Submitted:
24 July 2024
Posted:
25 July 2024
You are already at the latest version
Abstract
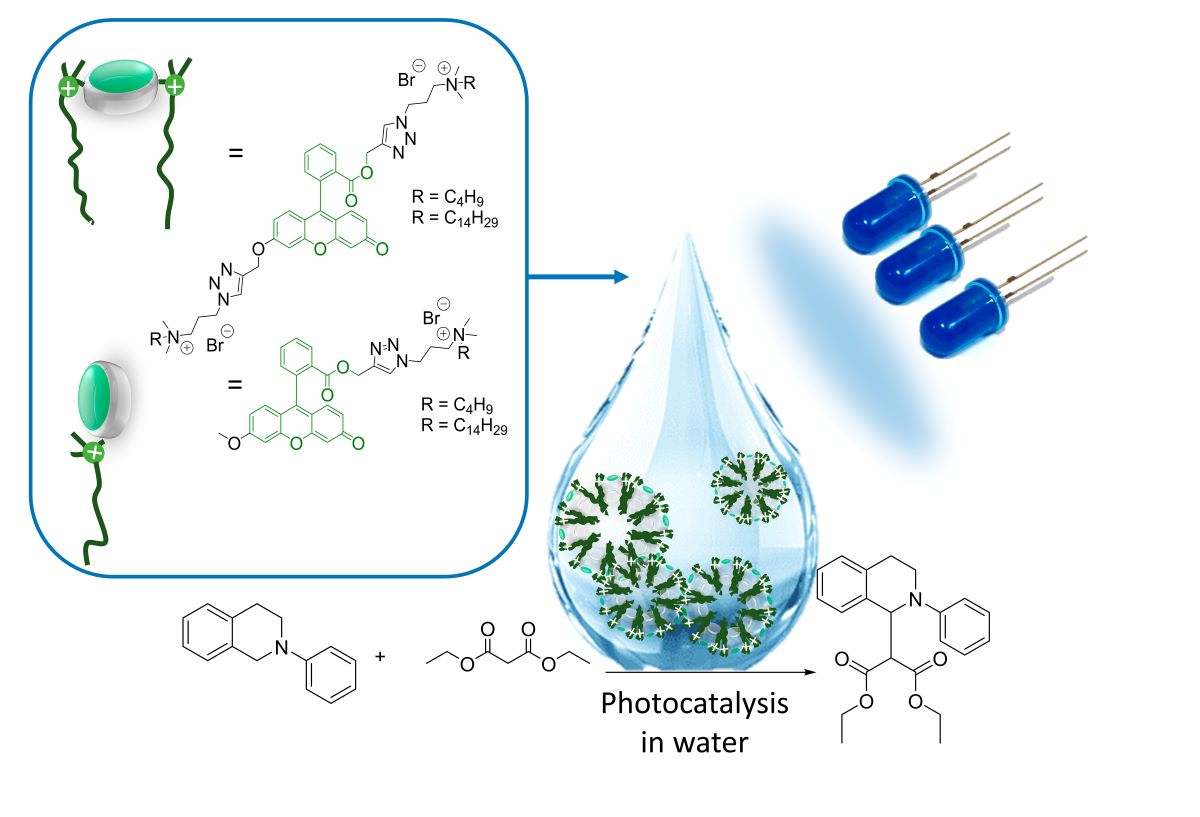
Keywords:
1. Introduction
2. Materials and Methods
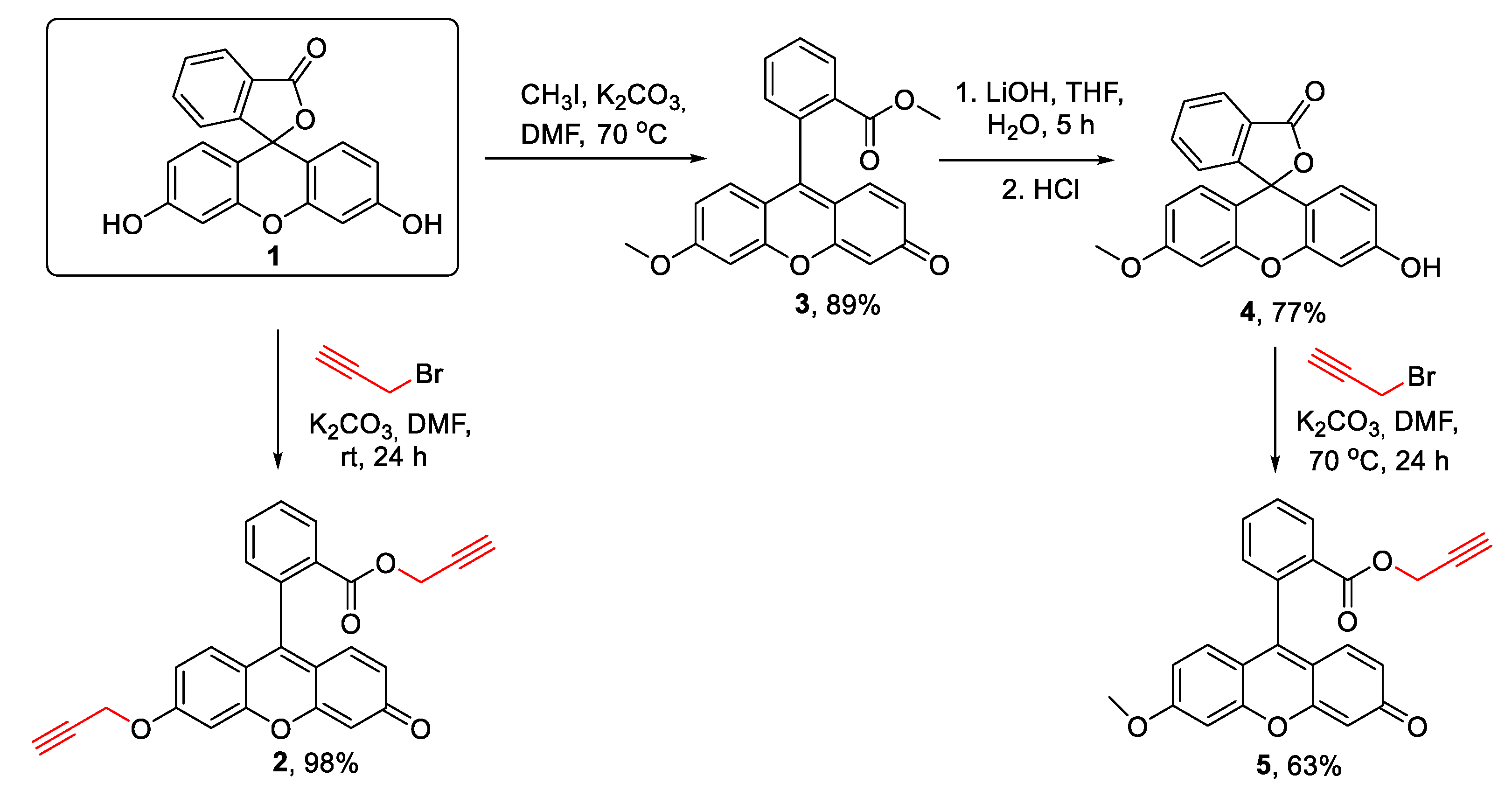
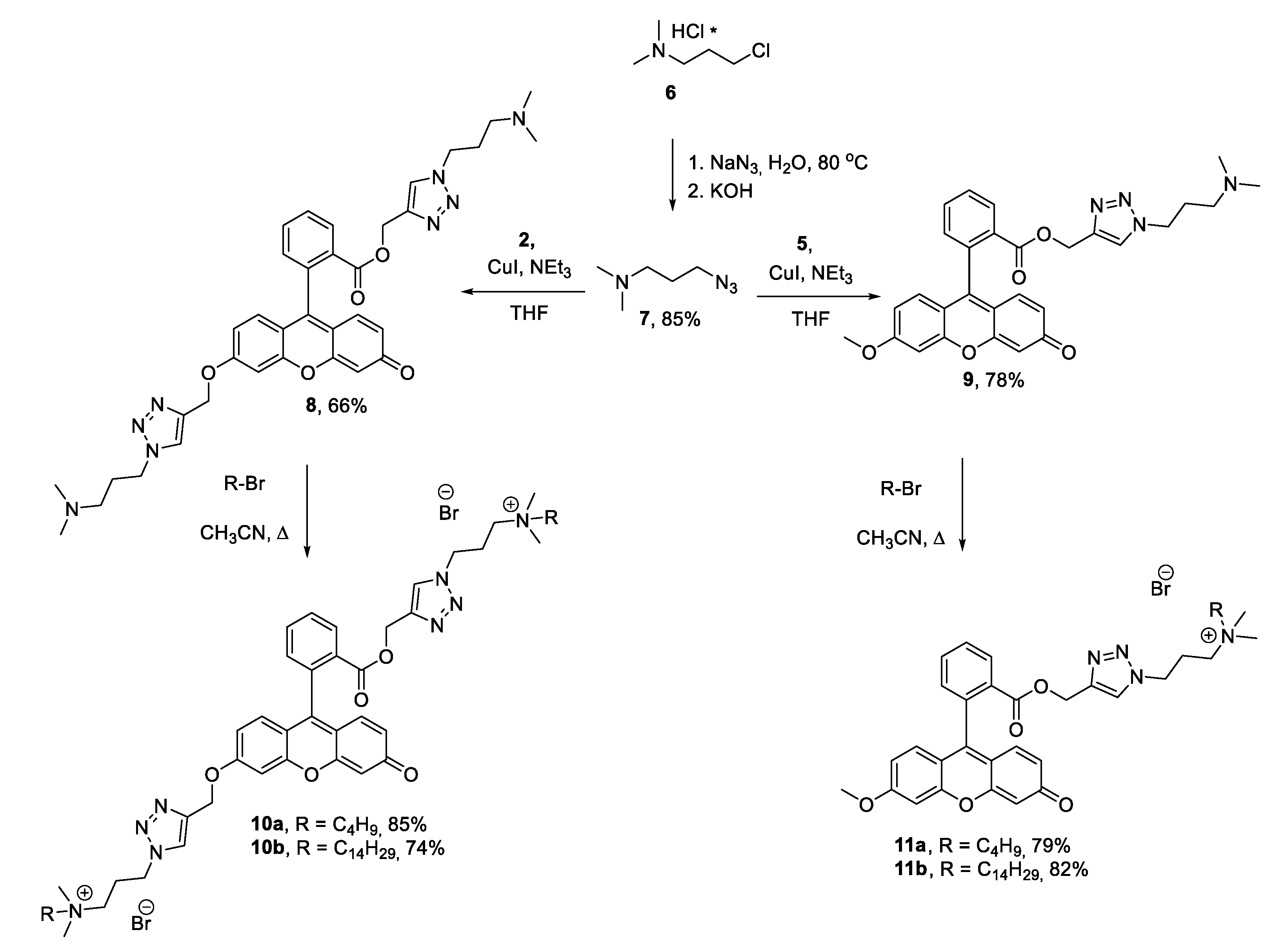
2.1. Synthesis
2.2. Photophysical and Self-Assembly Investigations
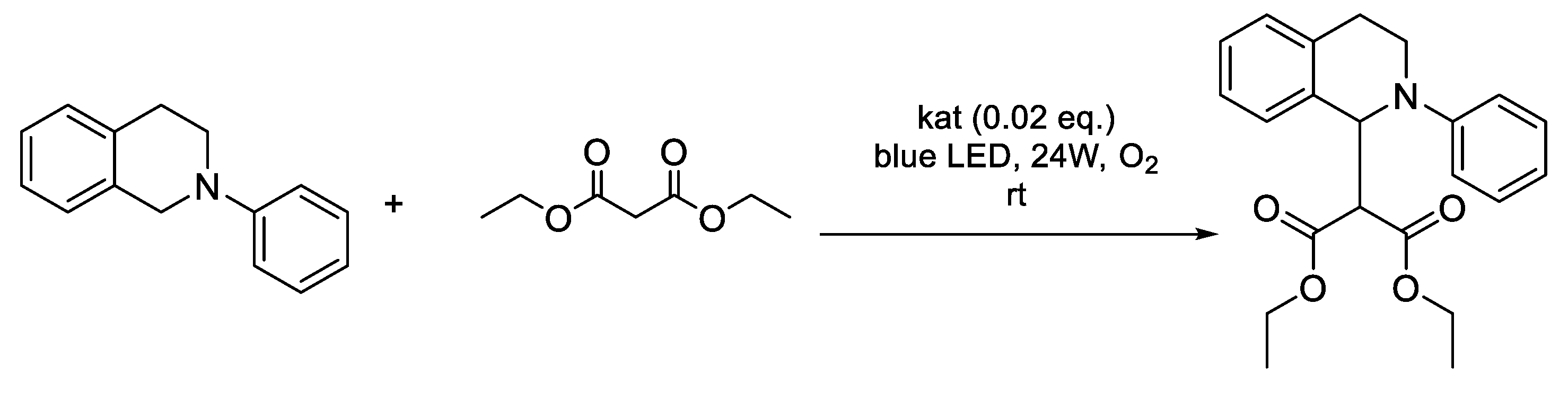
2.3. General Method for Photocatalytic Reaction
3. Results and Discussion
3.1. Synthesis of 1,2,3-Triazoles Containing Fragment of Fluorescein and Its Ammonium Derivatives
3.2. Photophysical and Self Assembly Properties
| System |
, % (water with 1% DMF) |
|---|---|
| 10a | 16 |
| 10b | 5 |
| 11a | 18 |
| 11b | 24 |
3.3. Photophysical and Self Assembly Properties
| 10a | 10b | 11a | 11b | |||||
|---|---|---|---|---|---|---|---|---|
| GCMS, % | 1H-NMR % | GCMS, % | 1H-NMR % | GCMS, % | 1H-NMR % | GCMS, % | 1H-NMR % | |
| MeCN | 87 | 94 | 86 | 93 | 86 | 91 | 84 | 87 |
| MeCN:Water, 50:50 | 87 | 95 | 86 | 94 | 87 | 91 | 87 | 89 |
| MeCN:Water, 10:90 | 92 | 94 | 84 | 90 | 66 | 71 | 95 | 100 |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ardila-Fierro, K.J.; Hernández, J.G. Sustainability Assessment of Mechanochemistry by Using the Twelve Principles of Green Chemistry. ChemSusChem 2021, 14, 2145–2162. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, K.N.; Zhang, D.; Miller, S.J.; Rossen, K.; Chirik, P.J.; Kozlowski, M.C.; Zimmerman, J.B.; Brooks, B.W.; Savage, P.E.; Allen, D.T.; et al. Green Chemistry: A Framework for a Sustainable Future. Org. Process Res. Dev. 2021, 25, 1455–1459. [Google Scholar] [CrossRef]
