1. Introduction
According to the latest statistical report by the European Biogas Association (EBA), gas import dependency in Europe has risen from 83% in 2021 to 97% in 2022. Therefore, it is necessary to enhance the production of alternatives to natural gas, an biogas is one of them [
1]. Biogas is produced by the decomposition of organic matter in a process called anaerobic digestion (AD) [
2]. During the process, lipids, carbohydrates, and proteins are digested into smaller compounds in the absence of oxygen. AD consists of four biochemical steps: hydrolysis, acidogenesis, acetogenesis, and methanogenesis, which are conducted by bacteria and archaea [
2]. The most commonly used organic matter in AD is maize silage, an edible component also used as livestock feed [
3]. Another option is lignocellulose biomass such as dedicated energy crops with biogas yields comparable to maize silage [
4]. In Mediterranean countries, an example of a promising substrate for energy production is
Miscanthus x giganteus (MxG) or
Arundo donax [
5]. The MxG was investigated as a potential biomass for biogas production because it is a non-edible and fast-growing perennial crop with high carbohydrate content that can be grown on marginal land. Moreover, cultivation is simple due to low nutrient and water requirements [
4]. In order to convert MxG crops into biogas, the crucial step is the presence of physiological groups of bacteria that will degrade the biomass to substrates (acetate, H
2 and CO
2) for further methanogenesis.
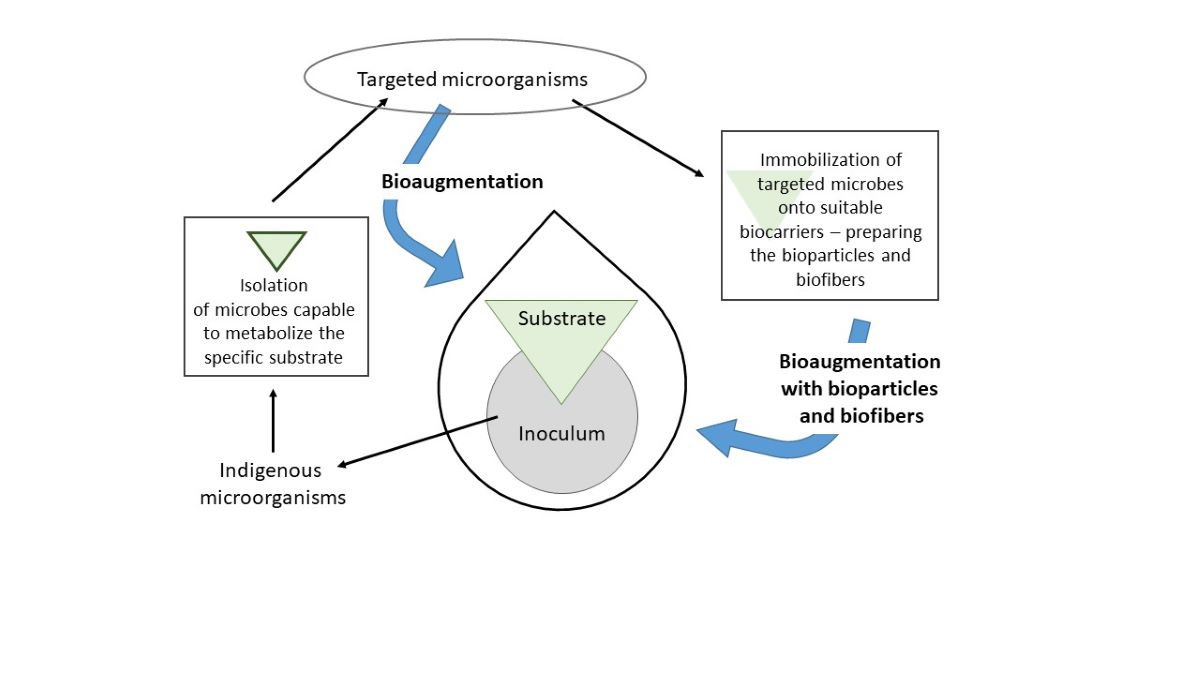
Immobilization of the selected bacterial species or microbial consortium onto solid materials as carriers have frequently been employed in biotechnological processes, especially in the bioremediation of soil or wastewater, biodegradation of food waste and different types of silage [
6]. The lignocellulose biomass is to a large scale quite resistant to microbial enzymatic biodegradation. Therefore, the immobilization of desired microbes accompanied by bioaugmentation is promising for enhancement of lignocellulose degradation [
3]. The usage of immobilized microbes provides many advantages, such as higher cell density and consequent metabolic activity, great stability, and protection from extreme environmental conditions, subsequently resulting in higher efficiency in bioremediation and biodegradation [
7]. However, not all materials are appropriate for the immobilization of microbes and selecting a suitable carrier is crucial for the successful immobilization. The properties of ideal carrier of microbes should be porous structure with rough and irregular surface for bacterial colonization, inexpensive and non-toxic [
7]. Numerous types of microbial carriers can be divided into organic and inorganic materials, synthetic and natural materials [
8]. The usage of natural materials has many ecological advantages against man-made plastic carriers. Natural organic materials such as corncob, straw, plant fibers, rice are promising, but their utilization in AD is limited due to low resistance to biodegradation and stability in different pH [
8]. Natural inorganic materials such as zeolite, perlite, and porous glass have high chemical and physical resistance [
8].
