, 246(5), 1051-1063. [CrossRef]
- Kumar, A., Pathak, R., Palfrey, H. A., Stone, K. P., Gettys, T. W., & Murthy, S. N. (2020). High levels of dietary methionine improves sitagliptin-induced hepatotoxicity by attenuating oxidative stress in hypercholesterolemic rats. Nutrition & Metabolism, 17(1), 2. [CrossRef]
- Liu, G., Yu, L., Fang, J., Hu, C.-A. A., Yin, J., Ni, H., Ren, W., Duraipandiyan, V., Chen, S., Al-Dhabi, N. A., & Yin, Y. (2017). Methionine restriction on oxidative stress and immune response in dss-induced colitis mice. Oncotarget, 8(27), 44511-44520. [CrossRef]
- Maddineni, S., Nichenametla, S., Sinha, R., Wilson, R. P., & Richie, J. P. (2013). Methionine restriction affects oxidative stress and glutathione-related redox pathways in the rat. Experimental Biology and Medicine, 238(4), 392-399. [CrossRef]
- Shah, V. O., Townsend, R. R., Feldman, H. I., Pappan, K. L., Kensicki, E., & Vander Jagt, D. L. (2013). Plasma Metabolomic Profiles in Different Stages of CKD. Clinical Journal of the American Society of Nephrology : CJASN, 8(3), 363-370. [CrossRef]
- Heredia Martinez, A., Rosa Diez, G., Ferraris, V., Coccia, P. A., Ferraris, J. R., Checa, A., Wheelock, C. E., Lundberg, J. O., Weitzberg, E., Carlström, M., & Krmar, R. T. (2020). Removal of nitrate and nitrite by hemodialysis in end-stage renal disease and by sustained low-efficiency dialysis in acute kidney injury. Nitric Oxide: Biology and Chemistry, 98, 33-40. [CrossRef]
- Kuwasawa-Iwasaki, M., Io, H., Muto, M., Ichikawa, S., Wakabayashi, K., Kanda, R., Nakata, J., Nohara, N., Tomino, Y., & Suzuki, Y. (2020). Effects of L-Carnitine Supplementation in Patients Receiving Hemodialysis or Peritoneal Dialysis. Nutrients, 12(11), 3371. [CrossRef]
- Guo, L., Chen, S., Ou, L., Li, S., Ye, Z.-N., & Liu, H.-F. (2022). Disrupted Alpha-Ketoglutarate Homeostasis: Understanding Kidney Diseases from the View of Metabolism and Beyond. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy, 15, 1961-1974. [CrossRef]
- Nakanishi, T., & Kuragano, T. (2024). Growing concerns about using hypoxia-inducible factor prolyl hydroxylase inhibitors for the treatment of renal anemia. Clinical Kidney Journal, 17(3), sfae051. [CrossRef]
- Zhang, J., Zhou, B., Sun, R., Ai, Y., Cheng, K., Li, F., Wang, B., Liu, F., Jiang, Z., Wang, W., Zhou, D., Chen, H., & Wu, Q. (2021). The metabolite α-KG induces GSDMC-dependent pyroptosis through death receptor 6-activated caspase-8. Cell Research, 31(9), 980-997. [CrossRef]
- Onishi, A., Fu, Y., Patel, R., Darshi, M., Crespo-Masip, M., Huang, W., Song, P., Freeman, B., Kim, Y. C., Soleimani, M., Sharma, K., Thomson, S. C., & Vallon, V. (2020). A role for tubular Na+/H+ exchanger NHE3 in the natriuretic effect of the SGLT2 inhibitor empagliflozin. American Journal of Physiology - Renal Physiology, 319(4), F712-F728. [CrossRef]
- Todd A. Swanson (Author), Sandra I. Kim (Author), Ph.D. Glucksman, Marc J. (Author),. (s. f.). BRS Biochemistry, Molecular Biology, and Genetics (5th ed.).