- Bobo, M.V.; Kuchta, J.J.; Vannucci, A.K. Recent advancements in the development of molecular organic photocatalysts. Org. Biomol. Chem. 2021, 19, 4816–4834. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Shi, W. Recent developments in visible-light photocatalytic degradation of antibiotics. Cuihua Xuebao/Chinese J. Catal. 2016, 37, 792–799. [Google Scholar] [CrossRef]
- Koe, W.S.; Lee, J.W.; Chong, W.C.; Pang, Y.L.; Sim, L.C. An overview of photocatalytic degradation: photocatalysts, mechanisms, and development of photocatalytic membrane. Environ. Sci. Pollut. Res. 2020, 27, 2522–2565. [Google Scholar] [CrossRef]
- Kumari, H.; Sonia; Suman; Ranga, R.; Chahal, S.; Devi, S.; Sharma, S.; Kumar, S.; Kumar, P.; Kumar, S.; et al. A Review on Photocatalysis Used For Wastewater Treatment: Dye Degradation; Springer International Publishing, 2023; Vol. 234; ISBN 0123456789. [CrossRef]
- Hari, D.P.; Hering, T.; König, B. Visible light photocatalytic synthesis of benzothiophenes. Org. Lett. 2012, 14, 5334–5337. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Wang, W.; Yue, J.-P.; Jiang, Y.-X.; Wei, M.-K.; Zhang, H.-P.; Yan, S.-S.; Liao, L.-L.; Yu, D. Visible-light photocatalytic di- and hydro-carboxylation of unactivated alkenes with CO2. Nat. Catal. 2022, 5, 1–7. [Google Scholar] [CrossRef]