In this research four different natural materials (natural zeolitized tuff, ZeoSand®, perlite, and crushed corncob) were tested as carriers of microbial consortium which is intended for bioaugmentation of anaerobic digesters using MxG as substrate.
2. Materials and Methods
2.1. Isolation of Microbial Consortium
The microbial consortium was previously isolated and conditioned for bioaugmentation of biogas production reactors that use MxG biomass as a substrate. The dried and ground biomass of MxG (1 g) with addition of 1 mL of activated sludge from wastewater treatment plant, was incubated at 37°C for one week, anaerobically, in tightly sealed Winkler bottles containing 100 mL of minimal salt medium (MSM) (composition in g L-1 of demineralized water; NaCl 2.5, K2HPO4 0.47, KH2PO4 0.56, MgSO4×7H2O 0.5, CaCl2×2H2O 0.1, NH4NO3 2.5, pH 7.0±0.2). In such system, the MxG biomass served as a sole carbon source. After one week, an aliquot of 0.1 mL was inoculated from the Winkler bottles to MSM agar plates (15 g L-1 of agar) and incubated for one week at 37°C. After such conditioning, the colonies grown on agar plates were presumably able to use the MxG biomass as a sole carbon source, and thus can be classified as MxG degraders. The alternating anaerobic/aerobic incubation was deliberately chosen to enable the growth of facultative anaerobic bacteria, which would be much simpler to cultivate in the subsequent steps, important from biotechnological point of view. From the surface of the plates, the grown biomass was scraped and bacterial solution was prepared, and transfered to MicrobankTM cryo-storage system (PRO-Lab Diagnostics, Richmond Hill, Canada) for future use. This suspension was labeled as microbial consortium.
2.2. Immobilization of Microbial Consortium on Carriers
Four different natural materials were tested as carriers. The natural zeolitized tuff (NZ) from a quarry in Donje Jesenje, Croatia, consisted of 50-55% clinoptilolite with lower amounts of celadonite, plagioclase feldspars, opal-CT (10-15% each), and traces of quartz and analcime [