- Lercher, A., Bhattacharya, A., Popa, A. M., Caldera, M., Schlapansky, M. F., Baazim, H., Agerer, B., Gürtl, B., Kosack, L., Májek, P., Brunner, J. S., Vitko, D., Pinter, T., Genger, J.-W., Orlova, A., Pikor, N., Reil, D., Ozsvár-Kozma, M., Kalinke, U., … Bergthaler, A. (2019). Type I Interferon Signaling Disrupts the Hepatic Urea Cycle and Alters Systemic Metabolism to Suppress T Cell Function. Immunity, 51(6), 1074-1087.e9. [CrossRef]
- Lee, J. S., Adler, L., Karathia, H., Carmel, N., Rabinovich, S., Auslander, N., Keshet, R., Stettner, N., Silberman, A., Agemy, L., Helbling, D., Eilam, R., Sun, Q., Brandis, A., Malitsky, S., Itkin, M., Weiss, H., Pinto, S., Kalaora, S., … Erez, A. (2018). Urea Cycle Dysregulation Generates Clinically Relevant Genomic and Biochemical Signatures. Cell, 174(6), 1559-1570.e22. [CrossRef]
- Zheng, H.-K., Zhao, J.-H., Yan, Y., Lian, T.-Y., Ye, J., Wang, X.-J., Wang, Z., Jing, Z.-C., He, Y.-Y., & Yang, P. (2018). Metabolic reprogramming of the urea cycle pathway in experimental pulmonary arterial hypertension rats induced by monocrotaline. Respiratory Research, 19, 94. [CrossRef]
- Li, T., Ning, N., Li, B., Luo, D., Qin, E., Yu, W., Wang, J., Yang, G., Nan, N., He, Z., Yang, N., Gong, S., Li, J., Liu, A., Sun, Y., Li, Z., Jia, T., Gao, J., Zhang, W., … Wang, H. (2021). Longitudinal Metabolomics Reveals Ornithine Cycle Dysregulation Correlates With Inflammation and Coagulation in COVID-19 Severe Patients. Frontiers in Microbiology, 12, 723818. [CrossRef]
