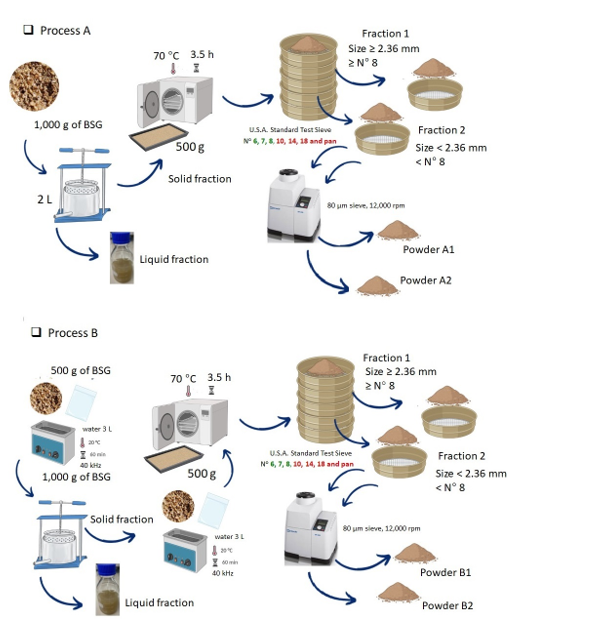
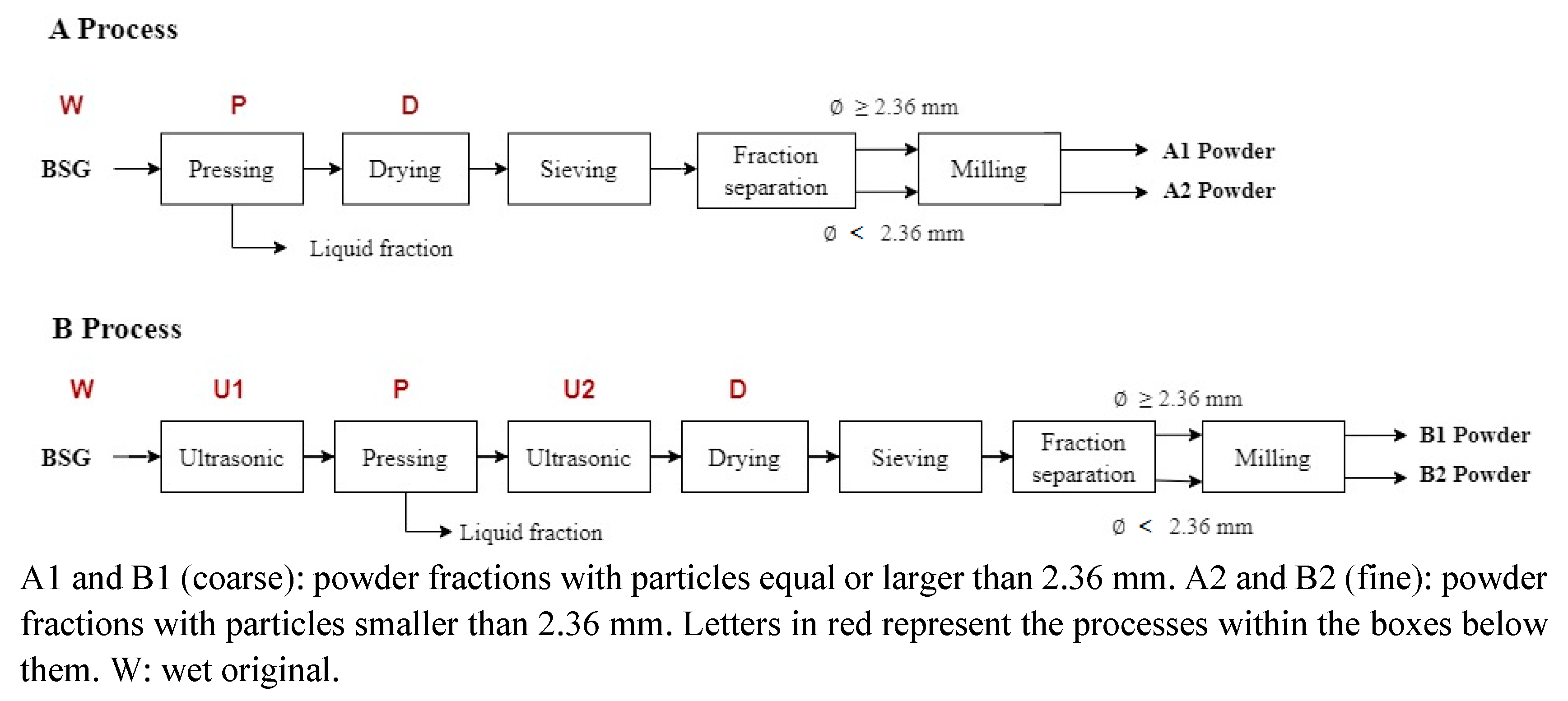
4.1.1. Monitoring of Moisture Content and Instrumental Color
The wet original BSG (W) used for both processes A and B show similar moisture levels, with 72.4 and 71.2%, respectively, that do not differ significantly (p > 0.05).
Table 1 show the monitoring of moisture content (% wet basis, w.b.) during processes A and B (
Figure 1) at the sampling points. These values agree with other studies that reported a moisture content ranging from 70 to 80% for fresh BSG [
1,
6,
12,
27] obtained immediately after the brewing process.
Process B include ultrasound operation before (U1) and after (U2) pressing. Ultrasound treatment has been used for assisted extraction of phenolic compounds and protein, enhancing saccharification, and structural modification of BSG and several biological materials [
9,
13,
16,
22,
28,
29,
30,
31].
The pressing (P) and drying (D) operations are common in both processes and the moisture contents achieved do not differ (p > 0.05) between A and B after these operations. In terms of yields, 47.2 ± 1.8 mL of liquid fraction /100g of original wet BSG (W) was obtained by pressing in process A and 53.7 ± 2.5 mL of liquid fraction /100g ultrasound BSG (U1) in process B. El-Shafey et al. [
32] proposed dewatering of brewer's spent grain using a membrane filter press, but they inform that there are processing plants for eliminate BSG water through a two-step process: pressing (to get a material with < 65% moisture) and drying (to get a material with < 10% moisture). Pressing produced a decrease of 12.6 (Process A) and 12.1 (Process B) percentage points in the moisture content of the wet original BSG (W). Drying carried out at 70 °C for 3.5 h (D) allowed BSG to be stabilized at a level of moisture content of 5.2 and 5.4% (
Table 1) similar or slightly higher than values reported by Fărcaş et al. [
3], Naibaho et al. [
5] and Thai et al. [
6]. A moisture content of 13% (wet basis) for BSG has been reported in order to avoid microbial proliferation, especially
Bacillus cereus [
33]. These levels of moisture content prevent the deterioration of BSG, which is one of the main problems for the use of this by-product of the brewing industry [
1].
For process B, ultrasonic applications U1 and U2, performed to the wet original (W) and pressed (P) BSG, respectively, produced no significant differences in moisture content (p > 0.05) between W and U1 as well as between P and U2. However, the slight increase in moisture content due to treatment with ultrasound could be attributed to some structural modification of BSG or water permeability of Ziploc bags which was non-visible. It was not possible to reach a lower moisture content of the BSG by drying (D) in process B (5.4%), due to the effect of using the second application of ultrasound (U2), compared to process A (5.2%). Possibly, its effect could have been observed on the drying curve, showing a decrease in the time to reach the moisture content for BSG stabilization in the process B compared to A, as it has been reported for other plant materials when ultrasound was used [
14,
15,
16]. Since the drying was carried out in a universal oven in this study, without the possibility of introducing a balance, data could not be obtained to construct the drying curve. This effect should be investigated in the future.