9]. The commercial mineral composition of the ZeoSand
® (Velebit Agro, Croatia) was a minimum of 80% clinoptilolite, and other mineral components were not listed in the safety data sheet. The expanded perlite was a commercial product intended for gardening (Special Mix B.V. manufactured by Gold Label, Aalsmeer, The Netherlands). The crushed corncob was from organic farming and not treated with pesticides and herbicides. The size fraction from 0.5–1 mm of NZ and ZeoSand
® were used. Particle size of perlite were 1-4 mm and crushed corncob were irregular particles between 2-6 mm in size. All materials were dry sterilized at 105°C/1h prior to testing.
The consortium was revitalized from MicrobankTM on Luria-Bertani (LB) agar plates (37°C / 24h). The grown biomass was scraped from the plate and suspended in 10 mL of sterile saline solution to obtain the consortium suspension. To immobilize the consortium on each individual carrier, 50 mL of sterile LB medium, 0.5 g of carrier, and 0.5 mL of consortium suspension were added to the sterile plastic vials. The control vial lacked the carrier. The vials were tightly sealed and incubated for 24 h at room temperature (23±1°C) on a rotatory shaker (5 rpm). Furthermore, the pure culture of E. indicum was immobilized on each carrier following the same procedure.
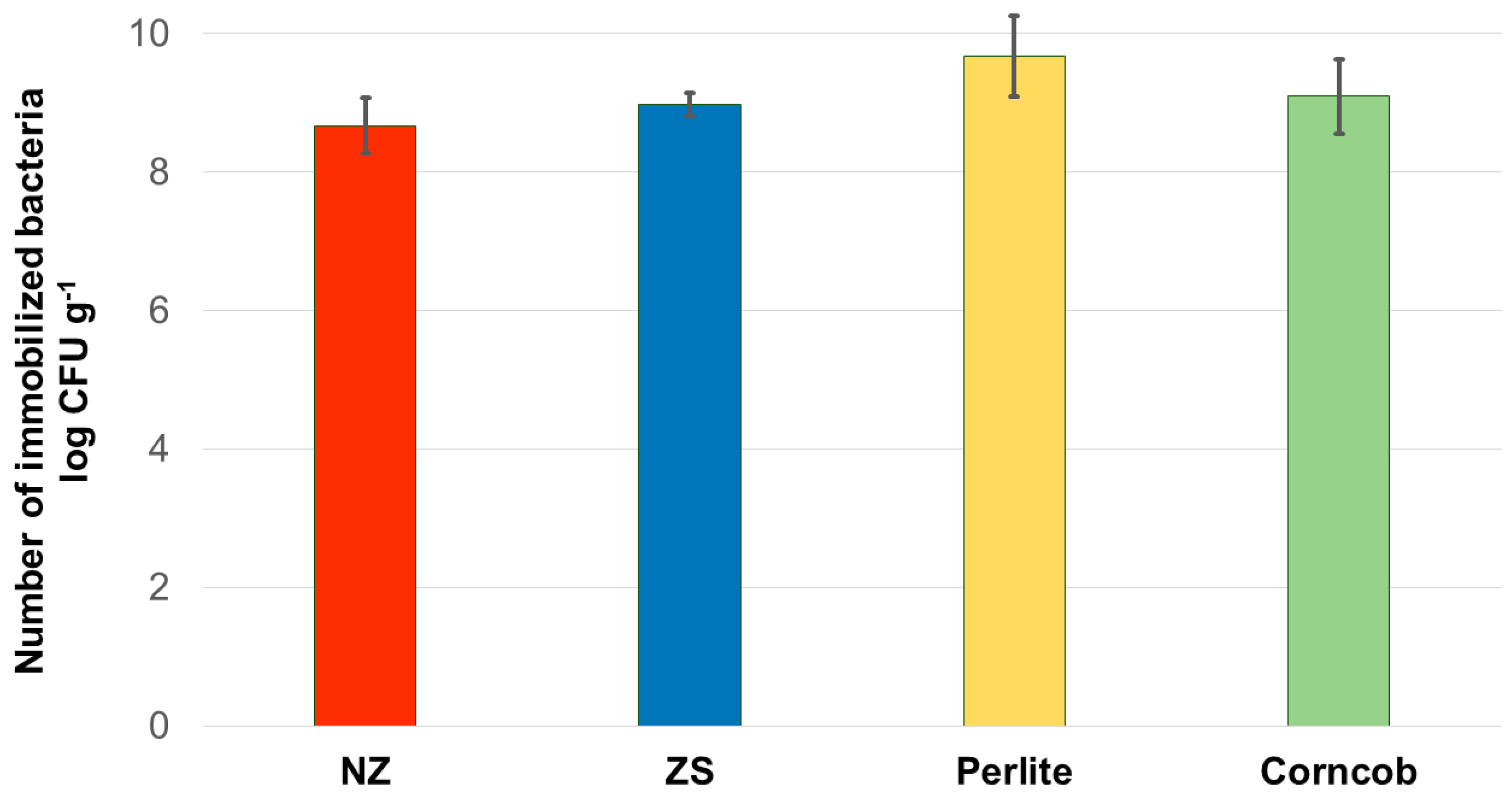
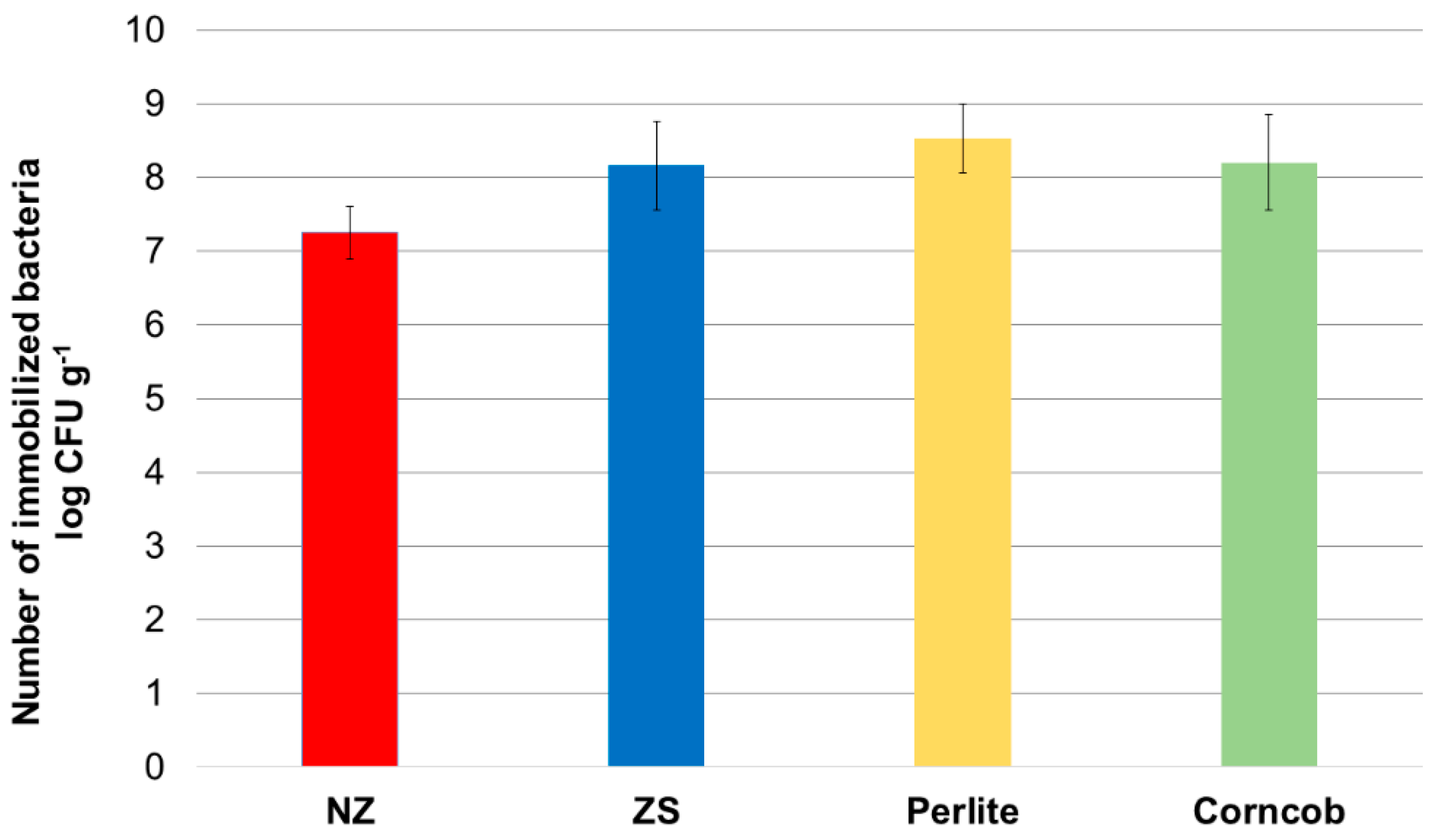
After the incubation, number of bacteria immobilized onto each carrier was determined as follows: a supernatant was decanted from vials, and the carriers were washed twice with sterile saline solution (0.3 %) to eliminate bacteria not attached to the carriers. Then, 20 mL of sterile saline was added, and the vials were vigorously shaken on a vortex (45 Hz/3 min). This way the immobilized bacteria detach from the carrier and remain as planktonic cells in the supernatant [
10]. The supernatant was serially diluted, inoculated on LB agar, and incubated at 37°C/24h. The grown colonies were counted, and the number of immobilized bacteria was expressed as log CFU g
-1 of the dry carrier.
