1. Introduction
Testicular cancer is a tumor arising from the pathological, uncontrolled growth of cells within one or both testicles. Testicular cancer is one of the most common malignant tumors in men. In Poland, the incidence of testicular cancer has increased more than three times over the last three decades. Every year, more than 1,000 new cases are detected (in 2017, 1,156 cases were diagnosed), unfortunately, about 100 patients die annually from testicular cancer. About one third of all germ cell tumors are seminomas, which affect young men aged 15 to 45 and are the most common malignancies in men in this age group, rapidly metastasizing to the retroperitoneal lymph nodes and then to internal organs, including the liver, lungs, brain and bones [
1]. Today we know that seminomas are solid germ cell tumours, and the main research efforts are aimed at the highest effectiveness of treatment based on the latest achievements in pathomorphology and molecular biology. It is not known exactly why seminomas develop. So far, several risk factors for this dangerous cancer have been identified. Disorders of gonocyte maturation and the related genome instability are one of the probable mechanisms of pathogenesis of seminomas [
2,
3,
4]. The CacyBP/SIP protein plays a very important role in many cellular processes, including proliferation, apoptosis, differentiation, as well as tumorigenesis and transcription regulation. It was first discovered in Ehrlich’s ascites tumor cells as a calcyclin-binding or Siah-1-binding protein. Distribution studies to date have shown the presence of this protein in various tissues and organs. Further studies showed variable expression of CacyBP/SIP in different tumor types [
5,
6,
7,
8]. Zhai and colleagues showed an increase in CacyBP/SIP expression in most tumor tissues of various organs, in contrast to the distribution of the protein in healthy tissues, where it was weakly expressed or barely detectable. In addition, the authors showed a large variation in the expression of CacyBP/SIP in tumors of various organs [
9]. The results of immunohistochemical studies showed that the main subcellular location of CacyBP/SIP is the cytoplasm, but it turns out that under certain conditions the protein is translocated to the cell nucleus Filipek et al. (2002) and Wu et al. (2003), [
10,
11,
12]. There are reports of the inhibitory effect of the CacyBP/SIP protein on cell apoptosis, which is closely related to its translocation to the nucleus. It has been shown that CacyBP/SIP can both promote apoptosis and inhibit cell death processes. Probably it may be related to the function of the examined organ. Careful analysis showed that CacyBP/SIP has a similar primary structure to some of the MAP family kinases (mitogen activated protein kinase). Two kinase interaction motifs responsible for reactions with these enzymes have also been identified in the sequence of this protein [
13,
14,
15]. The interaction of CacyBP/SIP with extracellular signal-regulated kinases ERK1/2 belonging to the MAP family causes their dephosphorylation and changes the expression of proliferation-related genes [
16,
17]. Recent studies show that the ligand of CacyBP/SIP may also be p38 kinase belonging to the MAP family, the dephosphorylation of which may affect signaling pathways related to cell survival and death. These data suggest that the CacyBP/SIP protein may play an important role in tumorigenesis by regulating the activity of ERK1/2 and p38 kinases [
18,
19]. Only a few literature reports present the results of the CacyBP/SIP protein as a phosphatase in cancers of a few organs. This function of CacyBP/SIP in testicular seminoma has not been studied so far. Our study was aimed at the immunohistochemical evaluation of the CacyBP/SIP protein in testicular seminomas and its interaction with ERK1/2 and p38 kinase [
20,
21,
22,
23]. In this work, we examined the expression of CacyBP/SIP in testicular tissues and analyzed the relationship between CacyBP/SIP and the expression of ERK1/2 and p38 kinases.
2. Materials and Methods
All research was performed in accordance with relevant guidelines/regulations. Research involving human research participants have been performed in accordance with the Declaration of Helsinki.
2.1. Sample Collection
After obtaining a positive opinion of the Independent Bioethics Committee for Scientific Research of the Medical University of Bialystok (Resolution number: APK. 002.360.2022), research was carried out using archival tissue material from 30 patients undergoing surgery due to testicular seminoma (cancer) in the years 2014-2023 in the Department of Urology of University Hospital in Bialystok.
The mean age of the patients at the time of operation was 37 years, with a range 23-63 years. All patients were classified into one group according to diagnosis and TNM classification (Tumour, Node, Metastasis). The material was taken directly from the tumour during surgery.
Immediately after collection, tissue fragments were fixed in 10% buffered formalin and routinely embedded in paraffin blocks. The material stored in the RNAlater solution was subjected to real-time PCR to evaluate the expression of the genes: Cacybp, Mapk3, Mapk1 and Mapk14 encoding CacyBP/SIP, ERK1/2 and p38. In order to detect CacyBP/SIP, ERK1/2 and p38 proteins, immunohistochemical reactions were performed using antibodies directed against the tested proteins. The slides were evaluated using an Olympus BX43 optical microscope with a built-in digital camera and connected to a computer. Color microscopic images of CacyBP/SIP, ERK1/2 and p38 at 200x magnification (at least 5 fields from each patient’s slide) were archived and saved in jpg format on a computer hard drive.