- Dissanayake, K.C.; Ebukuyo, P.O.; Dhahir, Y.J.; Wheeler, K.; He, H. A BODIPY-functionalized PdII photoredox catalyst for Sonogashira C-C cross-coupling reactions. Chem. Commun. 2019, 55, 4973–4976. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Yang, J.; Yang, B.; Han, Y.; Zhu, L.; Xue, X.S.; Zhu, F. Photoredox Nickel-Catalysed Stille Cross-Coupling Reactions. Angew. Chemie - Int. Ed. 2023, 62. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Lei, T.; Wei, X.Z.; Ye, C.; Liu, Z.; Chen, B.; Tung, C.H.; Wu, L.Z. Metal-free, redox-neutral, site-selective access to heteroarylamine via direct radical−radical cross-coupling powered by visible light photocatalysis. J. Am. Chem. Soc. 2020, 142, 16805–16813. [Google Scholar] [CrossRef] [PubMed]
- Jati, A.; Dey, K.; Nurhuda, M.; Addicoat, M.A.; Banerjee, R.; Maji, B. Dual Metalation in a Two-Dimensional Covalent Organic Framework for Photocatalytic C-N Cross-Coupling Reactions. J. Am. Chem. Soc. 2022, 144, 7822–7833. [Google Scholar] [CrossRef] [PubMed]
- Zavalishin, M.N.; Gamov, G.A.; Kiselev, A.N.; Nikitin, G.A. A fluorescein conjugate as colorimetric and red-emissive fluorescence chemosensor for selective recognition Cu2+ ions. Opt. Mater. (Amst). 2024, 153, 115580–10. [Google Scholar] [CrossRef]