- Smirnova, O. A., Isaguliants, M. G., Hyvonen, M. T., Keinanen, T. A., Tunitskaya, V. L., Vepsalainen, J., Alhonen, L., Kochetkov, S. N., & Ivanov, A. V. (2012). Chemically induced oxidative stress increases polyamine levels by activating the transcription of ornithine decarboxylase and spermidine/spermine-N1-acetyltransferase in human hepatoma HUH7 cells. Biochimie, 94(9), 1876-1883. [CrossRef]
- Wei, C., Xu, J., Liu, Y., Qadir, J., Zhang, S., & Yuan, H. (2023). Exogenous Spermidine Alleviates Diabetic Myocardial Fibrosis Via Suppressing Inflammation and Pyroptosis in db/db Mice. Balkan Medical Journal, 40(5), 333-343. [CrossRef]
- Yurdagul, A., Subramanian, M., Wang, X., Crown, S. B., Ilkayeva, O., Darville, L., Kolluru, G. K., Rymond, C. C., Gerlach, B. D., Zheng, Z., Kuriakose, G., Kevil, C. G., Koomen, J. M., Cleveland, J. L., Muoio, D. M., & Tabas, I. (2020). Macrophage Metabolism of Apoptotic Cell-Derived Arginine Promotes Continual Efferocytosis and Resolution of Injury. Cell metabolism, 31(3), 518-533.e10. [CrossRef]
- Lofft, Z., Taibi, A., Massara, P., Tokar, T., Paetau-Robinson, I., Khoo, C., & Comelli, E. M. (2022). Cranberry Proanthocyanidin and Its Microbial Metabolite 3,4-Dihydroxyphenylacetic Acid, but Not 3-(4-Hydroxyphenyl)-Propionic Acid, Partially Reverse Pro-Inflammatory microRNA Responses in Human Intestinal Epithelial Cells. Molecular Nutrition & Food Research, 66(8), e2100853. [CrossRef]