2.2. Immunohistochemistry
The immunohistochemical staining procedure was performed according to the following protocol [34]. The material was fixed in 10% buffered formalin and transferred to paraffin blocks in a routine manner. 4 μm thick sections were attached to adhesive slides, deparaffinized and rehydrated in ethanol of decreasing concentration. The sections of testis tissue were subjected to pretreatment in a pressure chamber and heated using Target Retrieval Solution Citrate pH=6.0 (Agilent Technologies, Inc. Santa Clara, CA, USA). After cooling down to room temperature, the sections were incubated with Dako REAL Peroxidase-Blocking Solution (Agilent Technologies, Inc. Santa Clara, CA, USA). Antibodies used: Rabbit polyclonal anti-CacyBP/SIP antibody (1:600) (Cat. No. ab190950; Abcam, Discovery Drive, Cambridge Biomedical Campus, Cambridge, CB2 0AX, UK), Rabbit polyclonal anti-p-ERK1/2 antibody (1:50) (Cat# 44-680G; Invitrogen; Thermo Fisher Scientific 168 Third Avenue Waltham, MA USA 02451) and Rabbit anti-p-p38 polyclonal antibody (1:50) (Cat# 44-684G; Invitrogen; Thermo Fisher Scientific 168 Third Avenue Waltham, MA USA 02451).The sections with the primary antibodies were incubated 24 hours at +4ºC in a humidified chamber. Procedure was followed by incubation with secondary antibody (REAL™ EnVision™ Detection System, Peroxidase/DAB, Rabbit/Mouse detection kit (K5007; Agilent Technologies Denmark Ap/S, Produktionsvej 42, 2600 Glostrup, Denmark). The bound antibodies were visualized by incubation with DAB Flex chromogen. Finally the testis sections were counterstained in hematoxylin QS (H-3404 Vector Laboratories, Burlingame, CA, USA) and observed under a light microscope. The seminoma sections were dehydrated and the specificity of the antibodies was confirmed using a negative control, where the antibodies were replaced by Antibody Diluent (S3022; Agilent Technologies Denmark Ap/S, Produktionsvej 42, 2600 Glostrup, Denmark). The staining results were evaluated in an Olympus BX43 microscope with an Olympus DP12 camera.
2.3. Real-Time PCR
Samples of testicular cancer and non-malignant testicular tissue taken from the material after operation were placed in an RNA-later solution. Total RNA was isolated using NucleoSpin® RNA Isolation Kit (Machery-Nagel). Quantification and quality control of total RNA was determined using a spectrophotometer – NanoDrop 2000 (ThermoScientific). An aliquot of 1 µg of total RNA was reverse transcribed into cDNA using iScript™ Advanced cDNA Synthesis Kit for RT-qPCR (BIO-RAD). Synthesis of cDNA was performed in a final volume of 20 μl using a Thermal Cycler (Model SureCycler 8800, Aligent Technologies). For reverse transcription, the mixtures were incubated at 46°C for 20 min, then heated to 95°C for 1 min and finally cooled quickly at 4°C. Quantitative real-time PCR reactions were performed using Stratagene Mx3005P (Aligent Technologies) with the SsoAdvanced™ Universal SYBER® Green Supermix (BIO-RAD). Specific primers for CacyBP/SIP (CACYBP), ERK1/2 (MAPK3, MAPK1), p38 (MAPK14) and GAPDH (GAPDH) were designed by BIO-RAD Company. The housekeeping gene GAPDH (GAPDH) was used as a reference gene for quantification. To determine the amounts of levels of test genes expression, standard curves were constructed for each gene separately with serially diluted PCR products. PCR products were obtained by cDNA amplification using specific primers as follows: CACYBP (qHsaCED0043669, BIO-RAD), MAPK3 (qHsaCID0010939, BIO-RAD), MAPK1 (qHsaCED0042738, BIO-RAD), MAPK14 (qHsaCED0043417, BIO-RAD) and GAPDH (qHsaCED0038674, BIO-RAD). QRT-PCR was carried out in a doublet in a final volume of 20 μl under the following conditions: 2 min polymerase activation at 95°C, 5 s denaturation at 95°C, 30 s annealing at 60°C for 35 cycles. PCR reactions were checked, including no-RT-controls, omitting of templates, and melting curve to ensure only one product was amplified. The relative quantification of gene expression was determined by comparing Ct values using the ΔΔCt method. All results were normalized to GAPDH.
2.4. Statistical Analysis
All data were analyzed for statistical significance using the Statistica version 13.3 computer software package. The mean values were computed automatically; significant differences were determined by one-way ANOVA test, p < 0.05 was considered significant.
3. Results
A total of 30 samples were considered for the study. The age range of the patients was 23-63 years, the average age was 37 years. A positive result of the immunohistochemical reaction in the form of a brown stain indicated the presence of the tested antigen.