- Wagay, S.A.; Alam, M.; Ali, R. Synthesis of two novel fluorescein appended dipyromethanes (DPMs): Naked-eye chemosensors for fluoride, acetate and phosphate anions. J. Mol. Struct. 2023, 1291, 135982–10. [Google Scholar] [CrossRef]
- Keerthana, S.; Sam, B.; George, L.; Sudhakar, Y.N.; Varghese, A. Fluorescein Based Fluorescence Sensors for the Selective Sensing of Various Analytes. J. Fluoresc. 2021, 31, 1251–1276. [Google Scholar]
- Li, Z.; Song, H.; Guo, R.; Zuo, M.; Hou, C.; Sun, S.; He, X.; Sun, Z.; Chu, W. Visible-light-induced condensation cyclization to synthesize benzimidazoles using fluorescein as a photocatalyst. Green Chem. 2019, 21, 3602–3605. [Google Scholar] [CrossRef]
- Tu, X.P.; Wei, L.L.; Zhang, K.X.; Chen, Y.; Zhou, M.D. Synthesis of fluorescein-containing polymeric heterogeneous photocatalyst and its applications. Tetrahedron 2024, 160, 134028–10. [Google Scholar] [CrossRef]
- Sun, W.; Chen, H.; Wang, K.; Wang, X.; Lei, M.; Liu, C.; Zhong, Q. Synthesis of benzothiazoles using fluorescein as an efficient photocatalyst under visible light. Mol. Catal. 2021, 510, 111693–10. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Zhou, Z.; Zhou, R.; Sun, Q.; Wu, P. Halo-fluorescein for photodynamic bacteria inactivation in extremely acidic conditions. Nat. Commun. 2021, 12, 526–10. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.; Llanos, A.; Gomila, R.M.; Frontera, A.; Rodríguez, L. Ligand and Gold(I) Fluorescein-AIEgens as Photosensitizers in Solution and Doped Polymers. Inorg. Chem. 2023, 62, 7131–7140. [Google Scholar] [CrossRef] [PubMed]