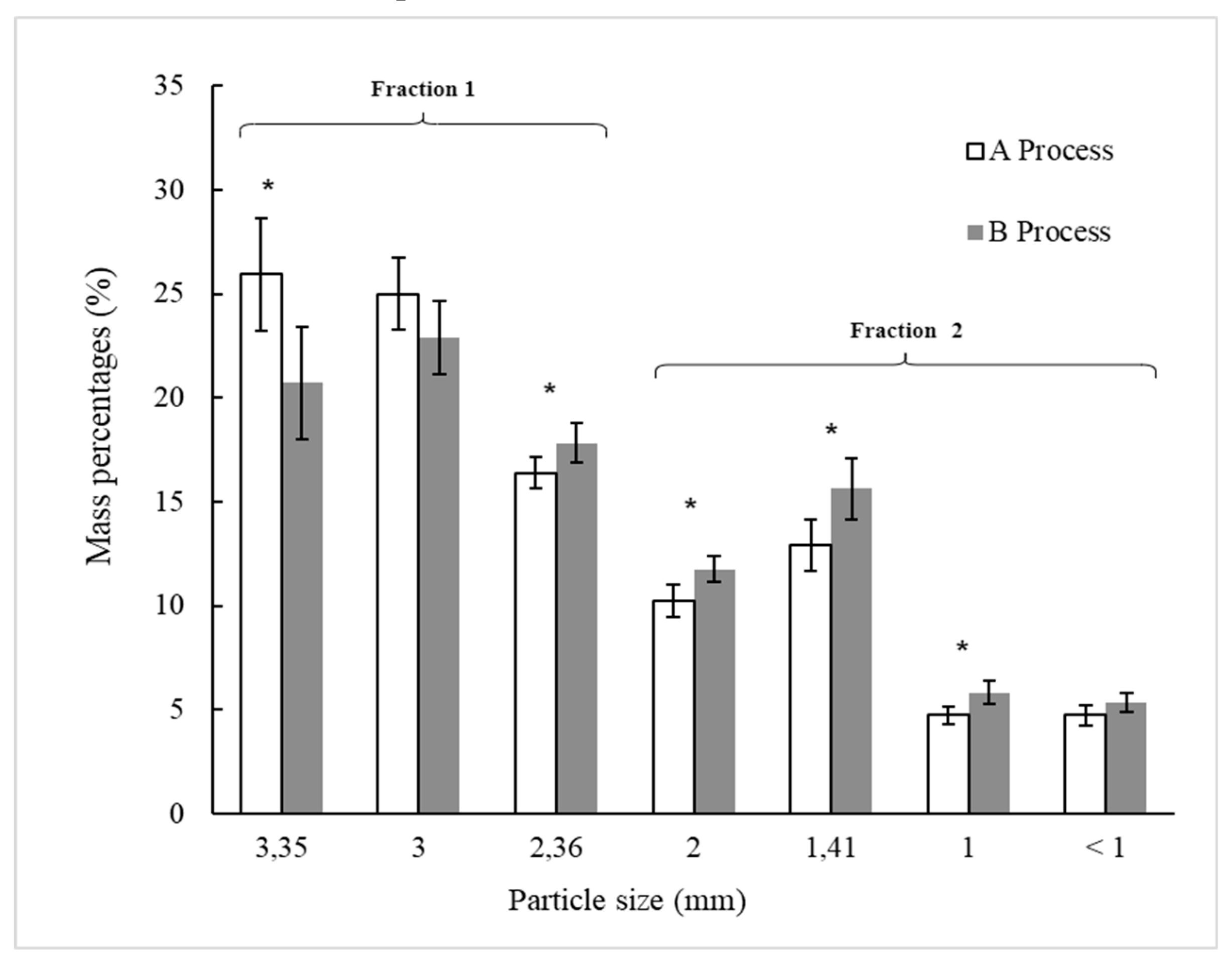
The moisture contents of powders did not differ significantly (p > 0.05) concerning the values of dried BSG (D) in each processes A and B (
Table 1). However, a slight increase is observed concerning D for the moisture contents of powders A1 and B1 (obtained from dried BSG with particles size equal or larger than 2.36 mm) and A2 and B2 (obtained from died BSG with particles sizes smaller than 2.36 mm). As shown at
Figure 1, the operation of sieving, fraction separation, and milling may have exposed dried BSG to the environmental air that could have contributed to a slight increasing in the moisture content of the powder. The moisture contents of powders (
Table 1) obtained from fractions 1 (Powders A1 and B1) are slightly higher than those obtained from fraction 2 (Powders A2 and B2) but did not differ statistically (p > 0.05) among them. This behavior agrees with the fact that larger particles have a greater water holding capacity compared to finer particles reported for leaves of white cabbage to produce dietary fiber powder, which could be attributed to the damage of the fiber matrix and the collapse of the pore during grinding [
34].