3.1. Immunohistochemical Evaluation
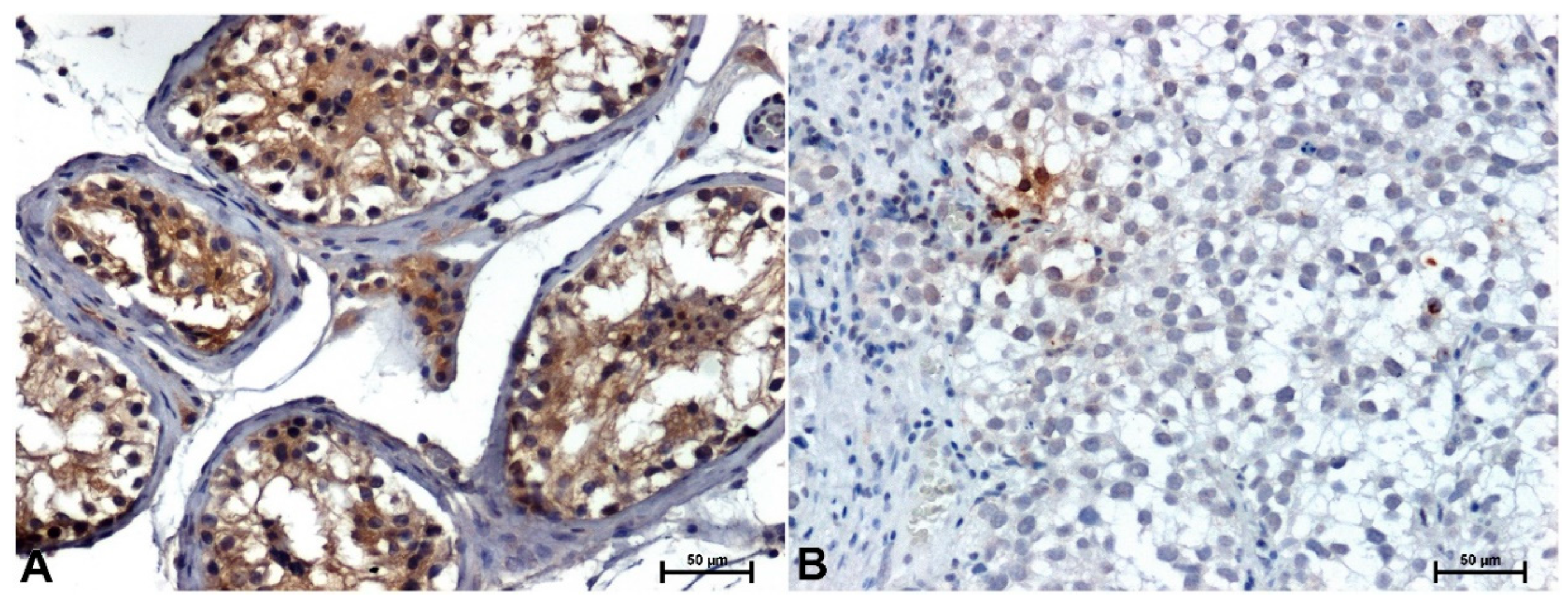
Strong CACYBP/SIP immunoreactivity was found in non-cancerous testicular tissue, mainly in the cytoplasm of the testicular tubule epithelial cells (
Figure 1A). CACYBP/SIP immunoreactivity in all seminomas was very low or negative. Nuclear or weak cytoplasmic localization of CACYBP/SIP was observed only in single tumor cells (
Figure 1B).
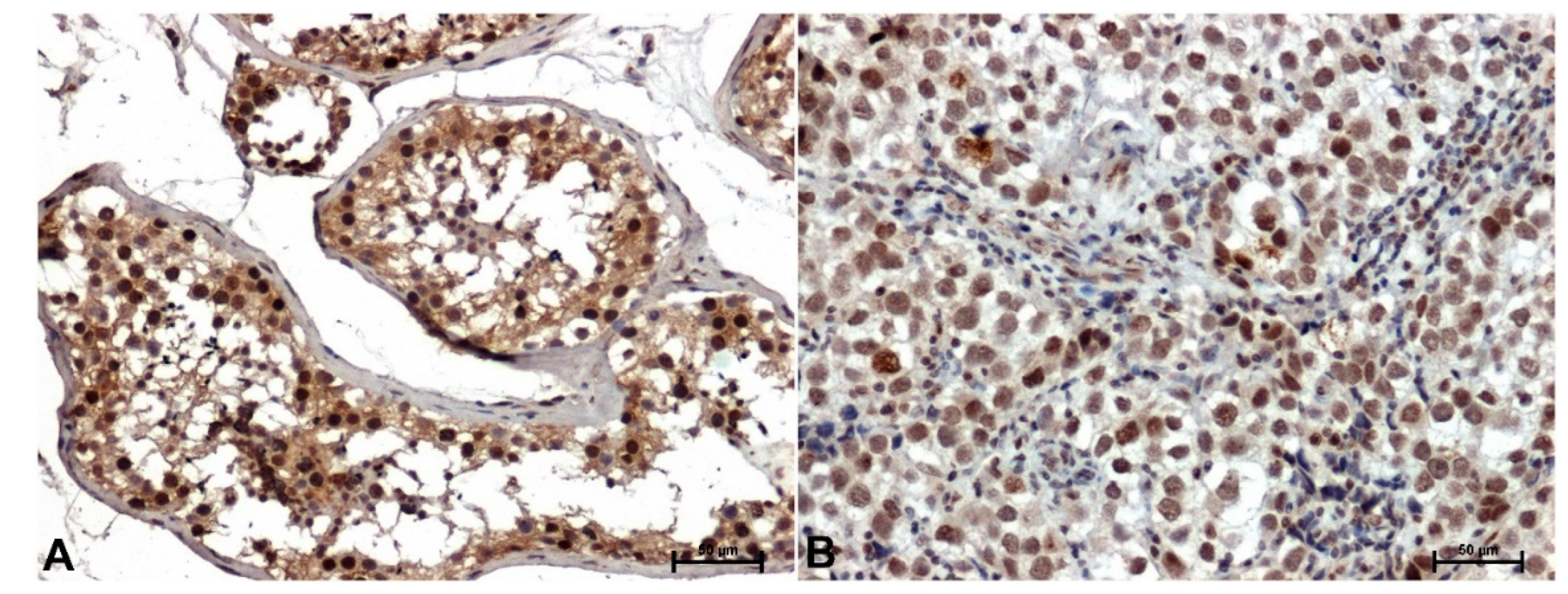
Immunohistochemical analysis showed moderate ERK 1/2 reactivity in both cytoplasm and nuclei of seminiferous tubular epithelial cells in non-neoplastic testicular tissues (
Figure 2A), whereas in seminomas only cell nuclei were reactive (
Figure 2B).