- Gaigalas, A. Measurement of the Fluorescence Quantum Yield Using a Spectrometer With an Integrating Sphere Detector. J. Res. Natl. Inst. Stand. Technol. 2008, 113. [Google Scholar] [CrossRef] [PubMed]
- Rajasekar, M. Recent development in fluorescein derivatives. J. Mol. Struct. 2021, 1224, 129085–10. [Google Scholar] [CrossRef]
- Russo, C.; Brunelli, F.; Tron, G.C.; Giustiniano, M. Visible-Light Photoredox Catalysis in Water. J. Org. Chem. 2023, 88, 6284–6293. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Clerget, M.; Yu, J.; Kincaid, J.R.A.; Walde, P.; Gallou, F.; Lipshutz, B.H. Water as the reaction medium in organic chemistry: From our worst enemy to our best friend. Chem. Sci. 2021, 12, 4237–4266. [Google Scholar] [CrossRef] [PubMed]
- Pallini, F.; Sangalli, E.; Sassi, M.; Roth, P.M.C.; Mattiello, S.; Beverina, L. Selective photoredox direct arylations of aryl bromides in water in a microfluidic reactor. Org. Biomol. Chem. 2021, 19, 3016–3023. [Google Scholar] [CrossRef] [PubMed]
- Artemenko, A.A.; Burilov, V.A.; Solov’eva, S.E.; Antipin, I.S. Covalent and Supramolecular Conjugates of Calixarenes with Some Fluorescent Dyes of the Xanthene Series. Colloid J. 2022, 84, 563–580. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT - integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A, Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G. A Short History of ShelX. Acta Crystallogr. A. