Introduction
In recent years, the operator's ability in performing carotid stenting (CAS) has improved clinical outcomes and, consequently, the number of patients treated [1]. However, more than 3% of patients need to be treated again after CAS [2].
In fact, most of the cases requiring further intervention are affected by in-stent restenosis, which usually occurs in the first 6-12 months after post-implantation [3]. In the case of ISR, the European Society for Vascular Surgery (ESVS) guidelines suggest reintervention at level A and class I for symptomatic patients with 50-99% ISR [2]. The recommended procedure for ISR treatment [4] [5] is the drug-coated balloons (DCB), which usually leads to successful and long-term flow restoration. The literature only reports small numbers of stent thrombosis after using DCB.
Furthermore, acute carotid stent thrombosis after CAS (ACST) is an infrequent but potentially fatal complication [5]. It may occur in stents nearly a decade after implant [6].
On the other hand, the literature reports a small number of carotid neoatherosclerosis cases that appeared after several years from stent procedure [3] [7]. The characteristic of neoatherosclerosis includes the formation of an unstable plaque prone to thrombus appearance consequent to a neo-plaque rupture [8]. Pathophysiology and risk factors of IRS neoatherosclerosis has been well documented by Nakamura [9]. Neoatherosclerosis has been already reported into carotid stents [7] [8] and is recommended to be treated using elective Micronet-covered stent [8].
Discriminating between the two types of ISR (hyperplasia or neoatherosclerosis) is not trivial, as illustrated by Garcia-Guimaraes [3], since Doppler PSV and Angiographic information may be similar. However, the correct identification of carotid neoatherosclerosis is critical for a positive outcome.
We describe a case in which a patient treated with carotid stenting 8 years before, was diagnosed with ISR. The patient was treated according to ESVS guidelines with a DCB device, obtaining an optimal restoration of the vessel lumen. A few minutes later, the patient suffered an acute in-stent thrombosis questioning the convenience of DCB treatment according to the initial hyperplasia diagnosis suggesting neoatherosclerosis. The emergency was successfully treated by implanting a CGuard MicroNet-covered stent, which isolates the thrombus obtained a normal endovascular reconstruction. We illustrate that CGuard may represent treatment-of-choice to protect against in-stent (neo)atherosclerosis complications, offering an innovative therapeutic option.
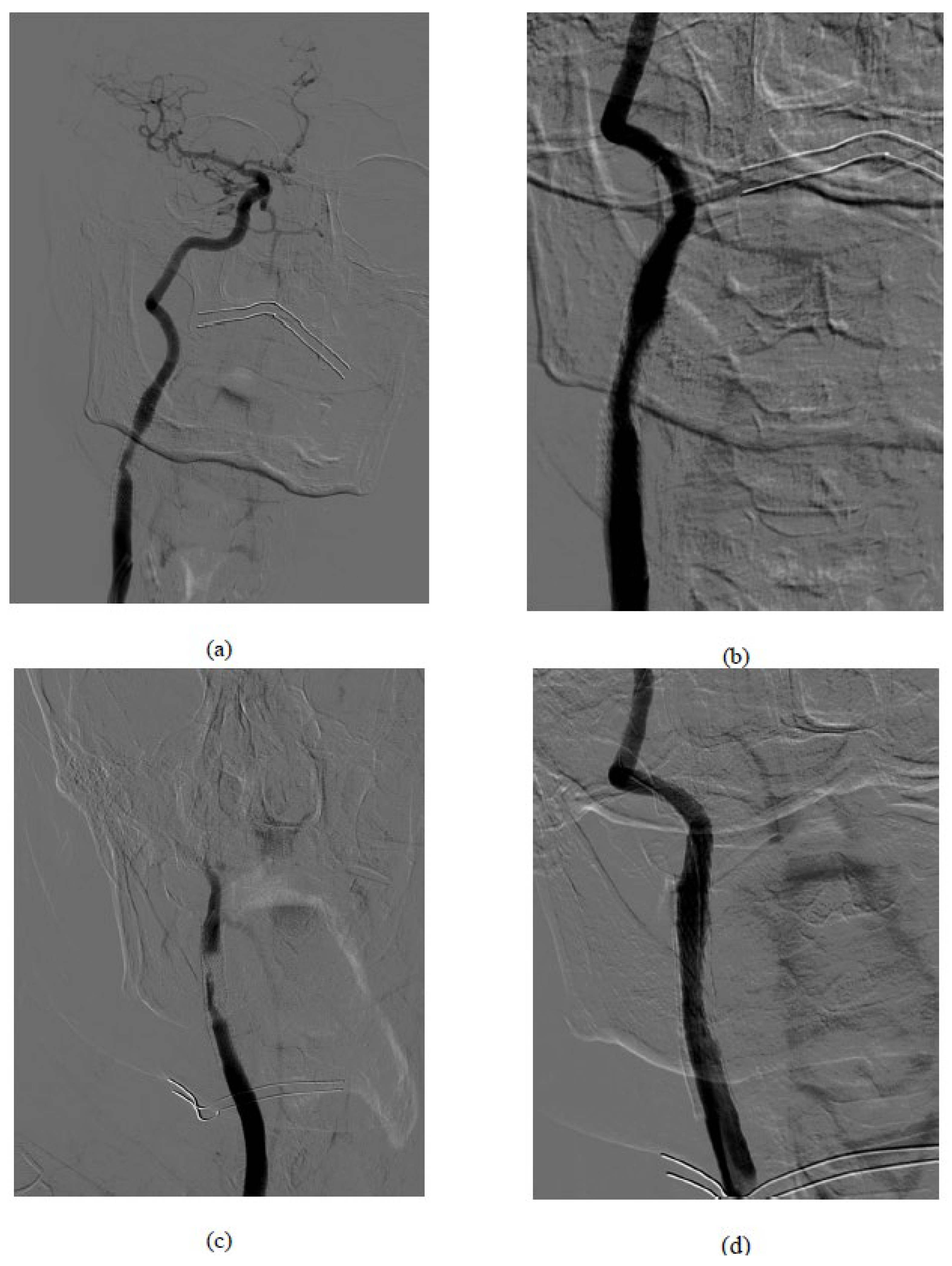
Figure 1.
Procedural images: (a) basal symptomatic in-stent restenosis (b) result after drug-coated balloons (c) acute carotid stent thrombosis after the DEBalloon (d) Final result after CGuard MicroNet-covered stent implantation.