- Cilleros, D. Á., López-Oliva, M. E., Martín, M. Á., & Ramos, S. (2020). (−)-Epicatechin and the colonic metabolite 2,3-dihydroxybenzoic acid protect against high glucose and lipopolysaccharide-induced inflammation in renal proximal tubular cells through NOX-4/p38 signalling. Food & Function, 11(10), 8811-8824. [CrossRef]
- Mohammed, N. N., Tadros, M. G., & George, M. Y. (2024). Empagliflozin repurposing in Parkinson’s disease; modulation of oxidative stress, neuroinflammation, AMPK/SIRT-1/PGC-1α, and wnt/β-catenin pathways. Inflammopharmacology, 32(1), 777-794. [CrossRef]
- Wu, C.-H., Huang, H.-W., Lin, J.-A., Huang, S.-M., & Yen, G.-C. (2011). The proglycation effect of caffeic acid leads to the elevation of oxidative stress and inflammation in monocytes, macrophages and vascular endothelial cells. The Journal of Nutritional Biochemistry, 22(6), 585-594. [CrossRef]
- Favero, F., Barberis, E., Gagliardi, M., Espinoza, S., Contu, L., Gustincich, S., Boccafoschi, F., Borsotti, C., Lim, D., Rubino, V., Mignone, F., Pasolli, E., Manfredi, M., Zucchelli, S., Corà, D., & Corazzari, M. (2022). A Metabologenomic approach reveals alterations in the gut microbiota of a mouse model of Alzheimer’s disease. PLoS ONE, 17(8), e0273036. [CrossRef]
- Vanholder, R., Baurmeister, U., Brunet, P., Cohen, G., Glorieux, G., Jankowski, J., & European Uremic Toxin Work Group. (2008). A bench to bedside view of uremic toxins. Journal of the American Society of Nephrology: JASN, 19(5), 863-870. [CrossRef]
- Zaware, N., & Zhou, M.-M. (2017). Chemical Modulators for Epigenome Reader Domains as Emerging Epigenetic Therapies for Cancer and Inflammation. Current opinion in chemical biology, 39, 116-125. [CrossRef]