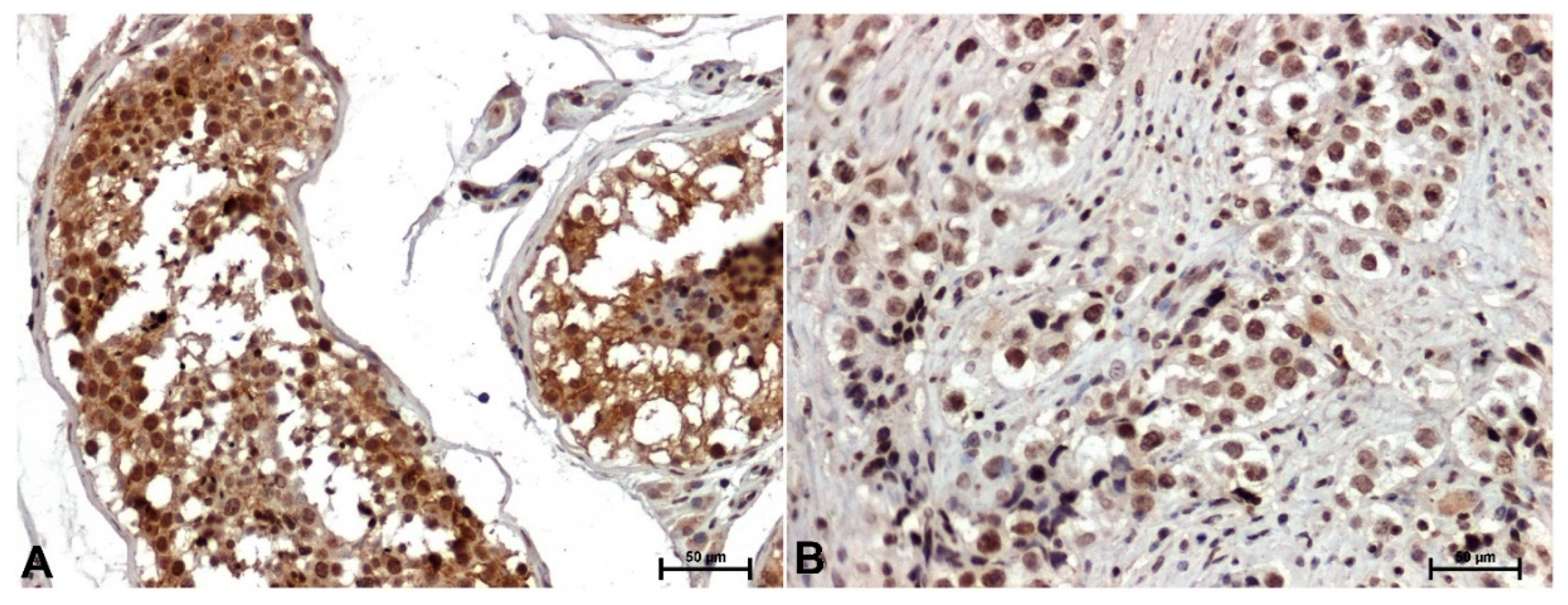
The immunoreactivity of p38 in seminomas was significantly attenuated compared to non-cancerous tissues (
Figure 3). In adjacent normal tissue, p38 staining was moderate to strong in both the nuclei and cytoplasm of seminiferous tubule cells (
Figure 3A). Significantly attenuated p38 immunoreaction with nuclear localization was observed in seminoma cells (
Figure 3B).