The moisture content of BSG powders (
Table 1) ranged from 5.9% to 6.6% (w.b.). They are within those reported by Shih et al. [
12], Okpala and Ofoedu [
35] and Baiano et al. [
36] for BSG flour: 12.2, 5.4-5.6 and 2.9-3.5%, respectively.
Table 2 and
Table 3 present the instrumental color parameters concerning wet original BSG (W) of processes A and B, respectively. Processes A and B present a significant increase (p < 0.05) in lightness (L*) from wet original BSG (W) to the powder BSG (A1, A2, B1 and B2). These results agree with Ahmed et al. [
37], Hejna et al. [
38] and Hejna et al. [
39], who informed that the lightness is the color parameter most significantly affected by particle size for water chestnut flour and extruded and ground BSG obtained by thermo-mechanical operation in a twin-screw extruder. Hejna et al. [
38] and Hejna et al. [
39] have been reported instrumental color ranges with general values of L* from 48.17 to 58.32, a* from 4.32 to 5.88, and b* from 12.14 to 14.82 for the BSG, different to L* and b* values of wet original (W) showed at
Table 2 and
Table 3. This could be attributed to differences in barley malts and beer type.
Process A (
Table 2), which includes the wet original BSG (W), pressing (P), drying (D), and powder (A1 and A2), shows significant differences (p < 0.05) in L*, a*, ΔE and h*color parameters. Lightness (L*) shows a significant increase throughout the process, with higher values for A1 and A2, which can be attributed to changes in the surface area of the material after milling. Smaller particles show higher lightness, which could be associated with an increase in the specific surface area that allows more light reflection [
38,
40,
41].
The a* values (
Table 2) decrease from P to D, indicating an alteration in the intensity of red or an increase in green, but remains stable after sieving and milling (
Figure 1) to obtain A1 and A2. The change in a* of BSG by drying (D) could be attributed to chemical reactions such as Maillard reaction and caramelization of reducing sugars present in this by-product [
12].
The total color difference (ΔE), respect to W, increases progressively, with greater changes after drying, sieving and milling to obtain the powders. According to the classification of Adekunte et al. [
22], the observed changes concerning wet original BSG color range from very different colors (ΔE > 3) for D, A1, and A2 to a small colors difference (ΔE < 1.5) for P.
Respect to h* (
Table 2), the BSG as wet original (W), pressed (P) and powder A2 showed a significant lesser (p < 0.05) yellow character in the assays [
21] than BSG after drying (D) and powder A1. Two factors probably affect this behavior of h* in the process A. Maybe, the first one would be the smaller particle size of fraction 1 then 2, associated with an increase in the specific surface area [
38,
40,
41], and the second would be the chemical reaction that led to browning associated to reducing sugars [
12].
To our knowledge, the mechanisms underpinning changes in BSG color during drying have not yet been reported. However, color changes of BSG during drying using hot-air dried, mainly at higher temperature (110 °C), is probably due to Maillard reaction and caramelization, generating a darker color product [
12]. Also, the Maillard reactions would be those that occurred in the extrusion grinding of BSG performed by thermo-mechanical treatment in the twin-screw extruder [
38,
39].