- Wang, T. J., Ngo, D., Psychogios, N., Dejam, A., Larson, M. G., Vasan, R. S., Ghorbani, A., O’Sullivan, J., Cheng, S., Rhee, E. P., Sinha, S., McCabe, E., Fox, C. S., O’Donnell, C. J., Ho, J. E., Florez, J. C., Magnusson, M., Pierce, K. A., Souza, A. L., … Gerszten, R. E. (2013). 2-Aminoadipic acid is a biomarker for diabetes risk. The Journal of Clinical Investigation, 123(10), 4309-4317. [CrossRef]
- Li, Y., Han, X., Tong, J., Wang, Y., Liu, X., Liao, Z., Jiang, M., & Zhao, H. (2023). Analysis of Metabolites in Gout: A Systematic Review and Meta-Analysis. Nutrients, 15(14), 3143. [CrossRef]
- Halade, G. V., Kain, V., Tourki, B., & Jadapalli, J. K. (2019). Lipoxygenase drives lipidomic and metabolic reprogramming in ischemic heart failure. Metabolism: Clinical and Experimental, 96, 22-32. [CrossRef]
- Zhou, B., Lou, B., Liu, J., & She, J. (2020). Serum metabolite profiles as potential biochemical markers in young adults with community-acquired pneumonia cured by moxifloxacin therapy. Scientific Reports, 10(1), 4436. [CrossRef]
- O’Neill, E., Chiara Goisis, R., Haverty, R., & Harkin, A. (2019). L-alpha-aminoadipic acid restricts dopaminergic neurodegeneration and motor deficits in an inflammatory model of Parkinson’s disease in male rats. Journal of Neuroscience Research, 97(7), 804-816. [CrossRef]