3.2. Real-Time PCR
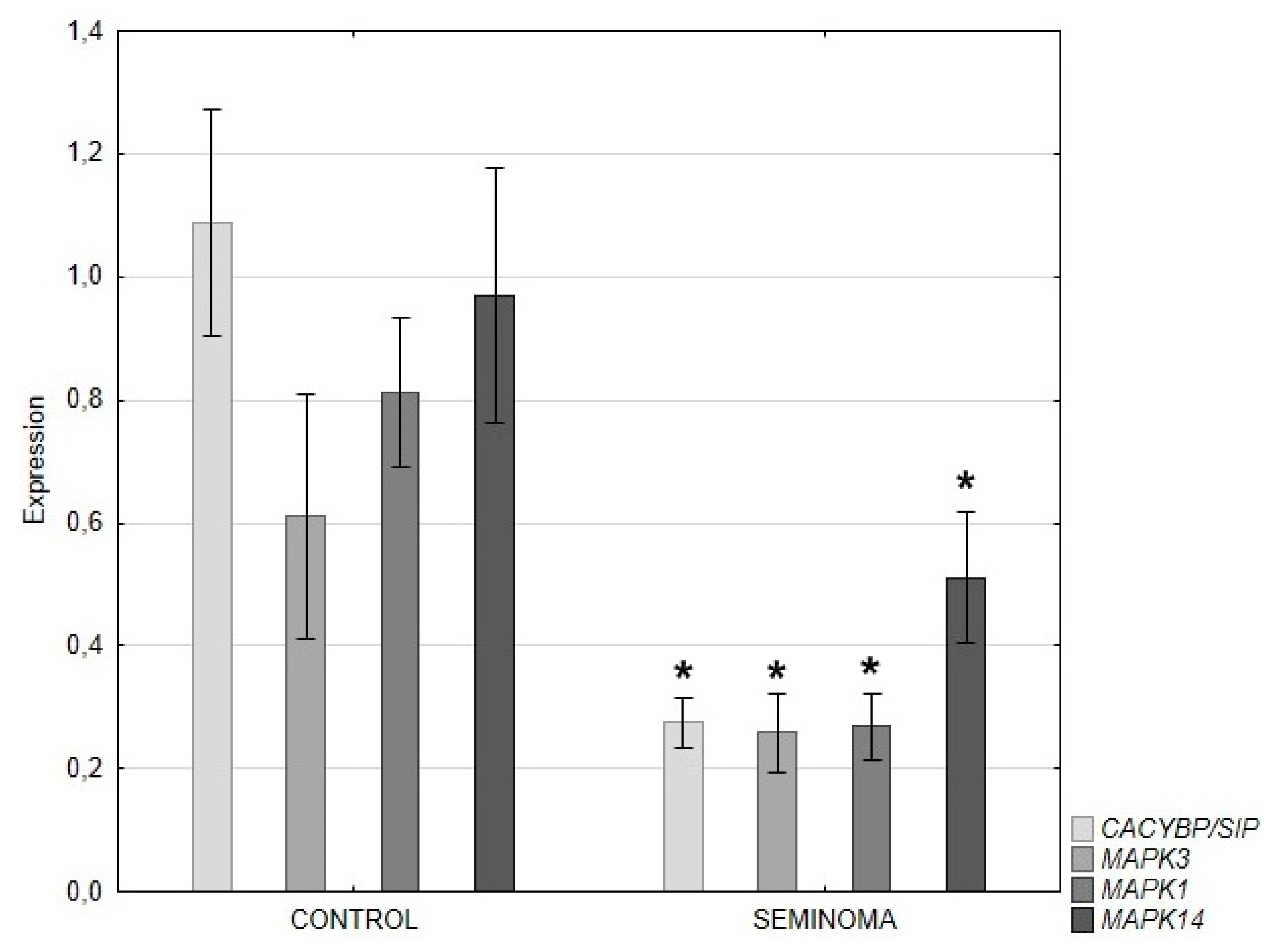
QRT-PCR analysis revealed a significant decrease in the expression of the CACYBP/SIP, ERK 1/2 and p38 genes in seminoma compared to control tissue (
Figure 4).
4. Discussion
According to the latest epidemiological data, testicular cancer accounts for 1.6% of all cancers in males, i.e. for 3-10 cases per 100,000 men/year, and unfortunately, the number of cases is increasing every year. The predominant type of cancer is germ cell carcinoma, including seminoma and non-seminoma. Treatment of testicular cancer starts with its removal. Then based on the results of histopathology tests, adjuvant treatment - chemotherapy may be necessary. There are no known factors that should be avoided to minimize the risk of testicular carcinoma. To improve the effectiveness of the prevention and treatment of this malignant tumor, we need to expand our knowledge about the mechanisms of formation and biology of testicular cancer. Considering the important role of CACYBP/SIP, ERK 1/2 and p38 in carcinogenesis, a study evaluating and comparing the expression of these proteins in testicular seminoma in comparison with healthy tissue seems to be justified. In this paper, we present for the first time the results of studies showing a significant decrease in the expression of the CacyBP/SIP protein in testicular seminoma and the potential function of this protein in relation to ERK1/2 and p38 kinas. Very weak but visible CACYBP/SIP immunoreactivity was found in tumor tissue primarily in the nuclear localization. Similarly, in the seminoma, attenuated expression of the studied kinases, especially p38, was found. Many published reports indicate the participation of CacyBP/SIP in various signalling pathways involved in the development of cancer. Previous studies have shown that CacyBP/SIP participates in processes related to ubiquitination, protein dephosphorylation, regulation of the cytoskeleton and gene expression, as well as cell proliferation and differentiation [8,9]. Data obtained from studies of many cancers indicate differential expression of the CACYBP/SIP protein in pathological processes related to oncogenesis [9–11]. There are reports regarding the CACYBP/SIP function, e.g. in cancer of colon, stomach, breast and pancreatic. Research results by Zhai et al. suggest that CACYBP/SIP may promote the growth of cancer cells in the course of colorectal cancer [6]. An increase in CACYBP/SIP protein expression with breast cancer progression was shown by Wang et al. [5]. Another study also showed that CACYBP/SIP is involved in promoting gastric cancer proliferation and cell cycle progression [7]. Other studies of renal cell carcinoma, gastric cancer, and chronic lymphocytic leukemia found decreased expression of CacyBP/SIP. In these cancers, the protein acted as a tumor suppressor [8–12]. The results of the cited studies confirm that CacyBP/SIP cannot be treated solely as an oncogene or tumor suppressor. In the available scientific literature, we have not found a single report referring to the role of the CACBP/SIP protein in testicular malignant tumor – seminoma. The extracellular signal-regulated kinase ERK1/2 has been analyzed in neoplastic processes, including the colon and breast cancer. Li et al. in their work obtained results proving that the promotion of cancer cell migration in breast cancer and local invasion takes place with a significant participation of ERK [24]. Similarly, in reports on colorectal cancer, results were obtained proving that carcinogenesis processes in this type of cancer occur with the participation of signalling pathways related to ERK1/2 [25–27]. The available literature lacks reports on the analysis of ERK1/2 in testicular cancer – seminoma.Previous studies have shown the role of p38 kinase as both a promoter and a suppressor of carciogenic processes. The involvement of p38 kinase in the inhibition of metastasis and tumor growth has been confirmed in prostate, thyroid and lung cancer [28–30]. Studies by Leelahavanichkul et al. showed hyperactivity of the p38 protein in 79% of cases in head and neck cancers [31]. As in the case of CACYBP/SIP and ERK1/2, p38 kinase has not been studied in seminomas so far. Not a single report related to this topic was found. The results of studies by other authors described above, relating to the proteins we analyzed, cancers other than testicular cancer. The lack of literature reports is the basic justification for conducting the