Figure 1.
Procedural images: (a) basal symptomatic in-stent restenosis (b) result after drug-coated balloons (c) acute carotid stent thrombosis after the DEBalloon (d) Final result after CGuard MicroNet-covered stent implantation.
Methods
A 55-year-old diabetic patient with hypertensive heart disease, who was treated with carotid stenting 8 years before, was referred to our facility because of a significant increase in PSV values in the last year, passing from 30 to 80% (currently, PSV at the eco-color-Doppler study was > 300 cm/sec). The same patient undergoes a coronary stent placement 3 months before the procedure, suggesting a generalised systemic progression of atherosclerosis. The patient was under dual antiplatelet therapy (plavix and cardioaspirin). However, in a neurological evaluation two weeks before the carotid intervention, the patient exhibited neurologic symptoms.
Medication was the dual platelet, including AAS and Clopidogrel. 5000 IU Heparine was given with ACT control pre intervention. Using an ultrasound-guided approach of the right common femoral, a 5F catheter (Terumo) was used to engage the right common carotid. With a curved 260 cm stiff guide (Terumo), a 40° Mach1 (Boston Scientific) guiding catheter was advanced proximal to the ISR. Angiographic control confirmed the presence of tight diffuse ISR affecting most of the stent length. After positioning the Epi-filter cerebral protection system (Boston Scientific), a dilation was performed with a 4 mm medicated balloon device (DCB) at the level of the restenosis. At the end of the procedure, the filter was removed, and the optimal restoration of the vessel lumen was documented without evidence of filling defects. The patient was assigned in good health to the recovery area.
Unexpectedly, ten minutes later, the patient initiates a state of confusion along with left hemiplegia, deviation of the gaze, and the buccal rim. The neurological evaluation informed an NIH score of 21. The immediate angiographic control documented acute in-stent thrombosis and slow-flow compromising the ipsilateral intracranial circulation.
This interventional unity disregarded stent aspiration, reserving thrombectomy bailout with thromboaspiration to the potential INR bailout, keeping focus to prompt carotid artery opening.
To tackle this emergency, an Epi-filter cerebral protection system was again placed, and a 9 x 40 mm micromesh stent (CGuard, InspireMD) was released slightly downstream from the end of the stenosed stent and gently post-dilated with a 5 mm balloon. The control angiography showed a resumption of the internal carotid flow with regular visualisation of the intracranial circulation.