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.; Edgington, P.; McCabe, P.; Pidcock, E.; Shields, G.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallogr. - J APPL CRYST 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Berscheid, R.; Nieger, M.; Vögtle, F. Konkave Farbstoffmoleküle vom Triphenylmethan-Typ. Chem. Ber. 1992, 125, 2539–2552. [Google Scholar] [CrossRef]
- Xiang, Y.; He, B.; Li, X.; Zhu, Q. The design and synthesis of novel “turn-on” fluorescent probes to visualize monoamine oxidase-B in living cells. RSC Adv. 2013, 3, 4876–4879. [Google Scholar] [CrossRef]
- Du, L.; Risinger, A.L.; Yee, S.S.; Ola, A.R.B.; Zammiello, C.L.; Cichewicz, R.H.; Mooberry, S.L. Identification of C-6 as a New Site for Linker Conjugation to the Taccalonolide Microtubule Stabilizers. J. Nat. Prod. 2019, 82, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Carboni, B.; Benalil, A.; Vaultier, M. Aliphatic amino azides as key building blocks for efficient polyamine syntheses. J. Org. Chem. 1993, 58, 3736–3741. [Google Scholar] [CrossRef]
- Cheptea, C.; Zara, A.; Ambrosi, E.; Morosanu, A.C.; Diaconu, M.; Miron, M.; Dorohoi, D.O.; Dimitriu, D.G. On the Solvatochromism of Fluorescein Sodium. Symmetry (Basel). 2024, 16, 673–10. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of fluorescence spectroscopy, 3rd Principles of fluorescence spectroscopy, Springer, New York, USA, 3rd edn, 2006.; 2006; ISBN 0387312781. 10.1007/978-0-387-46312-4.