research. Our work appears to be the first to evaluate the CacyBP/SIP protein and selected MAP kinases in this type of cancer. Due to the inability to relate our results to other authors, the comparison can only refer to similar determinations in other cancers. In our study, we demonstrated significant attenuation of CACYBP/SIP immunoreactivity in seminoma compared to non-cancerous tissue. The intensity of the reaction was confirmed by the expression of the gene encoding this protein. Tumors tested so far expression of CACYBP/SIP usually showed increased expression of this protein. Examples include pancreatic, gastric, colorectal and melanoma cancers [11,13]. Often, however, the increase in expression depended on the stage of the tumor. On the other hand, results regarding the role of CACYBP/SIP in breast or kidney cancer remain inconsistent [5,32–34]. Due to the different behaviour of CACYBP/SIP and the discrepancy of results obtained in different types of cancer, at the present stage of research it is difficult to precisely determine the exact role of this protein in testicular seminoma. Significant differences in the expression of CACYBP/SIP may indicate the participation of this protein in maintaining cellular homeostasis of testicular seminoma. Due to the invaluable role of ERK1/2 in transduction of intracellular signals, as well as the growing number of papers on its involvement in cancer processes, our analysis of this kinase in seminoma provides further, hitherto unknown evidence for its important role in this cancer as well. Weakened immunoreactivity and low expression of genes encoding ERK1/2 in cancer cells most likely means that seminoma cells inhibit the action of this kinase. On the other hand, the localization of ERK1/2, mainly in the nuclei of seminoma cells, may indicate the participation of this protein in processes related to protein transcription and coding. Kinase p38, involved in many processes accompanying neoplastic growth, is additionally a very interesting point to analysis in seminoma. As in the case of ERK1/2, p38 immunoreactivity mainly with nuclear localization was significantly reduced in seminoma cells, which was also reflected in the expression of genes encoding these proteins.
A recently discovered role of the CACYBP/SIP protein is its function as a phosphatase for MAP kinases. The unique structure of the CACYBP/SIP protein, as well as the not fully known characteristics of its substrates, currently do not allow to classify it to a specific family of phosphatases. It is generally believed that increased expression of CACYBP/SIP promotes decreased expression of the phosphorylated form of ERK1/2. In studies on colorectal cancer, the authors obtained results contrary to these assumptions [13,14]. Despite this discrepancy, it was also considered that this may be due to the phosphorylation state of the CACYBP/SIP protein itself in the cells studied. Considering the role of CACYBP/SIP as a phosphatase for ERK 1/2 and p38 kinases, our results should be analyzed both in terms of comparison of the control group with tumor tissue and the results in both groups separately. In the control material, the expression of ERK1/2 is significantly lower than CACYBP/SIP, which may indicate its phosphatase function. In neoplastic tissue, the expression of CACYBP/SIP and ERK1/2 remains at a similar level, which may indicate the inhibition of both proteins by seminoma cells or insufficient expression of CACYBP/SIP for phosphatase activity. On the other hand, p38 kinase expression is not significantly lower than CACYBP/SIP in control material and significantly higher in seminoma.
5. Conclusions
In conclusion, our study showed significant differences in the immunoreactivity and expression of CACYBP/SIP, ERK 1/2 and p38 genes in testicular seminoma compared to control material. Our findings suggest the involvement of the CacyBP/SIP protein in the ERK1/2 and p38 signalling pathways, which may be involved in the processes of testicular seminoma carcinogenesis. Literature data and the results of our own previous studies suggest that the expression of CacyBP/SIP depends on the type and degree of histological malignancy of the cancer. In addition, this study provides new, previously unpublished data on CacyBP/SIP activity in seminoma. More research is needed as understanding what determines the function of the CacyBP/SIP protein and its relationship to MAPK may be of great importance in the future treatment of testicular cancer.