Process B (
Table 3), which includes ultrasound treatments before (U1) and after (U2) pressing, presents similar patterns to process A (
Table 2), with significant differences (p < 0.05) in L*, a*, ΔE and h* color parameters. Hence, lightness (L*) is strongly affected by drying (D), sieving, and milling (
Figure 1), with higher values for powder B1 and B2. Moreover, the process B shows variations in a* after, indicating an impact of drying, sieving, and milling on red/green intensity with respect to W. Furthermore, the total color difference (ΔE) increases more by pressing (P) in the process B (
Table 3) than A (
Table 2). Nonetheless, similarly to process A, the greater changes in ΔE are observed after drying, sieving, and milling. Thus, according to Adekunte et al. [
22], the observed changes concerning W range from very different colors (ΔE > 3) for D, B1, and B2, to different colors (1.5 < ΔE < 3) for P and U2 and including small color difference (ΔE < 1.5) for U1. The process B affect significantly (p < 0.05) the values of h*, but the change in this color parameter occur in the pressing (P) operation, while in process A occur in the drying (D) operation. As mentioned above, the changes in color parameter in process B could be attributed to chemical modifications such as Maillard reaction and caramelization [
12,
38,
39] and to the decrease in particle size [
38,
40,
41] caused mainly by drying and milling, respectively.