- Peris-Fernández, M., Roca-Marugán, M., Amengual, J. L., Balaguer-Timor, Á., Viejo-Boyano, I., Soldevila-Orient, A., Devesa-Such, R., Sánchez-Pérez, P., & Hernández-Jaras, J. (2024). Uremic Toxins and Inflammation: Metabolic Pathways Affected in Non-Dialysis-Dependent Stage 5 Chronic Kidney Disease. Biomedicines, 12(3), 607. [CrossRef]
- Kalantar-Zadeh, K. (2007). Inflammatory Marker Mania in Chronic Kidney Disease: Pentraxins at the Crossroad of Universal Soldiers of Inflammation. Clinical Journal of the American Society of Nephrology, 2(5), 872. [CrossRef]
| Variable | Mean/frequency |
|---|---|
| Age (years) (mean ± SD) | 68.97 ± 14.18 |
| Female gender, n (%) | 16 (37%) |
| Height (cm) (mean ± SD) | 165.23 ± 8.87 |
| Weight (kg) (mean ± SD) | 75.03 ± 16.73 |
| BMI (mean ± SD) | 27.75 ± 4.76 |
| Smoker, n (%) | 23 (53,5%) |
| Type 2 diabetes, n (%) | 21 (48,8%) |
| Hypertension, n (%) | 40 (93%) |
| Dyslipidemia, n (%) | 34 (79,1%) |
| Urea (mg/dl) (mean ± SD) | 88.70 ± 25.59 |
| Uric acid (mg/dl) (mean ± SD) | 4.97 ± 1.22 |
| Corrected calcium for serum albumin (mg/dl) (mean ± SD) | 9.13 ± 0.77 |
| Phosphate (mg/dl) (mean ± SD) | 4.26 ± 1.13 |
| Sodium (mEq/L) (mean ± SD) | 139.84 ± 2.98 |
| Potassium (mEq/L) (mean ± SD) | 4.70 ± 0.69 |
| Chloride (mEq/L) (mean ± SD) | 106 ± 4.33 |
| CRP (mg/L) (mean ± SD) | 14.24 ± 57.29 |
| Hemoglobin (g/dl) (mean ± SD) | 9.5 ± 1.35 |
| Leukocytes (× 109/L) (mean ± SD) | 7.03 ± 2.30 |
| Platelets (× 109/L) (mean ± SD) | 198.53 ± 62.55 |
| AVF as a venous access, n (%) | 24 (55,8%) |
| Hemodiafiltration as KRT, n (%) | 32 (74,4%) |
| Average interdialysis weight gain (cc) (mean ± SD) | 1622,5 ± 1018,67 |
| Kt/V (mean ± SD) | 1,7 ± 0,5 |