After the procedure, the patient documented an NIH score of 0 with complete recovery of functions. A brain MRI was performed 24 hours later and showed no restriction area attributable to acute or sub-acute ischemia affecting the right cerebral hemisphere.
Discussion
This case report describes a patient diagnosed with ISR. The plaque appeared several years after the stent procedure and worsened significantly in a short time.
Despite the guidelines recommended ISR treatment with DCB, the patient suffered an acute thrombosis resulting from a plaque rupture induced by the balloon.
The combined unusual plaque evolution and the treatment complication suggested the presence of neoatherosclerosis instead of the initial hyperplasia diagnosis. Neoatherosclerosis is considered to have a low incidence in carotids. However, due to the increased population treated with carotid stents, the neoatherosclerosis will be more frequent in the future. The suspect of neoatherosclerosis must include restenosis in all patients with more the 3y implanted stents. The traditional treatment for In-stent Restenosis may produce iatrogenic stroke and acute stent thrombosis, like demonstrated in this case review. This may be relevant and might inform clinical practice.
Ethical Approval
The informed consent was obtained from the patient. This study was ethically approved by the Institutional Review Board (IRB).
Author Contributions
Introduction, case presentation, discussion, figures (MS, JR). Introduction, discussion, figures (SB). case presentation, patient intervention (MS, LM, LGC, GS). Manuscript writing (MS, JR, SB). Manuscript review (MS, LM, LGC, SB, JR, GS).
References
- Cole, T.S.; Mezher, A.W.; Catapano, J.S.; Godzik, J.; Baranoski, J.F.; Nakaji, P.; Albuquerque, F.C.; Lawton, M.T.; Little, A.S.; Ducruet, A.F. Nationwide trends in carotid endarterectomy and carotid artery stenting in the post-CREST era. Stroke 2020, 51, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Naylor, A.R.; Ricco, J.B.; de Borst, G.J.; Debus, S.; de Haro, J.; Halliday, A.; Hamilton, G.; Kakisis, J.; Kakkos, S.; Lepidi, S. ; others, "Management of atherosclerotic carotid and vertebral disease: 2017 clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg 2018, 55, 142. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Guimaraes, M.; Antuña, P.; Maruri-Sanchez, R.; Vera, A.; Cuesta, J.; Bastante, T.; Rivero, F.; Alfonso, F. Calcified neoatherosclerosis causing in-stent restenosis: Prevalence, predictors, and implications. Coronary Artery Disease 2019, 30, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liistro, F.; Porto, I.; Grotti, S.; Ventoruzzo, G.; Vergallo, R.; Bellandi, G.; Bolognese, L. Drug-eluting balloon angioplasty for carotid in-stent restenosis. Journal of Endovascular Therapy 2012, 19, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Xiromeritis, K.; Dalainas, I.; Stamatakos, M.; Katsikas, V.; Martinakis, V.; Stamatelopoulos, K.; Psarros, V. Acute carotid stent thrombosis after carotid artery stenting. Eur Rev Med Pharmacol Sci 2012, 16, 355–362. [Google Scholar] [PubMed]
- Iancu, A.; Grosz, C.; Lazar, A. Acute carotid stent thrombosis: Review of the literature and long-term follow-up. Cardiovascular Revascularization Medicine 2010, 11, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Yako, R.; Masuo, O.; Hirayama, K.; Uematsu, Y.; Nakao, N. A case of in-stent neoatherosclerosis 10 years after carotid artery stent implantation: Observation with optical coherence tomography and plaque histological findings. Neurologia medico-chirurgica 2014, 54, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Tekieli, L.; Mazurek, A.; Pieniazek, P.; Musialek, P. Symptomatic atherosclerotic plaque progression in a first-generation carotid stent—Case report: Management and 5-year clinical and imaging outcome. European Heart Journal-Case Reports, 2022.
- Nakamura, D.; Dohi, T.; Ishihara, T.; Kikuchi, A.; Mori, N.; Yokoi, K.; Shiraki, T.; Mizote, I.; Mano, T.; Higuchi, Y. ; others, "Predictors and outcomes of neoatherosclerosis in patients with in-stent restenosis. Eurointervention: Journal of Europcr in Collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology 2021, 17, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Köklü, E.; Arslan, Ş.; Yüksel, İ.Ö.; Bayar, N.; Koç, P. Acute carotid artery stent thrombosis due to dual antiplatelet resistance. Cardiovascular and interventional radiology 2015, 38, 1011–1014. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).