Author Contributions
Conceptualization, I.K., G.M.; Methodology, A.L., N.D., I.K.; Validation, N.D., A.L., A.N.; Formal analysis, A.L., A.N.; Investigation, G.M., N.D., I.K.; Resources, G.M., N.D., A.L., A.N., I.K.; Data curation, N.D., I.K.; Writing – original draft, G.M.; Writing – review & editing, I.K.; Visualization, A.L., A.N.; Supervision, I.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Science Centre (NCN) 2022/06/X/NZ5/00763.
Institutional Review Board Statement
The study was approved by the Bioethics Committee, of the Medical University of Bialystok. The code for our study is R-I-002/282/2019.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Due to patient privacy, no Data Availability Statement was provided for this article. All data generated or analysed during this study are included in this published article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Park, J.S.; Kim, J.; Elghiaty, A.; Ham, W.S. Recent global trends in testicular cancer incidence and mortality. Medicine (Baltimore) 2018, 97, e12390. [Google Scholar] [CrossRef] [PubMed]
- Looijenga, L.H.J.; Van der Kwast, T.H.; Grignon, D.; Egevad, L.; Kristiansen, G.; Kao, C.S.; Idrees, M.T. Report From the International Society of Urological Pathology (ISUP) Consultation Conference on Molecular Pathology of Urogenital Cancers: IV: Current and Future Utilization of Molecular-Genetic Tests for Testicular Germ Cell Tumors. Am. J. Surg. Pathol. 2020, 44, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Moch, H.; Amin, M.B.; Berney, D.M.; Compérat, E.M.; Gill, A.J.; Hartmann, A.; Menon, S.; Raspollini, M.R.; Rubin, M.A.; Srigley, J.R.; Hoon Tan, P.; Tickoo, S.K.; Tsuzuki, T.; Turajlic, S.; Cree, I.; Netto, G.J. The 2022 World Health Organization Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur. Urol. 2022, 82, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.D.; Gupta, M.; Cheaib, J.G.; Sharma, R.; Zhang, A.; Bass, E.B.; Pierorazio, PM. Testis-sparing surgery and scrotal violation for testicular masses suspicious for malignancy: A systematic review and meta-analysis. Urol. Oncol. 2020, 38, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Ma, Q.; Wang, Y.; Ma, G.; Zhai, H. CacyBP/SIP expression is involved in the clinical progression of breast cancer. World J. Surg. 2010, 34, 2545–2552. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H. ; Shi. Y.; Chen, X.; Wang, J.; Lu, Y.; Zhang, F.; Liu, Z.; Lei, T.; Fan, D. CacyBP/SIP promotes the proliferation of colon cancer cells. PLoS One 2017, 12, e0169959. [Google Scholar] [CrossRef]
- Zhai, H.; Meng, J.; Jin, H.; Li, Y.; Wang, J. Role of the CacyBP/SIP protein in gastric cancer. Oncol. Lett. 2015, 9, 2031–2035. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhang, R.Z.; Qi, D.W.; Chen, H.Y.; Zhang, G.C. CacyBP/SIP promotes tumor progression by regulating apoptosis and arresting the cell cycle in osteosarcoma. Exp. Ther. Med. 2020, 20, 1397–1404. [Google Scholar] [CrossRef]
- Zhai, H.; Shi, Y.; Jin, H.; Li, Y.; Lu, Y.; Chen, X.; Wang, J.; Ding, L.; Wang, X.; Fan, D. Expression of calcyclin-binding protein/Siah-1 interacting protein in normal and malignant human tissues: an immunohistochemical survey. J. Histochem. Cytochem. 2008, 56, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Filipek, A.; Jastrzebska, B.; Nowotny, M.; Kwiatkowska, K.; Hetman, M.; Surmacz, L.; Wyroba, E.; Kuznicki, J. Ca2+-dependent translocation of the calcyclin-binding protein in neurons and neuroblastoma NB-2a cells. J. Biol. Chem. 2002, 277, 21103–21109. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Tan, X.; Peng, X.; Yuan, J.; Qiang, B. Translocation and phosphorylation of calcyclin binding protein during retinoic acid-induced neuronal differentiation of neuroblastoma SH-SY5Y cells. J. Biochem. Mol. Biol. 2003, 36, 354–358. [Google Scholar] [CrossRef]