| Residual diuresis (cc) (mean ± SD) | 2097,67 ± 765,08 |
| Inflammed patients defined as CRP ≥ 2 mg/L, n (%) | 23 (67%) |
| Variable | Median inflammation =No | IQR Inflammation =No | Median Inflammation =Yes | IQR inflammation =Yes | Mann-Whitney statistic | p-value |
|---|---|---|---|---|---|---|
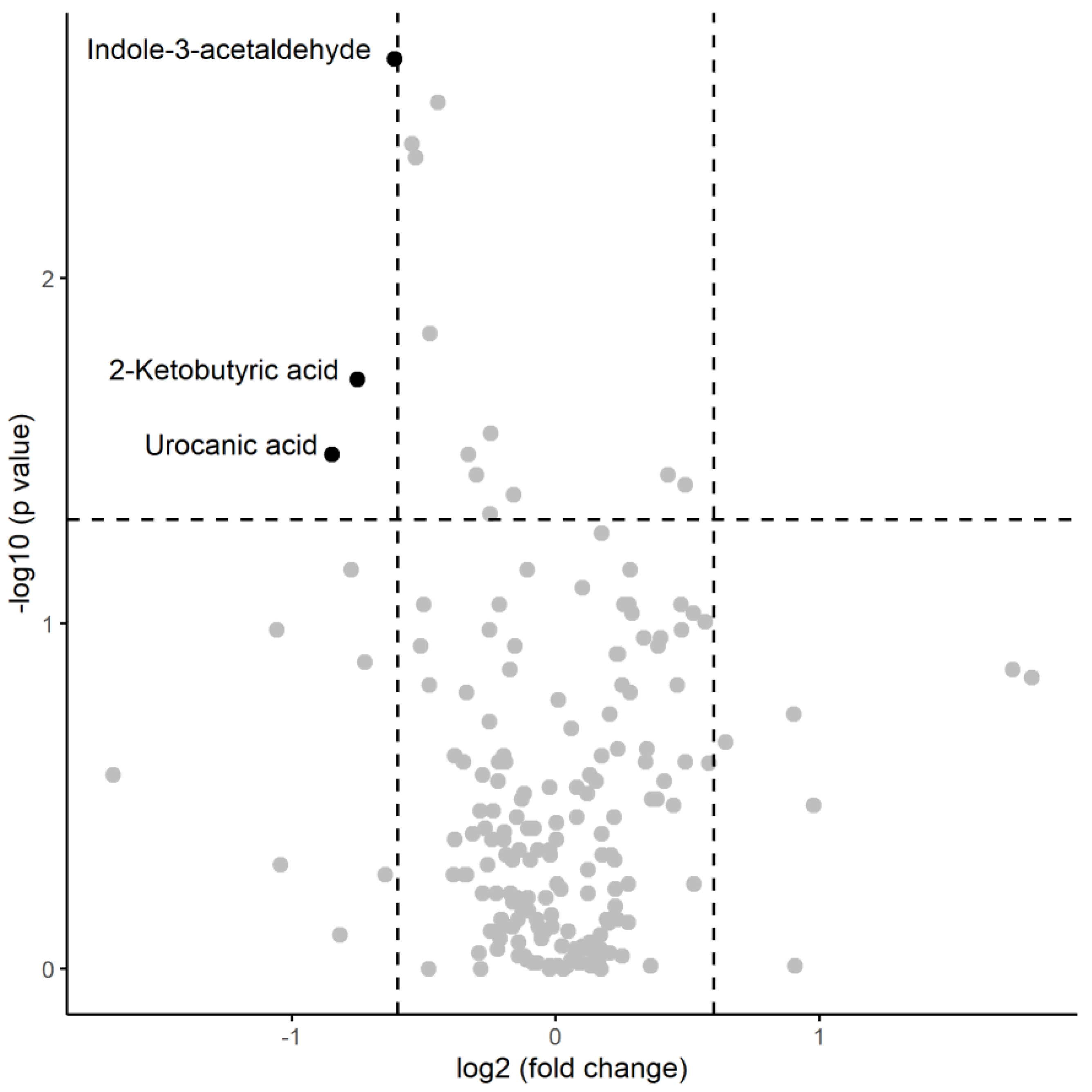
| Indole-3-acetaldehyde | 1190130 | 579521 | 778141 | 467437 | 316 | 0.0023 |
| tryptophan | 2988287 | 2263933 | 2191984 | 1182744 | 313 | 0.0031 |
| methionine | 937980 | 238906 | 641991 | 319903 | 310 | 0.0041 |
| Benzoic acid | 42742 | 18539 | 29556 | 14609 | 309 | 0.0045 |
| carnitine | 82227848 | 73058260 | 58997524 | 53464650 | 295 | 0.0145 |
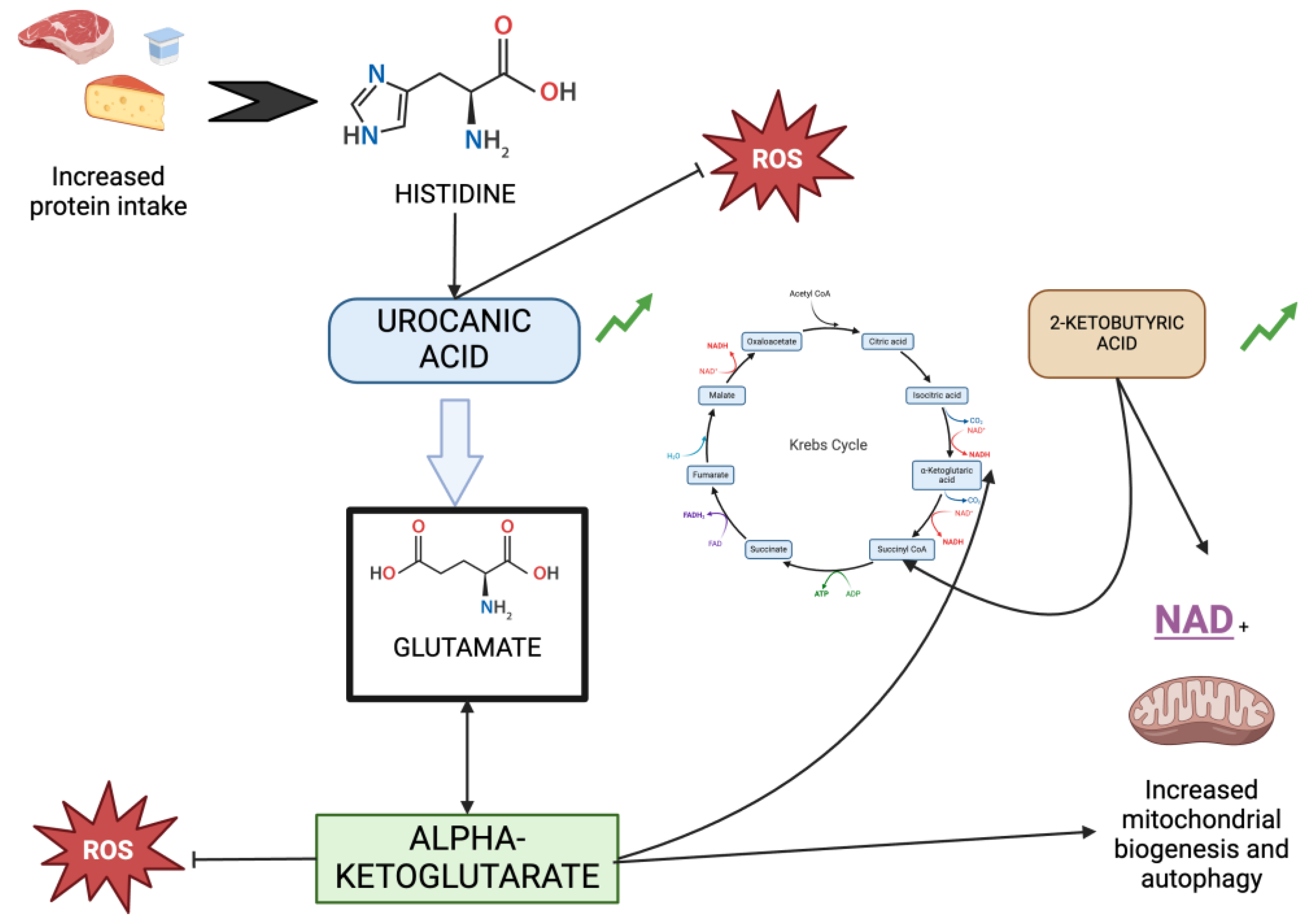
| 2-Ketobutyric acid | 872432 | 494891 | 517984 | 433389 | 291 | 0.0196 |
| alanine | 7982122 | 1829652 | 6727464 | 1840257 | 286 | 0.0281 |
| Urocanic acid | 15909 | 15612 | 8828 | 13043 | 284 | 0.0323 |
| 2-Methyl-3-ketovaleric acid | 65164816 | 25911640 | 51769852 | 22772580 | 284 | 0.0323 |
| trehalose | 74993 | 45349 | 100659 | 51935 | 123 | 0.0370 |
| D-2-Aminobutyric acid | 467418 | 132337 | 379133 | 248834 | 282 | 0.0370 |
| N-Acetyl-L-phenylalanine | 50225 | 36626 | 70619 | 56563 | 124 | 0.0396 |
| tyrosine | 2760424 | 852724 | 2470091 | 1027556 | 280 | 0.0423 |
| N-Acetylcarnitine | 31762998 | 18670840 | 26701374 | 14508230 | 278 | 0.0481 |
| Variables | Coef.estandarizados | Odds Ratio |
|---|---|---|
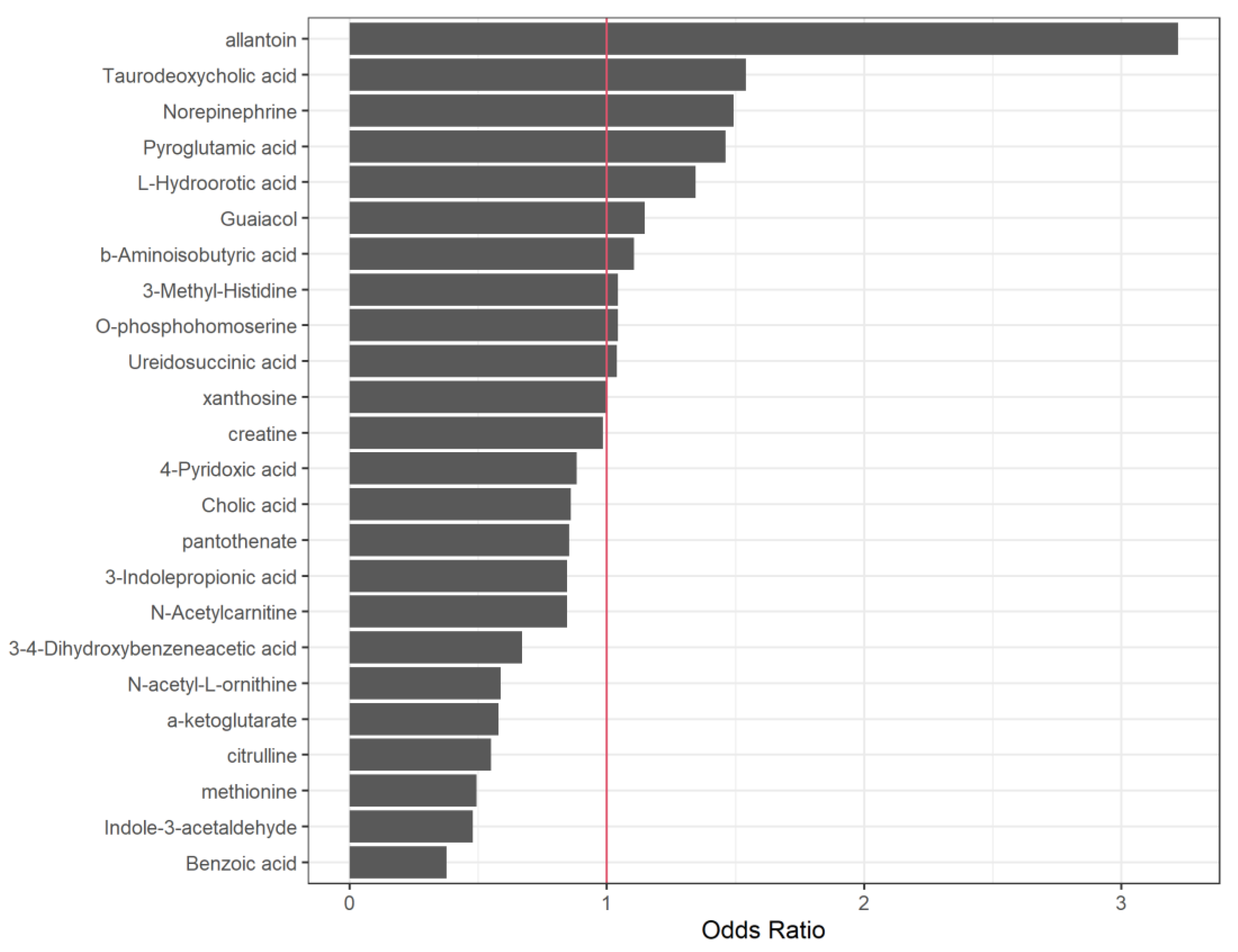
| Allantoin | 1.1695281 | 3.2204726 |