2.3. Scanning Electron Microscopy Analysis (SEM)
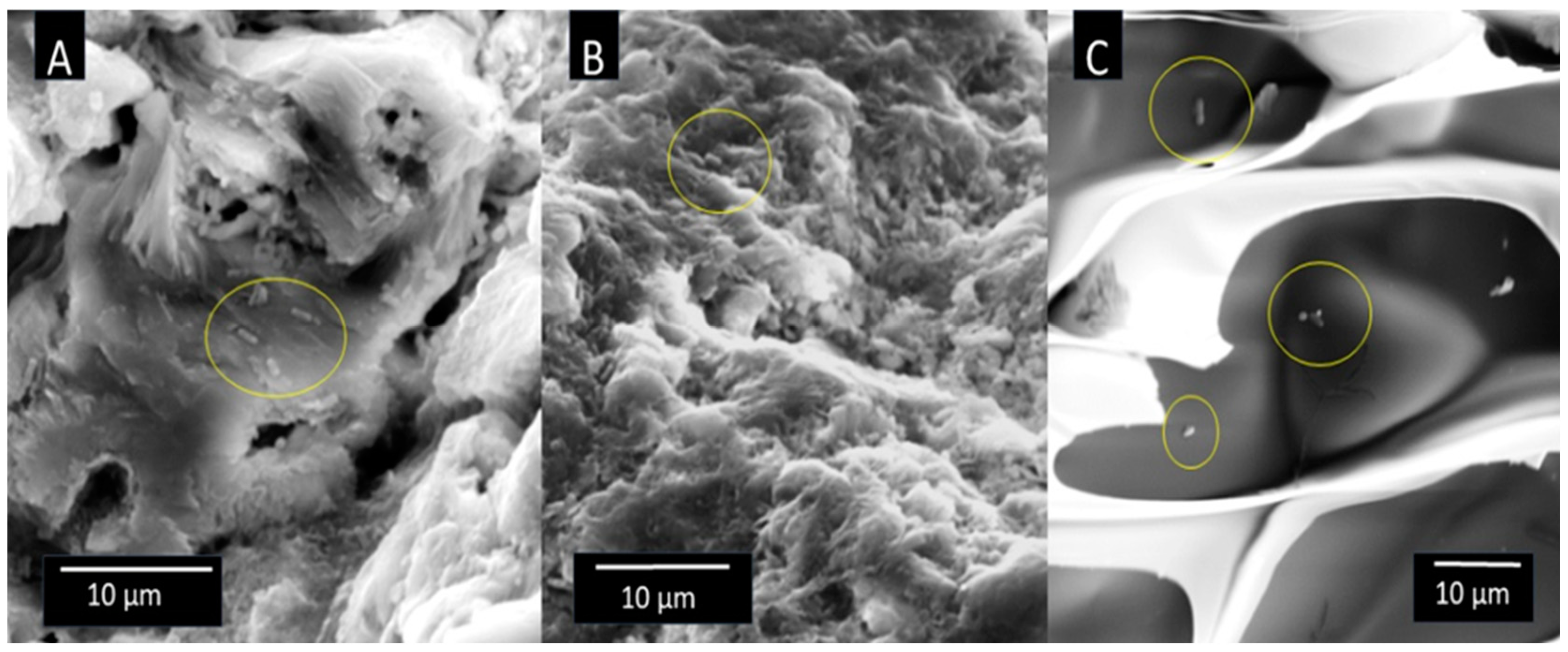
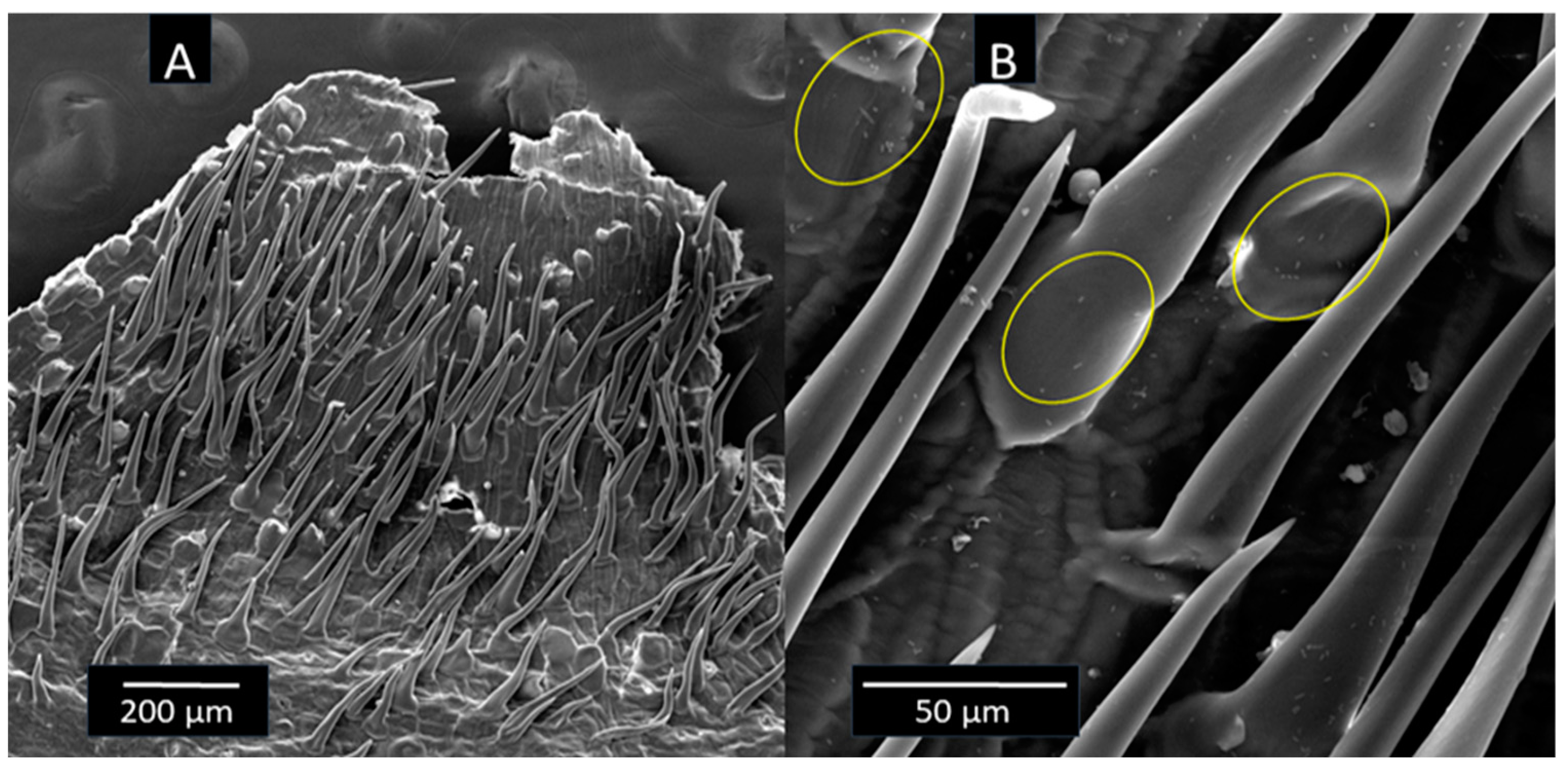
To perform the SEM analysis, after the incubation and rinsing with sterile saline solution, several particles of each carrier with immobilized bacteria were transferred to sterile vial containing 2% paraformaldehyde solution and kept at 4°C/24 h for cell fixation. Next, the samples were washed with sterile saline and dehydrated with ethanol as follows; 30% EtOH/2 min, 50%/2 min, 70%/5 min, 96%/5 min, 100%/5 min, 100%/5 min, and followed by drying for 30 min at 50°C. For imaging, a single bioparticle was coated with a plasma of gold and palladium for 180 s and imaged by TESCAN Vega3 EasyProbe SEM at an electron beam energy of 7 keV.