- Topolska-Woś, A.M.; Chazin, W.J.; Filipek, A. CacyBP/SIP--Structure and variety of functions. Biochim. Biophys. Acta. 2016, 1860, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, B.; Liu, Y.; Yu, X.; Cheng, G. Dual effects of active ERK in cancer: A potential target for enhancing radiosensitivity. Oncol. Lett. 2020, 20, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Kidger, A.M.; Sipthorp, J.; Cook, S.J. ERK1/2 inhibitors: New weapons to inhibit the RAS-regulated RAF-MEK1/2-ERK1/2 pathway. Pharmacol. Ther. 2018, 187, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Mebratu, Y.; Tesfaigzi, Y. How ERK1/2 activation controls cell proliferation and cell death: Is subcellular localization the answer? Cell Cycle 2009, 8, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, W.Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. Res. 2015, 35, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Wainstein, E.; Seger, R. The dynamic subcellular localization of ERK: mechanisms of translocation and role in various organelles. Curr. Opin. Cell Biol. 2016, 39, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Maik-Rachline, G.; Hacohen-Lev-Ran, A.; Seger, R. Nuclear ERK: Mechanism of Translocation, Substrates, and Role in Cancer. Int. J. Mol. Sci. 2019, 20, 1194. [Google Scholar] [CrossRef] [PubMed]
- Topolska-Woś, A.M.; Rosińska, S.; Filipek, A. MAP kinase p38 is a novel target of CacyBP/SIP phosphatase. Amino Acids 2017, 49, 1069–1076. [Google Scholar] [CrossRef]
- Martínez-Limón, A.; Joaquin, M.; Caballero, M.; Posas, F.; de Nadal, E. The p38 Pathway: From Biology to Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 1913. [Google Scholar] [CrossRef] [PubMed]
- Cargnello, M.; Roux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-Activated protein kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A.; Nebreda, A.R. Mechanisms and functions of p38 MAPK signalling. Biochem. J. 2010, 429, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Loba, A.; Manieri, E.; González-Terán, B.; Mora, A.; Leiva-Vega, L.; Santamans, A.M.; Romero-Becerra, R.; Rodríguez, E.; Pintor-Chocano, A.; Feixas, F.; et al. p38gamma is essential for cell cycle progression and liver tumorigenesis. Nature 2019, 568, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guo, Y.; Duan, L.; Hu, X.; Zhang, X.; Hu, J.; Huang, L.; He, R.; Hu, Z.; Luo, W.; et al. AKR1B10 promotes breast cancer cell migration and invasion via activation of ERK signalling. Oncotarget 2017, 8, 33694–33703. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Li, M.; Chen, W. FOXD1 predicts prognosis of colorectal cancer patients and promotes colorectal cancer progression via the ERK 1/2 pathway. Am. J. Transl. Res. 2018, 10, 1522–1530 PMID: 29887965; PMCID: PMC5992558. [Google Scholar] [PubMed] [PubMed Central]
- Urosevic, J.; Blasco, M.T.; Llorente, A.; Bellmunt, A.; Berenguer-Llergo, A.; Guiu, M.; Cañellas, A.; Fernandez, E.; Burkov, I.; Clapés, M.; Cartanà, M.; et al. ERK1/2 Signaling Induces Upregulation of ANGPT2 and CXCR4 to Mediate Liver Metastasis in Colon Cancer. Cancer Res. 2020, 80, 4668–4680. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Yang, J.; Song, P. Correlation of ERK/MAPK signaling pathway with proliferation and apoptosis of colon cancer cells. Oncol. Lett. 2019; 17, 2266–2270. [CrossRef]
- Yu-Lee, L.Y.; Yu, G.; Lee, Y.C.; Lin, S.C.; Pan, J.; Pan, T.; Yu, K.J.; Liu, B.; Creighton, C.J.; Rodriguez-Canales, J.; et al. Osteoblast-Secreted Factors Mediate Dormancy of Metastatic Prostate Cancer in the Bone via Activation of the TGFβRIII-p38MAPK-pS249/T252RB Pathway. Cancer Res. 2018, 78, 2911–2924. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.G.; Xiong, C.F.; Lv, Y.X. Kin17 facilitates thyroid cancer cell proliferation, migration, and invasion by activating p38 MAPK signaling pathway. Mol. Cell. Biochem. 2021, 476, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, J.; Liu, X. TRPV4 induces apoptosis via p38 MAPK in human lung cancer cells. Braz. J. Med. Biol. Res. 2021, 54, e10867. [Google Scholar] [CrossRef]
- Leelahavanichkul, K.; Amornphimoltham, P.; Molinolo, A.A.; Basile, J.R.; Koontongkaew, S.; Gutkind, J.S. A role for p38 MAPK in head and neck cancer cell growth and tumor-induced angiogenesis and lymphangiogenesis. Mol. Oncol. 2014, 8, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Ning, X.; Liu, J.; Liu, L.; Chen, Y.; Han, S.; Zhang, Y.; Liang, J.; Wu, K.; Fan, D. Overexpressed CacyBP/SIP leads to the suppression of growth in renal cell carcinoma. Biochem. Biophys. Res. Commun. 2007, 356, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, Ż.; Niezgoda, M.; Młynarczyk, G.; Acewicz, M.; Kasacka, I. Comparative Assessment of the WNT/β-Catenin Pathway, CacyBP/SIP, and the Immunoproteasome Subunit LMP7 in Various Histological Types of Renal Cell Carcinoma. Front. Oncol. 2020, 10, 566637. [Google Scholar] [CrossRef] [PubMed]
- Smereczańska, M.; Domian, N.; Młynarczyk, G.; Kasacka, I. The Effect of CacyBP/SIP on the Phosphorylation of ERK1/2 and p38 Kinases in Clear Cell Renal Cell Carcinoma. Int. J. Mol. Sci. 2023, 24, 10362. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).