| Benzoic acid | -0.9747088 | 0.3773022 |
| Indole-3-acetaldehyde | -0.7354908 | 0.4792702 |
| methionine | -0.7067793 | 0.4932302 |
| Citrulline | -0.5962893 | 0.5508519 |
| a-ketoglutarate | -0.5466127 | 0.5789074 |
| N-acetyl-L-ornithine | -0.5330003 | 0.5868416 |
| Taurodeoxycholic acid | 0.4326940 | 1.5414044 |
| 3-4-Dihydroxybenzeneacetic acid | -0.4005447 | 0.6699550 |
| Norepinephrine | 0.4000314 | 1.4918716 |
| Pyroglutamic acid | 0.3795375 | 1.4616085 |
| L-Hydroorotic acid | 0.2959347 | 1.3443824 |
| N-Acetylcarnitine | -0.1682766 | 0.8451200 |
| 3-Indolepropionic acid | -0.1674606 | 0.8458099 |
| pantothenate | -0.1585006 | 0.8534225 |
| Cholic acid | -0.1503721 | 0.8603877 |
| Guaiacol | 0.1375204 | 1.1474251 |
| 4-Pyridoxic acid | -0.1233154 | 0.8839848 |
| b-Aminoisobutyric acid | 0.1005446 | 1.1057730 |
| 3-Methyl-Histidine | 0.0429894 | 1.0439269 |
| O-phosphohomoserine | 0.0415483 | 1.0424235 |
| Ureidosuccinic acid | 0.0387375 | 1.0394976 |
| creatine | -0.0143093 | 0.9857926 |
| xanthosine | 0.0020708 | 1.0020730 |
| Variable | Median inflammation =No | IQR Inflammation =No | Median Inflammation =Yes | IQR inflammation =Yes | Mann-Whitney statistic | p-value |
|---|---|---|---|---|---|---|
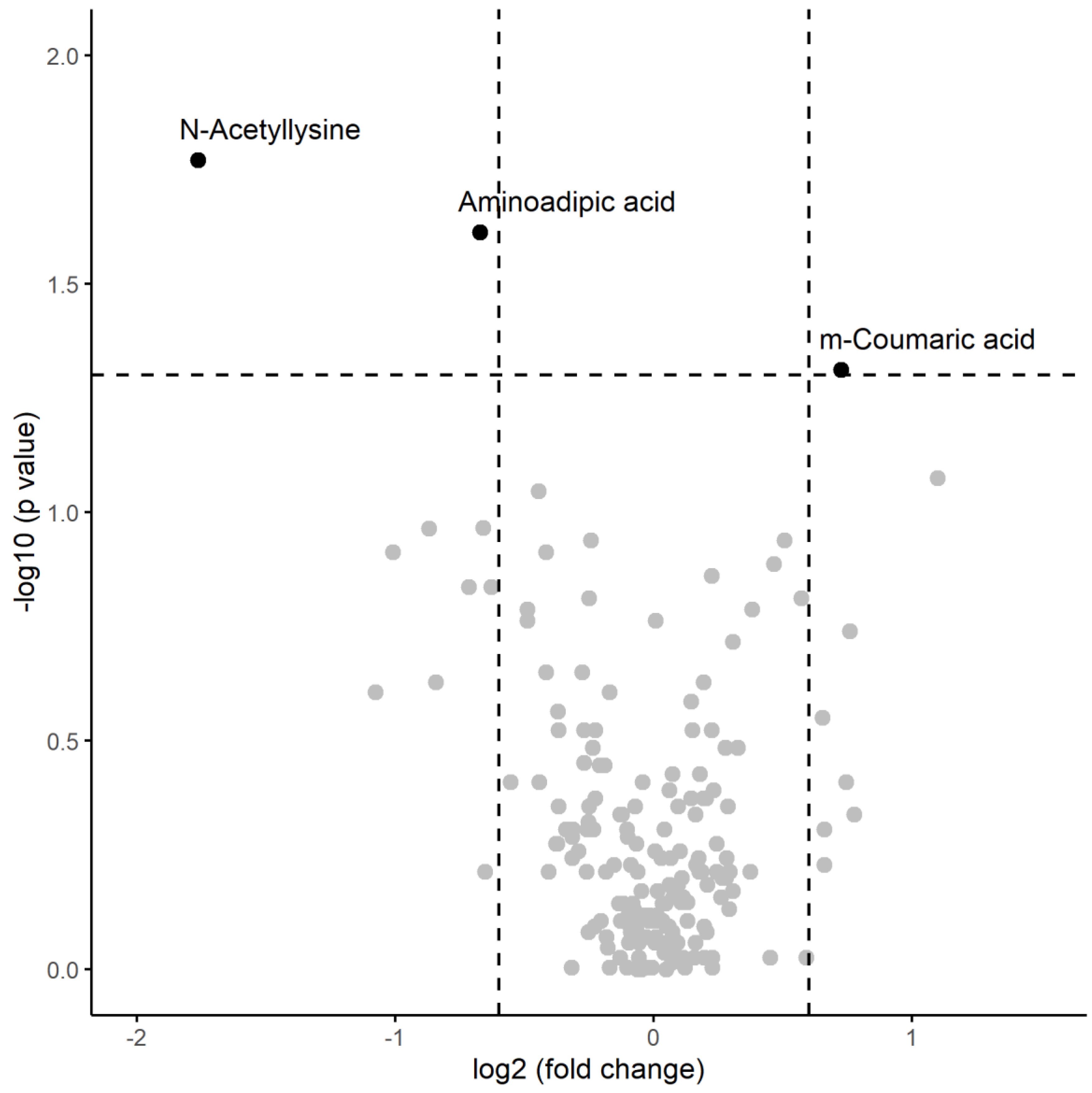
| N-Acetyllysine | 5267 | 9939 | 1550 | 3945 | 248 | 0.0170 |
| Aminoadipic acid | 33806 | 29710 | 21197 | 20038 | 244 | 0.0244 |
| m-Coumaric acid | 60008 | 50430 | 99180 | 82241 | 101 | 0.0487 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