2.4. Identification, Antibiotic Susceptibility Testing and Biochemical Characterization of Bacterial Species in the Microbial Consortium
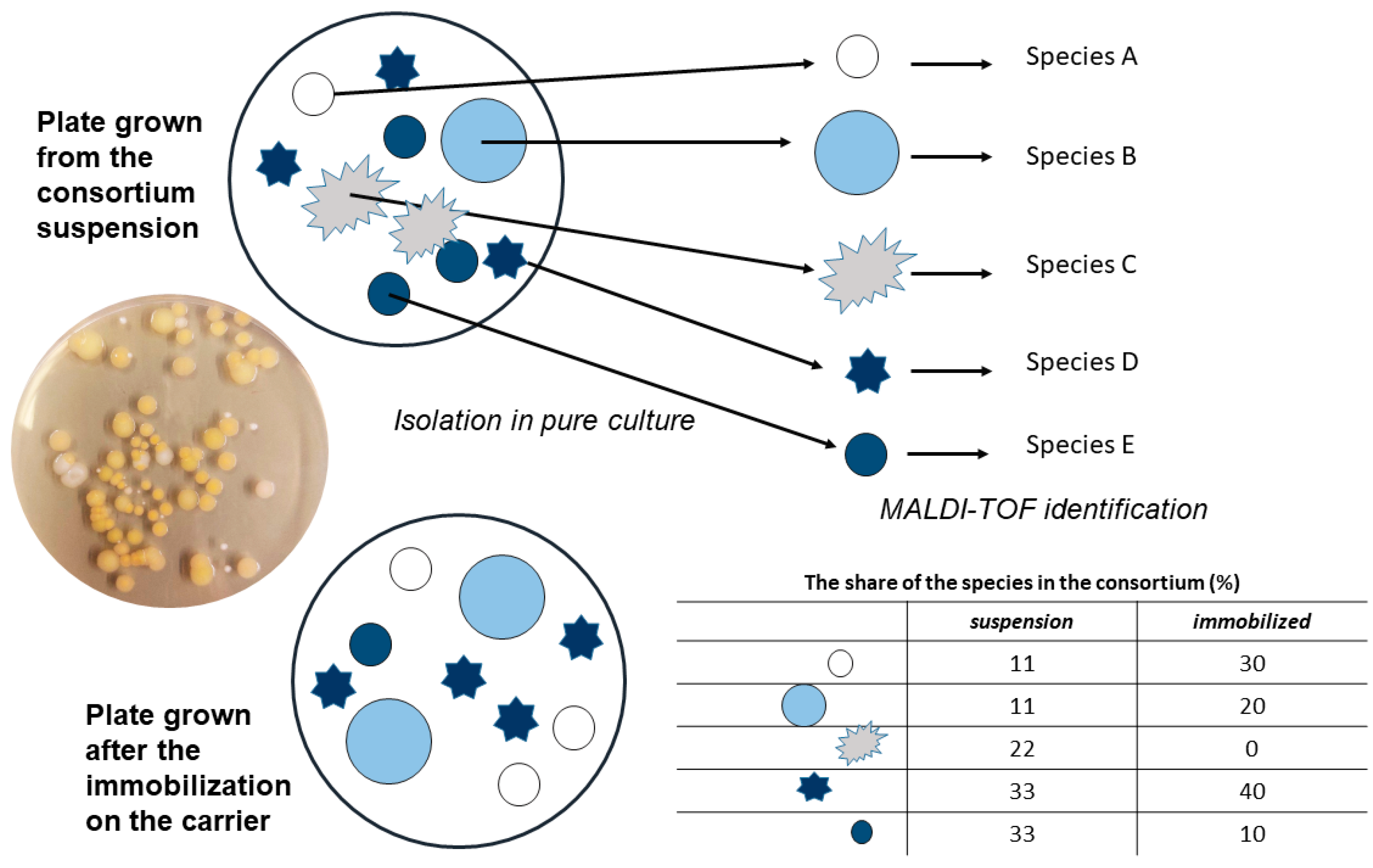
The consortium suspension was serially diluted up to 10
-7 and inoculated onto LB agar plates. On certain plates, single colonies were visible, and several types of morphologically different colonies were distinguishable (
Figure 1). By using this method, it is not possible to claim that these were the only bacterial species in the consortium, but it was safe to presume that these bacterial species were dominant in the consortium, since they remained in notable numbers after serial dilution. All the colonies that were clearly morphologically different were isolated in pure culture and subsequently identified with the MALDI-TOF mass spectrometry method (software version 3.0, Microflex LT, Bruker Daltonics).
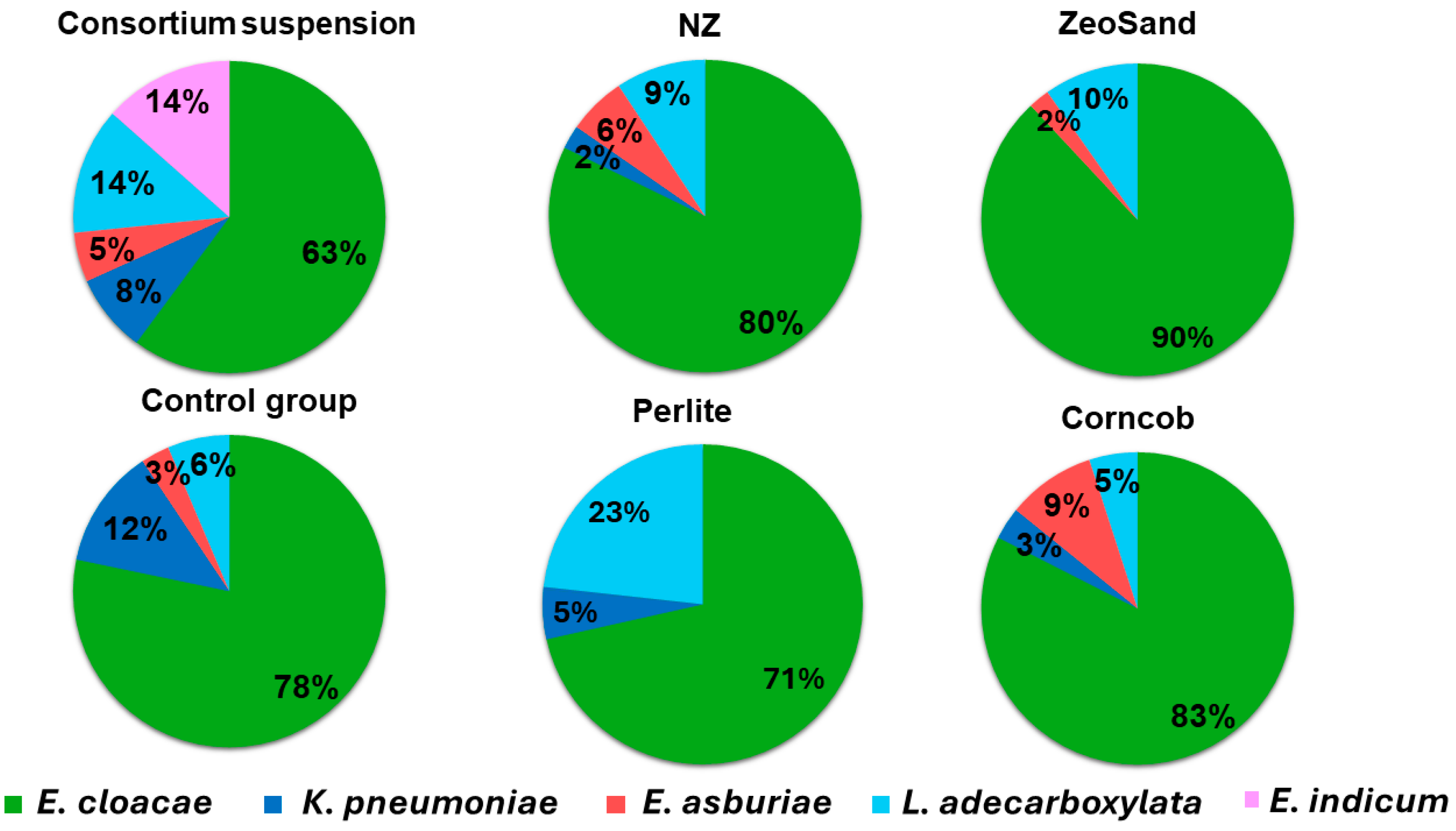
By counting the colonies at comparable plates, the share of each identified species in the consortium was determined and compared between consortium suspension, the control group and the bacteria immobilized on the carriers (
Figure 1).
All the experiments were done in triplicate and statistically compared by Student’s t-test with significance margin set at p = 0.05.
Biochemical determination, including catalase and oxidase tests, was conducted for each bacterial species. For the catalase test, biomass was suspended in 3% H2O2 on a microscope slide, and the appearance of oxygen bubbles was considered a positive result. The oxidase test was conducted by spreading biomass on strips (Microbiologie Bactident® Oxydase, Sigma-Aldrich Canada Co., Ontario, Canada); a positive result was indicated by the appearance of a purple color.
Susceptibility testing of
E. cloacae,
E. asburiae,
K. pneumoniae and
L. adecarboxylata to imipenem was determined by EUCAST standard disc diffusion assay [