- Shen, J.; Snook, R.D. Thermal lens measurement of absolute quantum yields using quenched fluorescent samples as references. Chem. Phys. Lett. 1989, 155, 583–586. [Google Scholar] [CrossRef]
- Kandi, D.; Mansingh, S.; Behera, A.; Parida, K. Calculation of relative fluorescence quantum yield and Urbach energy of colloidal CdS QDs in various easily accessible solvents. J. Lumin. 2021, 231, 117792. [Google Scholar] [CrossRef]
- Piñeiro, L.; Novo, M.; Al-Soufi, W. Fluorescence emission of pyrene in surfactant solutions. Adv. Colloid Interface Sci. 2015, 215, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Vasileva, L.; Gaynanova, G.; Valeeva, F.; Romanova, E.; Pavlov, R.; Kuznetsov, D.; Belyaev, G.; Zueva, I.; Lyubina, A.; Voloshina, A.; et al. Synthesis, Properties, and Biomedical Application of Dicationic Gemini Surfactants with Dodecane Spacer and Carbamate Fragments. Int. J. Mol. Sci. 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Dumur, F.; Lalevée, J. Recent Advances in Photoredox Catalysts. Catalysts 2024, 14, 1–5. [Google Scholar] [CrossRef]
- Romero, N.A.; Nicewicz, D.A. Organic Photoredox Catalysis. Chem. Rev. 2016, 116, 10075–10166. [Google Scholar] [CrossRef] [PubMed]