For both processes (
Table 2 and 3), the values of b*, and C* (chroma) are not affected after treatment applied to W, since no significant differences (p > 0.05) were observed. This behavior agrees with those reported by Shih et al. [
12], Hejna et al. [
38] and Hejna et al. [
39] for the effect of drying and extrusion on C* at different temperatures. This color parameter did not vary from fresh to dried or extruded BSG.
4.1.2. Ultrasonic Temperature Monitoring
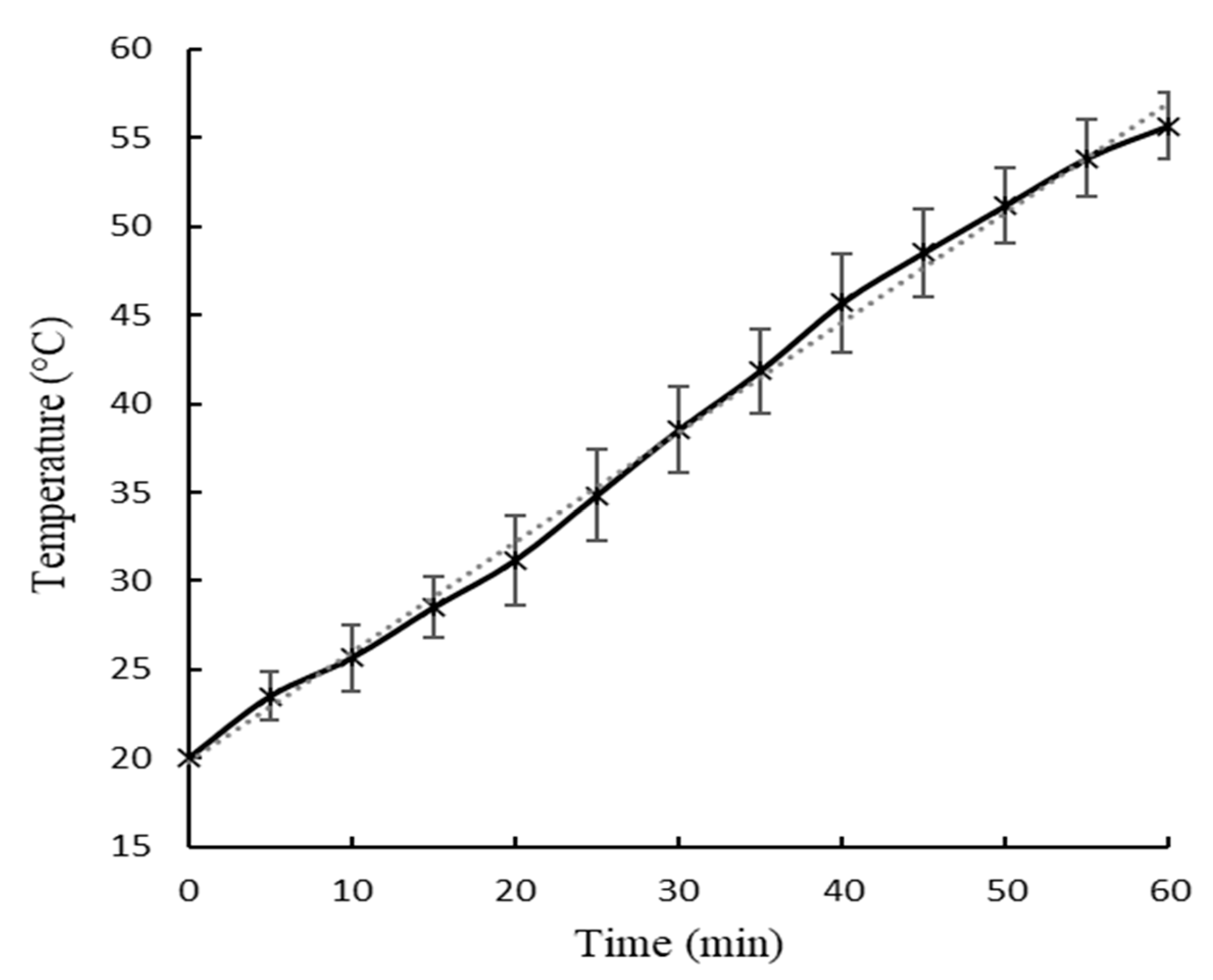
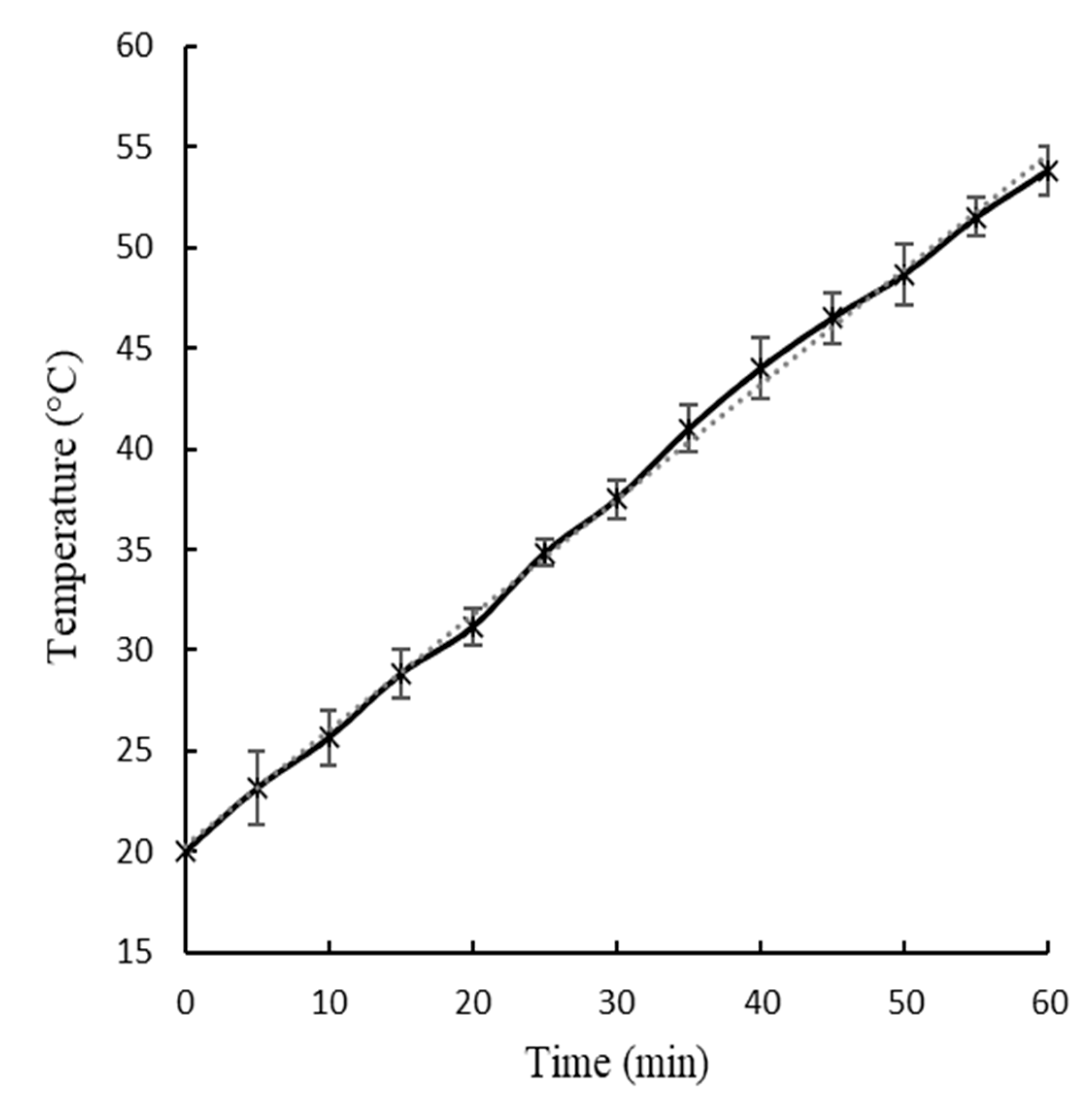
Figure 2 and
Figure 3 show the temperature increase of water ultrasonic bath during operations U1 and U2 applied for 60 min to wet original (W) and pressed (P) BSG, respectively. This increase in temperature can be attributed to energy release that occurs by acoustic cavitation in ultrasonic operations. Acoustical energy is generated by the transmission of ultrasound waves that consist of the rarefaction and compression cycles traveling through the liquid medium. When the cavities formed in the liquid collapse, small amounts of energy are released in the form of heat. In fact, the cavitation effects generate high temperatures, pressure, and violent shear forces, leading to the formation of the referred as "hot spots" [
28]. The final collapse phase is adiabatic
in nature and, thus, locally produces high-temperature and high-pressure conditions [
42].
The slope of the temperature-time curves showed in