11]. The bacterial suspensions (0.5 McFarland units / 10
8 CFU ml
-1) were smeared all over the Mueller-Hinton Agar plates (MH) (Biolife, Italy) with a sterile swab stick. The disc of 10 µg imipenem (Liofilchem
®, Italy) was placed on agar surface and plates were incubated for 24h at 37°C. After the incubation, antibiotic cut-off points were determined following the EUCAST protocol.
4. Discussion
Based on the results, all materials proved to be promising biocarriers for the microbial consortium intended to be used in biogas production. Perlite, an aluminosilicate material with high porosity, previously demonstrated great potential as a biocarrier for immobilization of phosphate-accumulating bacteria
Acinetobacter junii, resulting in high number of immobilized bacteria (12.65×10
9 CFU g
−1) [
12]. Its efficiency in augmenting biogas-producing reactors using olive waste as a substrate was also reported. High number of immobilized bacteria on perlite suggests its suitability for various applications in biogas production [
3].There was no statistical difference in the number of immobilized bacteria between the NZ and ZeoSand
®. The most obvious difference between the two zeolite samples is in the fraction of clinoptilolite mineral, 50-55 % in NZ and >80% in ZeoSand
®, suggesting that clinoptilolite content in the zeolitized tuff does not influence the immobilization of bacteria, already shown for other NZ samples [
10]. On NZ a great rate of immobilization was reported for bacteria such as
A. junii (1.27 × 10
10 CFU g
−1),
Escherichia coli (4.53 × 10
8 CFU g
−1) and
Enterococcus faecalis (1.3 × 10
9 CFU g
−1) [
9]. Immobilization of microorganisms on NZ has proven to be beneficial for biogas production. In the anaerobic digestion of grass silage, bacteria immobilized onto NZ enhanced the enzymatic activity of xylanase, which is responsible for the degradation of xylan, a cell wall component in grass biomass [
13].