- Franz, J.F.; Kraus, W.B.; Zeitler, K. No photocatalyst required-versatile, visible light mediated transformations with polyhalomethanes. Chem. Commun. 2015, 51, 8280–8283. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.O.; Jung, Y.J.; Kim, M.; Kim, H.; Li, J.; Ko, H.; Lee, H.I.; Lee, H.J.; Lee, J.K. Substituent Effects of Fluorescein on Photoredox Initiating Performance under Visible Light. ACS Omega 2023, 8, 40277–40286. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Yadav, R.K.; Kim, T.W.; Kumar, A.; Dwivedi, D.K. Chitosan-based fluorescein isothiocyanate film as a highly efficient metal-free photocatalyst for solar-light-mediated direct C-H arylation. Int. J. Energy Res. 2021, 45, 5964–5973. [Google Scholar] [CrossRef]
- Hari, D.P.; König, B. Eosin Y Catalyzed Visible Light Oxidative C–C and C–P bond Formation. Org. Lett. 2011, 13, 3852–3855. [Google Scholar] [CrossRef] [PubMed]
- Acharjee, A.; Rakshit, A.; Chowdhury, S.; Saha, B. Micelle catalysed conversion of ‘on water’ reactions into ‘in water’ one. J. Mol. Liq. 2021, 321, 114897–10. [Google Scholar] [CrossRef]
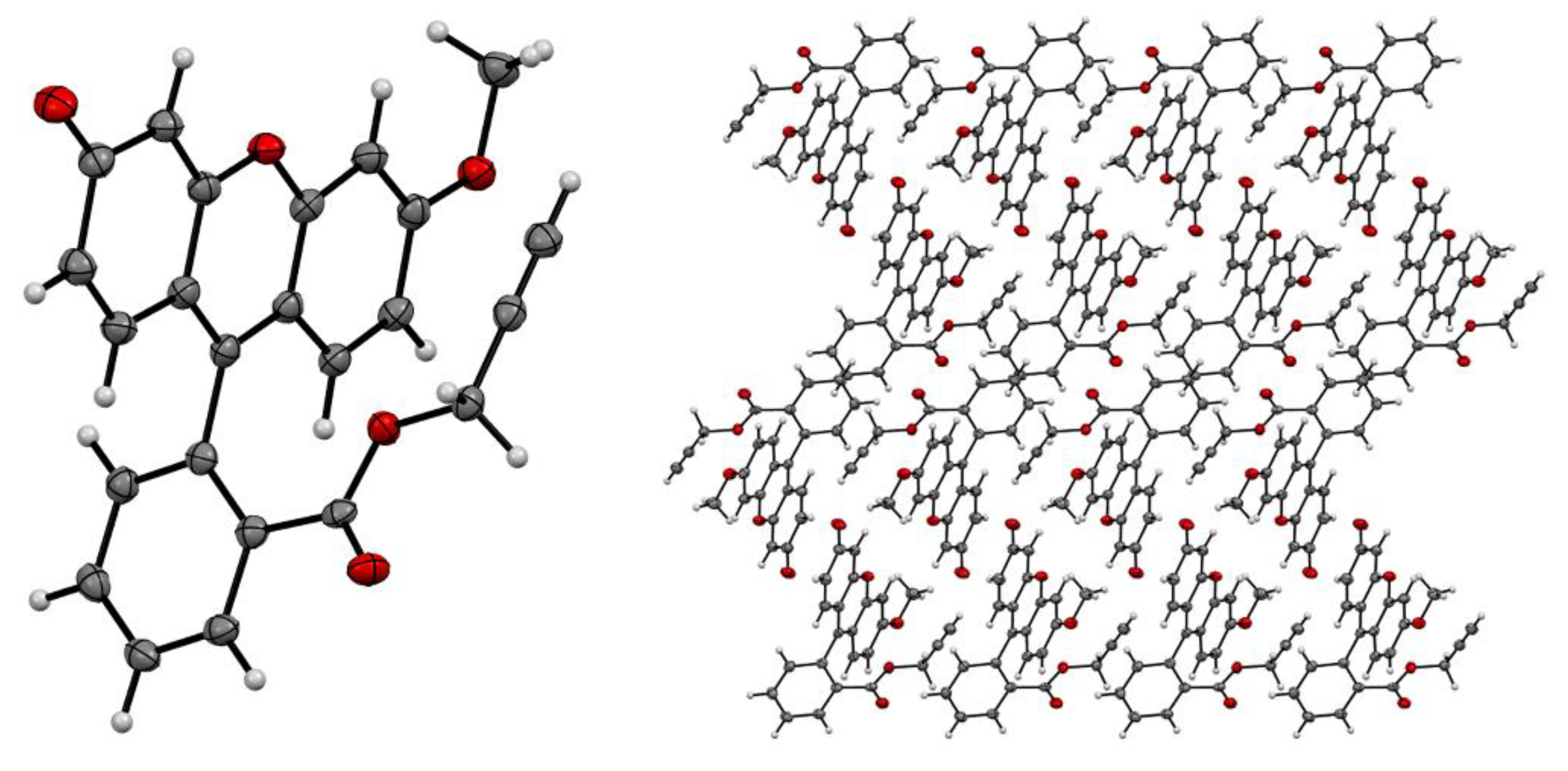
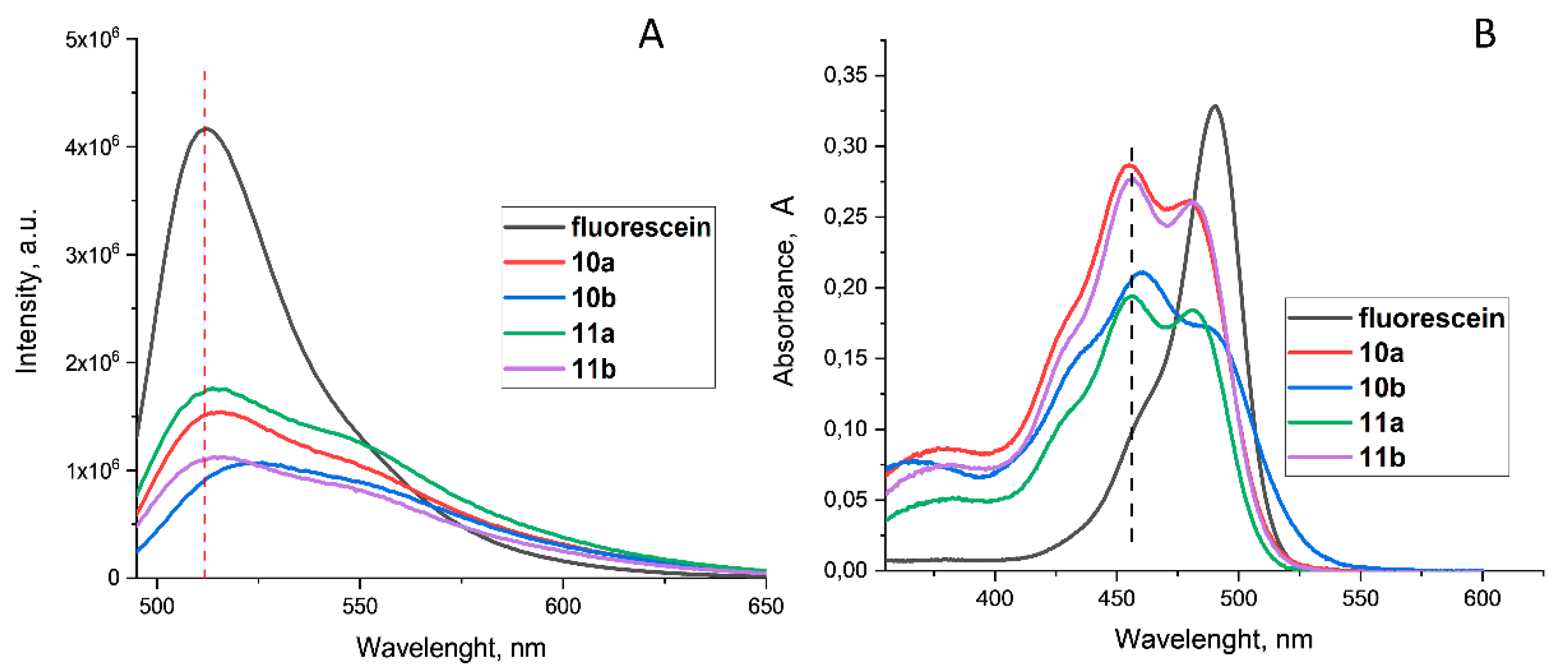
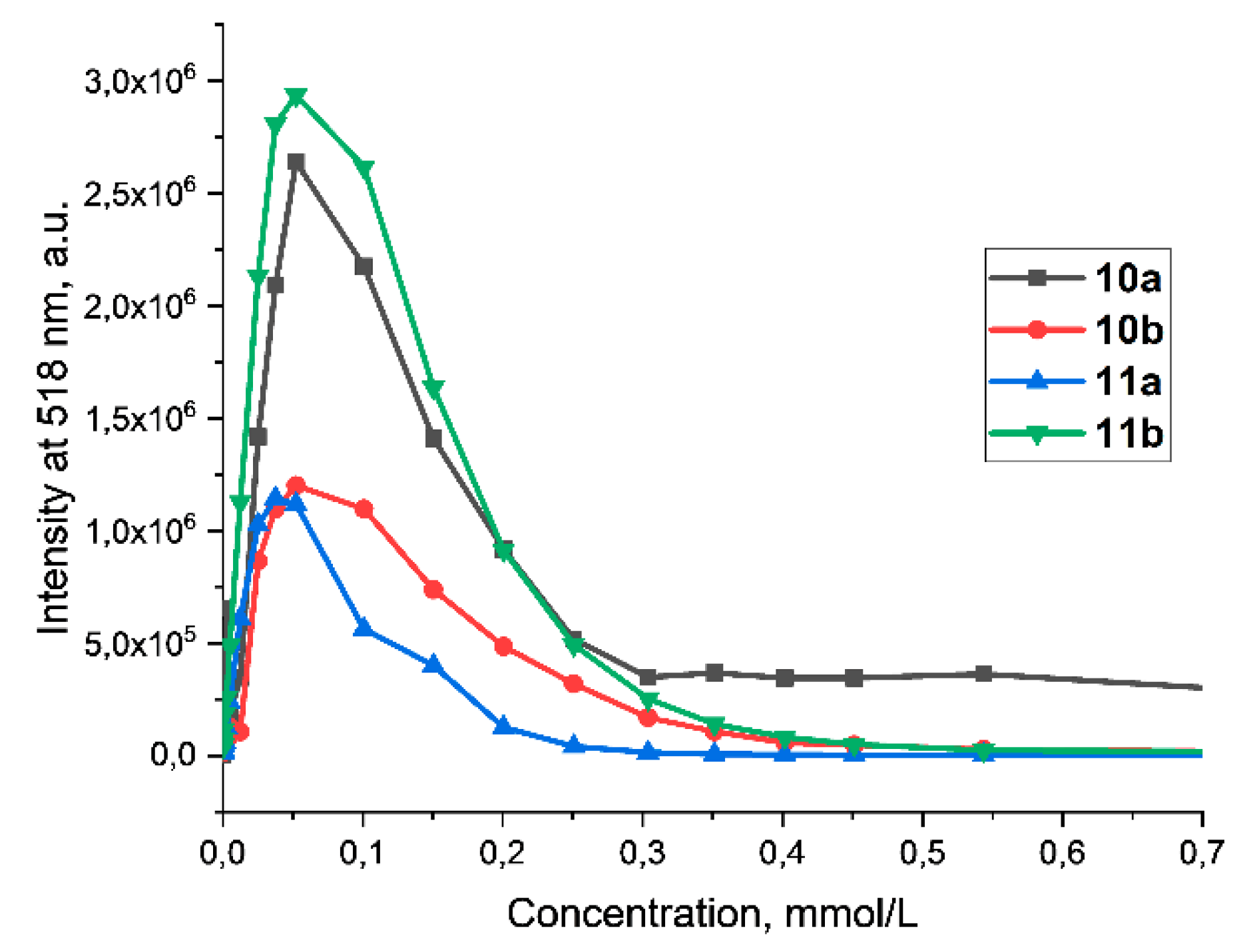
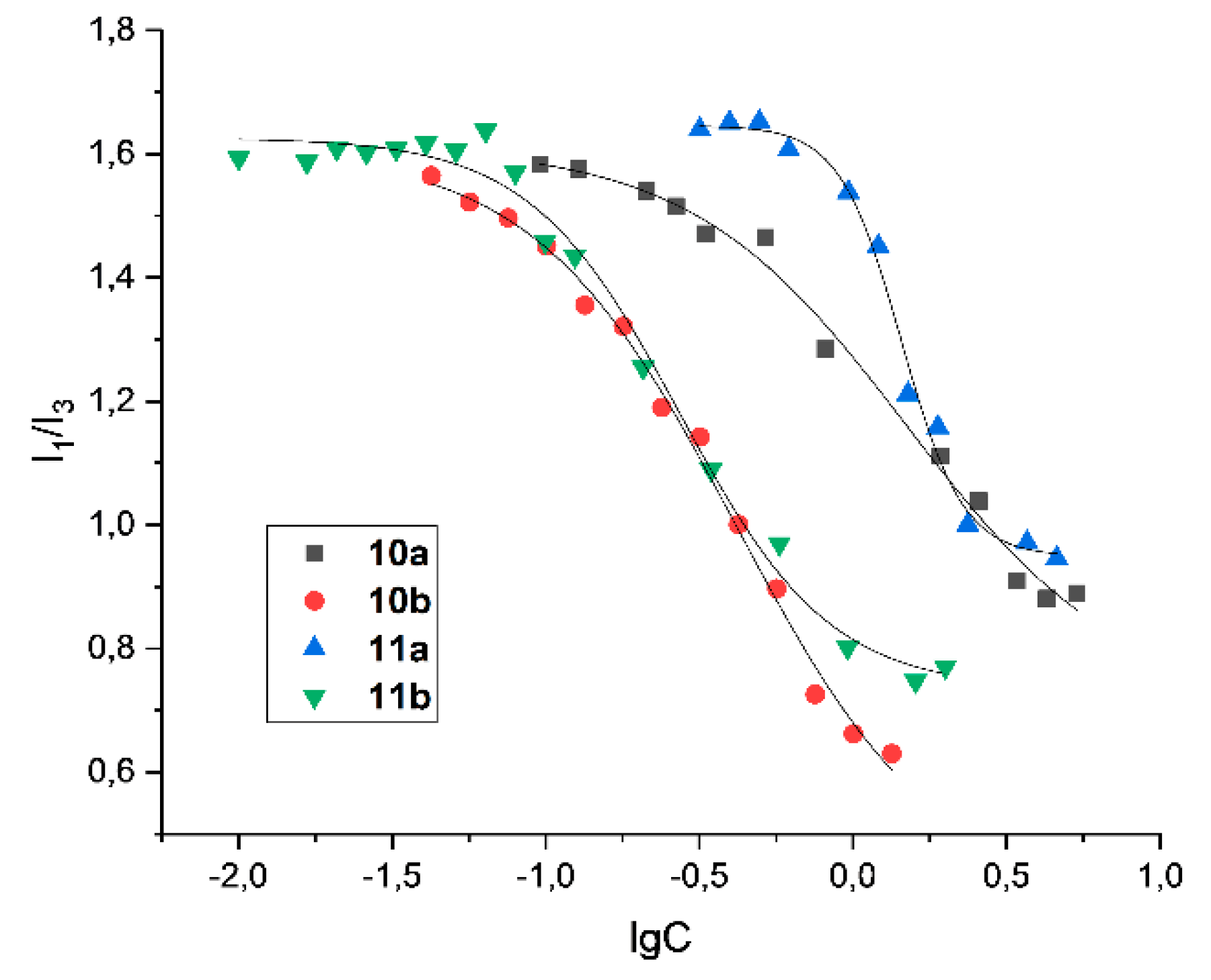
| System | 10a | 10b | 11a | 11b |
|---|---|---|---|---|
| CAC, mM | 1.43 | 0.43 | 0.82 | 0.28 |
| d, nm | 165±1 | 267±25 | 171±2 | 202±12 |
| PDI | 0.244±0.018 | 0.433±0.029 | 0.429±0.029 | 0.310±0.052 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