Figure 2 and
Figure 3, which represent the rate of water temperature rise at U1 and U2 were 0.62 ± 0.04 °C/min and 0.57 ± 0.05 °C/min, respectively. The maximum water temperatures reached during the ultrasound treatment were 55.8 ± 1.9 °C for U1 and 53.8 ± 1.2 °C for U2. No significant differences (p < 0.05) were observed in values of slop and maximum water temperatures between U1 and U2.
Increasing the temperature of water used into the ultrasonic equipment certainly produced a heating in the BSG, which was not measured due to the difficulty in introducing a thermocouple into the Ziploc® bag without water infiltration. However, the temperature reached by BSG in U1 and U2 probably did not exceed 55.8 and 53.8 °C, respectively. Therefore, we consider that no important chemical or structural changes should have occurred in the BSG due to the increase in the water temperature during ultrasound operation. Hassan et al. [
9] have applied ultrasound at temperatures of 20 to 60 °C for times of 20 to 60 min and after the optimized pretreatment of the native BSG, and following saccharification, 74% of sugars in BSG were recovered, while no degradation of lignin was observed. Conversely, Alonso-Riaño et al. [
29], aiming at obtaining polyphenol compounds from BSG, fixed 47 °C as the temperature during application of ultrasound assisted extraction for 30 min (60 min of total experiment) to avoid the degradation of these bioactive compounds.