Corncob is a material mostly made of lignin, cellulose, and hemicellulose. It has a high bulk density and provides a large amount of biodegradable carbon to immobilized bacteria [
14]. Moreover, SEM visualization revealed a perforated structure with outgrowths that increased surface area available for attachment of bacteria. Corncob was shown to be suitable carrier for ureolytic bacteria due to its wrinkled and porous structure that allows bacteria to immobilize and survive [
14]. However, it was confirmed that corncob is not an appropriate carrier for plant growth-promoting rhizobacteria due to high carbon content and poor adherence [
15]. Furthermore, corncob was not ideal for enriching anammox bacteria, but it could be used in research on denitrifying bacteria [
16].
In the view of this research, the isolated microbial consortium should not be considered biogas producers but biomass degraders. In the complex anaerobic digestion system, methane production is done by methanogenic archaea, which were not a part of the described consortium. This consortium's role was to help biodegrade complex organic biomolecules contained in MxG biomass, such as lignocellulose and cellulose, and to provide end-products that methanogens can metabolize. In this manner, it is very well known that microbial consortia, compared to single-species, are more advantageous for the degradation of complex compounds [
17]. Microbial consortia can be either isolated or constructed. For construction, a bottom-up approach is to determine the genomes of individual species of a potential consortium and reconstruct metabolic networks and microbial interactions. This should allow the design of microbiomes with specific properties, such as distributed pathways, modular species interactions, and resilience, that would be optimized for specific ecosystem function and stability [
17]. For actual bioaugmentation on an industrial scale, the approach must be as simple as possible and ready to be performed in a basic microbiological laboratory. That was the focus of the approach described here – to isolate the targeted consortia that can be adjusted to biodegrade various types of biomass, but in a simple and reproducible way, by focusing only on the bacteria that can be cultivated with ease, repeatedly for each new experiment. Using this parameter, we ended up with a consortium of five bacterial species that gave reproducible results and were identified with high certainty.
Detected species in the microbial consortium were
E. cloacae,
K. pneumoniae,
E. asburiae,
L. adecarboxylata and
E. indicum. These bacterial species are catalase-positive and oxidase-negative, meaning they are facultative anaerobic microbes [
18,
19]. Facultative anaerobic bacteria should be completely metabolically functional in the anaerobic digesters, and in the same time large biomass can be easily grown in aerobic conditions, without the use of specific anaerocultivators. The individual bacterial species from the bacterial suspension did not exhibit the same affinity for a specific carrier, whereas
E. cloacae demonstrated an ability to be immobilized on each tested carrier in huge percent (72-88%). The difference probably arises from either carrier affinity for
E. cloacae or the best adaptation of this species to competition with other species in tested conditions. In a study by Dermayati et al. [
20] a microbial consortium consisting of
Enterobacter cloacae,
Bacillus sp., and
Bacillus licheniformis was immobilized on perlite, zeolite, silica and vermiculite. Consortium was successfully immobilized on carriers, including
E. cloacae, although with lower cell loading capacity on zeolite (5.10 x 10
6 CFU g
-1) and perlite (5.37 x 10
6 CFU g
-1). The same microbial consortium immobilized on perlite improved the degradation of crude oil for 25% as compared to free-living cells [
21].
The most significant proportion for L. adecarboxylata was on perlite (23%), which is a higher result compared to the proportion in consortium suspension (13%). This indicates that perlite could be an appropriate carrier for L. adecarboxylata, but information regarding the immobilization on carriers or its potential implication in bioremediation is still lacking.
The
K. pneumoniae and
E. asburiae could be used in form of immobilized or free-living cells in the process of bioaugmentation. It was reported for
E. asburiae to be a promising approach for bioremediation of organochlorine pesticides in surface water [
22].
K. pneumoniae could be used for pre-treatment of food waste prior to anaerobic co-digestion with straw to decrease lipid content and enhance methane production [
23].
Although the proportion of E. indicum in bacterial suspension was 14%, after immobilization of consortium on each carrier, it was not detected. However, it was successfully immobilized as a pure culture on carriers, indicating that it was overgrown during the competition with other bacterial species. Furthermore, this was confirmed by the share of E. indicum in the control group being less than 1%.
One thing to consider in biotechnology is that it’s important not to use potentially pathogenic bacteria due to the risk of them leaking into the environment. Although,
K. pneumoniae was characterized as susceptible to imipenem, it is well known as a member of the 'ESCAPE' pathogens, a group of clinically significant and multi-drug-resistant bacteria [
24]. Instead of using potentially pathogenic bacteria, the microbial consortium can be adjusted to avoid risking public health. For further research,
K. pneumoniae could be removed or replaced with other bacterial species.
5. Conclusions
This research unequivocally demonstrated that the microbial consortium, consisting of several species intended for bioaugmentation of anaerobic digestion process, could successfully be immobilized onto natural biocarriers. All the tested materials showed similar capacity of bacterial immobilization and can be considered promising for reinforcing of biogas production. The experimental setup was designed to be used in real-life systems for anaerobic digestion, using easily cultivable species.
In this manner, further steps should be to identify the potential of each individual species for biomass degradation and maybe use principles of systemic biology to construct an optimal bacterial consortium immobilized onto various biocarriers.
Presumably, this technology could significantly increase the biogas yield by optimizing the degradation of complex organic compounds within the biomass, thereby enhancing the efficiency and rate of biogas production.